Abstract
As a vital antioxidant molecule, H2S can make an important contribution to regulating blood vessels and inhibiting apoptosis when present at an appropriate concentration. Higher levels of H2S can interfere with the physiological responses of the respiratory system and central nervous system carried out by mammalian cells. This is associated with many illnesses, such as diabetes, mental decline, cardiovascular diseases, and cancer. Therefore, the accurate measurement of H2S in organisms and the environment is of great significance for in-depth studies of the pathogenesis of related diseases. In this contribution, a new coumarin-carbazole-based fluorescent probe, COZ-DNBS, showing a rapid response and large Stokes shift was rationally devised and applied to effectively sense H2S in vivo and in vitro. Upon using the probe COZ-DNBS, the established fluorescent platform could detect H2S with excellent selectivity, showing 62-fold fluorescence enhancement, a fast-response time (<1 min), high sensitivity (38.6 nM), a large Stokes shift (173 nm), and bright-yellow emission. Importantly, the probe COZ-DNBS works well for monitoring levels of H2S in realistic samples, living MCF-7 cells, and zebrafish, showing that COZ-DNBS is a promising signaling tool for H2S detection in biosystems.
The probe COZ-DNBS displayed excellent selectivity, a fast response, high sensitivity, a large Stokes shift, and bright-yellow emission in response to H2S.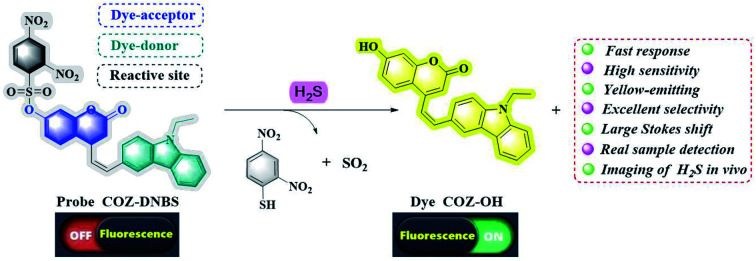
1. Introduction
In the process of maintaining biological homeostasis, reactive sulfur species (RSS), including hydrogen sulfide (H2S) and biological mercaptans (Cys, Hcy, and GSH), are essential thiol-containing molecules for resisting oxidative stress and maintaining the normal physiological function of organisms.1–3 Numerous research works have revealed that H2S is considered to be a third endogenous gaseous transmitter after nitric oxide (NO) and carbon monoxide (CO).4–6 As a vital antioxidant molecule, H2S can make an important contribution to the regulation of blood vessels and inhibition of apoptosis when present at an appropriate concentration.7–9 Reports in the literature have suggested that the level of intracellular H2S can be significantly upregulated during inflammatory events. Higher levels of H2S can interfere with the physiological responses of the respiratory system and central nervous system carried out by mammalian cells. This is associated with many illnesses, such as diabetes, mental decline, cardiovascular diseases, and cancer.10–13 Therefore, the accurate measurement of H2S in organisms and the environment is of great significance for in-depth studies on the pathogenesis of related diseases.
Fluorogenic assays utilizing small-molecule fluorescent sensors have attracted wide attention in pharmacology and the life sciences because of the obvious advantages of non-invasive detection, excellent selectivity, rapid response, and the lack of need for pretreatment.14–18 To date, numerous H2S-specific fluorescent probes have been established via exploiting various sensing mechanisms,19–24 such as the thiolysis of 2,4-dinitrobenzenesulfonamide and dinitrophenyl ether groups; the reduction of azide, hydroxylamine, and nitro groups; nucleophilic addition with C N+ groups; and the high affinity of S2− for Cu2+. Although big breakthroughs have been made, some problems still need to be overcome, such as slow responses, small Stokes shifts (<100 nm), synthetic complexity, and low sensitivity, which have restricted the application of these sensors in the fields of biochemistry and biomedicine. A fluorescent probe with a large Stokes shift is more preferred because it is relatively easy to reduce self-quenching and auto-fluorescence during fluorescence sensing.25–27 This method can remarkably improve the detection accuracy. Moreover, a rapid response is an important index for optical sensors used for the real-time monitoring and bio-imaging of H2S in biological systems. Thus, the development of a H2S-specific fluorescent probe with excellent properties is particularly necessary.
Combining all these considerations, in this work, a new coumarin-carbazole-based fluorescent probe, COZ-DNBS, with a rapid response and large Stokes shift was rationally devised and applied to effectively sense H2S in vivo and in vitro. The concrete synthesis route for COZ-DNBS is depicted in Scheme 1, and the structures of COZ-OH and COZ-DNBS were characterized via HRMS and 13C-NMR and 1H-NMR spectroscopy (Fig. S2–S7†). Structurally, in order to lengthen the emission wavelength, a carbazole group was modified onto the coumarin core to extend the π-conjugation structure. 2,4-Dinitrobenzenesulfonyl (DNBS), an outstanding fluorescence quenching moiety, served as the reactive site for H2S,28,29 while the newly synthesized coumarin-carbazole dye COZ-OH was employed as the fluorophore (Scheme 2). When using the probe COZ-DNBS, the established fluorescent platform could detect H2S with excellent selectivity, showing 62-fold fluorescence enhancement, a fast response (<1 min), high sensitivity (38.6 nM), a large Stokes shift (173 nm), and bright-yellow emission (Table S1†). Importantly, the probe COZ-DNBS works well when monitoring levels of H2S in realistic samples, living MCF-7 cells, and zebrafish, showing that COZ-DNBS is a promising signaling tool for H2S detection in biosystems.
Scheme 1. The synthetic route to the new fluorescent probe COZ-DNBS.
Scheme 2. The proposed mechanism involving the fluorescent probe COZ-DNBS for H2S detection.
2. Experimental
2.1. Instruments and reagents
HRMS (high-resolution mass spectroscopy) analysis was carried out using AB Sciex TripleTOF 4600 apparatus. 1H- and 13C-NMR (nuclear magnetic resonance) spectra were obtained using a Bruker Avance 600 MHz spectrometer. The UV-vis absorption and fluorescence spectra data were obtained using a Shimadzu UV-2450 spectrometer and HITACHI F-4600 fluorescence spectrophotometer. Fluorescence images of cells and zebrafish were acquired using a Zeiss LSM710 Wetzlar laser scanning confocal microscope. All chemicals were purchased from suppliers in China and used directly without further refining.
2.2. Synthesis of the dye COZ-OH
7-Hydroxycoumarin-4-acetic acid (110.0 mg, 0.5 mmol) and N-ethylcarbazole-3-carbaldehyde (116.4 mg, 0.5 mmol) were dissolved in 6.0 mL of CH3OH, and then piperidine (46.0 μL) was added to the above mixed solution. After stirring for 12.0 h at 75 °C, the reaction was completed. The cooled precipitate was washed with cold methanol (10.0 mL), whereafter the obtained solid was extracted using dichloromethane/brine (15.0 mL/50.0 mL) and dried over Na2SO4. The crude product was purified via column chromatography (CH2Cl2 : CH3OH = 50 : 1) to give the dye COZ-OH (97.2 mg, 51% yield). 1H NMR (600 MHz, DMSO) δ (ppm) 10.58 (s, 1H), 8.67 (s, 1H), 8.18 (dd, J = 41.8, 8.2 Hz, 2H), 7.96–7.63 (m, 5H), 7.50 (t, J = 7.6 Hz, 1H), 7.27 (t, J = 7.4 Hz, 1H), 6.87 (dd, J = 8.7, 1.8 Hz, 1H), 6.77 (d, J = 1.8 Hz, 1H), 6.58 (s, 1H), 4.48 (q, J = 7.0 Hz, 2H), 1.34 (t, J = 7.1 Hz, 3H). 13C NMR (151 MHz, DMSO) δ (ppm) 161.69, 161.34, 155.89, 151.00, 140.84, 140.57, 139.48, 127.45, 127.15, 126.90, 126.67, 123.10, 122.75, 120.97, 120.89, 119.81, 117.19, 113.27, 111.33, 110.01, 109.90, 103.88, 103.05, 37.63, 14.23. HRMS (ESI) m/z: calcd for [C25H20NO3]+, 382.1443; found, 382.1422.
2.3. Synthesis of the probe COZ-DNBS
COZ-OH (38.0 mg, 0.1 mmol) and 30.0 μL of Et3N were mixed in 8.0 mL of CH2Cl2 at room temperature for 10.0 min. Then, 2,4-dinitrobenzenesulfonyl chloride (32.0 mg, 0.12 mmol) was added into the mixture and it was stirred for 6.5 h. After natural cooling, the reaction solution was filtered and dried over Na2SO4. The crude product was purified via silica gel column chromatography using CH2Cl2 as the eluent to obtain COZ-DNBS (47.1 mg, 77% yield). 1H NMR (600 MHz, DMSO) δ (ppm) 9.14 (d, J = 2.3 Hz, 1H), 8.66 (s, 1H), 8.62 (dd, J = 8.7, 2.3 Hz, 1H), 8.42 (d, J = 8.9 Hz, 1H), 8.35 (d, J = 8.7 Hz, 1H), 8.19 (d, J = 7.7 Hz, 1H), 7.99–7.89 (m, 2H), 7.73–7.63 (m, 3H), 7.54–7.47 (m, 1H), 7.40 (d, J = 2.4 Hz, 1H), 7.30–7.24 (m, 2H), 6.89 (s, 1H), 4.49 (d, J = 7.2 Hz, 2H), 1.34 (t, J = 7.1 Hz, 3H). 13C NMR (151 MHz, DMSO) δ (ppm) 160.22, 154.56, 152.11, 150.53, 149.94, 148.63, 140.99, 140.63, 140.58, 134.19, 131.01, 128.12, 127.97, 127.29, 126.98, 126.74, 123.08, 122.71, 121.72, 121.18, 120.90, 119.90, 118.98, 118.37, 116.34, 111.27, 110.08, 109.98, 108.31, 37.65, 14.24. HRMS (ESI) m/z: calcd for [C31H22N3O9S]+, 612.1077; found, 612.1074.
2.4. Procedure for optical data measurements
COZ-DNBS was dissolved using CH3CN and a stock solution was prepared with a concentration of 3.0 mM. 10.0 mM analyte stock solutions (including Ala, Asn, Arg, Glu, Gln, Met, Ser, His, Pro, Thr, Ile, Cys, GSH, Hcy, Cl−, Br−, F−, I−, NO3−, CO32−, SO42−, Zn2+, H2O2, and NaHS) were made up in deionized water for immediate use. All solution tests were performed in pH 7.4 PBS/CH3CN (4 : 1, v/v). The fluorescence spectra were obtained at λex = 400.0 nm. COZ-DNBS was dissolved in DMSO for cell culture and zebrafish studies.
2.5. Cell cultures and imaging
Human breast carcinoma MCF-7 cells were plated in a confocal Petri dish with DMEM culture medium to adherence for 24 h. As a control, MCF-7 cells were immersed in 10.0 μM COZ-DNBS at 37 °C for 30 min. For the experiment groups, MCF-7 cells were pre-incubated with different concentrations of H2S (10.0, 20.0, and 50.0 μM, respectively) for 30 min and further treated with COZ-DNBS (10.0 μM) for another 30 min. After rinsing the confocal Petri dish with phosphate-buffered saline (PBS) three times to remove residue, the MCF-7 cells were imaged using a Zeiss LSM 710 laser scanning confocal microscope.
2.6. Zebrafish imaging
Four-day-old zebrafish were obtained from Eze-Rinka Company (Nanjing, China). Firstly, the zebrafish to be imaged were incubated with the probe COZ-DNBS (10.0 μM) at 37 °C for 30 min, and they were then imaged following three cycles of PBS washing. Subsequently, other zebrafish were pretreated with 50.0 μM H2S for 30 min and stained with COZ-DNBS (10.0 μM) for another 30 min. After washing with PBS three times, fluorescence imaging was performed using a confocal microscope.
2.7. Preparation of spiked samples
The river water, lake water, and tap water samples used in these experiments were obtained from the Nenjiang river, Laodong lake, and a laboratory in Qiqihar Medical University, respectively. After being filtered, the above real water samples were spiked with different concentrations of H2S (5.0, 10.0, and 20.0 μM), and then the fluorescence intensity changes of the mixtures were measured in triplicate.
3. Results and discussion
3.1. Spectral response
To understand the optical properties of COZ-DNBS when it reacted with H2S, fluorescence titration was carried out in pH 7.4 PBS/CH3CN (4 : 1, v/v) with various concentrations of H2S (Fig. 1). It was seen that the photoinduced electron transfer (PET)-based emission of COZ-DNBS resulted in “turn-off” fluorescence being observed in the absence of H2S. Upon the addition of H2S, COZ-DNBS showed dramatic fluorescence emission centered at 558 nm and with a large Stokes shift (173 nm) (Fig. S1†). With an increase in the H2S concentration, the fluorescence enhanced gradually and showed a 62-fold increase upon the addition of 60.0 μM H2S. Based on previous research,30,31 the above profound optical changes at 558 nm for COZ-DNBS in the presence of H2S should be ascribed to the formation of the dye COZ-OH. This sensing strategy was also verified based on the HRMS spectra. A mixture of COZ-DNBS and H2S (cal.: 382.1415) (Fig. S8†) has nearly the same molecular weight as COZ-OH (m/z = 382.1443) (Fig. S6†). Moreover, the regression equation was obtained according to the dose-dependent spectral response, and it displayed a good linear relationship between the fluorescence intensity and concentration of H2S (Fig. 2). In the concentration range of 0.0–5.0 μM, the limit of detection (LOD) for 10.0 μM COZ-DNBS was estimated to be 38.6 nM, suggesting that the probe COZ-DNBS had the potential to detect H2S qualitatively and quantitatively.
Fig. 1. Fluorescence spectra of COZ-DNBS (10.0 μM) in the presence of different amounts of H2S (0.0–60.0 μM); inset: the colorimetric changes of COZ-DNBS without (a) and with (b) H2S.
Fig. 2. The fluorescence intensity of COZ-DNBS at 558 nm as a function of the H2S dose (0.0–60.0 μM); inset: the linear relationship between the concentration of H2S (0.0–5.0 μM) and the fluorescence intensity at 558 nm.
3.2. Selectivity study
To prove the sensitivity and specificity of COZ-DNBS for identifying H2S, a series of analytes was employed and monitored in aqueous media. As depicted in Fig. 3, the fluorescence intensity of COZ-DNBS hardly changed upon adding various amino acids, ions, and biologically active materials (including Ala, Asn, Arg, Glu, Gln, Met, Ser, His, Pro, Thr, Ile, Cl−, Br−, F−, I−, NO3−, CO32−, SO42−, Zn2+, H2O2, Cys, GSH, and Hcy). However, a remarkable fluorescence response with an approximately 62-fold enhancement ratio at 558 nm was seen in the presence of H2S compared with the slight fluorescence behavior induced by biothiols (Cys, Hcy and GSH), which indicated that COZ-DNBS had good sensing abilities towards H2S. In addition, competition experiments, involving the coexistence of the aforementioned analytes (including Ala, Asn, Arg, Glu, Gln, Met, Ser, His, Pro, Thr, Ile, Cl−, Br−, F−, I−, NO3−, CO32−, SO42−, Zn2+, H2O2, Cys, GSH, and Hcy), were performed to explore the feasibility of using COZ-DNBS to detect H2S. It is found that the degree of fluorescence intensity change was similar as that in the presence of H2S alone, demonstrating that COZ-DNBS could selectively respond to H2S in complex biological environments.
Fig. 3. The fluorescence responses of COZ-DNBS (10.0 μM) incubated with various analytes (red bars; 60.0 μM for H2S (26) and 0.5 mM for 1–25, which were Ala, Asn, Arg, Glu, Gln, Met, Ser, His, Pro, Thr, Ile, Cl−, Br−, F−, I−, NO3−, CO32−, SO42−, Zn2+, H2O2, Cys, GSH, and Hcy, respectively); and the detection of H2S (60.0 μM) upon the addition of diverse coexisting competing analytes (gray bars; 0.5 mM for Ala, Asn, Arg, Glu, Gln, Met, Ser, His, Pro, Thr, Ile, Cl−, Br−, F−, I−, NO3−, CO32−, SO42−, Zn2+, H2O2, Cys, GSH, and Hcy (1–25, respectively)). The incubation time was 60 s.
3.3. Kinetics and pH studies
To obtain the optimal fluorescence sensing set-up for H2S detection, it is important to optimize the pH to obtain the highest response of COZ-DNBS towards H2S. In the present study, the effects of pH were explored in the range of pH 2 to 11. As shown in Fig. 4, the free probe COZ-DNBS exhibited a very weak emissive nature at 558 nm in diverse pH environments. After the addition of H2S, the fluorescence intensity of COZ-DNBS increased gradually from pH 2.0 to 5.0; a high fluorescence signal value emerged between 6.0 and 8.0, and this slightly decreased under alkaline conditions. The above results strengthen the possibility of using COZ-DNBS as a candidate probe for H2S detection under neutral conditions. Subsequently, the time-dependent fluorescence changes of COZ-DNBS in the presence of H2S were studied (Fig. 5). In the pH 7.4 PBS/CH3CN (4 : 1, v/v) system, the observed fluorescence intensity of COZ-DNBS alone remained unchanged, meaning that COZ-DNBS possessed excellent stability in the liquid state. However, the fluorescence signal of COZ-DNBS immediately increased (in less than 1 min) in this buffer system after adding H2S, and the bright fluorescence of the reaction product between COZ-DNBS and H2S was insensitive to the incubation time. This clearly indicates that COZ-DNBS has the real-time capability to detect H2S.
Fig. 4. The pH-dependence (2.0–11.0) of the fluorescence intensity of COZ-DNBS (10.0 μM) in H2O/CH3CN (4 : 1, v/v) without (black line) and with (red line) H2S (60.0 μM).
Fig. 5. The fluorescence intensity of COZ-DNBS (10.0 μM) over time at 558 nm before (blue line) and after (red line) the addition of H2S.
3.4. Imaging of H2S in living MCF-7 cells
Prompted by the favorable properties of COZ-DNBS, the bioimaging abilities of COZ-DNBS were studied via living cell analysis. Before intracellular imaging, the cytotoxicity of COZ-DNBS toward MCF-7 cells was assessed via MTT assays. The results showed that COZ-DNBS displayed low cytotoxicity in the range of 2–20.0 μM, with above 94% cell viability (Fig. 6), indicating that COZ-DNBS was biocompatible for performing imaging in living organisms. Subsequently, the probe COZ-DNBS was applied to monitor intracellular H2S in MCF-7 cells. As shown in Fig. 7, a slight fluorescence background signal emerged when MCF-7 cells were treated with COZ-DNBS alone, which maybe arise from the presence of a very small amount of H2S in the cells. However, the appearance of remarkable fluorescence was observed in the presence of H2S (Fig. 7B1–D1), and the fluorescence signal increased gradually with an increase (10.0–50.0 μM) in the H2S concentration. The above findings demonstrated that COZ-DNBS could be used to quantify the concentration of H2S based on the relative fluorescence intensity from images of live cells.
Fig. 6. The viability of MCF-7 cells incubated with different concentrations of COZ-DNBS (2.0–20.0 μM).
Fig. 7. Confocal imaging of MCF-7 cells: (A) MCF-7 cells incubated with 10.0 μM COZ-DNBS for 30 min; and (B–D) MCF-7 cells incubated with different concentrations of H2S (10.0, 20.0, and 50.0 μM), followed by treatment with 10.0 μM COZ-DNBS.
3.5. Imaging of H2S in living zebrafish
The potential application of imaging H2S in living zebrafish upon incubation with COZ-DNBS was then validated. As depicted in Fig. 8, incubation with COZ-DNBS (10.0 μM) led to only a weak fluorescence signal from H2S already present in the zebrafish in the yellow channel. In contrast, the fluorescence signal became much brighter after the addition of H2S (50.0 μM). It was advantageously confirmed that COZ-DNBS could be suitable for the detection of H2S in living zebrafish, showing excellent membrane permeability.
Fig. 8. Fluorescence imaging of H2S in zebrafish using COZ-DNBS: (a–c) zebrafish incubated with COZ-DNBS (10.0 μM) for 30 min; and (d–f) zebrafish incubated with H2S (50.0 μM) before staining with COZ-DNBS (10.0 μM).
3.6. Detection of H2S in spiked samples
To further gauge the feasibility of using the probe COZ-DNBS for practical applications, the quantitative analysis of real samples was investigated. In three different water samples (river water, lake water, and tap water), spiked H2S concentrations were measured using COZ-DNBS. As manifested via recovery experiments (Table S2†), moderate to good recovery rates were obtained when using COZ-DNBS to sensitively detect H2S, within the range of 95.6–103.4%, and the relative standard deviation (RSD) values were less than 3.0%. The above data showed that the proposed COZ-DNBS sensor exhibited excellent accuracy in actual water samples, proving the capacity of COZ-DNBS to act as a valid tool for the detection of H2S in environmental samples.
4. Conclusions
In conclusion, we have achieved a new fluorescent probe, COZ-DNBS, for H2S detection based on the novel coumarin–carbazole fluorescent dye COZ-OH. Upon reaction with H2S, COZ-DNBS exhibited high sensitivity (38.6 nM), excellent selectivity, and extraordinary fluorescence enhancement (62-fold) in the yellow region (λmaxem = 558 nm). Moreover, benefiting from the fast response (<1 min) and huge Stokes shift (173 nm), COZ-DNBS was successfully applied to the detection of H2S in realistic samples, living MCF-7 cells, and zebrafish, and it displayed great potential for H2S detection in biosystems.
Ethical statement
All animal procedures were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of Qiqihar Medical University and experiments were approved by the Animal Ethics Committee of Qiqihar Medical University (QMU-AECC-2020-63).
Conflicts of interest
There are no conflicts of interest to declare.
Supplementary Material
Acknowledgments
Thanks is extended for the financial support provided by the Clinical research fund project of Qiqihar Academy of Medical Sciences (No. QMSI2021L-21).
Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra00997h
References
- Rong Y. F. Niu P. X. Liu X. J. Chen W. Q. Wei L. H. Song X. Z. Double-channel based fluorescent probe for differentiating GSH and H2Sn (n > 1) via a single-wavelength excitation with long-wavelength emission. Sens. Actuators, B. 2021;344:130224. [Google Scholar]
- Li Y. Y. Zhang G. X. Ma C. J. Chen F. H. Dong J. Ge Y. Q. A simple dual-channel imidazo[1,5-a]pyridine-based fluorescent probe for the discrimination between Cys/Hcy and GSH. Dyes Pigm. 2021;191:109381. [Google Scholar]
- Zhang J. Wen G. H. Wang W. H. Cheng K. Guo Q. Tian S. Liu C. Hu H. R. Zhang Y. C. Zhang H. T. Wang L. D. Sun H. Y. Controllable cleavage of C–N bond-based fluorescent and photoacoustic dual-modal probes for the detection of H2S in living mice. ACS Appl. Bio Mater. 2021;4:2020–2025. doi: 10.1021/acsabm.0c00413. [DOI] [PubMed] [Google Scholar]
- Sun Y. Li C. Feng X. W. Wang C. F. Wang N. Zhu J. R. Wang T. Cui X. Y. Si-coumarin-based fluorescent probes for ultrafast monitoring H2S in vivo. Dyes Pigm. 2021;186:109059. [Google Scholar]
- Wang B. B. Wang X. Q. Zeng A. Q. Leng J. C. Zhao W. Engineering a mitochondria-targeted ratiometric fluorescent probe with a large stokes shift for H2S-specific assaying in foodstuffs and living cells. Sens. Actuators, B. 2021;343:130095. [Google Scholar]
- Zhang K. Q. Meng J. Q. Bao W. E. Liu M. Wang X. F. Tian Z. Y. Mitochondrion-targeting near-infrared fluorescent probe for detecting intracellular nanomolar level hydrogen sulfide with high recognition rate. Anal. Bioanal. Chem. 2021;413:1215–1224. doi: 10.1007/s00216-020-03086-6. [DOI] [PubMed] [Google Scholar]
- Zheng Y. L. Chai Z. H. Tang W. Yan S. Dai F. Zhou B. A multi-signal mitochondria-targetable fluorescent probe for simultaneously discriminating Cys/Hcy/H2S, GSH, and SO2 and visualizing the endogenous generation of SO2 in living cells. Sens. Actuators, B. 2021;330:129343. [Google Scholar]
- Zhong K. L. Hu X. L. Zhou S. Y. Liu X. Y. Gao X. Tang L. J. Yan X. M. Mitochondria-targeted red-emission fluorescent probe for ultrafast detection of H2S in food and its bioimaging application. J. Agric. Food Chem. 2021;69:4628–4634. doi: 10.1021/acs.jafc.1c00862. [DOI] [PubMed] [Google Scholar]
- Zhou L. Chen Y. Shao B. H. Cheng J. Li X. Recent advances of small-molecule fluorescent probes for detecting biological hydrogen sulfide. Front. Chem. Sci. Eng. 2022;16:34–63. [Google Scholar]
- Jia X. Li W. Guo Z. H. Guo Z. B. Li Y. Zhang P. Z. Wei C. Li X. L. An NBD-based mitochondrial targeting ratiometric fluorescent probe for hydrogen sulfide detection. ChemistrySelect. 2019;4:8671–8675. [Google Scholar]
- Zhou L. Yuan G. Hu S. A 4-hydroxy-1,8-naphthalimide-based turn-on two-photon fluorescent probe for hydrogen polysulfide sensing. J. Appl. Probab. 2020;86:1071–1076. [Google Scholar]
- Zhang Z. H. Li S. Q. Yan Y. Z. Qu J. B. Wang J. Y. A novel fast-responsive fluorescent probe based on 1,3,5-triazine for endogenous H2S detection with large stokes shift and its application in cell imaging. New J. Chem. 2021;45:9756–9760. [Google Scholar]
- Quan Y. Y. Fan L. L. Shen H. Y. Wu B. B. Kong S. S. Luo Y. S. Huang Z. S. Ye X. X. A multifunctional BODIPY based fluorescent probe for hydrogen sulfide detection and photodynamic anticancer therapy in HCT116 colon cancer cell. Dyes Pigm. 2022;197:109897. [Google Scholar]
- Chen S. Hou P. Sun J. W. Wang H. J. Liu L. Imidazo[1,5-α]pyridine-based fluorescent probe with a large stokes shift for specific recognition of sulfite. Spectrochim. Acta, Part A. 2020;225:117508. doi: 10.1016/j.saa.2019.117508. [DOI] [PubMed] [Google Scholar]
- Hou P. Sun J. W. Wang H. J. Liu L. Zou L. W. Chen S. TCF-imidazo[1,5-α]pyridine: a potential robust ratiometric fluorescent probe for glutathione detection with high selectivity. Sens. Actuators, B. 2020;304:127244. [Google Scholar]
- Hou P. Chen S. Liang G. L. Li H. M. Zhang H. G. A lysosome-targeted ratiometric fluorescent probe with a large blue shift for monitoring hypochlorous acid in living cells and zebrafish. Spectrochim. Acta, Part A. 2020;229:117866. doi: 10.1016/j.saa.2019.117866. [DOI] [PubMed] [Google Scholar]
- Hou P. Chen S. Liang G. L. Li H. M. Zhang H. G. Design of a facile fluorescent probe with a large stokes shift for hydrogen peroxide imaging in vitro and in vivo. Spectrochim. Acta, Part A. 2020;236:118338. doi: 10.1016/j.saa.2020.118338. [DOI] [PubMed] [Google Scholar]
- Chen S. Hou P. Sun J. W. Wang H. J. Liu L. A new long-wavelength emission fluorescent probe for imaging biothiols with remarkable stokes shift. Spectrochim. Acta, Part A. 2020;241:118655. doi: 10.1016/j.saa.2020.118655. [DOI] [PubMed] [Google Scholar]
- Yang M. W. Fan J. L. Du J. J. Peng X. J. Small-molecule fluorescent probes for imaging gaseous signaling molecules: current progress and future implications. Chem. Sci. 2020;11:5127–5141. doi: 10.1039/d0sc01482f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z. Y. Qin T. Y. Zhou X. F. Wang L. Liu B. Fluorescent probes with multiple channels for simultaneous detection of Cys, Hcy, GSH, and H2S. Trends Anal. Chem. 2019;121:115672. [Google Scholar]
- Li H. N. Fang Y. X. Yan J. J. Ren X. Y. Zheng C. Wu B. Wang S. Y. Li Z. L. Hua H. M. Wang P. Li D. H. Small-molecule fluorescent probes for H2S detection: advances and perspectives. Trends Anal. Chem. 2021;134:116117. [Google Scholar]
- Muthusam S. Rajalakshmi K. Zhu D. W. Zhao L. Wang S. Zhu W. A novel lysosome targeted fluorophore for H2S sensing: enhancing the quantitative detection with successive reaction sites. Sens. Actuators, B. 2020;320:128433. [Google Scholar]
- Shu W. Zang S. Wang C. Gao M. Jing J. Zhang X. An endoplasmic reticulum targeted ratiometric fluorescent probe for sensing of hydrogen sulfide in living cells and zebrafish. Anal. Chem. 2020;92:9982–9988. doi: 10.1021/acs.analchem.0c01623. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Chen Y. Bai Y. Xue X. He W. Guo Z. FRET-based fluorescent ratiometric probes for the rapid detection of endogenous hydrogen sulphide in living cells. Analyst. 2020;145:4233–4238. doi: 10.1039/d0an00531b. [DOI] [PubMed] [Google Scholar]
- Hou P. Wang J. Fu S. Liu L. Chen S. Highly sensitive fluorescent probe based on a novel phenothiazine dye for detection of thiophenols in real water samples and living cells. Anal. Bioanal. Chem. 2019;411:935–942. doi: 10.1007/s00216-018-1525-5. [DOI] [PubMed] [Google Scholar]
- Hou P. Wang J. Fu S. Liu L. Chen S. A new turn-on fluorescent probe with ultra-large fluorescence enhancement for detection of hydrogen polysulfides based on dual quenching strategy. Spectrochim. Acta, Part A. 2019;213:342–346. doi: 10.1016/j.saa.2019.01.081. [DOI] [PubMed] [Google Scholar]
- Zheng Y. Hou P. Li Y. Sun J. W. Cui H. X. Zhang H. Y. Chen S. A phenothiazine-HPQ based fluorescent probe with a large stokes shift for sensing biothiols in living systems. Molecules. 2021;26:2337–2349. doi: 10.3390/molecules26082337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang C. L. Wang H. Y. Ni T. J. Chang K. W. Ge C. P. A dicyanoisophorone-based near-infrared fluorescent probe with fast detection for H2S in living cells and zebrafish. J. Lumin. 2022;243:118669. [Google Scholar]
- Yang B. Su M. M. Xue Y. S. He Z. X. Xu C. Zhu H. L. A selective fluorescence probe for H2S from biothiols with a significant regioselective turn-on response and its application for H2S detection in living cells and in living Caenorhabditis elegans. Sens. Actuators, B. 2018;276:456–465. [Google Scholar]
- Zhou L. Cheng Z. Q. Li N. Ge Y. X. Xie H. X. Zhu K. Zhou A. Zhang J. Wang K. M. Jiang C. S. A highly sensitive endoplasmic reticulum-targeting fluorescent probe for the imaging of endogenous H2S in live cells. Spectrochim. Acta, Part A. 2020;240:118578. doi: 10.1016/j.saa.2020.118578. [DOI] [PubMed] [Google Scholar]
- Hu Z. Q. A fluorescent probe based on tetrahydro[5]helicene derivative with large stokes shift for rapid and highly selective recognition of hydrogen sulfide. Spectrochim. Acta, Part A. 2019;214:487–495. doi: 10.1016/j.saa.2019.02.079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.












