Abstract
Although the “skunky” odor characteristic of cannabis has been widely referenced, its cause has been historically misassigned to unspecified “skunky terpenes”. Recent reports from two independent research groups, the Koziel team (March and April 2021) and Oswald team (August and November 2021), have corrected this misassignment by linking the “skunky” character of industrial hemp and cannabis to 3-methyl-2-butene-1-thiol (321MBT). A recent USPTO patent application review clearly indicated that the Oswald team should take full credit for the discovery of this link with respect to cannabis. However, the August 19, 2021 publication of their patent application appears to be their formal public disclosure of 321MBT as the primary source odorant which is responsible for the targeted “skunky” odor. This date is well after the March and April 2021 public disclosures by the Koziel team for the 321MBT/“skunky” odor link relative to both cannabis and industrial hemp. This Viewpoint summarizes the investigative strategy leading to the public disclosure of this historically elusive link. It is presented from the perspective of the rapid multidimensional–gas chromatography–mass spectrometry–olfactometry (i.e., MDGC-MS-O) based odorant-prioritization “screening” approach, as applied by the Koziel team.
Environmental Odor Troubleshooting
The following comments are submitted in response to Oswald et al.1 The Oswald et al.1 integration of hyphenated techniques (i.e., GC × GC-TOF-MS/FID/SCD) represents an almost perfect tool for pushing the limits relative to compositional analysis. However, from the standpoint of a practical environmental odor mitigation strategy focus, multidimensional–gas chromatography–mass spectrometry–olfactometry (MDGC-MS-O)-based odorant prioritization “screening” to the smallest essential subset of compounds can be more instructive. Simply stated, with respect to environmental odor problem solving, “less can often be more”. A simplified, priority-odorant subset can, more directly, satisfy the “need-for-speed” relative to environmental odor problem solving.
While Oswald et al.1 appear to have an excellent and thorough treatment of the subject of “skunky” cannabis, we would like to alert readers to the Koziel et al.2,3 public disclosure of the relationship between 321MBT and the “skunky” characteristic of both hemp and cannabis. While the November 12, 2021 publication of Oswald et al.1 does cite the Rice and Koziel (2015)4 paper, it fails to cite the coauthor’s subsequent collaborative work (Byers Scientific, Don Wright & Associates, Iowa State University, and Volatile Analysis Corporation) and public disclosures of the connection between “skunky” cannabis and 321MBT.2,3 Public disclosure was by way of a March 22, 2021 Press Release2 and an April 19, 2021 Koziel et al.3 plenary lecture at the seventh NOSE International Conference on Environmental Odor Monitoring and Control. Two follow-up media articles were published on April 21 and June 22, 2021, respectively.5,6Table S1 (Supporting Information) provides a summary of our understanding of the chronological discovery1 and public disclosure2,3 timeline for the link between 321MBT and “skunky” cannabis/hemp odor. While these authors were disappointed that their public disclosure was not cited in the excellent Oswald et al.1 article, it was reassuring that these two independent and very different approaches to the “skunky” cannabis question yielded the same major conclusion.
We would like to offer the following Viewpoint to contrast these two disparate odor investigative strategies that arrive at the same major conclusion relative to the “skunky” cannabis odor, i.e., (a) vs (b):
-
(a)
The rapid screening approach; odorant prioritization by MDGC-MS-olfactometry; direct chemical/compositional and sensory analysis by Koziel et al.7
-
(b)
The comprehensive but more labor-intensive approach by GC × GC-TOF-MS/FID/SCD; indirect chemical/compositional followed by sensory analysis by Oswald et al.1
While this Viewpoint focuses on the skunky cannabis case, we believe it is applicable to a wide range of environmental odor-related questions and challenges.
MDGC-MS-Olfactometry-Based Odorant Prioritization and the “Need-for-Speed”
The MDGC-MS-O-based approach to rapid screening for character-defining odorants8 has emerged for these authors by way of many years of odor troubleshooting in the industry.9−12 A company faced with a crisis-driven odor/flavor quality defect in a commercial product, process, or environment must quickly respond to this quality excursion in order to protect the market share and customer base. Responding to such real-world crisis issues has taught us that mitigation strategies can often be focused on and resolved without resorting to comprehensive and time-consuming compositional analysis.
It is often the case that odor quality challenges can be quickly traced to character-defining odorants,7,9−12 single odorous VOCs that match the odor character of the quality “defect” and are deemed, therefore, primarily responsible for imparting that defect. Other cases may reflect somewhat greater complexity than the single “bad-actor” model, with a targeted odor defect traceable to a very small subset of compounds from the source’s total VOC emission profile. In either case, it is typical that such odor quality troubleshooting can be quickly focused onto the smallest impact-priority subset by integrating a direct sensory monitoring component (i.e., olfactometry) into the conventional GC-MS analytical system (Figure 1).
Figure 1.
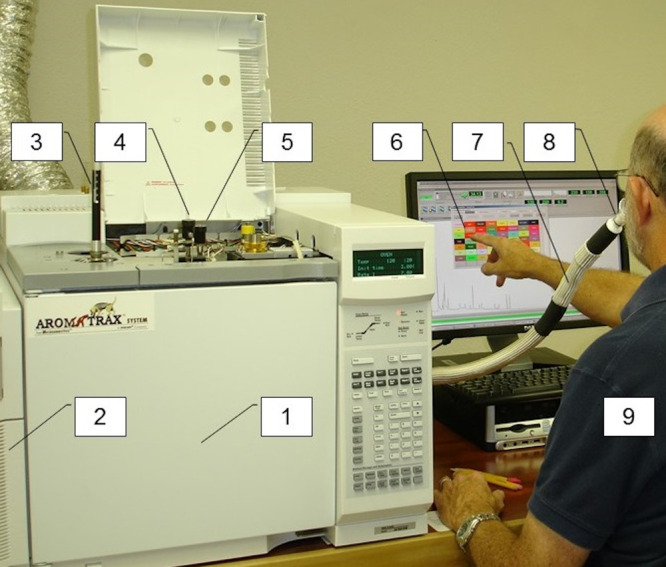
Overview of MDGC-MS-O systems integration: AromaTrax system from Volatile Analysis Corporation on an Agilent platform. Major components labeled as follows: (1) 6890 GC; (2) 5975B MSD; (3) manual SPME field sampler; (4) heart-cut valve; (5) cryo-trap valve; (6) Aromatrax data processing interface; (7) heated transfer line; (8) olfactory detector; and (9) human “sensor”.
This critical sensory integration permits direct “screening” of the source’s total VOC emission profile in a single GC-O run. Figure 1 illustrates the major integrated components of an MDGC-MS-O system, such as that used for the Koziel et al.2,3 odorant prioritization assessment of the industrial hemp environment. The AromaTrax system pictured is closely representative of the system which was used by the lead investigator in that investigation. Most importantly, this figure includes the integrated component, which these authors believe reflects the most significant difference between the Koziel et al.3 and Oswald et al.1 approaches: the “human sensor” operating in parallel with mass spectrometric detection. Our past publications cover MDGC-MS-O instrumentation, components, systems integration, and investigative strategies in great detail.7,13−17
MDGC-MS-O-Based Odorant Prioritization Applied to Cannabis/Industrial Hemp
The reported “skunky” odor character, historically described for cannabis and industrial hemp grow operations, proved to be an excellent candidate for MDGC-MS-O-based odorant prioritization screening.3 The “skunky” character had, historically, been attributed to undefined “skunky terpenes”.
These authors’ initial screening efforts were first directed at an industrial hemp growing operation in Central Texas. Industrial hemp was selected as a surrogate for cannabis, primarily for reasons of logistical convenience and accessibility to the Texas-based odor experts. The Texas legislature had only recently legalized the growing of industrial hemp, and the Pur Isolabs operation (Bergheim, TX) was, reportedly, one of the first permitted grantees in the state. The following Tables 1–3 reflect the first-approximation impact-priority odorant ranking profile which was derived from the initial SPME fiber-based indoor air sample collections from within the operation’s hemp drying trailer. The six air sample collections approximated a 3X serial dilution. Serial dilution was approximated by exposing the SPME fibers simultaneously to the environment for 1.8, 5.0, and 15 min, respectively.
Table 1. First-Tier (Group 1), Odor Impact-Priority Compoundsa.
| GC column retention time (min) | tentative chemical ID | odor descriptor | |
|---|---|---|---|
| group 1 | 12.0 | beta-myrcene | “characteristic”, “geranium leaf” |
| 10.4 | alpha-pinene | “characteristic”, “pine” | |
| 13.9 | unknown (likely a C4-substituted pyrazine) | “musty”, “nutty”, “foul” | |
| 16.7 | unknown (likely trans-2-nonenal) | “musty”, “cardboard”, “vegetable” | |
| 13.1 | d,l-limonene | “citrus” |
Dominant impact-priority subset at the time of on-site assessment and odor collection.
Table 3. Additional Possible Minor Impact VOCs in Group 3 That Are “Masked” by the Group 1 and 2 Odorants.
| GC column retention time (min) | tentative chemical ID | descriptor | |
|---|---|---|---|
| group 3 | 2.8 | methyl mercaptan | “fecal” |
| 18.8 | para-cresol | “barnyard” | |
| 8.3 | unknown (possibly methyl butanoate) | “fruity” | |
| 10.9 | camphene | “camphor” | |
| 13.2 | 1,8-cineole | “eucalyptus” | |
| ∼14.7+ | para-xylene and other alkyl benzenes | undefined | |
| ∼10.0 to 28.0 | a plethora of other terpenes beyond the above | various |
Table 2. Second-Tier (Group 2) Odor Modifier Compounds, Including 321MBT, “Masked” by the Group 1 Odorants.
| GC column retention time (min) | tentative chemical ID | odor descriptor | |
|---|---|---|---|
| group 2 | 11.8 | beta-pinene | “characteristic”, “pine” |
| 7.6 | 3-methyl-2-butene-1-thiol | “skunky”, “foul” | |
| 7.2 | hexanal | “grassy”, “green” | |
| 12.7 | unknown (possibly 1-octene-3-one) | “earthy”, “mushroom” | |
| 3.5 | diacetyl | “buttery” | |
| 8.7 | 2-hexenal | “grassy”, “herbaceous” |
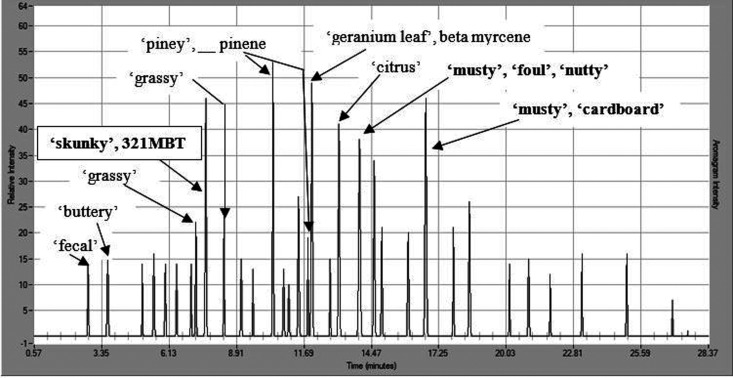
With respect to the “need-for-speed”, it is believed to be noteworthy that the overall odorant ranking profile (Tables 1–3) emerged for the single MDGC-MS-O investigator/panelist after only 4 person-days (i.e., including travel to/from the site and in-laboratory MDGC-MS-O analysis, on-site odor assessment, and SPME air sample collection). It is also noteworthy that the initial link between the dominant “skunky” odorant carrier and the 7.6 min GC column retention time was made during the fourth MDGC-MS-O run of the in-laboratory phase or approximately 4 person-hours from the start (Figure 2). In addition, as a result of prior knowledge of the expected retention time of the familiar “skunky” odor carrier compound (i.e., 321MBT),13,14,18 it was possible to make the tentative peak assignment hypothesis within that time as well (i.e., 4 person-hours from the start).
Figure 2.
GC-O odor profile (captured using the AromaTrax software from Volatile Analysis Corporation) enabled rapid linking of the “skunky” smell to 321MBT. Aromagram presents a GC-O odor profile overview of air samples collected, using a SPME fiber exposed for 15 min to the indoor environment of the hemp-drying room.
It should be noted that, in this case, the dominant “skunky” note @7.6 min (Figure 2) was relegated to the status of a second-tier odor modifier. This was, in fact, consistent with the associated on-site composite odor assessment which was carried out at this facility. Specifically, there was no perceived “skunky” odor character that was recognizable, by the investigator/panelist, at the time of the on-site assessment and SPME air sample collection. This indicated that the 321MBT concentration was below the recognition threshold and was therefore masked by the Group 1 impact-priority subset of odorants (Table 1). The “rolling unmasking effect” (RUE),7 as illustrated in Figure 3, reflects the odor impact-priority ranking of Tables 1–3. Under other varietal, seasonal, growing, or processing conditions, this ranking could reasonably be expected to change. By way of example, the downwind odor of large Texas bluebonnet fields (the state flower of Texas) is dominated by a single compound, 1,4-dimethoxybenzene, a “sweet”/“heavy”/“floral” aroma.7 However, this odor is only detectable at the peak of the flower’s growth cycle (i.e., just before seasonal die-off). Up until that time, the dense blue fields, while beautiful to look at, will not present with significant downwind odor impact. Likewise, dense prairie verbena colonies, like those of the Texas bluebonnet, are also dominated, at their seasonal environmental odor-impacting peak, by the nonterpene, semivolatile aromatic compound, p-cresol.7 As a result, in the case of the prairie verbena colonies, the downwind odor is dominated by a “barnyard”/“hog-truck” odor driven by p-cresol, the dominant odor rising above the masking capability of the dense, odorous terpene emissions at the source.7 This relationship and its investigation are explored in detail in the Supporting Information of a recent publication.7 Ironically, the 321MBT/“skunky” hemp link appears to reflect the same type of relationship as described for the Texas bluebonnet and prairie verbena. Specifically, a potent, nonterpene odorant (321MBT) can rise to odor impact-priority dominance (under seasonal or varietal conditions), emerging from the dense, odorous terpene background emissions near the source. The 321MBT/“skunky” hemp link hypothesis was subsequently validated and confirmed to exist for cannabis, as well, by the collaborative Byers Scientific research team, utilizing an odor-matching validation process as referenced below.2,3
Figure 3.
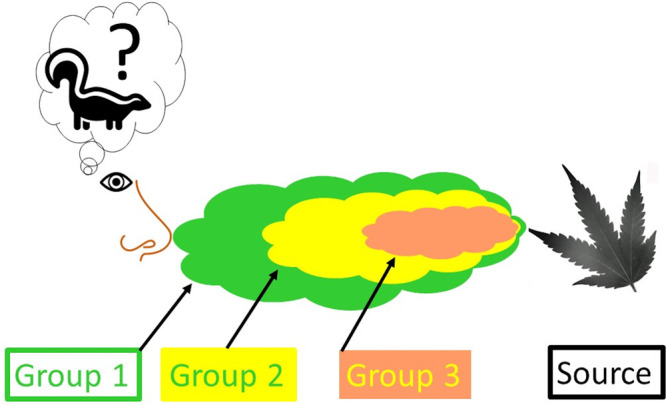
“Rolling unmasking effect” (RUE), i.e., group 1 “masking” groups 2 and 3 and group 2 “masking” group 3 (in the absence of group 1 odorants). Diagram illustrates, to a first approximation, the relationship between the odor impact-priority ranking profile from the industrial hemp drying room relative to the expected downwind odor impact. The RUE concept of environmental odor dispersion is examined in detail by Koziel et al.7
Odor-Matching Hypothesis Validation versus Reverse Engineering Hypothesis Development
The Koziel et al.3 “direct” approach and the “indirect” approach of Oswald et al.1 appear to be in agreement relative to the power of synthetic, odor-match formulations for focusing communication between “human sensors” regarding odor. In the case of Koziel et al.,3 synthetic odor matching is used to validate an impact-priority subset hypothesis arising directly from MDGC-MS-O-based odorant prioritization. In contrast, in the case of Oswald et al.,1 odor matching (i.e., which appears to be referred to by the authors as “reverse engineering”) is used to indirectly/sequentially develop and validate an impact-priority odorant hypothesis.
Communication between human “sensors” regarding an environmental odor of interest can be much more challenging when compared, for example, to the sense of color perception.7 With respect to color perception, color wheels can be very effective tools for developing consensus with respect to an “undefined” color of interest, whether the queried sensory “panelist” is a sensory professional or a layperson. Sensory professionals representing various industries have developed “descriptive-analysis”-based odor/aroma/flavor wheels which attempt to emulate the color-wheel as applied to color perception.19,20 The practical challenge for such odor wheels is that they rely on relatively subjective descriptors such as “grassy”, “herbaceous”, “skunky”, “fecal”, etc.
The odorant prioritization-based simplification of odor profiles to a single, character-defining odorant (or to the smallest subset of odorants) opens up the possibility of introducing a reconciling tool for odor that is more closely aligned with the simplicity of the color wheel for color. This tool was used in chemical odor matching.7,15 In its simplest form, the odor-match query asked of a lay panelist relative to a targeted environmental odor is a simple “YES/NO” when presented with a trace amount of a high-purity reference chemical. Utilizing this process, one can screen for a chemical’s odor-match fidelity to a “suspect”, character-defining odorant. As we argued earlier,7 this simplicity negates the requirement, on the part of the panelist, for extensive training, experience, or memory acuity relative to odor recognition. The only requirement is the usual application of his/her sense of smell (i.e., assumed or demonstrated to be properly functioning).
Odor-matching hypothesis validation can reflect a range of survey formalities and complexity. These surveys can range from (1) informal on-instrument demonstration/introductions of designated client representatives to a product’s “suspect” odor-defect source, to (2) informal on-instrument demonstration/introductions of a layperson sensory panel (e.g., volunteer representatives from impacted downwind citizenry) to a hypothesized link between an environmental odor issue and a “suspect” chemical odorant, to (3) formal odor-match fidelity grading by a professional sensory panel of a multicomponent synthetic odor-match formulation, to (4) formal odor-match fidelity grading of a multicomponent synthetic odor-match formulation by a seated jury in a civil trial.
Conclusion
Clearly, the work of Oswald et al.1 has generated excellent VOC compositional information regarding cannabis emissions (including a family of compounds related to 321MBT). The Oswald et al.1 integration of hyphenated techniques (i.e., GC × GC-TOF-MS/FID/SCD) represents an almost perfect tool for pushing the limits relative to odor compositional analysis. Clearly, there may be questions beyond odor impact, for which exhaustive emission compositional data may be more important (e.g., toxicology and atmospheric chemistry). However, from the standpoint of a practical environmental odor mitigation strategy focus, MDGC-MS-O-based odorant prioritization “screening” to the smallest essential subset of compounds can be more instructive.7,9−12 Simply stated, with respect to environmental odor problem solving and troubleshooting, “less can often be more”. A simplified, priority-odorant field can more directly satisfy the “need-for-speed” relative to environmental odor problem solving.
Acknowledgments
We would like to express appreciation to the Volatile Analysis Corporation for (1) allowing the panelist (D.W.W.) access to their AromaTrax software and instrumentation in this effort and (2) providing review and feedback on the manuscript. We would also like to express appreciation to Austin Rupple and Pur Isolabs in Bergheim, Texas, for allowing the research team access to their industrial hemp grow and processing facility.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c00517.
Table S1. Chronological summary of uncovering the link between 321MBT and “skunky” cannabis/hemp odor (PDF)
The authors declare the following competing financial interest(s): Three commercial entities are represented among the collaborative team (Byers Scientific, Don Wright & Associates, LLC, and Volatile Analysis Corporation).
Supplementary Material
References
- Oswald I. W. H.; Ojeda M. A.; Pobanz R. J.; Koby K. A.; Buchanan A. J.; Del Rosso J.; Guzman M. A.; Martin T. J. Identification of a New Family of Prenylated Volatile Sulfur Compounds in Cannabis Revealed by Comprehensive Two-Dimensional Gas Chromatography. ACS Omega 2021, 6 (47), 31667–31676. 10.1021/acsomega.1c04196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long E.Byers Scientific, Iowa State University, and Odor Experts Identify the Volatile Chemical Compound Responsible for Cannabis Odor Complaints; Business Wire (a Berkshire Hathaway Company): Bloomington, IN, USA, 22 March 2021. Available online: https://www.businesswire.com/news/home/20210322005837/en/Byers-Scientific-Iowa-State-University-and-Odor-Experts-Identify-the-Volatile-Chemical-Compound-Responsible-for-Cannabis-Odor-Complaints (accessed on 17 January 2022). [Google Scholar]
- Koziel J. A.; Guenther A.; Byers M.; Iwasinska A.; Parker D.; Wright D.. Update on the Development of a New ASTM Standard for Environmental Odor Assessment. Plenary Lecture at the NOSE2020. In Proceedings of the 7th International Conference on Environmental Odour Monitoring and Control, Virtual Conference, Online, 18–21 April 2021.
- Rice S.; Koziel J. A. Characterizing the smell of marijuana by odor impact of volatile compounds: An application of simultaneous chemical and sensory analysis. PLoS One 2015, 10, e0144160 10.1371/journal.pone.0144160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehr D.Why does cannabis smell like skunk? This Iowa State professor has answers; Ames Tribune. Published on April 21, 2021. Available online: https://www.amestrib.com/story/news/2021/04/19/weed-iowa-state-university-isu-participates-research-cause-skunky-skunk-why-marijuana-smell-pot/7010613002/ (accessed on March 02, 2022). [Google Scholar]
- Leigh C.Researchers May Have Discovered the Cause of the Skunky Smell from Cannabis; Veriheal. Published on June 22, 2021. Available online: https://www.veriheal.com/blog/researchers-may-have-discovered-the-cause-of-the-skunky-smell-from-cannabis/ (accessed on March 02, 2022). [Google Scholar]
- Wright D. W.; Koziel J. A.; Parker D. B.; Iwasinska A.; Hartman T. G.; Kolvig P.; Wahe L. Qualitative Exploration of the ‘Rolling Unmasking Effect’ for Downwind Odor Dispersion from a Model Animal Source. Int. J. Environ. Res. Public Health 2021, 18, 13085. 10.3390/ijerph182413085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGorrin R. J.Character-impact flavor compounds. In Sensory Directed Flavor Analysis; Marsili R., Ed.; Taylor & Francis Group: Boca Raton, FL, 2007; Chapter 9. [Google Scholar]
- Wright D. W.; Mahler K. O.; Ballard L. B. The Application of an Expanded Multidimensional G.C. System to Complex Fragrance Evaluations. J. Chromatogr. Sci. 1986, 24, 60–65. 10.1093/chromsci/24.2.60. [DOI] [Google Scholar]
- Wright D. W.; Nielsen L.; Eaton D.; Kuhrt F.; Koziel J. A.; Spinhirne J. P.; Parker D. B. Multidimensional GC-MS-olfactometry for identification and prioritization of malodors from confined animal feeding operations. J. Agric. Food Chem. 2005, 53, 8663–8672. 10.1021/jf050763b. [DOI] [PubMed] [Google Scholar]
- Nielsen L. T.; Eaton D. K.; Wright D. W.; Schmidt-French B. Characteristic odors of Tadarida braziliensis Mexicana chiroptera: Molossidae. J. Cave Karst Stud. 2006, 68, 27–31. [Google Scholar]
- Koziel J. A.; Cai L.; Wright D.; Hoff S. Solid phase microextraction as a novel air sampling technology for improved, GC-Olfactometry-based, assessment of livestock odors. J. Chromatogr. Sci. 2006, 44, 451–457. 10.1093/chromsci/44.7.451. [DOI] [PubMed] [Google Scholar]
- Eaton D. K.; Nielsen L. T.; Wright D. W.. An integrated MDGC-MS-Olfactometry Approach to Aroma and Flavor Analysis. In Sensory Directed Flavor Analysis; Marsili R., Ed.; Taylor and Francis Group LLC: New York, 2007; pp 81–110. [Google Scholar]
- Wright D. W.; Nielsen L.; Eaton D.; Kuhrt F.; Koziel J. A.; Cai L.; Parker D. B.. Animal Odor Assessment - Chickens, Pigs, Bats or Cats; it is Still Analytical Chemistry. In Proceedings of the National Poultry Waste Management Symposium; Springdale AK, USA, 23–25 Oct 2006.
- Wright D. W.; Eaton D. K.; Nielsen L. T.; Kuhrt F. W.; Koziel J. A.; Cai L.; Lo Y.; Parker D. B.; Buser Z.. Synthetic CAFO odor formulation; an effective technique for validation of odorant prioritizations. In Proceedings of the Ecological Society of America Conference; Washington, DC, USA, 5–8 June 2006.
- Wright D.Application of multidimensional gas chromatography techniques to aroma analysis. In Techniques for Analyzing Food Aroma; Ray M., Ed.; Marcel Dekker, Inc.: NY, 1997; Chapter 5. [Google Scholar]
- Bulliner E.A. IV.; Koziel J. A.; Cai L.; Wright D. Characterization of livestock odors using steel plates, solid phase microextraction, and multidimensional - gas chromatography-mass spectrometry- olfactometry. J. Air Waste Manage. Assoc. 2006, 56, 1391–1403. 10.1080/10473289.2006.10464547. [DOI] [PubMed] [Google Scholar]
- Lusk L. T.; Murakami A.; Nielsen L.; Kay S.; Ryder D. Beer photooxidation creates two compounds with aromas indistinguishable from 3-methyl-2-butene-1-thiol. J. of the Am. Soc. of Brew. Chemists. 2009, 67, 189–192. 10.1094/ASBCJ-2009-0910-02. [DOI] [Google Scholar]
- Torrice M. The scientists who sniff water. Chem. Eng. News 2017, 95, 16–19. 10.1021/cen-09527-scitech1. [DOI] [Google Scholar]
- Harbison M.(adapted from Dredge, M.). Daily infographics; beer edition: The beer flavor and aroma wheel. Pop. Sci. (Web Ed.) 2013, 10. Available online: https://www.popsci.com/science/article/2013-01/infographic-day-beersci-edition-beer-flavor-wheel/ (accessed on 17 January 2022). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.