Abstract
Background:
Individuals with unilateral transtibial amputation are at risk of abnormal mechanical joint loading and development of osteoarthritis on sound side joint structures.
Objectives:
This study describes the spatiotemporal and kinetic and kinematic parameters related to osteoarthritis in participants while using (A) a solid-ankle cushioned-heel prosthesis (SACH), (B) a conventional energy storage and return (ESAR) foot prosthesis, and (C) a novel ESAR (N-ESAR) foot prosthesis.
Study design:
A pragmatic randomized controlled trial.
Methods:
K3–K4 ambulators used three feet in a 2-week randomized cross-over order. Kinetics of vertical ground reaction forces (vGRFs) and 3D kinematics of joint angles were integrated to provide normalized parameters. Data were analyzed using one way and mixed model Analysis of variance (ANOVAs) (p < 0.05) and Cohen d statistic.
Results:
Twenty participants, aged 40 ± 16 years with body mass index of 24.7 ± 3.6 kg/m2, experienced minimal change in the spatiotemporal parameters between feet. Participants using the N-ESAR foot prosthesis experienced reduced peak knee external adduction moment (p = 0.030), peak vGRFs (p < 0.001), and peak loading rate of vGRFs (p = 0.030). Peak knee flexion moments only changed when using the solid-ankle cushioned-heel prosthesis, in a positive direction (p = 0.014). Using the N-ESAR prosthesis also increased peak distal shank power during late stance phase (p < 0.001).
Conclusions:
A novel ankle/foot ESAR prosthesis reduces loading on the sound side. With extended use of the N-ESAR foot prosthesis, these findings may provide the prosthesis user with improved outcomes related to sound side loading and development of osteoarthritis.
Keywords: amputees, gait, biomechanics, cross-over studies, osteoarthritis, RCT
Background
There is a strong association between mechanical loading and the development and progression of osteoarthritis (OA), although underlying mechanisms have not yet been fully understood.1-5 It has been postulated the joint’s articular cartilage and subchondral bone sustain microscopic damage from repetitive loading and over time, become stiff. This loss of elasticity limits the joint structures' ability to accept and distribute load during gait, putting the joint at further risk of degeneration.1,6 Biomechanical descriptors of knee joint loading include peak vertical ground reaction forces (PvGRFs) and loading rate of vGRFs (RvGRFs; vertical and shear loading of knee), peak knee external adduction moment (EAM; shear loading of medial knee in frontal plane), and peak knee flexion moment (FM; shear loading of medial knee in sagittal plane).7,8 Increased EAM and FM have been identified as surrogate measures of contact forces in the medial tibiofemoral compartment of the knee and directly linked to radiographic findings, pain, and cartilage damage.9-13 Interventions aimed at reducing the incidence, severity, and progression of OA have targeted EAM and FM as modifiable risk factors.9,14-17
Individuals with unilateral transtibial amputation (UTTA) are at increased risk of sound side knee pain and OA.18 Higher loading observed on the sound side limb has been proposed to be a significant contributor to the 17 times higher incidence of OA in this population.7,8 Furthermore, this loading may be the result of power deficiencies during simultaneous push-off from late stance phase of the prosthetic limb. Thus, by increasing energy return during stance phase, there may be a reduction in sound side loading.19,20 Our understanding of this relationship is limited by few studies and small sample sizes.
South Africa, similar to most developing countries, has both public and private health-care sectors. Clients with medical insurance are provided the best componentry for their individual needs. In the public sector, advanced componentry may not be available.21 Some active individuals with UTTA in diverse settings are provided with single-axis, low-cost feet. This is a multifaceted problem that low-income to middle-income countries are facing.22,23 Robust studies are required within these diverse settings to show the benefits of advanced feet.
This study described spatiotemporal and loading-associated kinetic and kinematic parameters of individuals with UTTA during self-paced walking, while using (1) a solid-ankle cushioned-heel (SACH) prosthesis, (2) an energy storage and return (ESAR) foot prosthesis, and (3) a novel ESAR (N-ESAR) foot prosthesis with a pivot linkage system at the ankle, in a randomized cross-over order. Owing to the ability of N-ESAR prosthesis' to provide the user with more energy during late stance phase, we hypothesized (1) decreased PvGRFs and RvGRFs on the sound side, (2) decreased EAM, with decreased or same FM, on the sound side, and (3) increased peak distal shank power (DSP) during late stance phase on the prosthetic side, while participants used the N-ESAR foot prosthesis.
Methods
Study design
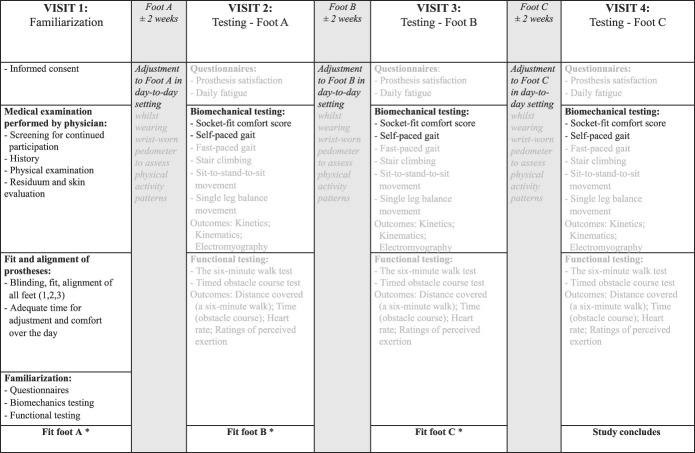
This study was a pragmatic randomized controlled cross-over trial (RCT; Pan African Clinical Trials Registry number: PACTR202006792038036).24 We chose a cross-over design so that each participant served as their own control, minimizing intergroup variation. Wherever possible, we sought to introduce blinding into this study by using measures to conceal prosthetic componentry from both the testing team and participants. Foot allocation randomization was achieved through manual random selection of concealed numbers, and the randomization was revealed only after the study was completed. Figure 1 outlines the full study design and methodology and which outcomes are included in this analysis (black text). The study was approved by the Health Research Ethics Committee of the University (number: N16/08/032).
Figure 1.
Design and testing protocol of the randomized controlled trial, with outcomes under current investigation in black text. *Foot A, B, and C provided in a randomized cross-over order.
Participants
Individuals with UTTA recruited from prosthetists in the local province in South Africa were prescreened to participate in the trial by their prosthetist. These prosthetists were highly experienced, selected primarily because of their established record of high-quality socket manufacture. In controlling for good socket fit, we were limited in our reach of suitable prosthetists, and thus recruited individuals in private care who were using ESAR prostheses at the time of this study. The prosthetist who fitted and aligned the feet for this study was a clinical specialist prosthetist with extensive experience. The inclusion criteria were as follows: K3–K4 activity level,25 men and women aged 18–80 years, UTTA at least one year before testing, medium length limb residuum, and use of a well-fitting and custom-made socket. The exclusion criteria were as follows: uncontrolled cardiovascular or metabolic disease, significant socket-residuum interface pathology or skin breakdown (including bruises, abrasions, lacerations, and infection), and musculoskeletal injury/surgery in the past year that might interfere with walking/exercise. Participants were informed about the study, including testing protocols. Written informed consent was obtained from participants before any further screening or testing commenced.
Testing procedures
Participants reported to the University's Central Analytics Facility for Neuromechanics on four occasions, each separated by two weeks.19,26 Visit 1 included informed consent, medical screening (including residuum skin evaluation), and protocol familiarization. Visits 2–4 comprised the same testing protocol. Before visits 2–4, the participants' socket comfort score was assessed using the socket fit comfort score,27 a validated 11-point scale that indicates the current fit and discomfort/comfort of the socket they are wearing. If the participants rated their socket comfort as less than 5, testing was postponed until the concern was resolved and they reported a score greater than 5. Each foot was fit at the end of visits 1–3 (Figure 1).
Sockets, suspension systems, and prostheses included in the study
Participants' own socket and suspension (liner) systems were retained for this study, and the pylon and foot componentry were changed for each foot. The same socket was used for the entire study period; thus, only individuals with well-fitting daily sockets (predetermined by their prosthetist) were recruited. Any cosmesis was removed before testing.
Three ankle/foot prostheses were used in this study: (1) the SACH foot (Kingsley), a rudimentary prosthesis still prescribed in South Africa (Figure 2(a)); (2) the Vari-Flex foot (Össur hf, Reykjavik, Iceland), a conventional passive ESAR prosthesis with a J-shaped carbon foot attached to a prosthetic heel (Figure 2(b))28-30; and (3) the Pro-Flex foot (Össur hf, Reykjavik, Iceland), a novel ESAR foot prosthesis with pivot linkage system between the forefoot, heel, and pylon, which increases carbon deformation, energy absorption, and power production at the ankle/foot complex (Figure 2(c)).26,31
Figure 2.

The three prosthetic feet prescribed in a randomized cross-over order for daily use over a two-week period in this study – (a) the conventional SACH foot (Kingsley) (b) a conventional energy storage and return (ESAR: Vari-Flex, Össur, Iceland) foot prosthesis, and (c) a novel energy storage and return (N-ESAR: Pro-Flex, Össur, Iceland) foot prosthesis.
The same clinical specialist prosthetist was responsible for all prosthetic blinding, fittings, and follow-ups required. The prostheses were masked from the participants and testing staff (study leader, biomedical engineers, and assistants) and participants. A black sock was secured onto the pylon with a plastic cable tie and the feet labeled “A”, “B,” and “C.” Fit and alignment were conducted as if in clinical practice, in accordance with procedures described by Blumentritt et al (1997).32 All three feet were fit during visit 1, and participants had adequate time to adjust to each prosthesis. Alignment was adjusted as required. Each foot's alignment was then preserved for easy attachment later in the study. Typically, prosthetic studies describing the mechanical properties of prostheses in a controlled setting use laser alignment. This study was conducted as a clinical RCT in a developing country setting, with the direct impact of the study on prosthesis users in mind. Thus, prosthetic fit was completed manually.
Biomechanical testing procedures
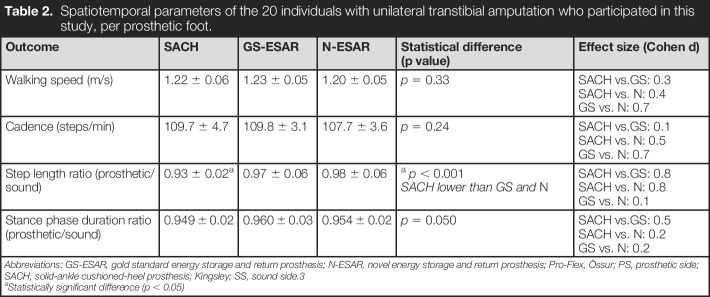
Participants walked 10 times across the laboratory at “a relaxed, normal walking pace.” Kinetics were collected at 1000 Hz using three floor-imbedded force plates (one Bertec FP9060 and two FP6040). Three-dimensional kinematics of the lower limbs and pelvis were recorded with a 10-camera stereophotogrammetry motion capture system (Vicon MX T-series running Nexus 1.8.5 software, UK). A modified Helen-Hayes marker set was used and marker placement on the prosthesis was performed in accordance with Heitzmann et al (2018).26 Integrated kinetic and kinematic data were analyzed using a modified Plug-In Gait model for all outcomes, except ankle/foot power, which was analyzed according to the unified deformable segment model DSP calculation proposed by Takahashi et al (2012)33 and Zelik and Hornert (2018).34 The authors identified this specific DSP calculation as the best for biomechanical analysis of prostheses. A forth-order 7Hz low-pass Butterworth filter was used before the model outputs were exported to Matlab 2018b (Mathworks, Massachusetts) for pre-processing, time normalization, and postprocessing (discrete outcome extraction). Timing of gait cycles was determined using a 20-N onset–offset threshold on the force plates. Spatiotemporal parameters (walking speed, cadence, step length ratio, and stance phase ratio; Table 2) and kinetic and kinematic parameters (EAM, FM, PvGRFs, RvGRFs, and DSP; Table 3) are presented for the SACH, ESAR, and N-ESAR.
Table 2.
Spatiotemporal parameters of the 20 individuals with unilateral transtibial amputation who participated in this study, per prosthetic foot.
| Outcome | SACH | GS-ESAR | N-ESAR | Statistical difference (p value) | Effect size (Cohen d) |
| Walking speed (m/s) | 1.22 ± 0.06 | 1.23 ± 0.05 | 1.20 ± 0.05 | p = 0.33 | SACH vs.GS: 0.3 SACH vs. N: 0.4 GS vs. N: 0.7 |
| Cadence (steps/min) | 109.7 ± 4.7 | 109.8 ± 3.1 | 107.7 ± 3.6 | p = 0.24 | SACH vs.GS: 0.1 SACH vs. N: 0.5 GS vs. N: 0.7 |
| Step length ratio (prosthetic/sound) | 0.93 ± 0.02a | 0.97 ± 0.06 | 0.98 ± 0.06 |
a
p < 0.001 SACH lower than GS and N |
SACH vs.GS: 0.8 SACH vs. N: 0.8 GS vs. N: 0.1 |
| Stance phase duration ratio (prosthetic/sound) | 0.949 ± 0.02 | 0.960 ± 0.03 | 0.954 ± 0.02 | p = 0.050 | SACH vs.GS: 0.5 SACH vs. N: 0.2 GS vs. N: 0.2 |
Abbreviations: GS-ESAR, gold standard energy storage and return prosthesis; N-ESAR, novel energy storage and return prosthesis; Pro-Flex, Össur; PS, prosthetic side; SACH, solid-ankle cushioned-heel prosthesis; Kingsley; SS, sound side.3
Statistically significant difference (p < 0.05)
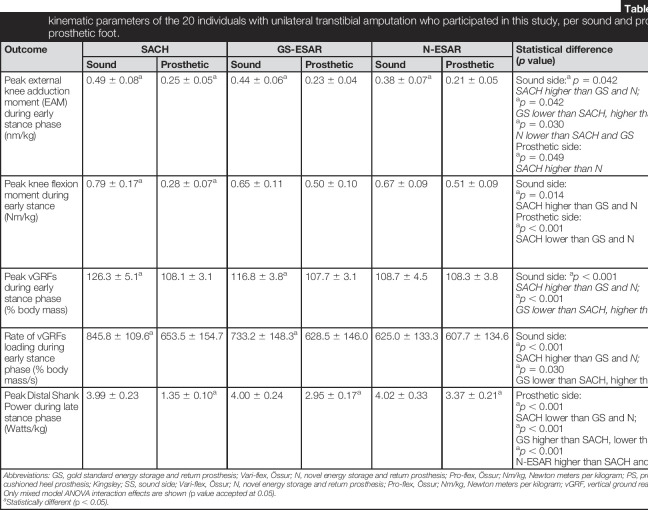
Table 3.
Kinetic and kinematic parameters of the 20 individuals with unilateral transtibial amputation who participated in this study, per sound and prosthetic sides for each prosthetic foot.
| Outcome | SACH | GS-ESAR | N-ESAR | Statistical difference (p value) | Effect size (Cohen d) | |||
| Sound | Prosthetic | Sound | Prosthetic | Sound | Prosthetic | |||
| Peak external knee adduction moment (EAM) during early stance phase (nm/kg) | 0.49 ± 0.08a | 0.25 ± 0.05a | 0.44 ± 0.06a | 0.23 ± 0.04 | 0.38 ± 0.07a | 0.21 ± 0.05 | Sound side:a
p = 0.042 SACH higher than GS and N; ap = 0.042 GS lower than SACH, higher than N; ap = 0.030 N lower than SACH and GS Prosthetic side: ap = 0.049 SACH higher than N |
Sound side: SACH vs. GS: 0.7 SACH vs. N: 1.4 GS vs. N: 0.9 Prosthetic side: SACH vs. GS: 0.7 SACH vs. N: 1.0 GS vs. N: 0.5 |
| Peak knee flexion moment during early stance (Nm/kg) | 0.79 ± 0.17a | 0.28 ± 0.07a | 0.65 ± 0.11 | 0.50 ± 0.10 | 0.67 ± 0.09 | 0.51 ± 0.09 | Sound side: ap = 0.014 SACH higher than GS and N Prosthetic side: ap < 0.001 SACH lower than GS and N |
Sound side: SACH vs.GS: 0.9 SACH vs. N: 0.9 GS vs. N: 0.1 Prosthetic side: SACH vs.GS: 2.4 SACH vs. N: 2.8 GS vs. N: 0.1 |
| Peak vGRFs during early stance phase (% body mass) | 126.3 ± 5.1a | 108.1 ± 3.1 | 116.8 ± 3.8a | 107.7 ± 3.1 | 108.7 ± 4.5 | 108.3 ± 3.8 | Sound side: ap < 0.001 SACH higher than GS and N; ap < 0.001 GS lower than SACH, higher than N |
Sound side: SACH vs.GS: 2.2 SACH vs. N: 3.7 GS vs. N: 1.9 |
| Rate of vGRFs loading during early stance phase (% body mass/s) | 845.8 ± 109.6a | 653.5 ± 154.7 | 733.2 ± 148.3a | 628.5 ± 146.0 | 625.0 ± 133.3 | 607.7 ± 134.6 | Sound side: ap < 0.001 SACH higher than GS and N; ap = 0.030 GS lower than SACH, higher than N |
Sound side: SACH vs.GS: 1.2 SACH vs. N: 2.3 GS vs. N: 1.3 |
| Peak Distal Shank Power during late stance phase (Watts/kg) | 3.99 ± 0.23 | 1.35 ± 0.10a | 4.00 ± 0.24 | 2.95 ± 0.17a | 4.02 ± 0.33 | 3.37 ± 0.21a | Prosthetic side: ap < 0.001 SACH lower than GS and N; ap < 0.001 GS higher than SACH, lower than N; ap < 0.001 N-ESAR higher than SACH and GS |
Prosthetic side: SACH vs.GS: 12.0 SACH vs. N: 13.2 GS vs. N: 2.3 |
Abbreviations: GS, gold standard energy storage and return prosthesis; Vari-flex, Össur; N, novel energy storage and return prosthesis; Pro-flex, Össur; Nm/kg, Newton meters per kilogram; PS, prosthetic side; SACH, solid ankle cushioned heel prosthesis; Kingsley; SS, sound side; Vari-flex, Össur; N, novel energy storage and return prosthesis; Pro-flex, Össur; Nm/kg, Newton meters per kilogram; vGRF, vertical ground reaction force.
Only mixed model ANOVA interaction effects are shown (p value accepted at 0.05).
Statistically different (p < 0.05).
Data analyses
Descriptive data are presented in mean ± standard deviation. Ratio calculations are described as prosthetic limb/sound limb. Statistical analyses are described by providing the p value (significance accepted at 0.05). Effect sizes (ES) were calculated using Cohen' d statistic (small effect: 0.2; medium effect: 0.5; and large effect: 0.8). The statistical analyses were performed using Statistica13 (TIBCO, 2018) software. The Shapiro–Wilk tests for normality were performed. The spatiotemporal parameters were found to be normally distributed and analyzed using one-way ANOVA with Tukey posthoc tests. The kinetic and kinematic parameters were found to be not normally distributed and analyzed using a mixed model ANOVA (foot x side) and covaried for prosthetic testing order, with the Fisher' least significant differences posthoc tests. The Kenward–Roger method addressed heteroskedasticity in the analyses.
Results
Participants
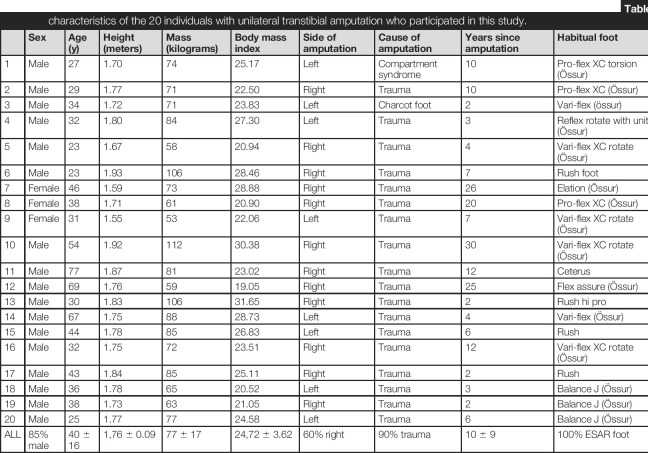
Twenty-one individuals were recruited, but one participant withdrew from the study in the first acclimatization period, before testing. The retained 20 participants (Table 1) included 17 men and 3 women, aged 40 ± 16 years, with body mass index (BMI) of 24.7 ± 3.6 kg/m2. The variation in participants' ages and morphometry (height and body mass) reflects recruitment of individuals in the region that matched our inclusion/exclusion criteria, rather than control for BMI/sex/time since amputation, etc. There were 18 trauma-related (motor vehicle-related, work-related, and gunshot-related) and two disease-related amputations (Charcot foot; compartment syndrome). The time from amputation was 2–26 years. All participants had experience of different prosthetic feet and were habitual ESAR foot prsothesis users at the time of this study. Significant socket-residuum interface pathologies were not recorded during gait. The socket fit comfort score scores were 8 ± 1 (SACH), 9 ± 1 (ESAR), and 9 ± 1 (N-ESAR).
Table 1.
Descriptive characteristics of the 20 individuals with unilateral transtibial amputation who participated in this study.
| Sex | Age (y) | Height (meters) | Mass (kilograms) | Body mass index | Side of amputation | Cause of amputation | Years since amputation | Habitual foot | Suspension (liner) system | |
| 1 | Male | 27 | 1.70 | 74 | 25.17 | Left | Compartment syndrome | 10 | Pro-flex XC torsion (Össur) | Suction (Össur) |
| 2 | Male | 29 | 1.77 | 71 | 22.50 | Right | Trauma | 10 | Pro-flex XC (Össur) | Pin-lock (synergy) |
| 3 | Male | 34 | 1.72 | 71 | 23.83 | Left | Charcot foot | 2 | Vari-flex (össur) | Pin-lock (össur) |
| 4 | Male | 32 | 1.80 | 84 | 27.30 | Left | Trauma | 3 | Reflex rotate with unity (Össur) | Suction (Össur) |
| 5 | Male | 23 | 1.67 | 58 | 20.94 | Right | Trauma | 4 | Vari-flex XC rotate (Össur) | Suction (Össur) |
| 6 | Male | 23 | 1.93 | 106 | 28.46 | Right | Trauma | 7 | Rush foot | Suction (Össur) |
| 7 | Female | 46 | 1.59 | 73 | 28.88 | Right | Trauma | 26 | Elation (Össur) | Suction (Össur) |
| 8 | Female | 38 | 1.71 | 61 | 20.90 | Right | Trauma | 20 | Pro-flex XC (Össur) | Suction (ottobock) |
| 9 | Female | 31 | 1.55 | 53 | 22.06 | Left | Trauma | 7 | Vari-flex XC rotate (Össur) | Suction (Össur) |
| 10 | Male | 54 | 1.92 | 112 | 30.38 | Right | Trauma | 30 | Vari-flex XC rotate (Össur) | Pin-lock (Össur) |
| 11 | Male | 77 | 1.87 | 81 | 23.02 | Right | Trauma | 12 | Ceterus | Pin-lock (Össur) |
| 12 | Male | 69 | 1.76 | 59 | 19.05 | Right | Trauma | 25 | Flex assure (Össur) | Suction (Össur) |
| 13 | Male | 30 | 1.83 | 106 | 31.65 | Right | Trauma | 2 | Rush hi pro | Pin-lock (Össur) |
| 14 | Male | 67 | 1.75 | 88 | 28.73 | Left | Trauma | 4 | Vari-flex (Össur) | Pin-lock (Össur) |
| 15 | Male | 44 | 1.78 | 85 | 26.83 | Left | Trauma | 6 | Rush | Suction (Össur) |
| 16 | Male | 32 | 1.75 | 72 | 23.51 | Right | Trauma | 12 | Vari-flex XC rotate (Össur) | Suction (Össur) |
| 17 | Male | 43 | 1.84 | 85 | 25.11 | Right | Trauma | 2 | Rush | Suction (Össur) |
| 18 | Male | 36 | 1.78 | 65 | 20.52 | Left | Trauma | 3 | Balance J (Össur) | Suction (Össur) |
| 19 | Male | 38 | 1.73 | 63 | 21.05 | Right | Trauma | 2 | Balance J (Össur) | Pin-lock (össur) |
| 20 | Male | 25 | 1.77 | 77 | 24.58 | Left | Trauma | 6 | Balance J (Össur) | Suction (Össur) |
| ALL | 85% male | 40 ± 16 | 1,76 ± 0.09 | 77 ± 17 | 24,72 ± 3.62 | 60% right | 90% trauma | 10 ± 9 | 100% ESAR foot | 100% silicon liner |
Spatiotemporal parameters
Spatiotemporal parameters of participants using the three feet are listed in Table 2. Differences between the feet were minimal: self-paced walking speed (range: 1.20–1.23 m/s); step cadence (range: 107.7–109.8 steps/min); step length ratio (range: 0.93–0.98), and stance phase duration ratio (range: 0.949–0.960).
Kinetic and kinematic parameters
Kinetic and kinematic parameters relating to both the sound and prosthetic sides of participants using the three feet are listed in Table 3. Early stance EAM (Table 3; Figure 3(a)) was reduced on the sound side of the participants using the N-ESAR prsothesis (0.38 ± 0.07 Nm/kg), compared with those using the ESAR prosthesis (0.44 ± 0.06 Nm/kg; p = 0.042; ES = 0.9) and the SACH (0.49 ± 0.08 Nm/kg; p = 0.03; ES = 1.4), compared with the prosthetic sides with similar values (range: 0.21–0.25 Nm/kg).
Figure 3.
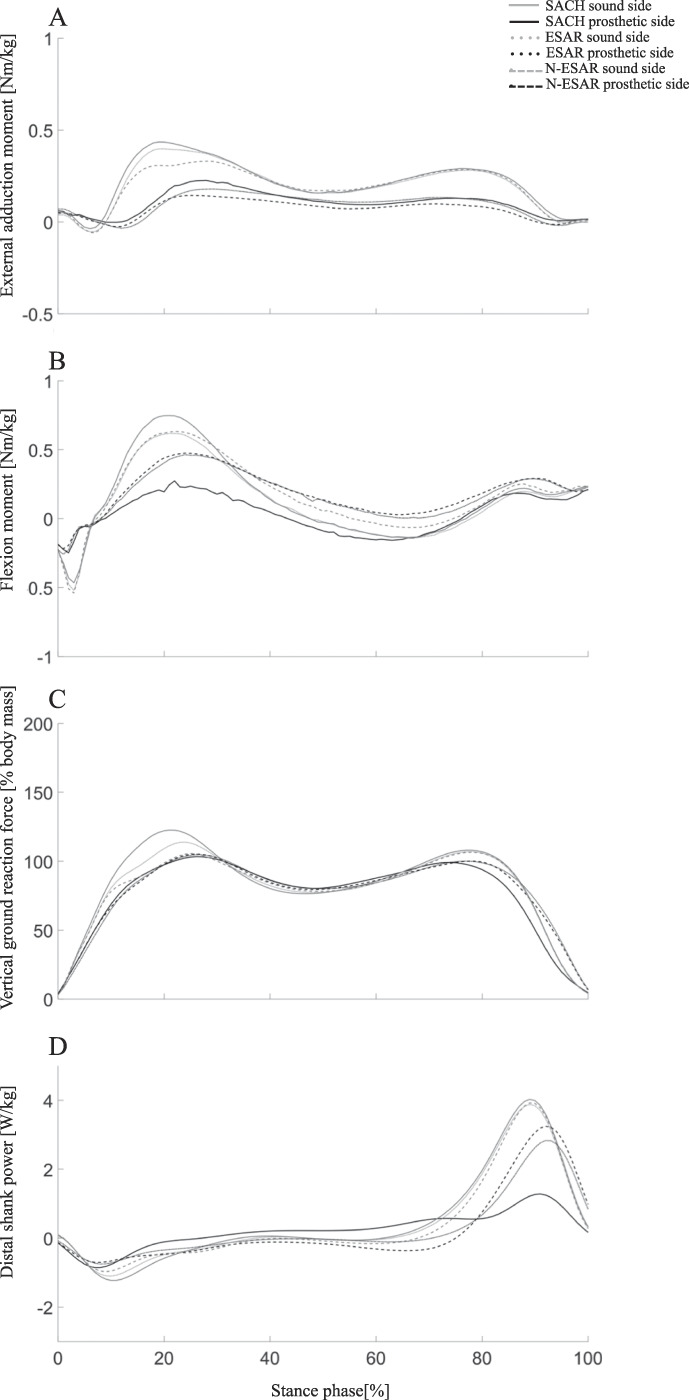
Peak knee external adduction moment (a) peak knee flexion moment (b) vertical ground reaction force (c) and distal shank power (d) during stance phase of gait in the 20 individuals with unilateral transtibial amputation who participated in this study, per sound and prosthetic sides of each prosthetic foot: solid-ankle cushioned-heel (SACH; Kingsley) foot prosthesis; conventional energy storage and return (ESAR; Vari-Flex, Össur, Iceland) foot prosthesis; and novel energy storage and return (N-ESAR) foot prosthesis; Pro-Flex, Össur, Iceland).
Early stance FM (Table 3; Figure 3(b)) was increased on the sound side (0.79 ± 0.17 Nm/kg, p = 0.014, ES = 0.9) and reduced on the prosthetic side (0.28 ± 0.07 Nm/kg, p ≤ 0.001, ES = 0.9) of participants while using the SACH prosthesis. Minimal differences were observed between ESAR and N-ESAR prostheses on the sound (0.65–0.67 Nm/kg; ES = 0.1) and prosthetic (0.50–0.51 Nm/kg; ES = 0.1) sides.
Early stance PvGRFs on the prosthetic side were similar across the three feet (Table 3; Figure 3(c); range: 107.7–108.3% body mass [BM]). There were similar PvGRFs values on the sound and prosthetic sides while participants used the N-ESAR prosthesis (108.7 ± 4.5% BM and 108.3 ±3.8% BM, respectively). However, there was an increase in PvGRFs on the sound side while using the ESAR prosthesis (116.76 ± 3.83 %BM; p ≤ 0.001; ES = 1.9) and the SACH (126.34 ± 5.06 %BM; p ≤ 0.001; ES = 3.7). Early stance RvGRFs (Table 3) were also similar on the prosthetic sides of participants (range: 607.7–653.5% BM/kg). Force developed at a quicker rate on the sound sides of participants while using the ESAR prosthesis (733.2 ± 148.3% BM/kg; p = 0.030; ES = 1.3) and SACH prosthesis (845.8 ± 109.6% BM/kg; p ≤ 0.001; ES = 2.3) compared with that while using the N-ESAR prosthesis (625.0 ± 133.3% BM/s).
Late stance DSP was similar on the sound sides of the participants wearing any of the feet (range: 3.99–4.02 W/kg, Table 3, Figure 3(d)). The N-ESAR prosthetic ankle produced greater DSP (3.37 ± 0.21 W/kg; p ≤ 0.001) than the ESAR prosthesis (2.95 ± 0.17 W/kg; p ≤ 0.001; ES = 4.3). The SACH prosthesis (1.35 ± 0.10 W/kg; p ≤ 0.001; ES = 23.8) generated almost no DSP.
Discussion
This study described spatiotemporal and loading-associated kinetic and kinematic parameters of a group of K3–K4 ambulators using three ankle/foot prostheses. The findings of the study support our hypothesis of reduced early stance mechanical loading (EAM, FM, PvGRFs, and RvGRFs) on the sound side, with increased late stance DSP on the prosthetic side, while using the N-ESAR prosthesis.
The N-ESAR prosthesis reduces mechanical loading on the sound side
Early stance EAM and FM have been described as surrogate biomechanical markers for knee contact forces and targeted as modifiable OA risk factors.4,10,11,17,35 While a causal relationship between these biomechanical outcomes and OA has not been established, there is evidence that EAM loading at baseline can predict radiographic progression of OA six years later, with a 1.9–6.0 times greater risk of OA progression for each degree of positive change in EAM at follow-up.5,36 Furthermore, by targeting EAM through knee bracing and orthotics, knee replacement surgery may be delayed.35,37 Secondary preventive programs aim to reduce EAM before OA diagnosis to reduce risk of OA development.38,39
In prosthesis users, increasing the power generation of the prosthesis has been proposed as the main mechanism by which loading may be reduced on the contralateral sound side.7,8,19,20 The N-ESAR prosthesis (Figure 2(c)) was designed with three carbon blades that work together to evenly distribute ground reaction forces. It uses a fulcrum-based linkage system at the ankle to produce greater ankle power during push-off, providing the user with a smoother step-to-step transition.26,31
In this study, the N-ESAR prosthesis provided 14% more power during late stance phase compared with the ESAR prosthesis (150% more than SACH foot; p < 0.001; d = 2.3–13.2; Figure 3(d)). Furthermore, there was a reduction in early stance phase loading in the leading sound side knee. These changes were observed in both direct vGRFs measures (7%–16% lower than ESAR and SACH feet; p < 0.001; d = 1.2–3.7; Figure 3(c)) and EAM (11-28% lower than ESAR and SACH feet; p = 0.030; d = 0.5–1.4 Figure 3(a)), whereas FM remained the same mostly for the ESAR feet (18% lower than SACH foot; d = 0.1–0.9; Figure 3(b)) for every step taken.9,11
It is interesting to note that this reduction was not observed over the whole stance phase, rather the change was primarily due to the flattened peak during early stance (±20% gait cycle), which occurred earlier in the stance phase than in the prosthetic side. This may indicate the ability of N-ESAR foot prosthesis' to accept and distribute the load through the foot and up the kinetic chain after heel strike, resulting in less shear forces transmitted through the knee joint.26,31 This finding was supported by RvGRFs, which showed a reduced peak and smoother step-to-step transition (Figure 3(c)) over the gait cycle. The combination of greater propulsion, smoother walking gait, and reduced loading may serve the user well, and this may be important clinically.20,38,39 It is also interesting to note that the changes in OA-related risk measures were observed in this study after a short (2 weeks) acclimatization period. Given that EAM is targeted in OA prevention programs,35,37,39 perhaps pre-emptive reductions may help protect prosthesis users from OA development and subsequent progression.10,24 However, this is currently speculative: this study was neither longitudinal nor with individuals with diagnosed OA. This suggestion needs to be supported by appropriate high-quality longitudinal studies.
Performance of the SACH and ESAR prostheses
In South Africa, it is not uncommon for individuals with UTTA treated through the public health-care sector to be prescribed SACH feet.22 In this study, participants were habitual ESAR prosthesis users managed by private sector prosthetists. Given challenges facing global and domestic economics, advanced prosthesis users in South Africa may no longer be able to afford private health care. They may be required to use public services and, potentially, SACH feet prosthesis. Our data show that the SACH demonstrated marked increases in EAM, FM, PvGRFs, and RvGRFs on the sound side, with almost no DSP on the prosthetic side. To provide adequate and equitable solutions in developing countries, it is important for governments to adopt patient-centered practices, including prescription of standard ESAR prostheses.
In this study, the ESAR prosthesis performed better than the SACH prosthesis in all outcomes. Although this report focuses on the N-ESAR prosthesis, the ESAR prosthesis also provided the participants in the study with marked reduction in sound side loading compared with the SACH. This foot has been shown to be cost-effective and functionally reliable in many trials.28-30 Indeed, given the costs associated with advanced prostheses, provision of a standard ESAR foot (in the range including this ESAR) may provide the most sustainable option for public health-care sectors in resource-constrained settings.
Strengths and limitations of the study
To the best of our knowledge, this study was the first large prosthetic RCT conducted in the setting of a developing country. The findings of the study are novel, but not without limitations. Bias may have been introduced into the study because participants were ESAR prosthesis users. This may have magnified negative results related to the SACH and our reason for including two ESAR feet for true comparison purposes. The acclimatization period was brief (two weeks), yet this period was based on previous studies, minimized drop-out, and allowed for financial feasibility.19,26 The prosthesis fit method may be considered a limitation. However, the blended pragmatic approach enabled us to conduct a rigorous study that has real-world application to K3–K4 ambulators in clinical practice.24 While it is almost impossible to “blind” prostheses, every effort was made to mask identification of the prosthetic componentry, and no discussion was entered into regarding the prostheses between participants and testing team. Finally, we acknowledge it is not standard clinical practice to fit K3–K4 ambulators with SACH feet, and these participants may not be very experienced at using these feet.25
Conclusion
Participants using a novel ESAR prosthesis experienced reduced EAM and FM on the sound side during early stance phase of gait. There was a concomitant increase in DSP generated by the prosthesis during late stance phase. With extended use of the N-ESAR prosthesis, these findings may provide the prosthesis user with improved outcomes related to OA. Longitudinal research is required to determine whether the effects observed in this study are maintained over time.
Acknowledgements
The authors thank the participants for their valuable time and effort in participating in this study, the specialist prosthetist and the other prosthetists who assisted with participant recruitment, and the prosthetists who helped with this study, Prof Nando Ferreira, Cara Mills, Tamsin Purkis, Sarah Arnold, Shannon Derman, Megan Oliver, Kyle Winik, Robyn Paulse, and Lisa Erasmus for their assistance with testing and Prof Daan Nel for his assistance with data analysis. The authors thank Össur for their financial and organizational support that made this investigation possible.
Footnotes
Associate Editor: Nachiappan Chockalingam
Contributor Information
John Cockcroft, Email: johnc@sun.ac.za.
Wayne Derman, Email: ewderman@iafrica.com.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by an Össur research funding grant; IOC Research Centre (South Africa) grant; and Claude Leon Foundation Postdoctoral Fellowship grant.
Declaration of conflicting interest
The authors disclosed no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental material
There is no supplemental material in this article.
References
- 1.Vincent KR, Conrad BP, Fregly BJ, et al. The Pathophysiology of Osteoarthritis: A Mechanical Perspective on the Knee Joint. Pharm Manag PM R 2012; 4: S3–S9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andriacchi TP, Mündermann A, Smith RL, et al. A Framework for the in Vivo Pathomechanics of Osteoarthritis at the Knee. Ann Biomed Eng 2004; 32: 447–457. [DOI] [PubMed] [Google Scholar]
- 3.Tanamas S, Hanna FS, Cicuttini FM, et al. Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis Care Res 2009; 61: 459–467. [DOI] [PubMed] [Google Scholar]
- 4.Foroughi N Smith R and Vanwanseele B. The association of external knee adduction moment with biomechanical variables in osteoarthritis: a systematic review. Knee 2009; 16: 303–309. [DOI] [PubMed] [Google Scholar]
- 5.Henriksen M, Creaby MW, Lund H, et al. Is there a causal link between knee loading and knee osteoarthritis progression? A systematic review and meta-analysis of cohort studies and randomised trials. BMJ Open 2014; 4: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin JA, Brown TD, Heiner AD, et al. Chondrocyte Senescence, Joint Loading and Osteoarthritis. Clin Orthop Relat Res 2004; 427S: S96–S103. [DOI] [PubMed] [Google Scholar]
- 7.Morgenroth DC Gellhorn AC and Suri P. Osteoarthritis in the Disabled Population: A Mechanical Perspective. Pharm Manag PM R 2012; 4: S20–S27. [DOI] [PubMed] [Google Scholar]
- 8.Morgenroth DC, Medverd JR, Seyedali M, et al. The relationship between knee joint loading rate during walking and degenerative changes on magnetic resonance imaging. Clin Biomech 2014; 29: 664–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richards RE, Andersen MS, Harlaar J, et al. Relationship between knee joint contact forces and external knee joint moments in patients with medial knee osteoarthritis: effects of gait modifications. Osteoarthritis Cartilage 2018; 26: 1203–1214. [DOI] [PubMed] [Google Scholar]
- 10.Kutzner I, Trepczynski A, Heller MO, et al. Knee adduction moment and medial contact force--facts about their correlation during gait. PLoS One 2013; 8: e81036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manal K, Gardinier E, Buchanan TS, et al. A more informed evaluation of medial compartment loading: the combined use of the knee adduction and flexor moments. Osteoarthritis Cartilage 2015; 23: 1107–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Creaby M. It's not all about the knee adduction moment: the role of the knee flexion moment in medial knee joint loading. Osteoarthritis Cartilage 2015; 23: 1038–1040. [DOI] [PubMed] [Google Scholar]
- 13.Koelewijn AD and van den Bogert AJ. Joint contact forces can be reduced by improving joint moment symmetry in below-knee amputee gait simulations. Gait Posture 2016; 49: 219–225. [DOI] [PubMed] [Google Scholar]
- 14.Favre J, Erhart-Hledik JC, Chehab EF, et al. General scheme to reduce the knee adduction moment by modifying a combination of gait variables. J Orthop Res 2016; 34: 1547–1556. [DOI] [PubMed] [Google Scholar]
- 15.Shull PB, Shultz R, Silder A, et al. Toe-in gait reduces the first peak knee adduction moment in patients with medial compartment knee osteoarthritis. J Biomech 2012; 46: 122–128. [DOI] [PubMed] [Google Scholar]
- 16.Cutti AG, Verni G, Migliore GL, et al. Reference values for gait temporal and loading symmetry of lower-limb amputees can help in refocusing rehabilitation targets. J NeuroEng Rehabil 2018; 15: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohd Sharif NA, Usman J, Wan Safwani WKZ, et al. Effects of simple knee sleeves on pain and knee adduction moment in early unilateral knee osteoarthritis. Proc IME H J Eng Med 2019; 233: 1132–1140. [DOI] [PubMed] [Google Scholar]
- 18.Norvell DC, Czerniecki JM, Reiber GE, et al. The Prevalence of Knee Pain and Symptomatic Knee Osteoarthritis Among Veteran Traumatic Amputees and Nonamputees. Arch Phys Med Rehabil 2005; 86: 487–493. [DOI] [PubMed] [Google Scholar]
- 19.Karimi MT, Salami F, Esrafilian A, et al. Sound side joint contact forces in below knee amputee gait with an ESAR prosthetic foot. Gait Posture. 2017; 58: 246–251. [DOI] [PubMed] [Google Scholar]
- 20.Morgenroth DC, Segal AD, Zelik KE, et al. The effect of prosthetic foot push-off on mechanical loading associated with knee osteoarthritis in lower extremity amputees. Gait Posture 2011; 34: 502–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dechambenoit G. Access to health care in sub-Saharan Africa. Surg Neurol Int 2016; 7: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andrysek J. Lower-limb prosthetic technologies in the developing world: A review of literature from 1994–2010. Prosthet Orthot Int 2010; 34: 378–398. [DOI] [PubMed] [Google Scholar]
- 23.Naidoo U and Ennion L. Barriers and facilitators to utilisation of rehabilitation services amongst persons with lower-limb amputations in a rural community in South Africa. Prosthet Orthot Int 2019; 43: 95–103. [DOI] [PubMed] [Google Scholar]
- 24.Nieuwenhuis JB, Irving E, Oude Rengerink K, et al. Pragmatic trial design elements showed a different impact on trial interpretation and feasibility than explanatory elements. J Clin Epidemiol 2016; 77: 95–100. [DOI] [PubMed] [Google Scholar]
- 25.Gailey RS, Roach KE, Applegate EB, et al. The Amputee Mobility Predictor: An instrument to assess determinants of the lower-limb amputee’s ability to ambulate. Arch Phys Med Rehabil 2002; 83: 613–627. [DOI] [PubMed] [Google Scholar]
- 26.Heitzmann DWW, Salami F, de Asha AR, et al. Benefits of an increased prosthetic ankle range of motion for individuals with a trans-tibial amputation walking with a new prosthetic foot. Gait Posture 2018; 64: 174–180. [DOI] [PubMed] [Google Scholar]
- 27.Hanspal RS Fisher K and Nieveen R. Prosthetic socket fit comfort score. Disabil Rehabil 2003; 25: 1278–1280. [DOI] [PubMed] [Google Scholar]
- 28.Barr AE, Siegel KL, Danoff Jv, et al. Biomechanical comparison of the energy-storing capabilities of SACH and Carbon Copy II prosthetic feet during the stance phase of gait in a person with below-knee amputation. Phys Ther 1992; 72: 344–354. [DOI] [PubMed] [Google Scholar]
- 29.Torburn L, Perry J, Ayyappa E, et al. Below-knee amputee gait with dynamic elastic response prosthetic feet: A pilot study. J Rehabil Res Dev 1990; 27: 369–384. [DOI] [PubMed] [Google Scholar]
- 30.Hsu MJ, Nielsen DH, Lin-Chan SJ, et al. The effects of prosthetic foot design on physiologic measurements, self-selected walking velocity, and physical activity in people with transtibial amputation. Arch Phys Med Rehabil 2006; 87: 123–129. [DOI] [PubMed] [Google Scholar]
- 31.Childers WL and Takahashi KZ. Increasing prosthetic foot energy return affects whole-body mechanics during walking on level ground and slopes. Sci Rep 2018; 8: 5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blumentritt S. A new biomechanical method for determination of static prosthetic alignment. Prosthet Orthot Int 1997; 21: 107–113. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi KZ Kepple TM and Stanhope SJ. A unified deformable (UD) segment model for quantifying total power of anatomical and prosthetic below-knee structures during stance in gait. J Biomech 2012; 45: 2662–2667. [DOI] [PubMed] [Google Scholar]
- 34.Zelik KE and Honert EC. Ankle and foot power in gait analysis: Implications for science, technology and clinical assessment. J Biomech 2018; 75: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moyer RF, Birmingham TB, Dombroski CE, et al. Combined effects of a valgus knee brace and lateral wedge foot orthotic on the external knee adduction moment in patients with varus gonarthrosis. Arch Phys Med Rehabil 2013; 94: 103–112. [DOI] [PubMed] [Google Scholar]
- 36.Miyazaki T, Wada M, Kawahara H, et al. Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Ann Rheum Dis 2002; 61: 617–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paul D, Lee YF, Lee PY, et al. Unloading knee brace is a cost-effective method to bridge and delay surgery in unicompartmental knee arthritis. Exercise Medicine 2017; 2: 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahmoudian A, Van Assche D, Herzog W, et al. Towards secondary prevention of early knee osteoarthritis. RMD Open 2018; 4: e000468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roos EM and Arden NK. Strategies for the prevention of knee osteoarthritis. Nat Rev Rheumatol 2016; 12: 92–101. [DOI] [PubMed] [Google Scholar]