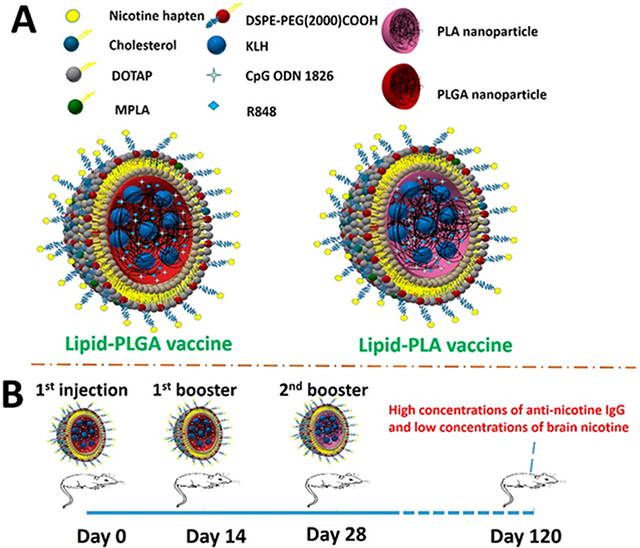
Abstract
Nicotine vaccines have been investigated to assist with smoking cessation. Because smoking cessation is a long process, past nicotine vaccines required multiple injections to achieve long-term efficacy. It would be of great significance if extended efficacy can be achieved with fewer injections. Here, we report the assembly of lipid-polylactic acid (PLA) and lipid-poly(lactic-co-glycolic acid) (PLGA) hybrid nanoparticle (NP) based nicotine vaccines. Mice immunized with the lipid-PLGA vaccine produced higher titers of nicotine-specific antibodies than the lipid-PLA vaccine in short-term. However, the lipid-PLA vaccine was found to induce long-lasting antibodies. Three months after the immunization, only mice that received first two injections of the lipid-PLGA vaccine and a third injection of the lipid-PLA vaccine achieved a significantly lower brain nicotine concentration of 65.13 ± 20.59 ng/mg than 115.88 ± 37.62 ng/mg from the negative controls. The results indicate that not only the stability of the vaccines but also the combination of the vaccines impacted the long-term efficacy of the immunization. Lastly, both the body weight and the histopathology study suggest that the vaccines were safe to mice. These findings suggest that long-term immunity against nicotine can be realized by a rational administration of nanovaccines of different levels of stability.
Keywords: nicotine vaccine, liposome hybrid nanoparticle, humoral response, smoking cessation, extended immunity
Graphical Abstract
1. INTRODUCTION
Cigarette smoking is a worldwide pandemic, which is responsible for more than 7 million deaths per year.1 Nicotine vaccines can elicit production of nicotine-specific antibodies (NSAs), reduce the entry of nicotine into the brain, and thus assist with smoking cessation.2,3 The effectiveness of a nicotine vaccine relies heavily on the concentration of NSAs in the bloodstream.4,5 It has been repeatedly shown in clinical trials that higher concentrations of nicotine-antibodies were associated with higher quitting rates.6,7 In addition, smoking cessation is a long-term process, which may take several months to reach the goal.8 Therefore, it is necessary for vaccines to induce a strong and long-lasting immune response against nicotine. A strong immune response requires a nicotine vaccine to be highly immunogenic, which may be achieved by selecting appropriate carrier proteins, nicotine epitopes, and adjuvants, etc.2,9,10 However, a prolonged immune response is difficult to attain with a limited number of injections, because nicotine on its own is not able to activate memory B cells, which means that the level of NSAs may rapidly drop without boosting injections.11 The most straightforward way to maintain a high level of NSAs is through repeated injections of the vaccines at certain intervals.4-6 However, this is not only costly, but also inconvenient to the users, which may dampen the willingness of smokers to use nicotine vaccines. Therefore, it is necessary to develop a nicotine vaccine or a vaccination scheme that can maintain a high level of antibodies against nicotine over a long period of time with fewer booster injections.
Our previous studies demonstrated that a lipid-PLGA hybrid NP-based nicotine vaccine (NanoNicVac) could more effectively and specifically induce the immune system to produce NSAs than a traditional carrier protein-based nicotine vaccine.12-16 The unique structure of this vaccine allows flexible modifications, including modulating its surface charge, particle size, and incorporating different molecular adjuvants, for stronger immunogenicity.
On the other hand, as we found in a previous study that the incorporation of a nanohorn as the core into liposomes produced nanohorn-liposome hybrid NPs, which had significantly better stability than the blank liposomes.17 The resulting hybrid NP-based nicotine vaccine was significantly more immunogenic than the liposome-based vaccine. In subsequent studies, nanohorn was substituted by biodegradable and biocompatible poly(lactic-co-glycolic acid) (PLGA) NPs for better safety.14 Since the better stability of a NP-liposome hybrid NP is attributed to the presence of the NP core, it is possible to improve the stability of a hybrid NP-based vaccine by strengthening the stability of the NP core. A nicotine vaccine assembled from such a stabilized hybrid NP may stay intact in the body for longer time, providing extended signal to the immune system and achieving a long-lasting immune response. It is reported that the stability of a PLGA NP is dependent on the ratio of lactic acid and glycolic acid in the polymer chain.18 At a ratio of 50:50 of lactic acid and glycolic acid, the resulting PLGA NPs have the fastest degradation rate, while the NPs composed of only polylactic acid (PLA) has the slowest degradation rate.19,20 It is possible that compared to lipid-PLGA nicotine vaccine, a lipid-PLA nicotine vaccine may circulate in the body for longer time and induce a prolonged immune response.
In this study, we assembled two nicotine vaccines from lipid- PLGA and lipid-PLA hybrid NPs, respectively. It was found that the lipid-PLA-based nicotine vaccine (PLA vaccine) has much better stability than the lipid-PLGA-based nicotine vaccine (PLGA vaccine) under both in vitro and in vivo conditions. However, mice immunized with either the PLA vaccine or the PLGA vaccine alone did not achieve high concentrations of long-lasting NSAs. Interestingly, it was the mice that received the first two injections of the PLGA vaccine, followed by a third injection of the PLA vaccine attained high concentrations of NSAs for up to 3 months and had significantly reduced brain nicotine levels.
2. RESULTS AND DISCUSSION
2.1. Characterization of Physicochemical Properties and Morphologies of Nicotine Vaccines.
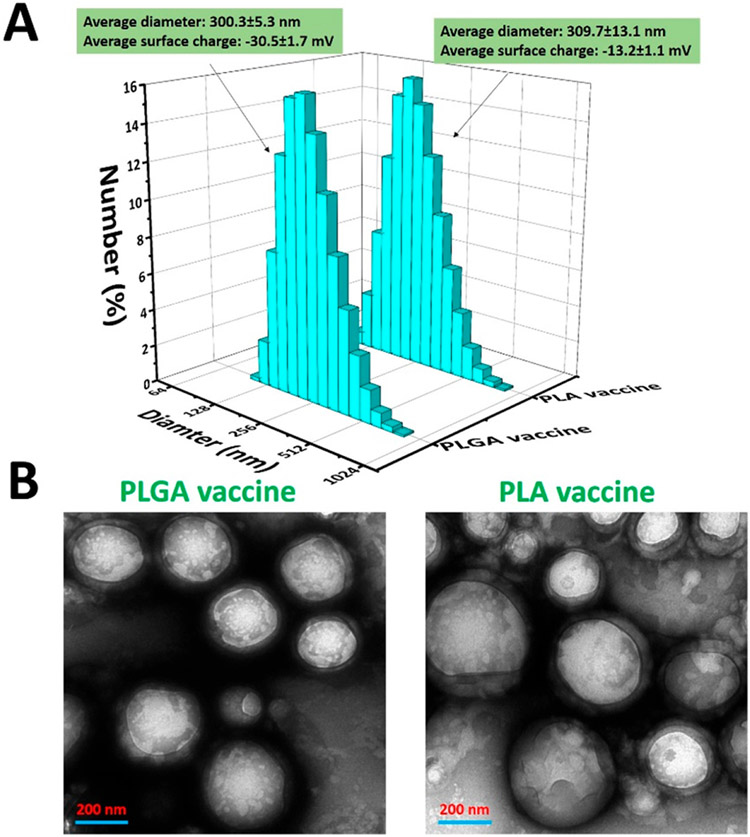
In this study, nicotine vaccines were assembled by conjugating nicotine haptens onto hybrid NPs containing either a PLGA core or a PLA core. The surface charge and size distribution of the vaccine particles were characterized. As shown in Figure 1A, the PLGA vaccine and the PLA vaccine had close average diameters of 300.3 and 309.7 nm, respectively. As shown in previous studies, nicotine vaccines with a nanosized dimension were efficiently internalized by immune cells.12-17,21 Particle surface charge, another important factor that can largely influence the cellular uptake of vaccine particles, was also measured for the vaccine particles.22-24 As shown in Figure 1A, the PLGA vaccine and the PLA vaccine had a zeta potential of −30.5 mV and −13.2 mV, respectively. Although, it was reported that vaccines that carried positive surface charges could be more easily captured by immune cells, our previous findings demonstrated that dendritic cells (DCs) were able to efficiently internalize anionic nicotine vaccines.14,25 In addition, compared to anionic NPs, cationic NPs were found to significantly disrupt plasma-membrane integrity and cause serious damage to mitochondria and lysosome.26 Therefore, anionic nicotine vaccines may be safer than cationic nicotine vaccines.
Figure 1.
Physiochemical properties of nano vaccines. (A) Physiochemical properties of the PLGA vaccine and the PLA vaccine. (B) TEM images of vaccine particles. The scale bars represent 200 nm
The morphologies of the vaccine particles were characterized using a transmission electron microscope (TEM). As shown in Figure 1B, both the PLGA vaccine and the PLA vaccine consisted of a nanosized core structure as well as a lipid shell surrounding the core. This is consistent with our previous findings that core–shell hybrid NPs could be assembled by sonicating a mixture of liposome and PLGA NPs.14,16 As discussed in previous studies, the core–shell structure not only allowed codelivery of antigen and adjuvants, but also facilitated the cellular uptake of the vaccine particles by immune cells.12,14,15,17 In addition, agreeing with the results in Figure 1A, the sizes of the two vaccine particles were around 300 nm. Overall, the two vaccine particles shared similar physicochemical properties and morphologies.
2.2. Cellular Uptake and Processing of Vaccine Particles by DCs.
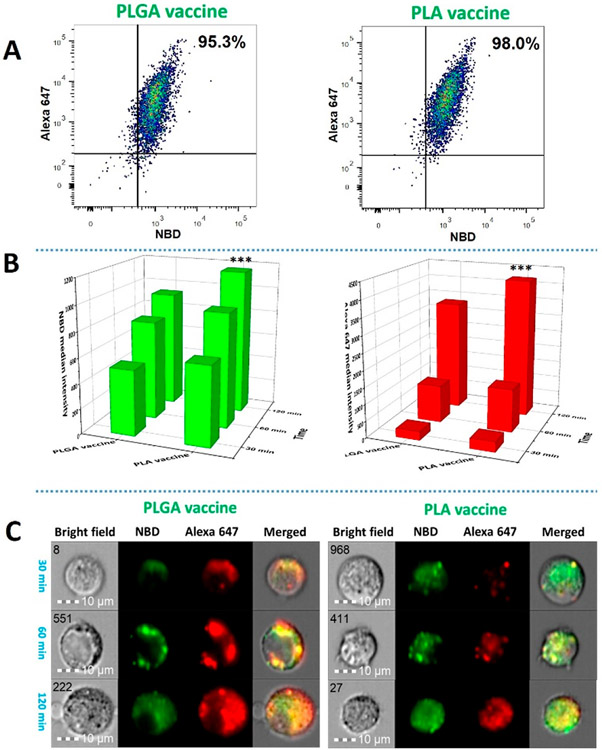
The development of a humoral immune response starts from the recognition and internalization of antigens by antigen presenting cells (APCs), such as DCs and B cells.27,28 Therefore, how efficiently a vaccine can be taken up by APCs may affect the quality of the resulting immune response. In our previous studies, we found that lipid-PLGA hybrid NPs were rapidly internalized by DCs.14,16 In this study, to monitor the uptake of the vaccine particles, keyhole limpet hemocyanin (KLH) in the core and the lipid layer of both the PLGA vaccine and the PLA vaccine were labeled with Alexa 647 (red) and NBD (green), respectively. As shown in Figure 2A, within 120 min, 95.3% and 98.0% of the DCs internalized the PLGA vaccine and the PLA vaccine, respectively. These results show that the two vaccine particles were rapidly captured by the DCs. Remarkably, both NBD and Alexa 647 were simultaneously detected in most of the cells. This demonstrates that the lipid layer and the core structure of the vaccine particles were concomitantly internalized by the cells. As discussed in a previous study, codelivery of the nicotine hapten on the lipid surface and the protein antigen in the core was essential for the development of nicotine-specific immune response.14 One of the advantages of the hybrid nanoparticle-based vaccines over the conventional carrier protein-nicotine conjugate vaccine is that they enable codelivery of antigens and adjuvants to the immune cells, which may produce stronger immune response and minimize systemic toxicity of the adjuvants.29,30 In addition, enclosing KLH inside the polymer core can reduce the exposure of the protein antigens to B cell receptors and decrease the number of KLH-specific antibodies, thereby improving the specificity of the vaccine. In contrast, the traditional protein-nicotine conjugate vaccines cannot avoid the production of antibodies against the carrier proteins 14.
Figure 2.
Cellular uptake and processing of nicotine vaccine particles by DCs. Two million DCs were incubated with 0.5 mg of PLGA nicotine vaccine or PLA nicotine vaccine, of which the lipid layer was labeled with NBD (green) and the KLH was labeled with Alexa 647 (Red), for 30, 60, and 120 min, respectively. The uptake and processing of vaccine particles by the DCs were quantitatively and qualitatively studied using a BD FACSARIA flow cytometer and an Amnis ImageStream flow cytometer, respectively. (A) The percentages of the cells that took up the PLGA and PLA vaccine particles within 120 min. (B) NBD and Alexa 647 median intensities from the cells that were incubated with the vaccine particles for a given period of time. (C) Representative images of processing of vaccine particles in the DCs. The scale bars represent 10 μm. *** denotes that significantly more PLA vaccine was taken up by DCs than PLGA vaccine at 120 min.
In addition, the relative quantities of the internalized vaccine particles were measured over time. As shown in Figure 2B, both the median intensities of Alexa 647 and NBD increased with time in the DCs that were treated with either the PLGA vaccine or the PLA vaccine. It is noteworthy that the intensities of the two fluorescence signals did not increase proportionally. This might be caused by the fact that Alexa 647 is less photosensitive than NBD.31 Although close percentages of cells captured the two vaccine particles within 120 min, significantly more PLA vaccine was detected in the DCs than the PLGA vaccine at 120 min. As shown in Figure 1A, the PLGA vaccine carried more negative charge than the PLA vaccine, this might cause elevated electrostatic repulsion between the vaccine particle and the negative charge on the cell membranes, leading to hindered cellular uptake of the PLGA vaccine.32
Following uptake, protein antigens in DCs need to be processed into antigenic peptides and presented on cell surface for the activation of T cells.33 Therefore, antigen processing is also a critical step in the development of an immune response.34 In this study, the processing of vaccines by DCs were studied using an ImageStream flow cytometer. As shown in Figure 2C, represented by the wide distribution of the red fluorescence, a large amount of Alexa 647 was released from the internalized PLGA vaccine within 30 min. In contrast, at 30 min, the red fluorescence from the PLA vaccine was confined in small vesicles, indicating the protein antigen (KLH) was contained in the PLA core without being processed. These results implied that the PLA vaccine was degraded slower than the PLGA vaccine in the cells. At 60 min, considerable amount of the red fluorescence was released from the PLA vaccine, indicating that part of the early internalized vaccine particles was degraded. Strikingly, by 120 min, most of the PLGA vaccine particles and PLA vaccine particles were degraded in the cells. Overall, these findings demonstrate that, despite the relatively lower uptake rate, PLGA vaccine was more efficiently processed by the DCs than the PLA vaccine. An efficient antigen processing may provide sufficient amount of processed antigenic peptides to T cells and facilitate the signaling between APCs and T cells, thereby promoting immune response. In contrast, an inefficient antigen processing may negatively affect the development of an immune response.35
2.3. In Vitro Stability of Vaccines.
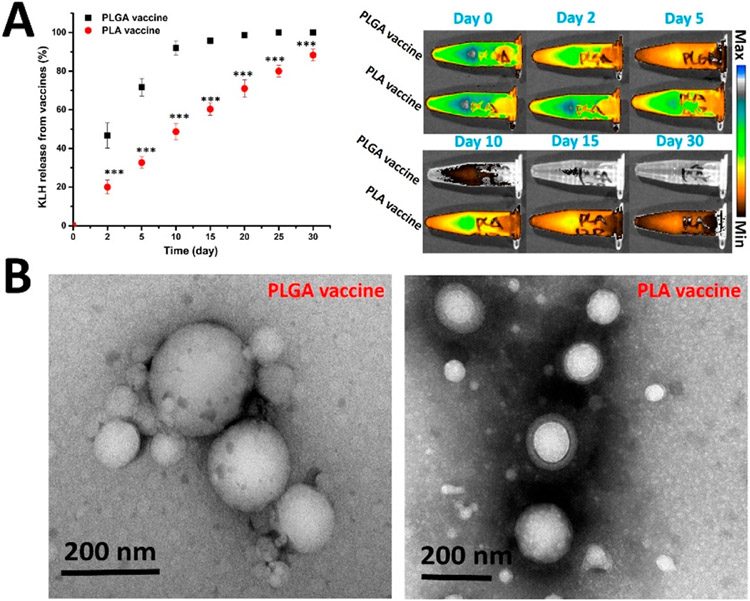
The stability of a vaccine can have a significant impact on its immunological performance.36,37 Better stability may increase the bioavailability of a vaccine to the immune system, thus enhancing the immune response. In addition, a stable vaccine can persist in the body for an extended time, leading to prolonged stimulation to immune cells. Moreover, stable vaccines may be more easily stored and transported with reduced cost.38 To compare the stability of the PLGA vaccine and the PLA vaccine, the two vaccines were suspended in human serum and the release of KLH was monitored. As shown in Figure 3A (left), at any studied time points, a significantly higher amount of KLH was released from the PLGA vaccine than that from the PLA vaccine. In addition, the PLGA vaccine released almost all the KLH within 15 days. In contrast, only 60% of KLH was released from the PLA vaccine within 15 days. Remarkably, as high as 12% of the KLH remained in the PLA vaccine after 30 days’ incubation. The decay of Alexa 647 shown in Figure 3A (right) also indicates that the PLGA vaccine lost KLH more rapidly than the PLA vaccine.
Figure 3.
In vitro degradation of PLGA and PLA vaccine particles. Two milligrams of PLGA or PLA vaccine particles that were suspended in 1 mL human serum (5% v/v, pH 7.4) was placed in a Spectra/Por Float-A-Lyzer G2 dialysis device and dialyzed against 30 mL of human serum (5% v/v, pH 7.4) at 37 °C. (A) Cumulative release of KLH from PLGA and PLA nicotine vaccines (left) and the remaining Alexa 647 fluorescence in the vaccine particles at given time points (right). (B) TEM images of vaccine particles after incubation in human serum for 30 days. The scale bars represent 200 nm. *** denotes that the amount of KLH release from the PLA vaccine was significantly less than that from the PLGA vaccine at the indicated time points.
The morphology of the vaccine particles after 30 days of incubation in human serum was studied using a TEM. As shown in Figure 3B, most of the PLGA vaccine particles were degraded, which was reflected by the loss of the lipid layer. In contrast, many of the PLA vaccine particles still retained the lipid layer. As discussed in a previous study, an intact lipid-NP hybrid structure was essential for codelivery of the nicotine antigen and T cell-adjuvant protein to induce an effective immune response.14 The loss of the lipid layer in the PLGA vaccine is likely to render this vaccine less immunogenic after long circulation in the body.
2.4. In Vivo Stability of Vaccines.
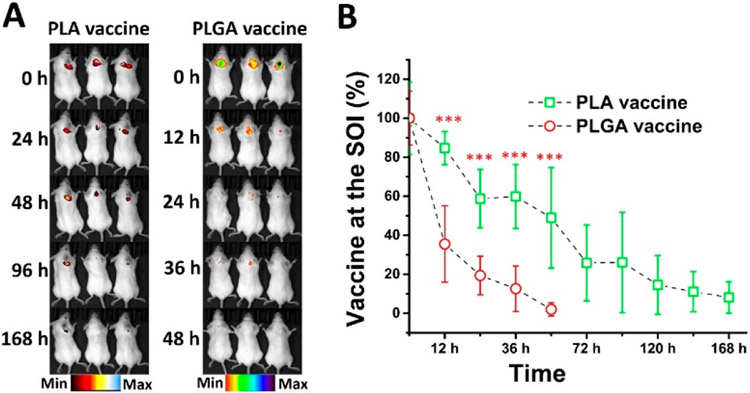
To evaluate the in vivo stability of the vaccines, mice were subcutaneously injected with Alexa 750-labeled vaccine particles, which were studied using an IVIS SpectrumCT. As shown in Figure 4A, the PLGA vaccine disappeared at the site of injection (SOI) much faster than the PLA vaccine. At 48 h after the injection, the PLGA vaccine was not detectable at the SOI. In contrast, the PLA vaccine stayed at the SOI for up to 168 h. As also shown in Figure 4B, as much as 84% of the PLA vaccine was detected at the SOI at 12 h, while only 35% of the PLGA vaccine was detected. In addition, the PLGA vaccine at the SOI was hardly detectable at 48 h, but up to 48% of the PLA vaccine was present. Post injection, there was a significantly higher amount of the PLA vaccine detected at the SOI than that of the PLGA vaccine at any studied time points. Although part of the decrease in the amount of the vaccines at the SOI might be attributed to cellular uptake of the particles, given the fact shown in Figure 2 that the immune cells internalized the PLA vaccine more efficiently than the PLGA vaccine, the faster loss of the PLGA vaccine at the SOI was more likely a result of faster degradation. Consistent with the in vitro stability of the vaccines, these in vivo results demonstrate that the PLA vaccine had better in vivo stability than the PLGA vaccine. Interestingly, both the PLGA and PLA vaccines tended to stay at the SOI instead of migrating away. This could form a vaccine depot at the SOI. One of the mechanisms that contribute the adjuvant effect of Alum is that Alum can retain vaccines at the SOI and form a vaccine depot.39,40 The slow degradation of the PLA vaccine at the depot will likely benefit the immunogenicity of the vaccine, because damages in the vaccines may render the vaccine less effective. Moreover, extended existence of the PLA vaccine might provide sustained signals to immune cells for prolonged immune response.
Figure 4.
In vivo degradation of vaccine particles in mice. The protein antigen KLH in the vaccine was labeled with Alexa 750 and 2 mg of the PLGA vaccine, or the PLA vaccine was subcutaneously injected into female BALB/c mice. The degradation of the vaccine particles in the mice was monitored using an IVIS SpectrumCT. (A) The fluorescence image of vaccine particles at the SOI. (B) The relative amount of nondegraded vaccine particles at the SOI. *** denotes that the amount of remaining PLA nicotine vaccine was significantly higher than that of the PLGA nicotine vaccine at the SOI.
2.5. Immunogenicity of Nanovaccines.
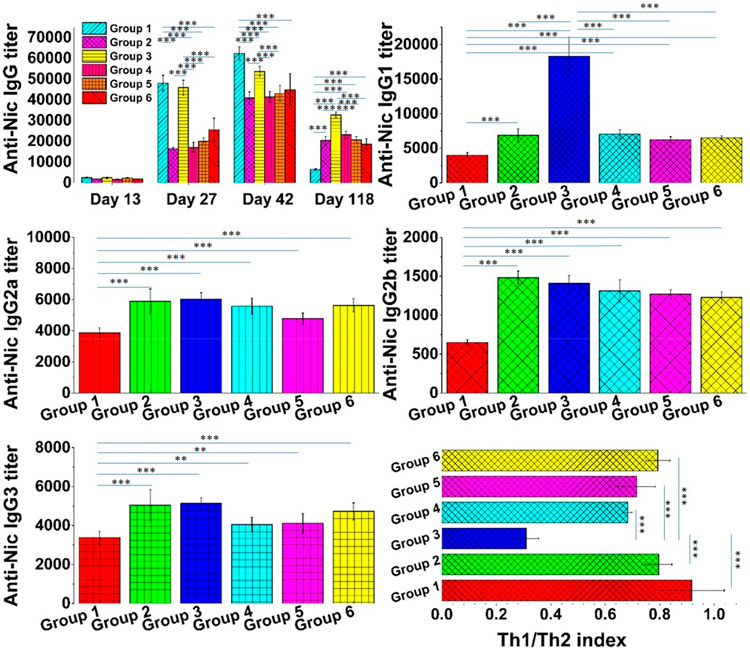
Anti-Nic antibody titer is one of the most important parameters for gaging the immunogenicity of nicotine vaccines.2,14,16 In preclinical trials, higher titers of anti-Nic antibodies were associated with lower brain nicotine concentrations.10,12,40 In clinical trials, people with higher anti-Nic antibodies in the blood were found to quit smoking more readily.6,41,42 Although a nicotine vaccine may induce production of many types of antibodies, such as IgA, IgG, and IgE, usually only the titer of IgG is evaluated because it is the most abundant serum antibody in humans.43,44 In this study, the titers of anti-Nic IgG from serum collected on days 13, 27, 42, and 118 were measured. As shown in Figure 5, mice injected with various combinations of the PLGA vaccine and the PLA vaccine, as illustrated in Table 1, produced markedly different levels of anti-Nic IgG. On day 13, minimal anti-Nic IgG titers of 2571 ± 282, 1818 ± 147, 2430 ± 352, 1657 ± 261, 2406 ± 167, and 1827 ± 52, which resulted from the first injection, were detected in groups 1, 2, 3, 4, 5, and 6, respectively. For humoral immune response, the first encounter of antigens usually does not result in high magnitude of antibody production, but instead, it primes the immune system for the next encounter with the antigens.14,23 It has been widely reported that when a primed immune system is challenged with a previous encountered antigen, the memory B cells can rapidly proliferate to produce high levels of antibodies.45,46 As expected, the first booster vaccine injection pronouncedly increased the titers to 48077 ± 3732, 16482 ± 696, 45875 ± 3654, 17138 ± 2367, 19984 ± 1708, and 25565 ± 5568 for groups 1, 2, 3, 4, 5, and 6, respectively. This was consistent with previous studies, in which booster injections significantly increased the concentrations of anti-Nic IgG.14-17,40,47 Interestingly, groups 1 and 3, which were administered with the PLGA vaccine both on days 0 and 14, show significantly higher anti-Nic IgG than those of other groups. This indicates that the PLGA vaccine was more immunogenic than the PLA vaccine. Although the underlying mechanism was unclear, it was possible that as shown in Figure 2C, the slower degradation of the PLA vaccine in DCs might not provide as many stimulating signals to the immune cells as the PLGA vaccine might, resulting in the suboptimal immunogenicity of the PLA vaccine. This hypothesis was also supported by comparing the antibody titers between group 2 and group 6. Both groups were injected with the PLA vaccine on day 0, but the PLGA vaccine injection in group 6 on day 14 produced a significantly higher level of anti-Nic IgG than group 2 which was injected with the PLA vaccine on day 14. Surprisingly, the second booster continued to increase the antibody titers to 62387 ± 3232, 40899 ± 2955, 53707 ± 2417, 41406 ± 2565, 43054 ± 3938, and 44872 ± 7673, respectively, on day 42. This was inconsistent with what was observed in a previous study, in which the second booster injected did not further promote the immune response.14 It was possible that the concomitant delivery of multiple molecular adjuvants, including MPLA, CpG ODN 1826, and R848, by the vaccines in this study provided more signals to the immune system for a stronger response.48,49 In addition, group 1, which received 3 injections of the PLGA vaccine, continued to show significantly higher antibody levels than other groups on day 42. In previous studies, only the short-term efficacy of the nicotine vaccines was evaluated.12,14,16,17,47 In this study, the long-term outcome of the vaccines was evaluated. As anticipated, the levels of anti-Nic IgG dropped significantly in all groups 3 months after the second booster injection. As IgG is known to have a half-life of 20 days, the decrease in the IgG level might partially result from the natural clearance of the antibodies.50 Interestingly, on day 118, mice in group 1 that received only the PLGA vaccines showed a significantly lower antibody titer of 6492 ± 340 than those of other groups that received at least one injection of the PLA vaccine, indicating that the PLA vaccine could maintain the immunity against nicotine longer than the PLGA vaccine. It has been reported that prolonged retention of antigens in the body resulted in long-term immune response.51,52 As shown in Figure 3 and Figure 4, the PLA vaccine showed significantly higher stability under both in vitro and in vivo conditions, which suggested that the PLA vaccine might stay in the body for a longer time and provide continuous stimulation to the immune system. The fact that the treatment groups 2, 3, 4, 5, and 6 with at least one injection of the PLA vaccine achieved significantly higher titers of anti-Nic IgG than the group 1 treated with only PLGA vaccine demonstrates that the efficacy of our previous nanovaccines built with PLGA can be extended by a combination use of the PLA vaccine.
Figure 5.
Titers of anti-Nic IgG, IgG1, IgG2a, IgG2b, and IgG3 and the Th1/Th1 index. Eight female BALB/c mice in each group were injected on days 0 (primary injection), 14 (first booster), and 28 (second booster) with either the PLGA vaccine or the PLA vaccine as shown in Table 1. Titers of anti-Nic IgG were measured for blood collected from each immunized group on days 13, 27, 42, and 118. Titers of subtype antibodies, including IgG1, IgG2a, IgG2b, and IgG3, were measured for blood collected from each group on day 118. The Th1/Th2 index, which represented the polarization of the immune response, was calculated according to the titers of subtype IgGs. ** indicates that the p-value was less than 0.05, and *** indicates that the p-value was less than 0.01.
Table 1.
Schedule of Immunization with Nicotine Vaccines in Mice
| 1st injection | 1st booster | 2nd booster | |
|---|---|---|---|
| control | PBS buffer | PBS buffer | PBS buffer |
| group 1 | PLGA vaccine | PLGA vaccine | PLGA vaccine |
| group 2 | PLA vaccine | PLA vaccine | PLA vaccine |
| group 3 | PLGA vaccine | PLGA vaccine | PLA vaccine |
| group 4 | PLA vaccine | PLA vaccine | PLGA vaccine |
| group 5 | PLGA vaccine | PLA vaccine | PLA vaccine |
| group 6 | PLA vaccine | PLGA vaccine | PLGA vaccine |
Adaptive immune response can be divided into either humoral or cell-mediated immune response.53,54 Usually, antigen can induce both humoral and cell-mediated immune response.55 Because the immunity against nicotine is based on the functions of NSAs, it is ideal to polarize the nicotine vaccine induced immune response to a humoral immune response. To evaluate the polarity of the immune response, the titers of subtype IgGs, including IgG1, IgG2a, IgG2b, and IgG3, were measured for blood collected on day 118, and the Th1/Th2 index was calculated. As shown in Figure 5, mice from groups 2, 3, 4, 5, and 6 produced significantly higher titers of anti-Nic IgG1, IgG2a, IgG2b, and IgG3 than mice from group 1. IgG1 and IgG2a represented the two most dominant subtype IgGs in all the immunized groups. In addition, mice in group 3 had a significantly higher titer of IgG1 than that of any other immunized groups. The Th1/Th2 index from groups 1, 2, 3, 4, 5, and 6 was 0.92 ± 0.12, 0.80 ± 0.05, 0.31 ± 0.05, 0.68 ± 0.02, 0.72 ± 0.07, and 0.79 ± 0.04, respectively. As reported in previous studies, a Th1/Th2 index that was less than 1 indicates that the immune response was dominated by the humoral immune response.14,17 The Th1/Th2 index in this study shows that both the PLA and the PLGA vaccines polarized the immune response to produce antibodies. These results were consistent with previous findings that the lipid-NP-based nicotine vaccine was mainly employed by the immune system to elicit production of NSAs instead of activating cytotoxic T cells.14,17
2.6. Brain Nicotine Level in the Immunized Mice.
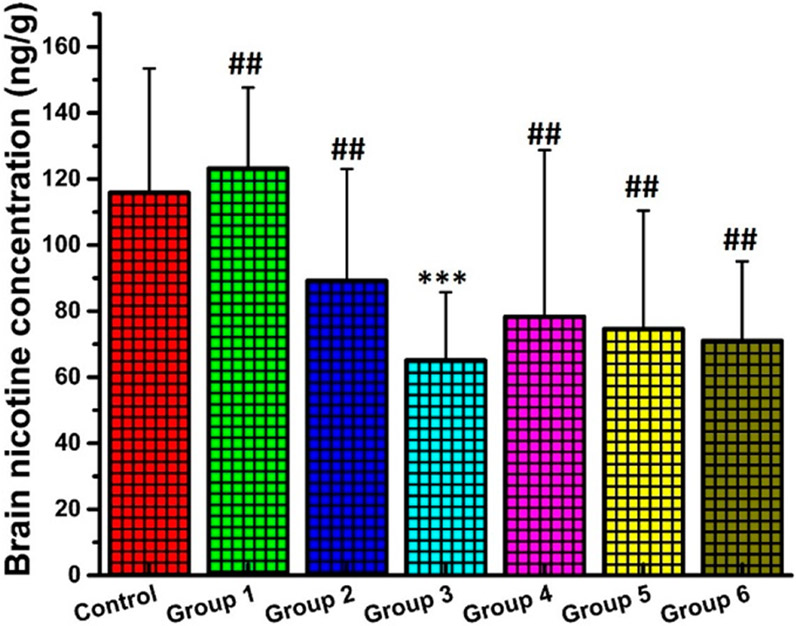
It has been widely believed that the efficacy of a nicotine vaccine primarily depends on its ability to reduce the entry of nicotine into the brain.42,56 In our previous research, nicotine pharmacokinetic studies were carried out shortly after booster injections, and, therefore, the results might only reflect the short-term efficacy of the vaccines.12,13,15-17,40 In this research, to evaluate the long-term efficacy of nicotine vaccines, the pharmacokinetic study was performed 3 months after the second vaccine booster injection. As shown in Figure 6, the control group and groups 1, 2, 3, 4, 5, and 6 achieved a brain nicotine concentration of 115.88 ± 37.62, 123.25 ± 24.44, 89.25 ± 33.81, 65.13 ± 20.59, 78.00 ± 50.37, 75.00 ± 35.79, and 71.00 ± 24.07 ng/g, respectively. In addition, only the mice in group 3 attained a significantly lower brain nicotine concentration than that of the control among all immunized mice. These results were consistent with the NSA levels in the mice measured using ELISA. In previous studies, lower brain nicotine concentrations were also correlated with higher NSA concentrations.12,13,15-17,40
Figure 6.
Brain nicotine concentrations. All mice that were treated with either nicotine vaccines or PBS buffer were subcutaneously administered with 0.07 mg/kg nicotine on day 120, and the brain nicotine concentrations were measured. *** denotes that the brain nicotine concentration in group 3 was significantly lower than that of the control (P-value is less than 0.05). ## denotes that the brain nicotine concentrations in groups 1, 2, 4, 5, and 6 were not significantly different from that of the control.
According to the data from other groups as well as our previous nanovaccines, the vaccinated animals were usually challenged with nicotine a few weeks post vaccination, and thus the long-term impact of these vaccines on the brain nicotine level is unknown.40,57-59 In other studies, conventional protein-nicotine vaccines require more injections to maintain long-term efficacy.60 In addition, the combination dosing of PLGA and PLA vaccine in this study clearly shows advantage over our previous PLGA vaccines in terms of long-term efficacy.
Although not significantly, the brain nicotine levels in all group immunized with at least one shot of the PLA vaccine were considerably lower than those of both the control group and group 1. This demonstrates that the PLA vaccine could maintain long-term immunological efficacy compared to the PLGA vaccine. In addition, the brain nicotine levels reveal that both the stability of vaccine and the combination of the vaccination influenced the long-term immunological outcome. Overall, these results demonstrate that it is possible to optimize the vaccine design and vaccination protocol with fewer injections for long-term immunity against nicotine. As discussed above, having a prolonged efficacy is very meaningful to a nicotine vaccine, because it may not only reduce the cost but also simplify the immunization process.
Because this is a proof-of-concept study, the efficacy of the vaccines were only tested in mice. It needs to be noted that humans may react differently to these vaccines due to the differences in physiological structure as well as the immune system. In addition, the adjuvants used in these hybrid vaccines may not be optimal for human use. Therefore, future studies in primates or human clinical trials are needed to validate the efficacy of these vaccines in this study.
2.7. Safety of the Nicotine Vaccines.
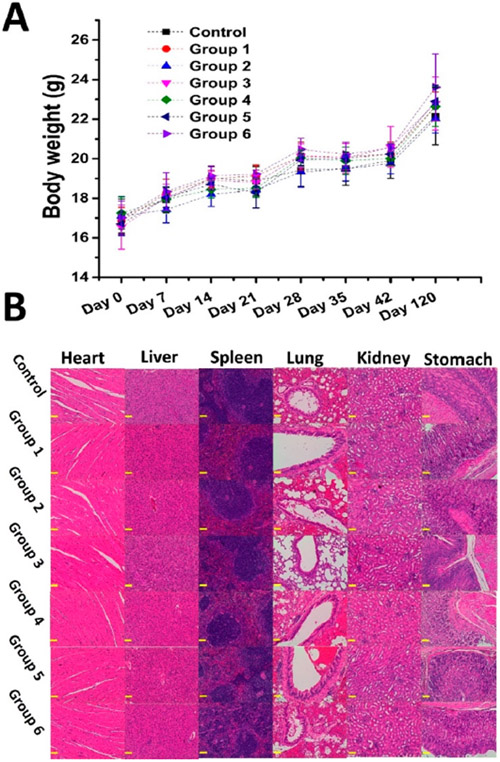
Safety is always the top concern during development of a vaccine.61 As considered from the very beginning of the vaccine design, all materials used in our nicotine vaccines were either clinically approved by the FDA or tested as safe in clinical trials.14,19 Previous findings revealed that these hybrid NP-based vaccines were safe to mice.13-16 However, this is the first time that we evaluated the long-term safety of the vaccines. As shown in Figure 7A, during a course of 120 days, mice immunized with either the PLGA vaccine or the PLA vaccine did not show significantly different body weight compared with the mice that received the PBS buffer. Moreover, we harvested the major organs from mice sacrificed on day 120 and conducted a histopathology study for these organs. As shown in Figure 7B, with the mice that received the PBS buffer as the control, no abnormity was detected in the organs, including heart, liver, spleen, lung, kidney, and stomach from the mice injected with nicotine vaccines. These results demonstrate that both the PLGA vaccine and the PLA vaccine are safe for an extended period of time. Although these vaccines demonstrate good safety profiles in mice, more comprehensive studies are needed to investigate their safety in humans due to the difference between humans and rodents.
Figure 7.
Safety of nicotine vaccines on mice. The body weight of the mice was monitored throughout the study, and the mice that received either the PBS buffer or nicotine vaccines were sacrificed on day 120. The major organs of mice, including heart, liver, spleen, lung, kidney, and stomach were harvested for a safety study. (A) Body weight of mice. (B) H&E staining of the sections of mice organs. Scale bars represent 200 μm.
3. CONCLUSIONS
In summary, the PLA vaccine was significantly more stable than the PLGA vaccine in both the in vitro and in vivo environment. The titers of anti-Nic IgG from the immunized mice demonstrate that the PLGA induced higher levels of immune response than the PLA vaccine in the short term. In contrast, the PLA vaccine was more capable of producing long-lasting antibody immune response. In addition, the Th1/Th2 index demonstrates that both the PLGA vaccine and the PLA vaccine dominantly induced humoral immune response. Finally, it was the first two injections of the PLGA vaccine and a third injection of the PLA vaccine achieved significantly lower brain nicotine levels in mice compared to the control 3 months after the second booster injection. These results imply that a rational administration of the two vaccines could improve the long-term efficacy of immunotherapy against nicotine addiction.
4. EXPERIMENTAL SECTION
4.1. Materials.
CpG oligonucleotides 1826 (CpG ODN 1826) and R848 VacciGrade were purchased from InvivoGen (San Diego, California). Monophosphoryl lipid A (MPLA), 1,2-diphytanoyl-sn-glycero-3-phosphoethanolamine-N-(7-nitro-2–1,3-benzoxadiazol-4-yl) (ammonium salt) (NBD PE), 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP), 1,2-distearoyl-sn-glycero-3-phosphoethanol-amine-N-[carboxy(polyethylene glycol)-2000] (ammonium salt) (DSPE-PEG(2000)COOH), and cholesterol were acquired from Avanti Polar Lipids, Inc. (Alabaster, AL). Lactel 50:50 PLGA and PLA were purchased from Durect Corporation (Cupertino, CA). Trypsin/EDTA, fetal bovine serum (FBS), alpha minimum essential medium, and granulocyte macrophage-colony stimulating factor (GM-CSF) recombinant mouse protein were purchased from Life Technologies Corporation (Grand Island, NY). TMB one component microwell substrate was procured from SouthernBiotech (Birmingham, AL). The antimouse IgG from goat and antigoat IgG-HRP were purchased from Alpha Diagnostic Intl (San Antonio, TX). Poly(vinyl alcohol) (PVA), bovine serum albumin (BSA), and dichloromethane (DCM) were acquired from Sigma-Aldrich Inc. (Saint Louis, MO). Alexa Fluor 750 dye (Alexa 750), Alexa Fluor 647 hydrazide (Alexa 647), keyhole limpet hemocyanin (KLH), 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC), and sulfo-NHS were purchased from Thermo Fisher Scientific Inc. (Rockford, IL). Ractrans 3′-aminomethyl nicotine (3′-AminoNic) was purchased from Toronto Research Chemicals Inc. (Toronto, Canada). JAWSII (ATCC CRL-11904) immature dendritic cells were purchased from ATCC (Manassas, VA). All other chemicals were of analytical grade.
4.2. Fabrication of PLA and PLGA NPs.
PLGA and PLGA NPs enclosing KLH and molecular adjuvants were formed according to a previously reported method with minor modifications.14,16,40 CpG ODN 1826 (1.6 mg) and R848 (0.8 mg) were included in 200 μL deionized water with 2 mg of KLH and mixing with PLGA or PLA solution. The NPs were prepared by closely following procedures described previously.16,40
4.3. Assembly of Nicotine Vaccines.
As described in a previous study, nicotine vaccines were assembled by conjugating 3′-AminoNic on to the surface of lipid-PLGA or lipid-PLA hybrid NPs via a reaction of carboxylic acid on the lipid layer with an amine on 3′-AminoNic.14 Lipids of MPLA (0.4 mg), DOTAP (3.77 mg), DSPE-PEG(2000)COOH (4.10 mg), and cholesterol (0.14 mg) were hydrated with 1 mL of 55 °C PBS buffer. Lipids-PLGA/PLA NPs and the conjugation of 3′-AminoNic were prepared following a previously described method.16
4.4. Synthesis of Nicotine-BSA Conjugate.
3′-AminoNic-BSA conjugate was synthesized via the method described previously.14
4.5. Synthesis of Alexa 647/Alexa 750-labeled KLH.
Alexa 647 or Alexa 750-labeled KLH was synthesized using a previously reported method.14
4.6. Characterization of Physicochemical Properties of Nicotine Vaccines.
The PLGA and the PLA vaccines were diluted ten times in PBS buffer (pH 7.0). The physicochemical properties including particle size (diameter, nm), size distribution, and surface charge (zeta potential, mV) were analyzed with a Malvern Nano-ZS zetasizer (Malvern Instruments Ltd., Worcestershire, United Kingdom).
4.7. Examination of Morphology of Nicotine Vaccines Using a Transmission Electron Microscope (TEM).
The morphologies of nicotine vaccines were examined using a TEM as described in previous studies.14,16,40
4.8. Study of In Vitro Uptake and Processing of Vaccines by Dendritic Cells (DCs).
NBD and Alexa 647 labeled vaccines were assembled using the same method as described above, except that these vaccines contained Alexa 647-labeled KLH and NBD in the lipid layer and did not contain molecular adjuvants. JAWSII (ATCC CRL-11904) immature DCs were cultured using a previously reported method.14 The PLGA vaccine or the PLA vaccine (500 μg) was incubated with 2 × 106 DCs for 30, 60, and 120 min, respectively. The methods to study cell uptake and cellular vaccine processing were described elsewhere.40
4.9. Study of In Vitro Release of KLH from Nicotine Vaccines.
Alexa 647-labeled PLGA vaccine or the PLA vaccine was assembled using the same method as described above, except that these vaccines contained Alexa 647-KLH and did not contain molecular adjuvants. The study of in vitro release of KLH followed that described previously.40
4.10. Study of Degradation of Nicotine Vaccines in Mice.
Alexa 750-labeled PLGA vaccine or PLA vaccine was assembled using the same method as described above, except that these vaccines contained Alexa 750-KLH and did not contain molecular adjuvants. Alexa 750-labeled vaccines (2 mg) were subcutaneously injected into female BALB/c mice (8–10 weeks). The degradation of the vaccines at the site of injection (SOI) was studied using an IVIS SpectrumCT (PerkinElmer, Waltham, MA). The in vivo fluorescence image was taken at given time points post vaccine injection, and fluorescence intensity from the SOI was defined manually.
4.11. Immunization of Mice with Nicotine Vaccines.
All animal studies were carried out following the National Institutes of Health (NIH) guidelines for animal care and use. Animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at Virginia Polytechnic Institute and State University. Female BALB/c mice (8–10 weeks, 16–20 g) were purchased from Charles River. Mice (n = 8) were immunized with nicotine vaccines according to a previously reported method with proper modifications.14 Briefly, the PLGA vaccine or the PLA vaccine containing 20 μg KLH was subcutaneously injected into female BALB/c mice (8 mice/group) on days 0 (primary injection), 14 (1st booster), and 28 (2nd booster) as shown in Table 1. Blood samples (~200 μL) were collected from each mouse on days −2, 13, 27, 42, and 118 via retro orbital puncture and processed following previously described procedures.14
4.12. Measurement of Titers of Antinicotine (anti-Nic) IgG and Subtype IgG.
For determining antibody titers, mice sera were analyzed according to the enzyme-linked immunosorbent assay (ELISA) procedure described in previous publications.14,16,40 The ELISA for measuring subtype anti-Nic IgGs, including IgG1, IgG2a, IgG2b, and IgG3 was carried out according to a previously reported protocol.14
4.13. Calculation of the Th1/Th2 Index.
Th1/Th2 index was calculated according to a previously reported equation: ([IgG2a + IgG3]/2)/(IgG1) for each group.14
4.14. Measurement of Brain Nicotine in Mice.
On day 120, mice either injected with PBS buffer or nicotine vaccines were subcutaneously administered with nicotine (0.07 mg/kg). All mice were sacrificed 4 min after injection of nicotine, and the brain tissues were collected. Brain nicotine concentrations were assayed by gas chromatography/mass spectrometry according to a method reported before.16,40
4.15. Histopathological Examination.
On day 120, major organs, including heart, liver, kidney, stomach, lung, and spleen were harvested from mice and fixed in 10% buffered formalin. These organs were treated with H&E staining according to the method described in previous studies.14,16,40 Tissue sections were examined with an Olympus CKX41 Inverted Microscope, and images were captured using an INFINITY 1 camera.14,16,40
4.16. Data Analysis.
As described in a previous study, data were compared among groups using one way ANOVA and t-tests.14
ACKNOWLEDGMENTS
This work was financially supported by National Institute on Drug Abuse (R21DA030083 and U01DA036850). We also thank Theresa Harmon and Dr. Paul R. Pentel from Minneapolis Medical Research Foundation for measuring the brain nicotine level.
Footnotes
The authors declare no competing financial interest.
Contributor Information
Yun Hu, Department of Biological Systems Engineering, Virginia Tech, Blacksburg, Virginia 24061, United States.
Zongmin Zhao, Department of Biological Systems Engineering, Virginia Tech, Blacksburg, Virginia 24061, United States.
Marion Ehrich, Department of Biomedical Sciences and Pathobiology, Virginia Tech, Blacksburg, Virginia 24061, United States.
Chenmin Zhang, Department of Biological Systems Engineering, Virginia Tech, Blacksburg, Virginia 24061, United States.
REFERENCES
- (1).Ahluwalia IB; Smith T; Arrazola RA; Palipudi KM; Garcia de Quevedo I; Prasad VM; Commar A; Schotte K; Garwood PD; Armour BS Current Tobacco Smoking, Quit Attempts, and Knowledge About Smoking Risks among Persons Aged > /=15 Years - Global Adult Tobacco Survey, 28 Countries, 2008–2016. MMWR Morb. Mortal. Wkly. Rep 2018, 67, 1072–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Pentel PR; LeSage MG New Directions in Nicotine Vaccine Design and Use. Adv. Pharmacol 2014, 69, 553–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Polosa R; Benowitz NL Treatment of Nicotine Addiction: Present Therapeutic Options and Pipeline Developments. Trends Pharmacol. Sci 2011, 32, 281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Raupach T; Hoogsteder PH; Onno van Schayck CP Nicotine Vaccines to Assist with Smoking Cessation: Current Status of Research. Drugs 2012, 72, e1–e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Hartmann-Boyce J; Cahill K; Hatsukami D; Cornuz J Nicotine Vaccines for Smoking Cessation. Cochrane Database Syst. Rev 2012, CD007072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Tonstad S; Heggen E; Giljam H; Lagerback PA; Tonnesen P; Wikingsson LD; Lindblom N; de Villiers S; Svensson TH; Fagerstrom KO Niccine(R), a Nicotine Vaccine, for Relapse Prevention: A Phase Ii, Randomized, Placebo-Controlled, Multicenter Clinical Trial. Nicotine Tob. Res 2013, 15, 1492–501. [DOI] [PubMed] [Google Scholar]
- (7).Shen XY; Orson FM; Kosten TR Vaccines against Drug Abuse. Clin. Pharmacol. Ther 2012, 91, 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Japuntich SJ; Leventhal AM; Piper ME; Bolt DM; Roberts LJ; Fiore MC; Baker TB Smoker Characteristics and Smoking-Cessation Milestones. Am. J. Prev. Med 2011, 40, 286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Lockner JW; Lively JM; Collins KC; Vendruscolo JC; Azar MR; Janda KD A Conjugate Vaccine Using Enantiopure Hapten Imparts Superior Nicotine-Binding Capacity. J. Med. Chem 2015, 58, 1005–11. [DOI] [PubMed] [Google Scholar]
- (10).McCluskie MJ; Pryde DC; Gervais DP; Stead DR; Zhang N; Benoit M; Robertson K; Kim IJ; Tharmanathan T; Merson JR; Davis HL Enhancing Immunogenicity of a 3′aminomethylnicotine-Dt-Conjugate Anti-Nicotine Vaccine with Cpg Adjuvant in Mice and Non-Human Primates. Int. Immunopharmacol 2013, 16, 50–6. [DOI] [PubMed] [Google Scholar]
- (11).Malin DH; Alvarado CL; Woodhouse KS; Karp H; Urdiales E; Lay D; Appleby P; Moon WD; Ennifar S; Basham L; Fattom A Passive Immunization against Nicotine Attenuates Nicotine Discrimination. Life Sci 2002, 70, 2793–8. [DOI] [PubMed] [Google Scholar]
- (12).Zhao Z; Hu Y; Hoerle R; Devine M; Raleigh M; Pentel P; Zhang C A Nanoparticle-Based Nicotine Vaccine and the Influence of Particle Size on Its Immunogenicity and Efficacy. Nanomedicine 2017, 13, 443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Zhao Z; Harris B; Hu Y; Harmon T; Pentel PR; Ehrich M; Zhang C Rational Incorporation of Molecular Adjuvants into a Hybrid Nanoparticle-Based Nicotine Vaccine for Immunotherapy against Nicotine Addiction. Biomaterials 2018, 155, 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Hu Y; Smith D; Frazier E; Hoerle R; Ehrich M; Zhang C The Next-Generation Nicotine Vaccine: A Novel and Potent Hybrid Nanoparticle-Based Nicotine Vaccine. Biomaterials 2016, 106, 228–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Zhao Z; Powers K; Hu Y; Raleigh M; Pentel P; Zhang C Engineering of a Hybrid Nanoparticle-Based Nicotine Nanovaccine as a Next-Generation Immunotherapeutic Strategy against Nicotine Addiction: A Focus on Hapten Density. Biomaterials 2017, 123, 107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Hu Y; Zhao Z; Harmon T; Pentel PR; Ehrich M; Zhang C Paradox of Pegylation in Fabricating Hybrid Nanoparticle-Based Nicotine Vaccines. Biomaterials 2018, 182, 72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Zheng H; Hu Y; Huang W; de Villiers S; Pentel P; Zhang J; Dorn H; Ehrich M; Zhang C Negatively Charged Carbon Nanohorn Supported Cationic Liposome Nanoparticles: A Novel Delivery Vehicle for Anti-Nicotine Vaccine. J. Biomed. Nanotechnol 2015, 11, 2197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Kapoor DN; Bhatia A; Kaur R; Sharma R; Kaur G; Dhawan S Plga: A Unique Polymer for Drug Delivery. Ther. Delivery 2015, 6, 41–58. [DOI] [PubMed] [Google Scholar]
- (19).Tyler B; Gullotti D; Mangraviti A; Utsuki T; Brem H Polylactic Acid (Pla) Controlled Delivery Carriers for Biomedical Applications. Adv. Drug Delivery Rev 2016, 107, 163–175. [DOI] [PubMed] [Google Scholar]
- (20).Reisch A; Runser A; Arntz Y; Mely Y; Klymchenko AS Charge-Controlled Nanoprecipitation as a Modular Approach to Ultrasmall Polymer Nanocarriers: Making Bright and Stable Nanoparticles. ACS Nano 2015, 9, 5104–16. [DOI] [PubMed] [Google Scholar]
- (21).An M; Li M; Xi J; Liu H Silica Nanoparticle as a Lymph Node Targeting Platform for Vaccine Delivery. ACS Appl. Mater. Interfaces 2017, 9, 23466–23475. [DOI] [PubMed] [Google Scholar]
- (22).Elci SG; Jiang Y; Yan B; Kim ST; Saha K; Moyano DF; Yesilbag Tonga G; Jackson LC; Rotello VM; Vachet RW Surface Charge Controls the Suborgan Biodistributions of Gold Nanoparticles. ACS Nano 2016, 10, 5536–42. [DOI] [PubMed] [Google Scholar]
- (23).Gause KT; Wheatley AK; Cui J; Yan Y; Kent SJ; Caruso F Immunological Principles Guiding the Rational Design of Particles for Vaccine Delivery. ACS Nano 2017, 11, 54–68. [DOI] [PubMed] [Google Scholar]
- (24).Pustulka SM; Ling K; Pish SL; Champion JA Protein Nanoparticle Charge and Hydrophobicity Govern Protein Corona and Macrophage Uptake. ACS Appl Mater. Interfaces 2020, 12, 48284–48295. [DOI] [PubMed] [Google Scholar]
- (25).Dobrovolskaia MA; McNeil SE Immunological Properties of Engineered Nanomaterials. Nat. Nanotechnol 2007, 2, 469–78. [DOI] [PubMed] [Google Scholar]
- (26).Frohlich E The Role of Surface Charge in Cellular Uptake and Cytotoxicity of Medical Nanoparticles. Int. J. Nanomed 2012, 7, 5577–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Kamphorst AO; Guermonprez P; Dudziak D; Nussenzweig MC Route of Antigen Uptake Differentially Impacts Presentation by Dendritic Cells and Activated Monocytes. J. Immunol 2010, 185, 3426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Lanzavecchia A Mechanisms of Antigen Uptake for Presentation. Curr. Opin. Immunol 1996, 8, 348–54. [DOI] [PubMed] [Google Scholar]
- (29).Petrovsky N Comparative Safety of Vaccine Adjuvants: A Summary of Current Evidence and Future Needs. Drug Saf. 2015, 38, 1059–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Pati R; Shevtsov M; Sonawane A Nanoparticle Vaccines against Infectious Diseases. Front. Immunol 2018, 9, 2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Ballard JL; Peeva VK; deSilva CJ; Lynch JL; Swanson NR Comparison of Alexa Fluor and Cydye for Practical DNA Microarray Use. Mol. Biotechnol 2007, 36, 175–83. [DOI] [PubMed] [Google Scholar]
- (32).Patil S; Sandberg A; Heckert E; Self W; Seal S Protein Adsorption and Cellular Uptake of Cerium Oxide Nanoparticles as a Function of Zeta Potential. Biomaterials 2007, 28, 4600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Mellman I; Steinman RM Dendritic Cells: Specialized and Regulated Antigen Processing Machines. Cell 2001, 106, 255–8. [DOI] [PubMed] [Google Scholar]
- (34).Banchereau J; Steinman RM Dendritic Cells and the Control of Immunity. Nature 1998, 392, 245–52. [DOI] [PubMed] [Google Scholar]
- (35).Moser M; Murphy KM Dendritic Cell Regulation of Th1- Th2 Development. Nat. Immunol 2000, 1, 199–205. [DOI] [PubMed] [Google Scholar]
- (36).Smith JD; Morton LD; Ulery BD Nanoparticles as Synthetic Vaccines. Curr. Opin. Biotechnol 2015, 34, 217–24. [DOI] [PubMed] [Google Scholar]
- (37).DeMuth PC; Moon JJ; Suh H; Hammond PT; Irvine DJ Releasable Layer-by-Layer Assembly of Stabilized Lipid Nanocapsules on Microneedles for Enhanced Transcutaneous Vaccine Delivery. ACS Nano 2012, 6, 8041–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Zaffran M; Vandelaer J; Kristensen D; Melgaard B; Yadav P; Antwi-Agyei KO; Lasher H The Imperative for Stronger Vaccine Supply and Logistics Systems. Vaccine 2013, 31, B73–80. [DOI] [PubMed] [Google Scholar]
- (39).Awate S; Babiuk LA; Mutwiri G Mechanisms of Action of Adjuvants. Front. Immunol 2013, 4, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Hu Y; Smith D; Zhao Z; Harmon T; Pentel PR; Ehrich M; Zhang C Alum as an Adjuvant for Nanoparticle Based Vaccines: A Case Study with a Hybrid Nanoparticle-Based Nicotine Vaccine. Nanomedicine 2019, 20, 102023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Hoogsteder PH; Kotz D; van Spiegel PI; Viechtbauer W; van Schayck OC Efficacy of the Nicotine Vaccine 3′-Amnic-Repa (Nicvax) Co-Administered with Varenicline and Counselling for Smoking Cessation: A Randomized Placebo-Controlled Trial. Addiction 2014, 109, 1252–9. [DOI] [PubMed] [Google Scholar]
- (42).Skolnick P Biologic Approaches to Treat Substance-Use Disorders. Trends Pharmacol. Sci 2015, 36, 628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Bellei E; Bergamini S; Monari E; Fantoni LI; Cuoghi A; Ozben T; Tomasi A High-Abundance Proteins Depletion for Serum Proteomic Analysis: Concomitant Removal of Non-Targeted Proteins. Amino Acids 2011, 40, 145–56. [DOI] [PubMed] [Google Scholar]
- (44).Vidarsson G; Dekkers G; Rispens T Igg Subclasses and Allotypes: From Structure to Effector Functions. Front. Immunol 2014, 5, 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Crotty S; Ahmed R Immunological Memory in Humans. Semin. Immunol 2004, 16, 197–203. [DOI] [PubMed] [Google Scholar]
- (46).Sallusto F; Lanzavecchia A; Araki K; Ahmed R From Vaccines to Memory and Back. Immunity 2010, 33, 451–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Hu Y; Zheng H; Huang W; Zhang C A Novel and Efficient Nicotine Vaccine Using Nano-Lipoplex as a Delivery Vehicle. Hum. Vaccines Immunother 2014, 10, 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Weeratna RD; Makinen SR; McCluskie MJ; Davis HL Tlr Agonists as Vaccine Adjuvants: Comparison of Cpg Odn and Resiquimod (R-848). Vaccine 2005, 23, 5263–70. [DOI] [PubMed] [Google Scholar]
- (49).McKay PF; King DF; Mann JF; Barinaga G; Carter D; Shattock RJ Tlr4 and Tlr7/8 Adjuvant Combinations Generate Different Vaccine Antigen-Specific Immune Outcomes in Minipigs When Administered Via the Id or in Routes. PLoS One 2016, 11, e0148984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Bernasconi NL; Traggiai E; Lanzavecchia A Maintenance of Serological Memory by Polyclonal Activation of Human Memory B Cells. Science 2002, 298, 2199–202. [DOI] [PubMed] [Google Scholar]
- (51).Harwood NE; Batista FD Antigen Presentation to B Cells. F1000 Biol. Rep 2010, 2, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Moyer TJ; Zmolek AC; Irvine DJ Beyond Antigens and Adjuvants: Formulating Future Vaccines. J. Clin. Invest 2016, 126, 799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Muyanja E; Ssemaganda A; Ngauv P; Cubas R; Perrin H; Srinivasan D; Canderan G; Lawson B; Kopycinski J; Graham AS; Rowe DK; Smith MJ; Isern S; Michael S; Silvestri G; Vanderford TH; Castro E; Pantaleo G; Singer J; Gillmour J; Kiwanuka N; Nanvubya A; Schmidt C; Birungi J; Cox J; Haddad EK; Kaleebu P; Fast P; Sekaly RP; Trautmann L; Gaucher D Immune Activation Alters Cellular and Humoral Responses to Yellow Fever 17d Vaccine. J. Clin. Invest 2014, 124, 3147–3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Bo R; Sun Y; Zhou S; Ou N; Gu P; Liu Z; Hu Y; Liu J; Wang D Simple Nanoliposomes Encapsulating Lycium Barbarum Polysaccharides as Adjuvants Improve Humoral and Cellular Immunity in Mice. Int. J. Nanomed 2017, 12, 6289–6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Lastuti NDR; Yuniarti WM; Hastutiek P; Suwanti LT; Chrismanto D Humoral and Cellular Immune Response Induced by Antigenic Protein of Sarcoptes Scabiei Var. Caprae. Vet. World 2018, 11, 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Fahim RE; Kessler PD; Kalnik MW Therapeutic Vaccines against Tobacco Addiction. Expert Rev. Vaccines 2013, 12, 333–42. [DOI] [PubMed] [Google Scholar]
- (57).Zhao Z; Hu Y; Harmon T; Pentel P; Ehrich M; Zhang C Rationalization of a Nanoparticle-Based Nicotine Nanovaccine as an Effective Next-Generation Nicotine Vaccine: A Focus on Hapten Localization. Biomaterials 2017, 138, 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Pryde DC; Jones LH; Gervais DP; Stead DR; Blakemore DC; Selby MD; Brown AD; Coe JW; Badland M; Beal DM; Glen R; Wharton Y; Miller GJ; White P; Zhang N; Benoit M; Robertson K; Merson JR; Davis HL; McCluskie MJ Selection of a Novel Anti-Nicotine Vaccine: Influence of Antigen Design on Antibody Function in Mice. PLoS One 2013, 8, e76557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Keyler DE; Roiko SA; Earley CA; Murtaugh MP; Pentel PR Enhanced Immunogenicity of a Bivalent Nicotine Vaccine. Int. Immunopharmacol 2008, 8, 1589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Roiko SA; Harris AC; Keyler DE; Lesage MG; Zhang Y; Pentel PR Combined Active and Passive Immunization Enhances the Efficacy of Immunotherapy against Nicotine in Rats. J. Pharmacol. Exp. Ther 2008, 325, 985–93. [DOI] [PubMed] [Google Scholar]
- (61).Wilson CB; Marcuse EK Vaccine Safety–Vaccine Benefits: Science and the Public’s Perception. Nat. Rev. Immunol 2001, 1, 160–5. [DOI] [PubMed] [Google Scholar]