We report here the case of a patient with hypertrophic lichen planus (LP) who was treated successfully with tofacitinib, and discuss the potential role of treating inflammatory skin diseases according to their immune response pattern, which opens therapeutic options for patients with rare inflammatory skin diseases.
CASE REPORT
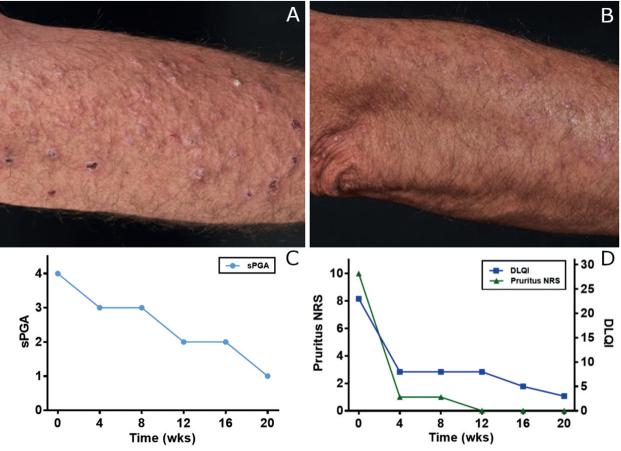
A 51-year-old male patient presented at our hospital with a 30-year history of erythematous papules, verrucous plaques, and crusts, located predominantly on his extremities (Fig. 1A, B). Histological samples revealed a lichenoid reaction pattern with interface dermatitis, including hypergranulosis, basal cell vacuolization, dyskeratosis, and a band-like lymphocytic infiltrate at the dermo-epidermal junction, leading to the diagnosis of hypertrophic LP (Fig. 2). Previous treatments included topical, intra-lesional and systemic corticosteroids, systemic retinoids, methotrexate, cyclosporine, mycophenolate mofetil, azathioprine, apremilast, ixekizumab, guselkumab, and photo(chemo)therapy, including psoralen plus ultraviolet A (PUVA), UVA1, and UVB-311 nm. None of the treatments controlled the disease sufficiently. When presenting at our department, the patient reported a high level of impairment of his quality of life (Dermatology Life Quality Index (DLQI) 23/30) and strong pruritus (10/10 on a numerical rating scale). The static Physician’s Global Assessment (sPGA) amounted to 4/4 (Fig. 1C, D).
Fig. 1.
Clinical images and scores over the course of therapy for lichen planus skin lesions at (A) week 0 and (B) week 20. (C) Static Physician’s Global Assessment (sPGA, 0–4). (D) Pruritus numerical rating scale (NRS, 0–10) and Dermatology Life Quality Index (DLQI, 0–30).
Fig. 2.
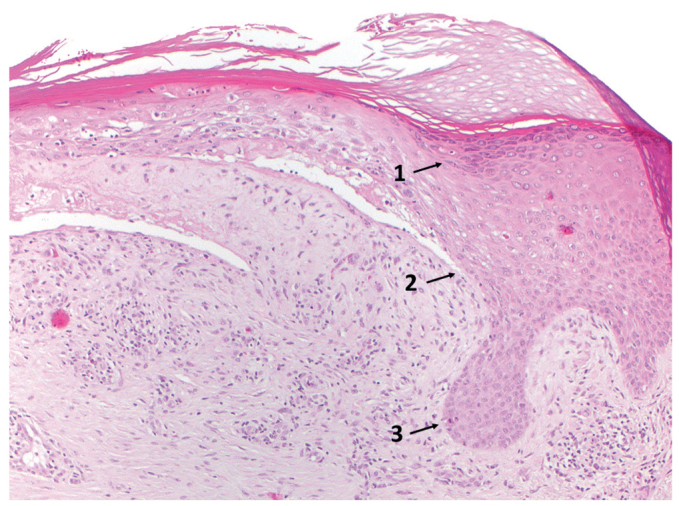
Histopathology of lesional skin. Lichenoid reaction pattern including hypergranulosis (1), basal cell vacuolization (2), dyskeratosis and a band-like lymphocytic infiltrate at the dermo-epidermal junction, leading to the diagnosis of hypertrophic lichen planus (Hematoxylin and eosin ×200x).
LP shows a type 1 immune response by inducing keratinocyte necroptosis and is associated with interface dermatitis (1, 2). Interferon-gamma (IFN-γ), derived from cytotoxic T cells, is a crucial cytokine for the promotion of epidermal apoptosis and necroptosis in LP lesions through a direct effect on keratinocytes via the JAK/STAT pathway (3). Therefore, we expected a positive therapeutic effect when inhibiting the JAK/STAT pathway in our patient. After obtaining informed consent, a therapy with the JAK1 and JAK3 inhibitor tofacitinib, 5 mg twice daily, was initiated. Drastic improvement was observed in all clinical parameters. sPGA reduced from 4/4 to 1/4, DLQI from 23/30 to 4/30, and pruritus NRS from 10/10 to 0/10 (Fig. 1C, D). No adverse events have been reported.
DISCUSSION
Amongst others, Okiyama & Fujimoto (4) and Shao et al. (3) proposed JAK inhibitors as potential drug targets for patients with lichenoid tissue reactions/interface dermatitis. Although the top predicted drug target for the cytotoxic responses to IFN-γ primed keratinocytes was tofacitinib, Shao et al. (3) treated keratinocytes with baricitinib, a selective JAK1 and JAK2 inhibitor, to show that they become completely resistant to cell-mediated cytotoxicity and upregulation of MHC class I. Baricitinib was selected instead of tofacitinib, as it is more selective for JAK1 and JAK2, in contrast with tofacitinib, which is selective for JAK1 and JAK3 (3). Other sources show that tofacitinib not only strongly inhibits JAK3, but also JAK1 and JAK2, albeit with 20-100-fold less potency (and even less tyrosine kinase 2) (5).
We treated our patient with tofacitinib, leading to an excellent clinical outcome and notable improvement in all observed parameters (Fig. 1C, D). This is remarkable, as it is not in agreement with the findings of Shao et al., showing that the IFN-γ mediated increase in susceptibility of keratinocytes to cytotoxic response is dependent mainly upon JAK2/STAT1, not JAK1/STAT2 (3). The response of our patient is in line with the model of Alves de Medeiros et al. (6), showing an upregulation of JAK1 and JAK3 in LP. Also, Damsky et al. (7) recently reported successful treatment of severe LP with tofacitinib and showed the activation of STAT1 in keratinocytes of patients with LP and erosive LP. Whether the role of other JAKs than JAK2 in LP is underestimated in the work of Shao et al. (3) or the JAK2 inhibiting potency of tofacitinib is higher than previously shown, is subject to further investigation.
These findings emphasize the JAK/STAT-pathway as a promising drug target for patients with type 1 inflammatory skin diseases, which are characterized by IFN-γ induced keratinocyte cell death and interface reaction, including LP, lupus erythematosus, alopecia areata, vitiligo, graft-versus-host disease, and pityriasis lichenoides chronica, amongst others (2). Besides these type 1/lichenoid diseases, there are 3 other major immune response patterns (type 2/eczematous and bullous, type 3/psoriatic, type 4/fibrogenic and granulomatous) (2). All diseases within a single pattern potentially respond to the specific treatment of that pattern, which opens a therapeutic door for patients with rare inflammatory skin diseases. Nevertheless, well-designed, multicentre, randomized, placebo-controlled, double-blind clinical trials have to be conducted to further evaluate the role of JAK inhibitors in interface dermatitis and other immune response pattern-related therapeutic agents.
ACKNOWLEDGEMENTS
All financial interests (including pharmaceutical and device products) and Employment: ACP received speakers’ fees or travel reimbursement from ALK Abelló, Abbvie. PS received speakers’ fee or travel reimbursement from Abbvie, BMS, Celgene, Janssen. FL received speakers’ fee or is member in advisory boards of Abbvie, Novartis, Leo Pharma, Lilly, Celgene, Roche, Sanofi, Almirall, Janssen. DB received travel reimbursement from Celgene, Pfizer. TB received speakers’ or consultant honoraria or research grants from ALK Abelló, Celgene, Mylan, Novartis, Sanofi. KE received speakers’ fee and is member in advisory boards of Abbvie, Almirall, BMS, Celgene, Hexal, Janssen, Lilly, Novartis, Sanofi.
REFERENCES
- 1.Lauffer F, Jargosch M, Krause L, Garzorz-Stark N, Franz R, Roenneberg S, et al. Type I immune response induces keratinocyte necroptosis and is associated with interface dermatitis. J Invest Dermatol 2018; 138: 1785–1794. [DOI] [PubMed] [Google Scholar]
- 2.Eyerich K, Eyerich S. Immune response patterns in noncommunicable inflammatory skin diseases. J Eur Acad Dermatol Venereol 2018; 32: 692–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shao S, Tsoi LC, Sarkar MK, Xing X, Xue K, Uppala R, et al. IFN-γ enhances cell-mediated cytotoxicity against keratinocytes via JAK2/STAT1 in lichen planus. Sci Transl Med 2019; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okiyama N, Fujimoto M. Clinical perspectives and murine models of lichenoid tissue reaction/interface dermatitis. J Dermatol Sci 2015; 78: 167–172. [DOI] [PubMed] [Google Scholar]
- 5.Könemann S, Dörr M, Felix SB. Cardiac immunomodulation. In: Nussinovitch U, editor. The heart in rheumatic, autoimmune and inflammatory diseases. Academic Press; 2017: p. 681–714. [Google Scholar]
- 6.Alves de Medeiros AK, Speeckaert R, Desmet E, Van Gele M, De Schepper S, Lambert J. JAK3 as an emerging target for topical treatment of inflammatory skin diseases. PLoS One 2016; 11: e0164080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damsky W, Wang A, Olamiju B, Peterson D, Galan A, King B. Treatment of severe lichen planus with the JAK inhibitor tofacitinib. J Allergy Clin Immunol 2020; 145: 1708–1710.e2. [DOI] [PubMed] [Google Scholar]