Abstract
Objectives:
To gain insight into the natural history and carcinogenesis pathway of Pancreatic Intraepithelial Neoplasia (PanIN) lesions by building a calibrated simulation model of PanIN progression to pancreatic ductal adenocarcinoma (PDAC)
Methods:
We revised a previously validated simulation model of solid PDAC, calibrating the model to fit data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results program and published literature on PanIN prevalence by age. We estimated the likelihood of progression from PanIN states (1, 2, and 3) to PDAC and the time between PanIN onset and PDAC (dwell time). We evaluated a hypothetical intervention to test for and treat PanIN 3 lesions to estimate the potential benefits from PanIN detection.
Results:
We estimated the lifetime probability of progressing from PanIN 1 to PDAC to be 1.5% (men), 1.3% (women). Progression from PanIN 1 to PDAC took 33.6 years and 35.3 years, respectively, and from PanIN 3 to PDAC took 11.3 years and 12.3 years. A hypothetical test for PanIN 3 detection and treatment could provide a maximum, average life expectancy gain of 40 days.
Conclusions:
Our modeling analysis estimates PanINs have a relatively indolent course to PDAC, supporting the feasibility of potential future early detection strategies.
Keywords: pancreatic cancer, pancreatic intraepithelial neoplasia, simulation modeling
1. Introduction
Defining the evolution of pancreatic ductal adenocarcinoma (PDAC) from precursor pancreatic intraepithelial neoplasia (PanIN) lesions is critically important. In particular, knowledge of the time periods between the onset of PanIN lesions and the development of PDAC – defined as “dwell times” – provide necessary insight into the feasibility of future early detection strategies that are focused on PanIN identification and treatment.
The rate and degree of dysplasia in PanINs has been observed to correlate with the presence of malignancy [1–3], and clinical information from retrospective studies of pancreatectomy specimens yields important insight into the natural history of PanINs. Published data on pancreatectomy and/or autopsy specimens suggests that, in general, higher-grade PanINs (e.g. PanIN 3) are more prevalent in: 1) older individuals [4]; 2) patients with PDAC [1]; and 3) patients at higher risk for PDAC (e.g. from chronic pancreatitis) [1,5]. Autopsy studies demonstrate that most patients with PanIN 3 lesions also have lower-grade PanINs, and confirm other observational data that indicate a higher likelihood of PanIN 3 with increasing age [4,6]. Most likely, these observations concerning PanINs correlate with a progressively increasing mutational burden [1,7–10].
PanIN lesions are not yet directly observable except in pathologic specimens; as such, observational data that elucidates their progression is absent. By using simulation modeling methods, it is possible to leverage the available data on PanIN prevalence [4,6], combined with SEER cancer registry data on age-based PDAC incidence and death [11,12], to infer PanIN dwell times and rates at which PanIN precursors transform. Our purpose was to gain quantitative insight into the natural history of PanIN lesions by building a calibrated simulation model of PanIN progression to PDAC.
2. Materials and methods
2.1. Model structure
We previously developed a simulation model of the natural history of PDAC in hypothetical populations, calibrated to data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program and published literature [4,6,11–16]. The current analysis was performed on an expanded version of this model. In our original model, we simulated progression along cystic and solid pathways to PDAC for both men and women, but did not explicitly consider PanIN precursor states [17]. In our updated model, we simulated PanIN development using three health states, defined by the highest grade of PanINs in the subject. The model, written in the programming language C++ (Microsoft Visual Studio 2015, Microsoft Corporation, Redmond WA), is a microsimulation model with a 1-month cycle length. It simulates cohorts of 10 million hypothetical patients from age 20 until death or age 100. A simplified schematic of the model is provided in Fig. 1.
Fig. 1. Model Schematic.
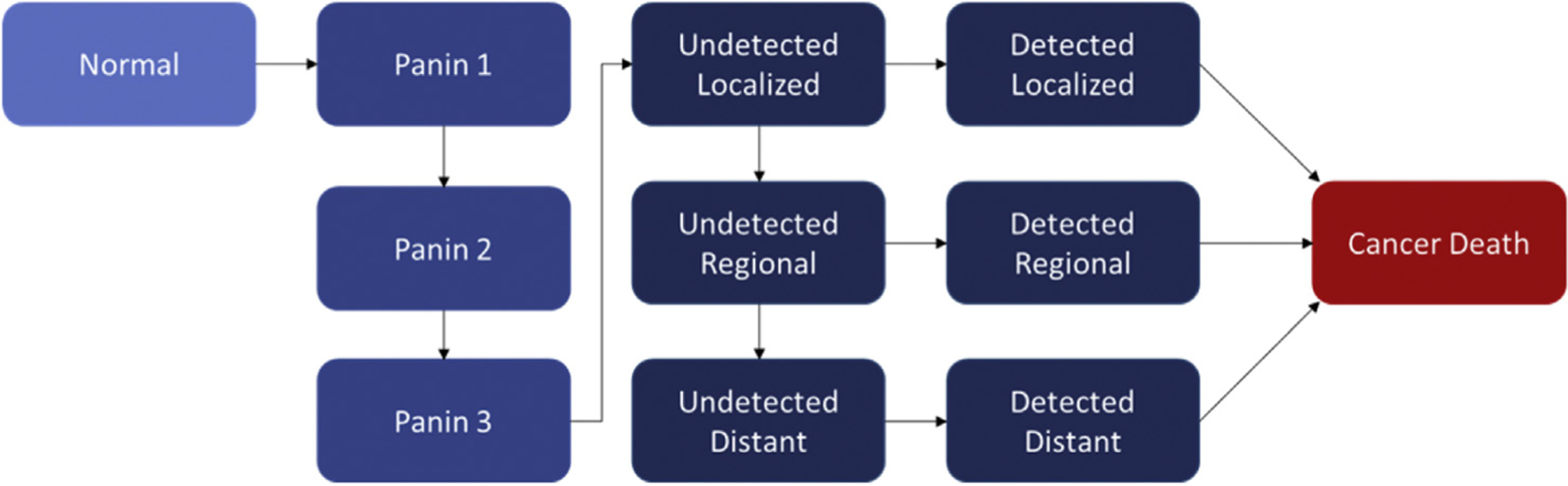
Simplified schematic of the Markov state transition model. In this pathway to solid-derived PDAC, subjects progress from Normal to having at least one PanIN 1 lesion. Subjects can transition to the PanIN 2 and then PanIN 3 cohorts if they develop at least one higher-grade PanIN lesion. From PanIN 3, subjects can transition to Undetected PDAC, which can then progress to wider Undetected disease or be Detected at each stage. Subjects with Detected PDAC are subject to stage-dependent estimates of cancer mortality.
We used this updated model in the current evaluation of the dominant, solid pathway of PDAC development [18]. Subjects could develop PanINs starting at grade 1 (i.e. “PanIN 1”) and progress through PanIN 2 and PanIN 3 to malignancy. We assumed that PanIN lesions could not regress or improve to less dysplastic forms. Once a subject reached the PanIN 3 category, they could transition to undetected malignancy, which could then be detected at the localized, regional, or distant disease stages. The “Undetected” malignancy state was defined as one in which malignant cells were present, but was not detectable through clinical (e.g. symptomatic) presentation. We assumed that there is a larger population of undetected PDAC than detected PDAC, given this broad definition of undetected cancer. Each model cycle (one month), a subject could remain in their current health state or progress to a new health state on the basis of calibrated transition probabilities. If diagnosed with PDAC, patients were subject to stage-dependent survival rates derived from SEER [11]. Patients were also subject to age and gender-specific all-cause mortality risks (i.e. non-PDAC-related) each cycle. Model outcomes were averaged over 10 million simulated subjects.
2.2. Model calibration
In our model, some transition probabilities address events that cannot be observed, such as a PanIN 1 to PanIN 2 transition, and therefore require estimation through a calibration process. In calibration, the model is run iteratively using multiple possible parameter estimates for unobservable parameters; the resulting outputs are compared to the calibration targets. Preferred parameter sets are determined by lowest χ2 goodness of fit (GOF) metric using a calibration method based on simulated annealing process with some modifications. Please see the Appendix for details of the calibration process.
Calibration targets for our model are shown in Table 1 and included: lifetime risk of PDAC, incidence of PDAC by age, lifetime risk of PDAC-associated death, the proportion of PDAC that arises from the solid pathway, PanIN prevalence by grade at ages 50 and 80, and stage of PDAC at diagnosis [4,6,11,13–15,18]. Some calibration targets were constant with age, while others varied. The development of PanIN 1 lesions was assumed to exponentially increase with age, reflecting the increase in prevalence observed in older age groups. Linear progression and power functions were tested and did not produce superior calibration results. Transitions from PanIN 1 to 2, PanIN 2 to 3, and PanIN 3 to Undetected PDAC were all modeled as linear functions with the freedom to increase with age; however, the calibration results were best if these transitions were constant.
Table 1.
Calibration targets and model inputs.
| Calibration Target/Model Input | Female | Male | Source |
|---|---|---|---|
| Lifetime prevalence of PDAC (%) | 1.06 | 1.13 | 11, 12 |
| Incidence of PDAC (age 20–29) (#/100K) | 0.11 | 0.11 | 11 |
| Incidence of PDAC (age 30–39) | 0.44 | 0.56 | 11 |
| Incidence of PDAC (age 40–49) | 2.89 | 3.67 | 11 |
| Incidence of PDAC (age 50–59) | 10.67 | 15.44 | 11 |
| Incidence of PDAC (age 60–69) | 27.89 | 38.44 | 11 |
| Incidence of PDAC (age 70–79) | 53.89 | 65.22 | 11 |
| Prevalence of PanIN 1 lesions, age 50 (%) | 20 | 20 | 4, 6 |
| Prevalence of PanIN 1 lesions, age 80 | 60 | 60 | 4, 6 |
| Prevalence of PanIN 2 lesions, age 80 | 15 | 15 | 4, 6 |
| Prevalence of PanIN 3 lesions, age 80 | 2 | 2 | 4, 6 |
| Proportion of PDAC diagnosed as localized disease (%) | 10.00 | 8.00 | 11 |
| … as regional disease | 28.00 | 27.00 | 11 |
| … as distant disease | 50.00 | 56.00 | 11 |
| … as unstaged disease | 12.00 | 9.00 | 11 |
| Proportion of PDAC from solid lesions (%) | 90 | 90 | 18 |
| Lifetime risk of PDAC-associated death (%) | 0.88 | 0.94 | 11, 12 |
| PDAC mortality from detected localized disease (monthly probability of death) | 0.0228 | 0.0242 | 11 |
| … from detected regional disease | 0.0393 | 0.0393 | 11 |
| … from detected distant disease | 0.0631 | 0.0631 | 11 |
| … from detected unstaged disease | 0.0522 | 0.0522 | 11 |
One of the key uncertainties in our model is the estimate of PanIN prevalence at ages 50 and 80. Available information to support these estimates is relatively less robust than information regarding cancer incidence and prevalence. To address this limitation, we tested two alternative cases for PanIN prevalence. We tested a low scenario, in which all PanIN prevalence estimates were adjusted down 30%, and a high scenario, in which these estimates were increased 30%. See Appendix for further details on the calibration process and results.
2.3. Outcome measures
The primary model outputs were the average life expectancy (LE) of the simulated population, the prevalence by age and gender of the population when stratified by highest-grade PanIN lesion, and the prevalence of undetected and detected PDAC. For the current analysis, primary measures of interest were the probabilities of progression from “normal” (i.e. no PanIN) to PanIN 1, PanIN 1 to 2, PanIN 2 to 3, PanIN 3 to Undetected PDAC, and Undetected to Detected PDAC, as well as the time elapsed between PanIN development (each grade) and Detected PDAC, an estimation of dwell time.
2.3. Exploratory analysis
In an exploratory analysis, we introduced a hypothetical strategy, defined by “perfect” detection and intervention, to determine the maximum life expectancy gains that could be achieved – at the population level – if future technologies were capable of PanIN 3 detection. In this strategy, subjects who have a PanIN 3 lesion were identified without the possibility of false-positive or false-negative findings, and an intervention was applied that was capable of removing their risk of developing PDAC without associated complication, morbidity, or mortality.
We specifically considered the following testing scenarios: a test that could identify PanIN3 lesions at the time they developed (i.e. any age); and a one-time test applied at age 50, 60 or 70. We considered a perfect treatment scenario: if a PanIN 3 lesion or Undetected PDAC was present at the time of testing, the hypothetical intervention removed the subject’s PDAC risk. We ran our test-and-treat analysis first without consideration of surgical mortality, and then again under the assumption that the hypothetical intervention would be associated with a 2% mortality rate, an estimate derived from published mortality rates associated with pancreatic surgery [19–21].
3. Results
3.1. Calibration results
The calibration process was successful; we were able to achieve a well-calibrated model under the constraints of the defined calibration targets. Specific results of the calibration process are detailed in the Appendix, including comparisons of calibration targets and model projections.
3.2. PanIN and PDAC prevalence
Fig. 2 shows the model-estimated prevalence of PanIN 1, PanIN 2, PanIN 3, and solid-pathway undetected and detected PDAC by age. The total prevalence of PanIN lesions increases slowly with age, such that by age 80 almost 74% of both men and women have at least a single PanIN 1 lesion.
Fig. 2. Pancreatic Intraepithelial Neoplasia (PanIN) and Pancreatic Ductal Adenocarcinoma (PDAC) Prevalence by Age (Male).
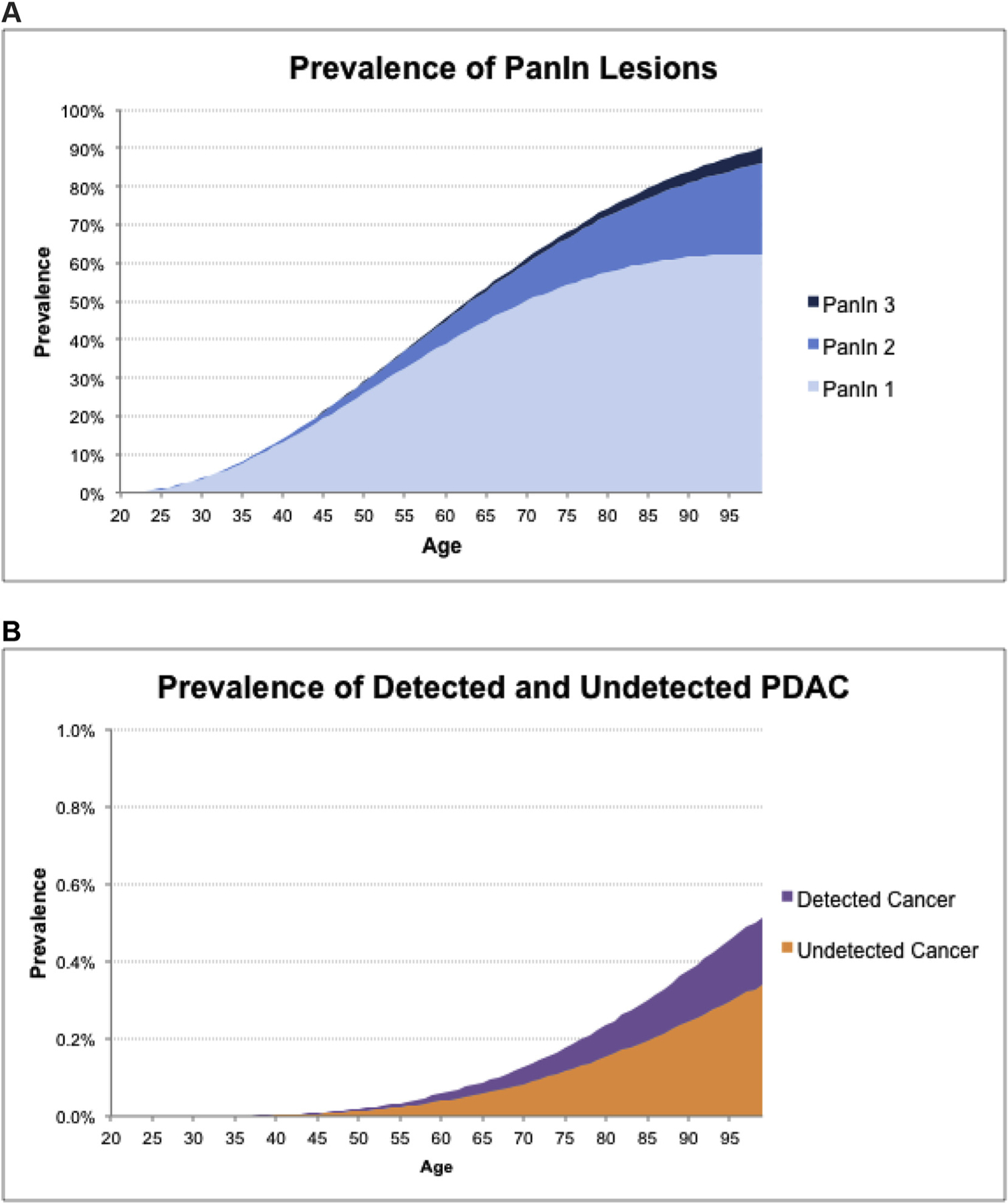
The prevalence of PanIN lesions (A) and the prevalence of undetected and detected PDAC (B) by age for males, as calculated by the simulation model. The incidence and lifetime prevalence of detected PDAC are calibrated to SEER data, PanIN prevalence at ages 50 and 80 are calibrated to available literature. All other prevalence values are calculated by the simulation model.
3.3. Progression probability
For men, over the course of a lifetime, we estimated the probability of transitioning from the PanIN 1 to the PanIN 2 cohort to be 24%, from the PanIN 2 to the PanIN 3 cohort to be 18%, and from the PanIN 3 cohort to Detected malignancy to be 33%. For women, these probabilities were 26%, 18%, and 28%, respectively. This information is summarized in Fig. 3. For both men and women, a substantial percentage of subjects in the PanIN 1 cohort did not progress to PanIN 2 (76% (men) and 74% (women)), and similarly a substantial percentage of subjects in the PanIN 2 cohort did not progress to PanIN 3 (82% (men and women)). The overall chance of progressing from PanIN 1 to Detected malignancy was 1.5% for men, and 1.3% for women.
Fig. 3. Conditional Probability of Progression.
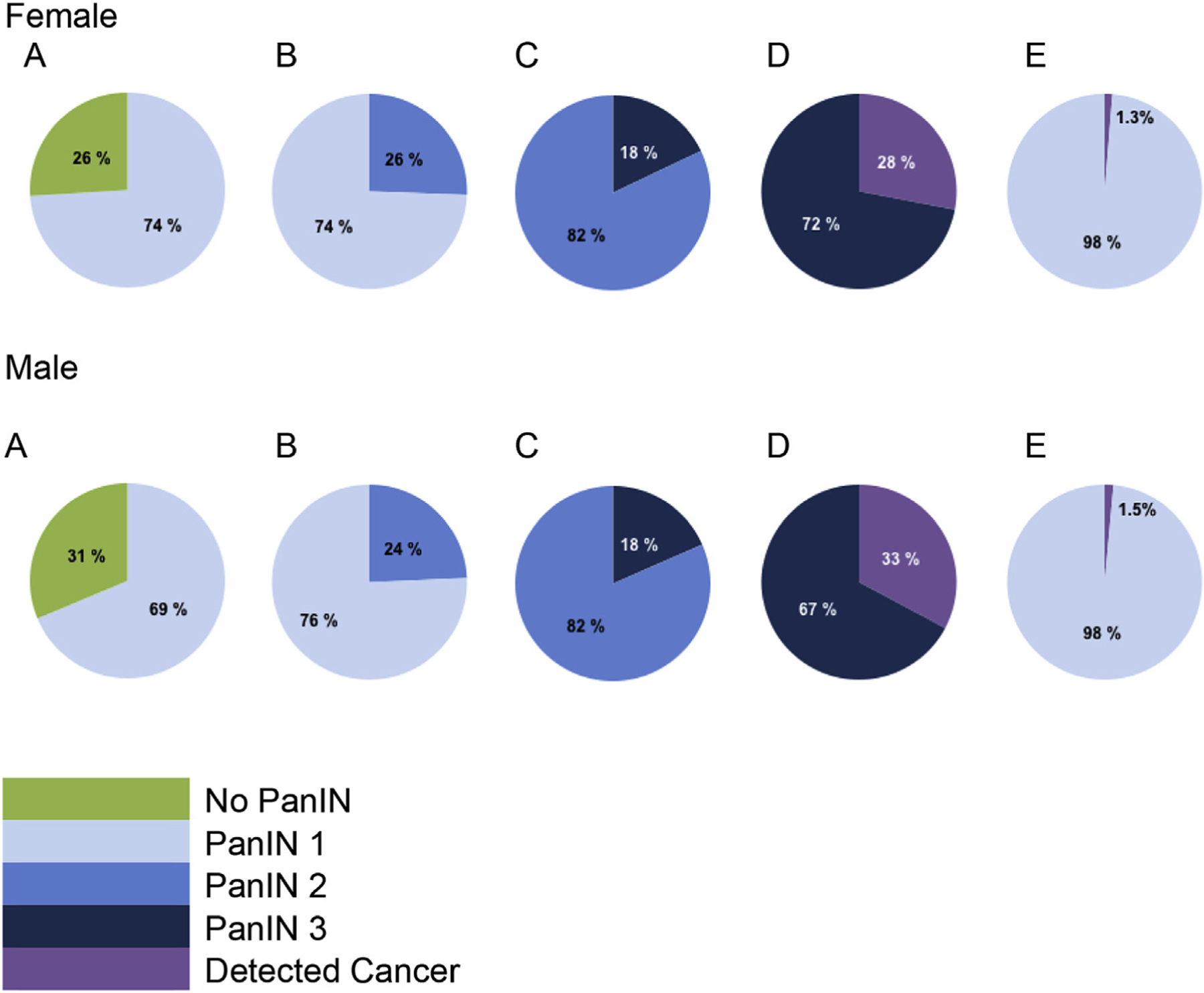
This figure shows the lifetime probability of progression conditional on a patient being in each PanIN state, from no PanIN to PanIN 1 (A), from PanIN 1 to PanIN 2 (B), from PanIN 2 to PanIN 3 (C), and from PanIN 3 to Detected PDAC (D). For example, for a woman, chart C shows the PanIN 2 population, for whom there is an 18% lifetime chance of progression to PanIN 3 and an 82% chance of remaining in PanIN 2. Undetected PDAC is not shown in these charts. The lifetime cumulative probability of progression from PanIN1 to Detected PDAC is shown in (E). Females are shown in the top sequence of charts, males in the bottom sequence.
3.4. Dwell times
On average, men spent 16.5 years (IQR Q1-Q3 6.00–24.3) in the PanIN 1 cohort before transitioning to the PanIN 2 cohort; women spent 17.4 (6.50–25.6) years. Men then spent 13.3 (4.58–19.3) years in the PanIN 2 cohort before transitioning to PanIN 3 (women 13.9 (4.83–20.3) years), and 9.0 (2.83–12.8) years in the PanIN 3 cohort before transitioning to undetected PDAC (women 10.0 (3.25–14.3) years). PDAC remained undetected for 2.56 (0.92–3.50) years (men) and 2.65 (1.00–3.58) years (women). If we include those subjects who died of other causes prior to progression, the time spent in the PanIN 1, 2, and 3 cohorts was 22.3 (9.42–32.8) years, 16.4 (6.00–24.1) years, and 10.0 (3.25–14.3) years for men) and 23.7 (10.3–34.9) years, 17.5 (6.50–25.7) years, and 11.3 (3.75–16.3) years for women, respectively. In total, men who developed PDAC spent 33.6 (24.0–42.6) years from the time they developed their first PanIN lesion until a PDAC diagnosis (women 35.3 (25.6–44.7) years). This dwell time analysis is summarized in Table 2.
Table 2.
Dwell time: Average time spent in each PanIN state.
| All Femalesa | Females Who Progress to the Next Stateb | All Malesa | Males Who Progress to the Next Stateb | |
|---|---|---|---|---|
| Time in PanIN 1 State | 23.7 | 17.4 | 22.3 | 16.5 |
| Average years (IQR Q1-Q3) | (10.3–34.9) | (6.50–25.6) | (9.42–32.8) | (6.00–24.3) |
| Time in PanIN 2 State | 17.5 | 13.9 | 16.4 | 13.3 |
| Average years (IQR Q1-Q3) | (6.50–25.7) | (4.83–20.3) | (6.00–24.1) | (4.58–19.3) |
| Time in PanIN 3 State | 11.3 | 10.0 | 10.0 | 9.0 |
| Average years (IQR Q1-Q3) | (3.75–16.3) | (3.25–14.3) | (3.25–14.3) | (2.83–12.8) |
| Time in Undetected PDAC | 2.65 | 2.56 | ||
| Average years (IQR Q1-Q3) | (1.00–3.58) | (0.92–3.50) | ||
| PanIN 1 to Detected PDAC | 35.3 | 33.6 | ||
| Average years (IQR Q1-Q3) | (25.6–44.7) | (24.0–42.6) | ||
| PanIN 3 to Detected PDAC | 12.3 | 11.3 | ||
| Average years (IQR Q1-Q3) | (5.75–16.8) | (5.25–15.4) |
The “All Females” and “All Males” columns show the average (IQR Q1-Q3) amount of time spent in the state for all subjects who enter that state, whether they progress to the next state or not.
The “Females Who Progress to the Next State” and “Males Who Progress to the Next State” columns show the average (IQR Q1-Q3) amount of time spent in each state for those subjects who eventually progress to the next state.
We tested the uncertainty surrounding PanIN prevalence at ages 50 and 80 using high and low scenarios. In the low scenario, the lifetime prevalence of a PanIN was decreased to 55%, with the result that the percentage of people with PanIN who eventually developed cancer increased to 1.7%. In this low scenario, the time from PanIN 1 development to Detected PDAC was slightly lower at 34.0 years, with each of the dwell times from PanIN 1 to cancer slightly shorter. In the high scenario, the lifetime prevalence of a PanIN was increased to 92%, with the result that the percentage of people with PanIN who eventually developed cancer decreased to 1.1%. The time from PanIN 1 development to Detected PDAC was slightly lower at 33.1 years, and each of the dwell times from PanIN 1 to cancer was again slightly shorter. The overall impact of changing PanIN prevalence estimates on dwell time results was small.
3.5. Exploratory analysis results: hypothetical “perfect” test and treatment
Our hypothetical perfect test-and-treat strategy for PanIN 3 lesions that allowed for continuous 100% risk reduction would have an expected benefit of 40 and 39 additional days of life for men and women, respectively. If such a strategy were applied only once (at age 50, 60, or 70), the expected risk reduction would drop to 12%, 23%, or 28% (ages 50, 60 or 70) for men and 11%, 23%, or 30% (ages 50, 60 or 70) for women. This would provide an attenuated life expectancy benefit of 7, 10, or 7 days (ages 50, 60, or 70) for men; 7, 10, or 8 days (ages 50, 60, or 70) for women. When introducing the risk of surgical mortality, the life expectancy benefit drops by approximately half a day across all cases. These trade-offs are summarized in Table 3.
Table 3.
Hypothetical “test and treat” intervention.a.
| Reduction in Lifetime Risk of PDAC (%)b | Female | Male |
|---|---|---|
| One time testing at age 50 | −11% | −12% |
| One time testing at age 60 | −23% | −23% |
| One time testing at age 70 | −30% | −28% |
| Continuous testing | −100% | −100% |
| Life Expectancy Gains (days) | ||
| With 0% Surgical Mortality c | Female | Male |
| One time testing at age 50 | 6.9 | 7.1 |
| One time testing at age 60 | 10 | 10 |
| One time testing at age 70 | 8.8 | 7.9 |
| Continuous testing | 39 | 40 |
| With 2% Surgical Mortality d | Female | Male |
| One time testing at age 50 | 6.4 | 6.7 |
| One time testing at age 60 | 9.2 | 9.3 |
| One time testing at age 70 | 7.7 | 7.0 |
| Continuous testing | 35 | 37 |
All benefits of the intervention are averaged across the entire simulated population.
In the first section, we show the average reduction in the lifetime risk of PDAC in the simulated population given a hypothetical perfect PanIN 3 test followed by perfect risk-reduction for future PDAC.
In the second section, we show the average change in life expectancy for the simulated population under each hypothetical “test and treat” scenario, with the assumption that there is no surgical mortality.
In the third section, we show the average change in life expectancy for the simulated population under the same hypothetical “test and treat” scenarios, with the addition of 2% surgical mortality.
4. Discussion
We found that PanIN 3 lesions are likely to have a higher probability of progression relative to PanIN 1 and 2 lesions, and to progress more rapidly. This would imply that PanIN 3 lesions, in addition to having a higher degree of dysplasia, are on average also more aggressive. We estimated the average dwell time from Undetected to Detected PDAC in our model to be just over three years, defining a potential window for cancer screening. It is important to note that not all undetected PDAC lesions may be within the detection threshold for imaging modalities, therefore the realistic window for clinical detection may be shorter. Also, the majority of PDACs are initially diagnosed within the pancreas or liver, rather than in vulnerable locations such as the CNS that might drive detection at a smaller size. This period of time is similar to the dwell time estimated for lung cancer in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) dataset (male smokers: 1.82 years, female smokers: 1.84 years) [22] and for colorectal cancer from the German screening cohort (4.5–5.8 years for all age groups in both men and women) [23]. Finally, the results of our model indicate a relatively long period from the development of PanIN 3 to malignancy (on average 11.3 years for men and 12.3 years for women). If true, this would allow a broad window for early detection.
Our hypothetical test-and-treat intervention – a test strategy that is perfect for the detection of PanIN 3 lesions – resulted in a gain of 9 days of life expectancy if applied once at age 60, about 30% of the value of a perfect test-and-treat applied continuously. Such an intervention would allow for the detection of a large number of pre-cancerous lesions prior to the onset of PDAC. At the age of 60, just under 50% of the population would have a PanIN lesion, but very few (0.1%) would have developed cancer. The mortality of surgical resection approaches 2% in most case series [19–21], and carries ongoing morbidity due to impaired insulin production, pancreatic insufficiency, and post-operative discomfort [24,25]. However, this must be weighed against the fact that most PDAC is discovered at an advanced stage when curative resection is not possible [26]. By way of comparison, colorectal cancer screening with colonoscopy has been shown to increase life expectancy 170 days among 50–54 year olds, and 41 days among 70–74 year olds [27]. Mammography has been estimated to increase life expectancy anywhere from 4–36 days depending on the frequency and age of screening [28]. These results indicate that the benefit of detecting and intervening on PanIN lesions may be within a reasonable range for a public health intervention if the performance characteristics of such a test were sufficiently high.
Our analysis has specific limitations that merit mention. First, we did not explicitly model the development of individual PanIN lesions, but rather the presence of at least one PanIN lesion within each cohort. It has been observed that some PDAC are associated with a relatively larger number of associated PanINs; the correlation between the number of PanINs and the relative risk of PDAC is unknown [29]. Our analysis does not allow for consideration of variability in the number of PanIN lesions, or of the correlation between the number of PanINs and the rate of progression. Second, it has been proposed that the medical community move from a three-tiered classification of PanIN lesions to a two-tiered system. In this revised description, PanIN 1 and 2 lesions (per WHO 2010) are grouped as low-grade PanINs, and PanIN 3 is classified as high-grade. This distinction was suggested to reflect the relatively low malignant potential of PanIN 1 and 2 lesions, relative to the higher risk behavior of PanIN 3 lesions [30]. In our analysis, we needed to retain the three-tiered classification because it was used in our primary data sources [4,6]. However, our results can be easily interpreted in the new classification schema by combining the PanIN 1 and PanIN 2 groups.
Finally, our analysis assumes an average-risk population. Several subpopulations of individuals have varied levels of elevated risk for PDAC. For example, smoking, type II diabetes, BRCA2 mutation positivity, and Peutz-Jeghers disease have been shown to confer risks for PDAC that are 2x, 2x, 3x, and 69x that of the average population [31–34]. Such individuals ultimately are likely to benefit more from PDAC screening than are average risk patients [17,35,36]. The current study does not address differences in the natural history of solid-derived PDAC or PanIN precursor lesions that are present in individuals at higher risk, and does not make corresponding projections in such subpopulations. However, the presented methods and analysis can be used to develop such projections in future work.
In conclusion, our simulation model describes the development of PDAC from PanIN lesions. PanIN lesions are a precursor to most PDAC, so knowledge of the dwell times between PanIN onset and the development of PDAC is critical to shaping future, successful screening strategies. We have shown that given this relatively long period for screening, an intervention that could detect and mitigate the risk from PanIN lesions could provide a population health benefit.
Supplementary Material
5. Acknowledgements
5.1. Disclosures
Peters: received honoraria from Bayer, travel support from Halozyme and AstraZeneca, outside the submitted work, no funding sources.
Eckel: none.
Mueller: none.
Tramontano: none.
Weaver: none.
Lietz: none.
Hur: received research funding from the National Cancer Institute (R01CA212086).
Kong: received research funding from the National Cancer Institute (R01CA212086).
Pandharipande: received research funding from the Medical Imaging and Technology Alliance, outside the submitted work.
5.2. Funding
The submitted work was supported by the American Cancer Society - New England Division - Ellison Foundation Research Scholar Grant (RSG-15–129-01CPHPS) and by the National Cancer Institute (R01CA212086).
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.pan.2018.07.009.
References
- [1].Andea A, Sarkar F, Adsay VN. Clinicopathological correlates of pancreatic intraepithelial neoplasia: a comparative analysis of 82 cases with and 152 cases without pancreatic ductal adenocarcinoma. Mod Pathol Offic J United States Can Acad Pathol Oct 2003;16(10):996–1006. [DOI] [PubMed] [Google Scholar]
- [2].Takaori K. Current understanding of precursors to pancreatic cancer. J Hepato-Biliary-Pancreatic Surg 2007;14(3):217–23. [DOI] [PubMed] [Google Scholar]
- [3].Takaori K, Hruban RH, Maitra A, Tanigawa N. Pancreatic intraepithelial neoplasia. Pancreas Apr 2004;28(3):257–62. [DOI] [PubMed] [Google Scholar]
- [4].Matsuda Y, Furukawa T, Yachida S, et al. The prevalence and clinicopathological characteristics of high-grade pancreatic intraepithelial neoplasia: autopsy study evaluating the entire pancreatic parenchyma. Pancreas May/Jun 2017;46(5):658–64. [DOI] [PubMed] [Google Scholar]
- [5].Konstantinidis IT, Vinuela EF, Tang LH, et al. Incidentally discovered pancreatic intraepithelial neoplasia: what is its clinical significance? Ann Surg Oncol Oct 2013;20(11):3643–7. [DOI] [PubMed] [Google Scholar]
- [6].Matsuda Y, Tanaka M, Sawabe M, et al. Relationship between pancreatic intraepithelial neoplasias, pancreatic ductal adenocarcinomas, and single nucleotide polymorphisms in autopsied elderly patients. Gene Chromosome Canc Jan 2018;57(1):12–8. [DOI] [PubMed] [Google Scholar]
- [7].Hruban RH, Goggins M, Parsons J, Kern SE. Progression model for pancreatic cancer. Clin Canc Res Offic J Am Assoc Cancer Res Aug 2000;6(8):2969–72. [PubMed] [Google Scholar]
- [8].Kanda M, Matthaei H, Wu J, et al. Presence of somatic mutations in most earlystage pancreatic intraepithelial neoplasia. Gastroenterology Apr 2012;142(4): 730–3. e739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Notta F, Chan-Seng-Yue M, Lemire M, et al. A renewed model of pancreatic cancer evolution based on genomic rearrangement patterns. Nature Oct 20 2016;538(7625):378–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yachida S, Iacobuzio-Donahue CA. Evolution and dynamics of pancreatic cancer progression. Oncogene Nov 7 2013;32(45):5253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Probability of Developing or Dying of Cancer Software. Version 6.7.3; statistical research and applications branch National Cancer Institute; 2005. Accessed 10/26/15, 2015, http://srab.cancer.gov/devcan. [Google Scholar]
- [12].Surveillance Research Program. Cancer statistics branch. Surveillance, Epidemiology, and End results results (SEER) program DevCan database 2015. https://surveillance.cancer.gov/devcan/.
- [13].Brugge WR, Lauwers GY, Sahani D, Fernandez-del Castillo C, Warshaw AL. Cystic neoplasms of the pancreas. N Engl J Med Sep 16 2004;351(12): 1218–26. [DOI] [PubMed] [Google Scholar]
- [14].Canto MI, Hruban RH, Fishman EK, et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology Apr 2012;142(4):796–804. quiz e714–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR (Am J Roentgenol) Sep 2008;191(3):802–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Arias E, Heron M, Xu JQ. National vital statistics reports. United States life tables, 2012, vol. 65. Hyattsville, MD: National Center for Health Statistics; 2016. no 8. [PubMed] [Google Scholar]
- [17].Pandharipande PV, Heberle C, Dowling EC, et al. Targeted screening of individuals at high risk for pancreatic cancer: results of a simulation model. Radiology Apr 2015;275(1):177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Poultsides GA, Reddy S, Cameron JL, et al. Histopathologic basis for the favorable survival after resection of intraductal papillary mucinous neoplasm-associated invasive adenocarcinoma of the pancreas. Ann Surg Mar 2010;251(3):470–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fernandez-del Castillo C, Morales-Oyarvide V, McGrath D, et al. Evolution of the whipple procedure at the Massachusetts general hospital. Surgery Sep 2012;152(3 Suppl 1):S56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med Jun 2 2011;364(22):2128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg Offic J Soc Surg Aliment Tract Nov 2006;10(9):1199–210. discussion 1210–1191. [DOI] [PubMed] [Google Scholar]
- [22].Wang DLB, Riley T, Wu D. Estimation of sojourn time and transition probability of lung cancer for smokers using the PLCO data. J Biometrics Biostat 2017;8(4):360. [Google Scholar]
- [23].Brenner H, Altenhofen L, Katalinic A, Lansdorp-Vogelaar I, Hoffmeister M. Sojourn time of preclinical colorectal cancer by sex and age: estimates from the German national screening colonoscopy database. Am J Epidemiol Nov 15 2011;174(10):1140–6. [DOI] [PubMed] [Google Scholar]
- [24].Huttner FJ, Fitzmaurice C, Schwarzer G, et al. Pylorus-preserving pancreaticoduodenectomy (pp Whipple) versus pancreaticoduodenectomy (classic Whipple) for surgical treatment of periampullary and pancreatic carcinoma. Cochrane Database Syst Rev Feb 16 2016;2, CD006053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cloyd JM, Tran Cao HS, Petzel MQ, et al. Impact of pancreatectomy on long-term patient-reported symptoms and quality of life in recurrence-free survivors of pancreatic and periampullary neoplasms. J Surg Oncol Feb 2017;115(2):144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shi S, Hua J, Liang C, et al. Proposed modification of the 8th edition of the AJCC staging system for pancreatic ductal adenocarcinoma. Ann Surg Jan 12 2018. 10.1097/SLA.0000000000002668. epub ahead of print. [DOI] [PubMed]
- [27].Inadomi JM, Sonnenberg A. The impact of colorectal cancer screening on life expectancy. Gastrointest Endosc May 2000;51(5):517–23. [DOI] [PubMed] [Google Scholar]
- [28].Myers ER, Moorman P, Gierisch JM, et al. Benefits and harms of breast cancer screening: a systematic review. Jama Oct 20 2015;314(15):1615–34. [DOI] [PubMed] [Google Scholar]
- [29].Miyazaki T, Ohishi Y, Miyasaka Y, et al. Molecular characteristics of pancreatic ductal adenocarcinomas with high-grade pancreatic intraepithelial neoplasia (PanIN) are different from those without high-grade PanIN. Pathobiology J Immunopathol Mol Cell Biol 2017;84(4):192–201. [DOI] [PubMed] [Google Scholar]
- [30].Basturk O, Hong SM, Wood LD, et al. A revised classification system and recommendations from the baltimore consensus meeting for neoplastic precursor lesions in the pancreas. Am J Surg Pathol Dec 2015;39(12):1730–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bosetti C, Rosato V, Li D, Silverman D, Petersen GM, Bracci PM, et al. Diabetes, antidiabetic medications, and pancreatic cancer risk: an analysis from the international pancreatic cancer case-control consortium. Ann Oncol Offic J Eur Soc Med Oncol 2014;25:2065–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 2013;144:1252–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Holter S, Borgida A, Dodd A, Grant R, Semotiuk K, Hedley D, et al. Germline rca mutations in a large clinic-based cohort of patients with pancreatic adenocarcinoma. J Clin Oncol Offic J Am Soc Clin Oncol 2015;33:3124–9. [DOI] [PubMed] [Google Scholar]
- [34].van Asperen CJ, Brohet RM, Meijers-Heijboer EJ, Hoogerbrugge N, Verhoef S, asen HF, et al. Cancer risks in brca2 families: estimates for sites other than breast and ovary. J Med Genet 2005;42:711–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Pandharipande PV, Jeon A, Heberle CR, Dowling EC, Kong CY, Chung DC, et al. Screening for pancreatic adenocarcinoma in brca2 mutation carriers: results of a disease simulation model. EBioMedicine 2015;2:1980–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Canto MI, Almario JA, Schulick RD, Yeo CJ, Klein A, Blackford A, et al. Risk of neoplastic progression in individuals at high risk for pancreatic cancer undergoing long-term surveillance. Gastroenterology 2018. In press, 10.1053/j.gastro.2018.05.035. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


