Abstract
Several extracellular enzymes that are responsible for plant tissue maceration were detected in culture supernatant of the psychrotrophic bacterium Chryseomonas luteola MFCL0. Isoelectrofocusing experiments showed that pectate lyase (PL) activity resulted from the cumulative action of three major isoenzymes, designated PLI, PLII, and PLIII. Cellulolytic activity was also detected in culture supernatants. These enzymes exhibited different behaviors with respect to growth temperature. PLII was not regulated by temperature, whereas PLI and PLIII were regulated similarly by growth temperature. Maximal levels of PLI and PLIII were produced at 14°C when cells were grown in polygalacturonate-containing synthetic medium and at around 20 to 24°C in nutrient broth. In contrast, thermoregulation of cellulolytic activity production differed from thermoregulation of PL. The level of cellulolytic activity was low in all media at temperatures up to 20°C, and then it increased dramatically until the temperature was 28°C, which is the optimal temperature for growth of C. luteola. Previously, we defined the critical temperature by using the modified Arrhenius equation to characterize bacterial behavior. This approach consists of monitoring changes in the maximal specific growth rate as a function of temperature. Our most striking result was the finding that the temperature at which maximum levels of PLI and PLIII were produced in two different media was the same as the critical temperature for growth observed in these two media.
The most general method for fresh food preservation is cold storage. The main drawback of this method is psychrotrophic bacteria that produce extracellular spoilage enzymes even at low temperatures (5). Psychrotrophic bacteria grow at a wide range of temperatures (from 0 to 35°C, with an optimum temperature of approximately 30°C according to Morita [23]). Chryseomonas luteola MFCL0 has been isolated from spoiled celeriac stored at low temperatures. This bacterium, which has not been described as a phytopathogen yet, is able to macerate plant tissue even at low temperatures, which suggests that extracellular spoilage enzymes are produced under these conditions.
Many fungi and bacteria cause soft rot of plant products during storage. These microorganisms produce pectinolytic and cellulolytic enzymes that macerate plant tissue (20, 24). Several pectinases have been characterized; these enzymes include pectate lyases (PL), pectin lyases, and polygalacturonases. PL and pectin lyases catalyze cleavage of the α-1,4 bond between galacturonic acid residues by β-elimination, generating unsaturated products (C4 to C5), while polygalacturonases hydrolyze their substrates. PL are distinguished from pectin lyases by their specificity for pectin that has been demethylated by pectin methyl esterases (8, 26, 28). Studies of cellulases (Cel) have revealed a number of cellulolytic enzymes that act together to degrade cellulose in plant cell walls (3, 29). These enzymes have been found previously in mesophilic bacteria, such as the well-known phytopathogenic erwiniae (2, 16), and in psychrotrophic bacteria, such as pseudomonads (10, 11, 21, 27), but not in members of the genus Chryseomonas.
It has been known for a long time that several extracellular enzymes of psychrotrophs may be produced preferentially at low temperatures (1). More recently, production of several enzymes by different strains of Pseudomonas fluorescens was described as being optimal at one temperature, 17°C (12, 15, 22). The physiological importance of this temperature was later emphasized when Guillou and Guespin-Michel showed that it separates two temperature domains in which the maximum specific growth rate depends on temperature (13). This was demonstrated when Arrhenius profiles were studied by plotting the natural logarithm of the specific maximal growth rate versus the inverse of the absolute temperature. Using the values obtained, the researchers drew two straight lines (the slopes of which represented the activation energies) that intersected at a point corresponding to 17°C. Results obtained with chemostat cultures (13) showed that the net production of proteins decreased as a function of temperature at temperatures above a “critical temperature” for this species. This led to coining of the term critical temperature. This term refers classically to the border between two temperature domains in which the effects of temperature on growth are different. However, in P. fluorescens MF0 the critical temperature coincides with the optimal temperature for production of several exported enzymes and with the temperature above which protein turnover may be increased. Although this set of processes is very different (some of the extracellular enzymes are produced only at the beginning of the stationary growth phase, for instance), whether this is a mere coincidence or reflects an important physiological property must be asked.
In this study we examined the physiological behavior of and production of extracellular enzymes (PL and Cel) by C. luteola MFCL0 in different growth media and at different temperatures. The aim of this work was to determine if this psychrotrophic strain has a critical temperature and if the coincidence described above concerning enzyme production occurs. Our findings could result in determining the importance of critical temperature in the regulation of some genes.
MATERIALS AND METHODS
Bacterial strain, growth media, and culture conditions.
C. luteola MFCL0 was obtained by screening psychrotrophic bacteria for the ability to grow on plant tissue and the ability to produce pectinolytic and cellulolytic activities on plates. The bacteria screened were obtained from a frozen (−80°C) collection of bacteria that had been isolated from celeriac (Apium graveolens) stored in a cold room. Celeriac is a tuber that is used in northern European countries for human alimentation.
Cells were grown in the following liquid media: polygalacturonate (PGA) synthetic medium, which contained (per liter) 11 g of K2HPO4, 5.5 g of KH2PO4, 1.2 g of (NH4)2SO4, 0.4 g of MgSO4, 0.15 g of CaCl2, and 4 g of PGA (Sigma) (pH 7); nutrient broth (NB) (Diagnostics Pasteur), which contained (per liter) 3 g of meat extract and 5 g of peptone (pH 7); and NB+PGA, which contained NB and 8 g of PGA per liter (pH 7). Batch cultures were grown in Erlenmeyer flasks at various temperatures between 8 and 28°C on a gyratory shaker at 180 rpm. In each case, the volume of the medium was 10% of the total flask volume. PGA solid medium was obtained by adding agar (15 g liter−1) and yeast extract (0.2 g liter−1) to the PGA liquid medium described above. Carboxymethyl cellulose (CMC) solid medium was obtained by adding agar (15 g liter−1), yeast extract (0.5 g liter−1), and CMC (4 g liter−1) to mineral salt medium containing (per liter) 1 g of NaNO3, 1 g of K2HPO4, 0.5 g of KCl, and 0.5 g of MgSO4 (pH 7).
Detection of pectinolytic and cellulolytic activities on plates.
Bacteria were grown on PGA solid medium at 28°C for 24 h, and the plates were flooded with a cetyltrimethylammonium bromide solution (10 g liter−1). The colonies that produced PL and/or polygalacturonases were surrounded by clear haloes as a result of substrate degradation. The Cel-producing colonies were identified by using CMC solid medium. After plates were incubated at 28°C for 24 h, Cel activities were revealed by staining the plates with a solution containing 0.1 g of Congo red dye (Sigma) per liter for 15 min. The plates were then washed with a 1 M NaCl solution to reveal Cel activity, which appeared as orange haloes on a red background.
Preparation of enzyme samples.
Cells were removed from each culture by centrifugation (10,000 × g, 15 min, Sorvall centrifuge), and the supernatants were sterilized by filtration through a 0.22-μm-pore-size Millipore filter. Cultures of C. luteola MFCL0 grown at 8, 17, and 28°C were centrifuged at appropriate times to determine the levels of PL and Cel activities in the supernatants during different growth phases. To determine PL and Cel activities spectrophotometrically, each culture was centrifuged in the early stationary phase. For sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and isoelectrofocusing (IEF), the filtered supernatants obtained from each culture were concentrated by reverse dialysis with polyethylene glycol 20,000 (Sigma) and then dialyzed in distilled water. All of the supernatants were divided into aliquots and frozen at −20°C until they were used.
Assay for PL activities.
PL activity was determined by monitoring the formation of C4 and C5 unsaturated products spectrophotometrically at 235 nm (9). The method which was described previously was modified as follows: 500 μl of 0.1 M Tris-HCl (pH 9) buffer containing 0.5 mM CaCl2 was rapidly mixed in a 1.5-ml cuvette with 370 μl of distilled water, 100 μl of a PGA solution (1% [wt/vol] in water), and 30 μl of supernatant. The reaction mixture was incubated at 30°C. One unit was defined as the amount of enzyme which produced 1 μmol of unsaturated product. Activity was expressed in micromoles of unsaturated product liberated per minute per milliliter of supernatant, and specific activity was expressed in micromoles of unsaturated product liberated per minute per unit of optical density at 580 nm.
Assay for Cel activity.
A soluble synthetic analog of cellulose, p-nitrophenyl-β-d-cellobioside, was used as a substrate in the Cel activity assay. The assay was performed in 50 mM HEPES buffer (pH 7.0) containing substrate at a concentration of 5 mM and 100 μl of supernatant (total volume, 250 μl). After 2 h of incubation at 35°C, the reaction was stopped by adding of 600 μl of 0.01 N NaOH. The amount of p-nitrophenol released was then measured spectrophotometrically at 405 nm. One unit corresponded to release of 1 μmol of p-nitrophenol per min.
Protein analysis.
Protein concentrations were determined with an ESL kit (Boehringer Mannheim). SDS-PAGE was performed by using a 5% stacking gel, a 15% separating gel, and the Laemmli (19) buffer system, except that no β-mercaptoethanol was added to the loading buffer. Molecular mass standards were obtained from Pharmacia. The gels were loaded with 5 μg of proteins. IEF was performed with a MultiphorII system (LKB Pharmacia) (4). pH 2 to 11 and pH 9 to 11 were obtained by using ampholytes as recommended by the manufacturer (Sigma). The anode strip was saturated with 0.5 M H3PO4, and the cathode strip was saturated with 0.5 M NaOH. The gels (thickness, 2 mm) were loaded with 5 μg of supernatant proteins and electrophoresed at 5 W for 6 h. The electrophoresis gels (SDS-PAGE and IEF gels) were first incubated in a 0.1 M Tris-HCl (pH 8.6) buffer for 30 min and then were blotted onto plates containing pectate agarose (4). Finally, the SDS-PAGE and IEF gels were silver stained (Bio-Rad kit), and the pectate agarose gels were stained with a 0.05% ruthenium red solution. Gel filtration chromatography was performed with a Sephacryl (Sigma) S-300 HR column (1 by 50 cm; Kontes-Scientific Glassware/Instruments). The concentrated and dialyzed supernatants (1 ml; 250 μg of protein) were applied to the column, which was equilibrated and eluted with 10 mM MES (morpholineethanesulfonic acid) buffer (pH 6.5) at a flow rate of 1 ml/min. The column was calibrated with a set of molecular mass standards (Sigma) under identical conditions. The 1-ml fractions collected were screened to determine whether proteins (A280) and PL activity were present.
RESULTS
Pectinolytic and cellulolytic activities of C. luteola MFCL0.
C. luteola MFCL0 was isolated from spoiled cold-stored celeriac, screened as described above, and identified by using API 20NE galleries. The extracellular enzymes that were responsible for postharvest spoilage of the vegetable and were secreted by this bacterium were first detected on solid media. The pectinolytic and cellulolytic activities produced by C. luteola MFCL0 appeared on PGA solid medium and CMC solid medium, respectively.
Effect of growth temperature and culture medium on growth.
To characterize the physiological behavior of C. luteola MFCL0, cultures were grown at different temperatures (8, 11, 14, 17, 20, 24, and 28°C) and in different media (PGA medium, NB, and NB+PGA). The maximum growth rate was determined under each set of conditions. The growth curves obtained in NB+PGA were diauxic, and NB was metabolized first (data not shown).
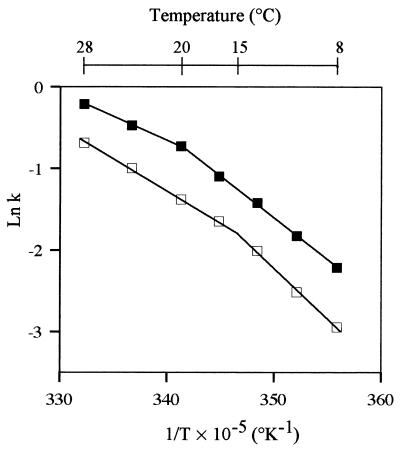
Figure 1 shows Arrhenius curves obtained by plotting the natural logarithm of the maximum specific growth rate versus the inverse of the absolute temperature. The Arrhenius plots were best fit by two linear segments whose convergence determined the critical temperature. The curves obtained when cells were grown in PGA medium and in NB were biphasic, but the temperatures that separated the two domains in the two media were different. The critical temperatures were approximately 15°C for cells grown in PGA medium and 20°C for cells grown in NB.
FIG. 1.
Arrhenius plots for growth of C. luteola MFCL0 (ln maximum specific growth rate [k] versus the reciprocal of the absolute temperature [T]). The maximum specific growth rate k is expressed in hours−1, and the temperature is expressed in degrees Kelvin. Each datum point represents a mean based on at least six independent determinations. The lines were drawn by calculating linear regressions for experimental data with the shareware Nonlin (r = 0.999). Symbols: □, PGA medium; ■, NB. The standard deviations were too small to be shown.
Effect of growth temperature and culture medium on production of PL.
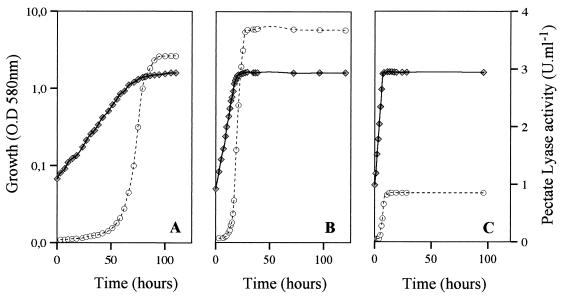
Figure 2 shows that PL activity appeared in PGA medium culture supernatants during late exponential growth and reached a maximum level at the beginning of the stationary phase. The level then remained stable for at least 50 h. The same results were obtained when cells were grown in NB and in NB+PGA.
FIG. 2.
PL production during growth of C. luteola MFCL0. Cells were grown in PGA medium at 8°C (A), 17°C (B), and 28°C (C). PL activity was assayed as described in the text. Each datum point represents a mean based on at least three independent determinations. Symbols: ⧫, growth; ○, PL activity. The maximum standard deviation for any enzymatic determination was 5%, and the maximum standard deviation for growth was 4%. O.D. 580 nm, optical density at 580 nm.
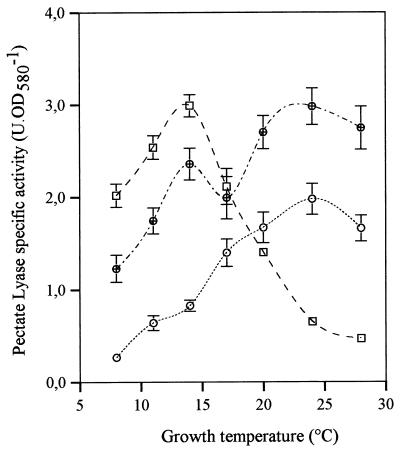
To study regulation of PL activity by temperature in different media, PL production was monitored over a broader temperature range (Fig. 3). In PGA medium (used because it has been shown that several PL are induced by PGA), PL production was maximal at temperatures around 14°C and then decreased rapidly as the temperature was increased to 28°C. In contrast, in NB medium PL production increased slowly until it reached a maximum value at temperatures around 20 to 24°C. It should be noted that these maxima are close to the critical temperatures observed in the media used (Fig. 1). Finally, maximum levels of production at these two temperatures were observed in the mixed medium, NB+PGA. These results are consistent with the hypothesis that the cells produce at least two PL, one that is inducible by PGA (maximum production occurs at temperatures around 15°C) and one that is inducible by NB (maximum production occurs at temperatures between 20 and 24°C).
FIG. 3.
PL specific activity of C. luteola MFCL0. Cells were grown in PGA medium, NB, and NB+PGA at 8, 11, 14, 17, 20, 24, and 28°C. PL activity was assayed as described in the text. Each datum point represents a mean based on at least six independent determinations. The error bars indicate standard deviations. Symbols: □, PGA medium; ○, NB; ⊕, NB+PGA. OD580, optical density at 580 nm.
Characterization of the different PL: effects of growth temperature and medium on enzyme production.
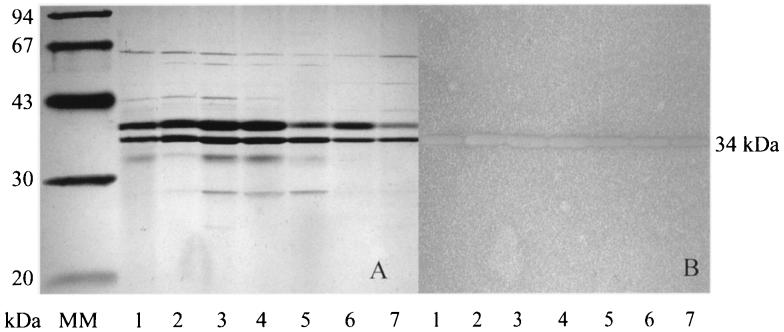
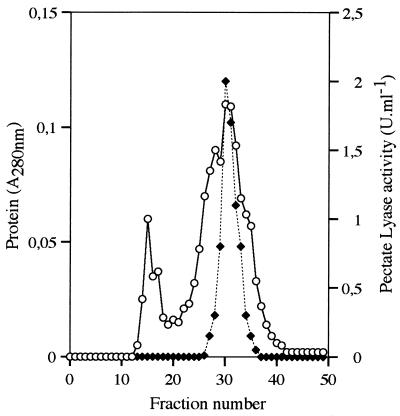
Supernatants of MFCL0 cultures were analyzed by SDS-PAGE, and PL activities were revealed by the pectate agarose overlay method. The molecular masses of PL were determined by comparing the clear spots on the pectate agarose gel with the silver-stained proteins. Figure 4 shows that the PL produced when cells were grown in PGA medium migrated as a single band at approximately 34 kDa. A band at 34 kDa was also observed when bacteria were grown in NB or NB+PGA (data not shown). To confirm that all of the PL produced under these conditions were present in a 34-kDa single band, the same concentrated and dialyzed supernatants were applied to a gel filtration column. Figure 5 shows that a protein peak (fractions 29 to 33) eluted at an apparent molecular mass of about 35 kDa, and this peak represented all of the PL activity detected.
FIG. 4.
Silver-stained SDS-PAGE gel (A) and PL activity-stained pectate agarose overlay (B). The SDS-PAGE gel was loaded with concentrated supernatants from cultures of C. luteola grown in PGA medium at different temperatures. Lane 1, 8°C; lane 2, 11°C; lane 3, 14°C; lane 4, 17°C; lane 5, 20°C; lane 6, 24°C; lane 7, 28°C; lane MM, molecular mass standards.
FIG. 5.
Gel filtration of a concentrated culture supernatant from C. luteola MFCL0 on a Sephacryl S-300 HR column. The column was loaded with a concentrated supernatant from a culture grown at 14°C in PGA medium. Fractions (1 ml) were collected and assayed to determine their protein contents (○) and PL activities (⧫) as described in the text. Calibration of the column showed that fraction 30 corresponded to approximately 35 kDa.
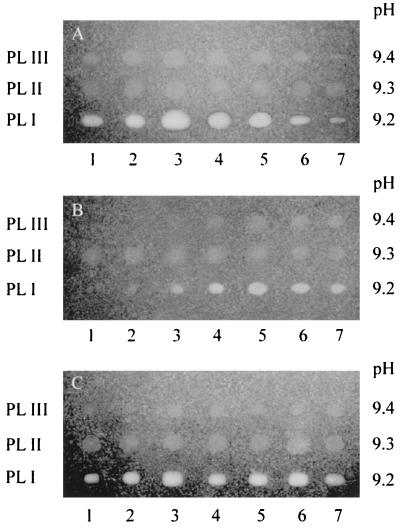
To separate and reveal the pectate lyases, MFCL0 supernatants were analyzed by IEF followed by staining for PL activity. The same amount of protein was loaded from each culture supernatant in order to compare the amounts of enzyme produced. No acidic PL was identified with pH 2 to 11 gels, and only a broad spot appeared in the alkaline parts of the gels. Figure 6 shows IEF (pH 9 to 11) profiles. The following three PL isoforms were found: PLI (pI 9.2), PLII (pI 9.3), and PLIII (pI 9.4). The size of the halo allowed us to estimate the relative production of each enzyme under each set of conditions. PLI and PLIII were regulated in the same way. The maximum amounts of both were produced at 14°C when PGA was the sole carbon source and at 20 to 24°C in NB. In NB+PGA, the maximum amounts of these two isoforms were produced at both of these temperatures. In contrast, production of PLII was almost constant under all of the conditions tested and did not appear to be affected by the temperature or by the medium used. The variation in the staining intensities of the haloes allowed us to show that at pH 8.6 (the pH of the pectate agarose) PLI was more active than PLII and PLIII, which might have different pH optima. Another hypothesis is that PLI activity is endolytic, while PLII and PLIII are exoenzymes. Hence, no PL activity was induced by PGA or NB, and the maximum production shown in Fig. 3 did not reflect the production of different PL in different media, as previously supposed.
FIG. 6.
IEF profiles of extracellular PL isoenzymes produced by C. luteola MFCL0 (pH 9 to 11). Concentrated supernatants from C. luteola cultures were prepared from cultures grown in PGA medium (A), NB (B), and NB+PGA (C). Each lane was loaded with 5 mg of proteins. Lane 1, 8°C; lane 2, 11°C; lane 3, 14°C; lane 4, 17°C; lane 5, 20°C; lane 6, 24°C; lane 7, 28°C. PL isoenzymes were activity stained with a pectate agarose overlay following IEF. The pH gradient of the corresponding IEF gel is shown.
As a control, we verified that regulation of PL enzyme production by the growth temperature is related to enzyme synthesis and not to secretion. This was demonstrated by the results of cell lysis experiments. The same level of PL activity was always found in culture supernatants before and after cell lysis (data not shown).
Effect of growth temperature on production of Cel by C. luteola.
Cel activity was also produced at the end of the exponential phase and could be assayed during the early stationary phase. It was not inducible by CMC or cellobiose and could be assayed in the three media utilized for PL production.
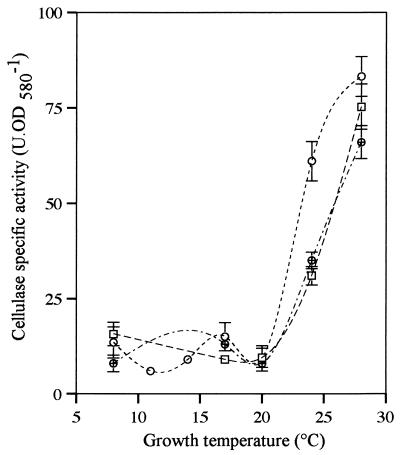
Regulation of Cel production by the growth temperature and the medium used (Fig. 7) was completely different from regulation of PL production since activity was negligible in all media at temperatures up to 20°C; at higher temperatures Cel activity increased dramatically.
FIG. 7.
Specific activity of cellulase produced by C. luteola MFCL0. Cells were grown in PGA medium, NB, and NB+PGA at 8, 11, 14, 17, 20, 24, and 28°C. Cellulase activity was assayed as described in the text. Each datum point represents a mean based on at least three independent determinations. The error bars indicate standard deviations. Symbols: □, PGA medium; ○, NB; ⊕, NB+PGA. OD580, optical density at 580 nm.
DISCUSSION
C. luteola is not a typical phytopathogen and has not previously been associated with vegetable spoilage. Nevertheless, like the well-known phytopathogen Erwinia chrysanthemi and several pseudomonads, C. luteola produces PL and Cel activities. In C. luteola, however, regulation of production of these enzymes seems to be somewhat different because neither PL nor Cel is directly inducible by their usual inducers, PGA and CMC, respectively. Instead, two PL activities and global Cel activity are regulated by growth temperature in completely different ways.
In E. chrysanthemi, PL are very sensitive to environmental conditions (17). Each condition affects expression of a particular set of pel genes; for example, expression of pelA, pelD, and pelE is modulated by anaerobiosis. It should also be noted that some genes which are not expressed in synthetic media can be expressed at high levels in macerated tissues (18). In C. luteola, PLII is not regulated by growth temperature, while PLI and PLIII are coregulated by temperature and by carbon and energy sources in a completely new way. This suggests that there is complex regulation by temperature that depends on the composition of the medium. In contrast, regulation of the production of Cel by temperature does not depend on the culture medium, and there is no temperature lower than the optimal temperature for growth at which maximum production of CelA occurs.
It was believed for a long time that the composition of the medium did not influence the effect of temperature on growth. However, Chablain et al. (7) demonstrated that in a psychrotrophic strain of Pseudomonas putida, the effect of growth temperature was different if the carbon source was toluene or benzoate. Our results provide a second example of a differential effect in a psychrotrophic strain. Moreover, our most striking result is the finding that the temperature at which maximum production of PLI and PLIII occurs on two different media coincides with the critical temperature for growth on these media. A coincidence between the temperature at which maximum production of extracellular enzymes occurs (which is lower than the temperature required for optimal growth) and the critical temperature for growth has been demonstrated only for strains of P. fluorescens (12, 13, 15, 22), for which only one critical temperature was observed. In addition, when each enzyme is considered, both PLI and PLIII have two different temperatures at which maximum production occurs, depending on the culture medium, which are the same temperatures as the two critical temperatures for growth. The similarity is, therefore, more than coincidence.
The results of studies in which P. fluorescens (6) and Pseudomonas fragi (14) were used suggest that some genes, possibly as many as 10% of the genes (25), are maximally expressed at 17°C, which is the critical temperature for these organisms.
All of the results described above must result in a new definition of the critical temperature. When related to the effect of temperature on the growth rate, the data suggest that the parameter limiting the growth rate should be changed. However, when all of the available data are examined together, it should be recognized that for some psychrotrophic strains at least, the critical temperature regulates some genes, depending on the carbon source.
ACKNOWLEDGMENTS
P.L. acknowledges the “Conseil Régional de Haute-Normandie,” the “Ministère de l'Agriculture,” and M. Frank Tonon of the Agro-Hall Association for supporting this work.
We especially thank V. Norris for his help.
REFERENCES
- 1.Andersson R E. Microbial lipolysis at low temperatures. Appl Environ Microbiol. 1980;39:36–40. doi: 10.1128/aem.39.1.36-40.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barras F, van Gijsegem F, Chatterjee A K. Extracellular enzymes and pathogenesis of soft-rot Erwinia. Annu Rev Phytopathol. 1994;32:201–234. [Google Scholar]
- 3.Beguin P, Aubert J P. The biological degradation of cellulose. FEMS Microbiol Rev. 1994;13:25–58. doi: 10.1111/j.1574-6976.1994.tb00033.x. [DOI] [PubMed] [Google Scholar]
- 4.Bertheau Y, Madgidi-Hervan E, Kotoujanski A, Nguyen-The C, Andro T, Coleno A. Detection of depolymerase isoenzymes after electrophoresis or electrofocusing, or in titration curves. Anal Biochem. 1984;139:383–389. doi: 10.1016/0003-2697(84)90022-8. [DOI] [PubMed] [Google Scholar]
- 5.Brocklehurst T F, Lund B M. Properties of pseudomonads causing spoilage of vegetables stored at low temperature. J Appl Bacteriol. 1981;50:259–266. [Google Scholar]
- 6.Burini J F, Gügi B, Merieau A, Guespin-Michel J F. Lipase and acidic phosphatase from the psychrotrophic bacterium Pseudomonas fluorescens: two enzymes whose synthesis is regulated by the growth temperature. FEMS Microbiol Lett. 1994;122:13–18. doi: 10.1111/j.1574-6968.1994.tb07136.x. [DOI] [PubMed] [Google Scholar]
- 7.Chablain P A, Philippe G, Groboillot A, Truffaut N, Guespin-Michel J F. Isolation of a soil psychrotrophic toluene-degrading Pseudomonas strain: influence of temperature on the growth characteristics on different substrates. Res Microbiol. 1997;148:153–161. doi: 10.1016/S0923-2508(97)87646-2. [DOI] [PubMed] [Google Scholar]
- 8.Collmer A, Keen N T. The role of pectic enzymes in plant pathogenesis. Annu Rev Phytopathol. 1986;24:383–409. [Google Scholar]
- 9.Collmer A, Ried J L, Mount M S. Assay methods for pectic enzymes. Methods Enzymol. 1988;161:329–399. [Google Scholar]
- 10.Dees C, Ringelberg D, Scott T C, Phelps T J. Characterization of the cellulose-degrading bacterium NCIMB 10462. Appl Biochem Biotechnol. 1995;51/52:263–273. [Google Scholar]
- 11.Gross D C, Cody Y S. Mechanisms of plant pathogenesis by Pseudomonas species. Can J Microbiol. 1985;31:403–410. [Google Scholar]
- 12.Gügi B, Orange N, Hellio F, Burini J F, Guillou C, Leriche F, Guespin-Michel J F. Effect of growth temperature on several exported enzyme activities in the psychrotrophic bacterium Pseudomonas fluorescens. J Bacteriol. 1991;173:3814–3820. doi: 10.1128/jb.173.12.3814-3820.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guillou C, Guespin-Michel J F. Evidence for two domains of growth temperature for the psychrotrophic bacterium Pseudomonas fluorescens. Appl Environ Microbiol. 1996;62:3319–3324. doi: 10.1128/aem.62.9.3319-3324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hebraud M, Dubois E, Potier P, Labadie J. Effect of growth temperatures on the protein levels in a psychrotrophic bacterium, Pseudomonas fragi. J Bacteriol. 1994;176:4017–4024. doi: 10.1128/jb.176.13.4017-4024.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hellio F C, Orange N, Guespin-Michel J F. Growth temperature controls the production of a single extracellular protease by Pseudomonas fluorescens MF0, in the presence of various inducers. Res Microbiol. 1993;144:617–625. doi: 10.1016/0923-2508(93)90064-9. [DOI] [PubMed] [Google Scholar]
- 16.Hugouvieux-Cotte-Pattat N, Condemine G, Nasser W, Reverchon S. Regulation of pectinolysis in Erwinia chrysanthemi. Annu Rev Microbiol. 1996;50:213–257. doi: 10.1146/annurev.micro.50.1.213. [DOI] [PubMed] [Google Scholar]
- 17.Hugouvieux-Cotte-Pattat N, Dominguez H, Robert-Baudouy J. Environmental conditions affect transcription of the pectinase genes of Erwinia chrysanthemi 3937. J Bacteriol. 1992;174:7807–7818. doi: 10.1128/jb.174.23.7807-7818.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelemu S, Collmer A. Erwinia chrysanthemi EC16 produces a second set of plant-inducible pectate lyase isozymes. Appl Environ Microbiol. 1993;59:1756–1761. doi: 10.1128/aem.59.6.1756-1761.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Liao C H. Analysis of pectate lyases produced by soft rot bacteria associated with spoilage of vegetables. Appl Environ Microbiol. 1989;55:1677–1683. doi: 10.1128/aem.55.7.1677-1683.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao C H, McCallus D E, Fett W F, Kang Y G. Identification of gene loci controlling pectate lyase production and soft-rot pathogenicity in Pseudomonas marginalis. Can J Microbiol. 1997;43:425–431. doi: 10.1139/m97-060. [DOI] [PubMed] [Google Scholar]
- 22.Merieau A, Gügi B, Guespin-Michel J F, Orange N. Temperature regulation of lipase secretion by Pseudomonas fluorescens MF0. Appl Microbiol Biotechnol. 1993;39:104–109. [Google Scholar]
- 23.Morita R Y. Psychrophilic bacteria. Bacteriol Rev. 1975;39:144–167. doi: 10.1128/br.39.2.144-167.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen-The C, Carlin F. The microbiology of minimally processed fresh fruits and vegetables. Crit Rev Food Sci Nutr. 1994;34:371–401. doi: 10.1080/10408399409527668. [DOI] [PubMed] [Google Scholar]
- 25.Regeard C, Mérieau A, Leriche F, Guespin-Michel J F. Genetic studies of a thermoregulated gene in the psychrotrophic bacterium P. fluorescens. Res Microbiol. 1999;150:1–11. doi: 10.1016/s0923-2508(99)00112-6. [DOI] [PubMed] [Google Scholar]
- 26.Rexova-Benkova L, Markovic O. Pectic enzymes. Adv Carbohydr Chem Biochem. 1976;33:323–385. doi: 10.1016/s0065-2318(08)60285-1. [DOI] [PubMed] [Google Scholar]
- 27.Schlemmer A F, Ware C F, Keen N T. Purification and characterization of a pectin lyase produced by Pseudomonas fluorescens W51. J Bacteriol. 1987;169:4493–4498. doi: 10.1128/jb.169.10.4493-4498.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sutherland I W. Polysaccharides lyases. FEMS Microbiol Rev. 1995;16:323–347. doi: 10.1111/j.1574-6976.1995.tb00179.x. [DOI] [PubMed] [Google Scholar]
- 29.Warren R A J. Microbial hydrolysis of polysaccharides. Annu Rev Microbiol. 1996;50:183–212. doi: 10.1146/annurev.micro.50.1.183. [DOI] [PubMed] [Google Scholar]