Abstract
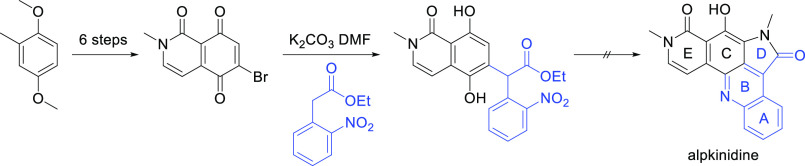
Model chemistry involving the bisannulation of 2,3-dichloro-1,4-naphthoquinone with the ester enolate derived from ethyl o-nitrophenylacetic acid, which rapid assembled the ABCD ring system of a pentacyclic pyrroloacridine, has been applied to the attempted synthesis of the marine natural product alpkinidine. The reaction of ethyl o-nitrophenylacetic acid with 6,7-dichloro-2-methylisoquinoline-1,5,8(2H)-trione, required to extend the model strategy to alpkinidine, was unfruitful, giving only complex mixtures. Efforts to direct the regiochemistry of the key Michael substitution step using 6-bromo-2-methylisoquinoline-1,5,8(2H)-trione afforded an adduct sharing the complete carbon skeleton of alpkinidine, but this could not be elaborated to the natural product.
Introduction
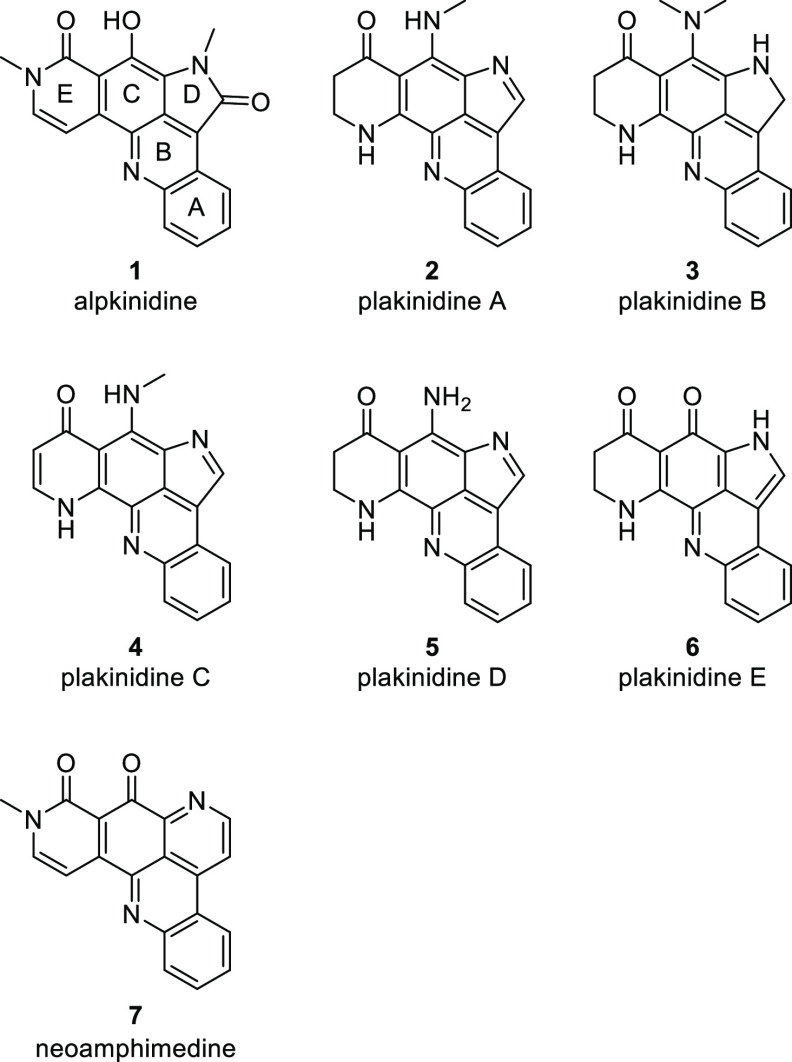
The natural product alpkinidine (1) (Figure 1), which was isolated from the Indonesian marine sponge Xestospongia carbonaria in 2002,1 is the only D-ring-oxygenated member of a small class of alkaloids possessing a rare pyrroloacridine core. The other congeners, plakinidines A–E (2–6), were also obtained from Indo-Pacific sponges2−4 and ascidians,5,6 although their biosynthetic origin is likely microbial.5
Figure 1.
Structures of alpkinidine and related marine alkaloids.
Alpkinidine was shown to be selectively toxic to solid tumor-derived cell lines over normal cells,1 and the plakinidines are cytotoxic to a variety of cancer cell lines.4 Plakinidines A and B also have anthelmintic activity.2 However, assessment of the biological activity of the pyrroloacridines is limited, presumably due to material scarcity. In contrast, the related pyridoacridines have been widely studied. Nearly all are cytotoxic as a result of their interactions with DNA,7 and a range of other biological activities have been described, including antibiotic,8 antifungal,9 antiviral,10 antiparasitic,11 insecticidal,12 and antitumor.13 Neoamphimedine (7) (Figure 1), which is a cometabolite of alpkinidine in X. carbonaria,1 is of particular relevance due to its structural similarity to alpkinidine and its promising biological activity. An inhibitor of DNA topoisomerase IIα, neoamphimedine (7), is cytotoxic to yeast and a wide variety of mammalian cell lines.1,14−16 In one study, it was equipotent with the clinical chemotherapeutic etoposide at inhibiting the growth of xenograft tumors in mice,16 making it a lead compound for cancer chemotherapy.
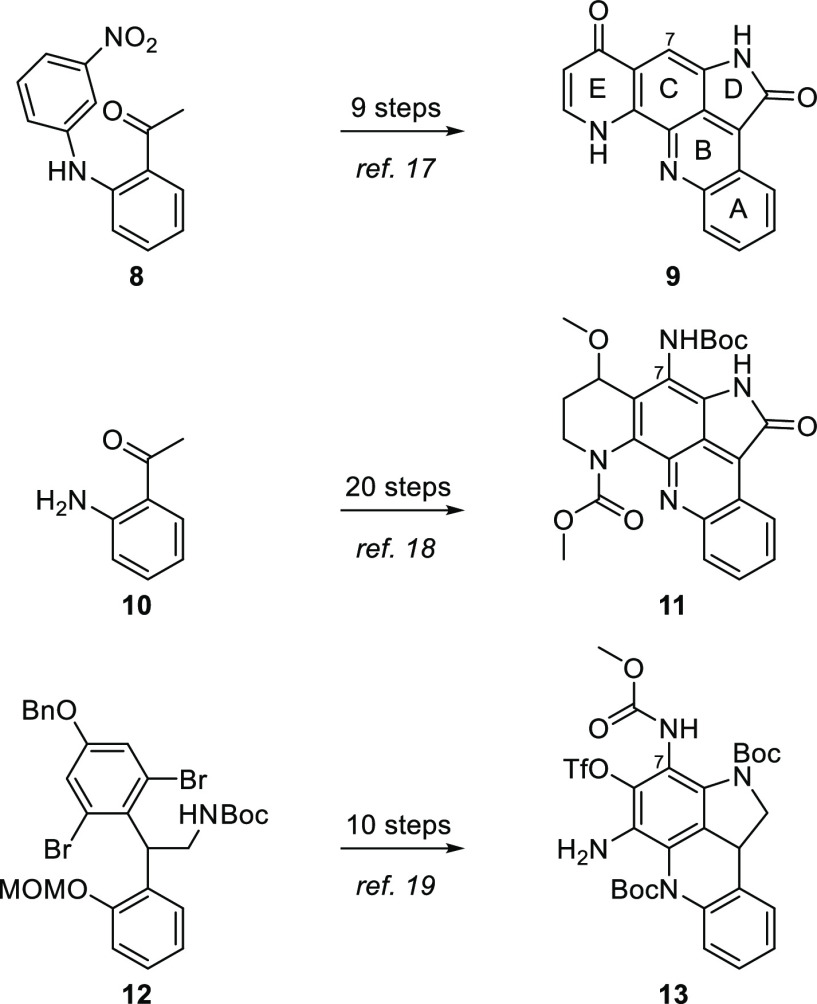
The potential for useful biological activity and the unique structures of pyrroloacridine natural products have attracted the interest of several synthetic chemists. In 2004, Kitahara et al.17 reported the synthesis of a hybrid pyrroloacridine 9 possessing the D ring of alpkinidine and the E ring of plakinidine C, but lacking the C7 substituent present in the natural products, in nine steps from 8 (Scheme 1). Beginning with o-aminoacetophenone (10), Fukuyama and co-workers detailed a 20-step synthesis of a similar hybrid, 11, which is nitrogenated at C7 but has an incompletely elaborated E ring.18 Tokuyama et al. have also tackled the plakinidine core, achieving the synthesis of a partially reduced ABCD ring system 13 in 10 steps from precursor 12.19
Scheme 1. Previous Syntheses of Pyrroloacridines Related to Alpkinidine and the Plakinidines.
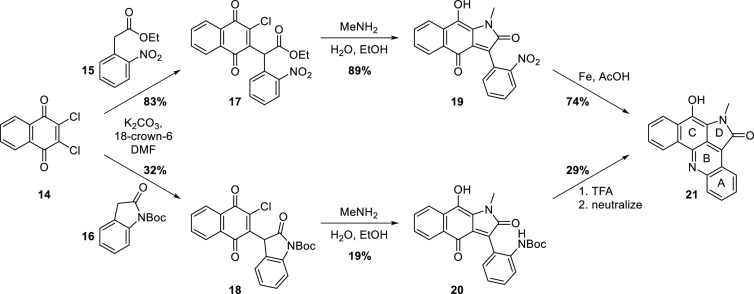
We previously reported the concise synthesis of the model compound 21, containing the ABCD ring system of alpkinidine, from 2,3-dichloronaphthoquinone (14) (Scheme 2).20 Conjugate substitution with the carbanions derived from ethyl o-nitrophenylacetate (15) or protected oxindole 16 gave intermediates 17 and 18, respectively. A cascade reaction involving conjugate substitution of methylamine, then intramolecular acyl transfer, provided lactams 19 and 20, which upon unmasking of the anilino group, cyclodehydrated to give 21.
Scheme 2. Construction of the ABCD Ring System of Alpkinidine20.
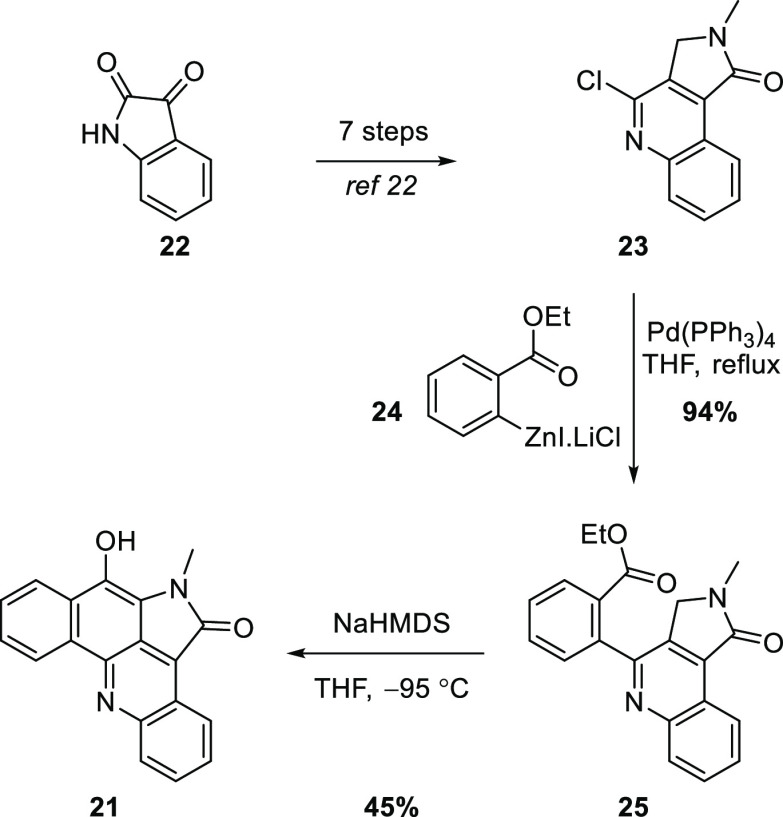
Tilve and co-workers subsequently developed a synthesis of 21 (Scheme 3).21 The quinolyl chloride 23, prepared from isatin (22) in seven steps,22 underwent a high-yielding Negishi coupling with arylzinc 24 to give biaryl 25. Dieckmann-like condensation then provided the target pentacycle 21.
Scheme 3. Tilve and Co-Workers’ Synthesis of 21(21).
Herein, we provide a full account of our efforts to develop and apply the model chemistry depicted in Scheme 2 to the synthesis of alpkinidine.
Results and Discussion
Model Chemistry
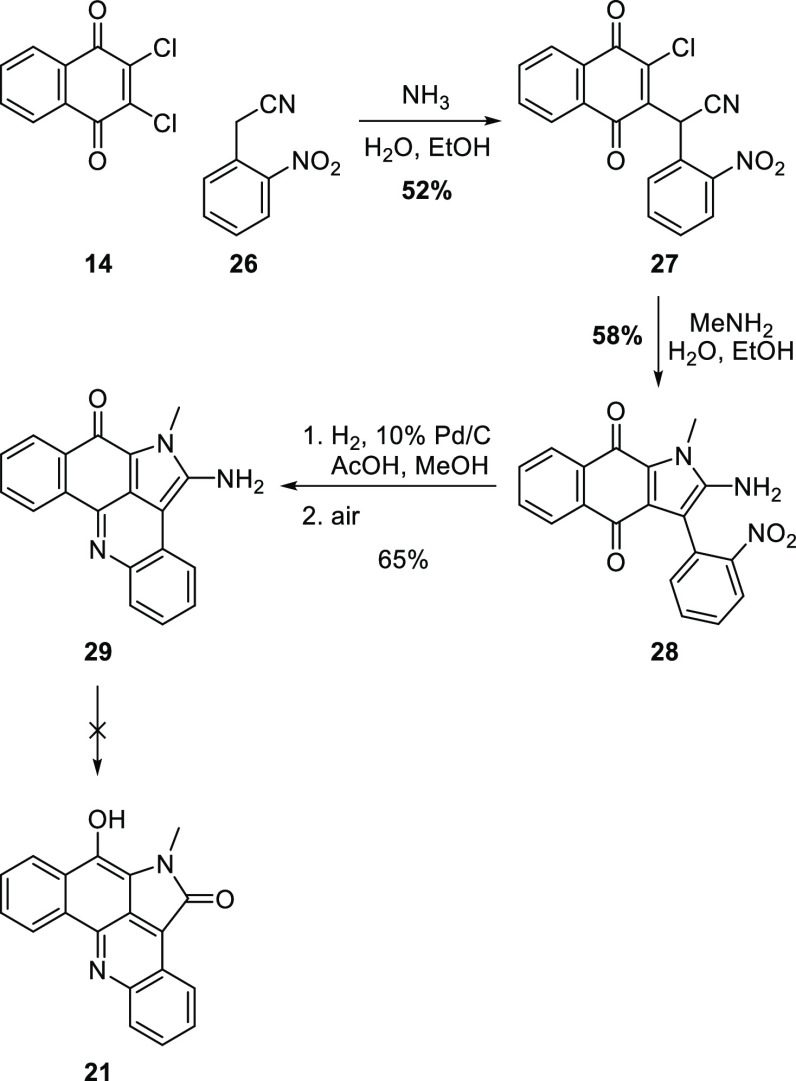
Our initial objective for this project was to efficiently access the ABCD ring system of alpkinidine. We established that this was possible through the chemistry depicted in Scheme 4.20 Conjugate substitution of 2,3-dichloronaphthoquinone (14) with the carbanion derived from nitrile 26, followed by treatment of the resultant adduct 27 with methylamine, afforded aminopyrrole 28. Reductive cyclization followed by aerial oxidation provided pentacycle 29, with the same ABCD ring scaffold as alpkinidine. However, attempts to oxodeaminate 29 to give the required pyrrolone D ring were unsuccessful. Hence, subsequent efforts focussed on nucleophiles that would more directly provide the D ring carbonyl group.
Scheme 4. First Route to the ABCD Ring System of Alpkinidine20.
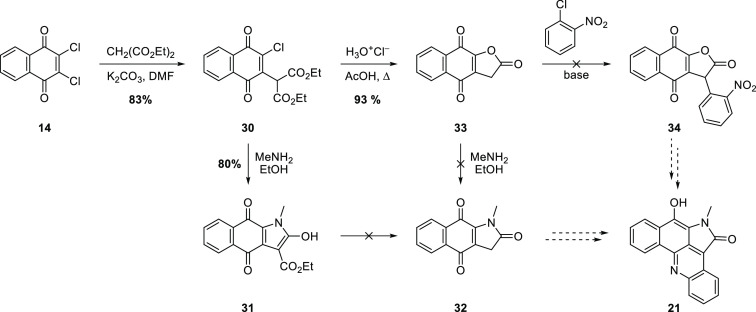
The reaction of 14 with diethyl malonate/sodium ethoxide was previously reported to give Michael substitution product 30 in only 27% yield.23 However, with a milder base in an aprotic solvent, the efficiency of this reaction was improved considerably (Scheme 5). Two lines of investigation were explored for the elaboration of adduct 30. First, substitution/lactamization with methylamine to give hydroxypyrrole 31 proceeded smoothly, as expected based on similar precedents.24,25 However, failure to effectively decarboxylate 31 to give 32 under a variety of conditions thwarted efforts to advance this intermediate. In contrast, acid-catalyzed decarboxylation/cyclization of 30 provided lactone 33 in good yield, but subsequent treatment with methylamine failed to give pyrrolone 32. Nucleophilic aromatic substitution of o-chloronitrobenzene with the carbanion derived from lactone 33 was also investigated but gave a complex mixture of products under a variety of conditions.
Scheme 5. Preliminary Efforts to Generate the D Ring of Alpkinidine.
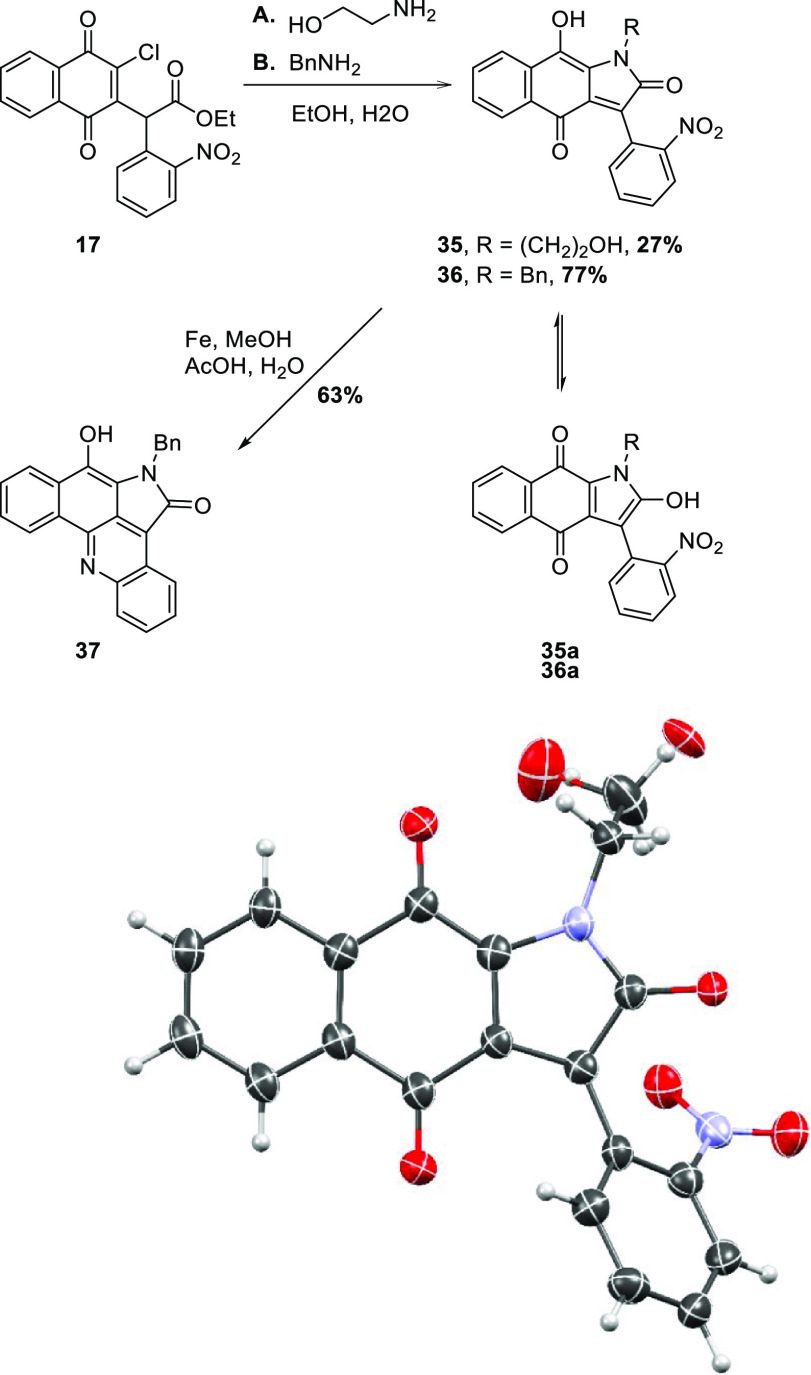
Attention then turned to the reaction of 14 with enolate nucleophiles already incorporating the A ring of alpkinidine. As mentioned in the Introduction section, this line of investigation was fruitful, providing two rapid approaches to the model compound 21 (Scheme 2). The reaction of intermediate 17 with other primary amines was also briefly investigated, providing the N-2-hydroxyethyl 35 and N-benzyl 36 analogues (Scheme 6). In the former case, the crude yield was good, but major losses during purification led to low recovery. An X-ray crystal structure of 35 was obtained (Scheme 6), revealing that the C2–O and C9–O bonds are of similar length and intermediate between standard phenolic C–OH and carbonyl bond lengths, as one might expect for the highly conjugated system. The position of the phenolic hydrogen was not placed, so tautomeric structure 35a cannot be ruled out, and ergo, 36a may also be a better representation than 36. The N-benzylpyrrolone 36 underwent reductive cyclization as expected to provide pentacycle 37. Like the N-methyl analogue 21,2037 was unstable in dimethyl sulfoxide (DMSO)/air but was able to be fully characterized before substantial decomposition occurred.
Scheme 6. Elaboration of 17 with Different Amines, Including a Representation of the X-Ray Crystal Structure of 35/35a.
The position of the phenolic proton in 35/35a could not be defined. The molecule is disordered about the ethanolamine portion, with two distinct alcohol environments being calculated for both with an associated molecule of MeOH (omitted for clarity). Displacement envelopes are at 50% probability amplitude, with hydrogen atoms assigned arbitrary radii.
Toward Alpkinidine
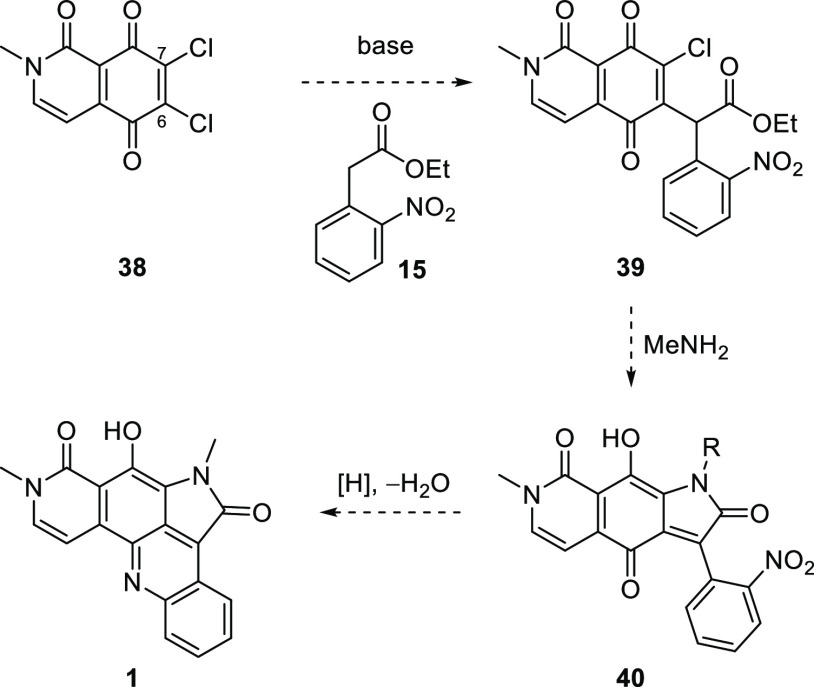
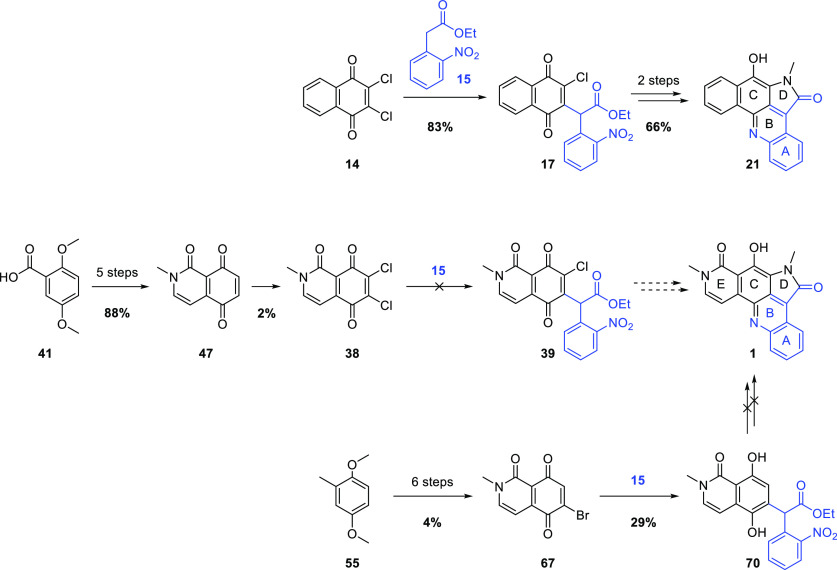
Application of the methodology described above to the synthesis of alpkinidine required dichloroisoquinolinetrione 38 (Scheme 7). The reduced symmetry of 38, compared to that of 1,2-dichloronaphthoquinone (14), necessitated a regioselective Michael substitution reaction with the enolate derived from 15, that is, to provide 39 preferentially. Simple resonance arguments suggest that C7 is likely the more electrophilic of the two chlorinated quinonoid carbons. Nevertheless, taking advantage of the chelating peri-dicarbonyl moiety with Lewis acid activation, it might be possible to reverse any such inherent bias. Should 39 be procured, it was anticipated that elaboration to alpkinidine (1) via 40 would proceed smoothly, based on the model chemistry.
Scheme 7. Key Precursor 38 and Proposed Route to Alpkinidine.
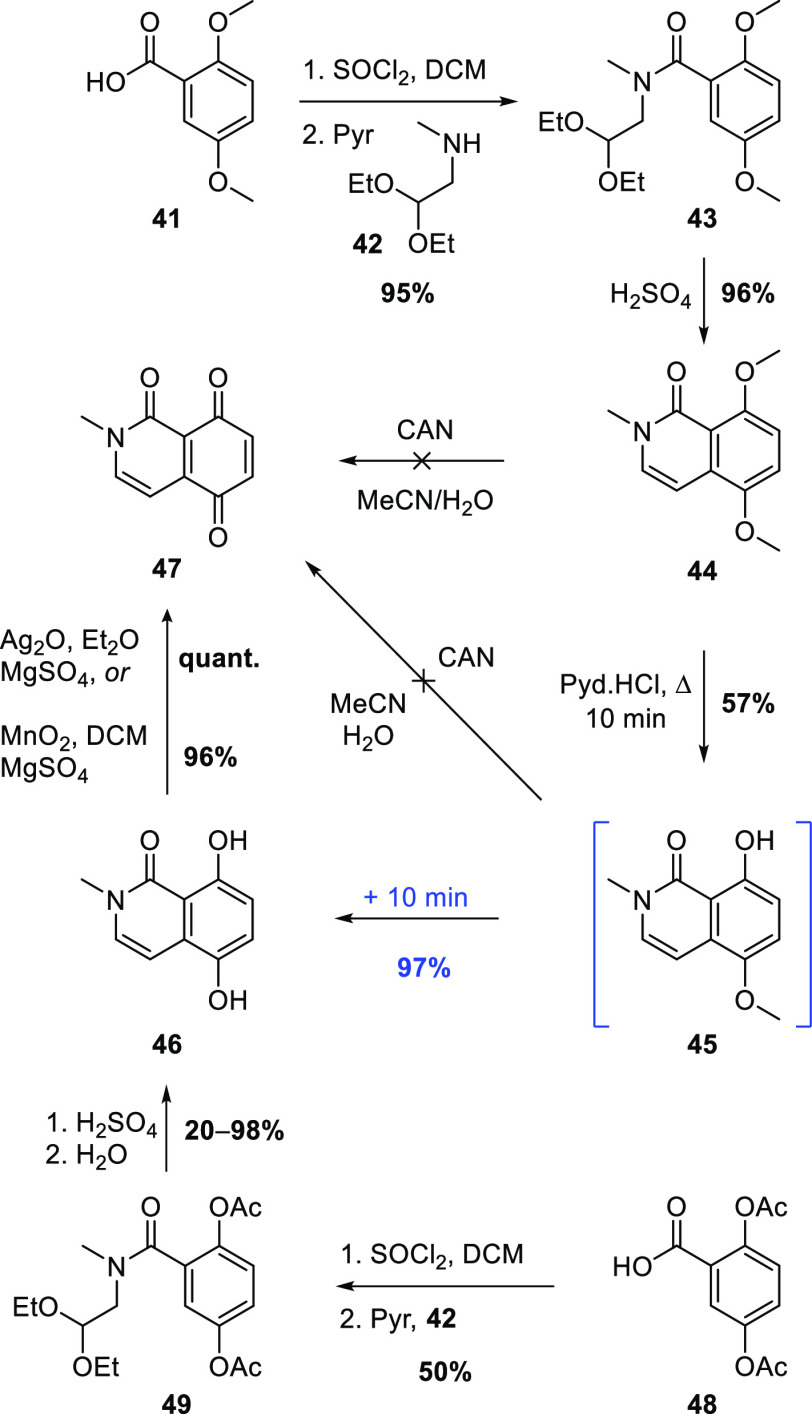
The synthesis of dichloroquinone 38 began with the commercial benzoic acid 41 (Scheme 8), which may also be conveniently prepared from the cheaper 2,5-dimethoxybenzaldehyde.26 Conversion to the acid chloride was followed by coupling with secondary amine 42, prepared in excellent yield by treatment of bromoacetal with excess methylamine. The resulting tertiary amide 43 underwent clean Pomeranz–Fritsch-like cyclization/aromatization27 to provide isoquinolone 44. Attempted oxidative demethylation of 44 with ceric ammonium nitrate (CAN) gave an intractable mixture of products; hence, stepwise demethylation then oxidation was pursued. A short treatment with molten pyridinium chloride28,29 (to avoid N-demethylation) provided monomethyl ether 45 in moderate yield, but again, CAN-mediated oxidative demethylation of this phenol was unsuccessful. Fortunately, a slightly longer Prey demethylation allowed very efficient conversion to hydroquinone 46, and quantitative oxidation to quinone 47 was achieved with silver(I) oxide.30,31 Subsequently, MnO232 was found to be nearly as effective for this oxidation.
Scheme 8. Synthesis of Quinone 47.
In an effort to circumvent the demethylation step, 2,5-diacetoxybenzoic acid (48)33 was amidated to give 49, which cyclized in concentrated sulfuric acid and deacetylated during aqueous work-up, providing hydroquinone 46. On one occasion, this cyclization gave a near quantitative yield of 46. However, on repetition, much lower yields were obtained, perhaps due to variability in the water content of the sulfuric acid.
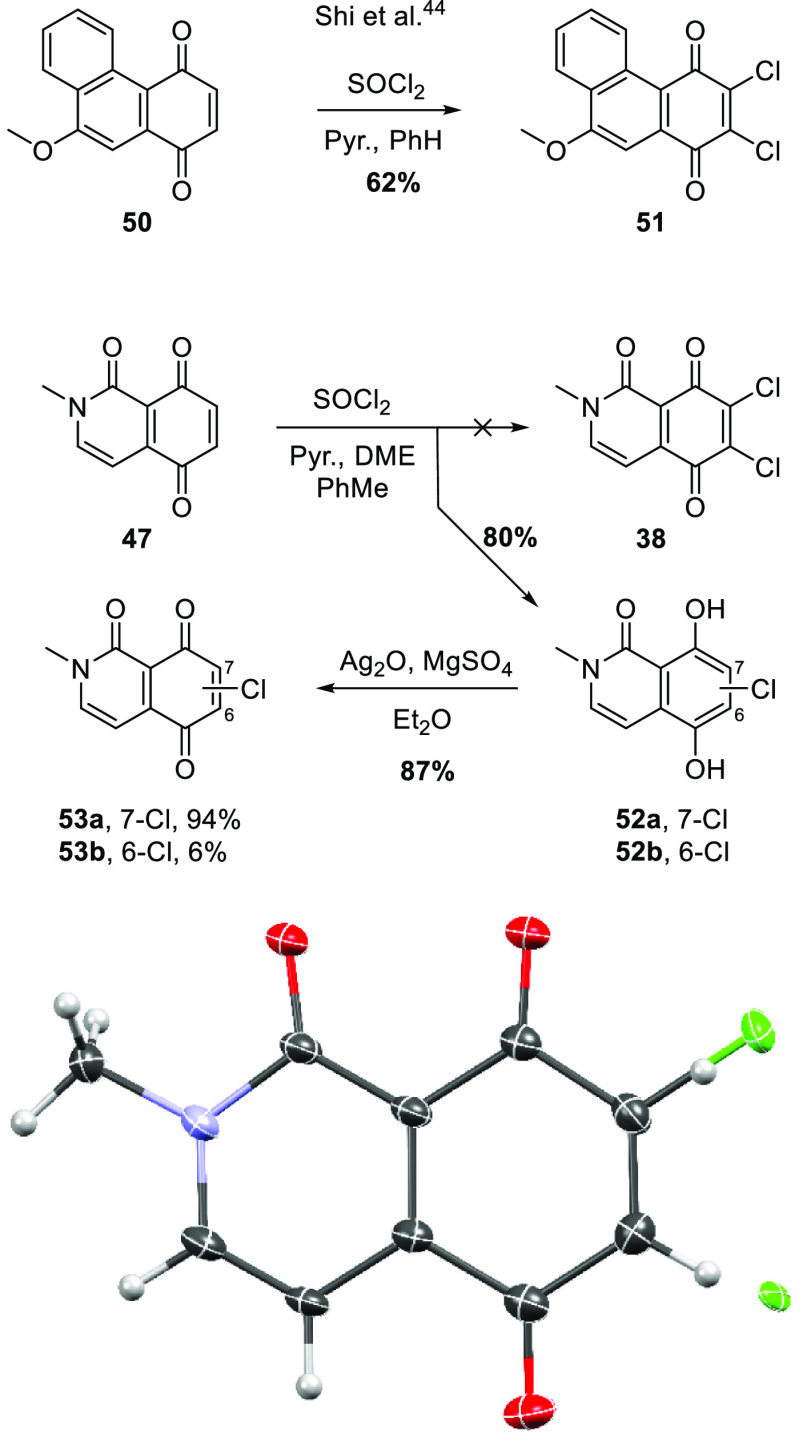
With 47 in hand, attention turned to the regioselective dichlorination of the quinone moiety. Shi et al.34 converted quinone 50 to dichloride 51 using thionyl chloride/pyridine (Scheme 9). Under similar conditions, 47 gave mainly chlorohydroquinone 52a, with a trace of the regioisomer 52b. The formation of the hydroquinone likely reflects the greater reduction potential of the electron-deficient quinone 47 (and its monochlorinated derivatives), relative to 50/51. The identity of the reductant is unclear, but may be sulfur monoxide, a byproduct of the chlorination reaction.34 The mixture of hydroquinones 52a/b was oxidized, and X-ray crystallography revealed a 94:6 cocrystal of quinones 53a/b, respectively (Scheme 9).
Scheme 9. Attempted Synthesis of 38, Including a Representation of the X-ray Crystal Structure of 53a/b (a 94:6 Cocrystal Structure).
Displacement envelopes are at 50% probability amplitude with hydrogen atoms assigned arbitrary radii.
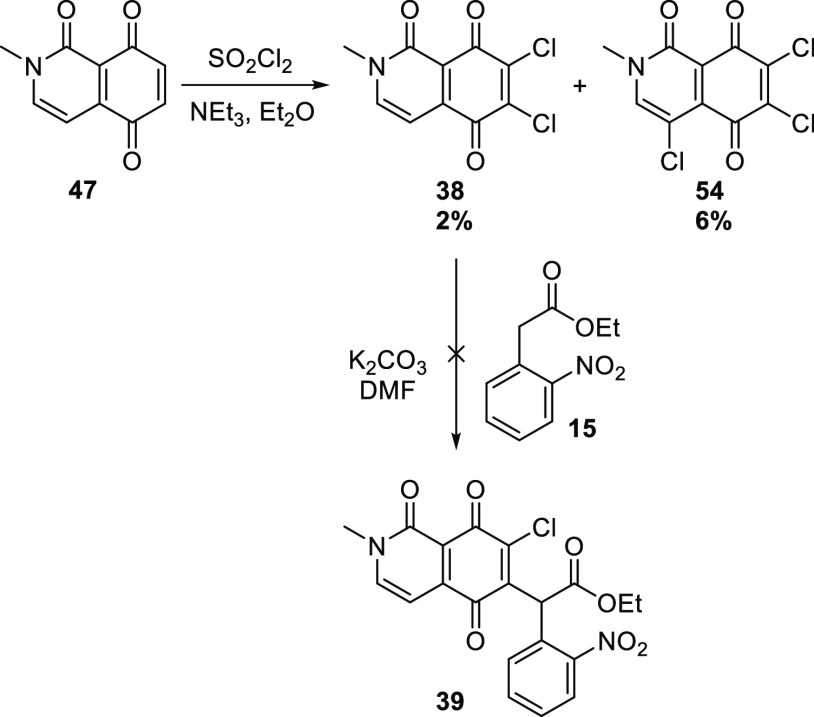
Quinone 47 reacted with sulfuryl chloride/triethylamine35 to give the desired dichloride 38, albeit in very poor yield, accompanied by trichloride 54 and an intractable mixture of other products (Scheme 10). Nevertheless, this provided enough material to test the key Michael substitution reaction. Alas, the reaction of 38 with the carbanion derived from ethyl o-nitrophenylacetate (15) gave none of the expected adduct 39. No other identifiable products were observed, providing little information as to why this reaction failed when the model chemistry (Scheme 2) worked so well.
Scheme 10. Low Yielding Chlorination and Failed Michael Substitution.
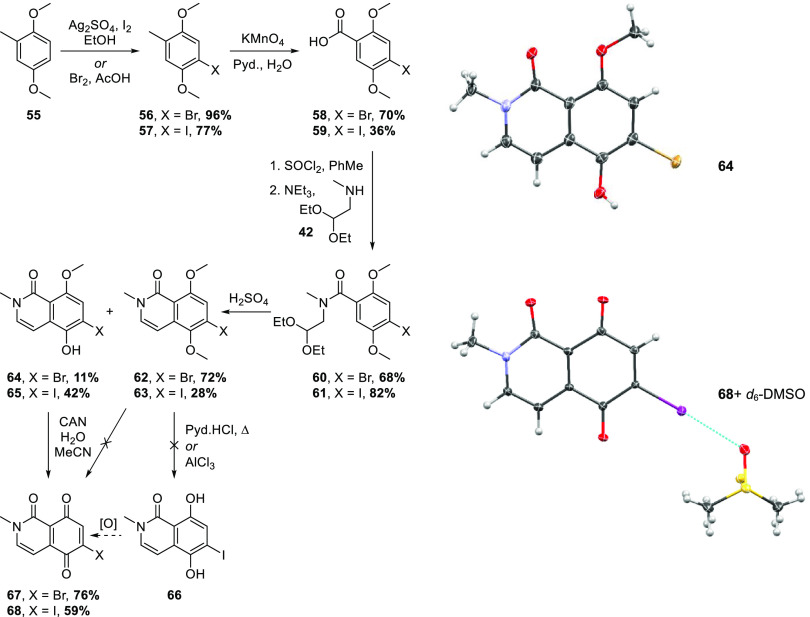
In an attempt to salvage this route and address the regiochemical challenge in linking the CE and ABD ring systems of alpkinidine, monohalogenated quinones, in which the halogen could direct Michael substitution to the 6-position, were targeted. Both bromide 67 and iodide 68 were prepared to test this hypothesis, as outlined in Scheme 11. Bromination or iodination36 of commercial 2,5-dimethoxytoluene (55) gave 56(37) and 57, respectively. Permanganate oxidation to benzoic acids 58(38)/59 was followed by amidation of the derived acid chlorides with methylaminoacetal 42, providing 60/61, respectively. Cyclization with sulfuric acid to give isoquinolones 62/63 was complicated by partial, regioselective demethylation, also affording 64/65, respectively. The structure of bromide 64 was confirmed by X-ray crystallography. The halogens clearly play a role here as no such demethylation was observed in reaction of the nonhalogenated analogue (43 → 44, Scheme 8). Precedents for similar demethylations of ortho-bromoanisoles by sulfuric acid exist.39−41 While initially annoying, this side reaction turned out to be fortuitous as attempts to oxidatively demethylate 62/63 gave complex mixtures of indiscernible products and efforts to fully demethylate iodide 63 under more standard conditions were similarly unsuccessful. In contrast, oxidative demethylation of monomethyl ethers 64/65 gave acceptable yields of the target quinones 67/68, respectively. Interestingly, iodide 68 crystallized from DMSO-d6 solution (NMR sample) as a monosolvate exhibiting halogen bonding (Scheme 11).
Scheme 11. Synthesis of 6-Haloisoquinolinetriones, including Representations of the X-Ray Crystal Structures of 64 and 68.
Displacement envelopes are at 50% probability amplitude with hydrogen/deuterium atoms assigned arbitrary radii. The I···O halogen bond between 68 and DMSO-d6 is indicated in light blue.
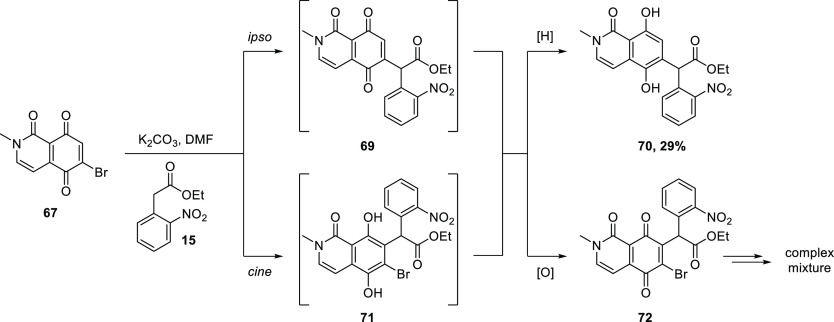
Unfortunately, the reaction of iodide 68 with ethyl o-nitrophenyl acetate (15) under basic conditions furnished neither the expected Michael substitution product 69 (Scheme 12) nor any other identifiable compounds. In contrast, the corresponding reaction of bromide 67 gave a discrete new spot by TLC. However, upon isolation, this product was identified as hydroquinone 70, which presumably arises by reduction of the expected Michael substitution product 69. The nature of the reductant can only be speculated upon, as no other products could be isolated or identified among the complex mixture. Reactions of bromoquinones with C-nucleophiles are known to be complicated by cine addition competing with ipso-substitution.42−47 Thus, cine addition of the carbanion derived from 15 to 67 would give rise to hydroquinone 71, which could reduce the ipso substitution product 69 to 70. The byproduct, quinone 72, might then take part in subsequent reactions, giving rise to the complex mixture of products observed.
Scheme 12. Unexpected Reductive Substitution of and Hypothetical Competing Cine Addition to 67.
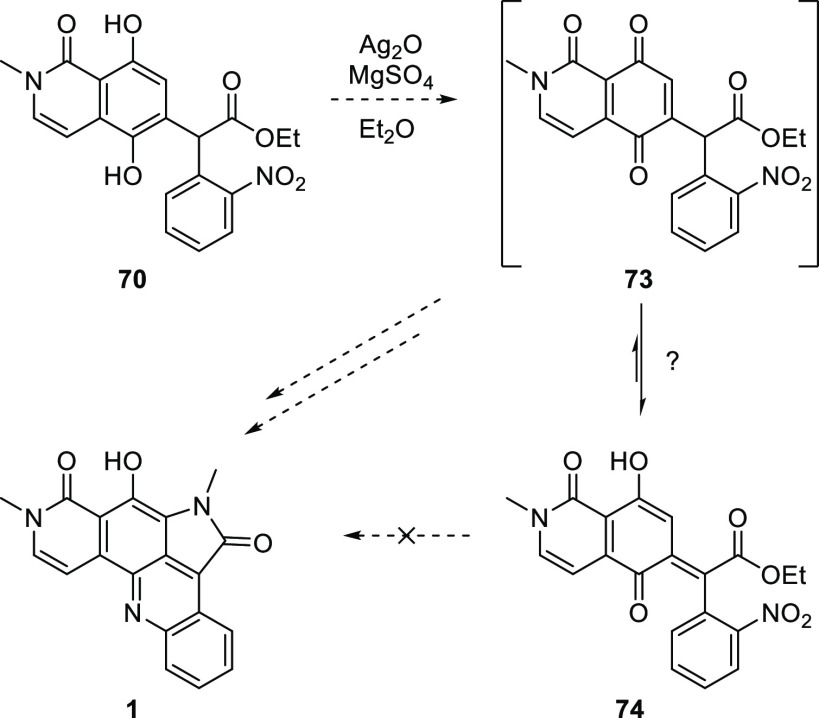
Despite the low yield, the production of 70 was a promising advance in developing a synthesis of alpkinidine using our bisannulation strategy. Hence, 70 was oxidized with silver(I) oxide with the expectation that quinone 73 might be elaborated to alpkinidine (1) (Scheme 13). However, 73 was not detected in the crude product of this reaction, as evidenced by the lack of a quinonoid methine resonance in the 1H NMR spectrum. The appearance of a downfield signal, consistent with a hydrogen-bonded phenolic hydroxyl, suggests that quinone 73 might have tautomerized to o-quinonemethide 74 (and or its Z isomer), but scarcity of material prevented conclusive identification of this product. Indeed, this complication, along with the disappointing yield of the previous step, and the perceived difficulty in carrying 74 forward to alpkinidine, made this route unappealing, and hence, it was abandoned.
Scheme 13. Oxidation of Hydroquinone 70 and Putative Tautomerization Preventing the Formation of the Alpkinidine D Ring.
Conclusions
Two approaches to connect the CE ring system and A ring of the pentacyclic pyrroloacridine natural product alpkinidine (1), through construction of the BD rings using Michael substitution of haloquinonoid isoquinolinetriones, have been explored (Scheme 14). Chemistry that efficiently afforded the model pentacyclic pyrroloacridine 21, lacking only the E ring of alpkinidine, failed to translate to the “real system”. The novel isoquinolinetrione 47, which may also prove useful for the synthesis of neoamphimedine and analogues, was prepared efficiently. This intermediate could be chlorinated, albeit in very low yield, but reactions of dichloroquinone 38 with 15 under basic conditions gave only complex mixtures.
Scheme 14. Summary of Key Approaches Investigated.
Attempts to direct the regiochemistry of Michael substitution reactions using 6-haloisoquinolinetriones (e.g., 67) were also explored. This strategy afforded adduct 70, comprising the complete carbon-connectivity of alpkinidine (1). However, attempts to elaborate this scaffold to the required heterocyclic ring system failed.
Given these setbacks, our attention turned to Michael additions (as opposed to substitutions) to construct the key carbon–carbon bond that is ultimately shared by the BD rings in alpkinidine (1). These endeavors are reported in the following paper.
Experimental Section
General
General experimental details are as reported previously.20,48
Synthesis
Diethyl 2-(3-Chloro-1,4-dioxo-1,4-dihydronaphthalen-2-yl)malonate (30)23
2,3-Dichloro-1,4-naphthoquinone (14) (1.14 g, 5.03 mmol) was added to a stirred suspension of diethylmalonate (1.6 mL, 10 mmol) and K2CO3 (1.4 g, 10 mmol) in dimethylformamide (DMF) (80 mL) and heated to 45 °C. After 1.5 h, the reaction mixture was cooled, acidified with 1 M HCl (20 mL), extracted with EtOAc (3 × 20 mL), dried and evaporated, and the crude residue was subjected to chromatography. Elution with 1:9 EtOAc/hexanes gave 30 (1.46 g, 83%) as a pale green solid, mp 102–104 °C [lit.23 102 °C]. 1H NMR (400 MHz, CDCl3): δ 8.21–8.12 (m, 2H, 2× ArH), 7.82–7.76 (m, 2H, 2× ArH), 5.12 (s, 1H, H2), 4.32–4.23 (m [app dq], 4H, 2× CH2), 1.28 (t, J = 7.1 Hz, 6H, 2× CH3). The 1H NMR data were consistent with the literature.23
Ethyl 2-Hydroxy-1-methyl-4,9-dioxo-4,9-dihydro-1H-benzo[f]indole-3-carboxylate (31)
Ethanolic MeNH2 (0.13 mL, 1.04 mmol) was added to a solution of 30 (0.17 g, 0.50 mmol) in EtOH (5 mL) at 0 °C. After 1.5 h, the solution was poured into 1 M HCl (10 mL), extracted with EtOAc (3 × 10 mL), dried, and evaporated. The crude solid was precipitated from dichloromethane (DCM)/hexanes to give 31 (0.12 g, 83%) as a pale-yellow/orange solid. 1H NMR (600 MHz): δ 11.4 (s, 1H, OH), 8.13 (m, 1H, H5 or H8), 8.07 (m, 1H, H5 or H8), 7.65 (m, 2H, H6 & H7), 4.70 (q, J = 7.2 Hz, 2H, OCH2CH3), 3.93 (s, 3H, NMe), 1.50 (t, J = 7.2 Hz, 3H, OCH2CH3). 13C NMR (150 MHz): δ 179.1 (C4 or C9), 176.2 (C4 or C9), 168.6 (CO2), 159.3 (C2), 133.4 (C4a or C8a), 133.1 (C6 or C7), 132.9 (C6 or C7), 132.5 (C4a or C8a), 126.7 (C5 or C8), 125.5 (C5 or C8), 125.6 (C9a), 123.5 (C3a), 91.2 (C3), 61.7 (OCH2CH3), 30.8 (NMe), 14.3 (OCH2CH3). This compound was synthesized in 1930 from the bromide analogue of 30 and described as yellow needles.49
Naphtho[2,3-b]furan-2,4,9(3H)-trione (33)
A suspension of 30 (0.44 g, 1.25 mmol) in AcOH (30 mL) and 6 M HCl (30 mL) was heated under reflux. After 24 h, the reaction mixture was cooled, neutralized with sat. aq NaHCO3 (100 mL), extracted with EtOAc (5 × 20 mL), dried, and evaporated. The crude solid was then washed with DCM/hexanes to give 33 (0.25 g, 93%) as a brown solid, mp 240–245 °C. Rf (1:9 MeOH/DCM) 0.2. IR (ATR) νmax cm–1: 1704 (C=O), 1668 (C=O). 1H NMR (600 MHz): δ 8.18 (m, 1H, ArH), 8.14 (m, 1H, ArH), 7.78 (m, 2H, ArH), 3.94 (s, 3H, H3). 13C NMR (150 MHz): δ 181.7 (C4 or C9), 177.4 (C4 or C9), 172.9 (C2), 145.6 (C9a), 140.4 (C3a or C4a or C8a), 134.6 (ArH), 134.4 (ArH), 131.4 (C3a or C4a or C8a), 131.4 (C3a or C4a or C8a), 127.5 (ArH), 127.3 (ArH), 33.5 (C3). HRMS (APCI): calcd for C12H7O4+ [M + H]+, 215.0348; found, 215.0339.
9-Hydroxy-1-(2-hydroxyethyl)-3-(2-nitrophenyl)-1H,2H,4H-benzo[f]indole-2,4-dione (35/35a)
A solution of ethanolamine (0.15 mL, 2.48 mmol) in H2O (1 mL) was added to a stirred solution of 17(20) (0.11 g, 0.27 mmol) in EtOH (5 mL). After 30 min, the reaction mixture was diluted with aqueous 10% citric acid (20 mL) and extracted with EtOAc (3 × 10 mL). The organic phase was extracted with sat. aq NaHCO3 (3 × 10 mL). The aqueous phase was acidified with 1 M HCl (50 mL), extracted with EtOAc (3 × 30 mL), dried, and evaporated to give 35/35a (27 mg, 27%) as a purple solid, mp 122–125 °C. Rf (1:20:80 AcOH/MeOH/DCM) 0.3. IR (ATR) νmax cm–1: 3500–2500 (OH), 1712 (C=O), 1658 (C=O), 1630 (C=O). 1H NMR (600 MHz, DMSO-d6): δ 8.09 (d, J = 8.4 Hz, 1H, ArH), 7.97 (d, J = 7.8 Hz, 1H, ArH), 7.79 (d, J = 7.2 Hz, 1H, ArH), 7.72 (m, 2H, ArH), 7.65 (dd [app. t], J1 = J2 = 7.2 Hz, 1H, ArH), 7.59 (dd [app. t], J1 = J2 = 7.2 Hz, 1H, ArH), 7.55 (d, J = 7.2 Hz, 1H, ArH), 4.46 (s, 1H, NCH2), 4.40 (s, 1H, NCH2), 3.72 (t, J = 6.6 Hz, 2H, CH2O). 13C NMR (150 MHz, DMSO-d6): δ 180.4 (C4), 171.3 (C9), 151.1 (C2), 149.1 (C2′), 133.8 (CH), 133.7, 133.5 (CH), 132.9 (CH), 132.5, 128.5 (CH), 127.2, 125.5 (CH), 124.3 (CH), 123.7, 122.5, 101.6 (C3), 59.7 (CH2), 45.4 (CH2). HRMS (APCI): calcd for C20H15N2O6+ [M + H]+, 379.0939; found, 379.0925.
1-Benzyl-9-hydroxy-3-(2-nitrophenyl)-1H,2H,4H-benzo[f]indole-2,4-dione (36/36a)
A solution of benzylamine (0.30 mL, 2.7 mmol) in H2O (1 mL) was added to a stirred solution of 17(20) (0.11 g, 0.27 mmol) in EtOH (5 mL). After 30 min, the reaction mixture was diluted with aqueous 10% citric acid (20 mL), extracted with EtOAc (3 × 10 mL), dried, and evaporated. Precipitation from a mixture of MeOH/DCM/hexanes gave 36/36a (89 mg, 77%) as a red solid, mp 110–113 °C. Rf (1:4 MeOH/DCM) 0.25. IR (ATR) νmax cm–1: 3500–2500 (OH), 1639 (C=O). 1H NMR (600 MHz, DMSO-d6): δ 8.12 (m [app. d], J = 5.4 Hz, 1H, ArH), 7.94 (m [app. dd], J1 = 4.2, J2 = 1.8 Hz, 1H, ArH), 7.81 (d, J = 7.2 Hz, 1H, ArH), 7.77–7.67 (m, 2H, ArH), 7.67–7.55 (m, 2H, ArH), 7.34 (dd [app. t], J1 = J2 = 7.2 Hz, 2H, PhH), 7.26 (m, 3H, PhH), 5.62 (s, 2H, CH2). 13C NMR (150 MHz, DMSO-d6): δ 180.5 (C4), 171.9 (C9), 151.0 (C2), 149.3 (C2′), 137.5, 134.1, 133.6, 133.1, 132.7, 128.7 (PhH), 127.3 (PhH), 126.8 (PhH), 125.6, 124.4, 124.1, 122.3, 101.6 (C3), 46.2 (CH2). HRMS (ESI–): calcd for C25H15N2O5– [M – H]−, 423.0989; found, 423.0986.
6-Benzyl-7-hydroxybenzo[c]pyrrolo[4,3,2-mn]acridin-5(6H)-one (37)
Iron powder (0.19 g, 3.4 mmol) was added to a vigorously stirred solution of 36 (0.13 g, 0.31 mmol) in AcOH (7 mL), H2O (3 mL), and MeOH (2 mL). After 2.5 h, the reaction mixture was diluted with H2O (20 mL), extracted with EtOAc (3 × 20 mL), dried, and evaporated. Precipitation from a mixture of MeOH/DCM/hexanes followed by recrystalization from EtOH gave 37 (74 mg, 63%) as a purple solid, mp 262–265 °C. Rf (1:19 MeOH/DCM) 0.25. IR (ATR) νmax cm–1: 3200–2800 (OH), 1661 (C=O), 1606 (C=O). 1H NMR (600 MHz, DMSO-d6): δ 7.87 (dd, J1 = 7.2, J2 = 3.0 Hz, 2H, ArH), 7.75 (d, J = 7.8 Hz, 1H, ArH), 7.64 (dd [app. t], J1 = J2 = 7.8 Hz, 1H, ArH), 7.58 (dd [app. t], J1 = J2 = 7.8 Hz, 1H, ArH), 7.33 (m, 3H, ArH), 7.30–7.24 (m, 4H, ArH), 7.21 (dd [app. t], J1 = J2 = 7.2 Hz, 1H, ArH), 5.52 (s, 2H, CH2). 13C NMR (150 MHz, DMSO-d6): δ 181.3 (C7), 159.7 (C5), 139.4 (C11b), 135.0, 133.9, 133.3, 132.6, 131.8, 130.5, 129.0, 128.7 (PhH), 127.8 (PhH), 127.3, 126.9, 126.0, 125.8, 125.3, 124.9, 123.0, 122.9, 101.4 (C4b), 45.2 (CH2). HRMS (ESI): calcd for C25H17N2O2+ [M + H]+, 377.1296; found, 377.1285.
2,2-Diethoxy-N-methylethanamine (42)
Bromoacetaldehyde diethyl acetal (15 mL, 97 mmol) was added to a stirred solution of 40% aqueous MeNH2 (90 mL, 0.11 mol) in MeOH (120 mL). The reaction mixture was heated under gentle reflux for 12 h before being cooled, diluted with brine (150 mL), and extracted with EtOAc (3 × 100 mL). The extracts were washed with brine and evaporated. Distillation of the residue at reduced pressure gave the secondary amine 42 as a clear, colorless oil (13.9 g, 94%), bp 77–79 °C@20 mm Hg. 1H NMR (400 MHz, CDCl3): δ 4.50 (t, J = 5.6 Hz, 1H, H2) 3.67–3.55 (m, 2H, CH2O), 3.51–3.40 (m, 2H, CH2O), 2.60 (d, J = 5.6 Hz, 2H, H1), 2.35 (s, 3H, NMe), 1.12 (t, J = 7.1 Hz, 6H, 2× CH3). 13C NMR (100 MHz, CDCl3): δ 101.9 (C2), 62.2 (2× CH2O), 54.1 (C1), 36.3 (NCH3), 15.2 (2× CH3). The synthesis of this compound has been described previously, but not by this method, and NMR data have not been reported.50
N-(2,2-Diethoxyethyl)-2,5-dimethoxy-N-methylbenzamide (43)
A solution of 2,5-dimethoxybenzoic acid (41)26 (5.71 g, 31.3 mmol) and SOCl2 (10 mL, 0.14 mol) in DCM (25 mL) was heated under reflux for 2 h before the solvent and excess SOCl2 were evaporated. The residue was cooled to 0 °C, and a solution of pyridine (10 mL, 0.12 mol) in DCM (10 mL) was added dropwise, followed by the dropwise addition of a solution of 42 (7.32 g, 49.7 mmol) in DCM (10 mL). The mixture was stirred at room temperature (rt) for 3 h before being diluted with H2O (50 mL) and extracted with DCM (3 × 20 mL). The extract was washed with sat. aq NaHCO3 (20 mL), dried, and evaporated, and the crude product was subjected to flash chromatography. Elution with 2:3 EtOAc/hexanes gave 43 (9.25 g, 95%) as a clear colorless oil. Rf (3:2 EtOAc/hexanes) 0.35. IR (ATR) νmax cm–1: 1634 (C=O). 1H NMR (600 MHz CDCl3; a 9:5 mixture of major and minor* rotamers): δ 6.87–6.80 (m, 2H, ArH), 6.78 *(d, J = 3.0 Hz, 1H, ArH), 6.76 (d, J = 3.0 Hz, 1H, ArH), 4.80 (t, J = 5.4 Hz, 1H, H2′), 4.42 *(t, J = 5.4 Hz, 1H, H2′), 3.81–3.77 (m, 2H, OCH2CH3), 3.76 (s, 3H, OMe), 3.75 (s, 3H, OMe), 3.74 *(s, 3H, OMe), 3.65–3.58 (m, 3H, OCH2CH3 & H1′), 3.57–3.50 *(m, 2H, OCH2CH3), 3.57–3.50 (m, 1H, H1′), 3.33 *(m, 2H, OCH2CH3), 3.29–3.26 *(m, 1H, H1′), 3.22–3.19 *(m, 2H, H1′), 3.15 *(s, 3H, NMe), 2.91 (s, 3H, NMe), 1.23 (t, J = 7.2 Hz, 6H, OCH2CH3), 1.12 *(t, J = 7.2 Hz, 3H, OCH2CH3). 13C NMR (150 MHz): δ 169.6 *(C=O), 169.4 (C=O), 153.8 (C2 or C5), 149.4 (C2 or C5), 149.1 *(C2 or C5), 127.2 (C1), 127.0 *(C1), 115.5 *(ArH), 115.4 (ArH), 113.6 *(ArH), 113.2 (ArH), 112.5 *(ArH), 112.3 (ArH), 102.1 *(C2′), 101.4 (C2′), 63.8 (OCH2CH3), 63.6 (OCH2CH3), 63.4 *(OCH2CH3), 63.2 *(OCH2CH3), 56.2 *(OMe), 56.1 (OMe), 55.9 (OMe), 53.6 *(C1′), 51.0 (C1′), 38.4 (NMe), 34.6 *(NMe), 15.5 (OCH2CH3), 15.3 *(OCH2CH3). HRMS (APCI): calcd for C16H26NO5+ [M + H]+, 312.1798; found, 312.1805.
5,8-Dimethoxy-2-methylisoquinolin-1(2H)-one (44)
Concentrated H2SO4 (10 mL) was added dropwise to neat 43 with stirring at 0 °C. After the addition was complete, the solution was warmed to 40 °C for 24 h under a CaCl2 guard tube. The solution was cooled, carefully neutralized with ice cold sat. aq NaHCO3 (∼50 mL), until effervescing ceased, then extracted with EtOAc (3 × 30 mL). The extract was dried and evaporated. Precipitation from EtOAc/hexanes gave 44 as an off-white solid (5.54 g, 96%), mp 143–148 °C. Rf (EtOAc) 0.15. IR (ATR) νmax cm–1: 1654 (C=O). 1H NMR (600 MHz, DMSO-d6): δ 7.38 (d, J = 7.8 Hz, 1H, H3), 7.14 (d, J = 9.0 Hz, 1H, H6 or H7), 6.89 (d, J = 9.0 Hz, 1H, H6 or H7), 6.64 (d, J = 7.8 Hz, 1H, H4), 3.84 (s, 3H, OMe), 3.74 (s, 3H, OMe), 3.38 (s, 3H, NMe). 13C NMR (150 MHz, DMSO-d6): δ 160.0 (C1), 153.8 (C5 or C8), 147.5 (C5 or C8), 134.2 (C3), 130.3 (C4a or C8a), 115.6 (C4a or C8a), 113.1 (C6 or C7), 109.2 (C6 or C7), 98.6 (C4), 56.5 (OMe), 56.3 (OMe), 36.9 (NMe). HRMS (APCI): calcd for C12H14NO3+ [M + H]+, 220.0959; found, 220.0968.
8-Hydroxy-5-methoxy-2-methylisoquinolin-1(2H)-one (45)
Neat 44 (45 mg, 0.20 mmol) was added to pyridine hydrochloride*; the mixture was heated under reflux for 10 min before being diluted with H2O (20 mL), extracted with EtOAc (3 × 10 mL), dried, and evaporated, and the crude product was subjected to flash chromatography. Elution with 3:2 EtOAc/hexanes gave 45 (24 mg, 57%) as an off-white solid, mp 75–78 °C. Rf (2:3 EtOAc/hexanes) 0.25. IR (ATR) νmax cm–1: 3200–2800 (OH), 1657 (C=O). 1H NMR (600 MHz, DMSO-d6): δ 12.4 (s, 1H, OH), 7.45 (d, J = 7.2 Hz, 1H, ArH), 7.19 (d, J = 8.4 Hz, 1H, ArH), 6.78 (d, J = 7.2 Hz, 1H, ArH), 6.76 (d, J = 8.4 Hz, 1H, ArH), 3.83 (s, 3H, OMe), 3.52 (s, 3H, NMe). 13C NMR (150 MHz, DMSO-d6): δ 164.9 (C1), 153.6 (C5 or C8), 145.7 (C5 or C8), 133.1 (ArH), 127.6 (C4a or C8a), 115.5 (ArH), 111.5 (C4a or C8a), 111.4 (ArH), 101.3 (ArH), 56.2 (OMe), 36.0 (NMe). HRMS (APCI): calcd for C11H12NO3+ [M + H]+, 206.0833; found, 206.0812.
*Pyridine hydrochloride was prepared from pyridine (2 mL) and concentrated HCl (2.5 mL) as detailed in the preparation of 46 below.
5,8-Dihydroxy-2-methylisoquinolin-1(2H)-one (46)
Method 1: Concentrated HCl (25 mL) was added dropwise to pyridine (20 mL) at 0 °C. After the addition was complete, excess H2O and pyridine were removed by distillation, leaving pyridine hydrochloride as a white solid. Neat 44 (0.58 g, 2.67 mmol) was added, and the mixture was heated under reflux for 20 min, then cooled, diluted with H2O (30 mL), and extracted with EtOAc (3 × 20 mL). The extract was dried and evaporated to give 46 (0.49 g, 97%) as an off-white solid, mp 180–185 °C. Rf (3:2 EtOAc/hexanes) 0.3. IR (ATR) νmax cm–1: 3200–3000 (OH), 1652 (C=O). 1H NMR (600 MHz, DMSO-d6): δ 12.3 (s, 1H, OH), 9.48 (s, 1H, OH), 7.38 (d, J = 7.8 Hz, 1H, ArH), 7.02 (d, J = 8.4 Hz, 1H, ArH), 6.77 (d, J = 7.8 Hz, 1H, ArH), 6.65 (d, J = 8.4 Hz, 1H, ArH), 3.50 (s, 3H, NMe). 13C NMR (150 MHz, DMSO-d6): δ 164.9 (C1), 152.5 (C5 or C8), 143.5 (C5 or C8), 132.0 (ArH), 126.1 (C4a or C8a), 118.8 (ArH), 111.7 (ArH), 111.2 (C4a or C8a), 101.8 (ArH), 35.7 (NMe). HRMS (APCI): calcd for C10H10NO3+ [M + H]+, 192.0641; found, 192.0655.
Method 2: Concentrated H2SO4 (2 mL) was added dropwise to 49 (205 mg, 0.558 mmol) with stirring at 0 °C. After the addition was complete, the solution was allowed to warm to rt, then stirring was continued at 50 °C overnight. After 20 h, H2O (5 mL) was added, and the solution was stirred for 1 h before being diluted with water (∼20 mL) and extracted with EtOAc (3 × 20 mL). The extract was dried and evaporated, and the residue was subjected to flash chromatography. Elution with 2:3 EtOAc/hexanes gave 46 (105 mg, 98%) as an off-white solid, which was identical to the material described above. Note: several attempts to repeat this outcome resulted in yields in the range 20–30%.
2-Methylisoquinoline-1,5,8(2H)-trione (47)
Ag2O (2.59 g, 11.2 mmol) was added to a stirred suspension of 46 (0.57 g, 2.47 mmol) and MgSO4 (1.52 g, 12.6 mmol) in Et2O (50 mL). After 20 min, the mixture was filtered through a plug of Celite and washed with DCM (3 × 10 mL). The volatiles were then removed to give 47 (0.47 g, quant.) as a bright red solid, mp 184–189 °C. Rf (EtOAc) 0.15. IR (ATR) νmax cm–1: 1661 (C=O). 1H NMR (600 MHz, DMSO-d6): δ 8.32 (d, J = 6.6 Hz, 1H, ArH), 6.97 (d, J = 9.6 Hz, 1H, ArH), 6.85 (d, J = 9.6 Hz, 1H, ArH), 6.63 (d, J = 6.6 Hz, 1H, ArH), 3 (s, 3H, NMe). 13C NMR (150 MHz, DMSO-d6): δ 185.4 (C5 or C8), 182.7 (C5 or C8), 157.4 (C1), 147.6 (ArH), 142.7 (C4a or C8a), 140.3 (ArH), 134.9 (ArH), 116.6 (C4a or C8a), 99.3 (ArH), 38.1 (NMe). HRMS (APCI): calcd for C10H8NO3+ [M + H]+, 190.0497; found, 190.0499.
N-(2,2-Diethoxyethyl)-N-methyl-2,5-diacetoxybenzamide (49)
A solution of 2,5-diacetoxybenzoic acid (48)33 (0.31 g, 1.29 mmol) and SOCl2 (0.30 mL, 4.1 mmol) in PhMe (20 mL) was heated under reflux for 2 h before the solvent and excess SOCl2 were removed by distillation. The residue was cooled to 0 °C, and a solution of NEt3 (0.5 mL, 3.61 mmol) in PhMe (10 mL) was added dropwise, followed by the dropwise addition of a solution of 42 (0.25 g, 1.7 mmol) in PhMe (10 mL). The mixture was stirred at rt for another 3 h before being diluted with H2O (50 mL) and extracted with EtOAc (3 × 20 mL). The extract was washed with sat. aq NaHCO3 (20 mL), dried, and evaporated, and the residue was subjected to flash chromatography. Elution with 2:3 EtOAc/hexanes gave 49 (0.24 g, 50%) as a pale-yellow oil. Rf (3:2 EtOAc/hexanes) 0.35. IR (ATR) νmax cm–1: 1763 (Ac C=O), 1638 (NC=O). 1H NMR (600 MHz, DMSO-d6; a 3:2 mixture of major and minor* rotamers): δ 7.29–7.19 (m, 2H, H3/4 both rotamers), 7.17 *(d, J = 2.4 Hz, 1H, H6), 7.12 (d, J = 2.4 Hz, 1H, H6), 4.67 (t, J = 5.4 Hz, 1H, H2′), 4.51 *(t, J = 5.4 Hz, 1H, H2′), 3.65 *(m, 2H, OCH2CH3), 3.51 (m, 3H, OCH2CH3 & H1′), 3.14 *(d, J = 5.4 Hz, 1H, H1′), 2.98 *(s, 3H, NMe), 2.82 (s, 3H, NMe), 2.27 (s, 3H, Ac Me), 2.22 (s, 3H, Ac Me), 2.19 *(s, 3H, Ac Me), 1.14 (t, J = 6.6 Hz, 6H, OCH2CH3), 1.05 *(t, J = 6.6 Hz, 6H, OCH2CH3). 13C NMR (150 MHz, DMSO-d6; a 3:2 mixture of major and minor* rotamers): δ 169.3 (C=O), 169.2 *(C=O), 168.9 (C=O), 168.8 *(C=O), 166.4 *(C=O), 166.0 (C=O), 147.8 (ArO), 147.6 *(ArO), 143.8 (ArO), 143.7 *(ArO), 130.8 (C1), 130.7 *(C1), 124.4 (ArH), 124.3 *(ArH), 123.5 (ArH), 123.4 *(ArH), 121.7 *(ArH), 120.9 (ArH), 100.5 *(C2′), 100.0 (C2′), 62.7 *(OCH2), 61.5 (OCH2), 52.9 *(C1′), 49.3 (C1′), 37.8 (NMe), 33.7 *(NMe), 20.9 (COCH3), 20.8 *(COCH3), 20.6 (COCH3), 20.5 *(COCH3), 15.3 (CH2CH3), 15.2 (CH2CH3). HRMS (APCI): calcd for C18H26NO7+ [M + H]+, 368.1712; found, 368.1704.
7-Chloro-5,8-dihydroxy-2-methylisoquinolin-1(2H)-one (52a)
SOCl2 (0.26 mL, 3.6 mmol) was added to a stirred solution of 47 (93 mg, 0.49 mmol) and pyridine (0.40 mL, 4.9 mmol) in PhMe (20 mL) and dimethoxyethane (DME) (5 mL) at 0 °C. The reaction was then heated to 50 °C for 20 min before being cooled, diluted with H2O (30 mL), and extracted with EtOAc (3 × 10 mL). The extract was dried and evaporated, and the residue was subjected to chromatography. Elution with 1:4 EtOAc/hexanes gave 52a, containing ∼6% of the regioisomer 6-chloro-5,8-dihydroxy-2-methylisoquinolin-1(2H)-one (52b) [NMR data not shown], as a yellow oil (89 mg, 80%). Rf (3:2 EtOAc/hexanes) 0.3. IR (ATR) νmax cm–1: 3100–2300 (OH), 1656 (C=O). 1H NMR (600 MHz, DMSO-d6): δ 9.97 (s, 1H, OH), 7.46 (d, J = 7.8 Hz, 1H, H3 or H4), 7.11 (s, 1H, H6), 6.79 (d, J = 7.8 Hz, 1H, H3 or H4), 3.53 (s, 3H, NMe). 13C NMR (150 MHz, DMSO-d6): δ 164.6 (C1), 148.3 (C5 or C8), 143.9 (C5 or C8), 132.8 (ArCH), 126.0 (C4a or C8a), 118.6 (ArH), 114.2 (C7), 112.1 (C4a or C8a), 101.8 (ArH), 36.2 (NMe). HRMS (APCI): calcd for C10H935ClNO3+ [M + H]+, 226.0257; found, 226.0265.
7-Chloro-2-methylisoquinoline-1,5,8(2H)-trione (53a)
Ag2O (0.40 g, 1.7 mmol) was added to a stirred suspension of 52a (69 mg, 0.31 mmol) and MgSO4 (0.25 g, 2.1 mmol) in Et2O (5 mL) and DME (1 mL). After 20 min, the suspension was filtered through a plug of Celite and washed with DCM (3 × 10 mL). The volatiles were then removed to give 53a, containing ∼6% of the regioisomer 6-chloro-2-methylisoquinoline-1,5,8(2H)-trione (53b), as a bright red solid (52 mg, 65%), mp 146–150 °C. Rf (1:19 MeOH/DCM) 0.40; IR (ATR) νmax cm–1: 1672 (C=O). 1H NMR (600 MHz, DMSO-d6): δ 8.38 (d, J = 6.6 Hz, 1H, H3 or H4), 7.44 (s, 1H, H6), 6.67 (d, J = 6.6 Hz, 1H, H3 or H4), 3.57 (s, 3H, NMe). 13C NMR (150 MHz, DMSO-d6): δ 182.8 (C5 or C8), 174.5 (C5 or C8), 157.2 (C1), 148.3 (ArH), 146.6 (C4a or C8a), 143.1 (C7), 132.9 (ArH), 116.3 (C4a or C8a), 99.5 (ArH), 38.2 (NMe). HRMS (APCI): calcd for C10H735ClNO3+ [M + H]+, 224.0112; found, 224.0109.
6,7-Dichloro-2-methylisoquinoline-1,5,8(2H)-trione (38) and 4,6,7-Trichloro-2-methylisoquinoline-1,5,8(2H)-trione (54)
A solution of 47 (0.26 g, 1.40 mmol) in Et2O (5 mL) was added dropwise to a solution of SO2Cl2 (0.45 mL, 5.33 mmol) and NEt3 (0.20 mL, 1.44 mmol) in Et2O (15 mL). Once the addition was complete, the reaction was heated under reflux for 20 h before being cooled, diluted with H2O (30 mL), and extracted with EtOAc (3 × 10 mL). The organic component was dried and evaporated, and the crude solid subjected to flash chromatography. Elution with 3:2 EtOAc/hexanes gave 54 as a red solid (25 mg, 6%), mp 216–220 °C. Rf (EtOAc) 0.25. IR (ATR) νmax cm–1: 1688 (C=O). 1H NMR (600 MHz): δ 7.99 (s, 1H, H3), 3.72 (s, 3H, NMe). 13C NMR (150 MHz): δ 175.2 (C5 or C8), 172.7 (C5 or C8), 156.4 (C1), 146.2 (ArH), 143.7 (C4a or C8a), 140.3 (C6 or C7), 138.9 (C6 or C7), 118.5 (C4a or C8a), 108.5 (C4), 39.4 (NMe). HRMS (APCI): calcd for C10H535Cl3NO3+ [M + H]+, 291.9332; found, 291.9330.
Further elution with EtOAc gave 38 (8 mg, 2%) as a red solid, mp 250–254 °C. Rf (1:99 MeOH/DCM) 0.1. 1H NMR (600 MHz, DMSO-d6): δ 8.38 (d, J = 6.6 Hz, 1H, H3 or H4), 6.76 (d, J = 6.6 Hz, 1H, H3 or H4), 3.56 (s, 3H, NMe). 13C NMR (150 MHz, DMSO-d6): δ 176.0 (C5 or C8), 172.5 (C5 or C8), 156.9 (C1), 148.0 (C3 or C4), 143.1 (C4a or C8a), 142.9 (C6 or C7), 138.7 (C6 or C7), 115.7 (C4a or C8a), 100.1 (C3 or C4), 38.1 (NMe). HRMS (APCI): calcd for C10H635Cl2NO3+ [M + H]+, 257.9721; found, 257.9719.
4-Iodo-2,5-dimethoxytoluene (57)
I2 (2.5 g, 10 mmol) was added to a stirred suspension of 2,5-dimethoxytoluene (55) (1.25 g, 8.21 mmol) and Ag2SO4 (5.4 g, 17 mmol) in EtOH (20 mL). After 24 h, the reaction mixture was filtered, diluted with H2O (30 mL), and extracted with EtOAc (3 × 20 mL). The extract was dried and evaporated, and the crude product was subjected to flash chromatography. Elution with 1:9 EtOAc/hexanes gave iodide 57 as a white solid (1.76 g, 77%). 1H NMR (500 MHz, CDCl3): δ 7.17 (s, 1H, H3 or H6), 6.67 (s, 1H, H3 or H6), 3.82 (s, 3H, OMe), 3.77 (s, 3H, OMe), 2.19 (Me). The 1H NMR data are identical to those reported.51
4-Bromo-2,5-dimethoxybenzoic Acid (58)38
KMnO4 (2.75 g, 17.4 mmol) was added to a stirred mixture of 1-bromo-2,5-dimethoxy-4-methylbenzene (56)37 (0.73 g, 3.16 mmol) in pyridine (10 mL) and H2O (10 mL). After 24 h, the reaction mixture was diluted with 1 M HCl (30 mL), extracted with EtOAc (3 × 20 mL), dried, and evaporated to give benzoic acid 58 as a white solid (0.58 g, 70%), which was used without further purification. 1H NMR (500 MHz, DMSO-d6): δ 12.81 (br s, CO2H), 7.37 (s, 1H, H3 or H6), 7.32 (s, 1H, H3 or H6), 3.81 (s, 3H, OMe), 3.78 (s, 3H, OMe). The 1H NMR spectrum matched the reported data.52
4-Iodo-2,5-dimethoxybenzoic Acid (59)
KMnO4 (6.05 g, 38.3 mmol) was added to a stirred mixture of 57 (1.76 g, 6.31 mmol) in pyridine (20 mL) and H2O (20 mL). After 24 h, the reaction mixture was diluted with 1 M HCl (50 mL) and extracted with EtOAc (3 × 20 mL). The extract was dried and evaporated. Precipitation from EtOAc/hexanes gave benzoic acid 59 as a yellow/white solid (0.69 g, 36%), mp 172–175 °C [lit.53 175–177 °C], which was used without further purification in the next step.
4-Bromo-N-(2,2-diethoxyethyl)-2,5-dimethoxy-N-methylbenzamide (60)
A solution of 58 (3.04 g, 11.7 mmol) and SOCl2 (1.5 mL, 21 mmol) in PhMe (40 mL) was heated under reflux for 2 h before the solvent and excess SOCl2 were removed by distillation. The residue was cooled to 0 °C, and a solution of pyridine (1.5 mL, 18 mmol) in PhMe (10 mL) was added dropwise, followed by the dropwise addition of a solution of 42 (2.34 g, 15.9 mmol) in PhMe (10 mL). The mixture was stirred at rt for another 2 h before being diluted with H2O (50 mL) and extracted with EtOAc (3 × 20 mL). The extract was washed with sat. aq NaHCO3 (20 mL), dried, and evaporated, and the residue was subjected to flash chromatography. Elution with 2:3 EtOAc/hexanes gave tertiary amide 60 (3.1 g, 68%) as a pale-yellow oil. Rf (2:3 EtOAc/hexanes) 0.15. IR (ATR) νmax cm–1: 1635 (C=O). 1H NMR (600 MHz, DMSO-d6; a 10:7 mixture of major and minor* rotamers): δ 7.33 (s, 1H, H3 or H6), 7.31 *(s, 1H, H3 or H6), 6.95 *(s, 1H, H3 or H6), 6.89 *(s, 1H, H3 or H6), 4.68 (t, J = 5.4 Hz, 1H, H2′), 4.48 *(t, J = 5.4 Hz, 1H, H2′), 3.78 (s, 3H, OMe), 3.78 *(s, 3H, OMe), 3.75 (s, 3H, OMe), 3.74 *(s, 3H, OMe), 3.71–3.45 (m, 6H, OCH2CH3 & H1′), 3.12 *(pseudo dd, J1 = 6.0 Hz, J2 = 4.2 Hz, 2H, H1′), 2.99 *(s, 3H, NMe), 2.80 (s, 3H, NMe), 1.15 (pseudo t [app. s], 6H, OCH2CH3), 1.04 *(t, J = 6.6 Hz, 6H, OCH2CH3). 13C NMR (150 MHz, DMSO-d6): δ 167.3 *(C=O), 167.2 (C=O), 149.6 (C2 or C5), 149.4 *(C2 or C5), 148.8 (C2 or C5), 148.7 *(C2 or C5), 126.1 (C1), 125.9 *(C1), 116.6 (C3 or C6), 116.4 *(C3 or C6), 112.2 (C4a or C8a), 111.3 (C3 or C6), 111.2 *(C3 or C6), 111.1 (C4a or C8a), 100.8 (C4), 100.7 *(C2′), 100.2 (C2′), 62.6 (OCH2CH3), 62.1 *(OCH2CH3), 61.8 (OCH2CH3), 60.9 *(OCH2CH3), 56.6 *(OMe), 56.5 (OMe), 56.3 (OMe), 56.2 *(OMe), 52.6 *(C1′), 49.6 (C1′), 37.3 (NMe), 33.5 *(NMe), 15.2 (OCH2CH3), 15.1 *(OCH2CH3). HRMS (ESI): calcd for C18H2779BrN2NaO5+ [M + Na + MeCN]+, 453.0986; found, 453.0996.
N-(2,2-Diethoxyethyl)-4-iodo-2,5-dimethoxy-N-methylbenzamide (61)
A solution of 59 (0.69 g, 2.24 mmol) and SOCl2 (5.0 mL, 69 mmol) in PhMe (20 mL) was heated under reflux for 2 h before the solvent and excess SOCl2 were removed by distillation. The residue was cooled to 0 °C, and a solution of NEt3 (8.0 mL, 58 mmol) in PhMe (10 mL) was added dropwise, followed by the dropwise addition of a solution of 42 (0.62 g, 4.21 mmol) in PhMe (5 mL). The mixture was stirred at rt for another 24 h before being diluted with H2O (50 mL) and extracted with EtOAc (3 × 20 mL). The extract was washed with sat. aq NaHCO3 (20 mL), dried, and evaporated, and the residue was subjected to flash chromatography. Elution with 1:4 EtOAc/hexanes gave tertiary amide 61 (0.81 g, 82%) as a pale-yellow oil. Rf (2:3 EtOAc/hexanes) 0.15. IR (ATR) νmax cm–1: 1630 (C=O). 1H NMR (600 MHz—isolated as a mixture of rotamers, with signals due to the minor rotamer denoted by asterisks): δ 7.32 (s, 1H, H3 or H6), 7.31 *(s, 1H, H3 or H6), 6.75 *(s, 1H, H3 or H6), 6.72 (s, 1H, H3 or H6), 4.80 (t, J = 5.4 Hz, 1H, H2′), 4.46 *(t, J = 5.4 Hz, 1H, H2′), 3.83 (s, 3H, OMe), 3.82 *(s, 3H, OMe), 3.78 (s, 3H, OMe), 3.77 *(s, 3H, OMe), 3.72–3.42 (m, 6H, OCH2CH3 & H1′), 3.38 *(m, 2H, OCH2CH3), 3.34–3.27 *(m, 1H, H1′), 3.24–3.17 *(m, 1H, H1′), 3.15 *(s, 3H, NMe), 2.92 (s, 3H, NMe), 1.24 (t, J = 7.2 Hz, 6H, OCH2CH3), 1.15 *(t, J = 7.2 Hz, 6H, OCH2CH3). 13C NMR (150 MHz): δ 168.9 *(C=O), 168.7 (C=O), 152.9 (C2 or C5), 152.8 *(C2 or C5), 149.7 (C2 or C5), 149.5 *(C2 or C5), 127.2 (C1), 127.0 *(C1), 122.8 *(C3 or C6), 122.7 (C3 or C6), 110.8 *(C3 or C6), 110.3 (C3 or C6), 101.6 *(C2′), 101.2 (C2′), 86.7 (C4), 86.6 *(C4), 63.4 (OCH2CH3), 63.0 (OCH2CH3), 61.8 *(OCH2CH3), 60.5 *(OCH2CH3), 57.1 (OMe), 57.0 *(OMe), 56.5 *(OMe), 56.4 (OMe), 53.5 *(C1′), 50.9 (C1′), 38.3 (NMe), 34.3 *(NMe), 15.5 (OCH2CH3), 15.3 *(OCH2CH3). HRMS (APCI): calcd for C16H25INO5+ [M + H]+, 438.0790; found, 438.0772.
6-Bromo-5,8-dimethoxy-2-methylisoquinolin-1(2H)-one (62) and 6-Bromo-5-hydroxy-8-methoxy-2-methylisoquinolin-1(2H)-one (64)
Concentrated H2SO4 (10 mL) was added dropwise to 60 (3.05 g, 7.82 mmol) with stirring at 0 °C under CaCl2 guard. After the addition was complete, the solution was allowed to warm to rt, then stirred at 50 °C for 24 h, before being diluted with H2O (30 mL), carefully neutralized with ice-cold sat. aq NaHCO3 (∼50 mL), then extracted with EtOAc (3 × 20 mL). The extract was dried and evaporated. Precipitation from EtOAc followed by recrystallization from MeOH gave 64 as white plates (0.24 g, 11%), mp 194–197 °C. Rf (1:19 MeOH/DCM) 0.15. IR (ATR) νmax cm–1: 3300–2700 (OH), 1643 (C=O). 1H NMR (600 MHz, DMSO-d6): δ 9.28 (s, 1H, OH), 7.46 (d, J = 7.8 Hz, 1H, H3), 7.05 (s, 1H, H7), 6.67 (d, J = 7.8 Hz, 1H, H4), 3.77 (s, 3H, OMe), 3.38 (s, 3H, NMe). 13C NMR (150 MHz, DMSO-d6): δ 158.9 (C1), 153.5 (C2 or C5), 141.6 (C2 or C5), 134.4 (C3), 131.2 (C4a or C8a), 114.6 (C4a or C8a), 113.9 (C6), 112.2 (C7), 98.4 (C4), 56.3 (OMe), 36.3 (NMe). HRMS (ESI): calcd for C13H1379BrN2NaO3+ [M + Na + MeCN]+, 347.0005; found, 347.0002.
The filtrate was evaporated to give isoquinolone 62 as a pale-yellow solid (1.67 g, 72%), mp 118–120 °C. Rf (1:19 MeOH/DCM) 0.35. IR (ATR) νmax cm–1: 1656 (C=O). 1H NMR (600 MHz, DMSO-d6): δ 7.54 (d, J = 7.8 Hz, 1H, H3), 7.11 (s, 1H, H7), 6.52 (d, J = 7.8 Hz, 1H, H4), 3.83 (s, 3H, OMe), 3.75 (s, 3H, OMe), 3.40 (s, 3H, NMe). 13C NMR (150 MHz): δ 158.9 (C1), 156.7 (C2 or C5), 144.4 (C2 or C5), 135.9 (C3), 134.4 (C4a or C8a), 119.8 (C4a or C8a), 114.8 (C6), 111.8 (C7), 97.6 (C4), 61.0 (OMe), 56.3 (OMe), 36.5 (NMe). HRMS (ESI): calcd for C12H1379BrNO3+ [M + H]+, 298.0095; found, 298.0073.
6-Iodo-5,8-dimethoxy-2-methylisoquinolin-1(2H)-one (63) and 5-Hydroxy-6-iodo-8-methoxy-2-methylisoquinolin-1(2H)-one (65)
Concentrated H2SO4 (10 mL) was added dropwise to 61 (0.81 g, 1.85 mmol) with stirring at 0 °C under CaCl2 guard. After the addition was complete, the solution was allowed to warm to rt, and stirring was continued at 60 °C for 24 h, before the reaction mixture was diluted with H2O (30 mL), carefully neutralized with ice cold sat. aq NaHCO3 (∼50 mL), then extracted with EtOAc (3 × 20 mL). The extract was dried and evaporated, and the residue was subjected to flash chromatography. Elution with EtOAc gave isoquinolone 63 as a pale-yellow solid (0.18 g, 28%), mp 50–52 °C. Rf (1:19 MeOH/DCM) 0.3. IR (ATR) νmax cm–1: 1647 (C=O). 1H NMR (500 MHz, DMSO-d6): δ 7.50 (d, J = 9.0 Hz, 1H, H3), 7.23 (s, 1H, H7), 6.47 (d, J = 9.0 Hz, 1H, H4), 3.80 (s, 3H, OMe), 3.70 (s, 3H, OMe), 3.38 (s, 3H, NMe). 13C NMR (125 MHz, DMSO-d6): δ 159.1 (C1), 156.6 (C2 or C5), 147.8 (C2 or C5), 135.8 (C3), 133.1 (C4a or C8a), 117.3 (C7), 115.4 (C4a or C8a), 98.0 (C4), 96.6 (C6), 61.0 (OMe), 56.3 (OMe), 36.6 (NMe). HRMS (APCI): calcd for C12H13INO3+ [M + H]+, 345.9938; found, 345.9935.
Further elution gave 65 (0.26 g, 42%) as a yellow/orange solid, mp 163–166 °C. Rf (1:19 MeOH/DCM) 0.2. IR (ATR) νmax cm–1: 3700–2700 (OH), 1646 (C=O). 1H NMR (500 MHz, DMSO-d6): δ 9.25 (s, 1H, OH), 7.45 (d, J = 9.0 Hz, 1H, H3), 7.21 (s, 1H, H7), 6.65 (d, J = 9.0 Hz, 1H, H4), 3.76 (s, 3H, OMe), 3.38 (s, 3H, NMe). 13C NMR (125 MHz, DMSO-d6): δ 159.2 (C1), 153.8 (C2 or C5), 144.8 (C2 or C5), 134.4 (C3), 129.9 (C4a or C8a), 118.0 (C7), 115.3 (C4a or C8a), 98.7 (C4), 91.9 (C6), 56.4 (OMe), 36.5 (NMe). HRMS (APCI): calcd for C11H11INO3+ [M + H]+, 331.9796; found, 331.9778.
6-Bromo-2-methylisoquinoline-1,5,8(2H)-trione (67)
A solution of CAN (0.29 g, 0.53 mmol) in H2O (1 mL) was added to a stirred solution of 64 (28 mg, 99 μmol) in MeCN (8 mL) at −30 °C. The reaction was allowed to warm to −20 °C over 30 min before being diluted with H2O (20 mL) and extracted with EtOAc (3 × 10 mL). The extract was dried and evaporated to give quinone 67 as a red solid (20 mg, 76%), mp 167–171 °C. Rf (1:19 MeOH/DCM) 0.15. IR (ATR) νmax cm–1: 1678 (C=O). 1H NMR (600 MHz, DMSO-d6): δ 8.31 (d, J = 7.2 Hz, 1H, H3), 7.45 (s, 1H, H7), 6.71 (d, J = 7.2 Hz, 1H, H4), 3.52 (s, 3H, NMe). 13C NMR (150 MHz): δ 180.2 (C5 or C8), 178.4 (C5 or C8), 157.3 (C1), 147.4 (C3), 142.6 (C4a or C8a), 141.2 (C7), 134.0 (C4a or C8a), 116.2 (C6), 100.4 (C4), 38.1 (NMe).
6-Iodo-2-methylisoquinoline-1,5,8(2H)-trione (68)
A solution of CAN (1.65 g, 3.01 mmol) in H2O (2 mL) was added to a stirred solution of 65 (0.18 g, 0.54 mmol) in MeCN (12 mL) at −30 °C. The reaction was allowed to warm to −20 °C over 20 min before being diluted with H2O (20 mL) and extracted with EtOAc (3 × 10 mL). The extract was dried and evaporated to give quinone 68 as a red solid (0.10 g, 59%), mp 218–220 °C. Rf (EtOAc) 0.1. IR (ATR) νmax cm–1: 1683 (C=O). 1H NMR (600 MHz, DMSO-d6): δ 8.27 (d, J = 6.6 Hz, 1H, H3), 7.69 (s, 1H, H7), 6.69 (d, J = 6.6 Hz, 1H, H4), 3.51 (s, 3H, NMe). 13C NMR (150 MHz, DMSO-d6): δ 180.1 (C5 or C8), 179.9 (C5 or C8), 157.4 (C1), 148.9 (C3), 147.3 (C7), 141.3 (C4a or C8a), 116.5 (C4a or C8a), 116.3 (C6), 100.8 (C4), 38.1 (NMe). HRMS (APCI): calcd for C10H7INO3+ [M + H]+, 315.9476; found, 315.9465.
Ethyl 2-(5,8-Dihydroxy-2-methyl-1-oxo-1,2-dihydroisoquinolin-6-yl)-2-(2-nitrophenyl)acetate (70)
K2CO3 (0.21 g, 1.54 mmol) was added to a stirred solution of 67 (92 mg, 0.34 mmol) and 15 (0.18 g, 0.87 mmol) in DMF (5 mL). The reaction was heated to 45 °C and maintained for 2 h before being cooled, diluted with 1 M HCl (20 mL), and extracted with EtOAc (3 × 10 mL). The extract was dried and evaporated, and the residue was subjected to flash chromatography. Elution with 3:2 EtOAc/hexanes gave 70 as a yellow-orange solid (39 mg, 29%). 1H NMR (600 MHz): δ 13.10 (s, 1H, OH), 8.07 (d, J = 7.2 Hz, 1H, H3″), 7.44 (dd, J1 = J2 = 7.2 Hz, 1H, H4″ or H5″), 7.42 (dd, J1 = J2 = 7.2 Hz, 1H, H4″ or H5″), 7.22 (d, J = 7.2 Hz, 1H, H6″), 7.09 (d, J = 7.2 Hz, 1H, H3′), 6.91 (d, J = 7.2 Hz, 1H, H4′), 6.34 (s, 1H, H2 or H7′), 5.52 (s, 1H, H2 or H7′), 4.22 (q, J = 6.6 Hz, 2H, OCH2CH3), 3.61 (s, 3H, NMe), 1.20 (t, J = 6.6 Hz, 3H, OCH2CH3). 13C NMR (150 MHz): δ 170.4 (C1), 165.3 (C1′), 153.3 (C5′ or C8′), 149.8 (C5′ or C8′), 138.7 (C2″), 133.3 (ArH), 133.2, 132.5, 130.7 (ArH), 128.3 (ArH), 126.4, 125.1, 125.0 (ArH), 119.2, 110.9, 102.5 (ArH), 61.9 (OCH2CH3), 47.1 (C2), 36.7 (NMe), 14.0 (OCH2CH3).
Acknowledgments
We thank the UWA Centre for Characterisation, Microscopy and Analysis, in particular Drs Lindsay Byrne and Gareth Nealon for assistance with NMR spectroscopy and Drs Anthony Reeder and Michael Clarke for help with mass spectrometry. The Australian Government is gratefully acknowledged for an Australian Postgraduate Award (M.R.B.) and Research Training Program (RTP) Scholarships (F.D. and L.T.). Waiver of tuition fees (F.D.) and a UWA—UQ Bilateral Research Collaboration Award from the University of Western Australia facilitated this work. C.M.W. thanks the University of Queensland for financial support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c02116.
CIF file for the crystal structure of 35/35a (CIF)
CIF file for the crystal structure of 53a/53b (CIF)
CIF file for the crystal structure of 64 (CIF)
CIF file for the crystal structure of 68 (CIF)
1H and 13C spectra annotated with skeleton-numbered structures and general crystallographic methods, tabulated data, and CCDC deposit numbers for compounds 35/35a, 53a/53b, 64, and 68 (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Thale Z.; Johnson T.; Tenney K.; Wenzel P. J.; Lobkovsky E.; Clardy J.; Media J.; Pietraszkiewicz H.; Valeriote F. A.; Crews P. Structures and cytotoxic properties of sponge-derived bisannulated acridines. J. Org. Chem. 2002, 67, 9384–9391. 10.1021/jo026459o. [DOI] [PubMed] [Google Scholar]
- Inman W. D.; O’Neill-Johnson M.; Crews P. Novel marine sponge alkaloids. 1. Plakinidine A and B, anthelmintic active alkaloids from a Plakortis sponge. J. Am. Chem. Soc. 1990, 112, 1–4. 10.1021/ja00157a001. [DOI] [Google Scholar]
- West R. R.; Mayne C. L.; Ireland C. M.; Brinen L. S.; Clardy J. Plakinidines: cytotoxic alkaloid pigments from the Fijian sponge Plakortis sp. Tetrahedron Lett. 1990, 31, 3271–3274. 10.1016/s0040-4039(00)89041-3. [DOI] [Google Scholar]
- Ralifo P.; Sanchez L.; Gassner N. C.; Tenney K.; Lokey R. S.; Holman T. R.; Valeriote F. A.; Crews P. Pyrroloacridine Alkaloids from Plakortis quasiamphiaster: Structures and Bioactivity. J. Nat. Prod. 2007, 70, 95–99. 10.1021/np060585w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford P. W.; Davidson B. S. Plakinidine, D a new pyrroloacridine alkaloid from the ascidian Didemnum rubeum. J. Nat. Prod. 1997, 60, 1051–1053. 10.1021/np970312o. [DOI] [PubMed] [Google Scholar]
- Smith C. J.; Venables D. A.; Hopmann C.; Salomon C. E.; Jompa J.; Tahir A.; Faulkner D. J.; Ireland C. M. Plakinidine D, a new pyrroloacridine alkaloid from two ascidians of the genus Didemnum. J. Nat. Prod. 1997, 60, 1048–1050. 10.1021/np970311w. [DOI] [PubMed] [Google Scholar]
- Marshall K. M.; Barrows L. R. Biological activities of pyridoacridines. Nat. Prod. Rep. 2004, 21, 731–751. 10.1039/b401662a. [DOI] [PubMed] [Google Scholar]
- Charyulu G. A.; McKee T. C.; Ireland C. M. Diplamine, a cytotoxic polyaromatic alkaloid from the tunicate Diplosoma sp. Tetrahedron Lett. 1989, 30, 4201–4202. 10.1016/s0040-4039(01)80689-4. [DOI] [Google Scholar]
- McCarthy P. J.; Pitts T. P.; Gunawardana G. P.; Kelly-Borges M.; Pomponi S. Antifungal activity of meridine, a natural product from the marine sponge Corticium sp. J. Nat. Prod. 1992, 55, 1664–1668. 10.1021/np50089a016. [DOI] [PubMed] [Google Scholar]
- Gunawardana G. P.; Kohmoto S.; Gunasekera S. P.; McConnell O. J.; Koehn F. E. Dercitine, a new biologically active acridine alkaloid from a deep water marine sponge, Dercitus sp. J. Am. Chem. Soc. 1988, 110, 4856–4858. 10.1021/ja00222a071. [DOI] [Google Scholar]
- Copp B. R.; Kayser O.; Brun R.; Kiderlen A. F. Antiparasitic activity of marine pyridoacridone alkaloids related to the ascididemins. Planta Med. 2003, 69, 527–531. 10.1055/s-2003-40640. [DOI] [PubMed] [Google Scholar]
- Eder C.; Schupp P.; Proksch P.; Wray V.; Steube K.; Mueller C. E.; Frobenius W.; Herderich M.; van Soest R. W. M. Bioactive pyridoacridine alkaloids from the Micronesian sponge Oceanapia sp. J. Nat. Prod. 1998, 61, 301–305. 10.1021/np9702704. [DOI] [PubMed] [Google Scholar]
- McDonald L. A.; Eldredge G. S.; Barrows L. R.; Ireland C. M. Inhibition of topoisomerase II catalytic activity by pyridoacridine alkaloids from a Cystodytes sp. ascidian: a mechanism for the apparent intercalator-induced inhibition of topoisomerase II. J. Med. Chem. 1994, 37, 3819–3827. 10.1021/jm00048a017. [DOI] [PubMed] [Google Scholar]
- de Guzman F. S.; Carte B.; Troupe N.; Faulkner D. J.; Harper M. K.; Concepcion G. P.; Mangalindan G. C.; Matsumoto S. S.; Barrows L. R.; Ireland C. M. Neoamphimedine: a new pyridoacridine topoisomerase II inhibitor which catenates DNA. J. Org. Chem. 1999, 64, 1400–1402. 10.1021/jo982047x. [DOI] [Google Scholar]
- Tasdemir D.; Marshall K. M.; Mangalindan G. C.; Concepcion G. P.; Barrows L. R.; Harper M. K.; Ireland C. M. Deoxyamphimedine, a new pyridoacridine alkaloid from two tropical Xestospongia sponges. J. Org. Chem. 2001, 66, 3246–3248. 10.1021/jo010153k. [DOI] [PubMed] [Google Scholar]
- Marshall K. M.; Matsumoto S. S.; Holden J. A.; Concepcion G. P.; Tasdemir D.; Ireland C. M.; Barrows L. R. The anti-neoplastic and novel topoisomerase II-mediated cytotoxicity of neoamphimedine, a marine pyridoacridine. Biochem. Pharmacol. 2003, 66, 447–458. 10.1016/s0006-2952(03)00209-0. [DOI] [PubMed] [Google Scholar]
- Kitahara Y.; Mizuno T.; Kubo A. Synthetic studies of benzo[b]pyrrolo[4,3,2-de][1,10]phenanthroline. Tetrahedron 2004, 60, 4283–4288. 10.1016/j.tet.2004.03.057. [DOI] [Google Scholar]
- Fukuyama T.; Komori T.; Yokoshima S. Synthetic studies on plakinidines. Synlett 2015, 26, 1537–1540. 10.1055/s-0034-1380689. [DOI] [Google Scholar]
- Tokuyama H.; Satoh T.; Adachi T.; Okano K.; Sakata J. Synthetic studies on plakinidines. Heterocycles 2019, 99, 310–323. 10.3987/com-18-s(f)26. [DOI] [Google Scholar]
- Buccini M.; Jeow S. Y.; Byrne L.; Skelton B. W.; Nguyen T. M.; Chai C. L. L.; Piggott M. J. Bisannulation of 2,3-dichloro-1,4-naphthoquinone with o-nitrophenylacetic acid derivatives: a succinct synthesis of the ABCD ring system of alpkinidine. Eur. J. Org. Chem. 2013, 3232–3240. 10.1002/ejoc.201300227. [DOI] [Google Scholar]
- Volvoikar P. S.; Tilve S. G.; Zubkov F. I. A concise approach for the synthesis of the ABCD ring system of alpkinidine. ChemistrySelect 2019, 4, 7187–7189. 10.1002/slct.201900357. [DOI] [Google Scholar]
- Cappelli A.; Gallelli A.; Manini M.; Anzini M.; Mennuni L.; Makovec F.; Menziani M. C.; Alcaro S.; Ortuso F.; Vomero S. Further studies on the interaction of the 5-hydroxytryptamine3 (5-HT3) receptor with arylpiperazine ligands. Development of a new 5-HT3 receptor ligand showing potent acetylcholinesterase inhibitory properties. J. Med. Chem. 2005, 48, 3564–3575. 10.1021/jm0493461. [DOI] [PubMed] [Google Scholar]
- Lee H.-J.; Suh M.-E.; Lee C.-O. Synthesis and cytotoxicity evaluation of 2-amino- and 2-hydroxy-3-ethoxycarbonyl-N-substituted-benzo[f]indole-4,9-dione derivatives. Bioorg. Med. Chem. 2003, 11, 1511–1519. 10.1016/s0968-0896(03)00062-2. [DOI] [PubMed] [Google Scholar]
- Salov B. V.; Shakhnovich A. I. 4,9-Dihydrobenz[f]indole-4,9-dione derivatives. Synthesis and properties of 2,3,4,9-tetrahydrobenz[f]indole-2,4,9-triones and 2,3,4,9-tetrahydrobenz[f]indole-2,3,4,9-tetrones. Khim. Geterotsikl. Soedin. 1976, 911–914. [Google Scholar]
- Shakhnovich A. I. Synthesis of 3-substituted 2-hydroxybenz[f]indolequinones and benz[f]isatinquinone—a new π-acceptor system. J. Heterocycl. Chem. 1991, 28, 33–39. 10.1002/jhet.5570280106. [DOI] [Google Scholar]
- Azadi-Ardakani M.; Wallace T. W. 3,6-Dimethoxybenzocyclobutenone: a reagent for quinone synthesis. Tetrahedron 1988, 44, 5939–5952. 10.1016/s0040-4020(01)81452-6. [DOI] [Google Scholar]
- Cody J. A.; Ahmed I.; Tusch D. J. Studies toward the total synthesis of eletefine: an efficient construction of the AB ring system. Tetrahedron Lett. 2010, 51, 5585–5587. 10.1016/j.tetlet.2010.08.058. [DOI] [Google Scholar]
- Prey V. Die Spaltung von Phenoläthern mit Pyridinhydrochlorid. Ber. Dtsch. Chem. Ges. B 1941, 74, 1219–1225. 10.1002/cber.19410740715. [DOI] [Google Scholar]
- De Silva S. O.; Watanabe M.; Snieckus V. General route to anthraquinone natural products via directed metalation of N,N-diethylbenzamides. J. Org. Chem. 1979, 44, 4802–4808. 10.1021/jo00394a012. [DOI] [Google Scholar]
- Beer R. J. S.; Clark K.; Davenport H. F.; Robertson A. Chemistry of the melanins. III. The synthesis of hydroxyindoles from p-benzoquinones. J. Chem. Soc. 1951, 2029–2032. 10.1039/jr9510002029. [DOI] [Google Scholar]
- Farina F.; Torres T. Polycyclic hydroxyquinones. Part III. Synthesis of 6-oxo-5,6,7,8-tetrahydro-1,4-naphthoquinone and derivatives and a new, convenient preparation of o-naphthazarin. Synthesis 1980, 753–755. 10.1055/s-1980-29202. [DOI] [Google Scholar]
- Cassis R.; Valderrama J. A. Studies on quinones. XI. Synthesis of quinones from hydroquinones by using manganese dioxide and acid-impregnated manganese dioxide. Synth. Commun. 1983, 13, 347–356. 10.1080/00397918308066988. [DOI] [Google Scholar]
- Kloetzel M. C.; Dayton R. P.; Abadir B. Y. Synthetic analogs of cortical hormones. I. Homogentisic acid and α,2,5-trihydroxyacetophenone derivatives from 2,5-diacetoxy-α-diazoacetophenone. J. Org. Chem. 1955, 20, 38–49. 10.1021/jo01119a008. [DOI] [Google Scholar]
- Shi S.; Katz T. J.; Yang B. V.; Liu L. Use of thiazyl chlorides, alkyl carbamates, and thionyl chloride to fuse 1,2,5-thiadiazoles to quinones and to oxidize, chlorinate, and aminate them. J. Org. Chem. 1995, 60, 1285–1297. 10.1021/jo00110a036. [DOI] [Google Scholar]
- Ferreira V. F.; Schmitz F. J. Improved preparation of 5,6-dichlorocyclohex-2-ene-1,4-dione. Org. Prep. Proced. Int. 1998, 30, 115–116. 10.1080/00304949809355271. [DOI] [Google Scholar]
- Sy W.-W.; Lodge B. A.; By A. W. Aromatic iodination with iodine and silver sulfate. Synth. Commun. 1990, 20, 877–880. 10.1080/00397919008052334. [DOI] [Google Scholar]
- Lanfranchi D. A.; Cesar-Rodo E.; Bertrand B.; Huang H.-H.; Day L.; Johann L.; Elhabiri M.; Becker K.; Williams D. L.; Davioud-Charvet E. Synthesis and biological evaluation of 1,4-naphthoquinones and quinoline-5,8-diones as antimalarial and schistosomicidal agents. Org. Biomol. Chem. 2012, 10, 6375–6387. 10.1039/c2ob25812a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttringhaus A.; Gralheer H. Über eine neue Art atropisomerer Verbindungen. Justus Liebigs Ann. Chem. 1942, 550, 67–98. 10.1002/jlac.19425500105. [DOI] [Google Scholar]
- Li C.; Lobkovsky E.; Porco J. A. Jr. Total synthesis of (±)-torreyanic acid. J. Am. Chem. Soc. 2000, 122, 10484–10485. 10.1021/ja005552w. [DOI] [Google Scholar]
- Li C.; Johnson R. P.; Porco J. A. Jr. Total synthesis of the quinone epoxide dimer (+)-torreyanic acid: application of a biomimetic oxidation/electrocyclization/Diels-Alder dimerization cascade. J. Am. Chem. Soc. 2003, 125, 5095–5106. 10.1021/ja021396c. [DOI] [PubMed] [Google Scholar]
- Bergman J.; Mason J.; Janosik T. A new approach to methoxyisatins leading to the total synthesis of ophiuroidine and other hydroxytryptanthrins. Synthesis 2009, 3642–3648. 10.1055/s-0029-1217004. [DOI] [Google Scholar]
- Moliterno M.; Cari R.; Puglisi A.; Antenucci A.; Sperandio C.; Moretti E.; Di Sabato A.; Salvio R.; Bella M. Quinine-catalyzed asymmetric synthesis of 2,2′-binaphthol-type biaryls under mild reaction conditions. Angew. Chem., Int. Ed. 2016, 55, 6525–6529. 10.1002/anie.201601660. [DOI] [PubMed] [Google Scholar]
- Chen Y.-H.; Cheng D.-J.; Zhang J.; Wang Y.; Liu X.-Y.; Tan B. Atroposelective synthesis of axially chiral biaryldiols via organocatalytic arylation of 2-naphthols. J. Am. Chem. Soc. 2015, 137, 15062–15065. 10.1021/jacs.5b10152. [DOI] [PubMed] [Google Scholar]
- Barker D.; Brimble M. A.; Do P.; Turner P. Addition of silyloxydienes to 2,6-dibromo-1,4-benzoquinone: an approach to highly oxygenated bromonaphthoquinones for the synthesis of thysanone. Tetrahedron 2003, 59, 2441–2449. 10.1016/s0040-4020(03)00291-6. [DOI] [Google Scholar]
- Lyubchanskaya V. M.; Alekseeva L. M.; Granik V. G. Synthesis of 2-(1-ethoxycarbonyl-2-aminopropen-1-yl)-3,5-dibromohydroquinones. Khim.-Farm. Zh. 1992, 26, 55–57. [Google Scholar]
- Hegedus L. S.; Mulhern T. A.; Mori A. Palladium(0)-catalyzed syntheses of indoloquinones. J. Org. Chem. 1985, 50, 4282–4288. 10.1021/jo00222a017. [DOI] [Google Scholar]
- Grandmaison J. L.; Brassard P. Reactions of ketene acetals. IX. The synthesis of naphthoquinones from benzoquinones. Tetrahedron 1977, 33, 2047–2053. 10.1016/0040-4020(77)80312-8. [DOI] [Google Scholar]
- Dhoro F.; Parkin-Gibbs J.; McIldowie M.; Skelton B. W.; Piggott M. J. Confirmation of the revised structure of samoquasine A and a proposed structural revision of cherimoline. J. Nat. Prod. 2018, 81, 1658–1665. 10.1021/acs.jnatprod.8b00319. [DOI] [PubMed] [Google Scholar]
- Kitasato Z.; Sone C. The synthesis of N-methylhydroxynaphthindolequinone and N-methylnaphthisatinquinone. Bull. Chem. Soc. Jpn. 1930, 5, 348–354. 10.1246/bcsj.5.348. [DOI] [Google Scholar]
- Jones R. G.; Kornfeld E. C.; McLaughlin K. C.; Anderson R. C.; Imidazoles I. V. The synthesis and antithyroid activity of some 1-substituted-2-mercaptoimidazoles. J. Am. Chem. Soc. 1949, 71, 4000–4002. 10.1021/ja01180a036. [DOI] [Google Scholar]
- Hubig S. M.; Jung W.; Kochi J. K. Cation radicals as intermediates in aromatic halogenation with iodine monochloride: solvent and salt effects on the competition between chlorination and iodination. J. Org. Chem. 1994, 59, 6233–6244. 10.1021/jo00100a025. [DOI] [Google Scholar]
- Gothard C. M.; Rao N. A.; Nowick J. S. Nanometer-sized Amino acids for the synthesis of nanometer-scale water-soluble molecular rods of preciselength. J. Am. Chem. Soc. 2007, 129, 7272. 10.1021/ja072648i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T. Estrogenic biphenyls. VII. Preparation and estrogenic action of methoxyl derivatives of 4-methoxybiphenyl-4′-carboxylic acid. Bull. Chem. Soc. Jpn. 1959, 32, 1292. 10.1246/bcsj.32.1292. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.