Abstract
Sex is a key risk factor for many types of cardiovascular disease. It is imperative to understand the mechanisms underlying sex differences to devise optimal preventive and therapeutic approaches for all individuals. Both biological sex (determined by sex chromosomes and gonadal hormones) and gender (social and cultural behaviors associated with femininity or masculinity) influence differences between men and women in disease susceptibility and pathology. Here we focus on the application of experimental mouse models that elucidate the influence of two components of biological sex—sex chromosome complement (XX or XY) and gonad type (ovaries or testes). These models have revealed that in addition to well-known effects of gonadal hormones, sex chromosome complement influences cardiovascular risk factors such as plasma cholesterol levels and adiposity, as well as the development of atherosclerosis and pulmonary hypertension. One mechanism by which sex chromosome dosage influences cardiometabolic traits is through sex-biased expression of X chromosome genes that escape X-inactivation. These include chromatin-modifying enzymes that regulate gene expression throughout the genome. The identification of factors that determine sex-biased gene expression and cardiometabolic traits will expand our mechanistic understanding of cardiovascular disease processes, and provide insight into sex differences that remain throughout the lifespan as gonadal hormone levels alter with age.
Subject Terms: Basic Science Research, Mechanisms, Cardiovascular Disease, Women, Sex, Gender
Introduction
Improved understanding of cardiovascular disease (CVD) development and prevention has led to a remarkable reduction in heart attack and stroke mortalities of >60% over the past four decades.1 Thousands of published studies describe the occurrence of sex differences in cardiovascular diseases, including atherosclerotic coronary artery disease (CAD), heart failure with preserved ejection fraction, SCAD (spontaneous coronary artery dissection), Takotsubo cardiomyopathy, and coronary microvascular disease.2,3 In studies in both humans and experimental models, females have been underrepresented. This exemplifies a missed opportunity to gain clearer insight into the mechanisms underlying cardiovascular diseases, since comparison of the two sexes may reveal molecular processes that lead to greater protection of one sex and could ultimately inform about the development of therapeutic strategies. A better understanding of the underlying causes of sex differences at the genetic and molecular levels is necessary for optimal diagnosis and treatment of both men and women.
Sex differences in CVD prevalence and pathologies
Sex differences in CVD have been documented in thousands of publications. A brief summary of some well-established sex differences in human CVD are shown in Table 1. Intrinsic differences in cardiac and vascular aging may contribute to sex differences in the development of vascular diseases.4 The most common type of heart disease in the U.S. is CAD. Prior to age 50, men have higher incidence of CAD, but women that experience acute coronary events have a worse prognosis than men.3 Following the menopause transition, the incidence of CAD in women increases and ultimately exceeds that in men.5,6 This implicates a role for estrogen in protection against CAD, but despite important roles for this hormone, estrogen cannot fully account for the sex differences in CAD prevalence.7
Table 1:
Examples of Sex Differences in Human CVD
| Disease | Differential Sex Incidence | Sex-biased Characteristics | References | |
|---|---|---|---|---|
| Women | Men | |||
| Coronary artery disease | <50 yrs: incidence F<M; mortality F>M | Non-obstructive CAD, microvascular dysfunction. Stable plaques rich in smooth muscle cells, prone to erosion. | Obstructive CAD. Lipid-rich plaques, prone to bleeding and rupture. | 3,8,9 |
| >50 yrs: F incidence increases | ||||
| Takotsubo cardiomyopathy | F>M (9:1) | Transient reversible acute heart failure. Characterized by apical ballooning. Triggered by physical or emotional stress, changes in hormone levels. | Rare in men; more common in Japanese men than other ethnic groups. | 10–12 |
| Spontaneous coronary artery dissection (SCAD) | F>M (9:1) | Most common cause of pregnancy-associated MI (>40%). Characterized by extra-coronary vascular abnormalities. Triggered by physical or emotional stress, often without cardiovascular risk factors. | Rare in men. | 13,14 |
| Abdominal aortic aneurysm | Incidence F<M (1:5) rupture F>M |
Diagnosed at later ages (protective effect of estrogen?). Increased rupture may be related to biomechanical properties of female aorta. | Occurs at earlier age than in women. Sex hormone signaling of renin-angiotensin-aldosterone system may promote male susceptibility. | 15–18 |
| Heart failure | HFpEF: F>M (2:1) | Heart failure with preserved ejection fraction (HFpEF) more common. Associated with non-ischemic disease, microvascular dysfunction, endothelial cell inflammation, altered nitric oxide signaling. | Heart failure with reduced ejection fraction (HFrEF) more common. Associated with ischemic heart disease and younger age of presentation. | 19–22 |
| HFrEF: F<M (1:2) | ||||
| Cardiac ischemic injury | <50 yrs: F<M | Better remodeling, decreased inflammatory cytokine production after ischemic heart injury in premenopausal women. However, estrogen replacement after menopause may increase incidence. | Lower cardiac tolerance to oxygen deprivation, reduced efficacy of cardiac remodeling. | 23,24 |
| >50 yrs: F incidence increases | ||||
| Aortic valve stenosis | F≠M presentation | Older at presentation. Characterized by sustained fibrosis. | Younger at presentation. Characterized by higher atherosclerotic burden and calcification. | 25,26 |
Beyond effects on CVD prevalence, sex influences the clinical pathology of multiple types of CVD. For example, men and women experience atherosclerotic CAD differently from one another. In men, atherosclerotic plaques are typically lipid-rich and unstable, subject to bleeding and rupture. By contrast, plaques in women are more stable, rich in smooth muscle cells, and undergo erosion rather than rupture.8,9 The events that lead to plaque rupture (men) vs. erosion (women) represent distinct molecular processes. Systems biology studies on atherosclerotic plaques from men and women with CAD have identified sex-specific driver genes that associate with male/female differences in plaque pathophysiology using human tissue banks.27 These studies showed that key driver genes of female CAD are expressed predominantly in smooth muscle cells, and are expressed at higher levels in female atherosclerotic plaques.
Heart failure occurs at a similar prevalence in men and women, but there are sex differences in pathophysiology and response to therapies.19–22 Men have a ~2:1 higher prevalence of heart failure with reduced ejection fraction (HFrEF), whereas women have a ~2:1 higher prevalence of heart failure with preserved ejection fraction (HFpEF). This has been attributed, in part, to the predisposition of men to macrovascular CAD and of women to microvascular dysfunction. Microvascular dysfunction in HFpEF is characterized by endothelial cell inflammation and altered activity of nitric oxide (NO) synthases and NO signaling, leading to downstream diastolic dysfunction in the left ventricle. A mouse model of HFpEF has been generated with a combination of high-fat diet feeding and inhibition of endogenous NO synthase production (with the chemical Nω-nitro-l-arginine methyl ester) and confirmed that nitrosative stress in cardiomyocytes is a pathogenic mechanism in HFpEF.28 To date only male mice have been studied with this HFpEF model, and it will be interesting to determine if this model can be used to identify mechanisms underlying greater predilection of females to HFpEF.
Women have a much higher predilection for two types of acute coronary syndromes that are often preceded by severe psychological or physical stress—Takotsubo cardiomyopathy, which occurs at a rate of 9:1 in women compared to men, and spontaneous coronary artery dissection (SCAD), which occurs overwhelmingly in women (upwards of 70%).10,13 The clinical presentation of these two conditions has similarities, mimicking acute coronary syndrome or myocardial infarction in individuals between 25 years old and middle age with few atherosclerotic disease risk factors. Takotsubo cardiomyopathy involves a sudden and transient weakening of cardiac muscle that leads to a characteristic apical ballooning and elevated catecholamine levels, but the mechanisms are not well understood. SCAD is characterized by a spontaneous arterial dissection that leads to hematoma within the artery wall, causing ischemia. SCAD is the most common cause of heart attack in pregnant and postpartum women, implicating vascular effects of estrogen and/or progesterone.13
Sex differences in many additional forms of cardiovascular disease have been previously reviewed.2,3 A key takeaway message from existing clinical and basic research studies is that much remains to be learned about the mechanisms that contribute to sex differences in CVD, and that inadequate attention is paid to inclusion of both sexes in clinical and preclinical studies.29–34
Determinants of biological sex that influence CVD
The most fundamental determinant of biological sex is the presence of XX or XY sex chromosomes, which are present in every cell of the body of female or male individuals, respectively. The presence of the Sry gene on the Y chromosome specifies the development of male gonads (testes); the lack of Sry leads to an alternate developmental program and the formation of female gonads (ovaries). Thus, presence of XX chromosomes are typically coupled with ovaries, and XY chromosomes with testes, making it difficult to tease apart the contributions of genetic vs. gonadal sex.
In human clinical studies, the biological sex component that has been primarily investigated is the contribution of gonadal hormones, particularly estrogen. This has been through assessment of cardiovascular diseases in pre- and postmenopausal women, and in women receiving hormone replacement therapy. The conclusions from these studies are inconsistent and likely reflect the presence of confounding variables in the groups of individuals studied. An in-depth discussion of the findings of the role of estrogen in CVD is beyond the intended scope of this article, but it is worth noting that the American Heart Association has suggested guidelines for assessing potential effects of hormone therapy in menopausal women on an individual basis.35 Menopausal hormone replacement is considered most beneficial in women with low risk for CVD (normal body weight and blood pressure, active lifestyle), when initiated soon after menopause, and with low doses of hormones. Menopausal hormone therapy is not recommended for women with CVD risk factors (diabetes, hypertension, smoking, obesity, hyperlipidemia) or known cardiovascular disease (including atherosclerotic CVD, stroke, clotting disorders, thrombotic disease, or breast cancer). In men, high levels of estrogens are associated with increased risk for CAD.36
The role of genetic sex (XX vs. XY chromosomes) in CVD has been difficult to assess in humans, in part due to the inability to disentangle XX and XY genotypes from the presence of the corresponding gonad type. CVD has been studied in individuals with gain or loss of entire sex chromosomes. For example, compared to XY men, Klinefelter men, who carry an additional copy of the X chromosome (XXY), have increased cardiovascular mortality.37 The role of the extra X chromosome in bringing about the increased CVD risk is unclear, since these individuals have hypogonadism (which may be treated with testosterone supplementation), may have congenital cardiovascular abnormalities, and are more susceptible to CVD risk factors such as visceral obesity and type 2 diabetes. Likewise, identifying the role of the sex chromosomes in CVD susceptibility in Turner syndrome (XO) patients is confounded by the high prevalence of congenital cardiovascular abnormalities (such as bicuspid aortic valve, left-handed heart obstructive disease, enlargement of the thoracic aorta) and early-onset hypertension, ischemic heart disease, and stroke.38
Studies of cardiovascular health in transgender individuals receiving hormone therapy (in which exogenous sex hormones typical of females are provided to XY individuals, and male hormones are provided to XX individuals) suggest an increased risk for myocardial infarction in transgender compared to age-adjusted cisgender individuals.39 While these studies are important for defining optimal health care for transgender individuals, limitations preclude meaningful conclusions regarding the role of sex chromosomes, gonadal hormones, or their interactions in CVD risk. These limitations include the small size and relatively low age of study populations such that small numbers of cardiovascular events are reported, and the much higher hormone levels in transgender individuals with exogenous hormone therapy compared to the intrinsic levels in cisgender individuals. Thus, major knowledge gaps remain regarding the role of genetic sex, and its interaction with gonadal sex, in CVD.
The impact of sex on gene expression and epigenetic regulation
Underlying sex differences in CVD physiology are pervasive differences in gene expression between males and females. Analysis of transcriptomic datasets in humans has revealed widespread sex differences in gene expression across tissues, including tissues with direct involvement in cardiovascular homeostasis.40–45 Critical in the interpretation of these data is a correction for sex differences in tissue cell-type abundance, which has been done in some studies but not in many. One such analysis of sex differences in gene expression across 44 human tissues that adjusted for cell-type composition revealed that >37% of the entire human transcriptome (including >35,000 transcripts for protein coding, long intergenic noncoding RNA and other transcripts across all autosomes and the X chromosome) is differentially expressed in males and females in at least one tissue.40 96% of the sex-biased transcripts are encoded on autosomes, and only 4% are from the X chromosome. Only 30 genes exhibit sex-biased expression across all 44 tissues, 22 of which are genes that escape X chromosome inactivation (described below). The sex-biased genes are enriched for proteins that are involved in deposition of epigenetic marks (such as histone modifications) and for genes that are adjacent to a subset of transcription recognition sequences, including hormone-related transcription factors.
Genome-wide transcriptome analyses described above implicate roles for hormones and sex-biased epigenetic mechanisms as determinants of sex differences in gene expression. One key sex determinant of epigenetic regulation is sex chromosome complement. The mammalian X chromosome harbors approximately 800 protein coding genes and dozens of non-coding RNAs, which have roles in many physiological processes ranging from immunity to brain development. The Y chromosome is much smaller and the male-specific region of Y harbors approximately 50 protein coding genes (the majority have roles in male reproduction), and contains a large amount of repetitive sequence that specifies few functional proteins or RNAs. To balance the dosage of X chromosome genes between XX and XY cells, during development XX mammalian cells undergo transcriptional inactivation of one X chromosome (specifically, genes lying outside of the pseudoautosomal region that also occurs on the Y chromosome). The choice of which X becomes inactivated in a given cell is random and typically leads to inactivation of each X in about 50% of the cells in the body (although this ratio may be skewed in cases of specific X chromosome mutations). Specific genes on the X chromosome, however, remain transcriptionally active from both copies in XX cells (Fig. 1A). The genes that escape X inactivation in mouse XX cells also escape inactivation in human cells. Human cells have additional genes that escape X inactivation, with evidence that at least 20% of X chromosome genes escape inactivation, although the levels of expression and tissue distribution varies.46 The “X escape genes” are expressed at higher levels in cells from XX compared to XY individuals, and therefore may contribute to sex differences. Particularly noteworthy are X escape genes that exert effects on gene expression across the genome and likely contribute to sex-biased gene expression levels. These include two histone demethylase enzymes, KDM5C and KDM6A, that modify methyl marks on histone tails and have the potential to regulate thousands of genes in a sex-biased manner (Fig. 1B). Additional X escape genes also have roles in gene expression, including RNA helicases and translation initiation factors.
Figure 1. Contributing mechanisms for sex differences in gene expression.
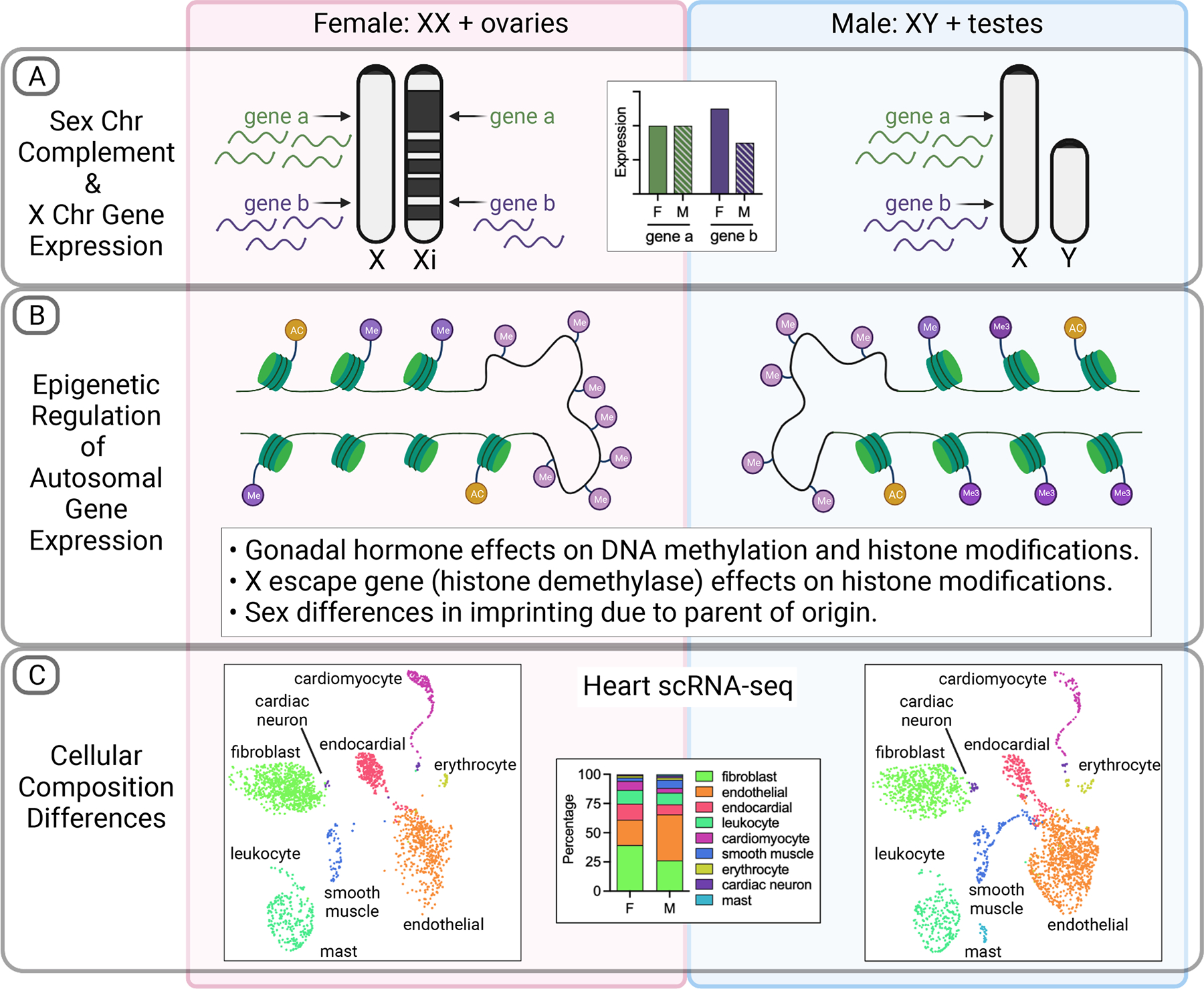
(A) The presence of two vs. one X chromosome in XX compared to XY cells leads to differential gene expression. While most genes on one X chromosome in XX cells become transcriptionally inactivated to equalize gene dosage between the sexes, specific genes consistently escape inactivation leading to higher expression levels in XX female (F) compared to XY male (M) cells. (B) Sex differences in genome-wide epigenetic modifications contribute to sex differences in gene expression. These include differences in the positions and prevalence of DNA methylation (lavender circles attached to DNA) and in modification of histones (green discs) by methylation (dark purple circles) and acetylation (yellow circles). Sex differences also occur in parent-of-origin imprinting (not illustrated here). (C) Sex differences in tissue gene expression result from differential cellular composition. This is evident from single-cell mRNA sequencing data from tissues such as heart. Data from adult heart from adult female (left) and male (right) mice indicate differences in proportion of cell populations such as endothelial cells (orange) and fibroblasts (bright green). Single-cell RNA-seq data from Tabula Muris Senis.99
Sex differences also occur in DNA methylation patterns, which influence transcriptional activity genome-wide (Fig. 1B).47–49 A meta-analysis of studies that investigated sex-biased DNA methylation and CVD traits identified data supporting connections between DNA methylation pattern and sex differences in blood lipid levels, stroke risk, and other cardiometabolic traits, and identified 31 genes with sex-biased DNA methylation and relationships to cardiometabolic disease.50 A better understanding of the mechanisms that promote sex differences in DNA methylation and the corresponding effects on gene expression and CVD is warranted.
Recent systems biology approaches have demonstrated differences in co-expression gene networks that allow the identification of genes that drive sex-biased gene expression.43,51 In a study that evaluated sex differences in gene expression across 29 human tissues, estrogen receptors and androgen receptor were not the top transcription factors driving the sex-biased gene expression in any tissue analyzed.41 One approach to interrogate transcriptomic sex differences is to identify intrinsic sex differences present at birth verses acquired sex differences later in life that are impacted by hormones and environmental factors. One study utilized umbilical vein endothelial cells from female/male twins that shared the same microenvironment (birth mother) and aortic endothelial cells from male and female adults to identify sex-dependent endothelial gene expression profiles at birth (intrinsic) and adulthood (acquired).52 Of the intrinsic sex differences identified at birth and maintained into adulthood, 87% of the differentially expressed genes were autosomal genes. For the acquired sex differences identified in adulthood, which were not present at birth, beta-estradiol and MYC were identified as upstream regulators for genes higher in females and higher in males, respectively. Differences in intrinsic and acquired sex-biased gene expression may reflect different sex factors and impact on regulating gene expression.
As described above, there are many levels at which sex components may influence gene expression and corresponding traits. The advent of single-cell RNA sequencing has made it possible to view aspects of cardiovascular development and disease at single-cell resolution. Single-cell RNA sequencing has been applied to both mouse and human cardiovascular tissues, including heart (fetal and adult), and vessels with atherosclerotic disease (reviewed in53–56). Exciting findings include the identification of previously undescribed cell populations that are involved in vascular remodeling and repair, and characterization of cellular subpopulations that distinguish healthy and pathological cardiovascular tissues. Single-cell RNA sequencing holds great promise to help elucidate sex differences in CVD by defining the effects of sex on the cell composition of healthy tissue (Fig. 1C) and sex-specific alterations that occur during progression of CVD.
Mouse models to elucidate mechanisms underlying sex differences
Mouse models have been valuable to elucidate cellular and molecular mechanisms of CVD pathogenesis. Mouse models allow the use of replicable genotypes and targeted manipulation of specific genes to evaluate their roles in cardiovascular physiology, which cannot be performed in humans. Despite the contributions of mouse models to understanding many aspects of CVD, there is underrepresentation of studies that allow direct comparisons between males and females within the same study such that biological and procedural variables are identical for both.57,58 Furthermore, studies of single sex or both sexes in different laboratories are difficult to compare due to differences in genetic background, age of mice, experimental diet, and experimental approach to quantify and characterize disease burden (reviewed in32). Thus, at present, only generalized conclusions can be made concerning sex differences in mouse atherosclerosis; beyond atherosclerosis research, the inclusion of both male and female mice within CVD studies is severely underrepresented making it difficult to draw conclusions.58
Studies of sex differences in both humans and traditional mouse models have limitations in the elucidation of how specific sex components contribute to disease processes. To allow mechanistic studies of biological sex components, mouse models that decouple the genetic and hormonal aspects of biological sex are valuable. Here we first describe mouse models for interrogation of the roles of chromosomal and gonadal sex. We then present a scheme that outlines the experimental application of these models. Finally, we describe findings using these models for studies relevant to CVD.
The most widely used model to assess the relative contributions of sex chromosomes and gonads to a trait of interest is the Four Core Genotypes (FCG) mouse model. In this model, the testis-determining gene, Sry, is dissociated from its normal position on the Y chromosome to an autosome (Chromosome 3) (Fig. 2A).59,60 The Y chromosome with deletion of the Sry gene is denoted Y−. The FCG model allows the generation of mice on an inbred C57BL/6 genetic background with four sex combinations that are equivalent to XX with ovaries, XX with testes, XY with ovaries, and XY with testes. Importantly, the presence of the Sry gene on XX and XY− genetic backgrounds leads to similar androgen levels and development of testosterone-sensitive traits in prenatal and adult animals, although the XX Sry animals cannot reproduce due to impaired spermatogenesis.59,61 A comparison of the four genotypes in a 2×2 design for a trait of interest reveals the contributions of XX vs. XY chromosomes, and of ovaries vs. testes (Fig. 2A, right panel). The data obtained with the four genotypes are best analyzed by two-way analysis of variance (ANOVA) using sex chromosome complement and gonad type as variables. An advantage of this analysis is that the combination of two genotypes for each group comparison increases the power to detect statistically significant differences. This analysis also allows the detection of interactions between genetic and gonadal sex. An interaction is apparent when there is a significant effect of gonad or chromosome type only in a specific context. For example, if a difference between mice with ovaries or testes occurs on an XX background but not an XY background, this would indicate an interaction between gonadal and chromosome type. Studies in FCG mice with n=6–8 mice per genotype allow group sizes of 12–16 for the 2-way ANOVA analysis, and have been successful in detecting chromosomal and gonadal effects, as well as interactions, for numerous metabolic traits, although this depends on the effect size.62–67
Figure 2. Chromosome composition and experimental comparisons of Four Core Genotypes (FCG) and XY* mouse models.
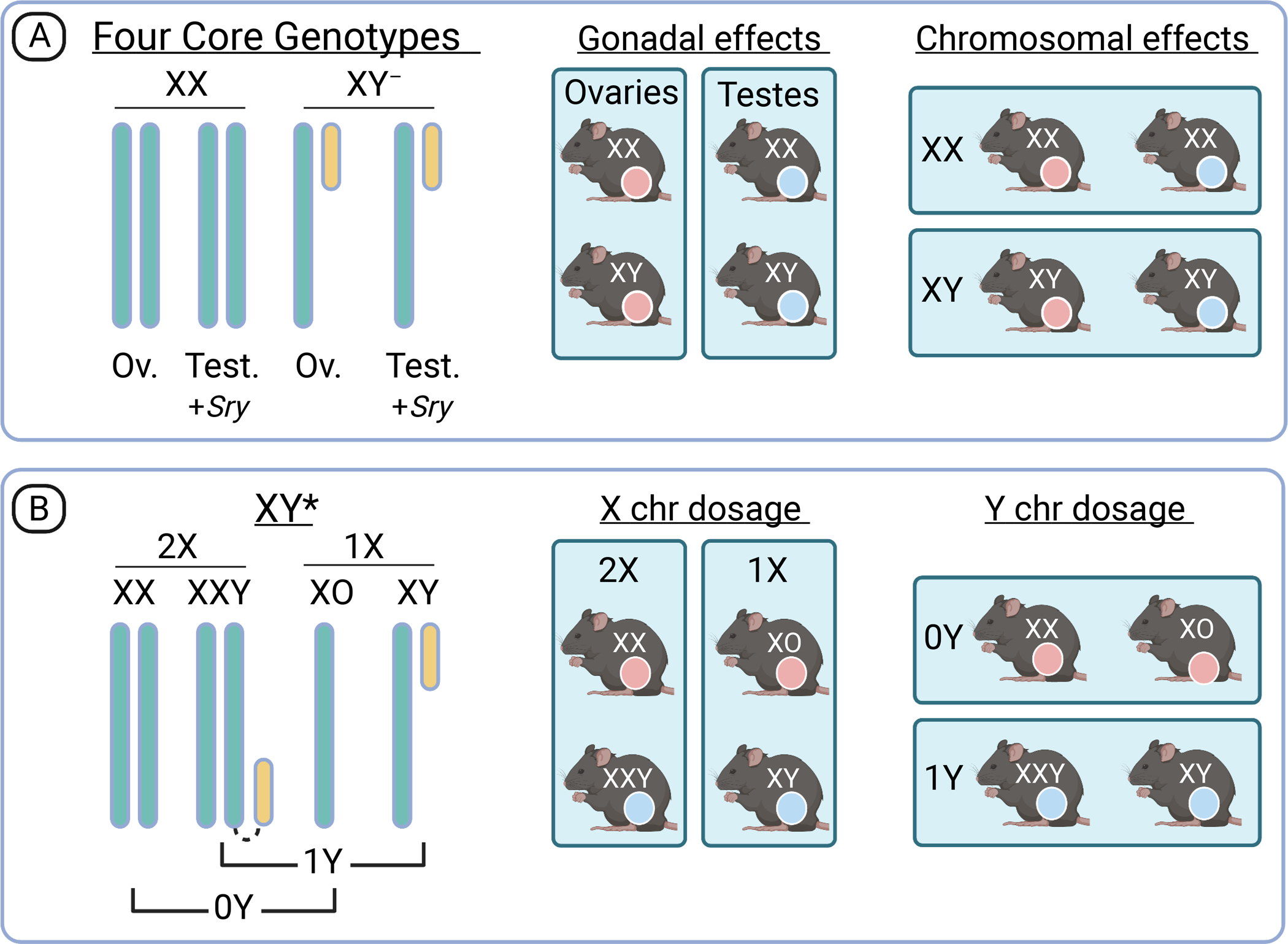
(A) FCG mice allow interrogation of the effects of ovaries and testes independently from the effects of sex chromosomes through dissociation of the Sry gene from the Y chromosome (denoted Y‒ chromosome) and migration of Sry to an autosome. There is independent segregation of gonadal type (ovaries denoted in pink and testes in blue) and chromosomal type (XX or XY). Comparison of the FCG in a 2×2 matrix allows detection of gonadal and chromosomal effects. (B) The XY* model is valuable after identification of a sex chromosome effect on a trait of interest. It allows the discrimination of X and Y chromosome dosage as a determinant, which is valuable in further investigation of the genes and pathways that determine a sex difference. Analysis of FCG and XY* models is best performed as a 2×2 comparison as shown. See text for additional details.
If sex chromosome complement influences a trait of interest, it is valuable to determine whether the mechanism results from differences in the number of X chromosomes (two in females vs. one in males) or the Y chromosome (absent in females and present in males). The genetic mechanism underlying a sex chromosome effect can be defined using the XY* model, which delineates the effects of X and Y chromosome copy number (Fig. 2B). The C57BL/6 XY* model includes four genotypes that are nearly equivalent to XX, XY, XXY, and XO.59 The Y* chromosome carries a duplication in the pseudoautosomal region (the region of homology between X and Y chromosomes that allows them to pair during meiosis). This allows unusual pairing of the X and Y chromosomes to generate the XXY genotype (which includes a hybrid chromosome with an entire X and Y chromosome) and XO genotypes (see reference for additional detail on the Y* chromosome)59. A 2×2 comparison of the four XY* genotypes reveals whether a trait is determined by presence of one or two X chromosomes, or by the presence/absence of a Y chromosome (Fig. 2B, right panel).
An additional consideration when assessing the sex components is the role of gonadal hormones during development (known as organizational effects) vs. their acute effects in adults (known as activational effects).68 As described above, it is well appreciated that the activational effects of circulating estrogen contributes to sex differences in many aspects of CVD. It is possible to study the activational effects of gonadal hormones by allowing mice to develop to adults (>8 weeks of age), surgically remove the gonads, and after a recovery period (~4 weeks), analyze the phenotype compared to sham operated mice that have gone through all procedures except gonad removal. This type of experiment may be performed in standard male and female mice, and also in FCG and XY* mice, if appropriate for the question that is being addressed.
Application of mouse models to identify biological sex determinants of a trait of interest
A schema to systematically investigate mechanisms underlying sex differences in a trait of interest is presented in Fig. 3. A good starting point is to assess the trait in FCG mice to identify chromosomal and/or gonadal contributions. This model is valuable even if prior studies have shown that a trait is influenced by gonadal hormones, as there also may be chromosomal effects that cannot be detected using standard males and females. Two of the possible outcomes are depicted. If the trait is similar between XX mice with ovaries and testes, which differ from XY mice with ovaries and testes, it suggests that the trait is influenced by sex chromosome complement (Fig. 3A). On the other hand, if the trait appears similar between XX and XY mice with ovaries, and these differ from XX and XY mice with testes, it suggests that gonadal sex is a key determinant (Fig. 3B). Traits may also exhibit an interaction between the sex chromosome and gonad type (not shown).
Figure 3. Application of mouse models to identify the biological sex components for a trait of interest.
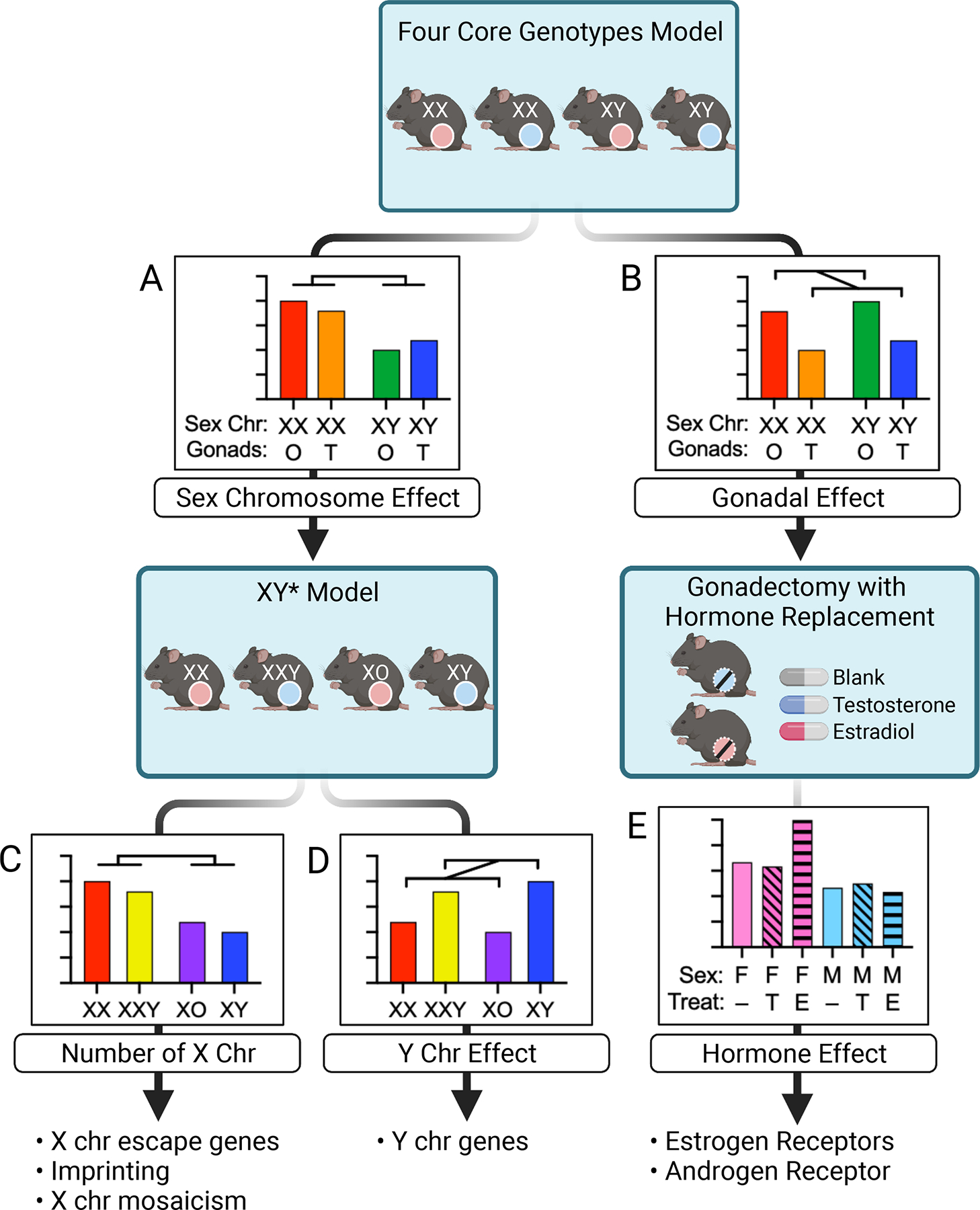
Phenotypic analysis in the Four Core Genotypes model may reveal an effect of sex chromosome complement (XX vs. XY) (A), gonad type (ovaries denoted by pink circles and testes by blue circles) (B), or a combination or interaction between the two (not shown). If sex chromosomes are implicated, subsequent studies in the XY* model differentiates the role of X chromosome number (C) from Y chromosome number (D). Follow-up studies may focus on X or Y chromosome genes. If data from FCG mice indicates a gonadal effect, subsequent gonadectomy and hormone replacement will confirm the gonadal effect and further delineate the role of hormones such as estradiol and testosterone (E). Follow-up studies may assess hormone receptor action.
If a sex chromosome effect is uncovered with FCG mice (Fig. 3A), it is valuable to determine whether the effect is dictated by X- or Y-chromosome copy number using the XY* model. Two possible outcomes of XY* analysis are depicted. If the trait of interest is determined by X chromosome number, mice with two X chromosomes (XX and XXY) will differ from those with one X chromosome (XY and XO) (Fig. 3C). If the trait is determined by the presence or absence of the Y chromosome, mice that lack a Y chromosome (XX and XO) will differ from mice that have a Y (XY and XXY) (Fig. 3D).
The distinction between an effect of X or Y chromosome dosage is significant because the two results suggest different mechanisms, and may even provide gene candidates to test for conferring the observed sex difference. If X chromosome dosage is implicated, likely mechanisms include roles for specific genes on the X chromosome that escape X chromosome inactivation, or differential imprinting of genes on the X chromosomes derived from the mother and father. If Y chromosome dosage is implicated, specific Y chromosome genes that have homologs on the X chromosome but have diverged in sequence (and possibly function) are likely candidates. In a subsequent section, we provide examples of cardiometabolic traits determined by these special classes of X- and Y-chromosome genes.
If FCG experiments suggest a primary effect of gonadal type (Fig. 3B), it is of interest to further investigate the role of gonadal hormones (typically estradiol and testosterone) in conferring the difference between males and females. A first step might be to test for a role of gonadal hormones acting in adult mice by gonadectomy followed by hormone replacement. This can be performed by subcutaneous placement of silastic tubes or hormone-containing pellets to deliver estradiol or testosterone over the course of several weeks; delivery of vehicle without hormone acts as a negative control.69,70 These experiments may also include sham-operated mice that maintain intact gonads to provide the baseline level for the trait. If removal of gonads abolishes the sex difference, and reconstitution with estradiol or testosterone reconstitutes the sex difference, it is good evidence that the relevant gonadal hormone is a determinant (Fig. 3E). Note that hormone action may differ on female and male (or XX and XY) backgrounds. Subsequent experiments might include assessing the role of relevant estrogen or androgen receptors by chemical or genetic manipulation. For example, gonadectomized mice could be administered commercially-available selective agonists for estrogen receptors ERα, ERβ, or the G-coupled estrogen receptor, or the androgen receptor.71 Additionally, the role of specific hormone receptors in mediating sex differences can be assessed in mouse models with knockout or floxed alleles for estrogen and androgen receptors (commercially available through suppliers such as The Jackson Laboratory).
Insights from mouse models regarding roles of chromosomal and gonadal sex in cardiovascular disease and risk factors
A goal of elucidating sex differences is to enhance our understanding of mechanisms that occur in one sex that are beneficial and which, ultimately, could be applied to disease prevention and treatment in both sexes. Here we describe examples of findings from approaches described in Fig. 3, and summarize key findings in Table 2.
Table 2:
Key Findings of Mouse Studies Evaluating Chromosomal and/or Gonadal Sex in CVD
| Mechanisms of Disease | Mouse Models (treatment) | Gonadal Status | Sex Factors Contributing to Disease Increase | References |
|---|---|---|---|---|
| Plasma lipid levels | C57BL/6 FCG (chow & atherogenic diets) | GDX | Elevated cholesterol levels: Two X chromosomes Ovarian hormones Elevated triglyceride levels: Testicular hormones Sex chromosome complement |
67 |
|
Ldlr−/−; FCG (western diet) |
Intact & GDX | 64 | ||
| Apoe−/−; FCG | GDX | 64 | ||
| Atherosclerosis | C57BL/6 FCG (atherogenic diet) | GDX | Two X chromosomes Ovarian hormones |
64 |
| Ldlr−/−; FCG (western diet) | Intact & GDX | 64 | ||
| Apoe−/−; FCG | GDX | 64 | ||
| Aneurysms & Aortic Dissections | Apoe−/− (angiotensin II) | Intact & GDX | Testicular hormones Single X chromosome |
72 |
| Apoe−/− (testosterone ± angiotension II) | Intact | 73 | ||
| Ldlr−/− (testosterone ± angiotension II) | Intact | 73 | ||
| C57BL/6 FCG (angiotensin II) | Intact & GDX | 65 | ||
| C57BL/6 XY* (angiotension II) | Intact | 74 | ||
| Ldlr−/−; XY* (angiotension II) | Intact & GDX | 74 | ||
| Myocardial ischemia/reperfusion injury | C57BL/6 FCG (I/R injury) | GDX | Two X chromosomes | 66 |
| C57BL/6 XY* (I/R injury) | GDX | 66 | ||
| Adiposity | C57BL/6 FCG (chow & high-fat diet) | GDX | Two X chromosome Higher Kdm5c gene dosage |
62 |
| C57BL/6 XY* | GDX | 62 | ||
| C57BL/6 FCG (chow & high-fat diet) | Intact | 63 | ||
| Kdm5c+/− (chow & high-fat diet) | Intact | 63 | ||
| Pulmonary arterial hypertension | C57BL/6 FCG (hypoxia) | GDX | Absence of Y chromosome | 75 |
| C57BL/6 XY* (hypoxia) | GDX | 75 |
Sex determinants of plasma lipid levels.
Sex differences in susceptibility to hyperlipidemia and atherosclerosis are well-established, and clinical studies have primarily assessed the role of estrogen. The investigation of sex determinants of plasma lipid levels with the FCG model have revealed a complex interplay between hormonal and chromosomal regulation. In mice fed a chow diet, the majority of circulating lipoproteins in the fasted state are classified as high-density lipoproteins (HDL), which differs from the more prevalent low-density lipoproteins (LDL) in fasted humans. Therefore, studies have been performed in mice fed diets, or undergoing genetic manipulations, that lead to elevated LDL to HDL ratios in the mouse to more closely mimic humans. When lipid levels were assessed in C57BL/6 FCG mice fed either chow or a cholesterol-enriched atherogenic diet, XX chromosome complement led to increased HDL cholesterol levels over those in XY mice, regardless of gonad type.76 The effects of XX chromosome complement on HDL cholesterol levels was evident in both gonadally intact and gonadectomized mice; intact animals fed an atherogenic diet also displayed higher HDL cholesterol levels in animals with ovaries compared to those with testes. In mice made hyperlipidemic by LDL receptor deficiency plus Western diet feeding, or by apolipoprotein E (apoE) deficiency, XX mice had elevated total cholesterol levels compared to XY mice.64
Studies in FCG mice have demonstrated that plasma triglyceride levels are regulated independently from cholesterol levels. The presence of male compared to female gonads led to two-fold higher triglyceride levels, and this sex difference was abolished by gonadectomy.76 The sex regulation of triglyceride levels was more complex when mice were made hyperlipidemic by diet or genetic deficiency. In FCG mice fed an atherogenic diet, triglyceride levels were higher in XY compared to XX mice, but on an LDL receptor-deficient background, XX mice had higher triglyceride levels than XY mice.64,76 Overall, studies of plasma lipids in FCG mice document that sex-biased regulation is complex, but some key patterns emerge. Sex chromosome complement influences total and HDL cholesterol levels in both gonadally intact and gonadectomized animals, although some differences occur depending on diet and genetic background. By contrast, gonadal sex is a key determinant of triglyceride levels, and this effect may be abolished in hypogonadal states, such as occur in both women and men at advanced ages.
Sex determinants of atherosclerosis.
Studies of atherosclerosis susceptibility in FCG mice made hyperlipidemic by diet, LDL receptor deficiency, or apoE deficiency revealed important effects of sex chromosome complement.64 Consistently in each of these three models (which include distinct genetic backgrounds and dietary treatments), XX animals had greater atherosclerotic lesion areas in the aortic sinus than XY animals. Furthermore, there were no differences in lesion areas in animals with male compared to female gonads. In animals that were assessed for both serum lipid levels and aortic lesions, XX animals exhibited both elevated cholesterol levels (total, LDL, and VLDL cholesterol levels) and increased lesion scores.64 When lesions were assessed in the aortic arch rather than the aortic sinus, interactions between gonadal and chromosomal sex were observed. For example, in mice with female gonads, XX animals had more lesions than XY animals, whereas in mice with male gonads, XX and XY mice had similar lesions. Mice with ovaries that underwent gonadectomy experienced increased lesion area if they had XX, but not XY, chromosomes; by contrast, mice with testes that underwent gonadectomy experienced reduced lesion area if they had XY, but not XX, chromosomes. This suggests interaction of ovarian hormones with XX genotype and interaction of testicular hormones with XY genotype.
It is appreciated that atherosclerosis occurs differently in mouse models and humans, where timespan and other environmental and physiological differences are substantial. Nevertheless, studies described above point to important considerations in understanding sex effects in CVD. These include the recognition that sex chromosomes may play an important role, and this may be context-dependent in terms of the type of gonadal hormones present and also the specific disease trait assessed (e.g., lesions in aortic sinus and aortic arch).
Sex determinants of aortopathies.
Aortopathies such as aortic dissections and aneurysms exhibit sexual dimorphism in clinical presentation and incidence rate, with higher rates of abdominal aneurysms in men compared to women.15–18 Mouse studies have demonstrated that both gonadal hormones and sex chromosome complement contribute to the manifestation and characterization of these conditions. Testosterone promotes aneurysm development as gonadectomy of male mice reduces angiotensin II (Ang-II)-induced aneurysms, and testosterone treatment of female mice increases Ang-II-induced aneurysms.72,73 However, the sex chromosome complement is an additional determinant of Ang-II-induced aortopathies. In FCG mice, XY mice exhibit more diffuse aortic aneurysms than XX mice, regardless of gonadal hormones.65 Studies with the XY* mouse model determined that reduced X chromosome copy number present in males compared to females, rather than the presence of the Y chromosome, contributes to increased abdominal aortic aneurysms in male mice. LDL receptor-deficient mice with one X chromosome (XO) had higher incidence of aneurysms than XX mice in both the descending and abdominal aorta.74 The aneurysm incidence for XO mice was either similar to or higher than XY mice, and gonadectomy of XO mice had no impact on the incidence rate. This is consistent with the ~6-fold higher rate of aortic dissections that occur in Turner syndrome women (XO) compared to XX women in the general population.77,78 These data raise the possibility that X chromosome escape genes, which are expressed at higher levels in XX compared to XY or XO individuals, may play a protective role in aortopathies.
Sex determinants of myocardial ischemia/reperfusion injury.
Women have lower rates of ischemia/reperfusion injury than age-matched men, but incidence in women increases after menopause.23,24 FCG and XY* mouse models were utilized in myocardial ischaemia and reperfusion injury studies to evaluate the contribution of sex chromosome complement and gonadal hormones. These studies demonstrated that the presence of two compared to one X chromosome, regardless of the presence of a Y chromosome, resulted in larger infarct size and lower recovery after reperfusion.66 However, the molecular mechanism by which XX genotype promotes increased infarct size after ischemia/reperfusion, and resultant effect on heart function, is still unclear.
Identification of genes on sex chromosomes that influence cardiometabolic traits
We now illustrate how findings from FCG and XY* models can lead to the discovery of specific genes that influence cardiometabolic traits. We describe the identification of an X-chromosome gene that influences sex differences in adipose tissue development and diet-induced obesity, and identification of the Y chromosome as a protective factor in pulmonary hypertension.
Identification of a sex-biased adiposity gene.
Increased adiposity is a risk factor for CVD. Men and women differ in fat distribution and in the properties of fat cells within specific anatomical depots.79,80 In general, men have more visceral adipose tissue than women, whereas women store more subcutaneous fat. Menopause tends to increase visceral fat storage in women, which has led to many investigations concerning the role of estrogen in fat storage. However, as with many cardiometabolic traits, gonadal sex is not the sole determinant of adipose tissue amount. Studies in FCG and XY* mice revealed that the X chromosome dosage is an important determinant of fat storage in two conditions that are associated with increased risk for CVD—a high-fat diet or reduced gonadal hormone levels (Fig. 4A). Thus, in FCG mice that were fed a diet rich in fat and carbohydrates, XX mice gained weight and fat tissue more rapidly than XY mice, leading to greater increases in body fat percentage.63 Similarly, removal of gonads from adult mice also led to enhanced weight and fat gain in XX compared to XY animals.62 In both conditions, enhanced weight gain was associated with increased food intake specifically during the inactive period of the circadian cycle, which is akin to increased nighttime snacking in humans.62,63,81
Figure 4. Identification of an X chromosome escape gene that promotes adiposity.
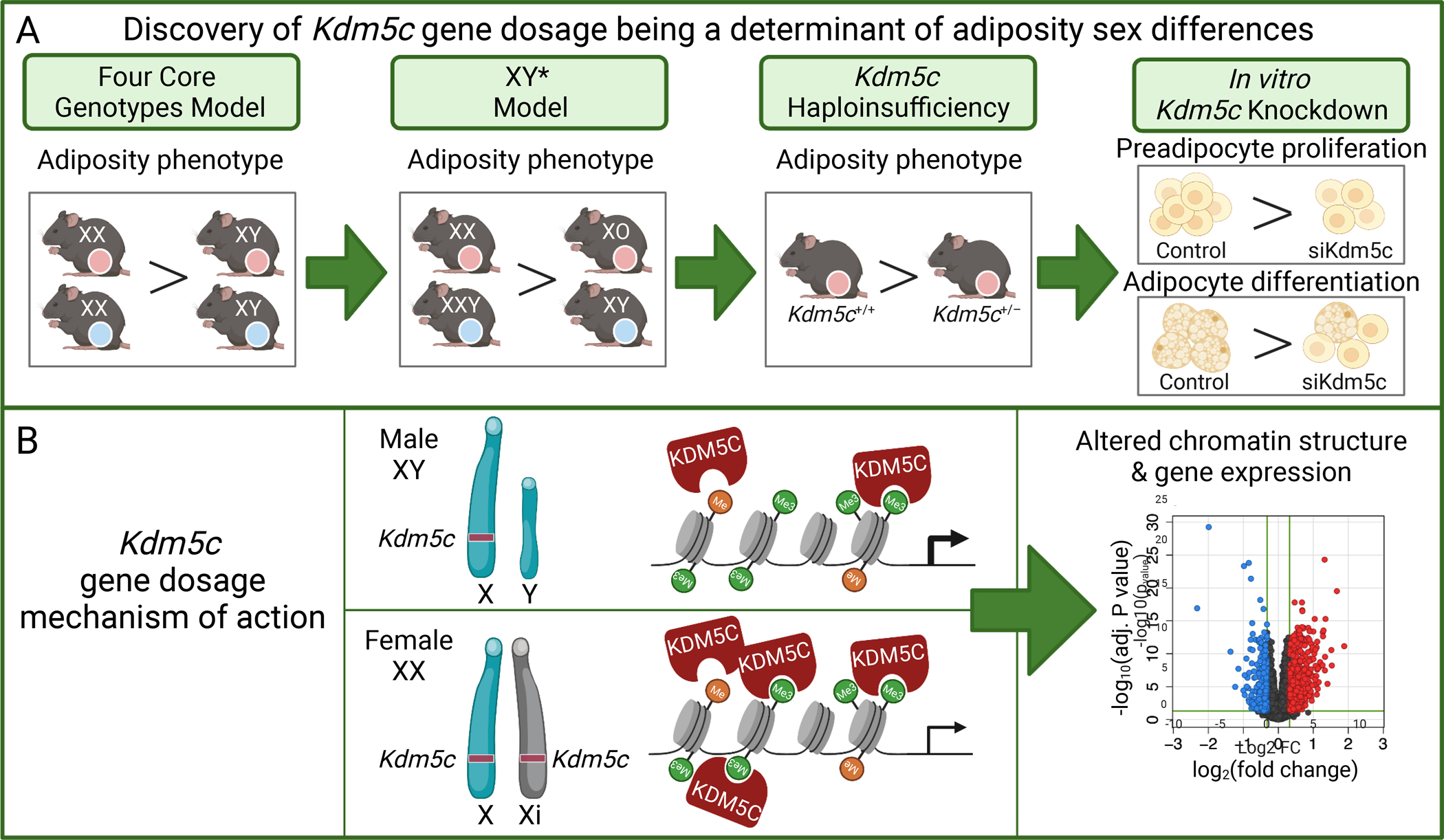
(A) Four Core Genotypes mice show greater adiposity in XX compared to XY mice in hypogonadal or high-fat diet conditions. XY* mice show greater adiposity in genotypes with two X chromosomes compared to those with one X chromosome, regardless of Y chromosome presence. This led to the hypothesis that an X chromosome escape gene may underlie this difference. Reduction of Kdm5c gene dosage in female mice to one copy present in male mice reduced adiposity. Reduced Kdm5c expression levels in cultured preadipocytes led to reduced adipocyte proliferation and differentiation in vitro. (B) Kdm5c encodes a histone demethylase that is expressed at higher levels in female XX cells compared to male XY cells due to escape from X inactivation. Modulation of Kdm5c expression in cultured preadipocytes leads to altered chromatin structure and gene expression levels, which contribute to the sex differences in adiposity. See text for additional details.
Studies with the XY* model revealed that presence of two X chromosomes promotes fat accrual, whereas Y chromosome dosage does not have an impact.62 This led to the hypothesis that increased levels of one or more genes that escape X chromosome inactivation may promote adiposity. One of the X chromosome genes that robustly escapes X inactivation in metabolic tissues in mice and humans is Kdm5c/KDM5C.62,63 Kdm5c encodes a histone 3 lysine 4 (H3K4) demethylase that modulates genome-wide chromatin structure. Previous work had demonstrated that H3K4 demethylases influence adipocyte differentiation,82 and Kdm5c/KDM5C was therefore tested as a contributor to X chromosome effects on adiposity. Altering the Kdm5c gene dosage in XX female mice to that normally found in XY male mice recapitulated the effects of X chromosome dosage on adiposity—female mice with two copies of Kdm5c gained more weight and fat mass than female mice with a single copy of Kdm5c (Kdm5c+/−) (Fig. 4A).63 Consistent with a role in genome-wide regulation of chromatin structure, modulation of Kdm5c gene dosage altered chromatin accessibility in cultured adipocytes, and altered expression levels of hundreds of autosomal genes in both mouse adipose tissue and in cultured adipocytes (Fig. 4B). Additionally, genetic polymorphisms within the KDM5C gene and KDM5C expression levels correlated with human adiposity.63 Recent studies have identified an additional X escape gene that encodes a histone demethylase, the KDM6A H3K27 demethylase, as a determinant of sex differences in mouse models of autoimmunity and Alzheimer’s disease.83,84
Protection from pulmonary arterial hypertension by Y chromosome genes.
Pulmonary arterial hypertension is characterized by increased blood pressure in the pulmonary artery, leads to right heart failure, and occurs more frequently in females than males.85 Due to the sex bias in disease prevalence, the role of estrogen has been extensively studied; however, both deleterious and advantageous effects of estrogens have been observed in pulmonary arterial hypertension in animal models and human subjects.86,87 Studies in the FCG and XY* models revealed a protective role of the Y chromosome in hypoxia-induced pulmonary arterial hypertension. In gonadectomized FCG mice, XX animals exhibited more severe pulmonary arterial hypertension than XY mice characterized by increased right ventricular systolic pressure and vascular remodeling in XX mice; this was true irrespective of the original gonad type.75 In XY* mice, hypoxia-induced pulmonary arterial hypertension was more severe in genotypes lacking a Y chromosome (XX and XO) compared to those with a Y chromosome (XY and XXY).75 An investigation of Y chromosome genes may facilitate the identification of mechanisms that protect against disease development in hypoxic conditions.
Sex-specific genetic loci for cardiometabolic traits
Genome-wide association studies (GWAS) are a powerful tool to identify specific genetic loci that influence a particular trait. With the advent of economical means to genotype millions of loci across the genome, GWAS has become a staple of geneticists to study complex traits, including CVD and associated risk factors. However, the majority of these studies have not performed stratified analyses for males and females separately, and even fewer have included an analysis of the X chromosome, which comprises 5% of the genome and carries more genetic material than half of the individual autosomes. One deterrent to sex stratification in GWAS studies is the necessity for very large sample sizes to achieve adequate power to identify sex-specific effects. The primary reason cited for not including X chromosome information in GWAS analyses is the fact that special computational considerations are required due to the presence of two alleles at each X locus in females and one allele in males (which requires adjustments in genotype calling), as well as X chromosome inactivation.88,89 Regardless of these issues, methods to account for the nuances of the X chromosome and its inclusion in GWAS analyses have been available for several years.90–93 GWAS that have integrated X chromosome analysis have identified X chromosome loci for numerous traits, including cardiovascular, lipid and inflammatory traits.89 However, a meta-analysis of CAD and X chromosome polymorphisms did not detect association between CAD and X chromosome variants.94 Although this meta-analysis was large, women were still underrepresented compared to men, and the criteria for CAD affected status among the studies in the meta-analysis were heterogeneous. It is important to note that the effects of sex chromosome dosage on CVD traits may not necessarily be revealed in studies of genetic polymorphism. To better assess how X chromosome genes contribute to sex differences in CVD and its risk factors, it is hoped that the practice of including the X chromosome in GWAS analyses will be widely adopted in the future.
Beyond assessing the contribution of the sex chromosomes, stratification of GWAS results by sex is a valuable approach and has revealed differential genetic architecture between males and females for several complex traits, including visceral adiposity, a risk factor for CVD. GWAS for visceral adiposity (assessed as waist-to-hip ratio adjusted for body mass index) identified 44 autosomal genetic loci with significant sex-specific effects.95 Some loci associated with visceral adiposity were female-specific, some were male-specific, and some loci were identified in both males and females but with opposite effects on waist-to-hip ratio.
Mouse models provide a useful adjunct to sex-stratified human GWAS. One approach is to study traits in male and female mice comprising a panel of more than 100 inbred mouse strains (the Hybrid Mouse Diversity Panel, HMDP).96,97 The HMDP strains are valuable for comparison of sex-specific genetic architecture because they provide an opportunity to perform analyses in males and females on the same genetic backgrounds; this would only be possible in humans with male/female twin pairs. Mouse GWAS studies also eliminate additional confounders that are present in human studies such as participation bias, gender effects, hormonal status, and environmental effects.
A study of sex-specific effects can be performed in the HMDP by assessing traits in males and females of the ~100 strains of inbred mice and performing genome-wide association analysis on each sex separately. A study of male and female HMDP cohorts demonstrated that the two sexes exhibit distinct genetic architecture for loci that influence body fat percent, subcutaneous fat content, hepatic cholesterol levels, and additional cardiometabolic traits.98 Further analysis of a locus that promotes increased body fat percent exclusively in females identified specific alleles of the Lyplal1 gene that provide protection against fat mass gain in response to diet in a female-specific manner. This approach can be applied to numerous phenotypes and provides the opportunity to generate a detailed catalog of male- and female-specific genetic determinants of metabolism.
Summary and prospects for the future
It is increasingly recognized that sex influences the expression of risk factors for cardiovascular disease development as well as CVD pathologies. It is necessary to understand these sex differences at both the clinical and basic biological levels in order to provide optimal preventive and therapeutic care for all individuals. As we have outlined, mouse models that permit the assessment of contributions by individual components of biological sex provide unique insights that focus attention on previously unexplored genes and mechanisms. In particular, effects of sex chromosome complement on plasma lipid levels, adiposity, and atherosclerosis suggest that mechanisms beyond gonadal hormone levels influence CVD risk. We recognize that there are challenges in the translation of findings from preclinical models to clinical practice. Nevertheless, mice and humans exhibit strong similarities in the fundamental determinants of sex: the mouse and human X and Y chromosomes are highly evolutionarily conserved and promote similar gonadal developmental processes; human and mouse X chromosome inactivation occurs via the same mechanism; genes that escape X chromosome inactivation in the mouse also escape in humans (additional genes also escape in humans, underscoring the importance of understanding X chromosome dosage in human disease); and the effects of XX gene dosage are amplified in the mouse by high-fat diet feeding and by hypogonadal state, both of which are associated with increased CVD in humans. Increased knowledge of fundamental mechanisms by which sex influences biology are a first step toward facilitating precision approaches to combat CVD. Findings available at present suggest that sex chromosome effects may be particularly relevant in assessing the risk for CVD in individuals during aging (as gonadal hormone levels wane), and in transgender individuals. It is clear that to enable the practice of personalized medicine, all aspects of biological sex and gender must be considered in the recommendations for maintenance of cardiovascular health and treatment of cardiovascular diseases.
Sources of Funding
This work was supported by U54 DK120342 from the National Institute of Diabetes and Digestive and Kidney Disease/Office of Research on Women’s Health (KR), The Iris Cantor-UCLA Women’s Health Center/National Center for Advancing Translational Sciences and UCLA Clinical and Translational Science Institute (KR), and American Heart Association post-doctoral fellowship 20POST35100000 (CBW).
Non-standard Abbreviations and Acronyms
- Ang-II
angiotensin II
- ANOVA
analysis of variance
- apoE
apolipoprotein E
- CAD
coronary artery disease
- CVD
cardiovascular disease
- ERa
estrogen receptor alpha
- ERb
estrogen receptor beta
- FCG
Four Core Genotypes
- GDX
gonadectomized
- GWAS
genome-wide association studies
- H3K4
histone 3 lysine 4
- HDL
high-density lipoproteins
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- HMDP
Hybrid Mouse Diversity Panel
- LDL
low-density lipoproteins
- Ldlr
low-density lipoproteins receptor gene
- NO
nitric oxide
- SCAD
spontaneous coronary artery dissection
Footnotes
Disclosures
None.
References
- 1.Nabel EG. Heart disease prevention in young women: sounding an alarm. Circulation 2015;132:989–991. doi: 10.1161/CIRCULATIONAHA.115.018352. [DOI] [PubMed] [Google Scholar]
- 2.Regitz-Zagrosek V, Kararigas G. Mechanistic pathways of sex differences in cardiovascular disease. Physiol Rev 2017;97:1–37. doi: 10.1152/physrev.00021.2015. [DOI] [PubMed] [Google Scholar]
- 3.Groban L, Lindsey SH, Wang H, Alencar AK. Sex and gender differences in cardiovascular disease. Sex Differ. Physiol, Elsevier Inc.; 2016, p. 61–87. doi: 10.1016/B978-0-12-802388-4.00005-7. [DOI] [Google Scholar]
- 4.Ji H, Kwan AC, Chen MT, Ouyang D, Ebinger JE, Bell SP, et al. Sex Differences in Myocardial and Vascular Aging. Circ Res 2022;130:566–577. doi: 10.1161/CIRCRESAHA.121.319902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freedman DS, Otvos JD, Jeyarajah EJ, Shalaurova I, Cupples LA, Parise H, et al. Sex and age differences in lipoprotein subclasses measured by nuclear magnetic resonance spectroscopy: the Framingham Study. Clin Chem 2004;50:1189–1200. doi: 10.1373/clinchem.2004.032763. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Magkos F, Mittendorfer B. Sex differences in lipid and lipoprotein metabolism: it’s not just about sex hormones. J Clin Endocrinol Metab 2011;96:885–893. doi: 10.1210/jc.2010-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold AP, Cassis LA, Eghbali M, Reue K, Sandberg K. Sex hormones and sex chromosomes cause sex differences in the development of cardiovascular diseases. Arterioscler Thromb Vasc Biol 2017;37:746–756. doi: 10.1161/ATVBAHA.116.307301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Jager SCA, Meeuwsen JAL, van Pijpen FM, Zoet GA, Barendrecht AD, Franx A, et al. Preeclampsia and coronary plaque erosion: Manifestations of endothelial dysfunction resulting in cardiovascular events in women. Eur J Pharmacol 2017;816:129–137. doi: 10.1016/j.ejphar.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Sato Y, Kawakami R, Sakamoto A, Cornelissen A, Mori M, Kawai K, et al. Sex Differences in Coronary Atherosclerosis. Curr Atheroscler Rep 2022. doi: 10.1007/s11883-022-00980-5. [DOI] [PubMed] [Google Scholar]
- 10.Lyon AR, Citro R, Schneider B, Morel O, Ghadri JR, Templin C, et al. Pathophysiology of takotsubo syndrome: JACC State-of-the-Art Review. J Am Coll Cardiol 2021;77:902–921. doi: 10.1016/j.jacc.2020.10.060. [DOI] [PubMed] [Google Scholar]
- 11.Agdamag AC, Patel H, Chandra S, Rao A, Suboc TM, Marinescu K, et al. Sex Differences in Takotsubo Syndrome: A Narrative Review. J Womens Health (Larchmt) 2020;29:1122–1130. doi: 10.1089/jwh.2019.7741. [DOI] [PubMed] [Google Scholar]
- 12.Gori T, Anadol R. Tako-Tsubo syndrome, spontaneous coronary dissection and microvascular disease: Sex-differences. Clin Hemorheol Microcirc 2018;70:375–379. doi: 10.3233/CH-189302. [DOI] [PubMed] [Google Scholar]
- 13.Hayes SN, Kim ESH, Saw J, Adlam D, Arslanian-Engoren C, Economy KE, et al. Spontaneous coronary artery dissection: current state of the science: A Scientific Statement From the American Heart Association. Circulation 2018;137:e523–e557. doi: 10.1161/CIR.0000000000000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim ESH, Saw J, Kadian-Dodov D, Wood M, Ganesh SK. FMD and SCAD: Sex-Biased Arterial Diseases With Clinical and Genetic Pleiotropy. Circ Res 2021;128:1958–1972. doi: 10.1161/CIRCRESAHA.121.318300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh K, Bønaa KH, Jacobsen BK, Bjørk L, Solberg S. Prevalence of and risk factors for abdominal aortic aneurysms in a population-based study : The Tromsø Study. Am J Epidemiol 2001;154:236–244. doi: 10.1093/aje/154.3.236. [DOI] [PubMed] [Google Scholar]
- 16.Lederle FA, Johnson GR, Wilson SE, Chute EP, Hye RJ, Makaroun MS, et al. The aneurysm detection and management study screening program: validation cohort and final results. Aneurysm Detection and Management Veterans Affairs Cooperative Study Investigators. Arch Intern Med 2000;160:1425–1430. doi: 10.1001/archinte.160.10.1425. [DOI] [PubMed] [Google Scholar]
- 17.Boese AC, Chang L, Yin K-J, Chen YE, Lee J-P, Hamblin MH. Sex differences in abdominal aortic aneurysms. Am J Physiol Heart Circ Physiol 2018;314:H1137–H1152. doi: 10.1152/ajpheart.00519.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lo RC, Bensley RP, Hamdan AD, Wyers M, Adams JE, Schermerhorn ML. Gender differences in abdominal aortic aneurysm presentation, repair, and mortality in the Vascular Study Group of New England. J Vasc Surg 2013;57:1261–1268, 1268.e1–5. doi: 10.1016/j.jvs.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Motiejunaite J, Akiyama E, Cohen-Solal A, Pietro Maggioni A, Mueller C, Choi DJ, et al. The association of long-term outcome and biological sex in patients with acute heart failure from different geographic regions. Eur Heart J 2020;41:1357–1364. doi: 10.1093/eurheartj/ehaa071. [DOI] [PubMed] [Google Scholar]
- 20.Lam CSP, Arnott C, Beale AL, Chandramouli C, Hilfiker-Kleiner D, Kaye DM, et al. Sex differences in heart failure. Eur Heart J 2019;40:3859–3868c. doi: 10.1093/eurheartj/ehz835. [DOI] [PubMed] [Google Scholar]
- 21.Pepine CJ, Merz CNB, El Hajj S, Ferdinand KC, Hamilton MA, Lindley KJ, et al. Heart failure with preserved ejection fraction: Similarities and differences between women and men. Int J Cardiol 2020;304:101–108. doi: 10.1016/j.ijcard.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Swaraj S, Kozor R, Arnott C, Di Bartolo BA, A Figtree G. Heart Failure with Reduced Ejection Fraction-Does Sex Matter? Curr Heart Fail Rep 2021;18:345–352. doi: 10.1007/s11897-021-00533-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy E, Steenbergen C. Gender-based differences in mechanisms of protection in myocardial ischemia-reperfusion injury. Cardiovasc Res 2007;75:478–486. doi: 10.1016/j.cardiores.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 24.Ostadal B, Ostadal P. Sex-based differences in cardiac ischaemic injury and protection: therapeutic implications. Br J Pharmacol 2014;171:541–554. doi: 10.1111/bph.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DesJardin JT, Chikwe J, Hahn RT, Hung JW, Delling FN. Sex Differences and Similarities in Valvular Heart Disease. Circ Res 2022;130:455–473. doi: 10.1161/CIRCRESAHA.121.319914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voisine M, Hervault M, Shen M, Boilard A-J, Filion B, Rosa M, et al. Age, Sex, and Valve Phenotype Differences in Fibro-Calcific Remodeling of Calcified Aortic Valve. J Am Heart Assoc 2020;9:e015610. doi: 10.1161/JAHA.119.015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartman RJG, Owsiany K, Ma L, Koplev S, Hao K, Slenders L, et al. Sex-stratified gene regulatory networks reveal female key driver genes of atherosclerosis involved in smooth muscle cell phenotype switching. Circulation 2021:713–726. doi: 10.1161/CIRCULATIONAHA.120.051231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schiattarella GG, Altamirano F, Tong D, French KM, Villalobos E, Kim SY, et al. Nitrosative stress drives heart failure with preserved ejection fraction. Nature 2019;568:351–356. doi: 10.1038/s41586-019-1100-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shapiro JR, Klein SL, Morgan R. Stop controlling for sex and gender in global health research. BMJ Glob Heal 2021;6:6–8. doi: 10.1136/bmjgh-2021-005714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller LR, Marks C, Becker JB, Hurn PD, Chen W-J, Woodruff T, et al. Considering sex as a biological variable in preclinical research. FASEB J 2017;31:29–34. doi: 10.1096/fj.201600781R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perrino C, Ferdinandy P, Bøtker HE, Brundel BJJM, Collins P, Davidson SM, et al. Improving translational research in sex-specific effects of comorbidities and risk factors in ischaemic heart disease and cardioprotection: Position paper and recommendations of the ESC Working Group on Cellular Biology of the Heart. Cardiovasc Res 2021;117:367–385. doi: 10.1093/cvr/cvaa155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Man JJ, Beckman JA, Jaffe IZ. Sex as a biological variable in atherosclerosis. Circ Res 2020:1297–1319. doi: 10.1161/CIRCRESAHA.120.315930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sylvester MA, Brooks HL. Sex-specific mechanisms in inflammation and hypertension. Curr Hypertens Rep 2019;21:53. doi: 10.1007/s11906-019-0959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clayton JA, Arnegard ME. Taking cardiology clinical trials to the next level: A call to action. Clin Cardiol 2018;41:179–184. doi: 10.1002/clc.22907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown HL, Warner JJ, Gianos E, Gulati M, Hill AJ, Hollier LM, et al. Promoting risk identification and reduction of cardiovascular disease in women through collaboration with obstetricians and gynecologists: A Presidential Advisory From the American Heart Association and the American College of Obstetricians and Gynecolog. Circulation 2018;137:e843–e852. doi: 10.1161/CIR.0000000000000582. [DOI] [PubMed] [Google Scholar]
- 36.Sudhir K, Komesaroff PA. Clinical review 110: Cardiovascular actions of estrogens in men. J Clin Endocrinol Metab 1999;84:3411–3415. doi: 10.1210/jcem.84.10.5954. [DOI] [PubMed] [Google Scholar]
- 37.Sesti F, Pofi R, Pozza C, Minnetti M, Gianfrilli D, Kanakis GA. Cardiovascular complications in patients with Klinefelter’s syndrome. Curr Pharm Des 2020;26:5556–5563. doi: 10.2174/1381612826666201102105408. [DOI] [PubMed] [Google Scholar]
- 38.Silberbach M, Roos-Hesselink JW, Andersen NH, Braverman AC, Brown N, Collins RT, et al. Cardiovascular health in Turner syndrome: A Scientific Statement From the American Heart Association. Circ Genomic Precis Med 2018;11:e000048. doi: 10.1161/HCG.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 39.Irwig MS. Cardiovascular health in transgender populations. Rev Endocr Metab Disord 2018;19:243–251. doi: 10.1007/s11154-018-9454-3. [DOI] [PubMed] [Google Scholar]
- 40.Oliva M, Muñoz-Aguirre M, Kim-Hellmuth S, Wucher V, Gewirtz ADH, Cotter DJ, et al. The impact of sex on gene expression across human tissues. Science 2020;369:eaba3066. doi: 10.1126/science.aba3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopes-Ramos CM, Chen C-Y, Kuijjer ML, Paulson JN, Sonawane AR, Fagny M, et al. Sex differences in gene expression and regulatory networks across twenty-nine human tissues. Cell Rep 2020;31:107795. doi: 10.1016/j.celrep.2020.107795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gershoni M, Pietrokovski S. The landscape of sex-differential transcriptome and its consequent selection in human adults. BMC Biol 2017;15:1–15. doi: 10.1186/s12915-017-0352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hartman RJG, Mokry M, Pasterkamp G, den Ruijter HM. Sex-dependent gene co-expression in the human body. Sci Rep 2021;11:1–10. doi: 10.1038/s41598-021-98059-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kassam I, Wu Y, Yang J, Visscher PM, McRae AF. Tissue-specific sex differences in human gene expression. Hum Mol Genet 2019;28:2976–2986. doi: 10.1093/hmg/ddz090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naqvi S, Godfrey AK, Hughes JF, Goodheart ML, Mitchell RN, Page DC. Conservation, acquisition, and functional impact of sex-biased gene expression in mammals. Science 2019;365:eaaw7317. doi: 10.1126/science.aaw7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tukiainen T, Villani AC, Yen A, Rivas MA, Marshall JL, Satija R, et al. Landscape of X chromosome inactivation across human tissues. Nature 2017;550:244–248. doi: 10.1038/nature24265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hall E, Volkov P, Dayeh T, Esguerra JLS, Salö S, Eliasson L, et al. Sex differences in the genome-wide DNA methylation pattern and impact on gene expression, microRNA levels and insulin secretion in human pancreatic islets. Genome Biol 2014;15:522. doi: 10.1186/s13059-014-0522-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yousefi P, Huen K, Davé V, Barcellos L, Eskenazi B, Holland N. Sex differences in DNA methylation assessed by 450 K BeadChip in newborns. BMC Genomics 2015;16:911. doi: 10.1186/s12864-015-2034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hartman RJG, Huisman SE, den Ruijter HM. Sex differences in cardiovascular epigenetics-a systematic review. Biol Sex Differ 2018;9:19. doi: 10.1186/s13293-018-0180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Asllanaj E, Zhang X, Ochoa Rosales C, Nano J, Bramer WM, Portilla-Fernandez E, et al. Sexually dimorphic DNA-methylation in cardiometabolic health: A systematic review. Maturitas 2020;135:6–26. doi: 10.1016/j.maturitas.2020.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Kurt Z, Barrere-Cain R, Laguardia J, Mehrabian M, Pan C, Hui ST, et al. Tissue-specific pathways and networks underlying sexual dimorphism in non-alcoholic fatty liver disease. Biol Sex Differ 2018;9:1–14. doi: 10.1186/s13293-018-0205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hartman RJG, Kapteijn DMC, Haitjema S, Bekker MN, Mokry M, Pasterkamp G, et al. Intrinsic transcriptomic sex differences in human endothelial cells at birth and in adults are associated with coronary artery disease targets. Sci Rep 2020;10:1–12. doi: 10.1038/s41598-020-69451-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamada S, Nomura S. Review of single-cell RNA sequencing in the heart. Int J Mol Sci 2020;21:8345. doi: 10.3390/ijms21218345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iqbal F, Lupieri A, Aikawa M, Aikawa E. Harnessing single-cell RNA sequencing to better understand how diseased cells behave the way they do in cardiovascular disease. Arterioscler Thromb Vasc Biol 2021;41:585–600. doi: 10.1161/ATVBAHA.120.314776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Depuydt MAC, Prange KHM, Slenders L, Örd T, Elbersen D, Boltjes A, et al. Microanatomy of the human atherosclerotic plaque by single-cell transcriptomics. Circ Res 2020;127:1437–1455. doi: 10.1161/CIRCRESAHA.120.316770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paik DT, Cho S, Tian L, Chang HY, Wu JC. Single-cell RNA sequencing in cardiovascular development, disease and medicine. Nat Rev Cardiol 2020;17:457–473. doi: 10.1038/s41569-020-0359-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu HS, Schmidt AM, Hegele RA, Mackman N, Rader DJ, Weber C, et al. Annual Report on Sex in Preclinical Studies: Arteriosclerosis, Thrombosis, and Vascular Biology Publications in 2018. Arterioscler Thromb Vasc Biol 2020;40:e1–e9. doi: 10.1161/ATVBAHA.119.313556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lindsey ML, Brunt KR, Kirk JA, Kleinbongard P, Calvert JW, de Castro Brás LE, et al. Guidelines for in vivo mouse models of myocardial infarction. Am J Physiol Heart Circ Physiol 2021;321:H1056–H1073. doi: 10.1152/ajpheart.00459.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burgoyne PS, Arnold AP. A primer on the use of mouse models for identifying direct sex chromosome effects that cause sex differences in non-gonadal tissues. Biol Sex Differ 2016;7:1–21. doi: 10.1186/s13293-016-0115-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Itoh Y, Arnold AP. Are females more variable than males in gene expression? Meta-analysis of microarray datasets. Biol Sex Differ 2015;6:1–9. doi: 10.1186/s13293-015-0036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen X, McClusky R, Chen J, Beaven SW, Tontonoz P, Arnold AP, et al. The number of X chromosomes causes sex differences in adiposity in mice. PLoS Genet 2012;8:e1002709. doi: 10.1371/journal.pgen.1002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Link JC, Wiese CB, Chen X, Avetisyan R, Ronquillo E, Ma F, et al. X chromosome dosage of histone demethylase KDM5C determines sex differences in adiposity. J Clin Invest 2020;130:5688–5702. doi: 10.1172/JCI140223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.AlSiraj Y, Chen X, Thatcher SE, Temel RE, Cai L, Blalock E, et al. XX sex chromosome complement promotes atherosclerosis in mice. Nat Commun 2019;10:2631. doi: 10.1038/s41467-019-10462-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alsiraj Y, Thatcher SE, Blalock E, Fleenor B, Daugherty A, Cassis LA. Sex chromosome complement defines diffuse versus focal angiotensin II-induced aortic pathology. Physiol Behav 2017;176:139–148. doi: 10.1016/j.physbeh.2017.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li J, Chen X, McClusky R, Ruiz-Sundstrom M, Itoh Y, Umar S, et al. The number of X chromosomes influences protection from cardiac ischaemia/reperfusion injury in mice: One X is better than two. Cardiovasc Res 2014;102:375–384. doi: 10.1093/cvr/cvu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Link JC, Chen X, Prien C, Borja MS, Hammerson B, Oda MN, et al. Increased high-density lipoprotein cholesterol levels in mice with XX versus XY sex chromosomes. Arterioscler Thromb Vasc Biol 2015;35:1778–1786. doi: 10.1161/ATVBAHA.115.305460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arnold AP. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm Behav 2009;55:570–578. doi: 10.1016/j.yhbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim JH, Meyers MS, Khuder SS, Abdallah SL, Muturi HT, Russo L, et al. Tissue-selective estrogen complexes with bazedoxifene prevent metabolic dysfunction in female mice. Mol Metab 2014;3:177–190. doi: 10.1016/j.molmet.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kudwa AE, Harada N, Honda S-I, Rissman EF. Regulation of progestin receptors in medial amygdala: estradiol, phytoestrogens and sex. Physiol Behav 2009;97:146–150. doi: 10.1016/j.physbeh.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mauvais-Jarvis F, Arnold AP, Reue K. A guide for the design of pre-clinical studies on sex differences in metabolism. Cell Metab 2017;25:1216–1230. doi: 10.1016/j.cmet.2017.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Henriques TA, Huang J, D’Souza SS, Daugherty A, Cassis LA. Orchidectomy, but not ovariectomy, regulates angiotensin II-induced vascular diseases in apolipoprotein E-deficient mice. Endocrinology 2004;145:3866–3872. doi: 10.1210/en.2003-1615. [DOI] [PubMed] [Google Scholar]
- 73.Zhang X, Thatcher SE, Rateri DL, Bruemmer D, Charnigo R, Daugherty A, et al. Transient exposure of neonatal female mice to testosterone abrogates the sexual dimorphism of abdominal aortic aneurysms. Circ Res 2012;110:e73–85. doi: 10.1161/CIRCRESAHA.111.253880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.AlSiraj Y, Thatcher SE, Blalock E, Saintilnord WN, Daugherty A, Lu HS, et al. Monosomy X in female mice influences the regional formation and augments the severity of angiotensin II-induced aortopathies. Arterioscler Thromb Vasc Biol 2021;41:269–283. doi: 10.1161/ATVBAHA.120.314407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Umar S, Cunningham CM, Itoh Y, Moazeni S, Vaillancourt M, Sarji S, et al. The Y chromosome plays a protective role in experimental hypoxic pulmonary hypertension. Am J Respir Crit Care Med 2018;197:952–955. doi: 10.1164/rccm.201707-1345le. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Link JC, Chen X, Prien C, Borja MS, Hammerson B, Oda MN, et al. Increased high-density lipoprotein cholesterol levels in mice with XX versus XY sex chromosomes SUPP DATA. Arterioscler Thromb Vasc Biol 2015;35:1778–1786. doi: 10.1161/ATVBAHA.115.305460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carlson M, Airhart N, Lopez L, Silberbach M. Moderate aortic enlargement and bicuspid aortic valve are associated with aortic dissection in Turner syndrome: report of the international turner syndrome aortic dissection registry. Circulation 2012;126:2220–2226. doi: 10.1161/CIRCULATIONAHA.111.088633. [DOI] [PubMed] [Google Scholar]
- 78.Matura LA, Ho VB, Rosing DR, Bondy CA. Aortic dilatation and dissection in Turner syndrome. Circulation 2007;116:1663–1670. doi: 10.1161/CIRCULATIONAHA.106.685487. [DOI] [PubMed] [Google Scholar]
- 79.Karastergiou K, Fried SK. Cellular mechanisms driving sex differences in adipose tissue biology and body shape in humans and mouse models. Adv Exp Med Biol 2017;1043:29–51. doi: 10.1007/978-3-319-70178-3_3. [DOI] [PubMed] [Google Scholar]
- 80.Karastergiou K, Fried SK, Xie H, Lee MJ, Divoux A, Rosencrantz MA, et al. Distinct developmental signatures of human abdominal and gluteal subcutaneous adipose tissue depots. J Clin Endocrinol Metab 2013;98:362–371. doi: 10.1210/jc.2012-2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen X, Wang L, Loh DH, Colwell CS, Taché Y, Reue K, et al. Sex differences in diurnal rhythms of food intake in mice caused by gonadal hormones and complement of sex chromosomes. Horm Behav 2015;75:55–63. doi: 10.1016/j.yhbeh.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brier ASB, Loft A, Madsen JGS, Rosengren T, Nielsen R, Schmidt SF, et al. The KDM5 family is required for activation of pro-proliferative cell cycle genes during adipocyte differentiation. Nucleic Acids Res 2017;45:1743–1759. doi: 10.1093/nar/gkw1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Itoh Y, Golden LC, Itoh N, Matsukawa MA, Ren E, Tse V, et al. The X-linked histone demethylase Kdm6a in CD4+ T lymphocytes modulates autoimmunity. J Clin Invest 2019;129:3852–3863. doi: 10.1172/JCI126250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Davis EJ, Broestl L, Williams G, Garay BI, Lobach I, Devidze N, et al. A second X chromosome contributes to resilience in a mouse model of Alzheimer’s disease. Sci Transl Med 2020;12:eaaz5677. doi: 10.1126/SCITRANSLMED.AAZ5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hoeper MM, Huscher D, Ghofrani HA, Delcroix M, Distler O, Schweiger C, et al. Elderly patients diagnosed with idiopathic pulmonary arterial hypertension: results from the COMPERA registry. Int J Cardiol 2013;168:871–880. doi: 10.1016/j.ijcard.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 86.Docherty CK, Harvey KY, Mair KM, Griffin S, Denver N, MacLean MR. The role of sex in the pathophysiology of pulmonary hypertension. Adv Exp Med Biol 2018;1065:511–528. doi: 10.1007/978-3-319-77932-4_31. [DOI] [PubMed] [Google Scholar]
- 87.Lahm T, Tuder RM, Petrache I. Progress in solving the sex hormone paradox in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2014;307:L7–26. doi: 10.1152/ajplung.00337.2013. [DOI] [PubMed] [Google Scholar]
- 88.Winham SJ, de Andrade M, Miller VM. Genetics of cardiovascular disease: Importance of sex and ethnicity. Atherosclerosis 2015;241:219–228. doi: 10.1016/j.atherosclerosis.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Khramtsova EA, Davis LK, Stranger BE. The role of sex in the genomics of human complex traits. Nat Rev Genet 2019;20:173–190. doi: 10.1038/s41576-018-0083-1. [DOI] [PubMed] [Google Scholar]
- 90.Gao F, Chang D, Biddanda A, Ma L, Guo Y, Zhou Z, et al. XWAS: A software toolset for genetic data analysis and association studies of the X chromosome. J Hered 2015;106:666–671. doi: 10.1093/jhered/esv059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.König IR, Loley C, Erdmann J, Ziegler A. How to include chromosome X in your genome-wide association study. Genet Epidemiol 2014;38:97–103. doi: 10.1002/gepi.21782. [DOI] [PubMed] [Google Scholar]
- 92.Wise AL, Gyi L, Manolio TA. eXclusion: toward integrating the X chromosome in genome-wide association analyses. Am J Hum Genet 2013;92:643–647. doi: 10.1016/j.ajhg.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hickey PF, Bahlo M. X chromosome association testing in genome wide association studies. Genet Epidemiol 2011;35:664–670. doi: 10.1002/gepi.20616. [DOI] [PubMed] [Google Scholar]
- 94.Loley C, Alver M, Assimes TL, Bjonnes A, Goel A, Gustafsson S, et al. No Association of Coronary Artery Disease with X-Chromosomal Variants in Comprehensive International Meta-Analysis. Sci Rep 2016;6:35278. doi: 10.1038/srep35278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Winkler TW, Justice AE, Graff M, Barata L, Feitosa MF, Chu S, et al. The influence of age and sex on genetic associations with adult body size and shape: A large-scale genome-wide interaction study. PLoS Genet 2015;11:e1005378. doi: 10.1371/journal.pgen.1005378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lusis AJ, Seldin MM, Allayee H, Bennett BJ, Civelek M, Davis RC, et al. The Hybrid Mouse Diversity Panel: a resource for systems genetics analyses of metabolic and cardiovascular traits. J Lipid Res 2016;57:925–942. doi: 10.1194/jlr.R066944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ghazalpour A, Rau CD, Farber CR, Bennett BJ, Orozco LD, van Nas A, et al. Hybrid Mouse Diversity Panel: a panel of inbred mouse strains suitable for analysis of complex genetic traits. Mamm Genome 2012;23:680–692. doi: 10.1007/s00335-012-9411-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Norheim F, Hasin-Brumshtein Y, Vergnes L, Chella Krishnan K, Pan C, Seldin MM, et al. Gene-by-sex interactions in mitochondrial functions and cardio-metabolic traits. Cell Metab 2019;29:932–949. e4. doi: 10.1016/j.cmet.2018.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Consortium Tabula Muris. A single-cell transcriptomic atlas characterizes ageing tissues in the mouse. Nature 2020;583:590–595. doi: 10.1038/s41586-020-2496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


