Abstract
Background
The literature has shown depression to be associated with an increased risk of dementia. In addition, hormone therapy can be a responsive treatment option for a certain type of depression. In this study, we examined the association between hormone therapy, including lifetime oral contraceptive (OC) use, and hormone replacement therapy (HRT) after menopause with the occurrence of dementia among female patients with depression.
Methods
The South Korean national claims data from January 1, 2005, to December 31, 2018, was used. Female subjects aged 40 years or older with depression were included in the analyses. Information on hormone therapy was identified from health examination data and followed up for the occurrence of dementia during the average follow-up period of 7.72 years.
Results
Among 209,588 subjects, 23,555 were diagnosed with Alzheimer’s disease (AD) and 3023 with vascular dementia (VD). Lifetime OC usage was associated with a decreased risk of AD (OC use for < 1 year: HR, 0.92 [95% CI, 0.88–0.97]; OC use for ≥ 1 year: HR, 0.89 [95% CI, 0.84–0.94]), and HRT after menopause was associated with a decreased risk of AD (HRT for < 2 years: HR, 0.84 [95% CI, 0.79–0.89]; HRT for 2–5 years: HR, 0.80 [95% CI, 0.74–0.88]; and HRT for ≥ 5 years : HR, 0.78 [95% CI, 0.71–0.85]) and VD (HRT < 2 years: HR, 0.82 [95% CI, 0.71–0.96]; HRT for 2–5 years: HR, 0.81 [95% CI, 0.64–1.02]; and HRT for ≥ 5 years: HR, 0.61 [95% CI, 0.47–0.79]).
Conclusions
In this nationwide cohort study, lifetime OC use was associated with a decreased risk of AD, and HRT after menopause was associated with a decreased risk of AD and VD among female patients with depression. However, further studies are needed to establish causality.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13195-022-01026-3.
Keywords: Hormone therapy, Hormone replacement therapy, Oral contraceptives, Depression, Alzheimer’s disease, Vascular dementia
Background
Depression is a common psychiatric disorder associated with significant morbidity [1]. Although the mechanism underlying depression is yet to be established, one potential etiology is a change in the levels of female sex hormones. Epidemiological studies have shown that women have about twice the risk of depression as men [2–4], and perimenopausal or postmenopausal women who experience a rapid decline in levels of female sex hormones have an increased risk of depression compared to premenopausal women [5]. A placebo-controlled study showed an increase in the depressive symptoms among premenopausal women who were rendered temporally hypogonadal by gonadotropin-releasing hormone agonists [6]. In addition, women can experience reproductive depression such as premenopausal dysphoric disorder and postpartum depression when rapid changes in the levels of estrogen occur. On the other hand, female sex hormones may help prevent and improve depressive symptoms; a cross-sectional study showed that women, aged ≥ 60 years, who received hormone replacement therapy (HRT) had lower rates of depressive symptoms than those not taking HRT [7]. Randomized controlled studies have likewise shown the effect of hormone therapy on perimenopausal women experiencing depressive disorders [8, 9]. In particular, hormonal therapy has been proven as an effective treatment option for reproductive depression [10].
Previous studies have suggested that depression is associated with an increased risk of dementia [11–13]. The Women’s Health Initiative Memory Study showed that baseline depressive disorder was associated with an increased risk of incident mild cognitive impairment (HR, 1.98) and probable dementia (HR, 2.03) among postmenopausal women without cognitive impairment aged 65 to 79 [14]. Similarly, prospective studies revealed that late-life depression is linked to dementia with a 2- to 5-fold increased risk [15–20]. Although fewer studies have focused on early-onset depression compared to late-life depression, early onset of depression was also associated with a 2- to 4-fold increased risk of dementia [21–24]. Depending on the type of dementia, depression was associated with a 1.2- to 4.6-fold increased risk of Alzheimer’s disease (AD) [13, 15, 21, 23, 25–27], and a 1.2 to 2.4-fold increased risk of vascular dementia (VD) [20, 25]. Overall, a recent meta-analysis study found that depression is one of the most significant risk factors for dementia, with a relative risk of 1.99 [28]. However, in these cases, the increased risk is not always the cause. Depression, for example, can be an actual risk factor or an early manifestation of dementia, and the underlying mechanism is yet unknown. Alternatively, it might be a case of pseudodementia, in which cognitive impairment caused by depression is misdiagnosed as dementia.
Previous studies have shown inconsistent results on the association between hormone therapy and dementia. In a longitudinal study, the risk of incident AD was reduced by about half in hormone replacement users compared to nonusers [29]. Hormone therapy, on the other hand, showed no significant effect on the prevention of dementia or the enhancement of cognition in elderly women who had been in menopause for several years. A randomized controlled trial showed that estrogen plus progestin therapy among postmenopausal women aged 65 years or older doubled the risk of probable dementia compared to placebo, and no protective effect on mild cognitive impairment was found [30]. In the Women’s Health Initiative Memory Study, estrogen plus progestin hormone therapy had a negative impact on verbal memory, but a positive impact on figural memory (committing objects to visual memory and then recognizing them when shown in a stream of different objects) was observed [31]. Additionally, there was no preventive effect of dementia on the estrogen-alone group [32]. When compared to placebo, 20 weeks of unopposed estradiol replacement therapy did not improve cognitive performance in women aged 70 or older [33].
The idea that estrogen has neuroprotective properties is a relatively new one [34, 35]. This research suggests that estrogen may affect both neurodegenerative disorders such as dementia and affective disorders such as depression. However, the association between depression, dementia, and estrogen is poorly understood, and there is still no evidence on the effect of hormone therapy on dementia among women with depression.
In this study, we used a national cohort of South Korea to examine the association between hormone therapy including oral contraceptives (OCs) and HRT with the risk of dementia in postmenopausal women. We hypothesized that (1) lifetime OC use is associated with the risk of dementia among patients with depression and (2) postmenopausal HRT is associated with the risk of dementia among patients with depression.
Methods
Data source
The database from the National Health Insurance Sharing Service (NHISS) of the National Health Insurance Service (NHIS) of South Korea was used [36, 37]. The NHIS is a public institution responsible for operating mandatory universal health insurance, and nearly 97% of the South Korean population is enrolled in this service, while the remaining 3% is covered by the Medical Aid Program. The NHISS contains medical service claims data such as admissions, emergency room visits, ambulatory care visits, and pharmaceutical services.
The database from the National Cancer Screening Program (NCSP) was also used [38]. The NCSP contains screening data for stomach, liver, colorectal, breast, and cervical cancers, and each screening examination was conducted based on the age of the participants. All South Korean women aged 40 years or older are encouraged to be screened for breast and cervical cancer biennially. Even though the screening program was voluntary, participation rates reached up to 70% [39].
The data of NHISS and NCSP were anonymized by using individual research numbers instead of social security numbers to protect the privacy of the individuals. The study protocol was approved by the Institutional Review Board of the Samsung Medical Center (IRB No. 2021-03-108).
Case identification
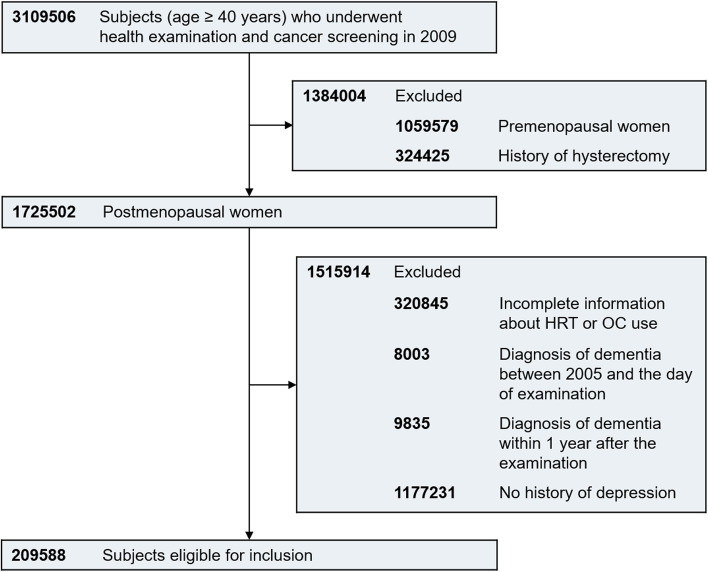
Among 3,109,506 female subjects aged 40 years or older who underwent health examination and screening for breast/cervical cancer on the same day from January 1, 2009, to December 31, 2009, 1,725,502 were identified as postmenopausal without a history of hysterectomy. Those with incomplete information about HRT or OC use (n = 320,845) and those with a previous diagnosis of dementia before the examination (n = 8003) were excluded to secure the first diagnosis of dementia. In addition, 9835 subjects who were diagnosed with dementia within 1 year after the examination were excluded to eliminate the effect of a temporary increase in the diagnosis of dementia by detecting it at the time of examination. After excluding 1,177,231 subjects with no history of depression, 209,588 subjects were deemed eligible for our study, and their medical records were followed until December 31, 2018 (Fig. 1). The diagnosis of depression (F32 and F33) was defined based on the International Statistical Classification of Disease and Related Health Problems 10th revision (ICD-10).
Fig. 1.
Flowchart
Lifetime uses of oral contraceptives
Information on the usage of OC was extracted from self-administered questionnaire data from the cancer screening program. The question was “Are you on or have you ever taken oral contraceptive pills?” Subjects were asked to choose a response among “never,” “use for less than 1 year,” “use for more than 1 year,” or “unknown.”
Hormone replacement therapy after menopause
Information on HRT was extracted from the self-administered questionnaire data from a cancer screening program. The question was “Are you on or have you ever taken hormonal agents to relieve postmenopausal symptoms?” Subjects were asked to choose a response among “never,” “use for less than 2 years,” “use of 2–5 years,” “use for more than 5 years,” or “unknown.”
Outcomes
The main outcome was the diagnosis of dementia (F00 and F30 for AD; F01 for VD; and F02, F03, and F31 for other dementia) and the prescription of one or more medications for dementia during the follow-up period. When a subject had more than one code for dementia diagnosis, the subject was classified based on the principal diagnosis. If both AD and VD codes were included in the additional diagnosis of a subject, the subject was classified based on the principal diagnosis of the next hospital visit, and if both AD and VD codes remained as additional diagnosis codes, the subject was classified as another dementia group. Prescribed medications for dementia included donepezil, rivastigmine, galantamine, or memantine.
Covariates
The body mass index (BMI) was calculated using the subjects’ weight and height measured on the day of the cancer screening examination. Lifestyle factors such as smoking, alcohol consumption, and exercise were identified from the NCSP self-questionnaire. Regular exercise was defined as performing a moderate physical activity for more than 30 min at least five times a week or vigorous physical activity for more than 20 min at least three times a week. Subjects were categorized into levels of income based on the payment of health insurance. Comorbid physical illnesses including hypertension, diabetes mellitus, and dyslipidemia were identified based on ICD-10 codes from past medical records.
Statistical analyses
Continuous variables were displayed as mean ± standard deviation (SD), while categorical variables were displayed as number and percentage. The Student t-test was used to compare the differences in individual factors between the groups. Cox proportional hazards regression analyses were conducted to identify the association between hormone therapy and the diagnosis of dementia, and censored for the occurrence of dementia or death. The proportional hazard assumption was visually tested using the Schoenfeld residuals plot and the log-log survival plot. The hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) revealed the magnitude of risk of dementia based on the duration of hormone therapy. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Table 1 shows the baseline characteristics of the subjects. The mean age of the subjects was 61.54 years (SD, 7.83) in the non-dementia group and 70.46 years (SD, 6.59) in the dementia group (p < 0.0001). In comparison with the non-dementia group, the dementia group had a lower proportion of alcohol consumption and regular exercise; a greater prevalence of hypertension, diabetes mellitus, and dyslipidemia; and lower usage of OC and experience with HRT. Table 2 shows the baseline characteristics of subjects according to the duration of hormone therapy. Regarding the use of OCs, longer OC users were more likely to have a higher BMI, lower total cholesterol and LDL levels, currently smoke, drink heavily, have the highest income level, have dyslipidemia, and experience menopause later in life. Regarding HRT, longer HRT users were more likely to exercise regularly; have lower diastolic blood pressure, fasting glucose, and total cholesterol; and have less dyslipidemia.
Table 1.
Baseline characteristics of study subjects
| Non-dementia group (n = 179,723) | Dementia group (n = 29,865) | P | |
|---|---|---|---|
| Age (years) | 61.54 ± 7.83 | 70.46 ± 6.59 | < 0.0001 |
| BMI (kg/m2) | 24.26 ± 3.14 | 24.19 ± 3.31 | < 0.001 |
| Systolic BP (mmHg) | 125.01 ± 15.81 | 128.73 ± 16.23 | < 0.0001 |
| Diastolic BP (mmHg) | 76.63 ± 9.98 | 77.77 ± 10.08 | < 0.0001 |
| Fasting glucose (mg/dL) | 99.89 ± 24.24 | 104.63 ± 30.56 | < 0.0001 |
| Total cholesterol (mg/dL) | 206.62 ± 46.60 | 205.47 ± 42.25 | < 0.0001 |
| HDL (mg/dL) | 57.50 ± 35.39 | 56.05 ± 35.84 | < 0.0001 |
| LDL (mg/dL) | 125.28 ± 77.35 | 123.31 ± 81.84 | < 0.0001 |
| Smoking status | 0.208 | ||
| Never | 172,482 (95.97) | 28,606 (95.78) | |
| Ex-smoker | 2121 (1.18) | 353 (1.18) | |
| Current smoker | 5120 (2.85) | 906 (3.03) | |
| Alcohol consumptiona | < 0.0001 | ||
| None | 160,398 (89.25) | 28,075 (94.01) | |
| Mild | 18,428 (10.25) | 1719 (5.76) | |
| Heavy | 897 (0.50) | 71 (0.24) | |
| Levels of income | < 0.0001 | ||
| Medical aid + 1st quartile (the lowest) | 37,606 (20.92) | 5645 (18.9) | |
| 2nd quartile | 31,760 (17.67) | 4842 (16.21) | |
| 3rd quartile | 46,044 (25.62) | 6739 (22.56) | |
| 4th quartile (the highest) | 64,313 (35.78) | 12,639 (42.32) | |
| Regular exercise | 34,103 (18.98) | 3792 (12.70) | < 0.0001 |
| Hypertension | 90,215 (50.20) | 20,248 (67.80) | < 0.0001 |
| Diabetes mellitus | 26,506 (14.75) | 7369 (24.67) | < 0.0001 |
| Dyslipidemia | 70,467 (39.21) | 12,847 (43.02) | < 0.0001 |
| Age at menarche (years) | 16.50 ± 1.82 | 16.92 ± 1.79 | < 0.0001 |
| Age at menopause (years) | 49.99 ± 4.07 | 49.27 ± 4.56 | < 0.0001 |
| Duration of fertility (years) | 33.49 ± 4.45 | 32.35 ± 4.95 | < 0.0001 |
| Duration of OC use (years) | < 0.0001 | ||
| Never | 149,286 (83.06) | 26,008 (87.09) | |
| < 1 | 17,771 (9.89) | 2156 (7.22) | |
| ≥ 1 | 12,666 (7.05) | 1701 (5.70) | |
| Duration of HRT (years) | < 0.0001 | ||
| Never | 140,749 (78.31) | 26,781 (89.67) | |
| < 2 | 22,097 (12.30) | 1735 (5.81) | |
| 2–5 | 9182 (5.11) | 663 (2.22) | |
| ≥ 5 | 7695 (4.28) | 686 (2.30) |
Data are expressed as the mean ± standard deviation, SD, or n (%)
Abbreviations: BMI body mass index, BP blood pressure, HDL high-density lipoprotein, LDL low-density lipoprotein, OC oral contraceptives, HRT hormone replacement therapy
aAlcohol consumption: mild = up to 30 g (equivalent to 3 drinks) a day; heavy = more than 30 g a day
Table 2.
Baseline characteristics of study subjects according to the duration of hormone therapy
| Duration of OC use (years) | Duration of HRT (years) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Never (n = 175,294) | < 1 (n = 19,927) | ≥ 1 (n = 14,367) | P | Never (n = 167,530) | < 2 (n = 23,832) | 2–5 (n = 9845) | ≥ 5 (n = 8381) | P | |
| Age (years) | 62.96 ± 8.45 | 61.59 ± 7.37 | 62.70 ± 7.12 | < 0.0001 | 63.62 ± 8.45 | 59.10 ± 6.87 | 59.35 ± 6.36 | 61.43 ± 60 | < 0.0001 |
| BMI (kg/m2) | 24.21 ± 3.18 | 24.35 ± 3.08 | 24.61 ± 3.12 | < 0.001 | 24.33 ± 3.22 | 24.00 ± 2.96 | 23.78 ± 2.86 | 23.85 ± 2.79 | < 0.0001 |
| Systolic BP (mmHg) | 125.55 ± 15.98 | 125.06 ± 15.71 | 126.15 ± 15.62 | < 0.0001 | 126.30 ± 16.04 | 122.65 ± 15.08 | 122.07 ± 15.01 | 122.77 ± 15.19 | < 0.0001 |
| Diastolic BP (mmHg) | 76.82 ± 10.01 | 76.55 ± 9.99 | 76.82 ± 9.95 | 0.0019 | 77.14 ± 10.04 | 75.59 ± 9.80 | 75.20 ± 9.74 | 75.04 ± 9.68 | < 0.0001 |
| Fasting glucose (mg/dL) | 100.52 ± 25.38 | 100.40 ± 24.65 | 101.41 ± 25.07 | 0.0002 | 101.29 ± 26.27 | 98.14 ± 20.76 | 97.26 ± 20.47 | 96.81 ± 20.59 | < 0.0001 |
| Total cholesterol (mg/dL) | 206.66 ± 46.53 | 205.79 ± 41.49 | 204.82 ± 45.48 | < 0.0001 | 207.16 ± 46.96 | 205.86 ± 44.10 | 202.37 ± 38.71 | 198.73 ± 38.30 | < 0.0001 |
| HDL (mg/dL) | 57.38 ± 36.31 | 56.87 ± 30.07 | 56.86 ± 31.51 | 0.0468 | 57.30 ± 36.50 | 57.68 ± 32.82 | 57.26 ± 28.92 | 56.09 ± 27.50 | 0.0059 |
| LDL (mg/dL) | 125.19 ± 80.79 | 124.31 ± 60.11 | 123.64 ± 64.17 | 0.0309 | 125.16 ± 74.84 | 125.18 ± 60.46 | 123.93 ± 113.57 | 122.51 ± 121.11 | 0.0105 |
| Smoking status | 0.208 | < 0.0001 | |||||||
| Never | 168,431 (96.08) | 19,019 (95.44) | 13,638 (94.93) | 161,079 (96.15) | 22,713 (95.30) | 9334 (94.81) | 7962 (95.00) | ||
| Ex-smoker | 1901 (1.08) | 320 (1.61) | 253 (1.76) | 1773 (1.06) | 371 (1.56) | 177 (1.80) | 153 (1.83) | ||
| Current smoker | 4962 (2.83) | 588 (2.95) | 476 (3.31) | 4678 (2.79) | 748 (3.14) | 334 (3.39) | 266 (3.17) | ||
| Alcohol consumptiona | < 0.0001 | < 0.0001 | |||||||
| None | 158,564 (90.46) | 17,410 (87.37) | 12,499 (87.00) | 152,177 (90.84) | 20,603 (86.45) | 8447 (85.80) | 7246 (86.46) | ||
| Mild | 15,998 (9.13) | 2390 (11.99) | 1759 (12.24) | 14,633 (8.73) | 3085 (12.94) | 1337 (13.58) | 1092 (13.03) | ||
| Heavy | 732 (0.42) | 127 (0.64) | 109 (0.76) | 720 (0.43) | 144 (0.60) | 61 (0.62) | 43 (0.51) | ||
| Levels of income | 0.0034 | < 0.0001 | |||||||
| Medical aid + 1st quartile (the lowest) | 36,339 (20.73) | 4079 (20.47) | 2833 (19.72) | 34,427 (20.55) | 5190 (21.78) | 2022 (20.54) | 1612 (19.23) | ||
| 2nd quartile | 30,713 (17.52) | 3454 (17.33) | 2435 (16.95) | 29,337 (17.51) | 4276 (17.94) | 1697 (17.24) | 1292 (15.42) | ||
| 3rd quartile | 44,113 (25.17) | 5060 (25.39) | 3610 (25.13) | 42,234 (25.21) | 5957 (25.00) | 2503 (25.42) | 2089 (24.93) | ||
| 4th quartile (the highest) | 64,129 (36.58) | 7334 (36.80) | 5489 (38.21) | 61,532 (36.73) | 8409 (35.28) | 3623 (36.80) | 3388 (40.42) | ||
| Regular exercise | 30,862 (17.61) | 4005 (20.10) | 3028 (21.08) | < 0.0001 | 28,011 (16.72) | 5330 (22.36) | 2372 (24.09) | 2182 (26.04) | < 0.0001 |
| Hypertension | 91,855 (52.40) | 10,346 (51.92) | 8262 (57.51) | < 0.0001 | 91,657 (54.71) | 10,477 (43.96) | 4322 (43.90) | 4007 (47.81) | |
| Diabetes mellitus | 28,077 (16.02) | 3155 (15.83) | 2643 (18.40) | < 0.0001 | 28,809 (17.20) | 2891 (12.13) | 1121 (11.39) | 1054 (12.58) | |
| Dyslipidemia | 69,087 (39.41) | 8101 (40.65) | 6126 (42.64) | < 0.0001 | 67,068 (40.03) | 9434 (39.59) | 3712 (37.70) | 3100 (36.99) | |
| Age at menarche (years) | 16.57 ± 1.83 | 16.49 ± 1.83 | 16.56 ± 1.81 | < 0.0001 | 16.63 ± 1.81 | 16.30 ± 1.86 | 16.25 ± 1.82 | 16.35 ± 1.84 | |
| Age at menopause (years) | 49.83 ± 4.15 | 50.13 ± 4.15 | 50.24 ± 4.19 | < 0.0001 | 49.88 ± 4.14 | 50.07 ± 4.00 | 50.04 ± 4.18 | 49.41 ± 4.78 | |
| Duration of fertility (years) | 33.26 ± 4.54 | 33.65 ± 4.52 | 33.68 ± 4.54 | < 0.0001 | 33.25 ± 4.55 | 33.78 ± 4.33 | 33.79 ± 4.43 | 33.06 ± 4.98 | |
| Duration of OC use (years) | < 0.0001 | ||||||||
| Never | 144,214 (86.08) | 17,651 (74.06) | 7295 (74.1) | 6134 (73.19) | |||||
| < 1 | 13,738 (8.20) | 3817 (16.02) | 1301 (13.21) | 1071 (12.78) | |||||
| ≥ 1 | 9578 (5.72) | 2364 (9.92) | 1249 (12.69) | 1176 (14.03) | |||||
| Duration of HRT (years) | < 0.0001 | ||||||||
| Never | 144,214 (82.27) | 13,738 (68.94) | 9578 (66.67) | ||||||
| < 2 | 17,651 (10.07) | 3817 (19.15) | 2364 (16.45) | ||||||
| 2–5 | 7295 (4.16) | 1301 (6.53) | 1249 (8.69) | ||||||
| ≥ 5 | 6134 (3.50) | 1071 (5.37) | 1176 (8.19) | ||||||
Data are expressed as the mean ± standard deviation, SD, or n (%)
Abbreviations: BMI body mass index, BP blood pressure, HDL high-density lipoprotein, LDL low-density lipoprotein, OC oral contraceptives, HRT hormone replacement therapy
aAlcohol consumption: mild = up to 30 g (equivalent to 3 drinks) a day; heavy = more than 30 g a day
The average follow-up length was 7.72 years (SD, 1.87). Overall, 29,865 patients were newly diagnosed with dementia during the follow-up period, and the incidence rate was 18.47 per 1000 person-years. Among them, 23,555 were classified as AD subgroup with an incidence rate of 14.57 per 1000 person-years, and 3023 were classified as VD subgroup with an incidence rate of 1.87 per 1000 person-years.
In the adjusted model, compared to those who had never used OC, those who had used OC showed a decreased risk of all dementia (OC use for < 1 year: HR, 0.92 [95% CI, 0.88–0.96]; OC use for ≥ 1 year: HR, 0.90 [95% CI, 0.86–0.95]). Although OC use was associated with a decreased risk of AD (OC use for < 1 year: HR, 0.92 [95% CI, 0.88–0.97]; OC use for ≥ 1 year: HR, 0.89 [95% CI, 0.84–0.94]), there was no significant association between OC use and VD.
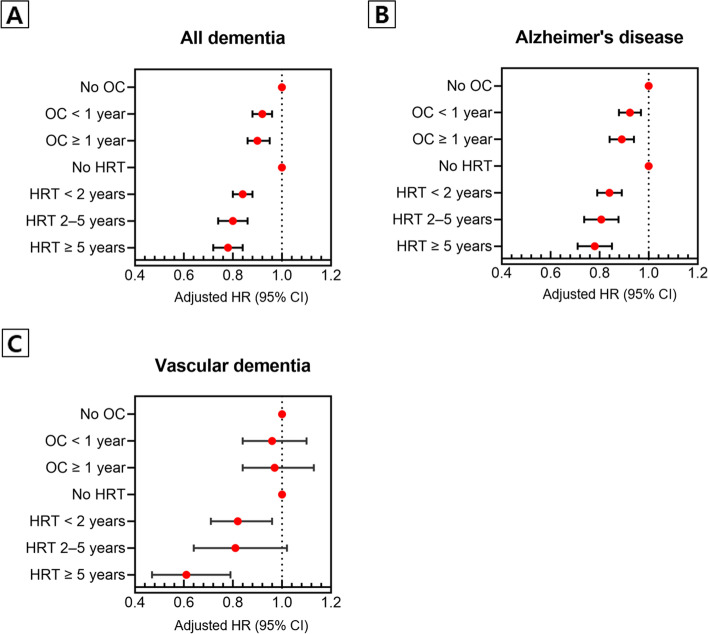
In the adjusted model, compared to those who had never received HRT, subjects who had received HRT showed a decreased risk of all dementia (HRT for < 2 years: HR, 0.84 [95% CI, 0.80–0.88]; HRT for 2–5 years: HR, 0.80 [95% CI 0.74–0.86]; and HRT for ≥ 5 years: HR, 0.78 [95% CI, 0.72–0.84]). The HRT was associated with a decreased risk of AD (HRT for < 2 years: HR, 0.84 [95% CI, 0.79–0.89]; HRT for 2–5 years: HR, 0.80 [95% CI, 0.74–0.88]; and HRT for ≥ 5 years : HR, 0.78 [95% CI, 0.71–0.85]) and VD (HRT < 2 years: HR, 0.82 [95% CI, 0.71–0.96]; HRT for 2–5 years: HR, 0.81 [95% CI, 0.64–1.02]; and HRT for ≥ 5 years: HR, 0.61 [95% CI, 0.47–0.79]) (Table 3 and Fig. 2).
Table 3.
Hazard ratios and 95% confidence intervals of the duration of hormone therapy on the diagnosis of dementia
| All dementia | Alzheimer’s disease | Vascular dementia | ||||
|---|---|---|---|---|---|---|
| Crude | Adjusteda | Crude | Adjusteda | Crude | Adjusteda | |
| Hazard ratio (95% confidence interval) | ||||||
| Duration of OC use (years) | ||||||
| Never | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| < 1 | 0.71 (0.68, 0.74) | 0.92 (0.88, 0.96) | 0.71 (0.67, 0.74) | 0.92 (0.88, 0.97) | 0.76 (0.66, 0.87) | 0.96 (0.84, 1.10) |
| ≥ 1 | 0.78 (0.74, 0.82) | 0.90 (0.86, 0.95) | 0.77 (0.73, 0.81) | 0.89 (0.84, 0.94) | 0.87 (0.75, 1.01) | 0.97 (0.84, 1.13) |
| Duration of HRT (years) | ||||||
| Never | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| < 2 | 0.43 (0.41, 0.45) | 0.84 (0.80, 0.88) | 0.43 (0.40, 0.45) | 0.84 (0.79, 0.89) | 0.44 (0.38, 0.51) | 0.82 (0.71, 0.96) |
| 2–5 | 0.40 (0.37, 0.43) | 0.80 (0.74, 0.86) | 0.39 (0.36, 0.43) | 0.80 (0.74, 0.88) | 0.42 (0.33, 0.53) | 0.81 (0.64, 1.02) |
| ≥ 5 | 0.48 (0.45, 0.52) | 0.78 (0.72, 0.84) | 0.48 (0.44, 0.52) | 0.78 (0.71, 0.85) | 0.39 (0.30, 0.51) | 0.61 (0.47, 0.79) |
Abbreviations: OC oral contraceptives, HRT hormone replacement therapy
aAdjusted for age, body mass index, level of income, current smoking, drinking status, regular exercise, diabetes mellitus, hypertension, and dyslipidemia
Fig. 2.
Forest plots showing the hazard ratios and corresponding 95% confidence intervals of hormone therapy on the diagnosis of dementia. OC, oral contraceptives; HRT, hormone replacement therapy
Supplementary Table 1 shows the results of Cox proportional hazards regression analyses with age as the time scale instead of risk time. As the result, the reduction in the risk of dementia according to the use of OCs and HRT was attenuated compared to that with risk time as the time-scale in the crude model, but after adjusting covariables, the magnitude of risk reduction was similar to that of main results.
Supplementary Table 2 presents the results of sensitivity analyses, which include those who were excluded from the main analyses because they had answered “unknown” to the question about the use of OC or HRT. Their associations with the risk of dementia were not statistically significant compared to those who had never used OC or HRT, respectively.
Supplementary Table 3 shows the results of sensitivity analyses excluding those who were diagnosed with dementia 3 years after baseline, and the patterns of results were similar to those of the main analyses.
Supplementary Table 4 shows the results of the regression model adding age at menopause as an adjusting variable in the main model, and the results were similar to those of the main analyses.
Discussion
This study showed an association between hormone therapy and the risk of dementia among female patients with depression. In this study, three major findings were presented. First, lifetime use of OCs was associated with a decreased risk of AD. Second, HRT was associated with a decreased risk of AD and VD. Third, the risk of dementia decreased with the increased duration of hormone therapy.
Our results showed that HRT prescribed to relieve postmenopausal symptoms was associated with a lower risk of dementia than lifetime OC use. Postmenopausal symptoms including vasomotor symptoms are prevalent across women’s late menopausal transition stage and early postmenopausal stage [40]. Considering the mean age of menopause of subjects was about 50 years, the users of HRT might have experienced menopausal symptoms in their late 40s or early 50s. The Women’s Health Initiative Memory Study, which reported that hormone therapy had no preventive effect or increased risk for dementia, targeted women over 65 years of age [30, 32]. The Baltimore Aging Study reported the preventive effect of estrogen therapy on AD in postmenopausal women in a longitudinal follow-up, and the study included community-dwelling adult women who were not restricted to a specific age group [29]. Overall, these findings suggest that hormone therapy conducted at a “critical period” in reproductive aging may reduce the risk of dementia in late life.
When comparing the results of a study that used the same database as our study but targeted the general population rather than patients with depression [41], the preventive effect of lifetime OC use on AD and VD was similar in the general population and depression patients. However, regarding HRT after menopause, there was a risk reduction of 13–19% in AD and 14–23% in VD among the general population, and our study showed a higher risk reduction both in AD and VD. This result suggests that HRT after menopause lowers the risk of dementia in patients with depression more than in the general population. Moreover, the reduced risk of VD in those who received HRT for more than 5 years was found to be more robust: 39% in patients with depression compared to 22% in the general population.
Our findings have implications for recognizing the preventive effect of hormone therapy on the occurrence of dementia in patients with depression, but several concerns need to be addressed in terms of causality. In a selected population of postmenopausal women with depression, a significant association between hormone therapy and dementia with a dose-response relationship suggests that hormone therapy could be a meaningful preventive factor for dementia. However, despite the fact that hormone therapy preceded the diagnosis of dementia, it is difficult to believe that it fits the temporality of evidence required to show a causal association between them, particularly in AD, because the initial pathologic change of AD, the accumulation of amyloid-β, is generally known to precede symptoms by 10–15 years [42]. Although the results were still significant after eliminating the occurrence of dementia up to 3 years after the diagnosis of depression in the sensitivity analyses, given the much longer preclinical time, a reversed causality of depression with early presentation of dementia cannot be ruled out.
Although the nature of the association between depression and dementia is unclear, potential biological mechanisms include vascular changes, alterations in glucocorticoid steroids, hippocampal atrophy, deposition of β-amyloid plaques, inflammatory changes, and deficits in neurotrophin nerve growth factors [43]. In addition to the direct neuroprotective actions of estrogen [44–46], the prominent HRT-associated reduction in risk of dementia in depression patients compared to the general population suggests that hormone therapy may affect this pathway linking depression to dementia. Previous studies suggest that hormones may affect the occurrence of dementia in patients with depression via these mechanisms: in a female rodent study, decreasing the level of estrogen by ovariectomy increased amyloid-β oligomers, while estrogen replacement in AD model mice decreased amyloid-β oligomers [47]; in a clinical study, patients with AD had a lower level of estradiol in the cerebrospinal fluid than controls, and the level of estradiol was inversely correlated with β-amyloid concentration, suggesting beneficial effects of HRT on the development and course of AD [48]; estrogen has been shown to act on glial cells to maintain neurovascular function and regulate neuroinflammation [49, 50]; and loss of female sex hormone exacerbated impaired cerebral blood flow and cognitive function via heightened vasoconstriction, reduced vasodilation, and impaired nitric oxide signaling [51].
To the best of our best knowledge, this is the first study to longitudinally follow the effect of hormone therapy on dementia among patients with depression. Our findings present real-world evidence that hormone therapy can be beneficial in the prevention of dementia in patients with depression.
Limitations
This study has several limitations. First, because the data on lifetime OC usage and HRT after menopause were identified retrospectively from responses on the self-administered questionnaires, there may be recall bias. Second, we could not verify information on hormone treatment formulation and dosage and the timing of therapy. Although the question for HRT included the phrase “to relieve postmenopausal symptoms,” subjects could perceive postmenopausal symptoms subjectively, and there was no information on when to start HRT after menopause. Third, we could not include several major risk factors of dementia in the analyses such as the ε4 allele of the Apolipoprotein E (APOE), hearing loss, history of traumatic brain injury, and the level of education [52]. Instead of the level of education, the level of income was included in the analyses as the level of education is a major contributor to income [53].
Conclusions
Among women with depression, lifetime OC use was associated with a decreased risk of AD, and HRT after menopause was associated with a decreased risk of AD and VD. In addition, as the duration of hormone therapy increased, the risk of dementia decreased. Our findings suggest that hormone therapy among patients with depression may be beneficial in the prevention of dementia, and this could represent valuable evidence for physicians’ clinical decision-making. Further prospective studies are needed to secure stronger causality and safety information.
Supplementary Information
Additional file 1: Supplementary Table 1. Hazard ratios and 95% confidence intervals of the duration of hormone therapy on the diagnosis of dementia with age as the time-scale. Supplementary Table 2. Hazard ratios and 95% confidence intervals of the duration of hormone therapy on the diagnosis of dementia including those who responded as “unknown” to the questions about hormone therapy. Supplementary Table 3. Hazard ratios and 95% confidence intervals of the duration of hormone therapy on the diagnosis of dementia excluding those who were diagnosed with dementia 3 years after the diagnosis of depression. Supplementary Table 4. Hazard ratios and 95% confidence intervals of the duration of hormone therapy on the diagnosis of dementia adding age at menopause as an adjusting variable.
Acknowledgements
Not applicable.
Authors’ contributions
HK contributed to the search for background literature, to the writing of the original draft of the manuscript, and to the review. JY and KH contributed to the formal analysis. HJJ contributed to the conceptualization, project administration, and supervision. All authors contributed to the writing and editing of the manuscript. The author(s) read and approved the final manuscript.
Funding
This research was supported by the Bio and Medical Technology Development Program of the National Research Foundation (NRF) funded by the Ministry of Science and ICT (No. 2021M3A9E4080784), the Healthcare AI Convergence Research & Development Program through the National IT Industry Promotion Agency of Korea (NIPA) funded by the Ministry of Science and ICT (No. S1601-20-1041) and by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), sponsored by the Ministry of Health and Welfare, Republic of Korea (No. HR21C0885).
Availability of data and materials
Publicly available datasets were analyzed in this study. This data can be found in the following: https://nhiss.nhis.or.kr/.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the Institutional Review Board of the Samsung Medical Center, and informed consent was not obtained from the subjects because the national claims data was used.
Consent for publication
Not applicable.
Competing interests
Dr. Mischoulon has received research support from Nordic Naturals and heckel medizintechnik GmbH. He has received honoraria for speaking from the Massachusetts General Hospital Psychiatry Academy. He also works with the MGH Clinical Trials Network and Institute (CTNI), which has received research funding from multiple pharmaceutical companies and NIMH.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Baldessarini RJ, Forte A, Selle V, Sim K, Tondo L, Undurraga J, et al. Morbidity in depressive disorders. Psychother Psychosom. 2017;86(2):65–72. doi: 10.1159/000448661. [DOI] [PubMed] [Google Scholar]
- 2.Gater R, Tansella M, Korten A, Tiemens BG, Mavreas VG, Olatawura MO. Sex differences in the prevalence and detection of depressive and anxiety disorders in general health care settings: report from the World Health Organization Collaborative Study on Psychological Problems in General Health Care. Arch Gen Psychiatry. 1998;55(5):405–413. doi: 10.1001/archpsyc.55.5.405. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51(1):8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 4.Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu H-G, et al. Cross-national epidemiology of major depression and bipolar disorder. JAMA. 1996;276(4):293–299. doi: 10.1001/jama.1996.03540040037030. [DOI] [PubMed] [Google Scholar]
- 5.Lin HL, Hsiao MC, Liu YT, Chang CM. Perimenopause and incidence of depression in midlife women: a population-based study in Taiwan. Climacteric. 2013;16(3):381–386. doi: 10.3109/13697137.2012.707706. [DOI] [PubMed] [Google Scholar]
- 6.Warnock JK, Bundren JC, Morris DW. Depressive symptoms associated with gonadotropin-releasing hormone agonists. Depress Anxiety. 1998;7(4):171–177. doi: 10.1002/(SICI)1520-6394(1998)7:4<171::AID-DA5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 7.Palinkas LA, Barrett-Connor E. Estrogen use and depressive symptoms in postmenopausal women. Obstet Gynecol. 1992;80(1):30–36. [PubMed] [Google Scholar]
- 8.Schmidt PJ, Nieman L, Danaceau MA, Tobin MB, Roca CA, Murphy JH, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol. 2000;183(2):414–420. doi: 10.1067/mob.2000.106004. [DOI] [PubMed] [Google Scholar]
- 9.Soares CN, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58(6):529–534. doi: 10.1001/archpsyc.58.6.529. [DOI] [PubMed] [Google Scholar]
- 10.Studd J, Nappi RE. Reproductive depression. Gynecol Endocrinol. 2012;28(Suppl 1):42–45. doi: 10.3109/09513590.2012.651932. [DOI] [PubMed] [Google Scholar]
- 11.Bassuk SS, Berkman LF, Wypij D. Depressive symptomatology and incident cognitive decline in an elderly community sample. Arch Gen Psychiatry. 1998;55(12):1073–1081. doi: 10.1001/archpsyc.55.12.1073. [DOI] [PubMed] [Google Scholar]
- 12.Geerlings MI, Schoevers RA, Beekman AT, Jonker C, Deeg DJ, Schmand B, et al. Depression and risk of cognitive decline and Alzheimer’s disease. Results of two prospective community-based studies in The Netherlands. Br J Psychiatry. 2000;176:568–575. doi: 10.1192/bjp.176.6.568. [DOI] [PubMed] [Google Scholar]
- 13.Wilson RS, Barnes LL, Mendes de Leon CF, Aggarwal NT, Schneider JS, Bach J, et al. Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology. 2002;59(3):364–370. doi: 10.1212/WNL.59.3.364. [DOI] [PubMed] [Google Scholar]
- 14.Goveas JS, Espeland MA, Woods NF, Wassertheil-Smoller S, Kotchen JM. Depressive symptoms and incidence of mild cognitive impairment and probable dementia in elderly women: the Women’s Health Initiative Memory Study. J Am Geriatr Soc. 2011;59(1):57–66. doi: 10.1111/j.1532-5415.2010.03233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersen K, Lolk A, Kragh-Sørensen P, Petersen NE, Green A. Depression and the risk of Alzheimer disease. Epidemiology (Cambridge, Mass) 2005;16(2):233–238. doi: 10.1097/01.ede.0000152116.32580.24. [DOI] [PubMed] [Google Scholar]
- 16.Saczynski JS, Beiser A, Seshadri S, Auerbach S, Wolf PA, Au R. Depressive symptoms and risk of dementia: the Framingham Heart Study. Neurology. 2010;75(1):35–41. doi: 10.1212/WNL.0b013e3181e62138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gatz JL, Tyas SL, St John P, Montgomery P. Do depressive symptoms predict Alzheimer’s disease and dementia? J Gerontol A Biol Sci Med Sci. 2005;60(6):744–747. doi: 10.1093/gerona/60.6.744. [DOI] [PubMed] [Google Scholar]
- 18.Byers AL, Covinsky KE, Barnes DE, Yaffe K. Dysthymia and depression increase risk of dementia and mortality among older veterans. Am J Geriatr Psychiatry. 2012;20(8):664–672. doi: 10.1097/JGP.0b013e31822001c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen R, Hu Z, Wei L, Qin X, McCracken C, Copeland JR. Severity of depression and risk for subsequent dementia: cohort studies in China and the UK. Br J Psychiatry. 2008;193(5):373–377. doi: 10.1192/bjp.bp.107.044974. [DOI] [PubMed] [Google Scholar]
- 20.Hébert R, Lindsay J, Verreault R, Rockwood K, Hill G, Dubois MF. Vascular dementia: incidence and risk factors in the Canadian study of health and aging. Stroke. 2000;31(7):1487–1493. doi: 10.1161/01.STR.31.7.1487. [DOI] [PubMed] [Google Scholar]
- 21.Green RC, Cupples LA, Kurz A, Auerbach S, Go R, Sadovnick D, et al. Depression as a risk factor for Alzheimer disease: the MIRAGE Study. Arch Neurol. 2003;60(5):753–759. doi: 10.1001/archneur.60.5.753. [DOI] [PubMed] [Google Scholar]
- 22.Dal Forno G, Palermo MT, Donohue JE, Karagiozis H, Zonderman AB, Kawas CH. Depressive symptoms, sex, and risk for Alzheimer’s disease. Ann Neurol. 2005;57(3):381–387. doi: 10.1002/ana.20405. [DOI] [PubMed] [Google Scholar]
- 23.Geerlings MI, den Heijer T, Koudstaal PJ, Hofman A, Breteler MM. History of depression, depressive symptoms, and medial temporal lobe atrophy and the risk of Alzheimer disease. Neurology. 2008;70(15):1258–1264. doi: 10.1212/01.wnl.0000308937.30473.d1. [DOI] [PubMed] [Google Scholar]
- 24.Dotson VM, Beydoun MA, Zonderman AB. Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology. 2010;75(1):27–34. doi: 10.1212/WNL.0b013e3181e62124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cankurtaran M, Yavuz BB, Cankurtaran ES, Halil M, Ulger Z, Ariogul S. Risk factors and type of dementia: vascular or Alzheimer? Arch Gerontol Geriatr. 2008;47(1):25–34. doi: 10.1016/j.archger.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Geerlings MI, Schmand B, Braam AW, Jonker C, Bouter LM, van Tilburg W. Depressive symptoms and risk of Alzheimer’s disease in more highly educated older people. J Am Geriatr Soc. 2000;48(9):1092–1097. doi: 10.1111/j.1532-5415.2000.tb04785.x. [DOI] [PubMed] [Google Scholar]
- 27.Lindsay J, Laurin D, Verreault R, Hébert R, Helliwell B, Hill GB, et al. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156(5):445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- 28.Arango C, Dragioti E, Solmi M, Cortese S, Domschke K, Murray RM, et al. Risk and protective factors for mental disorders beyond genetics: an evidence-based atlas. World Psychiatry. 2021;20(3):417–436. doi: 10.1002/wps.20894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zonderman AB. Predicting Alzheimer’s disease in the Baltimore longitudinal study of aging. J Geriatr Psychiatry Neurol. 2005;18(4):192–195. doi: 10.1177/0891988705281863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289(20):2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 31.Resnick SM, Maki PM, Rapp SR, Espeland MA, Brunner R, Coker LH, et al. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J Clin Endocrinol Metab. 2006;91(5):1802–1810. doi: 10.1210/jc.2005-2097. [DOI] [PubMed] [Google Scholar]
- 32.Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291(24):2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 33.Almeida OP, Lautenschlager NT, Vasikaran S, Leedman P, Gelavis A, Flicker L. A 20-week randomized controlled trial of estradiol replacement therapy for women aged 70 years and older: effect on mood, cognition and quality of life. Neurobiol Aging. 2006;27(1):141–149. doi: 10.1016/j.neurobiolaging.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 34.Arevalo M-A, Azcoitia I, Garcia-Segura LM. The neuroprotective actions of oestradiol and oestrogen receptors. Nat Rev Neurosci. 2015;16(1):17–29. doi: 10.1038/nrn3856. [DOI] [PubMed] [Google Scholar]
- 35.Zárate S, Stevnsner T, Gredilla R. Role of estrogen and other sex hormones in brain aging. Neuroprotection and DNA repair. Front Aging Neurosci. 2017;9:430. doi: 10.3389/fnagi.2017.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort profile: the National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2017;46(2):e15. doi: 10.1093/ije/dyv319. [DOI] [PubMed] [Google Scholar]
- 37.Shin DW, Cho B, Guallar E. Korean National Health Insurance Database. JAMA Intern Med. 2016;176(1):138. doi: 10.1001/jamainternmed.2015.7110. [DOI] [PubMed] [Google Scholar]
- 38.Yoo K-Y. Cancer control activities in the Republic of Korea. Jpn J Clin Oncol. 2008;38(5):327–333. doi: 10.1093/jjco/hyn026. [DOI] [PubMed] [Google Scholar]
- 39.Cheol Seong S, Kim Y-Y, Khang Y-H, Heon Park J, Kang H-J, Lee H, et al. Data resource profile: the National Health Information Database of the National Health Insurance Service in South Korea. Int J Epidemiol. 2016;46(3):799–800. doi: 10.1093/ije/dyw253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, et al. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause (New York, NY) 2012;19(4):387–395. doi: 10.1097/gme.0b013e31824d8f40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoo JE, Shin DW, Han K, Kim D, Won HS, Lee J, et al. Female reproductive factors and the risk of dementia: a nationwide cohort study. Eur J Neurol. 2020;27(8):1448–1458. doi: 10.1111/ene.14315. [DOI] [PubMed] [Google Scholar]
- 42.Drew L. An age-old story of dementia. Nature. 2018;559(7715):S2–S3. doi: 10.1038/d41586-018-05718-5. [DOI] [PubMed] [Google Scholar]
- 43.Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7(6):323–331. doi: 10.1038/nrneurol.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gingerich S, Kim GL, Chalmers JA, Koletar MM, Wang X, Wang Y, et al. Estrogen receptor α and G-protein coupled receptor 30 mediate the neuroprotective effects of 17β-estradiol in novel murine hippocampal cell models. Neuroscience. 2010;170(1):54–66. doi: 10.1016/j.neuroscience.2010.06.076. [DOI] [PubMed] [Google Scholar]
- 45.Vallés SL, Borrás C, Gambini J, Furriol J, Ortega A, Sastre J, et al. Oestradiol or genistein rescues neurons from amyloid beta-induced cell death by inhibiting activation of p38. Aging Cell. 2008;7(1):112–118. doi: 10.1111/j.1474-9726.2007.00356.x. [DOI] [PubMed] [Google Scholar]
- 46.Chowen JA, Torres-Alemán I, García-Segura LM. Trophic effects of estradiol on fetal rat hypothalamic neurons. Neuroendocrinology. 1992;56(6):895–901. doi: 10.1159/000126321. [DOI] [PubMed] [Google Scholar]
- 47.Ding F, Yao J, Zhao L, Mao Z, Chen S, Brinton RD. Ovariectomy induces a shift in fuel availability and metabolism in the hippocampus of the female transgenic model of familial Alzheimer’s. PLoS One. 2013;8(3):e59825. doi: 10.1371/journal.pone.0059825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schönknecht P, Pantel J, Klinga K, Jensen M, Hartmann T, Salbach B, et al. Reduced cerebrospinal fluid estradiol levels are associated with increased beta-amyloid levels in female patients with Alzheimer’s disease. Neurosci Lett. 2001;307(2):122–124. doi: 10.1016/S0304-3940(01)01896-1. [DOI] [PubMed] [Google Scholar]
- 49.Spence RD, Wisdom AJ, Cao Y, Hill HM, Mongerson CR, Stapornkul B, et al. Estrogen mediates neuroprotection and anti-inflammatory effects during EAE through ERα signaling on astrocytes but not through ERβ signaling on astrocytes or neurons. J Neurosci. 2013;33(26):10924–10933. doi: 10.1523/JNEUROSCI.0886-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johann S, Beyer C. Neuroprotection by gonadal steroid hormones in acute brain damage requires cooperation with astroglia and microglia. J Steroid Biochem Mol Biol. 2013;137:71–81. doi: 10.1016/j.jsbmb.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 51.Olver TD, Hiemstra JA, Edwards JC, Schachtman TR, Heesch CM, Fadel PJ, et al. Loss of female sex hormones exacerbates cerebrovascular and cognitive dysfunction in aortic banded miniswine through a neuropeptide Y-Ca(2+)-activated potassium channel-nitric oxide mediated mechanism. J Am Heart Assoc. 2017;6(11):e007409. doi: 10.1161/JAHA.117.007409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet (London, England) 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shin K-Y. A new approach to social inequality: inequality of income and wealth in South Korea. J Chin Sociol. 2020;7(1):17. doi: 10.1186/s40711-020-00126-7. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Table 1. Hazard ratios and 95% confidence intervals of the duration of hormone therapy on the diagnosis of dementia with age as the time-scale. Supplementary Table 2. Hazard ratios and 95% confidence intervals of the duration of hormone therapy on the diagnosis of dementia including those who responded as “unknown” to the questions about hormone therapy. Supplementary Table 3. Hazard ratios and 95% confidence intervals of the duration of hormone therapy on the diagnosis of dementia excluding those who were diagnosed with dementia 3 years after the diagnosis of depression. Supplementary Table 4. Hazard ratios and 95% confidence intervals of the duration of hormone therapy on the diagnosis of dementia adding age at menopause as an adjusting variable.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found in the following: https://nhiss.nhis.or.kr/.