Abstract
The insecticidal activity and receptor binding properties of Bacillus thuringiensis Cry1A toxins towards the forest pests Thaumetopoea pityocampa (processionary moth) and Lymantria monacha (nun moth) were investigated. Cry1Aa, Cry1Ab, and Cry1Ac were highly toxic (corresponding 50% lethal concentration values: 956, 895, and 379 pg/μl, respectively) to first-instar T. pityocampa larvae. During larval development, Cry1Ab and Cry1Ac toxicity decreased with increasing age, although the loss of activity was more pronounced for Cry1Ab. Binding assays with 125I-labelled Cry1Ab and brush border membrane vesicles from T. pityocampa first- and last-instar larvae detected a remarkable decrease in the overall Cry1Ab binding affinity in last-instar larvae, although saturable Cry1Ab binding to both instars was observed. Homologous competition experiments demonstrated the loss of one of the two Cry1Ab high-affinity binding sites detected in first-instar larvae. Growth inhibition assays with sublethal doses of Cry1Aa, Cry1Ab, and Cry1Ac in L. monacha showed that all three toxins were able to delay molting from second instar to third instar. Specific saturable binding of Cry1Ab was detected only in first- and second-instar larvae. Cry1Ab binding was not detected in last-instar larvae, although specific binding of Cry1Aa and Cry1Ac was observed. These results demonstrate a loss of Cry1Ab binding sites during development on the midgut epithelium of T. pityocampa and L. monacha, correlating in T. pityocampa with a decrease in Cry1Ab toxicity with increasing age.
The discovery of Bacillus thuringiensis has had the greatest impact on the use of biopesticides in forestry, as well as in crop systems and stored products. B. thuringiensis is a bacterium that produces proteinaceous insecticidal toxins in the form of inclusion bodies or crystals at sporulation. Each toxin specifically recognizes and binds to receptors in the insect gut, forming membrane pores that disrupt the selective permeability of the cells and eventually cause lysis of epithelial cells and insect death (5, 11, 22, 36).
Binding of toxins to midgut receptors is a key step in the mode of action of B. thuringiensis toxins (5, 6, 14, 15, 36, 37). Specific binding of lepidopteran-active toxins has been demonstrated by incubating midgut brush border membrane vesicles (BBMVs) (14, 15, 36, 37), tissue sections (5, 6), and BBMV protein blots (10, 26) with radiolabelled or biotin-labelled toxins. These studies aided in the characterization of the receptor system of many insect pests and identified several toxin-binding proteins (7, 12, 20, 21, 23, 30, 32).
The use of B. thuringiensis as a biopesticide has proven to be a viable alternative to chemical insecticides (25). There has been a major increase in the proportion of areas treated with B. thuringiensis in North America, particularly in Eastern Canada (33, 34), and in Eastern Europe, at the expense of chemical insecticides such as fenitrothion (Canada) and diflubenzuron and pyrethroids (Eastern Europe). This trend is expected to continue in the future, with at least 50% of forest areas being treated with microbial insecticides, of which B. thuringiensis will remain dominant (8). The environmental benefit of their increased use is their specificity and the possibility to use them in both rural and urban woods (25).
This paper analyzes the toxicity and binding of B. thuringiensis toxins in two important forest pests, Thaumetopoea pityocampa and Lymantria monacha, in an attempt to optimize the use of this microbial pathogen to control the two forest pests in the field.
T. pityocampa, processionary moth, is an important pine defoliator that represents a major endemic pest in Southern Europe. The larvae are covered with urticating hairs that can cause serious skin irritation in those living in close proximity to infested trees, and there is, thus, considerable public pressure to manage this moth population.
The nun moth, L. monacha, is one of the most severe insect pest species of European conifers, although this insect can also affect other host genera, such as Quercus, Fagus, and Betula, and even herbs. This is an epidemic pest distributed throughout Europe and parts of Asia, south of 60° latitude, thus occurring from Portugal to Japan.
We have previously demonstrated that Cry1Aa, Cry1Ab, and Cry1Ac specifically bound to the midgut brush border membrane of second-instar T. pityocampa larvae and that all three toxins compete for binding (28). In the present paper, we correlate the decrease in Cry1Ab toxicity during development with the loss of a binding site for this toxin in last-instar larvae. Similarly, in L. monacha we have found that binding of Cry1Ab to the larval midgut membrane was lost during the larval development of the insect.
MATERIALS AND METHODS
Biological material.
T. pityocampa S. and L. monacha L. larvae were used in all experiments. T. pityocampa larvae were collected from natural populations in Burjassot (Valencia, Spain). Larvae were fed on pine needles (Pinus sylvestris) at room temperature. L. monacha larvae were reared from egg masses collected from Orihuela del Tremedal (Teruel, Spain). Eggs were stored at 4°C until needed and then incubated at 25°C for hatching. At eclosion, larvae were transferred to petri dishes and reared, until third instar, on an artificial diet, according to the method of Grijpma et al. (13), except that aureomycin was omitted due to incompatibility with B. thuringiensis treatments (27). From third instar until pupation, larvae were fed on Pinus halepensis needles. Rearing conditions were as follows: 25°C, 70% relative humidity, and a 16-h–8-h (light-dark) photoperiod.
Insect toxicity assays.
Bioassays were carried out to determine the sensitivity of first-instar T. pityocampa larvae to Cry1A-type B. thuringiensis trypsin-activated toxins. Trypsin digestion and toxin purification were performed by the method of Höfte et al. (16). Each bioassay consisted of five doses of the corresponding activated crystal toxins (Cry1Aa, Cry1Ab, and Cry1Ac) prepared in phosphate-buffered saline (PBS) (8 mM Na2HPO4, 2 mM KH2PO4, 150 mM NaCl, pH 7.4) containing 0.1% bovine serum albumin (BSA) and a control of PBS (pH 7.4)–0.1% BSA. Fresh P. sylvestris needles were dipped into this suspension and allowed to air dry. Three groups of 20 larvae (1 day old) were placed on the coated pine needles for 4 days, after which the coated needles were replaced with fresh, untreated needles. Mortality was recorded 4 days later. Toxicity data were evaluated by probit analysis using the Polo PC program (LeOra Software, Berkeley, Calif.). Fifty percent lethal concentration (LC50) measurements refer to the concentration of crystal protein which, when applied uniformly on the needles, produces 50% mortality.
Toxicities of Cry1Ab (1 and 10 ng/μl) and of Cry1Ac (0.4 and 4 ng/μl) were also tested on third-instar T. pityocampa larvae as described above.
To study sublethal effects of B. thuringiensis Cry1A-type toxins on L. monacha development, freshly prepared artificial diet was dispensed in wells with 2 cm2 of surface area (Costar 24-well cluster plate), and dilutions of the corresponding toxin (50 μl; prepared in PBS [pH 7.4]–0.1% BSA) were uniformly applied over the food surface in each well and allowed to dry. Single larvae in the first day of the second instar were placed in each well; 20 larvae were used per dilution. Three dilutions of each trypsin-activated toxin (Cry1Aa, Cry1Ab, and Cry1Ac), corresponding to 2, 20, and 200 ng/cm2, were analyzed. After five days of toxin exposure, the percentage of larvae reaching third instar was scored. Experiments were duplicated, and controls with PBS were also included.
Binding to midgut tissue sections.
The midgut tissue preparation, sectioning, binding, and immunocytochemical staining were performed according to the method of Bravo et al. (5). Experiments involving binding of Cry1Ab and Cry1Ac trypsin-activated toxins to T. pityocampa tissue sections from first- and third-instar larvae were carried out as follows. Tissue sections were incubated with the appropriate toxin for 1 h by using 0.3 ml of a mixture of 10 μg of toxin per ml of TST buffer (10 mM Tris-HCl, 100 mM NaCl, 1 mM sodium ethylmercurisalicylate, 0.1% [vol/vol] Triton X-100, pH 7.6). Immunolocalization of toxins was performed by incubating tissue sections overnight with 0.3 ml of the monoclonal antibody 4D6 (Plant Genetic Systems, Ghent, Belgium) (0.1 μg/ml in TST buffer). Unbound antibody was rinsed with TST buffer, and an alkaline phosphatase-coupled secondary antibody was used to detect the binding. Color development was obtained by incubation in 0.5 ml of BCIP-nitroblue tetrazolium (NBT) solution (8 mM 5-bromo-4-chloro-3-indolyl phosphate and 9 mM 4-NBT chloride in 5 mM MgCl2, 100 mM Tris, 100 mM NaCl, pH 9.5) for 10 min. Color development was stopped by lowering the pH with glacial acetic acid-water (1:10, vol/vol). Slices were dehydrated by successive incubations in 70% and 100% ethanol and 100% xylol. Finally, tissue sections were covered with Entellan mounting medium (Merck, Darmstadt, Germany) and photographed.
Preparation of BBMVs.
BBMVs were prepared according to the method of Wolfersberger et al. (40) from T. pityocampa first- and early second-instar larvae, T. pityocampa last-instar larvae, L. monacha first- and early second-instar larvae, and L. monacha last-instar larvae. All BBMV preparations were from whole larvae of the corresponding instar, except for L. monacha last-instar larvae, from which dissected midguts were used. The final pellet was resuspended in ice-cold MET buffer (0.3 M mannitol, 5 mM EGTA, 17 mM Tris-HCl, pH 7.5), immediately frozen, and stored at −80°C.
The protein concentration of BBMVs was measured by Bradford's procedure (4) with a Bio-Rad kit (Richmond, Calif.), with BSA as the standard.
Labelling of toxins.
Cry1Aa, Cry1Ab, and Cry1Ac trypsin-activated toxins were iodinated by the chloramine-T method (17) as described by Van Rie et al. (36). The specific radioactivities of iodinated toxins, determined by a sandwich enzyme-linked immunosorbent assay technique, were 5 mCi/mg for Cry1Aa, 36 mCi/mg for Cry1Ab, and 4 mCi/mg for Cry1Ac.
Binding assays.
Immediately before the binding assay, the buffer of the BBMV suspension was replaced with PBS, pH 7.4, containing 0.1% BSA, by microcentrifuge centrifugation. The reaction volume was 0.1 ml, and all samples were duplicated. Other optimal assay conditions were as follows: 0.7 nM 125I-Cry1Ab, 4 μg of BBMV, and 120 min of incubation, for T. pityocampa first- and early second-instar larvae; 1.9 nM 125I-Cry1Ab, 12 μg of BBMV, and 60 min of incubation, for T. pityocampa last-instar larvae; 0.8 nM 125I-Cry1Ab and 60 min of incubation for L. monacha first- and early second-instar larvae; and 125I-labelled toxin (8.7 nM Cry1Aa or 1.3 nM Cry1Ac) and either 60 min (Cry1Aa experiments) or 120 min (Cry1Ac experiments) of incubation for L. monacha last-instar larvae. After incubation, the reaction mixtures were filtered through Whatman GF/F glass-fiber filters in a Millipore filtration manifold 1225 Unit (Millipore Corp., Bedford, Mass.). Filters were washed with 5 ml of ice-cold PBS, pH 7.4, containing 0.1% (wt/vol) BSA, and their radioactivity was measured in a 1282 Compugamma CS counter (LKB).
For homologous competition experiments, the reaction mixture contained the corresponding amount of BBMV and labelled toxin and increasing amounts of unlabelled toxin. Binding data were analyzed with the LIGAND program (24), which estimates the binding constants (equilibrium dissociation constant [Kd] and binding site concentration [Rt]). A Student t test was used to determine whether the mean values of the calculated binding constants were significantly different.
RESULTS
T. pityocampa toxicity assays.
Toxicity values for Cry1Aa, Cry1Ab, and Cry1Ac toxins to first-instar T. pityocampa larvae are presented in Table 1. Cry1Ac was most toxic, while Cry1Aa and Cry1Ab were equally toxic (95% fiducial limits overlap).
TABLE 1.
Toxicity of Cry1Aa, Cry1Ab, and Cry1Ac toxins to first-instar T. pityocampa larvaea
| Toxin | Slope ± SE | LC50, pg/μl (90% fiducial limits) |
|---|---|---|
| Cry1Aa | 1.86 ± 0.36 | 956 (586–1,552) |
| Cry1Ab | 3.55 ± 1.11 | 895 (694–1,687) |
| Cry1Ac | 2.68 ± 0.55 | 379 (279–543)b |
Slopes and LC50 values were determined by the POLO-PC program for dose-mortality curves, LeOra Software.
95% fiducial limits.
During processionary moth larval development, 1,000 pg of Cry1Ab per μl (concentration around the Cry1Ab LC50 value obtained in first-instar larvae) produced only 10% mortality when applied to either second- or third-instar larvae. However, 400 pg of Cry1Ac per μl (also corresponding to the Cry1Ac LC50 value obtained in first-instar larvae) caused 35% mortality in second-instar larvae and 10% mortality in third-instar larvae. Hence, the susceptibility of second-instar larvae to Cry1Ab and Cry1Ac toxins is markedly different. A concentration of 10,000 pg of Cry1Ab per μl (a toxin concentration 10-fold higher than the respective LC50 values in first-instar larvae, which produced 100% lethality in first-instar larvae) resulted in 10% mortality in second-instar larvae. Even a 4,000-pg/μl Cry1Ac concentration (corresponding to a 100% lethal concentration in first-instar larvae) caused between 40 and 60% mortality in second-instar larvae. These findings stress that second-instar larvae display a different susceptibility to Cry1Ab and Cry1Ac, Cry1Ab being less toxic than Cry1Ac.
Binding to T. pityocampa midgut brush border membrane.
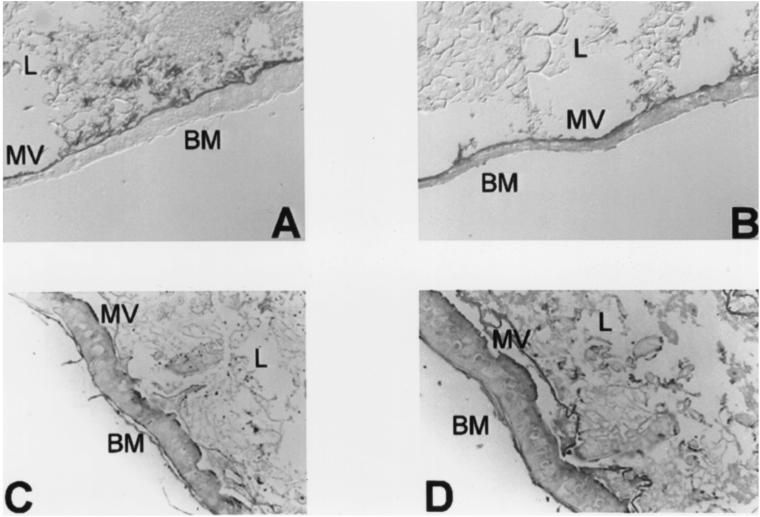
To analyze the cellular basis for the different toxin susceptibilities of different stages of T. pityocampa larval development, tissue sections from early first- and third-instar T. pityocampa larvae were prepared and binding of Cry1Ab and Cry1Ac toxins was analyzed. Cry1Ab and Cry1Ac both were able to specifically bind to the midgut brush border membrane of first- and third-instar larvae (Fig. 1), confirming the existence of specific binding sites for these B. thuringiensis toxins (28). Controls without toxin or primary antibody were also included (data not shown).
FIG. 1.
Binding of Cry1Ab and Cry1Ac to brush border membranes of first- and third-instar T. pityocampa larva midguts. (A) Cry1Ab binding to first-instar larva tissue sections; (B) Cry1Ac binding to first-instar larva tissue sections; (C) Cry1Ab binding to third-instar larva tissue sections; and (D) Cry1Ac binding to third-instar larva tissue sections. BM, basal membrane; MV, microvilli; L, lumen.
To investigate the biochemical basis of the observed developmental variations in Cry1Ab toxicity, binding saturation experiments with 125I-labelled Cry1Ab were performed. Cry1Ab binding to BBMVs from first- and last-instar T. pityocampa larvae followed sigmoidal kinetics (Fig. 2). Binding of Cry1Ab to BBMVs varied with larval development, maximum binding being shown to be between 80 and 150 μg of BBMV/ml, depending on the developmental stage analyzed (Fig. 2). The sigmoidal shape of the curve observed with last-instar larvae (Fig. 2B) indicated a decrease in binding affinity when compared with the hyperbolic curve obtained in first-instar larvae (Fig. 2A).
FIG. 2.
Specific binding of 125I-labelled Cry1Ab toxin as a function of BBMV concentration of first-instar T. pityocampa larvae (A) or last-instar T. pityocampa larvae (B). For each point, nonspecific binding (percentage of the total 125I-Cry1Ab counts that were bound to the BBMVs in the presence of a 500-fold excess of unlabelled Cry1Ab) was subtracted from total binding (percentage of the total 125I-Cry1Ab counts that were bound to the BBMVs).
Quantitative estimates of homologous binding competition experiments are shown in Fig. 3. Equilibrium dissociation constants and binding site concentrations for Cry1Ab binding to first- and last-instar T. pityocampa larvae were as follows. For first- and second-instar larvae, the Kd1 (± standard deviation [SD]) was 0.29 ± 0.17 nM; the Kd2 was 2.55 ± 1.62 nM. The Rt1 was 0.95 ± 0.41 pmol/mg; the Rt2 was 2.75 ± 1.74 pmol/mg. For last-instar larvae, the Kd (± SD) was 3.42 ± 0.66 nM, and the Rt was 1.29 ± 0.06 pmol/mg. In first-instar larvae, Cry1Ab exhibits one additional high-affinity site which is absent from last-instar larvae. The loss of the Cry1Ab high-affinity binding site during development is in accordance with the decrease in affinity observed in the saturation curve obtained in last-instar larvae (Fig. 2B) compared with that of first-instar larvae (Fig. 2A). In first-instar larvae, saturation is reached at around 80 μg of BBMV protein/ml, whereas, in last-instar larvae, only 25% of maximum binding was detected for that BBMV protein concentration.
FIG. 3.
Binding of 125I-labelled Cry1Ab toxin as a function of increasing concentration of nonlabelled Cry1Ab to BBMVs of first-instar T. pityocampa larvae (A) or last-instar T. pityocampa larvae (B). For each panel, data points correspond to one of at least three independent competition experiments performed.
The lack of Cry1Ab binding to the high-affinity site in last-instar larvae is consistent with the decrease in toxicity and binding to tissue sections of this toxin during processionary moth development.
Larval growth inhibition assays on L. monacha.
Preliminary bioassays with L. monacha showed a low toxicity of Cry1A toxins compared with that for other insects, such as T. pityocampa. Due to this fact, we performed larval growth inhibition experiments with Cry1Aa, Cry1Ab, and Cry1Ac toxins instead of dose-mortality curves. The effects of three sublethal concentrations of each toxin are shown in Fig. 4. In these experiments, as well as in controls with nontreated larvae, mortality never exceeded 5% and 87% of nontreated larva controls survived until the third instar. Results showed around 100% inhibition of molting from second to third instar when a 200-ng/cm2 concentration of Cry1A-type toxins was used. Fifty percent of the larvae did not molt when exposed to a 2-ng/cm2 concentration of Cry1Aa, while no significant differences with controls were found when the same Cry1Ab or Cry1Ac concentrations were applied. A 10-fold-higher concentration of Cry1Ac (20 ng/cm2) was needed to give rise to 50% molting inhibition, while for the same Cry1Ab concentration no significant differences with controls were observed.
FIG. 4.
Percentage of L. monacha second-instar larvae that molted to the third instar when sublethal concentrations of Cry1Aa, Cry1Ab, and Cry1Ac were applied on an artificial diet. Percentages were calculated over the total surviving larvae after treatment. In all experiments (including the control, nontreated larvae), mortality never exceeded 5%. The control, nontreated larvae, showed 87% molting to the third instar. Symbols: ▵, Cry1Aa; ●, Cry1Ab; and □, Cry1Ac.
In conclusion, larval growth was significantly affected by toxin concentration and the order of relative efficiency in molting inhibition was as follows: Cry1Aa > Cry1Ac > Cry1Ab.
Binding to L. monacha midgut BBMVs.
In order to determine whether the observed L. monacha larval growth inhibition can be related to the occurrence of specific binding of toxins to BBMV, binding experiments with radiolabelled toxins were carried out. Saturable binding of 125I-Cry1Aa and 125I-Cry1Ac to BBMVs prepared from last-instar L. monacha larvae was evident (Fig. 5A and B). However, no binding of 125I-Cry1Ab was detected at this developmental stage (Fig. 5C), which contrasted with the observed effect of Cry1Ab on molting inhibition. BBMVs from first- and second-instar larvae were then prepared, and binding saturation experiments with 125I-Cry1Ab were performed. As in the case of T. pityocampa with Cry1Ab, results showed saturable binding of Cry1Ab to BBMVs of early stages of L. monacha (Fig. 5D).
FIG. 5.
(A) Specific binding of 125I-Cry1Aa to increasing concentrations of BBMVs from last-instar L. monacha larvae. (B) Specific binding of 125I-Cry1Ac to increasing concentrations of BBMVs from last-instar L. monacha larvae. (C) Specific binding of 125I-Cry1Ab to increasing concentrations of BBMVs from last-instar L. monacha larvae. (D) Specific binding of 125I-Cry1Ab to increasing concentrations of BBMVs from first- and second-instar L. monacha larvae. In all cases, the reaction volume was 0.1 ml and for each point nonspecific binding was subtracted from total binding (see the legend to Fig. 2).
DISCUSSION
In this paper, the insecticidal activity of Cry1A-type B. thuringiensis toxins on T. pityocampa, and thus their suitability for processionary moth control, was demonstrated.
In addition, we demonstrated that the toxicity of Cry1Ab and Cry1Ac during larval development decreased with increasing age. A loss of toxicity in later instars is well documented and has been found for several insect pests in relation to B. thuringiensis (1–3, 9, 18, 19, 22, 29, 31, 35, 38), showing that the toxicity was mainly determined by the developmental stage of the larvae, i.e., it was significantly higher for earlier stages when compared with later stages. The biochemical basis of the reduced susceptibility among instars has so far been investigated only in Spodoptera spp. (19, 22), in which a variation of toxin receptor density during larval development has been proposed to account for the decrease in the capacity of Cry1C and Cry1D toxins to induce permeability changes on BBMVs in late instars (22).
In addition, experimental data indicated a loss or affinity reduction of one of the two Cry1Ab binding sites during development, which may account for the reduced Cry1Ab susceptibility with increasing T. pityocampa larval age. To our knowledge, T. pityocampa is the first insect pest in which changes in B. thuringiensis toxin binding sites during development have been reported. The occurrence of these changes may have a significant influence on the final efficacy of the formulations used against this insect, because larvae in the field are not synchronized and different instars coexist in infested areas. Thus, according to our results, formulations having a higher content of toxins whose activity is not readily lost during development would be more appropriate than those enriched in other toxins whose toxic effect decreases during larval development, as in the case of Cry1Ab in this insect.
On the other hand, in L. monacha, growth inhibition assays with sublethal doses of Cry1Aa, Cry1Ab, and Cry1Ac have shown that all three toxins were able to delay development from second to third instar. We have also demonstrated that Cry1Aa and Cry1Ac toxins specifically bound to last-instar L. monacha larva BBMVs whereas Cry1Ab did not, although Cry1Ab saturable binding to the brush border membrane of first-second instar L. monacha larvae was indeed observed. Therefore, the decrease in Cry1Ab toxicity during development due to the loss of binding sites in later instars could be a more general phenomenon, present not only in T. pityocampa but also in other insects.
A similar rationale could be applied to Lymantria dispar. In this insect, an inverse correlation has been reported between toxicity and binding properties of Cry1Ab and Cry1Ac to BBMV (39), Cry1Ab being significantly more toxic than Cry1Ac albeit binding with lower affinity. Surprisingly, when we reexamined the experiments of Wolfersberger (39) we realized that toxicity assays were performed on first-instar larvae whereas homologous competition experiments were done with BBMVs from last-instar larvae. Again, a decrease in or loss of Cry1Ab affinity for its binding sites during development could explain this disagreement between toxicity and binding data.
The finding of the variation of Cry1Ab binding sites during development can be explained by a differential expression pattern of the binding molecule in various larval stages. Alternatively, specific posttranslation modifications during development could also be responsible for the decrease in or loss of Cry1Ab binding. The overall effect of either of these changes could have important implications in the characterization of the toxin binding molecules as well as in the design and application of B. thuringiensis formulations appropriate for each insect pest.
ACKNOWLEDGMENTS
We are indebted to Gema Pérez-Guerra and Axel Gruppe for their technical support on the rearing of L. monacha. We also thank Plant Genetic Systems (Ghent, Belgium), Eduardo Obama, Laboratorio de Sanidad Vegetal de Mora de Rubielos (Teruel), and Servicio de Protección Vegetal de Silla (Valencia) for their expert technical assistance.
This work was supported by the Spanish Ministerio de Agricultura Pesca y Alimentación (Project No. AGF93-1171) and the Spanish Ministerio de Educación y Ciencia (Project No. PB95-1090). Carolina Rausell and Inmaculada García-Robles were supported by grants from the Spanish Ministerio de Educación y Ciencia and the Conselleria Valenciana d'Educació i Ciència, respectively.
REFERENCES
- 1.Ali A, Young S Y. Activity of Bacillus thuringiensis Berliner against different ages and stages of Helicoperva zea (Lepidoptera: Noctuidae) on cotton. J Econ Entomol. 1996;31:1–8. [Google Scholar]
- 2.Bauer L S. Response of the cottonwood leaf beetle (Coleoptera: Chrysomelidae) to Bacillus thuringiensis var. san diego. Environ Entomol. 1990;19:428–431. [Google Scholar]
- 3.Bauer L S. Response of the imported willow leaf beetle to Bacillus thuringiensis var. san diego on poplar and willow. J Invertebr Pathol. 1992;59:330–331. [Google Scholar]
- 4.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 5.Bravo A, Hendrickx K, Jansens S, Peferoen M. Immunocytochemical analysis of specific binding of Bacillus thuringiensis insecticidal crystal proteins to lepidopteran and coleopteran midgut membranes. J Invertebr Pathol. 1992;60:247–253. [Google Scholar]
- 6.Denolf P, Jansens S, Van Rie J, Degheele D, Peferoen M. Biotinylation of Bacillus thuringiensis insecticidal crystal proteins. Appl Environ Microbiol. 1993;59:1821–1827. doi: 10.1128/aem.59.6.1821-1827.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denolf P, Hendrickx K, Seurinck J, Vandamme J, Jansens S, Peferoen M, Van Rie J. Cloning and characterization of Manduca sexta and Plutella xylostella midgut aminopeptidase N related to Bacillus thuringiensis toxin-binding proteins. Eur J Biochem. 1997;248:748–761. doi: 10.1111/j.1432-1033.1997.t01-1-00748.x. [DOI] [PubMed] [Google Scholar]
- 8.Evans H F. British Crop Protection Council (ed.), Microbial insecticides: novelty or necessity? Symposium proceedings, no. 68. Nottingham, United Kingdom: Major Desing & Production Ltd.; 1997. The role of microbial insecticides in forest pest management; pp. 29–40. [Google Scholar]
- 9.Ferro D N, Lyon S M. Colorado potato beetle (Coleoptera: Chrysomelidae) larval mortality: operative effects of Bacillus thuringiensis subsp. san diego. J Econ Entomol. 1991;84:806–809. [Google Scholar]
- 10.Garczynski S F, Crim S W, Adang M J. Identification of putative insect brush border membrane-binding molecules specific to Bacillus thuringiensis δ-endotoxin by protein blot analysis. Appl Environ Microbiol. 1991;57:2816–2820. doi: 10.1128/aem.57.10.2816-2820.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gill S S, Cowles E A, Pietrantonio P V. The mode of action of Bacillus thuringiensis endotoxins. Annu Rev Entomol. 1992;37:615–636. doi: 10.1146/annurev.en.37.010192.003151. [DOI] [PubMed] [Google Scholar]
- 12.Gill S, Cowles E, Francis V. Identification, isolation and cloning of a Bacillus thuringiensis CryIA(c) toxin-binding protein from the midgut of the lepidopteran insect Heliothis virescens. J Biol Chem. 1995;270:20309–20315. doi: 10.1074/jbc.270.45.27277. [DOI] [PubMed] [Google Scholar]
- 13.Grijpma P, Belde J J M, van der Werf D C. Artificial diets and rearing of the nun moth, Lymantria monacha. Entomol Exp Appl. 1987;45:219–225. [Google Scholar]
- 14.Hofmann C, Lüthy P, Hütter R, Pliska V. Binding of the delta-endotoxin from Bacillus thuringiensis to brush-border membrane vesicles of the cabbage butterfly (Pieris brassicae) Eur J Biochem. 1988;173:85–91. doi: 10.1111/j.1432-1033.1988.tb13970.x. [DOI] [PubMed] [Google Scholar]
- 15.Hofmann C, Vanderbruggen H, Höfte H, Van Rie J, Jansen S, Van Mellaert H. Specificity of Bacillus thuringiensis δ-endotoxins is correlated with the presence of high affinity binding sites in the brush border membrane of target insect midguts. Proc Natl Acad Sci USA. 1988;85:7844–7848. doi: 10.1073/pnas.85.21.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Höfte H, De Greve H, Seurinck J, Jansens S, Mahillon J, Ampe C, Vanderkerckhove J, Vanderbruggen H, Van Montagu M, Zabeau M, Vaeck M. Structural and functional analysis of a cloned delta endotoxin of Bacillus thuringiensis Berliner 1715. Eur J Biochem. 1986;161:273–280. doi: 10.1111/j.1432-1033.1986.tb10443.x. [DOI] [PubMed] [Google Scholar]
- 17.Hunter W M, Greenwood F C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature (London) 1962;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- 18.James R R, Croft B A, Strauss S H. Susceptibility of the cottonwood leaf beetle (Coleoptera: Chrysomelidae) to different strains and transgenic toxins of Bacillus thuringiensis. Environ Entomol. 1999;28:108–115. [Google Scholar]
- 19.Keller M, Sneh B, Strizhov N, Prudovsky E, Regev A, Koncz C, Schell J, Zilberstein A. Digestion of delta-endotoxin by gut proteases may explain reduced sensitivity of advanced instar larvae of Spodoptera littoralis to Cry1C. Insect Biochem Mol Biol. 1996;26:365–373. doi: 10.1016/0965-1748(95)00102-6. [DOI] [PubMed] [Google Scholar]
- 20.Knight P, Knowles B H, Ellar D J. Molecular cloning of an insect aminopeptidase N that serves as a receptor for Bacillus thuringiensis CryIAc toxin. J Biol Chem. 1995;270:17765–17770. doi: 10.1074/jbc.270.30.17765. [DOI] [PubMed] [Google Scholar]
- 21.Lee M, You T, Young B, Cotrill J, Valaitis A, Dean D. Aminopeptidase N purified from gypsy moth brush border membrane vesicles is a specific receptor for Bacillus thuringiensis Cry1Ac toxin. Appl Environ Microbiol. 1996;62:2845–2849. doi: 10.1128/aem.62.8.2845-2849.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lorence A, Darszon A, Díaz C, Liévano A, Quintero R, Bravo A. δ-endotoxins induce cation channels in Spodoptera frugiperda brush border membrane vesicles in suspension and in planar lipid bilayers. FEBS Lett. 1995;360:217–222. doi: 10.1016/0014-5793(95)00092-n. [DOI] [PubMed] [Google Scholar]
- 23.Luo K, Lu Y-J, Adang M J. A 106 kDa form of aminopeptidase is a receptor for Bacillus thuringiensis CryIC δ-endotoxin in the brush border membrane of Manduca sexta. Insect Biochem Mol Biol. 1996;26:783–791. [Google Scholar]
- 24.Munson P J, Rodbard D. LIGAND: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- 25.Navon A. Control of lepidopteran pests with Bacillus thuringiensis. In: Entwistle P F, Cory J S, Bailey M J, Higgs S, editors. Bacillus thuringiensis, an environmental biopesticide: theory and practice. Chichester, England: John Wiley and Sons; 1993. pp. 125–146. [Google Scholar]
- 26.Oddou P, Hartmann H, Geiser M. Identification and characterization of Heliothis virescens midgut membrane proteins binding Bacillus thuringiensis δ-endotoxins. Eur J Biochem. 1991;202:673–680. doi: 10.1111/j.1432-1033.1991.tb16422.x. [DOI] [PubMed] [Google Scholar]
- 27.Pérez-Guerra G. Einfluβ der Nahrung auf die Empfindlichkeit von Nonnenlarven (Lymantria monacha Linné, 1758) (Lepidoptera: Lymantriidae) gegenüber Bacillus thuringiensis (Berliner, 1911) Mitt Dtsch Ges Allg Angew Entomol. 1995;10:147–150. [Google Scholar]
- 28.Rausell C, Martínez-Ramírez A C, García-Robles I, Real M D. The toxicity and physiological effects of Bacillus thuringiensis toxins and formulations to T. pityocampa, the processionary caterpillar. Pestic Biochem Physiol. 1999;65:44–54. [Google Scholar]
- 29.Salama H S, Ragaei M, Sabbour M. Larvae of Phthorimaea operculella (Zell) as affected by various strains of Bacillus thuringiensis. J Appl Entomol. 1995;119:241–243. [Google Scholar]
- 30.Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, Feitelson J, Zeigler D R, Dean D H. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol Mol Biol Rev. 1998;62:775–806. doi: 10.1128/mmbr.62.3.775-806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seyoum A, Abate D. Larvicidal efficacy of Bacillus thuringiensis var. israelensis and Bacillus sphaericus on Anopheles arabiensis in Ethiopia. World J Microbiol Biotechnol. 1997;13:21–24. [Google Scholar]
- 32.Vadlamudi R K, Weber E, Ji I, Ji T H, Bulla L A., Jr Cloning and expression of a receptor for an insecticidal toxin of Bacillus thuringiensis. J Biol Chem. 1995;270:5490–5494. doi: 10.1074/jbc.270.10.5490. [DOI] [PubMed] [Google Scholar]
- 33.Van Frankenhuyzen K. Development and current status of Bacillus thuringiensis for control of defoliating forest insects. For Chron. 1990;56:498–507. [Google Scholar]
- 34.Van Frankenhuyzen K. The challenge of Bacillus thuringiensis. In: Entwistle P F, Cory J S, Bailey M J, Higgs S, editors. Bacillus thuringiensis, an environmental biopesticide: theory and practice. Chichester, England: John Wiley and Sons; 1993. pp. 1–35. [Google Scholar]
- 35.Van Frankenhuyzen K, Gringorten J L, Milne R E, Gauthier D, Pusztai M, Brousseau B, Masson L. Specificity of activated CryIA proteins from Bacillus thuringiensis subsp. kurstaki HD-1 for defoliating forest lepidoptera. Appl Environ Microbiol. 1991;57:1650–1655. doi: 10.1128/aem.57.6.1650-1655.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Rie J, Jansens S, Höfte H, Degheele D, Van Mellaert H. Specificity of Bacillus thuringiensis δ-endotoxins: importance of specific receptors on the brush border membrane of the midgut of target insects. Eur J Biochem. 1989;186:239–247. doi: 10.1111/j.1432-1033.1989.tb15201.x. [DOI] [PubMed] [Google Scholar]
- 37.Van Rie J, Jansens S, Höfte H, Degheele D, Van Mellaert H. Receptors on the brush border membrane of the insect midgut as determinants of the specificity of Bacillus thuringiensis delta-endotoxins. Appl Environ Microbiol. 1990;56:1378–1385. doi: 10.1128/aem.56.5.1378-1385.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wierenga J M, Norris D L, Whalon M E. Stage-specific mortality of Colorado potato beetle (Coleoptera: Chrysomelidae) feeding on transgenic potatoes. J Econ Entomol. 1996;89:1047–1052. [Google Scholar]
- 39.Wolfersberger M G. The toxicity of two Bacillus thuringiensis delta-endotoxins to gypsy moth larvae is inversely related to the affinity of binding sites on midgut brush border membranes for the toxins. Experientia. 1990;46:475–477. doi: 10.1007/BF01954236. [DOI] [PubMed] [Google Scholar]
- 40.Wolfersberger M G, Luthy P, Maure A, Pareti P, Sacchi F V, Giordana B, Hanozet G M. Preparation and partial characterization of aminoacid transporting brush border membrane vesicles from the larval midgut of the cabbage butterfly (Pieris brassicae) Comp Biochem Physiol. 1987;86:301–308. [Google Scholar]