Abstract
Background:
Colorectal cancer is the third most common malignant disease and the second leading cause of death worldwide. Previous studies showed improved bioavailability and cytotoxicity of ginsenoside-modified nanostructured lipid carrier containing curcumin (G-NLC) in human colon cancer cell lines. This study aimed to evaluate the safety and tolerability with long-term survival rates in patients with colorectal cancer with unresectable metastases after treatment with first-line bevacizumab/FOLFIRI (folinic acid, bolus/continuous fluorouracil, and irinotecan) in combination with a dietary supplement of G-NLC.
Methods:
This study was a prospective, observational, single-group analysis. The enrolled patients had colorectal cancer with unresectable metastases and were administered bevacizumab and FOLFIRI in combination with daily oral G-NLC as first-line treatment. Overall survival, progression-free survival, tumor response, and adverse events were evaluated.
Results:
A total of 44 patients were enrolled between 2015 and 2019. The median age was 65 (range 45-81) years and the sex ratio was 31:13 (male:female). The primary tumor locations were the colon (31 patients) and rectum (13 patients). The metastatic sites included, liver only (n = 20), lung only (n = 6), both liver and lung (n = 12), and others (n = 6). The median duration of curcumin supply was 7.9 (range 0.9-16.6) months. The most common grade 3 or higher adverse events were neutropenia (n = 15, 34.1%), followed by nausea (n = 4, 9.1%) and vomiting (n = 4, 9.1%). Within the median follow-up period of 22.8 months, the median overall survival was 30.7 months, and the median progression-free survival was 12.8 months. None of the patients achieved complete response (CR); however, 9 patients showed partial response (PR), and 3 patients underwent conversion surgery.
Conclusions:
Bevacizumab/FOLFIRI with G-NLC as first-line chemotherapy in patients with colorectal cancer with unresectable metastases presented comparable long-term survival outcomes with acceptable toxicity outcomes. Additional randomized controlled studies are needed to establish definitive conclusions regarding this new regimen for metastatic colorectal cancer.
Keywords: curcumin, metastatic colorectal cancer, chemotherapy, survival, adverse event
Introduction
Colorectal cancer is the third most common malignant disease and the second leading cause of death worldwide. 1 Approximately 20% of patients with colorectal cancer are initially diagnosed with synchronous metastatic conditions, and 80% of these patients have unresectable metastases that require palliative systemic chemotherapy. 2 The current standard first-line palliative systemic chemotherapy for metastatic colorectal cancer is FOLFIRI (folinic acid, bolus/continuous fluorouracil, and irinotecan) and FOLFOX (folinic acid, bolus/continuous fluorouracil, and oxaliplatin) plus target agents, including bevacizumab or cetuximab. Despite the recent development of cytotoxic chemotherapy and targeted agents for the treatment of metastatic colorectal cancer, the survival outcomes are still poor. Based on the Aide et Recherche en Cancérologie (ARCAD) database, the median overall survival (OS) and the median progression-free survival (PFS) were only 16.4 and 7.9 months, respectively, in patients with metastatic colorectal cancer who were treated with systemic chemotherapy but did not undergo tumor resection. 3 This implies that patients with unresectable metastatic colorectal cancer have poor survival outcomes despite receiving standard palliative chemotherapy. Furthermore, current chemotherapy is often associated with various adverse events, including neutropenia, diarrhea, nausea, vomiting, and gastrointestinal hemorrhage or perforation.4,5 Therefore, there is an increasing interest in combination therapies that reduce toxicity and enhance the effectiveness of chemotherapeutic agents, thereby improving patients’ quality of life and survival rate.
Curcumin is a polyphenolic antioxidant derived from Curcuma longa, which is known as turmeric in English-speaking countries. 6 It has been widely used as a dietary supplement and flavoring in foods in Southeast Asia. Since curcumin has shown low inherent toxicity and various pharmacological effects, including antioxidant, anticancer, and anti-inflammatory activities, 7 it has increased interest as a complementary agent for chemoprevention. A recent study reported that curcumin-loaded liquid crystalline lipid nanoparticles were efficiently taken up by human colon cells and presented markedly enhanced anticancer activity. 8 Furthermore, our previous studies showed improving bioavailability and cytotoxicity of ginsenoside-modified nanostructured lipid carrier containing curcumin (G-NLC) in human colon cancer cell lines.9,10 Based on these potential therapeutic effects, this study aimed to evaluate long-term survival rates, tolerability and safety in colorectal cancer patients with unresectable metastases after treatment with first-line bevacizumab/FOLFIRI in combination with a dietary supplement of G-NLC.
Methods
Patient Selection
This study was a prospective, observational, single-group analysis. The enrolled patients had colorectal cancer with unresectable metastases and were treated with bevacizumab/FOLFIRI in combination with daily oral curcumin-containing supplement as first-line therapy after signing an informed consent. Patient enrollment was conducted between 2015 and 2019 at Gachon University Gil Medical Center, a tertiary referral hospital in Incheon, South Korea. The detailed inclusion criteria were as follows: age ≥20 years; histologically confirmed adenocarcinoma of the colon or rectum; patients with primary colon or rectal cancer and unresectable metastatic lesions; patients with no primary cancer-related symptoms; Eastern Cooperative Oncology Group (ECOG) performance status between 0 and 2; and appropriate organ functions (hepatic transaminases <5 times the normal range; bilirubin <2 times the normal range; serum creatinine <1.5 times the normal range; thrombocyte >100 000/μL; neutrophil >1500/μL). The exclusion criteria were as follows: colorectal cancer other than adenocarcinoma; patients who received adjuvant chemotherapy within the past 6 months; patients who received chemotherapy for metastatic colorectal cancer; patients planning to undergo curative surgery for metastatic lesions; patients with peritoneal carcinomatosis; patients with primary tumor-related complications; patients with chronic hepatitis or cirrhosis; and patients with another malignant tumor during the past 5 years. Institutional Review Board approval was obtained from the ethics committee of the same hospital from which the patients were enrolled (approval no. GAIRB2015-87), and the trial was registered at ClinicalTrials.gov (identifier: NCT02439385).
Treatment and Curcumin-Containing Supplement
The patients were treated with bevacizumab and FOLFIRI (treatment cycle every 14 days: bevacizumab 5 mg/kg on day 1, irinotecan 180 mg/m2 on day 1, leucovorin 200 mg/m2 on days 1 and 2, 5-fluorouracil bolus 400 mg/m2 on days 1 and 2, and 5-fluorouracil infusion 1200 mg/m2 on days 1 and 2) as first-line treatment. A curcumin delivery system (Aju Pharm, Korea), that is, G-NLC, was prepared as per our previous study 11 using the conventional melt emulsification technique. It mainly contained curcumin 100 mg, partially hydrolyzed ginsenoside 500 mg, phospholipid 2000 mg, and glycerin fatty acid ester 200 mg per bottle of 30 mL. The patients ingested the contents of a bottle twice a day after meals during first-line chemotherapy. Follow-up assessment was performed every 3 months using serum tumor markers and chest and abdominopelvic computed tomography.
Study Endpoints and Statistical Analysis
The primary endpoint was to evaluate the PFS of the enrolled patients. PFS was defined as the time from initial treatment with first-line chemotherapy and curcumin supplementation to disease progression or death. The secondary endpoints included OS, the best overall response rate (ORR), and adverse events. OS was defined as the time from the initial treatment with first-line chemotherapy and curcumin supplementation to death due to any cause. The best ORR was defined as the proportion of patients who achieved a partial or complete response to the treatment, which was the best record until disease progression or death. Tumor response was determined using Response Evaluation Criteria in Solid Tumors (RECIST) criteria version 1.1. 12 Adverse events were recorded based on the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. 13 Survival analysis was performed using the Kaplan–Meier method with IBM SPSS Statistics 23 for Windows (IBM SPSS Inc., Chicago, IL, USA).
Results
Patient Demographics
A total of 44 patients were enrolled in this study. The median age was 65 (range 45-81) years. Thirty-one male patients (70.5%) and 13 female patients (29.5%) were included. The primary tumor location was the colon in 31 patients and the rectum in 13 patients. The metastatic sites included: liver only (n = 20), lung only (n = 6), both liver and lung (n = 12), and others (n = 6). At the time of first-line chemotherapy with curcumin, 32 patients (72.7%) had synchronous metastases and 12 (27.3%) had recurrent disease. The patients who underwent resection of the primary tumor in cases of synchronous metastases were 30 in 32 patients (93.8%). There were 23 patients with wild-type K-RAS and 18 patients with K-RAS mutations. The median duration of curcumin supply was 7.9 (range 0.9-16.6) months. Baseline patient demographics are shown in Table 1.
Table 1.
Baseline Patient Demographics.
| Variables | Patients (n = 44) |
|---|---|
| Age, years | 65 (45-81) |
| Sex | |
| Male | 31 (70.5) |
| Female | 13 (29.5) |
| BMI, kg/m2 | 23.3 (17.2-30.5) |
| ASA score | |
| 1 | 7 (15.9) |
| 2 | 35 (79.5) |
| Unknown | 2 (4.5) |
| Underlying disease | |
| Diabetes | 7 (15.9) |
| Pulmonary disease | 7 (15.9) |
| Cardiovascular disease | 25 (56.8) |
| Primary tumor site | |
| Colon | 31 (70.5) |
| Rectum | 13 (29.5) |
| Metastatic site | |
| Liver only | 20 (45.5) |
| Lung only | 6 (13.6) |
| Liver and lung | 12 (27.3) |
| Others | 6 (13.6) |
| Metastatic status on first-line chemotherapy | |
| Synchronous | 32 (72.7) |
| Metachronous | 12 (27.3) |
| Resection of primary tumor a | |
| Yes | 30 (93.8) |
| No | 2 (6.2) |
| K-RAS status | |
| Wild-type | 23 (52.3) |
| Mutant-type | 18 (40.9) |
| Unknown | 3 (6.8) |
| MSI status | |
| MSS | 38 (86.4) |
| MSI-L | 2 (4.5) |
| Unknown | 4 (9.1) |
| Duration of curcumin supply, months | 7.9 (0.9-16.6) |
Continuous variables are listed as medians (range), and categorical variables are presented as n (%).
Abbreviations: BMI, body mass index; ASA, American Society of Anesthesiologist.
Among 32 synchronous metastatic patients.
Adverse Events
Table 2 shows the outcomes of adverse events. The most common adverse events were neutropenia (n = 21, 47.7%), followed by anemia (n = 17, 38.6%) and nausea (n = 13, 29.5%). Regarding serious adverse events, there were 2 (4.5%) grade 3 febrile neutropenia and 1 (2.3%) grade 3 gastrointestinal bleeding. The most common grade 3 or higher adverse events were neutropenia (n = 15, 34.1%), followed by nausea (n = 4, 9.1%) and vomiting (n = 4, 9.1%). Anemia, diarrhea, and stomatitis were grade 2 or less. Constipation, peripheral neuropathy, and alopecia presented as only grade 1.
Table 2.
Adverse Events.
| Adverse events | CTCAE | |||
|---|---|---|---|---|
| Patients (n = 44) | ||||
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Neutropenia | 1 (2.3) | 5 (11.4) | 8 (18.2) | 7 (15.9) |
| Febrile neutropenia | 0 | 0 | 2 (4.5) | 0 |
| Anemia | 12 (27.3) | 5 (11.4) | 0 | 0 |
| Thrombocytopenia | 0 | 0 | 0 | 0 |
| Nausea | 7 (15.9) | 2 (4.5) | 4 (9.1) | 0 |
| Vomiting | 5 (11.4) | 2 (4.5) | 4 (9.1) | 0 |
| Diarrhea | 3 (6.8) | 2 (4.5) | 0 | 0 |
| Constipation | 1 (2.3) | 0 | 0 | 0 |
| Peripheral neuropathy | 3 (6.8) | 0 | 0 | 0 |
| Stomatitis | 4 (9.1) | 2 (4.5) | 0 | 0 |
| Alopecia | 1 (2.3) | 0 | 0 | 0 |
| Gastrointestinal bleeding | 0 | 0 | 1 (2.3) | 0 |
Categorical variables are listed as n (%).
Abbreviation: CTCAE, Common Terminology Criteria for Adverse Events ver 4.0.
Tumor Response Rates
None of the patients achieved complete response (CR); however, 9 patients showed partial response (PR). The best ORR rate was 20% (9/44). Three patients underwent conversion surgeries in the PR group, with a conversion rate of 6.8%. Nineteen patients showed stable disease (SD), and 16 patients had progressive disease (PD). The disease control rate (CR + PR + SD) was 63.6% (28/44). The response rates are shown in Table 3.
Table 3.
Tumor Response Rate.
| Response | No. (%) | 95% CI |
|---|---|---|
| Complete response (CR) | 0 | — |
| Partial response (PR) a | 9 (20.5) | 9.1-31.8 |
| Stable disease (SD) | 19 (43.2) | 29.5-59.1 |
| Progressive disease (PD) | 16 (36.4) | 22.7-52.3 |
| Overall response b | 9 (20.5) | 9.1-31.8 |
| Disease control c | 28 (63.6) | 50.0-79.5 |
Abbreviation: CI, confidence interval.
Three patients underwent conversion surgery in the PR group.
Complete response and partial response (CR + PR).
Overall response and stable disease (CR + PR + SD).
Survival Rates
Within the median follow-up periods of 22.8 months, the median PFS was 12.8 months (95% confidence interval [CI], 10.6-15.1). The 1 and 2-year PFS rates were 57.1% and 8.0%, respectively (Figure 1). The median OS was 30.7 months (95% CI, 20.0-41.3). The 1 and 2-year OS rates were 87.4% and 63.6%, respectively (Figure 2). The most common disease progression site was the liver (n = 16, 36%), followed by the lungs (n = 11, 25%). Six (14%) patients showed no disease progression during the follow-up period (Figure 3).
Figure 1.
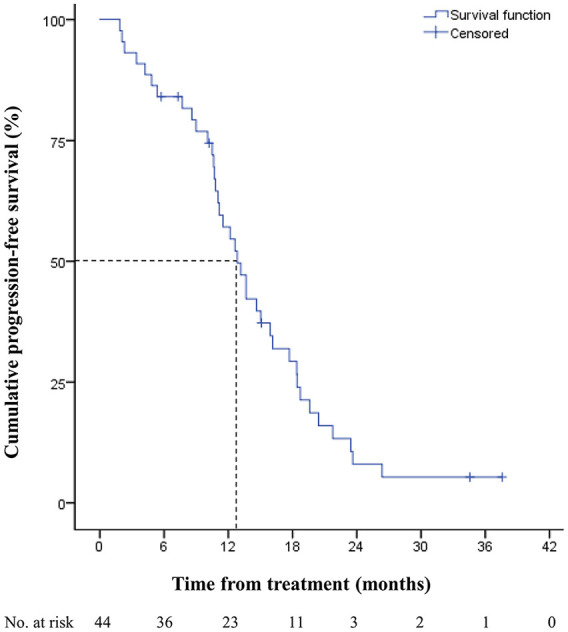
Progression-free survival of metastatic colorectal cancer patients treated with bevacizumab, FOLFIRI, and ginsenoside-modified nanostructured lipid carrier containing curcumin.
Figure 2.
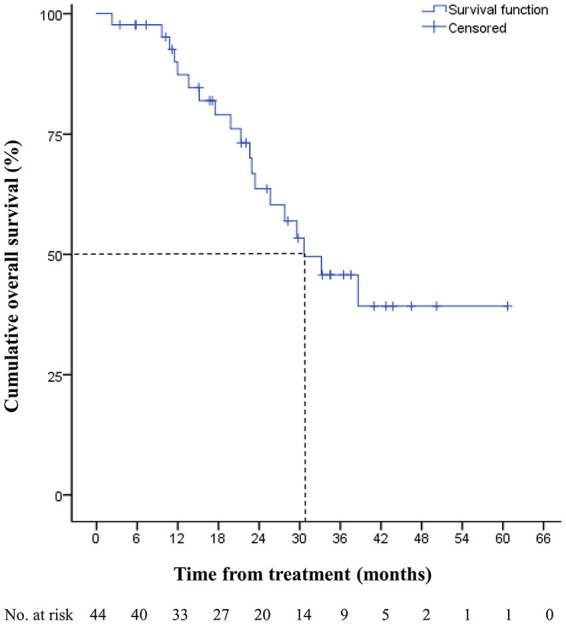
Overall survival of metastatic colorectal cancer patients treated with bevacizumab, FOLFIRI, and ginsenoside-modified nanostructured lipid carrier containing curcumin.
Figure 3.
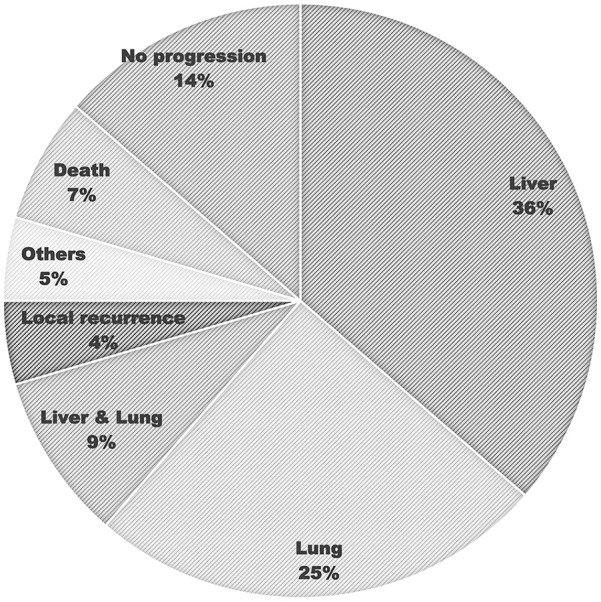
Site of cancer progression in metastatic colorectal cancer patients treated with bevacizumab, FOLFIRI, and ginsenoside-modified nanostructured lipid carrier containing curcumin.
Discussion
We investigated the tolerability and safety with long-term survival rates of bevacizumab/FOLFIRI with oral curcumin nanoformulations as a first-line treatment for unresectable metastatic colorectal cancer. There have been numerous studies regarding the anticancer effect of curcumin nanoformulations in colorectal cancer, showing suppressed tumor growth, antiproliferative activity, and enhanced cytotoxic effects. 14 However, these were mostly designed as in vitro or animal model studies. In human clinical research, several studies have reported the potential therapeutic benefits of curcumin in combination with chemotherapy in other malignancies, including breast, pancreatic, and prostate cancer.15-17 Although a number of clinical studies have suggested that curcumin has anticancer effects with low toxicity, there is still a lack of long-term survival results of treatment in combination with curcumin and standard chemotherapy in patients with metastatic colorectal cancer. To the best of our knowledge, this is the first clinical study conducted using a combination of bevacizumab/FOLFIRI and oral curcumin nanoformulations for unresectable metastatic colorectal cancer.
A recently published randomized phase II trial evaluated the efficacy of curcumin supplementation via survival outcomes for patients with metastatic colorectal cancer, including oral curcumin combined with FOLFOX (with or without bevacizumab) chemotherapy (CUFOX). 18 In the trial, comparing CUFOX (n = 18) with FOLFOX (n = 9), the median PFS was 171 days for FOLFOX and 291 days for CUFOX (hazard ratio: 0.571, 95% CI: 0.24-1.36; P = .2), and the median OS was 200 days for FOLFOX and 502 days for CUFOX (hazard ratio: 0.339, 95% CI: 0.141-0.815; P = .016). In our study, we obtained a median PFS of 12.8 months, and a median OS of 30.7 months. Although bevacizumab/FOLFIRI was similar PFS outcomes to bevacizumab/FOLFOX, 19 the PFS of 12.8 months in our study was better than 291 days in CUFOX study. However, the CUFOX cohort had 4 (22.2%) patients with peritoneal metastases, which are often associated with poor survival outcomes, 20 and only 8 (44.4%) patients received bevacizumab. In addition, the forms of the curcumin supplements were different. In the CUFOX study, the patients ingested a daily dose of 2 g of pure curcumin; meanwhile, in our study, the patients ingested a daily dose of 0.2 g of curcumin with ginsenoside modified lipid nanoformulation. The above differences may influence survival outcomes; however, the impact of these differences is not clear.
The representative randomized controlled trials regarding oncologic outcomes of first line chemotherapy using bevacizumab/FOLFIRI for patients with metastatic colorectal cancer were the WJOG4407G study in Japan 19 and the FIRE-3 study (AIO KRK-0306) in Germany.5,21 The WJOG4407G study showed that, among 197 patients with metastatic colorectal cancer who were treated with bevacizumab/FOLFIRI, the median OS was 31.4 months (95% CI, 27.6-36.4) and the median PFS was 12.1 months (95% CI, 11.0-13.8). 19 These results are comparable to our results. The FIRE-3 trial was conducted only for patients with K-RAS exon 2 wild-type metastatic colorectal cancer. The final long-term survival outcomes were reported in 2021, with a median OS of 25.6 months (95% CI, 23.2-28.8) and a median PFS of 10.4 months (95% CI, 9.8-11.7) for 201 patients. 21 The subgroup analysis of the AIO KRK-0306 study for patients with the K-RAS mutation showed that the median OS was 18.7 months, and the median PFS was 8.9 months for 45 patients. 22 Compared with the aforementioned German studies, our results showed better survival rates.
Despite its anticancer properties, oral bioavailability of curcumin is poor. This poor bioavailability of curcumin is due to its low water solubility, poor gastrointestinal tract absorption, rapid metabolism, and excretion. 23 These issues have led to the development of curcumin nanoformulation technologies, such as solid lipid nanoparticles, liposomes, micelles, polymeric nanoparticles, nanostructure lipid carriers, and phospholipid complexes, which were developed to improve curcumin delivery.14,24,25 In our previous study, we reported that curcumin-containing nanostructure lipid carriers with ginsenoside modification improved curcumin solubility, chemical stability, and cytotoxicity in colon cancer cells.9,10 We hypothesized that the enhanced bioavailability of curcumin in the G-NLC formulation would result in good long-term survival rates in the current study. However, this was not confirmed, since the evidence was insufficient to clarify the correlation between curcumin and survival benefits. There were no data on curcumin plasma levels in the enrolled patients and no comparative control group in this study. Therefore, further studies are necessary to confirm our results.
Chemotherapy is often associated with various toxicities. FOLFIRI plus target agents or FOLFOX with target agents are the usual anticancer drugs for metastatic colorectal cancer. FOLFIRI has a stronger relationship with gastrointestinal toxicities and alopecia than FOLFOX, whereas peripheral sensory neuropathy is more severe in FOLFOX than in FOLFIRI. 19 In addition, bevacizumab has side effects, including gastrointestinal hemorrhage, perforation, and thromboembolism. In our study, there were no other specific adverse events, and the toxicity outcomes associated with FOLFIRI and bevacizumab were reduced. Curcumin is not associated with severe toxicity; instead, several in vivo studies have reported that curcumin has a protective role in testicular tissue and heart tissue against irinotecan-induced damage.26,27 Compared with the WJOG randomized study, 9% patients had severe diarrhea above grade 3 and 3% patients had severe mucositis above grade 319; meanwhile, our results did not show a grade 3 or greater level of severe diarrhea and mucositis. As a result, patients’ compliance with chemotherapy may be improved with curcumin. In terms of low toxicity and enhanced anticancer properties, curcumin may be associated with improved survival rates and quality of life benefits when combined with current chemotherapeutic agents for patients with metastatic colorectal cancer.
Our study has certain limitations. First, it was a single-arm study with a small population. Although we obtained good long-term survival rates, the survival benefit is not clear since there was no comparative control group. Also, we did not assess the plasma levels of curcumin. Additionally, all enrolled patients were administered bevacizumab despite including patients with K-RAS wild-type mutation. Current guidelines recommend that patients with left-sided colon cancer with RAS wild-type be treated with FOLFIRI or FOLFOX with cetuximab as first-line chemotherapy. At the time of this study, cetuximab was not covered by the national health insurance in South Korea. Therefore, it is necessary to research a refined cohort based on the current guidelines. Despite the above limitations, this study is noteworthy since it is the first study on the long-term survival rates of the patients who had unresectable metastatic colorectal cancer treated with curcumin combined with bevacizumab/FOLFIRI chemotherapy.
Conclusions
Bevacizumab/FOLFIRI with G-NLC as first-line chemotherapy in colorectal cancer patients with unresectable metastases was associated with acceptable safety and tolerability with comparable long-term survival rates. Additional randomized controlled studies are needed to draw definite conclusions regarding this new regimen for metastatic colorectal cancer.
Acknowledgments
We would like to thank Editage (www.editage.co.kr) for English language editing.
Footnotes
Author Contributions: Youngbae Jeon—acquisition of data, data analysis and interpretation, drafting of manuscript, and critical revision. Sun Jin Sym—acquisition of data, drafting of manuscript, and critical revision. Bong Kyu Yoo—conception and design of study. Jeong-Heum Baek—conception and design of study, acquisition of data, drafting of manuscript, critical revision, and approval of final version of manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Trial Registration: The study was registered at ClinicalTrials.gov (identifier: NCT02439385).
ORCID iDs: Youngbae Jeon  https://orcid.org/0000-0003-1322-9536
https://orcid.org/0000-0003-1322-9536
Jeong-Heum Baek  https://orcid.org/0000-0001-9124-8041
https://orcid.org/0000-0001-9124-8041
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2. Cook AD, Single R, McCahill LE. Surgical resection of primary tumors in patients who present with stage IV colorectal cancer: an analysis of surveillance, epidemiology, and end results data, 1988 to 2000. Ann Surg Oncol. 2005;12:637-645. doi: 10.1245/ASO.2005.06.012 [DOI] [PubMed] [Google Scholar]
- 3. van Rooijen KL, Shi Q, Goey KKH, et al. Prognostic value of primary tumour resection in synchronous metastatic colorectal cancer: individual patient data analysis of first-line randomised trials from the ARCAD database. Eur J Cancer. 2018;91:99-106. doi: 10.1016/j.ejca.2017.12.014 [DOI] [PubMed] [Google Scholar]
- 4. Colucci G, Gebbia V, Paoletti G, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo oncologico Dell’Italia Meridionale. J Clin Oncol. 2005;23:4866-4875. doi: 10.1200/JCO.2005.07.113 [DOI] [PubMed] [Google Scholar]
- 5. Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065-1075. doi: 10.1016/S1470-2045(14)70330-4 [DOI] [PubMed] [Google Scholar]
- 6. Pricci M, Girardi B, Giorgio F, Losurdo G, Ierardi E, Di Leo A. Curcumin and colorectal cancer: from basic to clinical evidences. Int J Mol Sci. 2020;21:2364. doi: 10.3390/ijms21072364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mansouri K, Rasoulpoor S, Daneshkhah A, et al. Clinical effects of curcumin in enhancing cancer therapy: a systematic review. BMC Cancer. 2020;20:791. doi: 10.1186/s12885-020-07256-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baskaran R, Madheswaran T, Sundaramoorthy P, Kim HM, Yoo BK. Entrapment of curcumin into monoolein-based liquid crystalline nanoparticle dispersion for enhancement of stability and anticancer activity. Int J Nanomedicine. 2014;9:3119-3130. doi: 10.2147/IJN.S61823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vijayakumar A, Baskaran R, Maeng HJ, Yoo BK. Ginsenoside improves physicochemical properties and bioavailability of curcumin-loaded nanostructured lipid carrier. Arch Pharm Res. 2017;40:864-874. doi: 10.1007/s12272-017-0930-1 [DOI] [PubMed] [Google Scholar]
- 10. Vijayakumar A, Baskaran R, Baek JH, Sundaramoorthy P, Yoo BK. in vitro cytotoxicity and bioavailability of ginsenoside-modified nanostructured lipid carrier containing curcumin. AAPS PharmSciTech. 2019;20:88. doi: 10.1208/s12249-019-1295-1 [DOI] [PubMed] [Google Scholar]
- 11. Selvaraj K, Yoo BK. Curcumin-loaded nanostructured lipid carrier modified with partially hydrolyzed ginsenoside. AAPS PharmSciTech. 2019;20:252. doi: 10.1208/s12249-019-1467-z [DOI] [PubMed] [Google Scholar]
- 12. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 13. National Cancer Institute. Common terminology criteria for adverse events (CTCAE) v4.0. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed June 14, 2010.
- 14. Wong KE, Ngai SC, Chan KG, Lee LH, Goh BH, Chuah LH. Curcumin nanoformulations for colorectal cancer: a review. Front Pharmacol. 2019;10:152. doi: 10.3389/fphar.2019.00152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dhillon N, Aggarwal BB, Newman RA, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14:4491-4499. doi: 10.1158/1078-0432.CCR-08-0024 [DOI] [PubMed] [Google Scholar]
- 16. Bayet-Robert M, Kwiatkowski F, Leheurteur M, et al. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol Ther. 2010;9:8-14. doi: 10.4161/cbt.9.1.10392 [DOI] [PubMed] [Google Scholar]
- 17. Mahammedi H, Planchat E, Pouget M, et al. The new combination docetaxel, prednisone and curcumin in patients with castration-resistant prostate cancer: a pilot phase II study. Oncology. 2016;90:69-78. doi: 10.1159/000441148 [DOI] [PubMed] [Google Scholar]
- 18. Howells LM, Iwuji CO, Irving GR, et al. Curcumin combined with FOLFOX chemotherapy is safe and tolerable in patients with metastatic colorectal cancer in a randomized phase IIa trial. J Nutr. 2019;149:1133-1139. doi: 10.1093/jn/nxz029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yamazaki K, Nagase M, Tamagawa H, et al. Randomized phase III study of bevacizumab plus FOLFIRI and bevacizumab plus mFOLFOX6 as first-line treatment for patients with metastatic colorectal cancer (W. JOG4407G). Ann Oncol. 2016;27:1539-1546. doi: 10.1093/annonc/mdw206 [DOI] [PubMed] [Google Scholar]
- 20. Franko J, Shi Q, Meyers JP, et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the analysis and research in cancers of the digestive system (ARCAD) database. Lancet Oncol. 2016;17:1709-1719. doi: 10.1016/S1470-2045(16)30500-9 [DOI] [PubMed] [Google Scholar]
- 21. Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab or bevacizumab for advanced colorectal cancer: final survival and per-protocol analysis of FIRE-3, a randomised clinical trial. Br J Cancer. 2021;124:587-594. doi: 10.1038/s41416-020-01140-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stintzing S, Fischer von Weikersthal L, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer-subgroup analysis of patients with KRAS: mutated tumours in the randomised German AIO study KRK-0306. Ann Oncol. 2012;23:1693-1699. doi: 10.1093/annonc/mdr571 [DOI] [PubMed] [Google Scholar]
- 23. Prasad S, Tyagi AK, Aggarwal BB. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res Treat. 2014;46:2-18. doi: 10.4143/crt.2014.46.1.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Udompornmongkol P, Chiang BH. Curcumin-loaded polymeric nanoparticles for enhanced anti-colorectal cancer applications. J Biomater Appl. 2015;30:537-546. doi: 10.1177/0885328215594479 [DOI] [PubMed] [Google Scholar]
- 25. Vijayakumar A, Baskaran R, Jang YS, Oh SH, Yoo BK. Quercetin-loaded solid lipid nanoparticle dispersion with improved physicochemical properties and cellular uptake. AAPS PharmSciTech. 2017;18:875-883. doi: 10.1208/s12249-016-0573-4 [DOI] [PubMed] [Google Scholar]
- 26. Ciftci O, Turkmen NB, Taslıdere A. Curcumin protects heart tissue against irinotecan-induced damage in terms of cytokine level alterations, oxidative stress, and histological damage in rats. Naunyn Schmiedebergs Arch Pharmacol. 2018;391:783-791. doi: 10.1007/s00210-018-1495-3 [DOI] [PubMed] [Google Scholar]
- 27. Uyanık Ö, Gürbüz Ş, Çiftci O, et al. Curcumin protects against testis-specific side effects of irinotecan. Eur Rev Med Pharmacol Sci. 2021;25:7440-7448. doi: 10.26355/eurrev_202112_27441 [DOI] [PubMed] [Google Scholar]


