Abstract
Purpose
To investigate the effects and mechanisms of fenofibrate, a synthetic ligand of peroxisome proliferator-activated receptor α (PPAR-α), on autoimmune dacryoadenitis in a mouse model of Sjögren syndrome (SS) dry eye.
Methods
Male nonobese diabetic (NOD) mice were fed chow with or without 0.03% fenofibrate for 8 weeks, and clinical scores were determined by assessing tear secretion, fluorescein, and hematoxylin and eosin staining. Intracellular IFN-γ, IL-17, and Foxp3 in CD4+ T cells were measured by flow cytometry. The expressions of Th1, Th17, and Treg cell-related transcription factors and cytokines were detected by real-time PCR. The levels of PPAR-α and liver X receptor β (LXR-β) were detected with real-time PCR and Western blotting.
Results
Fenofibrate efficiently diminished the lymphocytic inflammation in lacrimal glands (LGs), increased tear secretion, and decreased corneal fluorescein staining in NOD mice. Meanwhile, treatment of fenofibrate evidently reduced the proportion of Th1 and Th17 cells and increased the proportion of Treg cells in vivo and vitro, together with decreased expression of T-bet, IFN-γ, RORγt, and IL-17, as well as increased expression of Foxp3 and TGF-β1 in LGs. Furthermore, fenofibrate significantly upregulated the expressions of PPAR-α and LXR-β at the protein and mRNA levels.
Conclusions
Fenofibrate potently attenuated LG inflammation in a model of autoimmune dry eye, and this effect might partially result from regulating Th1/Th17/Treg cell responses by activating PPAR-α/LXR-β signaling. These data suggest that fenofibrate may be a novel class of therapeutic agent for SS-associated dacryoadenitis.
Keywords: fenofibrate, autoimmune dacryoadenitis, Th1, Th17, Treg, LXR-β
Sjögren syndrome (SS) dry eye is a chronic, systemic autoimmune disease characterized by profound lymphocytic infiltration and tissue dysfunction of the tear-producing lacrimal glands (LGs), eventually resulting in impairment of vision.1 At present, there are limited options for treatment of SS dry eye.2 Artificial tears for symptomatic relief have insufficient effect, and steroids for reduction of ocular surface inflammation are linked to unwanted side effects, including cataracts and glaucoma, during long-term treatment.3 In order to more effectively prevent the progression of SS dry eye, it is essential to further clarify its pathogenesis and explore potential therapeutics.
A well-established mouse model for studying SS dry eye is nonobese diabetic (NOD) mice, which spontaneously develop ocular surface dryness and autoimmune dacryoadenitis that recapitulate characteristics of human SS dry eye.4 It is well known that CD4+ T cells play a critical role in the pathology of SS dry eye.5 Elevated frequencies of Th1 and Th17 cells and remarkable reduction of Treg numbers were observed in peripheral blood of patients with SS.6–8 Moreover, defective LG-protective Treg cells contributed to autoimmune dacryoadenitis in NOD mice,9 and recruiting endogenous Treg cells through local release of CCL22 in the LG could mitigate SS dry eye progression.10 Thus, strategies that inhibit Th1 and Th17 cell differentiation and promote the generation of Treg cells would be expected to suppress disease progression.
Fenofibrate is a synthetic peroxisome proliferator-activated receptor α (PPAR-α) agonist widely used clinically for its effectiveness in decreasing cholesterol and triglyceride levels.11 Recent studies have demonstrated that fenofibrate could improve several autoimmune diseases due to its anti-inflammatory effects. For example, Cheng et al.12 reported that fenofibrate inhibited inflammation in rats with experimental autoimmune myocarditis (EAM) by inhibiting Th17 cell development and promoting Treg differentiation. Moreover, it has been reported that fenofibrate repressed IFN-γ and IL-17 expression in splenocytes and improved colitis in IL-10–deficient mice.13 However, to date, the role of PPAR-α agonist fenofibrate in SS dry eye remains unknown.
The present study aimed to determine whether fenofibrate could alleviate SS-associated dacryoadenitis and explore its underlying mechanisms with an emphasis on the Th1, Th17, and Treg responses in NOD mice.
Materials and Methods
Animal Study
Male nonobese diabetic (NOD/ShiLtJ) mice with Sjögren-like disease used in the experiment were purchased from GemPharmatech Co., Ltd (Nanjing, China). All animal experimental procedures were conducted in accordance with the guidelines of the Laboratory Animal Care and Use Committee of Tianjin Medical University and conformed to the ARVO Statement on Use of Animals in Ophthalmic and Vision Research. At 15 weeks of age, NOD mice were randomly divided into two groups: one fed regular chow and the other fed special chow containing 0.03% fenofibrate (Trophic Animal Feed High-Tech Co., Ltd, Nantong, China) for another 8 weeks.14
Measurement of Tear Secretion
Tear production was measured daily at a same time point (8 am) with phenol red cotton threads (Jingming Co., Ltd, Tianjin, China). To avoid any contribution from the lacrimal lake, absorbent paper was used to remove the tear fluid inside the conjunctival sac. Then threads were applied in the lower conjunctival fornix of both eyes for 30 seconds at a position that was one-third of the lower eyelid distance from the lateral canthus. The length of thread wetting by basal tears was recorded in millimeters. The average value from both eyes was determined.
Fluorescein Ocular Surface Staining
Three artificial blinks after 1 µL of 2% liquid sodium fluorescein was instilled onto the ocular surface. Ninety seconds later, corneal epithelial damage was examined using a cobalt blue light under the slit-lamp microscope (Kanghua Science & Technology, Chongqing, China). Subsequently, the cornea staining was recorded and scored from both eyes as described previously.15 Corneal staining scores from both eyes are reported as an average.
Histologic Analysis of Lacrimal Glands
Mice were sacrificed on week 8 after treatment, and body weight was measured. LGs were dissected carefully, fixed in 10% formalin, and embedded in paraffin using standard methods. LG sections were stained with hematoxylin-eosin (H&E) to evaluate inflammation. H&E-stained sections from each experiment group were analyzed and photographed using light microscopy (BX51; Olympus Corporation, Tokyo, Japan). Images of three nonconsecutive whole-gland cross sections were obtained for each LG. The area of lymphocytic infiltration was calculated by using ImageJ software (National Institutes of Health, Bethesda, MD, USA).16
Intracellular Cytokine Staining and Flow Cytometry
A single-cell suspension was stimulated for 4 hours at 37°C with phorbol myristic acetate (50 ng/mL), ionomycin (1 mg/mL), and brefeldin A (1 mg/mL) (Sigma-Aldrich, St. Louis, MO, USA); then, cells were fixed, permeabilized overnight, and intracellularly stained with antibodies. Tregs were detected directly without stimulations. Cell surface proteins were stained with PE-anti-CD4 (cat. 116006; BioLegend, San Diego, CA, USA) or FITC-anti-CD4 (cat. 130308; BioLegend) antibodies for 20 minutes at 4°C, and intracellular cytokines were stained with APC-anti-IFN-γ antibody (cat. 505810; BioLegend) for Th1 cells, FITC-anti-IL-17 antibody (cat. 506908; BioLegend) for Th17 cells, or PE-anti-Foxp3 antibody (cat. 12-5773-82; eBioscience, San Diego, CA, USA) for Tregs for 1 hour at room temperature. Stained cells were collected on a flow cytometer (FACS Calibur; BD Biosciences, San Jose, CA, USA). Data were analyzed with FlowJo software (TreeStar, Ashland, OR, USA).
CD4+ T-Cell Isolation and Polarization
CD4+ T cells were purified from spleens or draining lymph nodes of NOD mice by positive selection using a combination of PE-conjugated anti-mouse CD4 antibodies (100408; BioLegend) and anti-PE antibody-coated microbeads (130-048-801; Miltenyi Biotec, Bergisch Gladbach, Germany), followed by separation using an autoMACS Separator Column (Miltenyi Biotec) according to the manufacturer's protocol. Isolated cells then were stimulated with 5 µg/mL plate-coated anti-CD3 (16-0031-86; eBioscience) and 2 µg/mL soluble anti-CD28 (16-0281-86; eBioscience). For Th1 polarization, 20 ng/mL IL-12 (419-ML; R&D Systems, Minneapolis, MN, USA), 20 ng/mL IL-2 (402-ML; R&D Systems), and 10 µg/mL anti-IL-4 (16-7041-85; eBioscience) were added. For Th17 polarization, 10 µg/mL anti-IL-4, 10 µg/mL anti-IFN-γ (16-7312-85; eBioscience), 5 ng/mL TGF-β (7666-MB; R&D Systems), 40 ng/mL IL-6 (406-ML; R&D Systems), and 20 ng/mL IL-23 (1887-ML; R&D Systems) were added into the medium. For Treg polarization, 20 ng/mL IL-2 and 5 ng/mL TGF-β were added. The polarized cells were added to a 48-well plate (250 µL, 6 × 105 cells per well) and medium was changed on the second day. Fenofibrate was dissolved in dimethyl sulfoxide (DMSO), and cells were incubated with the indicated concentrations. In control groups, DMSO was used at amounts similar to those of treatment groups. After 3 days of culture, cells were collected and assessed by flow cytometry.
Quantitative Real-Time PCR
Total RNA was isolated from LGs using the EZ-press RNA Purification Kit (EZBioscience, Roseville, CA, USA) according to the manufacturer's instructions and reverse transcribed (1000 ng) to cDNA using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, USA). Quantitative RT-PCR (qRT-PCR) analysis was then performed using FastStart Universal SYBR Green Master (Rox) on a LightCycler 480 II system (Roche Diagnostics GmbH, Mannheim, Germany). The gene-specific primer sequences are listed in the Table. Gene expression was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and expressed relative to the control group according to the 2−ΔΔCt method. PCR was run at 50°C for 2 minutes and 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute.
Table.
Sequences of Primers in This Study for Real-Time qRT-PCR
| Gene | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|
| GAPDH | 5′-CATGGCCTTCCGTGTTCCTA-3′ | 5′-GCGGCACGTCAGATCCA-3′ |
| IL-17A | 5′-CCTGGCGGCTACAGTGAAG-3′ | 5′-TTTGGACACGCTGAGCTTTG-3′ |
| RORγt | 5′-CCTCAGCGCCCTGTGTTTT-3′ | 5′-GAGAACCAGGGCCGTGTAGA-3′ |
| IFN-γ | 5′-TTGGCTTTGCAGCTCTTCCT-3′ | 5′-TGACTGTGCCGTGGCAGTA-3′ |
| T-bet | 5′-ACCTGTTGTGGTCCAAGTTCAA-3′ | 5′-GCCGTCCTTGCTTAGTGATGA-3′ |
| TGF-β1 | 5′-CCGCAACAACGCCATCTATG-3′ | 5′-CTCTGCACGGGACAGCAAT-3′ |
| Foxp3 | 5′-CCCTGCCCTTGACCTCAA-3′ | 5′-GCCTCAGTCTCATGGTTTTGG-3′ |
| PPAR-α | 5′-GAACCGGAACAAATGCCAGT-3′ | 5′-CTTCAGGTAGGCTTCGTGGA-3′ |
| LXR-α | 5′-AACAGCTCCCTGGCTTCCTA-3′ | 5′-CAGAAGCATGACCTCGATTGC-3′ |
| LXR-β | 5′-CGCTCCTCTCCTACACGAG-3′ | 5′-TCTTGTCCTGGAGTCGCAAT-3′ |
Immunohistochemistry
Lacrimal gland sections were deparaffinized, rehydrated, treated with 3% H2O2 in methanol for 30 min, and then blocked with 5% (w/v) fat-free dry milk for 1 hour. The primary antibody CD4 (1:1000, cat. ab183685; Abcam, Cambridge, UK) was incubated overnight in a 4°C refrigerator. After washing, polyclonal goat anti-rabbit secondary antibody (1:1000, cat. ab6721; Abcam) was applied for 1 hour at room temperature. The stained sections were examined under a virtual microscope (BX51; Olympus Corporation, Tokyo, Japan). The immunohistochemical images were quantified on 10 complete LG cross sections of each mouse, and five high-power fields (×400) were selected from each section randomly. For CD4 analysis, the area of positive signal was calculated by using ImageJ software.
Western Blotting Analysis
Lacrimal gland tissue was extracted with RIPA Lysis Buffer (Beyotime, Shanghai, China) containing PMSF (Solarbio, Beijing, China) on ice for 30 minutes. Lysates were centrifuged and the protein concentration of the supernatant was quantified using a BCA Protein Assay kit (Solarbio). Equal amounts of protein (50 µg) were resolved by electrophoresis on 10% SDS-PAGE and then transferred onto a PVDF membrane. After blocking in 5% nonfat milk for 2 hours, the membranes were incubated overnight at 4°C with primary antibodies to PPAR-α (1:1000, cat. PA1-822A; Thermo Fisher Scientific), liver X receptor β (LXR-β) (1:500, cat. ab28479; Abcam), and β-actin (1:2000, cat. TA-09; ZSGB BIO, Beijing, China). An appropriate horseradish peroxidase–-conjugated secondary antibody (1:4000, cat. 7076; Cell Signaling Technology, Danvers, MA, USA) was used to detect the bound antibodies for 2 hours at room temperature. The protein bands were visualized by Multispectral Imaging System (UVP, Upland, CA, USA). Quantitative analysis of target protein level was analyzed using Quantity One software (Bio-Rad, Hercules, CA, USA).
Statistical Analysis
All statistical analyses were analyzed using GraphPad Prism software (GraphPad Software, San Diego, CA, USA). All the data are presented as mean ± SD. The normality of the data was tested using the Shapiro–Wilk test. For normally distributed data, comparisons were analyzed using Student's t-test, whereas the Mann-Whitney test was used for nonparametric analysis. A P value of 0.05 or less was considered statistically significant.
Results
Fenofibrate Improves Clinical Signs in Autoimmune-Mediated Dry Eye
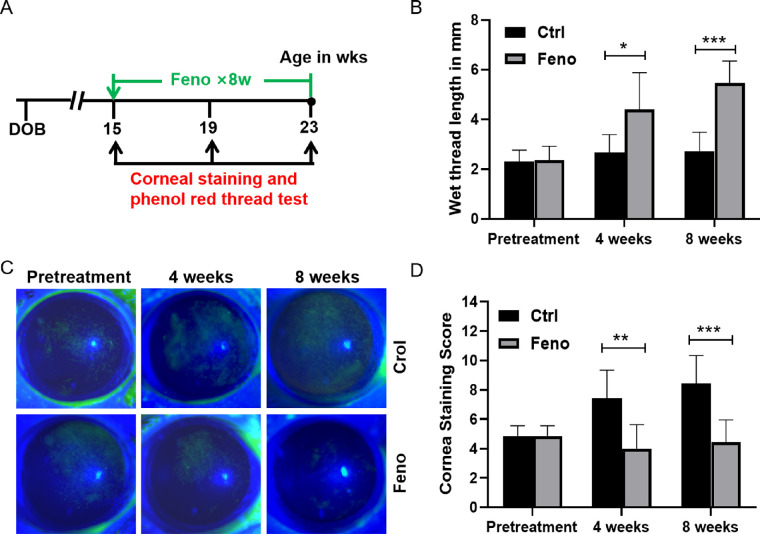
To investigate the effect of fenofibrate on SS dry eye pathogenesis, we treated NOD mice with chow containing 0.03% fenofibrate for 8 weeks, starting at 15 weeks of age when SS-like autoimmune dacryoadenitis was fully established.17 Tear production and corneal fluorescein staining were measured at three consecutive time points: pretreatment at week 0 (15 weeks old) and then 4 and 8 weeks posttreatment (Fig. 1A). Tear secretion measured by wetting threads was significantly increased in fenofibrate group after 4 weeks of treatment compared with the control group (Fig. 1B). This improvement in tear secretion continued even after 8 weeks of fenofibrate treatment. As shown in Figures 1C and 1D, in the control group, corneal fluorescein scores gradually increased from baseline to week 8, and fenofibrate significantly decreased corneal fluorescein staining at week 4 and week 8 in the fenofibrate group. After 8 weeks of fenofibrate, there was no significant body weight change in the fenofibrate group compared with the control group (Supplementary Fig. S1).
Figure 1.
Fenofibrate reduced clinical signs of SS-associated dry eye. (A) Timeline depicting treatment of NOD mice starting at 15 weeks of age. Treatments (0.03% fenofibrate) were given over 8 weeks. (B) Phenol red cotton thread measurement of tear secretion in control (Ctrl) and fenofibrate (Feno) group. (C) Representative images of the fluorescein-stained corneal surface of the control and fenofibrate group at 4 and 8 weeks posttreatment. (D) Corneal fluorescein grading scores. Data are representative of three independent experiments. Data are presented as mean ± SD (n = 7, Student's t-test, *P < 0.05, **P < 0.01, ***P < 0.001).
Fenofibrate Efficiently Alleviates Autoimmune Dacryoadenitis Histologically
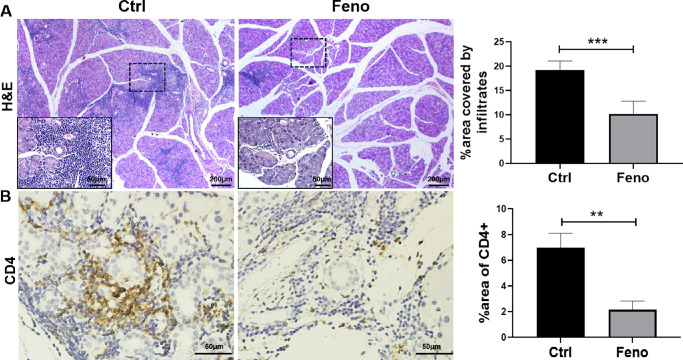
Male NOD mice exhibit inflammatory cell infiltration in LGs, accompanied by LG dysfunction and decreased tear secretion.16 To evaluate the potential effect of fenofibrate on the level of inflammatory infiltrates in LGs, a histologic assessment was performed at week 8 posttreatment. As seen in Figure 2A, H&E-labeled sections and image analysis quantifying the extent of inflammatory cell infiltration showed a significant reduction in the fenofibrate group after 8 weeks of treatment as compared with the control group. Immunohistochemical analysis revealed that the LGs from the fenofibrate group had only rare CD4+ cell infiltrates, whereas those from the control group were heavily infiltrated and the immune cells were frequently concentrated around the ducts and venules (Fig. 2B).
Figure 2.
Fenofibrate treatment suppressed inflammatory cell infiltration in LGs. (A) Male NOD mice were treated with and without fenofibrate-supplemented diet for 8 weeks starting at 15 weeks of age. Lacrimal gland sections from treated mice were stained with H&E, and representative sections are shown. Higher magnifications are displayed in the lower left of each H&E-stained image. Lower magnification bars are 200 µm, while higher magnification bars are 50 µm. (B) Immunohistochemical staining of CD4 displayed CD4+ T cells. Scale bar: 50 µm. Inflammatory cell infiltration was quantified as described in the Materials and Methods section. Image analysis of the LG shows the percentage of inflammatory cell infiltration area relative to the entire LG section. Data are representative of three independent experiments. Data are presented as mean ± SD (n = 3–7, Student's t-test, **P < 0.01, ***P < 0.001).
Fenofibrate Inhibits Th1/Th17 Responses but Promotes Treg Response In Vivo
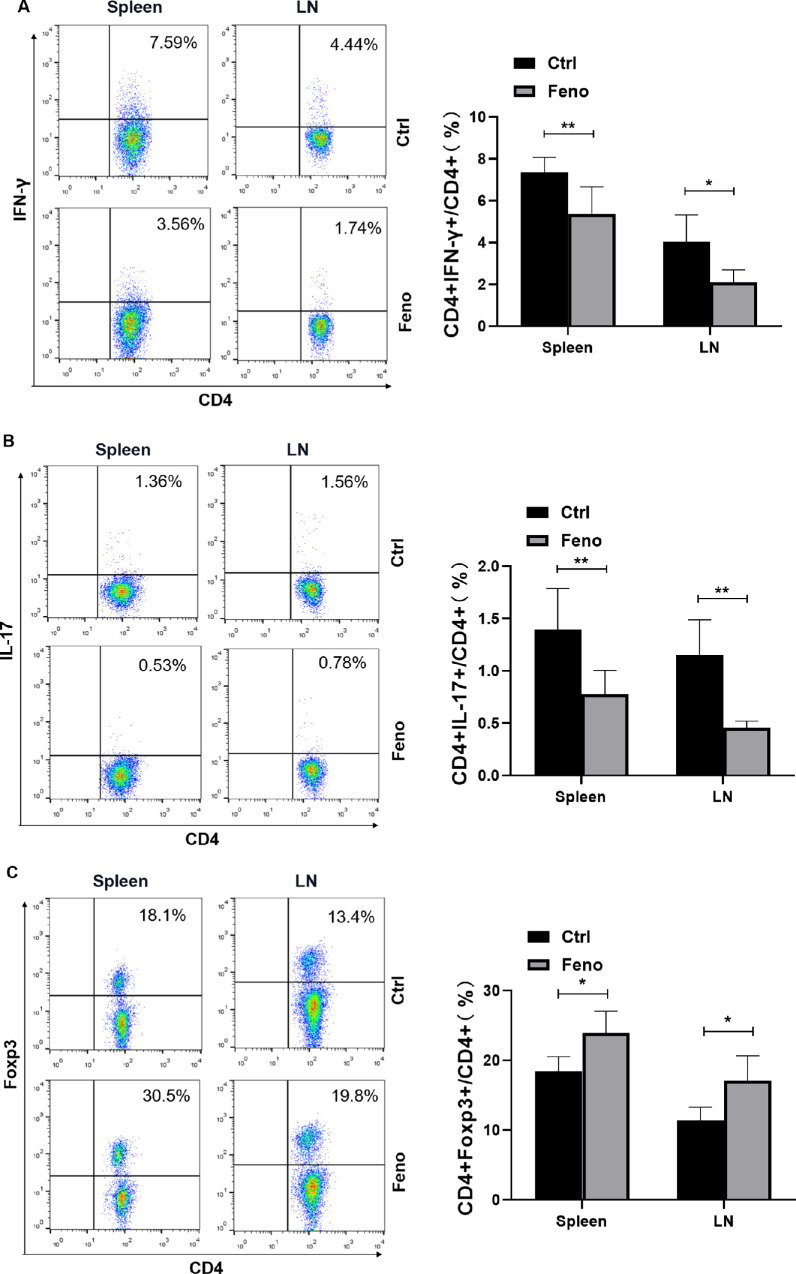
Previous studies suggest that CD4+ T-cell subsets are closely associated with the development of SS dry eye.5 To address whether fenofibrate treatment could regulate CD4+ T-cell responses, a single-cell suspension isolated from the fenofibrate or control group was analyzed by intracellular flow cytometry at week 8 posttreatment. As shown in Figures 3A–C, the proportions of Th1 and Th17 cells in the spleens and cervical lymph nodes in the fenofibrate group were lower than those in the control group, while the proportion of Treg cells was significantly higher. These results suggest that fenofibrate inhibits the differentiation of Th1 and Th17 cells and promotes the differentiation of Treg cells in NOD mice.
Figure 3.
Fenofibrate regulated Th1, Th17, and Treg responses in NOD mice. Flow cytometry for (A) IFN-γ (Th1), (B) IL-17 (Th17), and (C) Foxp3 (Treg) expression in spleens and cervical lymph nodes of the control and fenofibrate group. Data are representative of three independent experiments. Data are presented as mean ± SD (n = 5–6, Student's t-test, *P < 0.05, **P < 0.01).
Fenofibrate Suppresses the Polarization of Th1/Th17 Cells but Enhances the Expansion of Treg Cells In Vitro
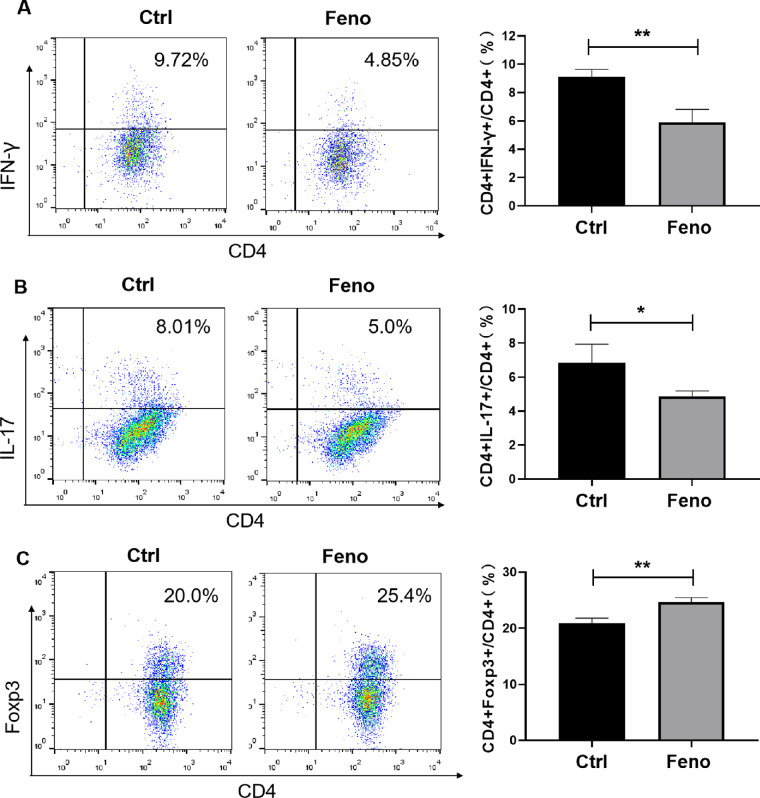
Having observed the modulation of fenofibrate in Th1, Th17, and Treg cells in vivo, we next determined whether fenofibrate could affect the expansion of these Th subsets directly. To this end, CD4+ T cells isolated from NOD mice were stimulated with anti-CD3 and anti-CD28 antibodies and cultured under Th1-, Th17- and Treg-polarizing conditions in the presence or absence of fenofibrate. Three days later, the cells were collected and subjected to fluorescence activated cell sorting (FACS) analysis. As shown in Figures 4A–C, treatment with fenofibrate led to a decrease in the frequencies of Th1 and Th17 cells and an increase in the proportion of Treg cells.
Figure 4.
Fenofibrate suppressed Th1 and Th17 induction while promoting Treg differentiation. (A–C) CD4+ T cells stimulated with anti-CD3 and anti-CD28 mAbs were cultured under Th1 (A), Th17 (B), or Treg (C) polarizing conditions for 3 days in the presence of fenofibrate (2 µM) or a control, respectively. Frequencies of Th1, Th17, and Treg cells were detected by flow cytometry. Data are representative of three independent experiments. Data are shown as mean ± SD (n = 3, Student's t-test, *P < 0.05, **P < 0.01).
Fenofibrate Modulates Expression of Th1, Th17, and Treg Cell-Related Transcription Factors and Cytokines in LGs
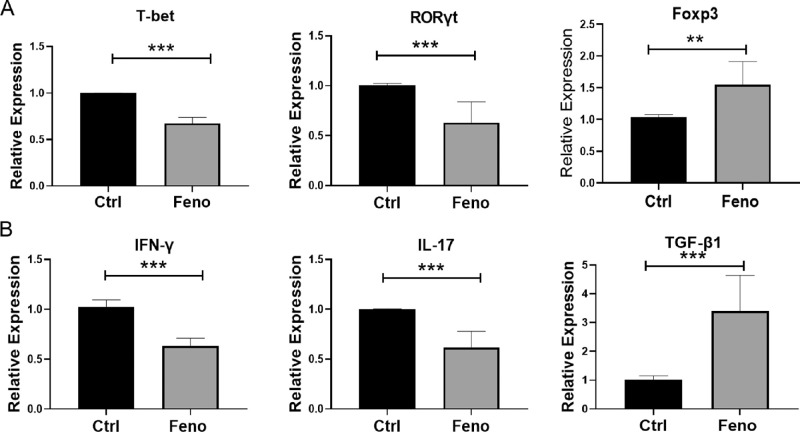
T-bet, RORγt, and Foxp3 are lineage-specific transcription factors for Th1, Th17, and Treg cells, respectively. To examine whether fenofibrate treatment altered the gene expression of these transcription factors, we collected LGs from NOD mice treated with or without fenofibrate and determined relative mRNA levels by real-time qRT-PCR analysis. As shown in Figure 5A, the expressions of T-bet and RORγt were reduced markedly, and the expression of Foxp3 was significantly increased in the fenofibrate group compared with the control group. Consistent with the changes in transcription factors, the IFN-γ and IL-17 mRNA levels in the LGs were lower and the TGF-β1 mRNA level was higher in fenofibrate-treated mice (Fig. 5B).
Figure 5.
Fenofibrate regulated the gene expression of Th1, Th17, and Treg cell-related transcription factors and cytokines in LGs. (A) qRT-PCR analysis of T-bet, RORγt, and Foxp3 mRNA levels. (B) IFN-γ, IL-17, and TGF-β1 mRNA levels. Data are representative of three independent experiments. Data are presented as mean ± SD (n = 3–6, Student's t-test, **P < 0.01, ***P < 0.001).
Fenofibrate Upregulates mRNA and Protein Levels of PPAR-α and LXR-β
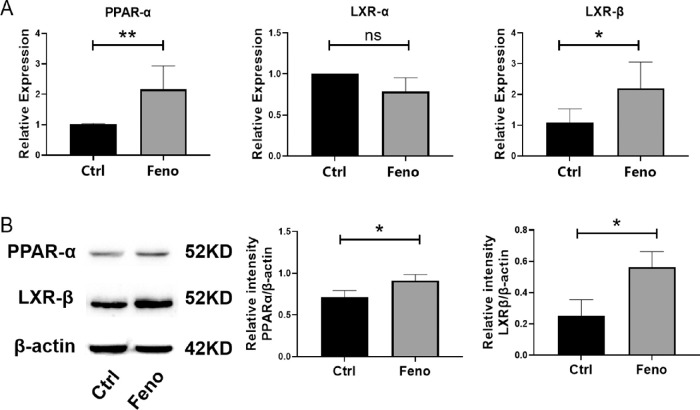
We next defined the underlying molecular mechanism through which fenofibrate modulated Th1, Th17, and Treg cell responses. Considering that PPAR-α plays a vital role in the differentiation of Th1, Th17, and Treg cells and fenofibrate is a PPAR-α agonist,18 we wondered whether fenofibrate could enhance the expression of PPAR-α in SS dry eye. To test this, LGs were collected from the fenofibrate or control group after 8 weeks of fenofibrate treatment, and real-time qRT-PCR and Western blot were performed to determine the expression of the mRNA and protein expressions of PPAR-α. As shown in Figures 6A and 6B, PPAR-α level in the fenofibrate group was higher than in the control group. Furthermore, fenofibrate has been reported to increase the expression of LXR-β,19 a physiologic regulator of lipid and cholesterol metabolism, which enhances and inhibits the polarization of Th17 and Treg cells, respectively.20,21 We further validated whether the effect of fenofibrate was associated with LXR-β. After fenofibrate administration, both protein and mRNA levels of LXR-β were upregulated (Figs. 6A, 6B). These results suggest that fenofibrate treatment may regulate Th1, Th17, and Treg cell responses by enhancing the PPAR-α and LXR-β levels in NOD mice.
Figure 6.
Fenofibrate increased the expression of PPAR-α and LXR-β in LGs. (A) Gene expression of PPAR-α, LXR-α, and LXR-β in LG. (B) Western blot assay for PPAR-α and LXR-β expression. Images shown are from one experiment representative of three independent experiments. Relative protein level is quantified by ratio of PPAR-α and LXR-β to β-actin. Data are representative of three independent experiments. Data are presented as mean ± SD (n = 3–5, *P < 0.05, **P < 0.01; NS, not significant).
Discussion
Emerging evidence showed that fenofibrate is a negative regulator of inflammatory responses in a variety of tissues, including the eye.22,23 The potent anti-inflammatory effect of fenofibrate has been evidenced in patients with diabetic retinopathy, as reported independently by two large, longitudinal clinical studies.24,25 A recent study, including topical application of fenofibrate to dry eye model induced by benzalkonium chloride, showed an improvement in ocular surface staining, tear film breakup time, and a reduction in inflammatory response in the cornea.26 Moreover, He et al.27 reported that fenofibrate might provide therapeutic benefit in dry eye induced by hyperlipidemia. However, no prior study has focused on the effect of fenofibrate on SS-associated dacryoadenitis. Here, we demonstrated that systemic administration of fenofibrate after disease onset efficiently suppressed autoimmune-mediated LG inflammation and improved ocular surface integrity and tear secretion in NOD mice during an observation period of 8 weeks. The beneficial effects of fenofibrate might be mediated through the inhibition of Th1 and Th17 cells and promotion of Treg cells, probably in part via the upregulation of PPAR-α/LXR-β signaling.
It has been acknowledged that Th1 and Th17 cells play important roles in the pathogenesis of various autoimmune diseases, including SS dry eye.28,29 In patients with primary Sjögren Syndrome (pSS), percentages of Th1 and Th17 cell populations were significantly increased in peripheral circulation,30,31 and elevated levels of IFN-γ and IL-17 were detected in tear fluid.32 Recently, using a murine model of EAM, Chang et al.33 demonstrated that fenofibrate ameliorated EAM by suppressing Th17 cell differentiation through decreasing the expression of RORγt and IL-17. Moreover, fenofibrate reduced the production of IFN-γ by CD4+ T cells in vitro.34 Similarly, we found that fenofibrate led to a reduction in the frequencies of Th1 and Th17 cells in the fenofibrate-treated group, as well as the downregulation of IFN-γ and IL-17 levels. Furthermore, we also observed that fenofibrate treatment significantly suppressed the expression of transcription factors T-bet and RORγt, which are critical for Th1 and Th17 cell differentiation, respectively.35 These findings suggest that fenofibrate treatment may inhibit Th1/Th17 responses in vivo and thus ameliorate disease development.
Tregs are essential for maintaining self-tolerance and controlling the occurrence of autoimmune diseases,36 and decreased frequency and dysfunction of Tregs are associated with patients with SS.37 Previous studies have revealed that fenofibrate promotes Treg response in vivo and in vitro.12,38 In line with these reports, we observed an increased proportion of Tregs and expression of Foxp3 in the fenofibrate group compared with the control group. Furthermore, Tregs can suppress autoreactive lymphocytes by releasing the soluble mediator TGF-β1,39 and TGF-β1 has also been found to be pivotal in the generation of Tregs.40 Our data showed that the expression of TGF-β1 was significantly upregulated in the LGs of NOD mice treated with fenofibrate, indicating that fenofibrate may enhance Treg suppressive function. The augmented Tregs can restrain Th1 or Th17 responses,41 leading to alleviated autoimmune dacryoadenitis in fenofibrate-treated NOD mice.
Fenofibrate is known to suppress inflammation and immune response because of its potential to activate PPAR-α.27,42 By activating PPAR-α, fenofibrate increased the gene expression of Foxp3 in isolated CD4+ T cells from EAM rats33 and decreased corneal epithelial staining scores in a dry eye model induced by topical benzalkonium chloride.26 In addition, fenofibrate has also been found to inhibit nitric oxide synthase (iNOS) expression and nitric oxide (NO) release in infected cardiomyocytes in a PPARα-independent manner.43 In our study, we found that after fenofibrate treatment, the protein and mRNA levels of PPAR-α were significantly higher, suggesting that fenofibrate might ameliorate LG inflammation via its PPAR-α agonistic effect.
In the process of high-density lipoprotein biogenesis, activation of PPAR-α by its agonist fenofibrate has been shown to enhance the expression of LXR-β,19 which is considered a key regulator of CD4+ T-cell development and differentiation.44–46 Knockout of LXR-β increased Th17 induction in mouse CD4+ T cells and suppressed Treg cell functionality in mouse bone marrow.46,47 Additionally, several studies have reported that LXR activator T0901317 reduced the expression of IFN-γ in vitro.44,48 Consistent with previous studies, we also observed that LXR-β expression was dramatically increased in the fenofibrate group. Activation of PPAR-α via fenofibrate might directly promote the transcription of LXR-β through interacting with a positive responsive element upstream of the LXR promoter region.49 The augmented LXR-β expression may lead to decreased Th1/Th17 responses and increased Treg generation, resulting in alleviated autoimmune dacryoadenitis in fenofibrate-treated NOD mice. However, the crosstalk between PPAR-α and LXR-β and the detailed molecular mechanisms underlying the action of PPAR-α/LXR-β signaling in modulating Th1/Th17 and Treg cells within the context of autoimmune disorders need further investigation.
In summary, fenofibrate treatment efficiently suppressed inflammation and restored LG function in SS-like dacryoadenitis, and this effect may be partially ascribed to modulating Th1/Th17/Treg cell responses via activating PPAR-α/LXR-β signaling. Therefore, our finding suggests that PPAR-α agonist fenofibrate might be a promising candidate to alleviate SS dry eye.
Supplementary Material
Acknowledgments
Supported by the National Natural Science Foundation of China (81970793, 82070929), the Scientific Research Foundation for the Returned Overseas Chinese Scholars, the State Education Ministry (No. 48), and the Tianjin Clinical Key Discipline Project (TJLCZDXKT003).
Disclosure: X. Guo, None; W. Dang, None; N. Li, None; Y. Wang, None; D. Sun, None; H. Nian, None; R. Wei, None
References
- 1. Craig JP, Nichols KK, Akpek EK, et al.. TFOS DEWS II definition and classification report. Ocul Surf. 2017; 15: 276–283. [DOI] [PubMed] [Google Scholar]
- 2. Foulks GN, Forstot SL, Donshik PC, et al.. Clinical guidelines for management of dry eye associated with Sjogren disease. Ocul Surf. 2015; 13: 118–132. [DOI] [PubMed] [Google Scholar]
- 3. Baer AN, Walitt B.. Update on Sjogren syndrome and other causes of sicca in older adults. Rheum Dis Clin North Am. 2018; 44: 419–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chiorini JA, Cihakova D, Ouellette CE, Caturegli P.. Sjogren syndrome: advances in the pathogenesis from animal models. J Autoimmun. 2009; 33: 190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Verstappen GM, Kroese FGM, Bootsma H.. T cells in primary Sjogren's syndrome: targets for early intervention. Rheumatology (Oxford). 2019; 60(7): 3088–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Verstappen GM, Corneth OBJ, Bootsma H, Kroese FGM.. Th17 cells in primary Sjogren's syndrome: pathogenicity and plasticity. J Autoimmun. 2018; 87: 16–25. [DOI] [PubMed] [Google Scholar]
- 7. Sudzius G, Mieliauskaite D, Butrimiene I, Siaurys A, Mackiewicz Z, Dumalakiene I.. Activity of T-helper cells in patients with primary Sjogren's syndrome. In Vivo. 2013; 27: 263–268. [PubMed] [Google Scholar]
- 8. Miao M, Hao Z, Guo Y, et al.. Short-term and low-dose IL-2 therapy restores the Th17/Treg balance in the peripheral blood of patients with primary Sjogren's syndrome. Ann Rheum Dis. 2018; 77: 1838–1840. [DOI] [PubMed] [Google Scholar]
- 9. Lieberman SM, Kreiger PA, Koretzky GA.. Reversible lacrimal gland-protective regulatory T-cell dysfunction underlies male-specific autoimmune dacryoadenitis in the non-obese diabetic mouse model of Sjogren syndrome. Immunology. 2015; 145: 232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ratay ML, Glowacki AJ, Balmert SC, et al.. Treg-recruiting microspheres prevent inflammation in a murine model of dry eye disease. J Control Release. 2017; 258: 208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ahmed W, Ziouzenkova O, Brown J, et al.. PPARs and their metabolic modulation: new mechanisms for transcriptional regulation? J Intern Med. 2007; 262: 184–198. [DOI] [PubMed] [Google Scholar]
- 12. Cheng H, Xi Y, Chi X, Wu Y, Liu G. Fenofibrate treatment of rats with experimental autoimmune myocarditis by alleviating Treg/Th17 disorder. Cent Eur J Immunol. 2016; 41: 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee JW, Bajwa PJ, Carson MJ, et al.. Fenofibrate represses interleukin-17 and interferon-gamma expression and improves colitis in interleukin-10-deficient mice. Gastroenterology. 2007; 133: 108–123. [DOI] [PubMed] [Google Scholar]
- 14. Pearsall EA, Cheng R, Matsuzaki S, et al.. Neuroprotective effects of PPARalpha in retinopathy of type 1 diabetes. PLoS One. 2019; 14: e0208399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xiao X, Luo P, Zhao H, et al.. Amniotic membrane extract ameliorates benzalkonium chloride-induced dry eye in a murine model. Exp Eye Res. 2013; 115: 31–40. [DOI] [PubMed] [Google Scholar]
- 16. Shah M, Edman MC, Janga SR, et al.. A rapamycin-binding protein polymer nanoparticle shows potent therapeutic activity in suppressing autoimmune dacryoadenitis in a mouse model of Sjogren's syndrome. J Control Release. 2013; 171: 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hunger RE, Carnaud C, Vogt I, Mueller C.. Male gonadal environment paradoxically promotes dacryoadenitis in nonobese diabetic mice. J Clin Invest. 1998; 101: 1300–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Christofides A, Konstantinidou E, Jani C, Boussiotis VA.. The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metabolism. 2021; 114: 154338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ogata M, Tsujita M, Hossain MA, et al.. On the mechanism for PPAR agonists to enhance ABCA1 gene expression. Atherosclerosis. 2009; 205: 413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Parigi SM, Das S, Frede A, et al.. Liver X receptor regulates Th17 and RORgammat(+) Treg cells by distinct mechanisms. Mucosal Immunol. 2021; 14: 411–419. [DOI] [PubMed] [Google Scholar]
- 21. Korf H, Vander Beken S, Romano M, et al.. Liver X receptors contribute to the protective immune response against Mycobacterium tuberculosis in mice. J Clin Invest. 2009; 119: 1626–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nakano Y, Arima T, Tobita Y, Uchiyama M, Shimizu A, Takahashi H.. Combination of peroxisome proliferator-activated receptor (PPAR) alpha and gamma agonists prevents corneal inflammation and neovascularization in a rat alkali burn model. Int J Mol Sci. 2020; 21(14): 5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Elkjaer AS, Lynge SK, Grauslund J.. Evidence and indications for systemic treatment in diabetic retinopathy: a systematic review. Acta Ophthalmol. 2020; 98: 329–336. [DOI] [PubMed] [Google Scholar]
- 24. Keech AC, Mitchell P, Summanen PA, et al.. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007; 370: 1687–1697. [DOI] [PubMed] [Google Scholar]
- 25. Group AS, Group AES, Chew EY, et al.. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010; 363: 233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. He H, Liang M, Li L, et al.. PPAR-alpha agonist fenofibrate suppressed the formation of ocular surface squamous metaplasia induced by topical benzalkonium chloride. Invest Ophthalmol Vis Sci. 2020; 61: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. He X, Zhao Z, Wang S, et al.. High-fat diet-induced functional and pathologic changes in lacrimal gland. Am J Pathol. 2020; 190: 2387–2402. [DOI] [PubMed] [Google Scholar]
- 28. Chen YH, Lightman S, Calder VL.. CD4(+) T-cell plasticity in non-infectious retinal inflammatory disease. Int J Mol Sci. 2021; 22(17): 9584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen Y, Dana R.. Autoimmunity in dry eye disease—an updated review of evidence on effector and memory Th17 cells in disease pathogenicity. Autoimmun Rev. 2021; 20: 102933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Verstappen GM, Meiners PM, Corneth OBJ, et al.. Attenuation of follicular helper T cell-dependent B cell hyperactivity by abatacept treatment in primary Sjogren's syndrome. Arthritis Rheumatol. 2017; 69: 1850–1861. [DOI] [PubMed] [Google Scholar]
- 31. Szodoray P, Gal I, Barath S, et al.. Immunological alterations in newly diagnosed primary Sjogren's syndrome characterized by skewed peripheral T-cell subsets and inflammatory cytokines. Scand J Rheumatol. 2008; 37: 205–212. [DOI] [PubMed] [Google Scholar]
- 32. Chen X, Aqrawi LA, Utheim TP, et al.. Elevated cytokine levels in tears and saliva of patients with primary Sjogren's syndrome correlate with clinical ocular and oral manifestations. Sci Rep. 2019; 9: 7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chang H, Zhao F, Xie X, et al.. PPARalpha suppresses Th17 cell differentiation through IL-6/STAT3/RORgammat pathway in experimental autoimmune myocarditis. Exp Cell Res. 2019; 375: 22–30. [DOI] [PubMed] [Google Scholar]
- 34. Zhang MA, Ahn JJ, Zhao FL, et al.. Antagonizing peroxisome proliferator-activated receptor alpha activity selectively enhances Th1 immunity in male mice. J Immunol. 2015; 195: 5189–5202. [DOI] [PubMed] [Google Scholar]
- 35. Zhou L, Littman DR.. Transcriptional regulatory networks in Th17 cell differentiation. Curr Opin Immunol. 2009; 21: 146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eggenhuizen PJ, Ng BH, Ooi JD.. Treg enhancing therapies to treat autoimmune diseases. Int J Mol Sci. 2020; 21(19): 7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Szodoray P, Papp G, Horvath IF, et al.. Cells with regulatory function of the innate and adaptive immune system in primary Sjogren's syndrome. Clin Exp Immunol. 2009; 157: 343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhou Z, Liang Y, Gao Y, Kong W, Feng J, Wang X.. Fenofibrate enhances the in vitro differentiation of foxp3(+) regulatory T cells in mice. PPAR Res. 2012; 2012: 529035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bommireddy R, Doetschman T.. TGFbeta1 and Treg cells: alliance for tolerance. Trends Mol Med. 2007; 13: 492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen W, Jin W, Hardegen N, et al.. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003; 198: 1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Miyara M, Ito Y, Sakaguchi S.. TREG-cell therapies for autoimmune rheumatic diseases. Nat Rev Rheumatol .2014; 10: 543–551. [DOI] [PubMed] [Google Scholar]
- 42. Tang L, Wang X, Wu J, et al.. Sleep deprivation induces dry eye through inhibition of PPARalpha expression in corneal epithelium. Invest Ophthalmol Vis Sci. 2018; 59: 5494–5508. [DOI] [PubMed] [Google Scholar]
- 43. Cevey AC, Mirkin GA, Donato M, et al.. Treatment with fenofibrate plus a low dose of benznidazole attenuates cardiac dysfunction in experimental Chagas disease. Int J Parasitol Drugs Drug Resist. 2017; 7: 378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Solt LA, Kamenecka TM, Burris TP.. LXR-mediated inhibition of CD4+ T helper cells. PLoS One. 2012; 7: e46615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Herold M, Breuer J, Hucke S, et al.. Liver X receptor activation promotes differentiation of regulatory T cells. PLoS One. 2017; 12: e0184985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cui G, Qin X, Wu L, et al.. Liver X receptor (LXR) mediates negative regulation of mouse and human Th17 differentiation. J Clin Invest. 2011; 121: 658–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Michaels AJ, Campbell C, Bou-Puerto R, Rudensky AY.. Nuclear receptor LXRbeta controls fitness and functionality of activated T cells. J Exp Med. 2021; 218(4): e20201311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Walcher D, Kummel A, Kehrle B, et al.. LXR activation reduces proinflammatory cytokine expression in human CD4-positive lymphocytes. Arterioscler Thromb Vasc Biol. 2006; 26: 1022–1028. [DOI] [PubMed] [Google Scholar]
- 49. Chawla A, Boisvert WA, Lee CH, et al.. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell. 2001; 7: 161–171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.