Abstract
Monkeypox (MPX) is a viral zoonosis with lesions like smallpox. Though rare in Nigeria, sporadic outbreaks have been reported in 17 states since September 2017. Unfortunately, the COVID-19 pandemic has further reduced surveillance and reporting of MPX disease. This study seeks to assess the effect of an enhanced surveillance approach to detect MPX cases and measure the cumulative incidence of MPX in priority states in Nigeria. We identified three priority states (Rivers, Delta and Bayelsa) and their Local Government Areas (LGAs) based on previous disease incidence. We also identified, trained, and incentivized community volunteers to conduct active case searches over three months (January to March 2021). We supported case investigation of suspected cases and followed up on cases in addition to routine active surveillance for MPX in health facilities and communities. Weekly and monthly follow-up was carried out during the same period. Out of the three states, 30 hotspots LGAs out of the 56 LGAs (54%) were engaged for enhanced surveillance. We trained three state supervisors, 30 LGA surveillance facilitators and 600 Community informants across the three priority states. Overall, twenty-five (25) suspected cases of MPX were identified. Out of these, three (12%) were confirmed as positive. Enhanced surveillance improved reporting of MPX diseases in hotspots LGAs across the priority states. Extension of this surveillance approach alongside tailored technical support is critical intra and post-pandemic.
Key words: Monkeypox, COVID-19, Zoonosis, Enhanced Surveillance, Nigeria
Introduction
Monkeypox (MPX) is an infectious zoonotic viral disease with lesions like smallpox and was first discovered in 1958 in research monkeys.1 Its first human case was reported in 1970 in the Democratic Republic of Congo (DRC) during an intensified effort to eliminate smallpox.1 In Nigeria, the first reported human case was in 1971.2 After a while the advent of the disease seems to have receded until 2017 when there was a resurgence with 88 confirmed cases.3,4 Since then, Monkeypox has been endemic in the country especially in the Southern region. As of 31st December 2021, Nigeria recorded a total of 226 Monkeypox cases.4
Though the global burden of MPX is uncertain either due its rarity or to paucity of research, nonetheless, human cases of MPX have been documented in several Central and Western African nations giving rise to two distinct groups known as Central and West African clades.5 Unlike the Central clade, the West African clade is associated with milder disease, fewer deaths, and limited human-to-human transmission. Nigeria recorded the largest documented outbreak of West African clade in 2017 after which sporadic cases have been reported across 17 states.5 Rivers, Bayelsa, Lagos and Delta states have reported the highest number of cases in Nigeria. Although rodents are thought to be the primary source of MPX introduction into human populations, the virus’s natural reservoir remains unknown.5,6 Transmission usually occurs when a person comes in contact with MPX virus from an animal, a human, or contaminated objects.6 The virus enters the body either through a break in the skin, the respiratory tract, or mucous membranes (eyes, nose, or mouth).6
Surveillance has been identified as a strategic, focused, and scalable intervention in mopping cases of sporadic outbreaks of several infectious diseases.7,8 For example, detecting measles, polio and dengue outbreaks have been made possible using this method.9-11 In some instances, enhanced surveillance, which is the collection of data with more effort than is needed for routine surveillance, has also played a significant role in curbing transmission of infectious diseases.11 Its effectiveness has been shown to increase detection of influenza.12 However, enhanced surveillance (i.e., active) is marked by massive cost with a high yield of cases.
In Nigeria, the outbreak of COVID-19 impeded on the lives and livelihood of citizens. Importantly, the healthcare system was exposed for its fragility, with most of the resources allocated to diseases of public health importance being channeled to combat COVID-19, hence resulting in a de-prioritization of other infectious diseases including Monkeypox. This resulted in a rapid decrease in the number of reported cases of MPX as compared to previous years. It is not clear if the reduction in case reporting was due to decreased identification of cases or de-prioritization due to COVID-19 outbreak. It was therefore imperative to conduct enhanced surveillance to improve the detection of cases especially in states where there was high reporting of cases prior to COVID-19 outbreak. This study aimed to assess the true burden of Monkeypox in priority states in Nigeria during COVID-19 outbreak.
Materials and Methods
Nigeria is a country consisting of 36 states and the Federal Capital Territory (FCT). Earlier cases of Monkeypox have been reported from 32 states and predominantly among states in the Southern region of Nigeria such as Rivers, Bayelsa, Delta, Cross River and Edo States. We selected three states namely Rivers, Bayelsa and Delta with the highest number of cases historically but with a contrary low reportage of the incidence of Monkeypox likely due to the COVID-19 outbreak from 2020 to 2021 for enhanced surveillance. Within the selected states, 30 Local Government Areas (LGA) referred as priority were identified and engaged for this project. Selection criteria for the LGA includes high burden LGAs within the states using records from previous years. We conducted an enhanced surveillance for a period of 3 months between January and March 2021. The case definitions are on Table 1. A defined geographical description (i.e., population size, area of land and community classification) of these participating LGAs is shown, more so, defined community classification was used (Table 1). The enhanced surveillance was coordinated by epidemiologists from NCDC as well as Disease Surveillance and Notification Officers (DSNOs) at the LGA level with support from the state Epidemiologists and State DSNOs. The DSNOs helped identify surveillance officers and community volunteers within the selected LGAs using defined criteria for selection. All engaged personnel were assigned clearly defined terms of reference and trained on the project using the NCDC Monkeypox training manual, which was developed from the NCDC Monkeypox Guidelines.
Terms of reference for Community Health Workers
Support contact tracing, follow-up and monitoring in the ward(s).
Assist in case identification in the ward(s) especially among contacts
Support active case search in the health care facilities
Assist in the distribution of IEC materials to the community
Transmit key health messages including basic elements of mental health and psychosocial support to families through house-to-house visits
Prompt dissemination of information to state surveillance team
Institute prompt feedback from the surveillance team to the community
Serve as liaison officer between the community and the response team
Participate in data collection from the community and health care facilities
Linking suspected cases to sample collection and management centers
Collation of data from community members that require sample collection
Participate in other activities as assigned by supervisors
Criteria for engaging Community Health Workers (Volunteers)
Community Health Workers (Volunteers) are personnel, who will work at ward levels in the LGAs across selected states to support community-based case finding and reporting. To be engaged as a community health worker (CHW) or volunteer, the following attributes were required:
They may be volunteers or receive a salary. They are generally not, however, civil servants or professional employees of the Ministry of Health
They must be resident of the state and LGA of support
Must reside in or are willing to stay in ward of deployment
Respect the culture of the community
Speaks fluently the local language
Must possess a minimum of a healthrelated diploma certificate (preferably in community health)
Well known to the community / ward /LGA where they will work with community persons
Respected person in the community
Should possess basic knowledge of MPX
Understand and speak English
We scheduled and ensured regular meetings between the DSNOs, the surveillance officers and community informants to encourage them to identify suspected patients with monkeypox and to collect swabs, blood, and crust samples from them for diagnostic testing. Samples collected from suspected cases in the 3 states were shipped and analyzed for MPX at the National Reference Laboratory in Abuja.
Regular meetings were also held between the epidemiologist at NCDC and the participating LGA DSNOs and surveillance supervisors. These individuals at the LGA level were responsible for training community informants and supervising patient enrolment. Monthly meetings were held to discuss and review the previous month data and to share experiences, best practices as well as provide feedback to the participating LGAs. Necessary information was also conveyed periodically by the monkeypox national surveillance supervisor to the participating LGAs in the selected states through phone calls, WhatsApp messages and virtual meetings.
Table 1.
Definition of key terms.
| S/No | Term | Definition |
|---|---|---|
| 1. | Suspected case | Any person with acute illness, fever >38.3oC, intense headache, lymphadenopathy, back pain, myalgia, and intense asthenia followed one to three days later by a progressively developing rash often beginning on the face (most dense) then spreading elsewhere on the body, including soles of feet and palms of hand |
| 2. | Confirmed case | Any suspected case with laboratory confirmation (viral identification by polymerase chain reaction: PCR, antibody detection, viral isolation) |
| 3. | Probable case | A suspected case with epidemiological linkage with a confirmed case in whom laboratory testing could not be carried out for confirmation |
| 4. | Urban | An LGA was classified as urban if it is State capital has an approximate population size of 20 000, >75% of its population is engaged in non-agricultural occupations, and availability of infrastructure, good transportation system and a broad array of economic, social, and recreational activities |
| 5. | Rural | An LGA was classified as rural if it is not capital has an approximate population size of less than 20 000, >75% of its population is engaged in agricultural occupations, and unavailability of infrastructure, good transportation system and a broad array of economic, social, and recreational activities |
| 6. | Peri-urban | LGAs that stand in the interphase of urban and rural |
Once suspected cases of monkeypox are identified, samples were collected in addition to demographic information and information about the clinical signs and symptoms. The information collected were filled into the case investigation forms (CIFs) which are then uploaded to the Surveillance Outbreak Response Management and Analysis System (SORMAS), a surveillance outbreak tool and database of the NCDC. The samples collected with the CIFs were shipped to the National Reference Laboratory via TRANEX, a sample collection and transporting company within 24 hrs. All collected samples were tested for Monkeypox using the RT-PCR method. All samples tested for Monkeypox were also tested for varicella-zoster virus (VZV).
Ethical considerations
This study was not designed for research purpose in the initial stage but for Monkeypox intervention in the respective states. However, participants were asked to provide oral consent before allowing their samples to be taken. As a secondary data, approval was not sort from the National Health Research Ethics committee (NHREC), but permission was gotten from NCDC for the use of the data.
Statistical analysis
Data analysis was done using excel and results were presented as frequencies, as well as simple tables and charts. The age group of all suspected and confirmed cases are represented using age-sex pyramid. The number of suspected cases per epidemiological week are represented by histogram. The trends of Monkeypox cases from 2017-2021 are represented by bar charts.
Results
Out of the 3 states (Rivers, Bayelsa and Delta State) where this study took place, 30 hotspots LGAs out of a total of 56 LGAs (54%) were engaged for enhanced surveillance. We trained a total of 483 persons. These included 3 state supervisors, 30 LGA surveillance facilitators and 450 community informants across the three priority states.
Over the study period (i.e., January to March 2021), a total of twenty-five (25) suspected cases of monkeypox in humans were identified as shown in Table 2. Following PCR laboratory testing of these samples, 3 (12%) cases were positive, 22 (88%) cases were negative. Bayelsa and Delta states contributed the highest number of suspected cases with 9 (36%) cases each. Two (8%) of the confirmed cases were from Sapele LGA in Delta State and the third confirmed case (4%) was from Southern Ijaw LGA, in Bayelsa State. Out of the 25 suspected cases, only 19 had their age and gender recorded. Out of this number ten were females (10, 40%) and the median age of the suspected cases was 27 years (Figure 1). The three confirmed cases include 2 females and 1 male with their corresponding age being, 27, 21 and 41years respectively. The mean age of the confirmed cases was 30 years while the median age was 27 years. The highest number of suspected cases was reported in week 5 with 5 (20%) suspected cases, each while in weeks 9 and 12, no suspected cases were recorded (Figure 2). The confirmed cases recorded were reported in weeks 1, 2 and 5 with 1 (4%) confirmed cases in each week.
The cumulative risk of MPX declined over the years between 2017 and 2021 (Figure 3). The number of suspected and confirmed cases were highest in 2017 and lowest in 2020 and 2021 respectively.
Figure 1.
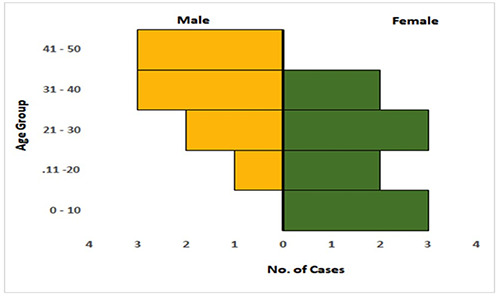
Age/ sex pyramid of suspected cases of Monkeypox. A representation of the age and gender of suspected and confirmed cases of Monkeypox.
Figure 2.
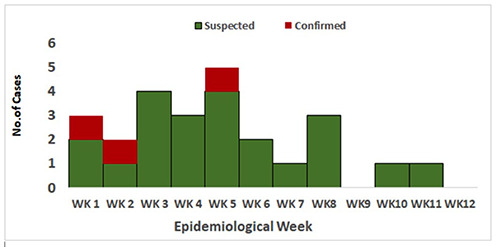
Epicurve among suspected cases of MPX. A distribution of Monkeypox infection among residents of Bayelsa, Rivers and Delta states, within 12 weeks of enhanced surveillance.
Discussion
We embarked on the enhanced surveillance study during the COVID-19 pandemic where we reported 25 suspected cases and 3 confirmed cases among persons living within the selected LGAs in Rivers, Delta and Bayelsa states over a period of 3 months (January - March 2021). We report a monthly average of one case per month. The highest number of the suspected cases were reported in the second month.
In recent years from 2017-2020 the risk of Monkeypox in Nigeria has declined considerably, although the number of suspected cases rose comparably in 2021. Our intervention of enhanced surveillance seems to mop up cases of MPX that would have been missed because of the COVID- 19 pandemic. The monthly average of 3 confirmed cases is higher than the previous year (2020), where we had 8 cases over the 12-month period with a monthly average of 0.67 cases. Compared to the preceding year (2020), the number of suspected cases within the short period is quite high and is projected to increase before the year ends, if the sensitized and trained key actors in the enhanced surveillance in each of the implementing states continue to identify and report MPX after the program has ended. This observed and forecasted increase in suspected cases may be due to an increase in population density or because of unknown animal reservoirs encroaching human settlements within selected communities. 5 Moreso, the potential increase here may be due to the activation of the active case search through the enhanced surveillance implemented. Other reports have also shown the benefit of this approach in successfully detecting the true burden of diseases.13
Figure 3.
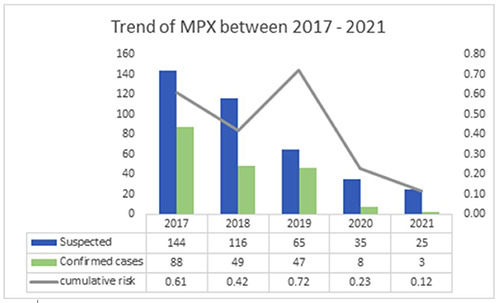
Trend of confirmed Monkeypox cases from 2017-2021. A representation of the trend of Monkeypox infection in suspected and confirmed cases in Nigeria between 2017 and 2021.
Table 2.
Suspected cases of monkeypox in hotspot LGAs of Bayelsa, Delta and Rivers States.
| S/No | Targeted Territory | Area, Km2 | Population size Bayelsa | Community classification | No. of cases |
|---|---|---|---|---|---|
| 1 | Ogbia | 1,692 | 179,926 | Rural | 1 suspected case |
| 2 | Southern Ijaw | 2,682 | 319,413 | Rural | 1 Confirmed case |
| 3 | Yenegoa | 1,698 | 352,285 | Urban | 5 suspected cases |
| 4 | Brass | 1,404 | 185,049 | Rural | 1 suspected case |
| 5 | Ekeremor | 1,910 | 270,257 | Rural | No case |
| 6 | Nembe | 760 | 130,931 | Rural | No case |
| 7 | Sagbama | 945 | 187,146 | Rural | No case |
| 8 | Kolokuma | 361 | 77,292 | Rural | No case |
| Rivers | |||||
| 9 | Degema | 1,011 | 249,467 | Rural | 2 suspected cases |
| 10 | Port Harcourt | 98.49 | 538,558 | Urban | 1 suspected case |
| 11 | Gokana | 126 | 233,813 | Rural | 2 suspected cases |
| 12 | Obio/Akpor | 260 | 462,350 | Rural | No case |
| 13 | Bonny | 646 | 214,983 | Peri-Urban | 2 suspected cases |
| 14 | Andoni | 233 | 217,924 | Rural | No case |
| 15 | Eleme | 138 | 190,194 | Rural | No case |
| 16 | Etche | 774.7 | 249.939 | Rural | No case |
| 17 | Emohua | 831 | 201,057 | Rural | No case |
| 18 | Tai | 159 | 120,308 | Rural | No case |
| Delta | |||||
| 19 | Isoko North | 479 | 143,559 | Rural | 1 suspected case |
| 20 | Sapele | 580.3 | 174,273 | Peri-urban | 2 confirmed cases |
| 21 | Ughelli North | 832.7 | 320,687 | Rural | 2 suspected cases |
| 22 | Ndokwa East | 1,614 | 103,224 | Rural | 2 suspected cases |
| 23 | Ika Northeast | 475.3 | 182,819 | Rural | 1 suspected case |
| 24 | Udu | 137 | 142,480 | Rural | 1 suspected case |
| 25 | Ethiope East | 378.6 | 200,942 | Rural | No case |
| 26 | Aniocha South | 867.4 | 142,045 | Rural | No case |
| 27 | Ukuani | 414.2 | 119,034 | Rural | No case |
| 28 | Uvwie | 92.41 | 188,728 | Rural | No case |
| 29 | Warri Southwest | 1,279 | 116,538 | Peri-urban | No case |
| 30 | Warri South | 542.2 | 311,970 | Peri-urban | No case |
In previous years, between 2018 and 2019, reports show that the number confirmed cases of Monkeypox rose above 10 cases.4 However, the number of confirmed cases in 2020 reduced drastically to below 10 with only 8 confirmed cases for the entire year. This is supposedly due to the COVID-19 pandemic which deprioritized intervention for other diseases including MPX. COVID-19 has taken the center stage in matters concerning infections disease in Nigeria, with most resources including human, materials and financial originally budgeted for other diseases being redirected to curb the colossal disease.4,19 In a similar pattern, the number of confirmed cases in 2021 has been below 10 (i.e., 3 cases over 3 months). Alternatively, this result may not imply reduced incidence of the disease but may be due to reduced case reporting. In some instances, many people infected with MPX prefer to hide themselves due to the stigmatization as documented by Godfrey et al., in their study.20 In addition, some suspected cases usually decline consent for samples to be taken during surveillance activities.
Several individual factors such as age, gender, and occupation may be responsible for acquisition of MPX. In our study, the median age among those infected with monkeypox was 27 years. Energetic activities such as partying and clubbing especially among young people may have led to interacting with infected persons in their community. Again, amid the fragile economic situation in the country, many young people may have been involved in poaching activities. Though illegal, it has been proven to be a lucrative business and many youths must have contracted the disease through their interaction with animals.15 Similar to our findings, the World Health Organization has reported that people below the age of 40 are mostly infected with the disease and that this could be a result of the discontinuance of smallpox vaccination.16,17 Like our findings, another study has shown infection with monkeypox occurs in the population of people less than 40 years, with the median age of infection being 31 years.18 Our study also shows higher infection among females, suggesting higher prevalence among this gender, although the number of infected cases is too low to conclude. However, other studies have reported that the disease is more prevalent in males as against females.17
The increase in the number of confirmed cases in 2021 compared to 2020 highlights the importance of the enhanced surveillance deployed and indicates the need for more vigorous and sustained surveillance programs, early case identification and reporting which may result in timely containment of the disease in the affected areas. Otherwise, there could be a resurgence of MPX which could potentially be exported to other parts of the country and inadvertently to other regions of the world considering how easily COVID-19 was transmitted.21
Limitations
The limitation of this study includes the fact that it essentially uses the vertical approach which is cash intensive and thus not a sustainable project. This stand-alone vertical project does not adhere strictly to the principles of Integrated Disease Surveillance guidelines, which seeks to address more diseases that affect a population. Moreso, the short time involved in this study really limited the number of cases reported. In addition, there were missing information on the demographic of suspected cases because of incomplete Case Investigation Forms (CIFs).
Conclusions
Enhanced surveillance improved reporting of MPX diseases in hotspots LGAs across the priority states where the ES was conducted. It provided timely and essential information about the burden of monkeypox infection during the COVID-19 outbreak in Nigeria. The findings here will prove useful to public health officers and institutions in the country especially as they plan to contain infectious disease in the priority states. Extending the surveillance approach employed here alongside tailored technical support is critical during and post COVID-19 pandemic. We recommend the support of community activities to emphasis early presentation of MPX cases and treatment centers be dedicated and equipped to manage MPX cases
Acknowledgements
The authors wish to appreciate all the members of NCDC Monkeypox Technical Working Group; State Epidemiologists and Disease Surveillance and Notification Officers who contributed to collecting data for this study.
Funding Statement
Funding: The funding for this project was provided by the Nigeria Centre for Disease Control, Abuja, Nigeria.
References
- 1.Marennikova SS, Seluhina EM, Mal’ceva NN, Ladnyj ID. Poxviruses isolated from clinically ill and asymptomatically infected monkeys and a chimpanzee. Bull World Health Organ 1972;46:613. [PMC free article] [PubMed] [Google Scholar]
- 2.Eke RA. Monkey-pox in a four-year old girl: case report. West Afr Med J 1972;21:21–2. [PubMed] [Google Scholar]
- 3.Yinka-Ogunleye A, Aruna O, Ogoina D, et al. Reemergence of human monkeypox in Nigeria, 2017. Emerg Infect Dis 2018;24:1149–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NCDC. Nigeria Centre for Disease Control, Monkeypox. 2021. https://www.ncdc.gov.ng/diseases/sitreps/?cat=8&name=An Update of Monkeypox Outbreak in Nigeria. Assessed 6 February 2022 [Google Scholar]
- 5.Beer EM, Bhargavi Rao V. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl Trop Dis 2019;13:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CDC. Transmission - Monkeypox - Poxvirus - CDC. 2021. Available from: https://www.cdc.gov/poxvirus/monkeypox/transmission.html. Assessed 7 February 2022 [Google Scholar]
- 7.Milinovich GJ, Williams GM, Clements ACA, Hu W. Internet-based surveillance systems for monitoring emerging infectious diseases. Lancet Infect Dis 2014;14:160–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thacker SB, Choi K, Brachman PS. The Surveillance of Infectious Diseases. JAMA 1983;249:1181–5. [PubMed] [Google Scholar]
- 9.Tikhonova NT, Bichurina MA, Gerasimova AG, et al. Enhanced surveillance for measles in low-incidence territories of the Russian Federation: defining a rate for suspected case investigation. Epidemiol Infect 2011;139:239–46. [DOI] [PubMed] [Google Scholar]
- 10.Wen N, Fan CX, Fu JP, et al. Enhanced surveillance of acute flaccid paralysis following importation of wild poliovirus in Xinjiang Uygur Autonomous Region, China. BMC Infect Dis 2014;14:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hales CM, Fryar CD, Carroll MD, et al. Differences in obesity prevalence by demographic characteristics and urbanization level among adults in the United States, 2013-2016. J Am Med Assoc 2018;319:2419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leventhal A, Ramlawi A, Belbiesi A, et al. Enhanced surveillance for detection and management of infectious diseases: Regional collaboration in the middle east. Emerg Health Threats J 2013;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craig AT, Joshua CA, Sio AR, et al. Enhanced surveillance during a public health emergency in a resource-limited setting: Experience from a large dengue outbreak in Solomon Islands, 2016-17. PLoS One 2018;13:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alakunle E, Moens U, Nchinda G, et al. Monkeypox Virus in Nigeria: Infection Biology, Epidemiology, and Evolution. Virology 2020;12:1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson-Wilde L. Wildlife crime: a global problem. Forensic Sci Med Pathol 2010;6:221–2. [DOI] [PubMed] [Google Scholar]
- 16.WHO. Monkeypox. 2019. Available from: https://www.who.int/newsroom/fact-sheets/detail/monkeypox. Accessed 6 February 2022. [Google Scholar]
- 17.Oladoye MJ. Monkeypox: A Neglected Viral Zoonotic Disease. Eur J Med Educ Technol 2021;14:2108. [Google Scholar]
- 18.Van Bavel JJ, Cichocka A, Capraro V, et al. National identity predicts public health support during a global pandemic. Nat Commun 2022;13:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nwozor A, Okolie C, Okidu O, Oshewolo S. The Looming Dangers of Explosion in Community Transmissions of COVID-19 in Nigeria. Ann Glob Health 2020;86:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Godfrey B. Tangwa, Akin Abayomi Samuel J. Ujewe Nchangwi Syntia Munung. Socio-cultural Dimensions of Emerging Infectious Diseases in Africa. Springer Nature Switzerland AG. 2019 [Google Scholar]
- 21.Mauldin MR, McCollum AM, Nakazawa YJ, et al. Exportation of Monkeypox Virus From the African Continent. J Infect Dis 2020;2005–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


