Abstract
Background
Understanding why patients with severe asthma do not follow healthcare provider (HCP) advice to adjust treatment is critical to achieving personalised disease management.
Methods
We reviewed patient choice to follow HCP advice to adjust asthma treatment in a UK-based randomised, controlled, single-blind (study participant), multicentre, parallel group 48-week clinical study comparing biomarker-directed treatment adjustment with standard care in severe asthma.
Results
Of 1572 treatment advisories (291 participants), instructions were followed in 1377 cases (87.6%). Patients were more likely to follow advice to remain on treatment (96.7%) than to either reduce (70.3%) or increase (67.1%) their treatment, with 64% of patients following all treatment advice. Multivariate analysis associated belonging to an ethnic minority group (OR 3.10, 95% CI 1.68–5.73) and prior study medication changes (two or more changes: OR 2.77, 95% CI 1.51–5.10) with failure to follow treatment advice. In contrast, emergency room attendance in the prior year (OR 0.54, 95% CI 0.32–0.92) was associated with following treatment advice. The largest effect was seen with transition onto or off oral corticosteroids (OR 29.28, 95% CI 16.07–53.36) when compared with those requested to maintain treatment. Centre was also an important determinant regarding the likelihood of patients to follow treatment advice.
Conclusions
Belonging to an ethnic minority group and multiple prior treatment adjustments were associated with not following HCP treatment advice. Patients also responded differently to HCP advice across UK specialist centres. These findings have implications for the generalisability of models of care in severe asthma and require further focused studies.
Short abstract
Belonging to a minority ethnic group, multiple prior medication changes, being treated at a specific clinical centre, introduction of systemic corticosteroids and increased asthma symptoms were associated with resistance to asthma treatment modification https://bit.ly/3gYb66S
Introduction
Asthma treatment guidelines advocate that treatment is increased to reduce symptoms and risk of asthma exacerbations, with consideration of treatment reduction when asthma is controlled for a period of at least 3 months [1]. This strategy requires partnership between patients and healthcare professionals (HCPs) to adjust treatment when appropriate, particularly among those patients on high-dose corticosteroid treatment [2–4].
In a recent study, we investigated two strategies for adjusting corticosteroid therapy (both inhaled corticosteroids (ICS) and oral corticosteroids (OCS)) in patients with severe asthma: type 2 inflammation (T2) biomarker adjustment versus adjustment using current symptoms and recent asthma exacerbation history [5]. The study was designed primarily to explore the impact of corticosteroid treatment reduction in T2 biomarker-low participants. Clear clinical benefits were seen using biomarker-based treatment adjustment in patients who followed the study treatment algorithms (pre-specified per-protocol analysis), which included a greater proportion of patients on lower dose of ICS and reduced risk of exacerbation [5]. Despite these benefits, a large proportion of patients did not follow HCP recommendations to modify treatment. A reluctance to change therapy was anticipated for treatment increase, often meaning starting OCS, but not to reduce therapy in a study where patients were advised the primary aim was to reduce corticosteroid treatment.
Asthma guidelines distil an extensive scientific literature into evidence-based treatment pathways. However, effective implementation depends on patient engagement. We explored demographic and clinical factors to identify patient barriers to following HCP advice to adjust treatment.
Methods
Patients and study design
We performed a post hoc secondary analysis of data from our UK-based randomised, controlled, single-blind (study participant), multicentre, parallel group 48-week clinical study in patients with severe asthma (Global Initiative for Asthma Steps 4 and 5 classification of asthma severity) (ClinicalTrials.gov: NCT02717689) [5, 6]. The study team included a Patient Input Platform (PIP), a panel of patients with an insight into clinical trials who provided direction regarding patient needs and how to facilitate their understanding of study aims, objectives and requirements. This group, recruited by Asthma UK, was embedded in all discussions relating to study design and implementation. The protocol was reviewed and approved by the Office for Research Ethics Northern Ireland (NI0158) and obtained local National Health Service Research and Development approval for individual sites. All patients provided written informed consent for study participation.
Study procedures
Following randomisation, patients attended the clinic every 8 weeks for review of asthma control and treatment, and the electronic case report form software processed the study algorithms to generate a treatment advisory in both treatment arms to decrease, maintain or increase treatment. In brief, we compared a composite biomarker-based adjustment of corticosteroid therapy (using a composite index of blood eosinophil count, serum periostin concentration and fractional exhaled nitric oxide (FENO)) with adjustments in the control arm based on asthma symptoms, lung function and recent exacerbation history. The term “advisory” was specifically chosen as it was anticipated that some patients would not follow treatment advice, e.g. progression to OCS. In keeping with the pragmatic nature of the study, patients were permitted to stay in the study if treatment advice was not followed, although the reasons for this were recorded at the subsequent study visit. Treatment advisories which were not followed due to reasons allowed for in the study protocol (patient on lowest allowed ICS dose or low cortisol preventing prednisolone reduction) or where other external factors influenced the patient's choice to adjust treatment (clinician decision to override treatment adjustment or site logistical error) were interpreted as the patient following HCP advice (described in supplementary table E1a and b). When a treatment advisory could not be generated (primarily due to a missing biomarker measurement), a default “maintain treatment” advisory was generated.
Statistical analyses
Descriptive statistics are presented as mean±sd, median (interquartile range) or count (percentage) as appropriate. Comparisons between patients who followed all treatment advice during the study and those who did not follow at least one advisory were made using the t-test (normally distributed variables), Mann–Whitney U-test (nonnormally distributed variables) or Chi-squared test (categorical variables). Initial univariate logistic regression models were used to assess the association for a broad range of demographic and clinical variables that could plausibly impact the patient's decision to follow treatment advisories. A final multivariate model was selected using a modified form of backward selection. Our initial models investigated all advisories combined; however, we fitted separate models estimating the probability of following a reduce, maintain or increase advisory. To investigate potential outcome misclassification (due to intentional or unintentional patient misreporting) we compared reported medication adjustment with change in T2 biomarkers, known to be highly corticosteroid sensitive [7, 8].
Supplementary analysis compared exacerbation risk among those with a disassociated symptom/biomarker profile. A subgroup of patients with low symptoms and moderate/high biomarkers was identified, as was a separate subgroup with high symptoms and moderate/low biomarkers (supplementary material). The outcome measure was time to first exacerbation within the 8-week study period with patients considered “at risk” from the date of the study visit until the day prior to the next study visit (follow-up truncated at 56 days). Comparisons were investigated using Kaplan–Meier plots, and Cox regression models adjusted for age, gender and treatment centre were used to conduct hypothesis tests. Full details of the statistical methods are provided in the supplementary material. Analyses were conducted using Stata version 16 (StataCorp, College Station, TX, USA).
Results
Patients (n=301) undertook 1629 visits during the course of the study; of these, 25 visits had missing data. There was no information on whether or not the treatment advice was followed for 26 visits (26 patients). In six further cases it was unclear why treatment advice was not followed. Of the remaining 1572 treatment advisories issued (291 patients), 1377 (87.6%) were followed. Patients were more likely to follow advice to remain on current treatment (96.7%) than advice to either reduce (70.3%) or increase (67.1%) treatment (supplementary table E1a and b). Where treatment advisories were reported as either followed or not followed, change in individual T2 biomarkers was consistent with accurate self-reporting of treatment (supplementary table E1c).
Baseline demographic and clinical factors in patients who followed all treatment advice (n=186 (64%)) and those who decided not to follow at least one treatment advisory (n=105 (36%)) are summarised in table 1. Minority ethnic group (13.3% versus 4.3%; p=0.005) and higher intensity ICS dose (2418±873 versus 2151±608 μg beclomethasone dipropionate equivalent; p=0.002) were associated with not following treatment advice, whereas patients on maintenance OCS at study entry (41.4% versus 28.6%; p=0.029) and having an emergency room attendance in the 12 months before randomisation (25.8% versus 15.2%; p=0.037) were more likely to follow advice. There was wide variation in the way treatment advisories were followed by patients at different clinical centres (supplementary table E2 and supplementary figure E1), which may be partly related to cross-site differences in the characteristics of patients enrolled (e.g. ethnicity and corticosteroid treatment intensity), although differences were also seen between sites in gender, primary care asthma attendance and asthma control (supplementary table E2).
TABLE 1.
Baseline demographic and clinical factors in the randomised population who followed all treatment advice and those who did not follow at least one treatment advisory
| Followed treatment advice (n=186) | Chose not to follow treatment advice (n=105) | p-value | |
| Age (years) | 55.6±13.7 | 56.1±12.4 | 0.7790 |
| Male | 58 (31.2) | 41 (39.0) | 0.1739 |
| Ethnic minority group | 8 (4.3) | 14 (13.3) | 0.0051 |
| BMI (kg·m−2) | 32.1±7.3 | 31.3±7.0 | 0.3568 |
| Smoking status | 0.5193 | ||
| Never-smoker | 141 (75.8) | 76 (72.4) | |
| Ex-smoker | 45 (24.2) | 29 (27.6) | |
| Medical history | |||
| Atopic disease | 130 (70.3) | 71 (67.6) | 0.6380 |
| Hospital admission for asthma (prior year) | 34 (18.3) | 21 (20.0) | 0.7189 |
| ER attendance for asthma (prior year) | 48 (25.8) | 16 (15.2) | 0.0366 |
| GP attendance for asthma (prior year) | 107 (57.5) | 50 (47.6) | 0.1034 |
| Rescue OCS (prior year) | 2.0 (1.0–4.0) | 2.0 (1.0–4.0) | 0.8711 |
| ER asthma admission (ever) | 34 (18.3) | 28 (26.7) | 0.0933 |
| Ventilated (ever) | 15 (44.1) | 15 (53.6) | 0.4585 |
| Comorbidities | |||
| Rhinitis | 128 (68.8) | 73 (69.5) | 0.9003 |
| Eczema | 67 (36.0) | 31 (29.5) | 0.2600 |
| Nasal polyps | 43 (23.1) | 27 (25.7) | 0.6188 |
| Previous nasal surgery | 40 (21.5) | 27 (25.7) | 0.4128 |
| Oesophageal reflux | 115 (61.8) | 60 (57.1) | 0.4331 |
| Aspirin sensitivity | 29 (15.6) | 16 (15.2) | 0.9362 |
| Depression/anxiety | 60 (32.3) | 31 (29.5) | 0.6290 |
| Hypertension | 59 (31.7) | 33 (31.4) | 0.9590 |
| Osteoporosis/osteopenia | 47 (25.3) | 18 (17.1) | 0.1100 |
| Osteoarthritis | 54 (29.0) | 23 (21.9) | 0.1856 |
| Hypercholesterolaemia | 39 (21.0) | 14 (13.3) | 0.1051 |
| Diabetes | 21 (11.3) | 12 (11.4) | 0.9715 |
| Cataracts | 20 (10.8) | 13 (12.4) | 0.6740 |
| Obstructive sleep apnoea | 11 (5.9) | 6 (5.7) | 0.9444 |
| Ischaemic heart disease | 9 (4.8) | 3 (2.9) | 0.4143 |
| Peptic ulcer | 4 (2.2) | 3 (2.9) | 0.7056 |
| Stroke | 2 (1.1) | 4 (3.8) | 0.1150 |
| Chronic kidney disease | 2 (1.1) | 5 (4.8) | 0.0487 |
| Glaucoma | 4 (2.2) | 0 (0.0) | 0.1302 |
| Myocardial infarction | 2 (1.1) | 1 (1.0) | 0.9206 |
| Lung function | |||
| FEV1 (% pred) | 75.1±20.2 | 76.4±17.7 | 0.5692 |
| FVC (% pred) | 90.4±17.1 | 92.1±16.7 | 0.4325 |
| FEV1/FVC | 0.66±0.12 | 0.66±0.11 | 0.8747 |
| PEF (L) | 366.1±120.3 | 386.5±138.4 | 0.1934 |
| Laboratory values | |||
| Sputum eosinophils (%) | 1.5 (0.4–7.0) | 1.0 (0.3–8.3) | 0.9419 |
| FENO (ppb) | 20 (13–28) | 21 (13–29) | 0.8503 |
| Blood eosinophils (×109 L−1) | 0.20 (0.11–0.32) | 0.24 (0.10–0.37) | 0.3364 |
| Periostin (ng·mL−1) | 52.0±13.8 | 54.6±20.1 | 0.2021 |
| Corticosteroid treatment | |||
| Maintenance OCS user | 77 (41.4) | 30 (28.6) | 0.0293 |
| OCS dose (mg) | 0 (0–8) | 0 (0–5) | 0.0519 |
| ICS dose (µg BDP equivalent) | 2151±608 | 2418±873 | 0.0024 |
| Questionnaires | |||
| ACQ-7 score | 2.1±1.1 | 1.8±1.2 | 0.0875 |
| AQLQ total score | 4.8±1.3 | 5.0±1.5 | 0.5433 |
Data are presented as mean±sd, n (%) or median (interquartile range), unless otherwise stated. BMI: body mass index; OCS: oral corticosteroid; ER: emergency room; GP: general practitioner; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; PEF: peak expiratory flow; FENO: fractional exhaled nitric oxide; ICS: inhaled corticosteroid; BDP: beclomethasone dipropionate; ACQ: Asthma Control Questionnaire; AQLQ: Asthma Quality of Life Questionnaire.
Univariate associations with all candidate variables are provided in supplementary table E3. In multivariate analysis, belonging to an ethnic minority group (OR 3.10, 95% CI 1.68–5.73) and prior medication changes during the course of the study (two or more changes: OR 2.77, 95% CI 1.51–5.10) were associated with failure to follow treatment advice, whereas an emergency room attendance in the prior year (OR 0.54, 95% CI 0.32–0.92) was associated with following treatment advice (table 2). The largest effect in adjusting treatment was seen with transition onto or off OCS (OR 29.28, 95% CI 16.07–53.36), although patients advised to amend OCS or ICS dose (OR 11.75, 95% CI 6.97–19.78) or add/remove a long-acting muscarinic antagonist (OR 9.84, 95% CI 4.20–23.02) were also less likely to follow treatment advice than those asked to maintain treatment. Thus, after adjusting for other factors in the model, predictions suggest that 42.8% (95% CI 32.6–53.1%) of patients decided not to initiate/discontinue OCS versus only 3.6% (95% CI 2.2–4.9%) who decided not to maintain treatment (difference 39.3%, 95% CI 28.9–49.6%). Study centre was an important determinant regarding the likelihood of patients to follow treatment advice, in particular patients from Site B (OR 7.54, 95% CI 3.46–16.41) were much less likely to follow treatment advisories than those from other centres. For example, model predictions suggest that 28.0% (95% CI 23.1–32.9%) of advisories were not followed at Site B versus just 3.9% (95% CI 0.5–0.7%) at Site C (difference 24.1%, 95% CI 17.9–30.3%) after adjusting for other factors in the model.
TABLE 2.
Multivariate analysis of factors associated with not following treatment advice (all advisories combined)
| Univariate | Multivariate | |||
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Centre | ||||
| Site A | Reference | Reference | ||
| Site B | 7.44 (3.43–16.14) | 0.001 | 7.54 (3.46–16.41) | 0.001 |
| Site C | 0.46 (0.15–1.44) | 0.184 | 0.37 (0.12–1.18) | 0.094 |
| Site D | 1.31 (0.46–3.74) | 0.609 | 1.80 (0.62–5.29) | 0.282 |
| Site E | 1.94 (0.84–4.49) | 0.119 | 1.57 (0.64–3.86) | 0.322 |
| Site F | 1.57 (0.62–3.97) | 0.341 | 1.01 (0.38–2.69) | 0.987 |
| Other | 2.15 (0.98–4.69) | 0.056 | 1.39 (0.62–3.10) | 0.428 |
| Ethnic minority group | 2.06 (0.95–4.46) | 0.068 | 3.10 (1.68–5.73) | 0.001 |
| Ex-smoker | 1.11 (0.69–1.80) | 0.664 | 1.31 (0.81–2.11) | 0.265 |
| ER visit (prior year) | 0.58 (0.32–1.04) | 0.070 | 0.54 (0.32–0.92) | 0.024 |
| ACQ-7 >1.5 | 0.94 (0.63–1.39) | 0.757 | 1.16 (0.79–1.70) | 0.459 |
| Previous changes | ||||
| 0 | Reference | Reference | ||
| 1 | 1.67 (1.08–2.59) | 0.021 | 1.89 (1.18–3.02) | 0.008 |
| 2+ | 2.07 (1.19–3.62) | 0.010 | 2.77 (1.51–5.10) | 0.001 |
| Treatment adjustment | ||||
| Maintain treatment | Reference | Reference | ||
| Amend ICS/OCS dose | 11.58 (7.05–19.02) | 0.001 | 11.75 (6.97–19.78) | 0.001 |
| Add/remove LAMA | 9.95 (4.82–20.53) | 0.001 | 9.84 (4.20–23.02) | 0.001 |
| Add/remove OCS | 24.36 (13.62–43.58) | 0.001 | 29.28 (16.07–53.36) | 0.001 |
ER: emergency room; ACQ: Asthma Control Questionnaire; OCS: oral corticosteroid; ICS: inhaled corticosteroid; LAMA: long-acting muscarinic antagonist.
We explored factors associated with not following treatment advice (figure 1 and supplementary table E4). Minority ethnic group and multiple prior treatment changes consistently reduced the probability of a patient following treatment adjustment across all advisories. Patients with poorer asthma control (OR 3.40, 95% CI 1.62–7.16) and ex-smokers (OR 2.23, 95% CI 1.01–4.91) were more likely to not follow reduce advisories, although there was little effect of these factors on following maintain or increase advisories. Patients were more likely to refuse initiation of OCS (OR 3.93, 95% CI 1.52–10.17), which was an anticipated predefined secondary outcome, when compared with advice to increase corticosteroid dose. Conversely, there was evidence of patients being more likely to follow treatment advice when asked to discontinue OCS (OR 0.39, 95% CI 0.13–1.19; p=0.097). Centre effects were broadly consistent across advisories to reduce, maintain or increase treatment, with patients treated at Site B least likely to follow advisories.
FIGURE 1.
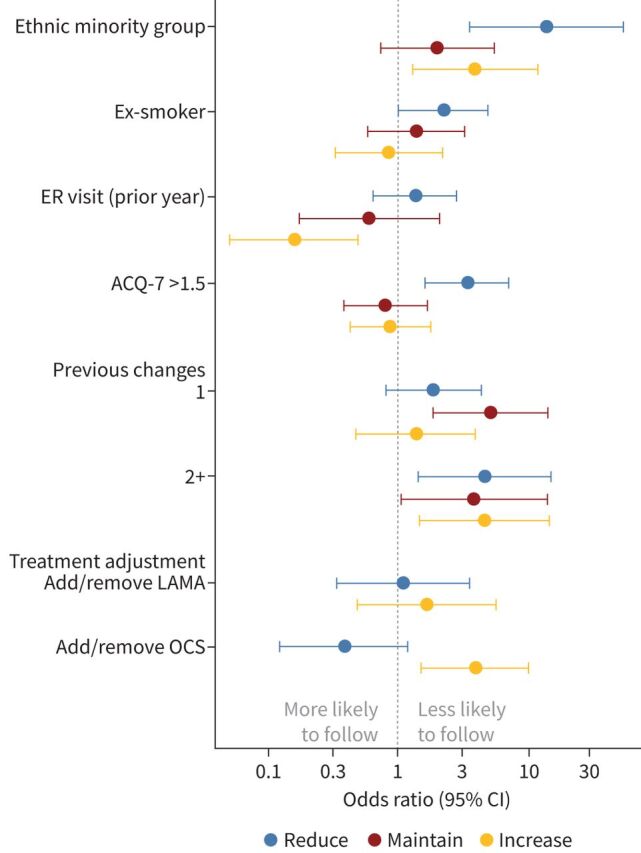
Comparison of multivariate analysis for reduce, maintain and increase advisories. ER: emergency room; ACQ: Asthma Control Questionnaire; OCS: oral corticosteroid; ICS: inhaled corticosteroid; LAMA: long-acting muscarinic antagonist.
The diagnostic accuracy of the multivariate model for following treatment advice demonstrated an area under the curve (AUC) of 0.870 (95% CI 0.842–0.899) (supplementary figure E2), and was consistent for advice to both reduce (AUC 0.826, 95% CI 0.79–0.883) and increase (AUC 0.830, 95% CI 0.780–0.880) treatment (supplementary figure E2). The internally cross-validated AUC was similar for all analyses (e.g. all treatment advisories combined: AUC 0.858, 95% CI 0.814–0.882), suggesting low test error.
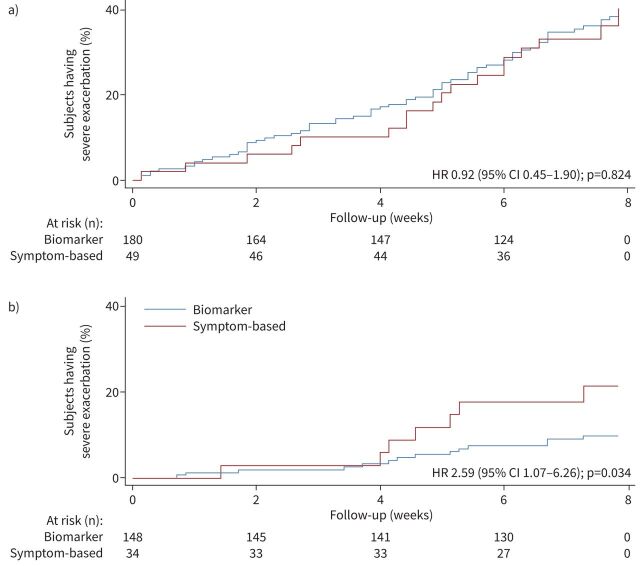
As current asthma symptoms impacted decisions to reduce corticosteroid treatment adversely, we analysed the impact of this decision by exploring exacerbation risk in patients where T2 biomarkers were dissociated from asthma symptoms. The symptom-based and biomarker-based algorithms are shown in supplementary table E5. Univariate analysis and demographic comparisons are shown in supplementary tables E6 and E7; in patients with high symptoms and low/moderate T2 biomarkers, exacerbation risk was no different (hazard ratio (HR) 0.92, 95% CI 0.45–1.90) when patients were managed according to symptoms (advised to increase treatment) or biomarkers (advised to maintain or reduce treatment) despite 59% of patients increasing their corticosteroid dose in the symptom-based arm versus only 4% in the biomarker-based arm (p<0.001) (figure 2a). However, in patients with low symptoms and moderate/high biomarkers, there was a significantly increased risk of exacerbation (HR 2.59, 95% CI 1.07–6.26) (figure 2b) when patients were managed according to symptoms (advised to reduce treatment) compared with those treated according to biomarker score (advised to maintain or increase treatment).
FIGURE 2.
Exacerbation outcome in patients with dissociated symptoms and type 2 inflammation biomarkers. a) High symptoms/low type 2 inflammation biomarkers. We observed no benefit with increased corticosteroid dose. b) Low symptoms/high type 2 inflammation biomarkers. We observed substantial benefit with increased corticosteroid dose. HR: hazard ratio.
Discussion
We explored reasons for patients not following HCP advice in a clinical trial comparing T2 biomarker-directed corticosteroid treatment with standard care in people with severe asthma. We reclassified any scenario where an external factor (e.g. advice from HCP to not follow the advice) interfered with the patient decision to follow the treatment, thus ensuring a focus on patient-directed decisions. Detailed patient information about the study explicitly described how treatment adjustment would be based on biomarker treatment in 80% taking part in the study (4:1 randomisation to T2 biomarker-directed treatment). It is generally assumed that adherence to treatment in clinical trials is high [9]; however, extensive evidence exists to the contrary [10]. The possible reluctance to initiate OCS was anticipated, thus adherence with all treatment adjustments was carefully captured at sequential study visits, allowing further analysis of the factors influencing this behaviour [5], and change in T2 biomarkers was consistent with accurate patient self-reporting of treatment.
Belonging to a minority ethnic group was consistently associated with patients not following treatment advice irrespective of type. Poorer asthma outcomes and different patterns of health service use have previously been described in UK patients with asthma from ethnic minority backgrounds [11]. It is generally accepted that ethnic diversity is inadequately reflected in clinical trials and potentially limits the applicability of results to the wider population [12, 13]. All patients were approached to take part in the clinical trial after assessment by investigators to ensure they understand the study aims and could comply with the study protocol. In this context, issues such as language barriers and comprehension of the goals of the study seem unlikely to explain ethnic differences in patient adherence to treatment guidance and further work is required to explain our observation.
Prior medication changes during the course of the study were associated with patients subsequently deciding not to follow further treatment adjustment and, importantly, this was consistent whether treatment was being increased or reduced. Where treatment is frequently adjusted, it seems logical to assume that patients decide further adjustments will not be beneficial, particularly if advice contradicts prior changes. It is also recognised that in severe asthma, more complex multimodality drug regimens are associated with treatment nonadherence [14]. Adherence with advice to maintain treatment was extremely high (97%); however, we did not predict the low level of adherence with treatment advice observed during our study and hence this is a post hoc analysis with no pre-specified analysis plan. Consequently, our results should be interpreted as exploratory, but future studies of biomarker-directed treatment in asthma should include detailed analysis of adherence with study advice and patient adherence to this advice. The apparent reluctance to adjust established treatment has potential implications for asthma guidelines, which currently suggest treatment reduction after periods of asthma stability and treatment increases if asthma control deteriorates [1]. It should be noted that while individual components of these guidelines have been formally tested, the overall benefit of a stepwise model of care included in asthma guidelines has not been formally validated in a controlled clinical trial. A more successful strategy, particularly in cases of more severe asthma that could progress to high-dose ICS, may be to target treatment using predictive biomarkers of therapeutic response on first presentation and maintain patients on this “correct” treatment when stable [15, 16].
Marked variation in patients following treatment advice was seen across different clinical centres (particularly one centre), which occurred consistently irrespective of the type of advice given. Some of the variability may be explained by centre differences in the patient population; it also suggests that patient willingness to adjust treatment was substantially different between centres. This heterogeneity is surprising considering the specialist nature of the centres and since the intention to adjust treatment had been communicated clearly to patients as a core study aim. It may reflect a patient's belief that they were currently on “optimal” treatment and lack of confidence that the provided advice was correct. Whatever the mechanism, it is an important observation as even under the tight constraints of a clinical trial, with standardised algorithms and training of site staff relating to treatment adjustment, patient behaviour differed markedly between clinical centres. Further work is needed to clarify why there was such disparity between centres. Prior patient experience of biomarker-directed care is a potential factor, particularly when these dissociate from symptoms. There are clearly implications around implementation of any future models of asthma care that require treatment adjustment as this may be differentially acceptable to individual patients being managed at different clinical centres.
Recent emergency room attendance was associated with patients following treatment advice, potentially as this recent “watershed” event may make patients more engaged in treatment advice and more open to the benefits of changing treatment. Poor adherence with asthma treatment has consistently been shown to be associated with increased emergency room visits [17], but our findings suggest that a prior emergency room visit is also associated with a greater willingness to increase treatment, suggesting this may be a bidirectional relationship. Not following advice to initiate OCS was anticipated, reflecting patient dislike of their well-recognised side-effects [2, 18, 19]. However, lack of engagement with treatment adjustment was evident across all therapeutic changes, consistent with a general reticence to adjust any form of treatment.
The accuracy of the multivariate model examining patient adherence with treatment advice supports our understanding that most important variables affecting patient treatment decisions were captured; however, further in-depth qualitative studies exploring the roots of these associations are required to aid the design of effective interventions. Indeed, a literature review identified six key factors that contribute to intentional nonadherence among older adults (illness beliefs, perceived treatment risks, benefits and necessity of potential treatments, patient–practitioner relationship, poly-pharmacy/regimen complexity, and inter-current physical/mental illness) and all of the factors identified in our analysis can be mapped onto these areas [20]. The study team took advice on study design from a panel of expert patients (PIP) who advised that patients would be enthusiastic about both biomarker-directed treatment and achieving low doses of corticosteroid. However, UK patient/public advisory groups in health research are often unrepresentative of the wider patient population, skewing towards those who are White, middle-class and older aged. This risks overlooking some of the key barriers to study design identified in our study and, specifically, the impact of ethnicity on patient decisions, must inform PIP selection criteria for future programmes.
As the study focused on reducing corticosteroid treatment, we explored patients’ reasons for choosing not to reduce treatment, and identified being an ex-smoker and having uncontrolled asthma as key factors. The latter finding suggests that some study participants would have benefited from a more thorough explanation of the dissociation between symptoms and corticosteroid dose when T2 biomarkers are low. As prominent asthma symptoms adversely impacted on advice to reduce corticosteroid treatment, we examined exacerbation risk in patients where T2 biomarkers were dissociated from asthma symptoms. Among those with high symptoms and low/moderate biomarkers, exacerbation risk was not different when patients were asked to reduce/maintain corticosteroid treatment based on T2 biomarkers, whereas in those with high/moderate T2 biomarkers and low symptoms, exacerbation risk was higher when biomarkers were ignored when determining the treatment advisory. This increased risk of exacerbation has been described previously in sputum-guided treatment adjustment where symptom-low/sputum eosinophil-high patients had a 10-fold reduction in exacerbation risk when treatment was increased according to sputum eosinophilia [21]. Taken together, high levels of symptoms are associated with patients deciding not to reduce treatment where this is appropriate (T2 biomarker-low) and treatment adjustment based on low symptoms (and ignoring high T2 biomarkers) is associated with increased risk. However, this study demonstrates that while T2 biomarkers provide prognostic information and correct corticosteroid treatment advice, many symptomatic patients will decide not to follow appropriate advice to reduce corticosteroid treatment.
In conclusion, we identified factors associated with patients not following HCP treatment advice within a robustly conducted randomised controlled trial, which may be important in improving patient engagement with HCP-directed advice in the routine management of severe asthma. Factors such as minority ethnic group and clinical centre require further focused studies to explore the underlying reasons for their importance. Irrespective of the outcomes, these factors have implications for the generalisability of any model of care in severe asthma.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary appendix ERJ-00768-2021.Supplement (466KB, pdf)
Shareable PDF
Acknowledgements
We would like to thank the members of the Trial Steering Committee for all their support and assistance with study delivery: Martyn Partridge (Chair), Mike Morgan, Anne Millar, Mark Stafford-Watson (patient representative; during his tenure on the Trial Steering Committee, Mark sadly passed away and we gratefully acknowledge his significant contribution to this programme and other patient involvement in research projects) and Gabriella Cooper. We are grateful to Niche Science & Technology Ltd (London, UK) for assistance with study delivery to all the patients who volunteered for the study, and to the clinical and research teams at all the participating clinical and academic centres. We thank Sofia Mosesova (Genentech, San Francisco, CA, USA), Chris Patterson and Nicola Gallagher (Queen's University Belfast, Belfast, UK) for statistical advice during study set-up and analysis. We also thank Amgen Inc. (Thousand Oaks, CA, USA), AstraZeneca (London, UK), Jannsen Research & Development LLC (London, UK) and Vitalograph Inc. (Ennis, Ireland) for supporting the RASP-UK Consortium.
Footnotes
This study is registered at ClinicalTrials.gov with identifier number NCT02717689. Following publication of the primary and secondary analyses, individual de-identified patient data, including a data dictionary, will be made available via our data sharing portal eTRIKS indefinitely delivered via Imperial College London. This will allow for maximum utilisation of the data to improve patient care and advance medical knowledge. The trial protocol and statistical analysis plan will also be made available. L.G. Heaney (the manuscript's guarantor) affirms that the manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Conflict of interest: J. Busby has nothing to disclose.
Conflict of interest: J.G. Matthews has nothing to disclose.
Conflict of interest: R. Chaudhuri reports grants, personal fees for advisory board work and nonfinancial support for meeting attendance from AstraZeneca, personal fees for advisory board work from GlaxoSmithKline and Novartis, personal fees for advisory board work and nonfinancial support for meeting attendance from Chiesi, nonfinancial support for meeting attendance from Napp Pharmaceuticals, outside the submitted work.
Conflict of interest: I.D. Pavord reports personal fees for lectures, advisory board honoraria, sponsorship to attend scientific meetings, and payments for organising educational events from AstraZeneca, personal fees for lectures, advisory board honoraria, and sponsorship to attend scientific meetings from Boehringer Ingelheim and GlaxoSmithKline, personal fees for lectures from Aerocrine and Chiesi, personal fees for lectures and advisory board honoraria from Almirall and Novartis, advisory board honoraria from Genentech, Regeneron, Sanofi, Circassia and Knopp, personal fees for lectures, payments for organising educational events, and sponsorship to attend scientific meetings from Teva, grants from the National Institute for Health Research, outside the submitted work.
Conflict of interest: T.C. Hardman has nothing to disclose.
Conflict of interest: J.R. Arron has patents “Diagnosis and treatments relating to Th2 inhibition” (US 9,684,000 B2) and “Diagnosis and treatments relating to Th2 inhibition” (US 9,995,755 B2) issued with rights assigned to Genentech, a member of the Roche group, and is an employee of Genentech, Inc. and owns stocks/options in the Roche group.
Conflict of interest: P. Bradding reports grants from Genentech, other (consultancy work on behalf of the University of Leicester) from Roche and Boehringer Ingelheim, outside the submitted work.
Conflict of interest: C.E. Brightling reports grants and personal fees from GlaxoSmithKline, AstraZeneca, Sanofi, Novartis, Chiesi, Genentech, Gossamer, Mologic and 4D Pharma, all paid to the institution and outside the submitted work.
Conflict of interest: D.F. Choy is an employee of Genentech, Inc. and owns stocks/options in the Roche group.
Conflict of interest: D.C. Cowan has nothing to disclose.
Conflict of interest: R. Djukanovic reports receiving fees for lectures at symposia organised by Novartis, AstraZeneca and Teva, consultation for Teva and Novartis as member of advisory boards, and participation in a scientific discussion about asthma organised by GlaxoSmithKline; he is a co-founder and current consultant, and has shares in Synairgen, a University of Southampton spin-out company.
Conflict of interest: C.E. Hanratty has nothing to disclose.
Conflict of interest: T.W. Harrison reports grants, personal fees and nonfinancial support from AstraZeneca, grants and personal fees from GlaxoSmithKline, personal fees from Vectura, Synairgen and Chiesi, outside the submitted work.
Conflict of interest: C.T. Holweg is an employee and stock owner of Genentech Inc., a member of the Roche Group, outside the submitted work.
Conflict of interest: P.H. Howarth reports employment by GlaxoSmithKline.
Conflict of interest: S.J. Fowler reports personal fees for lectures and support to attend an international conference from AstraZeneca, grants and personal fees for lectures from Boehringer Ingelheim, personal fees for lectures, participation in an advisory board, and support to attend an national conference from Chiesi, personal fees for lectures from GlaxoSmithKline, Novartis and Teva, outside the submitted work.
Conflict of interest: J.L. Lordan has nothing to disclose.
Conflict of interest: A.H. Mansur reports personal and institution payment for talks, advisory board meetings, sponsorship to attend conferences and education grants for service developments from GlaxoSmithKline, AstraZeneca, Novartis, Sanofi, Teva and others, outside the submitted work.
Conflict of interest: A. Menzies-Gow reports grants and personal fees from AstraZeneca, personal fees from Novartis, GlaxoSmithKline, Sanofi and Vectura, personal fees and nonfinancial support from Teva and Boehringer Ingelheim, outside the submitted work.
Conflict of interest: R.M. Niven has received lecture fees, advisory board activity and been supported to attend international meetings/conferences in the last 5 years by the following companies: AstraZeneca, Boehringer, Boston Scientific, Chiesi, Napp, Novartis and Teva. None of these have any relevance to the manuscript; R.M. Niven owns no shares or stocks and is not personally or in any family way connected to any pharmaceutical companies.
Conflict of interest: D.S. Robinson has nothing to disclose.
Conflict of interest: S.M. Walker has nothing to disclose.
Conflict of interest: A. Woodcock reports personal fees for lectures from GlaxoSmithKline, personal fees for lectures and consultancy from Novartis, personal fees for consultancy from Chiesi, other (chairing research projects) from Reacta Biotech, Axalbion and Medicines Evaluation Unit, outside the submitted work.
Conflict of interest: L.G. Heaney reports other (sponsorship for meeting attendance) from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline and Napp Pharmaceutical, personal fees for lectures and advisory board work from Novartis, Hoffman la Roche/Genentech Inc., Sanofi, GlaxoSmithKline, AstraZeneca, Teva, Theravance and Circassia, grants from Medimmune, Novartis UK, Roche/Genentech Inc., and GlaxoSmithKline, and is academic lead for the Medical Research Council Stratified Medicine UK Consortium in severe asthma, which involves industrial partnerships with Amgen, Genentech/Hoffman la Roche, AstraZeneca, Medimmune, GlaxoSmithKline, Aerocrine and Vitalograph, outside the submitted work.
Support statement: This study was part of the MRC Refractory Asthma Stratification Programme, and programme support was obtained from Hoffman la Roche/Genentech (periostin assay and sample biobanking) and Circassia (FENO measurements (reduced pricing for machines and test kits)) for in-kind support within that Consortium. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Global Initiative for Asthma (GINA) . Global Strategy for Asthma Management and Prevention. 2019. Available from: http://ginasthma.org/
- 2.Sweeney J, Patterson CC, Menzies-Gow A, et al. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the Optimum Patient Care Research Database and the British Thoracic Difficult Asthma Registry. Thorax 2016; 71: 339–346. doi: 10.1136/thoraxjnl-2015-207630 [DOI] [PubMed] [Google Scholar]
- 3.Bleecker ER, Menzies-Gow AN, Price DB, et al. Systematic literature review of systemic corticosteroid use for asthma management. Am J Respir Crit Care Med 2020; 201: 276–293. doi: 10.1164/rccm.201904-0903SO [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heffler E, Madeira LNG, Ferrando M, et al. Inhaled corticosteroids safety and adverse effects in patients with asthma. J Allergy Clin Immunol Pract 2018; 6: 776–781. doi: 10.1016/j.jaip.2018.01.025 [DOI] [PubMed] [Google Scholar]
- 5.Heaney LG, Busby J, Hanratty CE, et al. Composite type-2 biomarker strategy versus a symptom–risk-based algorithm to adjust corticosteroid dose in patients with severe asthma: a multicentre, single-blind, parallel group, randomised controlled trial. Lancet Respir Med 2021; 9: 57–68. doi: 10.1016/S2213-2600(20)30397-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanratty CE, Matthews JG, Arron JR, et al. A randomised pragmatic trial of corticosteroid optimization in severe asthma using a composite biomarker algorithm to adjust corticosteroid dose versus standard care: study protocol for a randomised trial. Trials 2018; 19: 5. doi: 10.1186/s13063-017-2384-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busby J, Khoo E, Pfeffer PE, et al. The effects of oral corticosteroids on lung function, type-2 biomarkers and patient-reported outcomes in stable asthma: a systematic review and meta-analysis. Respir Med 2020; 173: 106156. doi: 10.1016/j.rmed.2020.106156 [DOI] [PubMed] [Google Scholar]
- 8.Busby J, Holweg CTJ, Chai A, et al. Change in type-2 biomarkers and related cytokines with prednisolone in uncontrolled severe oral corticosteroid dependent asthmatics: an interventional open-label study. Thorax 2019: 74: 806–809. doi: 10.1136/thoraxjnl-2018-212709 [DOI] [PubMed] [Google Scholar]
- 9.Vrijens B, Urquhart J. Methods of measuring, enhancing, and accounting for medication adherence in clinical trials. Clin Pharmacol Ther 2014; 95: 617–626. doi: 10.1038/clpt.2014.59 [DOI] [PubMed] [Google Scholar]
- 10.Czobor P, Skolnick P. The secrets of a successful clinical trial: compliance, compliance, and compliance. Mol Interv 2011; 11: 107–110. doi: 10.1124/mi.11.2.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Netuveli G, Hurwitz B, Levy M, et al. Ethnic variations in UK asthma frequency, morbidity, and health-service use: a systematic review and meta-analysis. Lancet 2005; 365: 312–317. doi: 10.1016/S0140-6736(05)17785-X [DOI] [PubMed] [Google Scholar]
- 12.Hussain-Gambles M, Atkin K, Leese B. South Asian participation in clinical trials: the views of lay people and health professionals. Health Policy 2006; 77: 149–165. doi: 10.1016/j.healthpol.2005.07.022 [DOI] [PubMed] [Google Scholar]
- 13.Oakley A, Wiggins M, Turner H, et al. Including culturally diverse samples in health research: a case study of an urban trial of social support. Ethn Health 2003; 8: 29–39. doi: 10.1080/13557850303554 [DOI] [PubMed] [Google Scholar]
- 14.Barr RG, Somers SC, Speizer FE, et al. National Asthma Education and Prevention Program (NAEPP). Arch Intern Med 2002; 162: 1761–1768. doi: 10.1001/archinte.162.15.1761 [DOI] [PubMed] [Google Scholar]
- 15.Lee LA, Bailes Z, Barnes N, et al. Efficacy and safety of once-daily single-inhaler triple therapy (FF/UMEC/VI) versus FF/VI in patients with inadequately controlled asthma (CAPTAIN): a double-blind, randomised, phase 3A trial. Lancet Respir Med 2021; 9: 69–84. doi: 10.1016/S2213-2600(20)30389-1 [DOI] [PubMed] [Google Scholar]
- 16.Pavord ID, Beasley R, Agusti A, et al. After asthma: redefining airways diseases. Lancet 2018; 391: 350–400. doi: 10.1016/S0140-6736(17)30879-6 [DOI] [PubMed] [Google Scholar]
- 17.Engelkes M, Janssens HM, de Jongste JC, et al. Medication adherence and the risk of severe asthma exacerbations: a systematic review. Eur Respir J 2015; 45: 396–407. doi: 10.1183/09031936.00075614 [DOI] [PubMed] [Google Scholar]
- 18.Price DB, Trudo F, Voorham J, et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J Asthma Allergy 2018; 11: 193–204. doi: 10.2147/JAA.S176026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bloechliger M, Reinau D, Spoendlin J, et al. Adverse events profile of oral corticosteroids among asthma patients in the UK: cohort study with a nested case-control analysis. Respir Res 2018; 19: 75. doi: 10.1186/s12931-018-0742-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mukhtar O, Weinman J, Jackson SH. Intentional non-adherence to medications by older adults. Drugs Aging 2014; 31: 149–157. doi: 10.1007/s40266-014-0153-9 [DOI] [PubMed] [Google Scholar]
- 21.Haldar P, Pavord ID, Shaw DE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med 2008; 178: 218–224. doi: 10.1164/rccm.200711-1754OC [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary appendix ERJ-00768-2021.Supplement (466KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-00768-2021.Shareable (435.5KB, pdf)