We recently described four distinct types of plexiform lesions in human idiopathic and familial pulmonary arterial hypertension (PAH) [1], visualising the three-dimensional lesion structure using synchrotron-based phase-contrast micro-computed tomography (SPµCT). Two types, 1 and 2, are shunt-type lesions that connect pulmonary arteries to the bronchial circulation: type 1 to the vasa vasorum, and type 2 to peribronchial vessels. Type 3 lesions are found peripherally in the lung as spherical structures abruptly terminating the distal pulmonary artery/arteriole, and type 4 lesions are characterised by recanalisation of an occluded artery/arteriole. Our observation of type 1 and type 2 lesions in PAH supports previous work that demonstrated intrapulmonary bronchopulmonary anastomoses (IBAs) connected to plexiform lesions in human PAH, suggesting that shunting of blood can occur within lesions in the setting of supra-systemic pulmonary arterial pressure [2]. Further haemodynamic studies of distinct subtypes of plexiform lesions have been hampered by the lack of available animal models with plexiform lesions representative of the full range of lesion types found in human disease. Plexiform lesions have previously been described in the Sugen5416/hypoxia rat model of pulmonary hypertension when time until sacrifice following hypoxia is extended to 13–14 weeks. Initially plexiform lesions were identified within the pulmonary artery, as well as in the form of aneurysm-like lesions projecting outside the vessel lumen [3], and recently the latter type was shown to form in supernumerary arteries [4]. However, neither study observed plexiform lesions communicating with the bronchial circulation, possibly because of methodological limitations of the histological analysis.
Short abstract
Human like plexiform lesions identified in the prolonged Sugen5416/hypoxia rat model, visualised by synchrotron tomography imaging https://bit.ly/3KQvDHg
To the Editor:
We recently described four distinct types of plexiform lesions in human idiopathic and familial pulmonary arterial hypertension (PAH) [1], visualising the three-dimensional lesion structure using synchrotron-based phase-contrast micro-computed tomography (SPµCT). Two types, 1 and 2, are shunt-type lesions that connect pulmonary arteries to the bronchial circulation: type 1 to the vasa vasorum, and type 2 to peribronchial vessels. Type 3 lesions are found peripherally in the lung as spherical structures abruptly terminating the distal pulmonary artery/arteriole, and type 4 lesions are characterised by recanalisation of an occluded artery/arteriole. Our observation of type 1 and type 2 lesions in PAH supports previous work that demonstrated intrapulmonary bronchopulmonary anastomoses (IBAs) connected to plexiform lesions in human PAH, suggesting that shunting of blood can occur within lesions in the setting of supra-systemic pulmonary arterial pressure [2]. Further haemodynamic studies of distinct subtypes of plexiform lesions have been hampered by the lack of available animal models with plexiform lesions representative of the full range of lesion types found in human disease. Plexiform lesions have previously been described in the Sugen5416/hypoxia rat model of pulmonary hypertension when time until sacrifice following hypoxia is extended to 13–14 weeks. Initially plexiform lesions were identified within the pulmonary artery, as well as in the form of aneurysm-like lesions projecting outside the vessel lumen [3], and recently the latter type was shown to form in supernumerary arteries [4]. However, neither study observed plexiform lesions communicating with the bronchial circulation, possibly because of methodological limitations of the histological analysis.
Here, we set out to further characterise the range of plexiform lesion types in the prolonged Sugen5416/hypoxia rat model using SPµCT combined with injections of a radiopaque dye. As previously suggested by our group and others [5], SPµCT grants superior three-dimensional imaging of biological structures with low and homogenous attenuation and is an emerging tool in the fields of pulmonary vascular physiology and digital pathology.
The well-established Sugen5416/hypoxia model, combining vascular endothelial growth factor receptor 2 inhibitor injections with a second hit of hypoxia, was used as initially described [6], but sacrifice was delayed until 15 weeks post injections. No animals died during the study period. Following removal of the heart–lung block, the main pulmonary artery was injected with a radiopaque green dye (CDI's Tissue Marking Dye; Cancer Diagnostics, Durham, NC, USA), diluted with an equal volume of tap water to ensure low viscosity. Super-resolution x-ray radiographies of paraffin-embedded lobes were acquired using a laboratory setup as previously described [7], confirming the presence of contrast agent (radiopaque green dye) and thus enabling tracing of corresponding areas between individuals for subsequent high-resolution SPµCT imaging, which was performed at the X02DA TOMCAT beamline of the Swiss Light Source at the Paul Scherrer Institute, Switzerland [1, 5]. In short, 4× scan volumes (4.2×4.2×3.5 mm3) with an effective voxel size of (1.63 μm)3 were acquired from selected areas of interest. The algorithm of Paganin et al. [8] and the gridrec algorithm [9] were applied for phase retrieval and tomographic reconstruction, respectively, using a Fiji plugin available at the beamline. Three-dimensional analysis was performed in Fiji [10] and Amira version 2020.2.
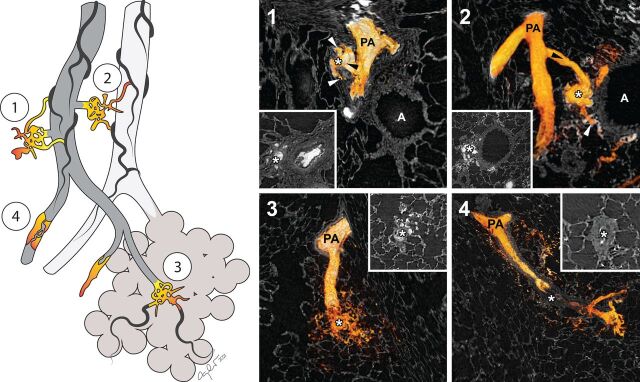
The imaging revealed vascular pathology similar to human PAH. All four types of plexiform lesions previously identified in human PAH [1] were observed (figure 1). Segmentation of dye-filled vessels confirmed that plexiform lesions do communicate with the bronchial circulation in Sugen5416/hypoxia rats. As in human PAH, type 1 and type 2 plexiform lesions communicated with the vasa vasorum and peribronchial vessels, respectively. Neointimal thickening and complete arterial occlusions were present, but quite distal in the pulmonary vascular tree compared to human PAH. Type 4 lesions were observed, but as with the neointima, they were found in relatively small vessels. Patent IBAs without plexiform lesion formation were also observed (not shown).
FIGURE 1.
Three-dimensional reconstructions of the four types of plexiform lesions in the rat model. All four types of plexiform lesions previously described in human pulmonary arterial hypertension were identified (1–4) and the injected radiopaque dye was used to three-dimensionally reconstruct the vascular lumen of the different lesions. White asterisks mark the three-dimensional reconstructed plexiform lesions and, in insets 1–4, the same lesions in two-dimensional cross sections, as they would have been seen histologically. Type 1 lesions derive from monopodial branches/supernumerary arteries (black arrowhead), with connections to the vasa vasorum (white arrowheads). Type 2 lesion are best described as tortuous transformations of IBAs (black arrowhead), connected to peribronchial vessels (white arrowheads). Type 3 are found at abrupt ends of distal, intra-acinar, pulmonary arteries/arterioles and type 4 lesions are blocked pulmonary arteries/arterioles with re-canalisation. All four types of plexiform lesions are also illustrated in multi-dimensional video format (supplementary videos 1–4). PA: pulmonary artery; A: airway.
In summary, SPµCT imaging of the prolonged Sugen5416/hypoxia rat model revealed patent IBAs, vascular remodelling and plexiform pathology similar to human idiopathic and familial PAH. Furthermore, it is our belief that the aneurysm-type plexiform lesions observed in the initial study [4] likely correspond to type 1 and 2 lesions in our material, as they were observed to originate from monopodial branches (supernumerary arteries). The prolonged model is very promising as it can be used to study the temporal and spatial regulation of PAH lesion formation, to establish a pathological timeline. Since the imaging method used here is non-destructive it can also be combined with subsequent sectioning and methods like immunohistochemistry and in situ hybridisation to decipher molecular mechanisms.
Although many novel pressure-lowering therapies have been introduced over the past two decades, no treatment currently available prevents the formation of PAH specific vascular pathology. We firmly believe that increased knowledge on the temporal aspects and underlying processes driving the formation of plexiform lesions, combined with insights regarding the haemodynamic roles of the different types of lesions, will bring us closer to developing novel treatments for the disease. This description of the vascular pathology of the Sugen5416/hypoxia rat is an essential first step in using the model to understand the biology of plexiform lesion formation and function and will hopefully be useful for many groups in the field.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary video 1 ERJ-02802-2021.Video_1 (19.1MB, mp4)
Supplementary video 2 ERJ-02802-2021.Video_2 (11.1MB, mp4)
Supplementary video 3 ERJ-02802-2021.Video_3 (12.7MB, mp4)
Supplementary video 4 ERJ-02802-2021.Video_4 (14.3MB, mp4)
Shareable PDF
Acknowledgements
We acknowledge the Paul Scherrer Institute, Villigen, Switzerland, for provision of synchrotron radiation beamtime at the X02DA TOMCAT beamline of the Swiss Light Source and Anne Bonnin for excellent technical assistance. We also acknowledge Phan-Kiet Tran for schematic illustrations.
Footnotes
Conflict of interest: C. Westöö declares a regional salary grant for medical resident researchers from ALF Forskningsutrymme för ST-läkare. T. Dreier declares an industrial PhD salary from Excillum AB, and payment to their institution from the Swedish Foundation for Strategic Research (SSF) in connection with the present manuscript. M.E. Kumar declares American Heart Association Scientist Development Grant AHA 16SDG30030006, Stanford Pediatrics startup funds 1246483-200-JHAJH, Stanford Spectrum Child Health Research Institute 1170805-100-GHEBG, Stanford Maternal and Child Health Research Institute Pilot Grant 1220318-108-JHACT, Vera Moulton Wall Center for Pulmonary Vascular Research grant 1144545-401-GHDCK and a Bravo Family Endowed Faculty Scholarship, in connection with the present manuscript. E. Spiekerkoetter declares that they have a research scholarship from Vera Moulton Wall Center for Pulmonary Vascular Disease, in connection with the present manuscript; and that they have received grants from the National Institutes of Health (National Heart, Lung and Blood Institute grants R01HL158868 and R01 HL128734) and Department of Defense (grants PR161256 and PR 181774) in the 36 months prior to manuscript submission; and that they receive royalties from Stanford University for Method of use patent “Use of FK506 for the treatment of Pulmonary Arterial Hypertension”; that they have a provisional US patent for “Enzastaurin and Fragile Histidine Trial (FHIT) Increasing Agents for the Treatment of Pulmonary Hypertension”; and that they are chair of the American Heart Association 3CPR Early Career Committee, and a member of the Stanford University Institutional Review Board. K. Tran-Lundmark declares funding from the Swedish Heart-Lung Foundation, the Crafoord Foundation, the Swedish Society of Medicine, the Knut and Alice Wallenberg Foundation and the Skåne County Council, in connection with the present manuscript; as well as roles as American Thoracic Society (ATS) Pulmonary Circulation Program Committee member (unpaid); American Heart Association (AHA) 3CPR Early Career Committee (unpaid) and Association for European Paediatric and Congenital Cardiology (AEPC) Councillor in the Working Group for Pulmonary Hypertension, Heart Failure and Transplantation (unpaid). All other authors declare no competing interests.
Support statement: This work was supported by the Swedish Heart-Lung Foundation (K. Tran-Lundmark), the Swedish Society of Medicine (K. Tran-Lundmark), the Crafoord Foundation (K. Tran-Lundmark), the Knut and Alice Wallenberg foundation (K. Tran-Lundmark) and the Skåne County Council (K. Tran-Lundmark), as well as the Swedish Foundation for Strategic Research ID17-0097 (T. Dreier), American Heart Association 16SDG30030006 (M.E. Kumar) and Vera Moulton Wall Center for Pulmonary Vascular Disease research (M.E. Kumar), Wall Center Faculty Research Scholar Award (E. Spiekerkoetter), R01 HL128734 National Institute of Health/National Heart Lung Blood Institute (E. Spiekerkoetter) and the Department of Defense – PR161256 (E. Spiekerkoetter). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Westoo C, Norvik CC, Peruzzi N, et al. . Distinct types of plexiform lesions identified by synchrotron-based phase contrast micro-CT. Am J Physiol Lung Cell Mol Physiol 2021; 321: L17–L28. doi: 10.1152/ajplung.00432.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galambos C, Sims-Lucas S, Abman SH, et al. . Intrapulmonary bronchopulmonary anastomoses and plexiform lesions in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2016; 193: 574–576. doi: 10.1164/rccm.201507-1508LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abe K, Toba M, Alzoubi A, et al. . Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation 2010; 121: 2747–2754. doi: 10.1161/CIRCULATIONAHA.109.927681 [DOI] [PubMed] [Google Scholar]
- 4.Oshima K, Crockett ES, Joshi SR, et al. . Aneurysm-type plexiform lesions form in supernumerary arteries in pulmonary arterial hypertension: potential therapeutic implications. Am J Physiol Lung Cell Mol Physiol 2019; 317: L805–L815. doi: 10.1152/ajplung.00121.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norvik C, Westoo CK, Peruzzi N, et al. . Synchrotron-based phase-contrast micro-CT as a tool for understanding pulmonary vascular pathobiology and the 3-D microanatomy of alveolar capillary dysplasia. Am J Physiol Lung Cell Mol Physiol 2020; 318: L65–L75. doi: 10.1152/ajplung.00103.2019 [DOI] [PubMed] [Google Scholar]
- 6.Taraseviciene-Stewart L, Kasahara Y, Alger L, et al. . Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J 2001; 15: 427–438. doi: 10.1096/fj.00-0343com [DOI] [PubMed] [Google Scholar]
- 7.Dreier T, Lundström U, Bech M. Super-resolution X-ray imaging with hybrid pixel detectors using electromagnetic source stepping. J Instrum 2020; 15: C03002–C03002. doi: 10.1088/1748-0221/15/03/C03002 [DOI] [Google Scholar]
- 8.Paganin D, Mayo SC, Gureyev TE, et al. . Simultaneous phase and amplitude extraction from a single defocused image of a homogeneous object. J Microsc 2002; 206: 33–40. doi: 10.1046/j.1365-2818.2002.01010.x [DOI] [PubMed] [Google Scholar]
- 9.Marone F, Stampanoni M. Regridding reconstruction algorithm for real-time tomographic imaging. J Synchrotron Radiat 2012; 19: 1029–1037. doi: 10.1107/S0909049512032864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods 2012; 9: 676–682. 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary video 1 ERJ-02802-2021.Video_1 (19.1MB, mp4)
Supplementary video 2 ERJ-02802-2021.Video_2 (11.1MB, mp4)
Supplementary video 3 ERJ-02802-2021.Video_3 (12.7MB, mp4)
Supplementary video 4 ERJ-02802-2021.Video_4 (14.3MB, mp4)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-02802-2021.Shareable (331.2KB, pdf)