Abstract
The standard method of producing recombinant proteins such as immunotoxins (rITs) in large quantities is to transform gram-negative bacteria and subsequently recover the desired protein from inclusion bodies by intensive de- and renaturing procedures. The major disadvantage of this technique is the low yield of active protein. Here we report the development of a novel strategy for the expression of functional rIT directed to the periplasmic space of Escherichia coli. rITs were recovered by freeze-thawing of pellets from shaking cultures of bacteria grown under osmotic stress (4% NaCl plus 0.5 M sorbitol) in the presence of compatible solutes. Compatible solutes, such as glycine betaine and hydroxyectoine, are low-molecular-weight osmolytes that occur naturally in halophilic bacteria and are known to protect proteins at high salt concentrations. Adding 10 mM glycine betaine for the cultivation of E. coli under osmotic stress not only allowed the bacteria to grow under these otherwise inhibitory conditions but also produced a periplasmic microenvironment for the generation of high concentrations of correctly folded rITs. Protein purified by combinations of metal ion affinity and size exclusion chromatography was substantially stabilized in the presence of 1 M hydroxyecotine after several rounds of freeze-thawing, even at very low protein concentrations. The binding properties and cytotoxic potency of the rITs were confirmed by competitive experiments. This novel compatible-solute-guided expression and purification strategy might also be applicable for high-yield periplasmic production of recombinant proteins in different expression systems.
Recombinant proteins such as antibodies, recombinant bispecific antibodies (diabodies) (21), or immunotoxins are increasingly being used to selectively destroy undesired cells in malignant diseases. Recombinant immunotoxins are chimeric proteins composed of a truncated, binding-deficient, catalytically active toxin directly linked to a recombinant single-chain antibody fragment (scFv) (33). These fusion gene products are highly homogeneous, much easier to modify and more economical to produce than chemical conjugates (29). However, bacterially expressed single-chain immunotoxins vary tremendously in their stability, and some have developed a strong tendency to aggregate (6). The standard method for the isolation and purification of recombinant immunotoxins was developed by Pastan and coworkers (9). Their recombinant immunotoxins were expressed under the control of a T7 late promoter in transformed Escherichia coli BL21(DE3) and induced after the addition of isopropyl β-d-thiogalactoside (IPTG). The proteins remained intracellular and appeared to be primarily associated with inclusion bodies (15). Chimeric recombinant protein was purified after careful de- and renaturation procedures by Q-Sepharose, Mono-Q, and size exclusion chromatography on a TSK 250 column (9). Under optimized conditions, only 5 to 10% of the input protein was properly folded and active.
Our group has evaluated the monoclonal antibody (MAb) RFT5, binding to the interleukin-2 receptor α (CD25), and MAb Ki-4, binding to the human CD30 receptor, for their potential value in specifically targeting malignant lymphoma cells (4, 5, 27). In order to develop an optimized bacterial expression system, we subsequently constructed pBM1.1, a new pET-based vector for PelB-directed periplasmic expression of recombinant immunotoxins (31). Selected RFT5 and Ki-4 scFv's were directionally inserted into this plasmid and thus genetically fused to a modified Pseudomonas aeruginosa exotoxin A (ETA′) gene with a deletion of domain Ia. The problem of production of sufficient amounts of pure and fully active recombinant immunotoxins, however, still remains an obstacle for clinical application. We therefore used a new strategy for optimized periplasmic expression under osmotic stress. To cope with these conditions imposed by saline environments, most halophilic and halotolerant Bacteria and Eucarya control cytoplasmic osmolarity by accumulation of a range of organic osmolytes, referred to as compatible solutes (8, 16). We used protein-stabilizing compatible solutes (namely, glycerol, sorbitol, glycine betaine, and hydroxyectoine) during the production phase and/or in the course of purification and storage to optimize the functionality and stability of the proteins. To our knowledge, this is the first study demonstrating highly efficient compatible-solute-assisted periplasmic production of recombinant chimeric molecules.
MATERIALS AND METHODS
Bacterial strains, oligonucleotides, and plasmids.
Bacteria and plasmids used in this study are summarized in Table 1. E. coli XL1-Blue (Stratagene, Amsterdam, The Netherlands) was used as the host for cloning and sequencing. E. coli BL21(DE3) (36), purchased from Novagen (Abingdon, United Kingdom), was used for the synthesis of recombinant immunotoxins. E. coli TG1 and E. coli HB2151 (Pharmacia, Freiburg, Germany) were used as hosts for synthesis of scFv-displaying phages and soluble scFv, respectively. Phagemid vector pCANTAB6 (32) was used for N-terminal fusion of SfiI/NotI-scFv fragments to the minor coat protein p3 of filamentous phage M13, allowing the selection of antigen-binding phage (22). Plasmid pBM1.1 (31), derived from the pET27b vector (Novagen), was used for N-terminal fusion of scFv's to ETA′ (39). Synthetic oligonucleotides were synthesized by Eurogentec (Seraing, Belgium). Plasmid pcDNA3 (Invitrogen, Groningen, The Netherlands) was used for eukaryotic expression of recombinant soluble CD30 receptor. Purified MAb Ki-4 was supplied by Boehringer GmbH (Mannheim, Germany), and hydroxyectoine was supplied by Bitop GmbH (Witten, Germany). Plasmids were prepared by the alkaline lysis method and purified using plasmid kits from Qiagen (Hilden, Germany). Restriction fragments or PCR products were separated by horizontal agarose gel electrophoresis and extracted with Qiaex II (Qiagen). Cloning into plasmid vectors was performed by standard methods (35).
TABLE 1.
Plasmids, bacteria, and eukaryotic cells used in this study
| Designation | Function |
|---|---|
| Plasmids | |
| pcDNA3 | Eukaryotic expression of recombinant soluble CD30 receptor |
| pCANTAB6 | Phagemid vector used for the synthesis of filamentous phages displaying recombinant scFv's on their surfaces |
| pBM1.1 | Bacterial vector for periplasmic expression of recombinant immunotoxins |
| Bacteria | |
| XL1-Blue | Cloning and sequencing |
| TG1 | Synthesis of scFv-displaying phages |
| HB2151 | Expression of soluble scFv |
| BL21(DE3) | Periplasmic expression of recombinant immunotoxins |
| Cells | |
| COS-1 | Expression of soluble CD30 receptor |
| Ki-4 | Anti-CD30 MAb producing hybridoma |
| RFT5 | Anti-CD25 MAb producing hybridoma |
| HD-MyZ | CD25− CD30− Hodgkin-derived cell line |
| L540Cy | CD25+ CD30+ Hodgkin-derived cell line |
Cell culture.
All cell lines (Table 1), including the CD25+ CD30+ Hodgkin-derived cell line L540Cy (25) and the CD25− CD30− Hodgkin-derived cell line HD-MyZ (3) (kindly provided by B. Dörken, Berlin, Germany), as well as the hybridoma cell lines RFT5 (13) and Ki-4 (23) and the simian COS-1 cells, were cultivated in complex medium (RPMI 1640) supplemented with 10% (vol/vol) heat-inactivated fetal calf serum, 50 μg of penicillin/ml, 100 μg of streptomycin/ml, and 2 mM l-glutamine. All cells were cultured at 37°C in an atmosphere of 5% CO2 in air.
Plasmid construction.
Total cellular RNA was isolated from 107 hybridoma cells using RNazol solution (Gibco, Eggenstein, Germany) as described by the manufacturer. cDNA was synthesized using 5 μg of freshly prepared RNA and 10 μl of random hexamer primers (10 μM) from the RiboClone cDNA Synthesis Systems kit (Promega, Mannheim, Germany) in a 50-μl reaction mixture. Immunoglobulin variable-region genes (VH and VL) were PCR amplified from 5 μl of cDNA, assembled, and cloned into pCANTAB6 as described elsewhere (27). Plasmids were transformed into 50 μl of E. coli TG-1 by electroporation as described elsewhere (11). Binding RFT5 scFv and Ki-4 scFv were selected on CD25- and CD30-positive Hodgkin-derived L540Cy cells as published recently (27), and their genes were released from phagemid vector pCANTAB6 by SfiI/NotI digestion and inserted into SfiI/NotI-restricted expression vector pBM1.1 containing an ETA′ gene (31). The resulting plasmids were transformed into E. coli BL21(DE3). For cloning of the recombinant human CD30 receptor (rhCD30) gene fused to a poly-His tag (20), cDNA of the extracellular part of CD30, including its signal sequence (GenBank accession no. M83554 [12]), was amplified by reverse transcription-PCR (30 cycles of 94°C for 1 min, 55°C for 2 min, and 72°C for 2 min) using proofreading Pfu DNA polymerase (Stratagene, La Jolla, Calif.) and the oligonucleotide primers CD30-HisBack (5′-g-cta-gag-cgg-ccg-ccc-acc-ATG-CGC-GTC-CTC-CTC-GCC-GCG-CTG-3′ [the NotI consensus is underlined, and the CD30 coding region is shown in capital letters]) and CD30-HisFor (5′-gcc-gcg-ccc-tct-aga-tta-atg-atg-atg-atg-atg-atg-cgc-agc-tgc-CTT-CCC-CGT-GGA-GGA-GAG-AGC-GAC-3′ [the XbaI consensus is underlined, the His6 tag coding region is double underlined, and the CD30 coding region is shown in capital letters]). The resulting PCR fragment was ligated into the NotI/XbaI-linearized eukaryotic expression vector pcDNA3 (Invitrogen) and transformed into E. coli XL1-Blue. A 60-μg portion of the sCD30-His-pcDNA3 plasmid was used for stable transformation of 2 × 107 COS-1 cells using DOTAP (Boehringer) according to the manufacturer's instructions. Transformed COS cells were subcloned by limited dilutions in medium supplemented with 2 mg of G418 (Boehringer)/ml.
Periplasmic expression and purification of the recombinant immunotoxins.
Recombinant immunotoxins were expressed under the control of the IPTG-inducible T7 lac promoter in E. coli BL21(DE3). Bacteria were grown overnight at 26°C in Terrific Broth (TB) (35) containing 50 μg of kanamycin/ml and 0.5 mM ZnCl2, since it has been shown earlier that periplasmic proteolysis can be dramatically reduced upon addition of this salt (2). The culture was diluted 30-fold in 200 ml of the same medium. At an optical density at 600 nm (OD600) of 2, it was supplemented with 0.5 M sorbitol, 4% NaCl, and 10 mM glycine betaine and was then incubated at 26°C for additional 30 to 60 min. Thereafter, immunotoxin production was induced by the addition of 2 mM IPTG at 26°C. Fifteen hours later, cells were harvested by centrifugation at 3,700 × g for 10 min at 4°C. For all the following steps, tubes were kept on ice. The bacterial pellet was centrifuged, and its wet weight was determined. Cells were frozen at −196°C. After thawing, the cells were resuspended in 75 mM Tris-HCl (pH 8)–300 mM NaCl–1 capsule of protease inhibitors/50 ml (Complete; Roche Diagnostics, Mannheim, Germany)–5 mM dithiothreitol–10 mM EDTA–10% (vol/vol) glycerol and were sonicated six times for 30 s at 200 W. The periplasmic fraction was recovered after centrifugation at 21,000 × g for 30 min at 4°C and was transferred to 75 mM Tris-HCl (pH 8)–1 M NaCl–10% (vol/vol) glycerol using Hitrap desalting columns (Pharmacia). Recombinant immunotoxin was partially purified by immobilized metal ion affinity chromatography (IMAC) with nickel-nitriloacetic acid (Ni2+-NTA) chelating Sepharose (Qiagen) on a BioLogic workstation (Bio-Rad, Munich, Germany). Bound protein was eluted with 250 mM imidazole in 75 mM Tris-HCl (pH 8)–1 mM NaCl–10% (vol/vol) glycerol. Fractions containing the RFT5 or Ki-4 scFv fused to the ETA′ protein [RFT5(scFv)-ETA′ or Ki-4(scFv)-ETA′] were pooled and concentrated by ultrafiltration. Because the anticipated sizes of the recombinant immunotoxins were about 70 kDa, they were finally purified using size exclusion chromatography with Bio-Prep SE-100/17 columns (Bio-Rad) on a BioLogic workstation. Protein was eluted with phosphate-buffered saline (PBS) (pH 7.4)–1 M NaCl, analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and quantified by densitometry (GS-700 Imaging Densitometer; Bio-Rad) after Coomassie staining in comparison with bovine serum albumin (BSA) standards.
SDS-PAGE and Western blotting.
SDS-PAGE and Western blotting were performed as described previously (4). GroEL was detected by polyclonal anti-GroEL polyclonal rabbit antibodies (Sigma), and immunotoxin was detected by the mouse anti-ETA′ MAb TC-1 (kindly provided by D. R. Galloway, Columbus, Ohio), combined with an alkaline-phosphatase-conjugated sheep anti-rabbit immunoglobulin G (IgG) MAb and an anti-mouse IgG MAb (Sigma), respectively.
Immunoprecipitation.
Extracts of identical wet weights of bacteria cultured in TB or Luria-Bertani (LB) medium (35) at 26°C were prepared as described above. Particulate matter was removed by centrifugation at 21,000 × g for 30 min. Clarified soluble extracts were incubated with gentle shaking for 1 h at 4°C with 1 μg of rabbit polyclonal anti-GroEL (Sigma) antibody. The samples were treated with 5 mg of swollen protein A-agarose (Sigma) and mixed at 4°C for an additional hour. The immunoprecipitates were washed, resuspended in 50 μl of reducing 2× SDS loading buffer (Roth, Karlsruhe, Germany), and heated at 95°C for 5 min. Supernatants were resolved on duplicate 10% SDS gels and transferred to polyvinylidene difluoride membranes. GroEL and Ki-4(scFv)-ETA′ were visualized by incubation of the membranes with polyclonal anti-GroEL and TC-1, respectively. Immunoblotting was performed as described above.
Binding assays.
Enzyme-linked immunosorbent assays (ELISA) were performed as described previously (4) in 96-well Maxisorb microtiter plates (Nunc, Wiesbaden, Germany) coated at 4°C with 100 μl of either 125-ng/ml recombinant human interleukin-2 receptor-α (Biermann, Bad Nauheim, Germany) or rhCD30 in coating buffer (0.2 M Na2CO3–0.2 M NaHCO3) (Merck, Darmstadt, Germany)/well. Bound immunotoxin was detected with the anti-ETA′ MAb TC-1 and F(ab′)2 fragments of peroxidase-coupled goat anti-mouse IgG (Boehringer) (1:5,000 in Tris-buffered saline containing 0.5% BSA and 0.05% Tween 20). Flow cytometry was performed on cell suspensions containing 5 × 106 L540Cy cells/ml as described previously (27). Bound immunotoxin was documented using MAb TC-1 and fluorescein isothiocyanate (FITC)-labeled goat anti-mouse immunoglobulin on a FACScan (Becton Dickinson, Heidelberg, Germany). Binding specificity was verified by competition with excess parenteral MAbs (10 μg/ml).
Colorimetric cell viability assay.
The metabolism of the yellow tetrazolium salt XTT to a water-soluble orange formazan dye was determined according to the manufacturer's instructions (Roche Molecular Biochemicals, Mannheim, Germany). Various dilutions of the recombinant toxins were distributed in 100-μl aliquots in 96-well plates. Target cells (2 × 104 to 4 × 104) in 100-μl aliquots of complete medium were added, and the plates were incubated for 48 h at 37°C. Afterwards, the cell cultures were pulsed with 100 μl of fresh culture medium supplemented with XTT and N-methyl dibenzopyrazine methyl sulfate (PMS) (final concentrations, 1.49 and 0.025 mM, respectively) for 4 h. The spectrophotometrical absorbances of the samples were measured at 450 and 650 nm (reference wavelength) with an ELISA reader (MWG Biotech, Ebersberg, Germany). The concentration required to achieve a 50% reduction in cell viability relative to that of untreated control cultures (50% inhibitory concentration [IC50]) was calculated. All measurements were carried out in triplicate.
Stress experiments.
Freeze-thawing experiments were performed as described elsewhere (30). The residual “after-stress” binding activities in at least three independent ELISA experiments were determined, and the results were expressed as percentages of “prestress” binding activities.
RESULTS
Construction of recombinant immunotoxins.
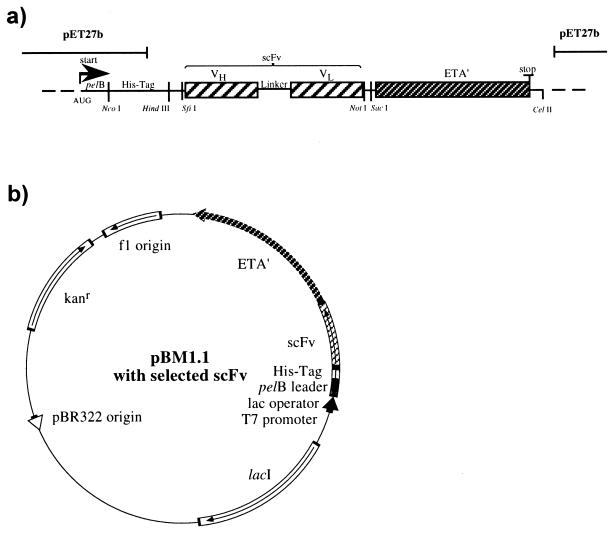
Recombinant immunotoxins directed against the lymphoid activation markers CD25 and CD30 for potential use in malignant lymphoma were developed. mRNAs were isolated from the hybridoma cell lines RFT5 (anti-CD25) and Ki-4 (anti-CD30). After first-strand cDNA synthesis, PCR-amplified coding regions of the light- and heavy-chain variable domains were assembled to single-chain variable fragments, inserted into the phagemid vector pCANTAB6, and expressed as minilibraries for display on filamentous phage. Functional scFv's were obtained by selection of binding phage on the Hodgkin lymphoma-derived CD25- and CD30-expressing cell line L540Cy. The selected recombinant scFv's were shown to specifically bind to their respective target antigens with binding kinetics similar to those of the original antibodies (27). The selected scFv's were inserted into the pET27b-derived expression vector pBM1.1 containing an IPTG-inducible lac operator, a pelB signal peptide followed by a His10 tag, and ETA′ (Fig. 1) (31). The deleted domain Ia of Pseudomonas exotoxin responsible for nonspecific cell recognition was thus replaced by the scFv's. The recombinant immunotoxins constructed were designated RFT5(scFv)-ETA′ and Ki-4(scFv)-ETA′, as described elsewhere (4, 27).
FIG. 1.
Cloning scheme of the bacterial expression vector pBM1.1. The expression module (a) is composed of the signal peptide of the pectate lyase gene (pelB), the IPTG-inducible T7 lac operon from the original pET vector (36), the synthetic His10 cluster (His) (20), the variable-region genes (VH and VL) connected by (Gly4Ser)3 (linker), and the ETA′ gene. Plasmid pBM1.1 (b) contains the expression module, a kanamycin resistance gene (Kanr), an E. coli origin of replication (pBR322 origin), an M13 origin of replication (f1 origin), and the lactose repressor gene (lacI). The binding single-chain variable fragments (scFv) are fused to ETA′.
Expression and purification.
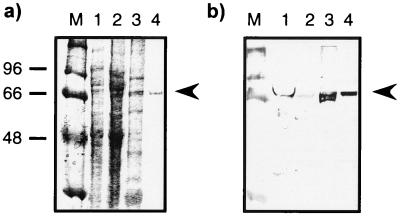
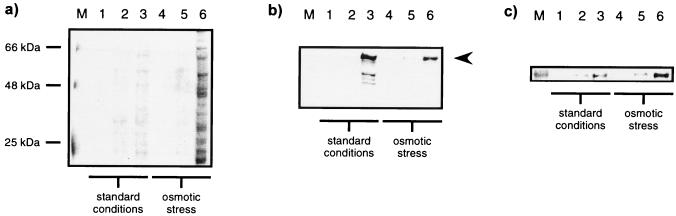
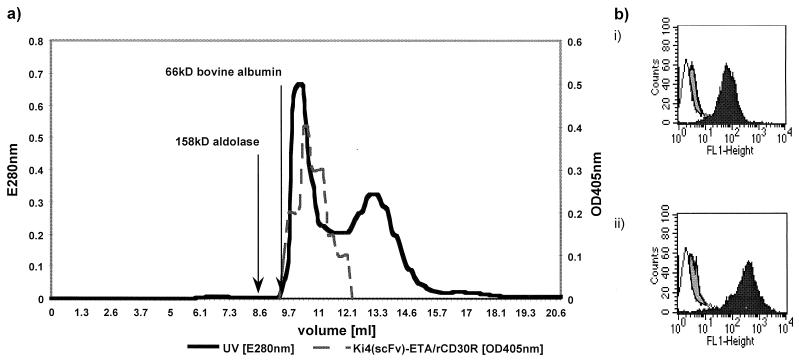
Expression plasmids were transformed into E. coli BL21(DE3). In a first series of experiments, E. coli shaking cultures were grown to an OD600 of 2.0 at salt concentrations of 4% NaCl plus 0.5 M sorbitol, supplemented with 0, 2.5, 5, 10, or 20 mM glycine betaine. Recombinant immunotoxins were directed to the periplasmic space after induction with 2 mM IPTG, and the highest concentrations of biologically active proteins were recovered in the presence of 10 mM glycine betaine (data not shown). RFT5(scFv)- and Ki-4(scFv)-ETA′ were purified by a combination of IMAC and molecular size chromatography in the presence of 10% glycerol. Recombinant immunotoxins were identified by Western blotting and visualized with a MAb specific for correctly folded Pseudomonas exotoxin A (17) (Fig. 2). Recently, it has been shown that functionally active scorpion toxin Cn5 can be purified by means of immobilized cytoplasmic and periplasmic chaperones such as GroEL, disulfide isomerase, and peptidyl propyl cis-trans isomerase (1). Thus, we compared our new method with a previously used periplasmic expression protocol (26) by analysis of polyclonal anti-GroEL-immunoprecipitated whole-cell protein fractions (Fig. 3). The most striking difference is documented by SDS-PAGE analysis: 2 h after induction of immunotoxin expression, there is a dramatic increase in the levels of precipitated proteins under osmotic stress conditions in the presence of 10 mM glycine betaine (Fig. 3a, lane 6) compared to those under standard growth conditions (Fig. 3a, lane 3). By Western blotting, full length Ki-4(scFv)-ETA′ was shown to be complexed in these chaperone precipitates using the anti-ETA′ MAb TC-1 (Fig. 3b). The presence of GroEL was confirmed with the polyclonal anti-GroEL antibody described above (Fig. 3c). The amounts of TC-1-detectable fragmented protein were two- and threefold higher under standard conditions than under osmotic stress conditions without (data not shown) and with ZnCl2 (Fig. 3b, lanes 3 and 6), respectively. In addition, the ratio of Ki-4(scFv)-ETA′ to GroEL estimated by densitometric scanning was 0.45 under osmotic stress conditions and 1.3 under standard conditions. IMAC was performed under high-salt conditions in order to prevent nonspecific binding of the charged groups to the affinity matrix via an ion-exchange effect (14). The recombinant immunotoxins were substantially stabilized during purification by 1 M NaCl, eluted from Ni2+-NTA columns by 250 mM imidazole, and afterwards separated by size exclusion chromatography between 20 and 100 kDa (Fig. 4a). As can be seen in the elution profile, only proteins smaller than 100 kDa, but no aggregates, were collected. These data were also confirmed by nonreducing SDS-PAGE and Western blotting; additionally, immunotoxin aggregates were not detectable during protein preparation (Fig. 2b). Densitometry of SDS-PAGE gels and Western blots as well as a receptor ELISA were used to help establish the purity of the prepared proteins. The functional immunotoxins, Ki-4(scFv)-ETA′ and RFT5(scFv)-ETA′, were enriched to more than 95%. By exporting the immunotoxins to the periplasm of E. coli, the final yield of functional protein varied around 1.1 mg/4.1 g of cell paste from bacterial shaking cultures (Table 2). In contrast to our former standard periplasmic expression and extraction protocol, the functionality was preserved after the extraction procedure. In addition, other ETA′-based toxins fused to different binding structures (CD30 ligand [5], interleukin-9 [26], three more anti-CD30 scFv's, and four additional scFv's targeting neuroblastoma, colon, and mammary carcinomas) were efficiently expressed by this new method (data not shown).
FIG. 2.
Purification of Ki-4(scFv)-ETA′. (a) 10% SDS-PAGE gel at different steps of purification. Lanes: M, molecular mass markers (with values in kilodaltons shown on the left); 1, soluble periplasmic content; 2, IMAC flowthrough; 3, IMAC eluate eluted with 250 mM imidazole; 4, Ki-4(scFv)-ETA′ after size exclusion chromatography. The gel was stained with Coomassie brilliant blue. (b) Corresponding Western blot. The recombinant immunotoxin was detected using the anti-ETA′ MAb TC-1 combined with alkaline-phosphatase-conjugated anti-mouse IgG MAbs and fast red. Arrowheads indicate the positions of Ki-4(scFv)-ETA′.
FIG. 3.
Comparative analyses of polyclonal anti-GroEL immunoprecipitates. (a) 10% SDS-PAGE of precipitated proteins; (b and c) Western blot analyses of Ki-4(scFv)-ETA′ and GroEL, respectively. Results are shown for standard growth conditions (LB medium with 150 mM NaCl) (lanes 1 through 3) and osmotic-stress growth conditions in the presence of 10 mM glycine betaine (lanes 4 through 6). Lanes 1 and 4, before stress and IPTG induction; lane 2, 1/2 h before IPTG induction; lane 5, 1/2 h after supplementation with NaCl, sorbitol, and glycine betaine; lanes 3 and 6, 2 h after IPTG induction. Arrowhead indicates the position of Ki-4(scFv)-ETA′.
FIG. 4.
Functional activity of purified recombinant immunotoxins after size exclusion chromatography using Bio-Prep SE-100/17 columns. (a) Elution profile monitored at 280 nm (solid line) combined with binding activity of the eluted fractions as documented by ELISA (dashed line). Immobilized rhCD30 receptor was incubated with dilutions of fractions from the column. Specifically bound Ki-4(scFv)-ETA′ was detected after incubation with the anti-ETA′ MAb TC-1 followed by alkaline-phosphatase-conjugated anti-mouse IgG. Converted substrate (o-phenylenediamine-dihydrochloride) was measured as absorbance at 405 nm. (b) Cell-binding activities of RFT5(scFv)- and Ki-4(scFv)-ETA′ as evaluated by flow cytometry analysis. (i) CD25+ CD30+ Hodgkin-derived L540Cy cells were incubated with PBS (open), and CD25− CD30− Hodgkin-derived HD-MyZ cells (shaded) or L540Cy cells (solid) were incubated with RFT5(scFv)-ETA′, for 15 min at 4°C. (ii) L540Cy cells were incubated with PBS (open), and HD-MyZ (shaded) or L540Cy (solid) were incubated with Ki-4(scFv)-ETA′, for 15 min at 4°C. Cells were stained with TC-1, mouse anti-TC-1, and goat anti-mouse FITC-conjugated antibody. Immunofluorescence (FL1 channel) was measured by flow cytometry, using a FACScan.
TABLE 2.
Recovery of Ki-4(scFv)-ETA′ from E. coli BL21(DE3) cultured under osmotic-stress conditions in the presence of 10 mM glycine betaine
| Step | Purification dataa
|
Relative biological activity (RU/mg)b under the indicated conditions
|
|||
|---|---|---|---|---|---|
| Total vol (ml) | Protein concn (mg/ml)c | Total protein (mg) | Osmotic stress + compatible solutes | Standard conditions | |
| Periplasmic extract | 50 | 12 (±0.85) | 600 (±42.5) | 0.006 | 0.001 |
| IMAC eluate | 2 | 2.85 (±0.13) | 5.7 (±0.26) | 0.6 | Not detectable |
| SECd eluate | 3 | 0.37 (±0.06) | 1.11 (±0.18) | 3.6 | Not detectable |
Recoveries are based on 4.1 g of E. coli (wet weight) obtained from 1 liter of shaking culture. After IMAC, the 250 mM imidazole fractions were pooled, and after SEC, the fractions with the highest binding activity were pooled.
Calculated by measuring the extinction at 405 nm (determined by ELISA) per milligram of protein in the pooled fractions.
Mean concentrations from five different experiments.
SEC, size exclusion chromatography.
Functionality.
The binding properties of the recombinant proteins against the CD25 and CD30 antigens were demonstrated by ELISA analyses using MAb TC-1 (Fig. 4a). The recombinant immunotoxins bound to their respective recombinant human target antigens. Additionally, binding of the final preparation was also documented by flow cytometry as shown for Ki-4(scFv)-ETA′ and RFT5(scFv)-ETA′. The recombinant proteins bound to the CD25+ CD30+ cell line L540Cy but not to the antigen-negative cell line HD-MyZ (Fig. 4b). Antigen specificity was documented by competitive ELISA experiments. The binding of RFT5(scFv)-ETA′ and Ki-4(scFv)-ETA′ against both CD25 or CD30 antigen-positive cell membrane fractions and recombinant CD25 or rhCD30 receptor was inhibited by 10 μg of soluble CD25 receptor (Fig. 5a, inset) or MAb Ki-4 (data not shown)/ml, respectively.
FIG. 5.
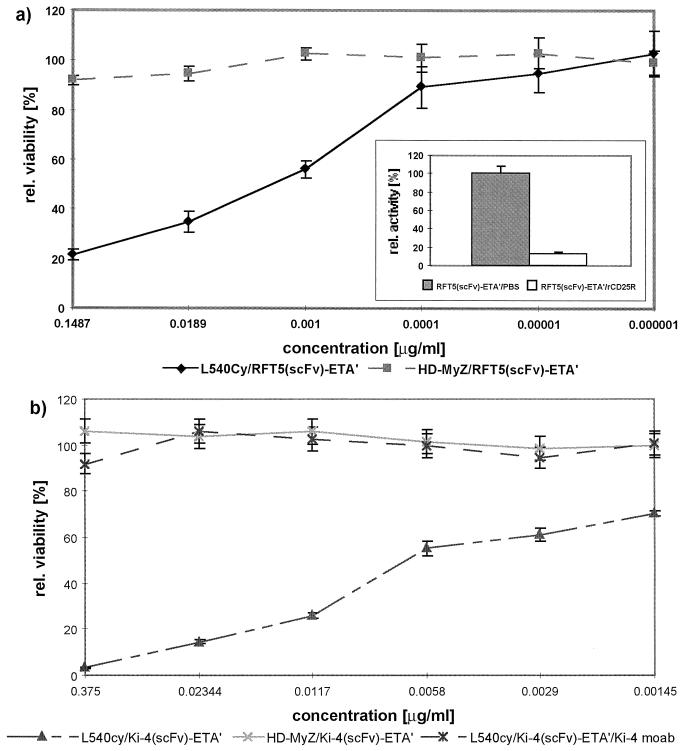
Growth inhibition of Hodgkin-derived cell lines after incubation with recombinant immunotoxins as documented by cell viability assays. L540Cy (CD25+ CD30+) and HD-MyZ (CD25− CD30−) cells were treated with the immunotoxins, and their abilities to metabolize the tetrazolium salt XTT to a water-soluble formazan salt (formed by mitochondrial dehydrogenase activity) were measured as absorbance at 450 and 650 nm (reference wavelength) in a cell viability assay (24). (a) Cytotoxic activities of 1:10 dilutions of RFT5(scFv)-ETA′ on L540Cy cells. (Inset) RFT5(scFv)-ETA′ (10 μg/ml in PBS) showed 100% binding activity on immobilized CD25 receptor; binding was reduced to <15% after the addition of 10 μg of soluble CD25 rCD25R receptor/ml. (b) Cytotoxic activity of Ki-4(scFv)-ETA′ on L540Cy cells (competition with MAb Ki-4).
To characterize the cytotoxic activity of the recombinant immunotoxins in vitro, we measured their ability to reduce the viability of target cells (Fig. 5). Both Ki-4(scFv)-ETA′ and RFT5(scFv)-ETA′ were specifically cytotoxic to L540Cy cells, with calculated IC50s of 6 and 12 ng/ml, respectively. The CD30− CD25− cell line HD-MyZ was not affected at recombinant protein concentrations up to 10 μg/ml. Again, antigen specificity was documented by competition with 10 μg of MAb Ki-4 (Fig. 5b) or RFT5 (data not shown)/ml in cell viability assays.
Stability.
To analyze possible effects of self-stabilization, increasing protein concentrations were tested for residual binding activity after one freeze-thawing step (data not shown). Experiments with the unmodified parental Ki-4 MAb and recombinant immunotoxin Ki-4(scFv)-ETA′ confirmed that increasing protein concentrations are correlated with enhanced resistance against fast freezing and slow thawing. Immunotoxins were much more sensitive in these experiments than MAbs. Based on these results, subsequent experiments were performed at protein concentrations of 1 μg/ml.
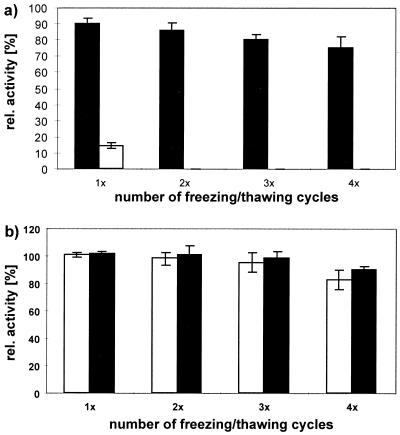
The binding activities of MAb Ki-4 and recombinant immunotoxin with respect to the frequency of freezing and thawing are depicted in Fig. 6a. Repeated freezing and thawing was correlated with losses of as much as 30% of the binding activity for MAb Ki-4 and 99% for the immunotoxin.
FIG. 6.
Cryoprotection and stabilization of 1 μg of CD30-binding proteins/ml by compatible solutes were measured as relative binding activity by a CD30 receptor ELISA. Specifically bound Ki-4(scFv)-ETA′ was detected after incubation with the anti-ETA′ MAb TC-1 followed by alkaline-phosphatase-conjugated anti-mouse IgG, and bound MAb Ki-4 was detected by alkaline-phosphatase-conjugated anti-mouse IgG. Converted phosphatase substrate (o-phenylenediamine-dihydrochloride) was measured as absorbance at 405 nm. (a) Relative binding activity of Ki-4(scFv)-ETA′ (open bars) compared to that of MAb Ki-4 (solid bars) after several rounds of freeze-thawing. (b) Relative binding activities of Ki-4(scFv)-ETA′ (open bars) and MAb Ki-4 (solid bars) in the presence of 1 M hydroxyectoine.
In order to assess the efficiency of compatible solutes as stabilizers of binding structures and enzymes (30), the freeze-thaw experiments were repeated in the presence of 1 M hydroxyectoine. Generally, supplementation with compatible solutes resulted in significantly higher activity of the proteins investigated. Using hydroxyectoine, the stabilizing properties ranged between 100 and 89% for MAb Ki-4 after one to four cycles of freezing and thawing (Fig. 6b). For Ki-4(scFv)-ETA′, the most obvious protection (100%) was achieved after one cycle of freeze-thawing compared to 16% without compatible solutes; after four cycles in the presence of hydroxyectoine the residual binding activity was 89%, compared to 0% without hydroxyectoine. Control experiments with 1 M NaCl or 10% glycerin resulted in 50%-reduced stability in contrast to experiments with hydroxyectoine (data not shown).
Hydroxyectoine-stabilized Ki-4(scFv)-ETA′ which had undergone four cycles of freeze-thawing (ca. 90% residual binding activity) was tested for its cytotoxic activity against L540Cy cells. The IC50 was slightly reduced (7 ng/ml) compared to that for unfrozen protein; 10 mM hydroxyectoine alone and identically treated Ki-4(scFv)-ETA′ probes without protectants had no influence on cell viability (data not shown).
DISCUSSION
In this study, we report the development of a new protocol for periplasmic expression of recombinant proteins. The major findings emerging from our study are as follows. (i) Both recombinant immunotoxins examined were directionally expressed into the periplasmic space of E. coli BL21(DE3) and functionally purified by a combination of metal ion affinity and molecular size chromatography. (ii) Functional protein in sufficient amounts was generated only under osmotic stress (high-salt, low-water) conditions in the presence of compatible solutes. (iii) The protein-stabilizing functions of the compatible solutes were documented under storage conditions in PBS by freeze-thawing experiments at low protein concentrations.
The standard method used hitherto to purify Pseudomonas exotoxin-based recombinant proteins is to accumulate these proteins in inclusion bodies by coprecipitation in a denatured state together with ribosomes, nucleic acids, or other cytoplasmic proteins. The proteins are then recovered by de- and renaturation as originally described by Buchner et al. (9). A combination of factors relating to the physiological state of the cell and the growth conditions determine the formation of inclusion bodies. The reducing environment in the cytoplasm prevents the formation of disulfide bonds. Thus, proteins requiring disulfide bonds to assume their native conformation should be directed into the nonreducing periplasmic compartment of E. coli (37).
The use of compatible solutes as a means to counteract the insolubility of recombinant proteins has been described for recombinant ferritin. In these experiments, expression at 25°C in the presence of sorbitol and glycine betaine gave perfectly assembled and functional cytoplasmic protein (38). Similarly, E. coli transformed with the high-level expression vector pMON5525 formed insoluble complexes of recombinant dimethylallyl pyrophosphatase-AMP transferase at 27°C (7). When the same experiment was performed under osmotic stress (170 mM NaCl) in the presence of 1 M sorbitol and glycine betaine (2.5 mM), large amounts of active, soluble cytoplasmic protein were obtained. The use of compatible-solute-assisted stress conditions in our hands resulted in unprecedented yields of functional recombinant immunotoxins. At 4% NaCl, 0.5 M sorbitol, and 10 mM glycine betaine, more than 95% functional protein was accumulated in the periplasm in contrast to less than 10% under standard conditions. Importantly, neither aggregation products nor unprocessed proteins were detectable.
The underlying principle of hyperosmotic-stress-governed and solute-assisted expression is poorly understood. Combined effects are thought to influence productivity in the following ways: (i) by induction of heat shock proteins by stress, (ii) by establishing a stabilizing microenvironment in the periplasm by high salt concentrations, (iii) by enabling bacterial growth under the described osmotic-stress conditions in the presence of compatible solutes, and (iv) by direct interaction of compatible solutes with recombinant proteins after active uptake via proP and proU at high osmolarity. It is known, however, that under the conditions used (4% NaCl plus 0.5 M sorbitol, equivalent to an osmolarity of approximately 2 osM), glycine betaine and hydroxyectoine are efficiently taken up by E. coli via the proP and proU transport systems (10). One would therefore expect a concentration of at least 500 mM compatible solutes in the cells and subsequent modification of the cytosolic environment of the expression system. This salt stress situation alone, in combination with a considerably reduced metabolic rate, may be sufficient to explain the observed effect on the folding of recombinant proteins. However, one should keep in mind that moderate salt stress as applied in our experiments would have far-reaching effects on cellular metabolism. As has been pointed out by Hengge-Aronis (19) and others, the addition of salt and/or sucrose increases the intracellular level of sigma factor S and, as a consequence, induces a whole range of stress response mechanisms, including thermotolerance and starvation survival. It may therefore well be possible that additional elements of protein protection include the induction of chaperones and/or indirect effects on the presence and activity of periplasmic proteolytic enzymes during cultivation. In fact, our immunoprecipitation experiments documented a Ki-4(scFv)-ETA′/GroEL ratio of 0.45 and reduced proteolysis resulting in higher yields of functional immunotoxins by optimized concentrations of immunotoxin-bound chaperones under low-water conditions. This is in accordance with the data of Phillips and Silhavy, who demonstrated that the cytoplasmic solubility and/or secretion of a number of aggregation-prone heterologues can be improved by the overproduction of components of either the DanK-DnaJ-GrpE or the GroEL-GroES chaperone complex (34).
The results presented in this paper show that the compatible solutes tested, including glycine betaine and hydroxyectoine, efficiently protected recombinant fusion proteins against degradation, aggregation, misfolding, and freezing. Our data are supported by findings of Lippert and Galinski (30), who compared the effects of freeze-thawing, heating, and freeze-drying on the enzymatic activities of lactic dehydrogenase and phosphofructokinase. The enzymes tested were highly sensitive to each of these three destabilizing factors but retained more than 90% of their activities when stabilized with hydroxyectoine after four cycles of freeze-thawing. The prime importance of glycine betaine and hydroxyectoine as protein protectants has recently been emphasized (18, 28).
Compatible-solute-supported periplasmic expression may offer an attractive new tool for the high-yield production of functional and stable recombinant proteins. In addition, the major principles of the new method described in this paper, i.e., high-salt–low-water conditions and supplementation with compatible solutes, might also be generally applicable to other expression systems.
ACKNOWLEDGMENTS
We thank Darrell R. Galloway for providing the monoclonal anti-ETA′ hybridoma TC-1. We also thank Silke Drillich for cloning assistance and Gisela Schön for performing the toxicity assays.
This work was supported in part by the Deutsche Forschungsgemeinschaft, grants SFB502 and TFB 6-98.
S.B. and M.H. contributed equally to this work.
REFERENCES
- 1.Altamiro M M, Garcia C, Possani L D, Fersht A R. Oxidative refolding chromatography: folding of the scorpion toxin Cn5. Nat Biotechnol. 1999;17:187–191. doi: 10.1038/6192. [DOI] [PubMed] [Google Scholar]
- 2.Baneyx F, Georgiou G. Degradation of secreted proteins in Escherichia coli. Ann N Y Acad Sci. 1992;665:301–308. doi: 10.1111/j.1749-6632.1992.tb42593.x. [DOI] [PubMed] [Google Scholar]
- 3.Bargou R C, Mapara M Y, Zugck C, Daniel P T, Pawlita M, Dohner H, Dorken B. Characterization of a novel Hodgkin cell line, HD-MyZ, with myelomonocytic features mimicking Hodgkin's disease in severe combined immunodeficient mice. J Exp Med. 1993;177:1257–1268. doi: 10.1084/jem.177.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barth S, Huhn M, Wels W, Diehl V, Engert A. Construction and in vitro evaluation of RFT5(scFv)-ETA′, a new recombinant single-chain immunotoxin with specific cytotoxicity toward CD25+ Hodgkin-derived cell lines. Int J Mol Med. 1998;1:249–256. doi: 10.3892/ijmm.1.1.249. [DOI] [PubMed] [Google Scholar]
- 5.Barth S, Matthey B, Huhn M, Diehl V, Engert A. CD30L-ETA′: a new recombinant immunotoxin based on the CD30 ligand for possible use against human lymphoma. Cytokines Cell Mol Ther. 1999;5:69–78. [PubMed] [Google Scholar]
- 6.Benhar I, Pastan I. Cloning, expression and characterization of the Fv fragments of the anti-carbohydrate mAbs B1 and B5 as single-chain immunotoxins. Protein Eng. 1994;7:1509–1515. doi: 10.1093/protein/7.12.1509. [DOI] [PubMed] [Google Scholar]
- 7.Blackwell J R, Horgan R. A novel strategy for production of a highly expressed recombinant protein in an active form. FEBS Lett. 1991;295:10–12. doi: 10.1016/0014-5793(91)81372-f. [DOI] [PubMed] [Google Scholar]
- 8.Brown A D. Microbial water stress. Bacteriol Rev. 1976;40:803–846. doi: 10.1128/br.40.4.803-846.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchner J, Pastan I, Brinkmann U. A method for increasing the yield of properly folded recombinant fusion proteins: single-chain immunotoxins from renaturation of bacterial inclusion bodies. Anal Biochem. 1992;205:263–270. doi: 10.1016/0003-2697(92)90433-8. [DOI] [PubMed] [Google Scholar]
- 10.Csonka L, Epstein W. Osmoregulation. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C.: ASM Press; 1996. pp. 1210–1223. [Google Scholar]
- 11.Dower W J, Miller J F, Ragsdale C W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988;16:6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durkop H, Latza U, Hummel M, Eitelbach F, Seed B, Stein H. Molecular cloning and expression of a new member of the nerve growth factor receptor family that is characteristic for Hodgkin's disease. Cell. 1992;68:421–427. doi: 10.1016/0092-8674(92)90180-k. [DOI] [PubMed] [Google Scholar]
- 13.Engert A, Martin G, Amlot P, Wijdenes J, Diehl V, Thorpe P. Immunotoxins constructed with anti-CD25 monoclonal antibodies and deglycosylated ricin A-chain have potent anti-tumour effects against human Hodgkin cells in vitro and solid Hodgkin tumours in mice. Int J Cancer. 1991;49:450–456. doi: 10.1002/ijc.2910490324. [DOI] [PubMed] [Google Scholar]
- 14.Essen L, Skerra A. Single-step purification of a bacterially expressed antibody Fv fragment by immobilized metal affinity chromatography in the presence of betaine. J Chromatogr A. 1993;657:55–61. doi: 10.1016/0021-9673(93)83034-p. [DOI] [PubMed] [Google Scholar]
- 15.FitzGerald D, Pastan I, Robertus J. Clinical applications of immunotoxins. Introduction. Curr Top Microbiol Immunol. 1998;234:1–11. [PubMed] [Google Scholar]
- 16.Galinski E. Osmoadaptation in bacteria. Adv Microb Physiol. 1995;37:272–328. [PubMed] [Google Scholar]
- 17.Galloway D R, Hedstrom R C, Pavlovskis O R. Production and characterization of monoclonal antibodies to exotoxin A from Pseudomonas aeruginosa. Infect Immun. 1984;44:262–267. doi: 10.1128/iai.44.2.262-267.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Göller K, Galinski E A. Protection of a model enzyme (lactate dehydrogenase) against heat, urea and freeze-thaw treatment by compatible solute additives. J Mol Catal B Enzymatic. 1999;7:37–45. [Google Scholar]
- 19.Hengge-Aronis R. Back to log phase: sigma S as a global regulator in the osmotic control of gene expression in Escherichia coli. Mol Microbiol. 1996;21:887–893. doi: 10.1046/j.1365-2958.1996.511405.x. [DOI] [PubMed] [Google Scholar]
- 20.Hochuli E, Dobeli H, Schacher A. New metal chelate adsorbent selective for proteins and peptides containing neighbouring histidine residues. J Chromatogr. 1987;411:177–184. doi: 10.1016/s0021-9673(00)93969-4. [DOI] [PubMed] [Google Scholar]
- 21.Holliger P, Winter G. Diabodies: small bispecific antibody fragments. Cancer Immunol Immunother. 1997;45:128–130. doi: 10.1007/s002620050414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoogenboom H R, Marks J D, Griffiths A D, Winter G. Building antibodies from their genes. Immunol Rev. 1992;130:41–68. doi: 10.1111/j.1600-065x.1992.tb01520.x. [DOI] [PubMed] [Google Scholar]
- 23.Horn-Lohrens O, Tiemann M, Lange H, Kobarg J, Hafner M, Hansen H, Sterry W, Parwaresch R, Lemke H. Shedding of the soluble form of CD30 from the Hodgkin-analogous cell line L540 is strongly inhibited by a new CD30-specific antibody (Ki-4) Int J Cancer. 1995;60:539–544. doi: 10.1002/ijc.2910600419. [DOI] [PubMed] [Google Scholar]
- 24.Jost L M, Kirkwood J M, Whiteside T L. Improved short- and long-term XTT-based colorimetric cellular cytotoxicity assay for melanoma and other tumor cells. J Immunol Methods. 1992;147:153–165. doi: 10.1016/s0022-1759(12)80003-2. [DOI] [PubMed] [Google Scholar]
- 25.Kapp U, Wolf J, von Kalle C, Tawadros S, Rottgen A, Engert A, Fonatsch C, Stein H, Diehl V. Preliminary report: growth of Hodgkin's lymphoma derived cells in immune compromised mice. Ann Oncol. 1992;3(Suppl. 4):21–23. doi: 10.1093/annonc/3.suppl_4.s21. [DOI] [PubMed] [Google Scholar]
- 26.Klimka A, Barth S, Drillich S, Wels W, van Snick J, Renauld J C, Tesch H, Bohlen H, Diehl V, Engert A. A deletion mutant of Pseudomonas exotoxin-A fused to recombinant human interleukin-9 (rhIL-9-ETA′) shows specific cytotoxicity against IL-9-receptor-expressing cell lines. Cytokines Mol Ther. 1996;2:139–146. [PubMed] [Google Scholar]
- 27.Klimka A, Barth S, Matthey B, Roovers R C, Lemke H, Hansen H, Arends J W, Diehl V, Hoogenboom H R, Engert A. An anti-CD30 single-chain Fv selected by phage display and fused to Pseudomonas exotoxin A (Ki-4(scFv)-ETA′) is a potent immunotoxin against a Hodgkin-derived cell line. Br J Cancer. 1999;80:1214–1222. doi: 10.1038/sj.bjc.6690488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knapp S, Ladenstein R, Galinski E A. Thermal stabilisation of bovine ribonuclease A by the naturally occurring osmolytes beta-hydroxyectoine and betaine. Extremophiles. 1999;3:191–198. doi: 10.1007/s007920050116. [DOI] [PubMed] [Google Scholar]
- 29.Kreitman R, Pastan I. Recombinant toxins. Adv Pharmacol. 1994;28:193–219. doi: 10.1016/s1054-3589(08)60496-2. [DOI] [PubMed] [Google Scholar]
- 30.Lippert K, Galinski E A. Enzyme stabilization by ectoine-type compatible solutes: protection against heating, freezing and drying. Appl Microbiol Biotechnol. 1992;37:61–65. [Google Scholar]
- 31.Matthey B, Engert A, Klimka A, Diehl V, Barth S. A new series of pET-derived vectors for high efficiency expression of Pseudomonas exotoxin-based fusion proteins. Gene. 1999;229:145–153. doi: 10.1016/s0378-1119(99)00038-4. [DOI] [PubMed] [Google Scholar]
- 32.McCafferty J, Fitzgerald K J, Earnshaw J, Chiswell D J, Link J, Smith R, Kenten J. Selection and rapid purification of murine antibody fragments that bind a transition-state analog by phage display. Appl Biochem Biotechnol. 1994;47:157–171. doi: 10.1007/BF02787932. [DOI] [PubMed] [Google Scholar]
- 33.Pastan I, FitzGerald D. Recombinant toxins for cancer treatment. Science. 1991;254:1173–1177. doi: 10.1126/science.1683495. [DOI] [PubMed] [Google Scholar]
- 34.Phillips G, Silhavy T. Heat-shock proteins DnaK and GroEL facilitate export of LacZ hybrid proteins in E. coli. Nature. 1990;344:882–884. doi: 10.1038/344882a0. [DOI] [PubMed] [Google Scholar]
- 35.Sambrook J, Fritsch E, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 36.Studier F W, Moffatt B A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986;189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- 37.Talmadge K, Gilbert W. Cellular location affects protein stability in Escherichia coli. Proc Natl Acad Sci USA. 1982;79:1830–1833. doi: 10.1073/pnas.79.6.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Wuytswinkel O, Savino G, Briat J. Purification and characterization of recombinant pea-seed ferritins expressed in Escherichia coli: influence of N-terminus deletions on protein solubility and core formation in vitro. Biochem J. 1995;305:253–261. doi: 10.1042/bj3050253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wels W, Harwerth I, Mueller M, Groner B, Hynes N. Selective inhibition of tumor cell growth by a recombinant single-chain antibody-toxin specific for the erbB-2 receptor. Cancer Res. 1992;52:6310–6317. [PubMed] [Google Scholar]