Abstract
Cardiac arrhythmias are a significant cause of morbidity and mortality worldwide, accounting for 10–15% of all deaths. Although most arrhythmias are due to acquired heart disease, inherited channelopathies and cardiomyopathies disproportionately affect children and young adults. Arrhythmogenesis is complex, involving anatomical structure, ion channels and regulatory proteins, and the interplay between cells in the conduction system, cardiomyocytes, fibroblasts, and the immune system. Animal models of arrhythmia are powerful tools for studying not only molecular and cellular mechanism of arrhythmogenesis, but also more complex mechanisms at the whole heart level, and for testing therapeutic interventions. This review summarizes basic and clinical arrhythmia mechanisms followed by an in-depth review of published animal models of genetic and acquired arrhythmia disorders.
Keywords: Arrhythmias, Atrial Fibrillation, Animal Models of Human Disease, Basic Science Research Electrophysiology
Introduction
Cardiac arrhythmias affect ~2% of community-dwelling adults, with an incidence of ~0.5% per year.1 Arrhythmias can manifest as relatively benign entities, such as atrial and ventricular premature beats, or as life-threatening arrhythmias such as ventricular tachycardia (VT) and ventricular fibrillation (VF), which can lead to sudden cardiac death (SCD), accounting for 20% of all deaths in the United States. Atrial fibrillation (AF) accounts for the greatest arrhythmia burden and is associated with stroke and heart failure, fueling huge healthcare costs. Arrhythmia treatment approaches focused on risk factor reduction, drug therapy, catheter ablation, device implantation, or a combination of these strategies has improved morbidity and mortality over the last 20 years, but treatment with antiarrhythmic drugs is often ineffective or increases mortality long-term.2, 3 A more thorough understanding of the pathophysiology of arrhythmia initiation and maintenance is important for improving clinical outcomes.
The mechanisms underlying arrhythmogenesis at the cellular level involve ion channels and electrogenic transporters that are altered via biogenic (synthesis, processing, trafficking, and degradation), biochemical (post-translation modification, phosphorylation), and biophysical (gating, permeation) processes (reviewed here4). The interplay between ion channels and transporters controls the action potential duration (APD), effective refractory period (ERP), and Ca2+ cycling to coordinate excitation-contraction coupling and normal myocyte function; dysregulation leads to abnormal cardiomyocyte electrical activity.5 Structural and hemodynamic parameters contribute to further cardiac remodeling, increasing the risk for arrhythmia development and maintenance.6–9
To study underlying arrhythmia mechanisms and evaluate treatment approaches, multiple in vitro systems and in vivo models have been developed. This review focuses on animal models that have informed our understanding of arrhythmia pathophysiology and have been used to develop new therapeutic approaches. An ideal model would recapitulate human anatomic, electrophysiologic, and hemodynamic parameters. Currently, no single model can accomplish this feat. However, animal models have enabled the discovery of new treatment strategies for humans with genetic arrhythmia disorders. For example, mouse models demonstrated the efficacy of flecainide in catecholaminergic polymorphic ventricular tachycardia10 and mexiletine in long QT type 3.11 When choosing an animal model of cardiac arrhythmia, researchers must consider the most appropriate model to address a specific scientific question based on cost, complexity, ease of handling, access to diagnostic and surgical expertise, and the ability for genetic modification.
Here, we provide the reader with a brief overview of basic and clinical cardiac electrophysiology, followed by an in-depth review of existing animal models of cardiac arrhythmias. Animal models are classified as either genetic (i.e., arrhythmia risk caused by gene mutation) or acquired (i.e., arrhythmia risk caused by non-genetic heart diseases such as myocardial infarction, metabolic abnormalities, or cardiac hypertrophy).
I. PRINCIPLES OF CARDIAC ELECTROPHYSIOLOGY
The cardiac conduction system
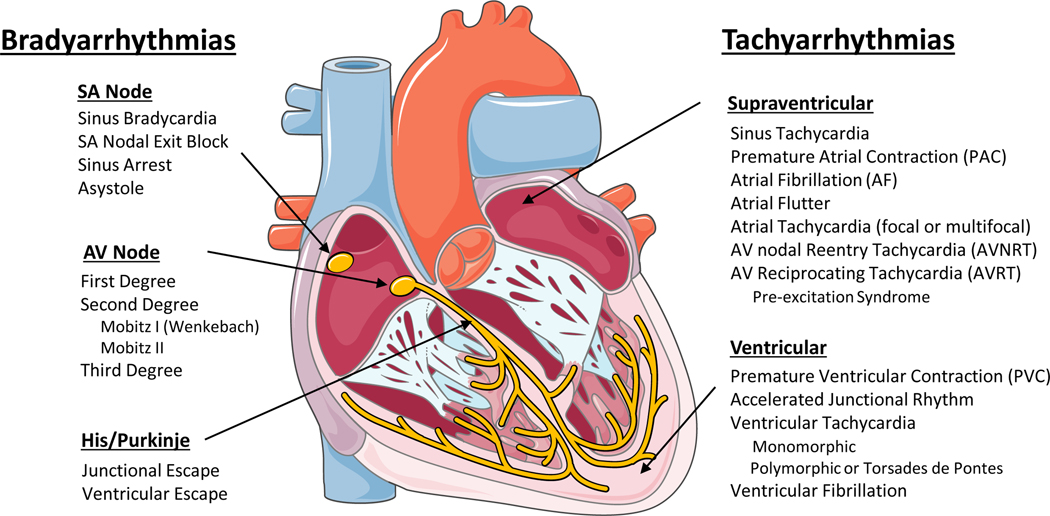
Normal heart rhythm is generated and regulated in the specialized cardiac conduction system, which consists of the sinoatrial (SA) node, the atrioventricular (AV) node, and the HIS-Purkinje system (Figure 1). Electrical impulses are initiated in the SA node and spread through the atria to the AV node. After a slight delay (0.12–0.20 seconds), excitation continues through the bundle of His, the right and left bundle branches, and finally the Purkinje fibers, which then excite the working myocardium. The delay in the AV node allows the atria to contract earlier than the ventricles and provides adequate time for optimal ventricular filling.12 The specialized cells within the SA node, AV node, and His-Purkinje system are capable of spontaneous depolarization that is regulated by both the sympathetic and parasympathetic nervous system. Conduction through the heart depends on electrical coupling between cells, which is mediated by gap junctions.
Figure 1: Schematic of the cardiac conduction system and clinical classification of cardiac arrhythmias.
SA – Sinoatrial; AV – Atrioventricular (Illustration credit: Ben Smith)
Species differences in the cardiac action potential and cardiac Ca2+handling
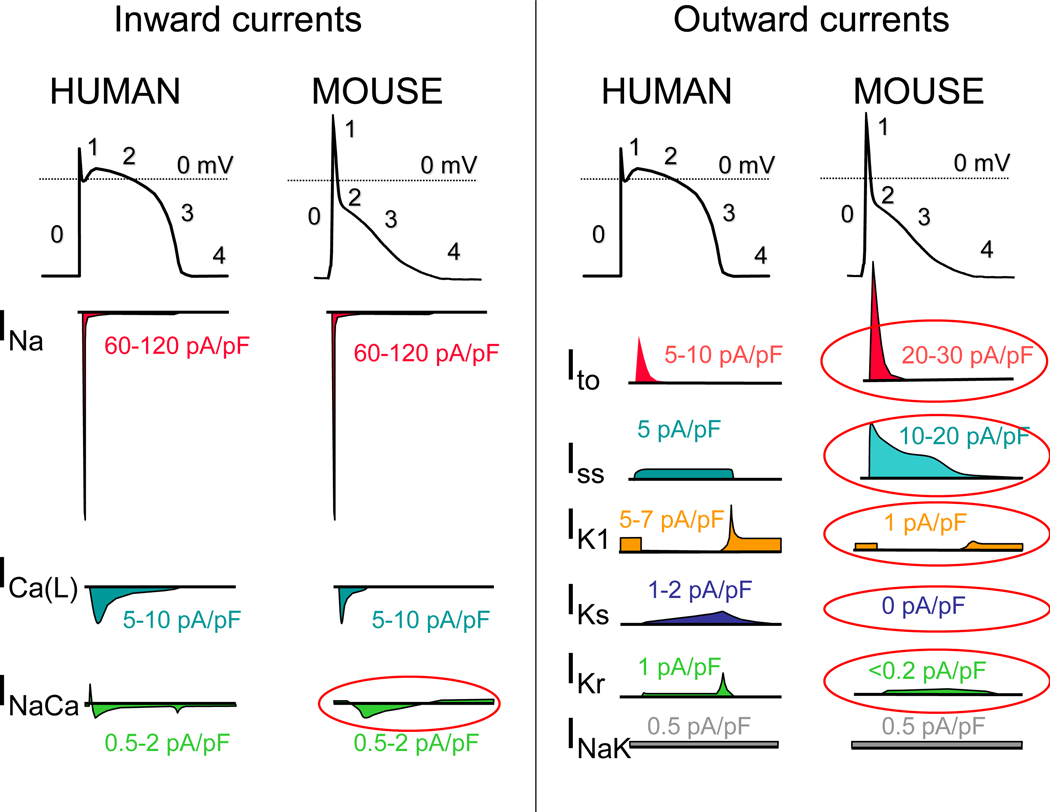
The cardiac action potential (AP) results from the opening and closing of ion channels and electrogenic transporters in the plasma membrane of individual cardiomyocytes (see13 for details). Figure 2 illustrates AP wave forms and underlying membrane currents for ventricular cardiomyocytes of humans and mice. When choosing an animal model for arrhythmia research, it is important to recognize species differences in cardiac AP and membrane currents, which are the result of species-specific expression of ion channels and transporters. For example, unlike humans, mice and rats have a low AP plateau at approximately −40 mV membrane potential (Figure 2). This is primarily the result of differential expression in repolarizing transient K-currents, as illustrated in Figure 2. On the other hand, rabbits and guinea pigs have a more positive AP plateau analogous to humans.14 For a more detailed comparison of ionic currents in different species, the reader is referred to here.15
Figure 2: The ventricular action potential and ionic currents in humans and mice.
Note the differences in action potential shape, which is caused primarily by differences in ionic currents circled in red. INa – Na current; ICa(L) – L-type Ca current, ICa(T) – T-type Ca current; INaCa – Na-Ca-Exchange current; Ito – Transient outward K-current; Iss – Rapidly activating steady-state K-currents; IK1 – Inward rectifier K-current; IKs – slowly activating delayed rectifier K-current; IKr – rapidly activating delayed rectifier K-current; INaK – NaK-ATPase pump current. Please note that current densities (pA/pF) measured in single cells vary drastically with experimental conditions and voltage clamp protocols. Current densities were chosen to reflect relative contributions to the AP.
As with the AP, there are important species differences in cardiac Ca2+ handling. For example, mice and rats primarily (>90%) utilize SR-mediated Ca2+ cycling (via the cardiac ryanodine receptor [RyR2] and the SR Ca uptake pump [SERCA2a]) for excitation-contraction coupling, whereas in humans, dogs, and rabbits the SR accounts for approximately 65%, with the remainder coming from outside the cell via the L-type Ca channel (CaV1.2) and the Na/Ca exchanger (NCX).16 For a more detailed comparison of species differences in Ca2+ handling, the reader is referred to here.17
Pathophysiology of cardiac arrhythmias
The main mechanisms of arrhythmogenesis can be divided into either abnormal impulse generation or abnormal impulse propagation. Disorders of impulse generation and propagation, regulation of the AP duration, and cellular substrates can all contribute to three categories of arrhythmias; enhanced automaticity, triggered ectopic beats, and reentry. Each arrhythmia category is explained briefly below. A more detailed review can be found in here.5
Automaticity
Automaticity is the ability of cells to generate their own AP.18 The intrinsic depolarization rate of the SA node is faster than the rest of the cardiac conduction system and overdrives pacemaking in the AV node and His-Purkinje system. However, automaticity in the AV node and His-Purkinje system can become dominant in SA nodal dysfunction. The SA node is more sensitive to increased sympathetic and parasympathetic tone, leading to sinus tachycardia and bradycardia, respectively. Under normal conditions, atrial and ventricular cardiomyocytes display either no or very slow intrinsic depolarization that are easily suppressed by the faster, coordinated impulses from the SA node through the conduction system. Increased automaticity in the atria can lead to focal and multifocal atrial tachycardia (AT, MAT) and AF. Specifically, the pulmonary vein sleeve, where the left atria myocytes transition to the tunica media of the pulmonary veins, is known to harbor tissue with increased automaticity,19 and is a target for catheter based ablation by pulmonary vein isolation for AT and AF. Increased automaticity in the ventricle is less common but can lead to ventricular tachycardia (VT) or accelerated idioventricular rhythms.
Afterdepolarizations and triggered arrhythmia
Triggered arrhythmias are due to spontaneous membrane depolarization of atrial or ventricular myocytes that precede the next sinus beat. Membrane depolarizations that occur within or follow the cardiac AP are referred to as afterdepolarizations. Two classes are traditionally recognized: early and delayed. An early afterdepolarization (EAD) interrupts the repolarization during phase 2 or early phase 3 of the cardiac AP, whereas a delayed afterdepolarization (DAD) occurs after full repolarization in Phase 4. When an EAD or DAD brings the membrane to its threshold potential, a spontaneous AP is referred to as a triggered response. These triggered events can give rise to premature extrasystolic complexes in the atria (PACs) or the ventricle (PVCs), precipitating tachyarrhythmias. In general, any unbalanced increased inward current (i.e., gain-of-function mutations in Na+ or Ca2+ channels) or decreased outward currents (i.e., loss-of-function mutations in K+ channels) will depolarize the cell membrane and can lead to EADs or DADs.20 Specifically, a major cause of triggered arrhythmia is spontaneous RyR2-mediated SR Ca2+ release, driving inward Na+ current via the NCX, leading to EAD and in particular DAD formation, which are important cellular arrhythmia mechanisms in AF, VT and SCD.21
Reentrant arrhythmia
In reentry, a group of myocardial cells that are not activated during the early stage of depolarization can resume excitability before the impulse vanishes. In this situation, they may connect to re-excite zones that were previously depolarized but were recovered from the refractory period of the initial wave. Two crucial factors predisposing reentry are prolonged conduction time and shortened refractory period. Reentry is the dominant mechanism of arrhythmias in the clinical setting and occurs due to anatomical and functional factors.22
Classification of clinical arrhythmias
Clinically, cardiac arrhythmias are usually classified as bradyarrhythmias and tachyarrhythmias (Figure 1). Both types can reduce cardiac output, resulting in hypotension and ultimately can cause death, but have different underlying mechanisms. Figure 1 lists the major clinical types of brady and tachyarrhythmias. Briefly, bradyarrhythmias reduce the heart rate either by reducing spontaneous depolarization within the SA node, slowing conduction through the conduction system, or increasing parasympathetic tone. Sinus bradycardia, sinus node exit block, sinus arrest and asystole are caused by dysfunction within the sinus node itself, due to destruction of the pacemaker cells, fibrosis of the SA node, or increased parasympathetic tone. AV nodal block prolongs the conduction above, within or below the AV node. Depending on the severity of AV block, it is classified as first-degree, second-degree, or complete (third-degree) heart block (Figure 1).
Tachyarrhythmias are accelerated rhythms that originate from either above (supraventricular, SVT) or below the AV node (ventricular arrhythmia). The most common SVTs are sinus tachycardia and AF. Premature atrial contractions (PACs) and atrial tachycardia (AT) are commonly caused by automatic foci within the atria. Reentrant atrial arrhythmias include atrial flutter, AV nodal reentry tachycardia (AVNRT), and AV reciprocating tachycardia (AVRT). Atrial flutter is a macroreentrant loop, typically involving the tricuspid annulus limited by anatomical barriers such as the superior and inferior cava veins, the coronary sinus and crista terminalis.23 AVNRT is a microreentry related to differences in the refractory period of the slow and fast pathway within the AV node.24 AVRT, also known as pre-excitation syndrome, occurs due to the presence of an accessory pathway, most notably the Bundle of Kent leading to Wolf-Parkinson-White syndrome, which can prematurely conduct impulses between the atria and ventricles.
Ventricular arrhythmias include premature ventricular contractions (PVCs), ventricular tachycardia (VT), and ventricular fibrillation (VF). PVCs are single premature beats due to EADs or DADs in myocardial cells and benign, unless they trigger VT or VF. VT and VF are usually reentrant arrhythmias, and if not treated rapidly, can lead to sudden cardiac death. While a majority of cases of VT are due to reentry around the scar in structural heart disease, 10% of VT occurs in structurally normal hearts due to non-reentrant mechanisms such as catecholaminergic polymorphic VT (CPVT), fascicular VT, left or right outflow tract VT, mitral and tricuspid annular VT, long QT, and Brugada syndrome.25
Animal models
An important consideration for selecting an animal model to study cardiac arrhythmias is how closely the species resembles human cardiac physiology. Caenorhabditis elegans and Drosophila melanogaster both develop heart tubes and have been primarily used to screen gene function and examine development and cardiac structure. Zebrafish have a two chambered heart with some similarities in AP electrophysiology to humans and provide advantages for understanding cardiogenesis. Zebrafish embryos are transparent, enabling optical viewing, fluorescent protein expression, and optogenetic pacing; they have large clutch sizes with a rapid embryonic stage lasting only 3–4 days post fertilization; are amenable to drug absorption; and genes are easily manipulated. Mouse hearts are anatomically similar to human hearts with four chambers and comparable development,26 albeit differences in coronary anatomy.27 However, there are major differences in heart rate, cardiac AP, and membrane currents (Figure 2). These differences influence ion channel function, refractoriness, and arrhythmia susceptibility. Additionally, the small size of the mouse heart may contribute to the frequently observed self-termination of reentrant arrhythmias or lack of spontaneous arrhythmias in many models. Nevertheless, the mouse has been the primary animal model for cardiac arrhythmia studies of inherited cardiomyopathies and channelopathies, and many models faithfully capture cardiac disease. Rabbits more closely recapitulate the human AP compared to rats and mice. The rabbit AP has a sustained Ca2+ current-driven plateau phase and the major repolarizing K+ currents are similar to humans. Rabbit heart size and beating rate is between that of mice and humans. Dogs, pigs, and goats have a similar cardiac anatomy, size, and beating rate (slightly higher in dogs) as humans. Their cardiac electrophysiology, APs, and ionic currents are all fairly comparable to humans, and their primary limitations as an animal model for research come from their cost, size, and time to breed and reach sexual maturity.
II. ANIMAL MODELS OF GENETIC ARRHYTHMIA DISORDERS
Genetic arrhythmia disorders are either caused by or associated with identifiable gene mutations. Genetic arrhythmia syndromes can be subdivided into channelopathies without structural heart disease (e.g., CPVT, LQTS, Brugada Syndrome) and genetic arrhythmia syndromes associated with structural heart disease (e.g., HCM, DCM, ARVC, AF). Tables 1 and 2 list published animal models of genetic arrhythmia syndromes. Most animal arrhythmia models are mice, given the ease of genetic manipulation. Despite differences between rodent and human cardiac electrophysiology (Figure 2), mouse models have enabled the study of human genetic diseases, identification of pathogenic mutations, characterization of disease pathophysiology, and testing/screening of therapeutic interventions. Advances in gene editing technology such as CRISPR/Cas9 have provided faster, easier, and cheaper methods to develop genetic models in animals and cells. Compared to cellular models such as human induced pluripotent stem cells, animal models provide a distinct advantage in modeling cardiac arrhythmias where the anatomy of the heart is relevant.28 In the following section, each of the major genetic arrhythmia syndromes are introduced, followed by examples of animal models that have informed disease pathophysiology and treatment approaches.
Table 1. Animal models of genetic arrhythmia channelopathies.
Models are separated by disease, listing the animal species, orthologous human gene and protein, mutation, notable arrhythmia phenotypes/findings, and reference. Abbreviations: wild type (WT), double knockout (DKO), knockout (KO), homozygous (hom), heterozygous (het), heart rate (HR), monomorphic/polymorphic ventricular tachycardia (MVT, PVT, VT), nonsustained ventricular tachycardia (NSVT), supraventricular tachycardia (SVT), premature ventricular complexes (PVCs), isoproterenol (iso), caffeine (caff), ventricular fibrillation (VF, VF), atrial fibrillation (AF), programmed electrical stimulation (PES), loss of function (LoF), gain of function (GoF), dominant negative (DN), action potential duration (APD), early afterdepolarization (EAD), delayed afterdepolarization (DAD), atrioventricular (AV), action potential duration (AP, APD), ischemia-reperfusion (IR), heart failure (HF), right bundle branch block (RBBB).
| Disease, Type, Animal | Human Ortholog Gene (Protein) | Mutation | Notes | Ref. |
|---|---|---|---|---|
| Catecholaminergic polymorphic ventricular tachycardia (CPVT) | ||||
| CPVT1 | ||||
| Mouse | RYR2 (RyR2) | R4496C+/− | Catecholamine/exercise-induced arrhythmia; phenotype not as penetrant as some other models | 31 |
| Mouse | RYR2 (RyR2) | V2475F+/− | Homs are embryonic lethal, catecholamine-induced arrhythmia | 343 |
| Mouse | RYR2 (RyR2) | R2474S+/− | Seizures, exercise-induced arrhythmia, sudden death | 344 |
| Mouse | RYR2 (RyR2) | P2328S+/− | Iso-induced PVCs, VT, VF | 345 |
| Mouse | RYR2 (RyR2) | R2474S/V3599K | GoF + LoF; protective- no arrhythmia | 346 |
| Mouse | RYR2 (RyR2) | R4496C+/− with E4872Q+/− | GoF + LoF; protective- no arrhythmia | 32 |
| Mouse | RYR2 (RyR2) | L433P+/−, N2386I+/− | Mutations from patients with CPVT; no report of CPVT in mouse models; mice also develop AF with pacing protocol | 347 |
| Mouse | RYR2 (RyR2) | R176Q+/− | CPVT-like phenotype; AF | 348 |
| Mouse | RYR2 (RyR2) | exon3del+/− | Homs embryonic lethal, hets did not develop CPVT; patients with exon3del have exercise-induced VT | 33 |
| C. elegans |
RYR2 (RyR2), CASQ2 (Casq2) |
R4743C, Casq2 KO |
Enables optogenetic pacing, observed defect with mutations | 349 |
| CPVT2 | ||||
| Mouse | CASQ2 (Casq2) | KO | Severe CPVT, ultrastructure changes, catecholamine/exercise-induced arrhythmia | 38 |
| Mouse | CASQ2 (Casq2) | D307H/D307H, D309deltaE9/D309deltaE9 (Casq2−/−) | Both mutations result in loss of Casq2 protein, catecholamine/exercise-induced arrhythmia | 350 |
| Mouse | CASQ2 (Casq2) | ventricular/Purkinje KO | Subtissue-selective knockout | 351 |
| Mouse | CASQ2 (Casq2) | ventricular/Purkinje KO | Subtissue-selective knockout | 352 |
| Mouse | CASQ2 (Casq2) | R33Q/R33Q | Reduction of Casq2 protein, ultrastructure changes, arrhythmia | 353 |
| Mouse | CASQ2 (Casq2) | K180R+/− | Autosomal dominant inheritance; iso-induced arrhythmias | 354 |
| CPVT4 | ||||
| Zebrafish | CALM (CaM) | N53I and N97S | Increased iso-induced HR (indicated dominant negative effect) | 355 |
| Zebrafish | CALM (CaM) | E105A | Arrhythmias, tachycardia, altered RyR2 binding | 356 |
| CPVT5 | ||||
| Mouse | TRDN (Triadin) | KO | Ultrastructure changes; iso-induced arrhythmias; overlap syndrome with LQT? | 36 |
| CPVT? | ||||
| Mouse | KCNJ2 (Kir2.1) | R67Q+/−, cardiac-specific | Structurally normal heart, iso-induced VT, no LQT | 357 |
| Calcium release deficiency syndrome (CRDS) | ||||
| Mouse | RYR2 (RyR2) | A4860G+/− | VF with sympathetic stimulation, homs embryonic lethal | 45 |
| Mouse | RYR2 (RyR2) | D4646A+/− | RyR2 Ca2+ release deficiency syndrome (CRDS); homs are embryonic lethal | 44 |
| Long QT syndrome (LQTS) | ||||
| LQT1 | ||||
| Mouse | KCNQ1 (Kv7.1) | KO | Deaf, shaker/waltzer phenotype; LQT | 53 |
| Mouse | KCNQ1 (Kv7.1) | truncated isoform, cardiac-specific overexpression | LQT, sinus node dysfunction, occasional AV block | 358 |
| Mouse | KCNQ1 (Kv7.1) | A340V−/− | Homs have LQT, hets do with gene dose dependence; PVCs after feeding (linked to diabetes) | 359 |
| Rabbit | KCNQ1 (Kv7.1) | Y315S cardiac-specific overexpression | LQT; sympathetic stimulation induces EADs and VT; rabbits die within 3 weeks of AV node ablation; | 54 |
| LQT2 | ||||
| Rabbit | KCNH2 (Kv11.1/hERG) | G628S cardiac-specific overexpression | LQT, spontaneous PVT, sudden death; prolonged APD | 54 |
| Zebrafish | KCNH2 (Kv11.1/hERG) | I462R, M521K | 2:1 block (phenotype) | 360 |
| Zebrafish | KCNH2 (Kv11.1/hERG) | I59S−/− | 2:1 block (phenotype); prolonged APD; EADs | 361 |
| Zebrafish | KCNH2 (Kv11.1/hERG) | Morpholino KD of WT + expression of hERG +/− mutants | Tested 40 pathogenic and 10 non-pathogenic hERG mutants in the zERG background | 55 |
| LQT3 | ||||
| Mouse | SCN5A (Nav1.5) | ΔKPQ/+ | 1505–1507 deletion; prolonged QT/QTc; prominent T wave; prolonged APD; arrhythmias; sudden death | 57 |
| Mouse | SCN5A (Nav1.5) | ΔQKP/+ | 1507–1509 deletion; long QTc, wide QRS, AV block; spontaneous PVCs, VT, and VF with sudden death; no atrial arrhythmias | 56 |
| Mouse | SCN5A (Nav1.5) | N1325S cardiac-specific overexpression | LQT; arrhythmia; sudden death; also other non-LQT3 features like shorter PR and elevated heart rate | 362 |
| Mouse | SCN5A (Nav1.5) | 1795insD | LQT and Brugada in family; homs embryonic lethal; sinus node dysfunction, conduction slowing, bradycardia, and LQT | 76 |
| Guinea Pig | SCN5A (Nav1.5) | cellular model, isolated cells tx | Isolated cardiomyocytes treated with anthopleurin; rescued by mexillitine | 58 |
| Minor Types | ||||
| Mouse | ANK2 (Ankyrin-B) | KO | LQT with HR deceleration, sinus node dysfunction | 62 |
| Mouse | ANK2 (Ankyrin-B) | +/− | Bradycardia, variable HR, slow conduction, AV dissociation, long QTc; iso/exercise-induced PVT & death | 63 |
| Mouse | KCNA1 (Kv1.1) | N-term fragment overexpression | Long QTc; spontaneous PVC, couplets, ventricular tachycardia | 67 |
| Mouse | KCNA5 (Kv1.5) | W461F cardiac-specific overexpression | Long QTc | 363 |
| Mouse | KCNB1 (Kv2.1) | N216 cardiac-specific overexpression | Long QTc | 364 |
| Mouse | KCND2 (Kv4.2) | W362F cardiac-specific overexpression | Subtle bradycardia, prolonged QTc | 365 |
| Mouse | KCND2 x KCNA4 | W362F x KO | QRS widening, prolonged QTc, AV block/dropped beats, spontaneous ventricular arrhythmia | 366 |
| Rabbit | KCNE1 (minK) | G52R dominant negative overexpression | Long QTc; drug-induced arrhythmia (Torsades) | 65 |
| Mouse | KCNE1 (minK) | KO | LQT with HR deceleration | 64 |
| Mouse | KCNE2 (MiRP1) | KO | Long QTc with age, mice had hyperkalemia | 367 |
| Mouse | KCNE3 (MiRP2) | KO | Females have long QTc at 9 months (hyperaldosteronism); increased susceptibility to IR arrhythmias | 69 |
| Mouse | KCNJ2 (Kir2.1) | KO | Bradycardia, LQT | 60 |
| Mouse | KCNJ2 (Kir2.1) | T75R cardiac-specific overexpression | Long QTc; spontaneous VT; iso-induced PVCs, VT, atrial flutter/fibrillation | 61 |
| Mouse | KCNIP2 (KChIP2) | KO | Significant reduction in Ito; elevated ST segment, atrial flutter and VT with PES; prolonged APD in cells | 368 |
| Mouse | CACNA1C (Cav1.2) | −/−, +/− | KO embryonic lethal, hets survive and are just like WT | 369 |
| Mouse | CACNA1C (Cav1.2) | G406R cardiac-specific overexpression | Long QTc, exercise-induced PVCs and Torsades; crossing with AKAP150 KO protected against all phenotypes of the G406R mutant | 66 |
| Zebrafish | CALM (CaM) | D129G | Bradycardia; conduction abnormality; LQT | 370 |
| Mouse | SCN1B (Scn β1) | KO | Bradycardia, prolonged APD/QTc, slowed repolarization; sodium channel expression increased | 59 |
| Mouse | SLC18A2 (VMAT2) | +/− | LQT | 371 |
| Mouse | ATP1A3 (NaK ATPase α3) | human isoform overexpression | LQT, steeper QT rate dependence, T wave alternans, VT | 372 |
| Short QT syndrome (SQTS) | ||||
| SQTS1 | ||||
| Rabbit | KCNH2 (Kv11.1/hERG) | N588K cardiac-specific overexpression | Shortened APs and QTc, normal T wave height, ex vivo perfused hearts inducible VT/VF | 73 |
| Zebrafish | KCNH2 (Kv11.1/hERG) | L499P | SQT | 71 |
| SQTS8 | ||||
| Zebrafish | SLC4A3 (AE3) | Knockdown | SQT and systolic duration; WT SLC4A3 expression rescued phenotype, but R370H did not | 72 |
| Mouse | SLC8A1 (NCX) | KO | SQT | 373 |
| Mouse | CAV3 (Caveolin 3) | WT cardiac-specific overexpression | Short QTc, bradycardia, prolonged PR | 374 |
| Kangaroo | Unknown | None Reported | Kangaroos have LV hypertrophy, SQT, and are highly susceptible to VF and sudden death, especially under light anesthesia | 375 |
| Brugada Syndrome (BrS) | ||||
| Pig | SCN5A (Nav1.5) | E558X+/− | Conduction abnormalities, QRS widening, reduced conduction velocity, ex vivo hearts have increased susceptibility to VF | 375 |
| Mouse | SCN5A (Nav1.5) | +/− | KO is embryonic lethal; hets have sick sinus, slowed conduction, pacing-induced VT; QTS widening & fibrosis with age | 74 |
| Mouse | SCN5A (Nav1.5) | 1795insD | LQT and BrS in family; hom mice embryonic lethal; sinus node dysfunction, conduction slowing, bradycardia, and LQT | 76 |
| Mouse | SCN5A (Nav1.5) | ΔSIV/+ | C-term truncation; SA, AV, and His conduction slowing; one human patient with V2016M diagnosed with Brugada | 376 |
Table 2. Animal models of genetic arrhythmia syndromes with structural heart disease.
Models are separated by disease, listing the animal species, orthologous human gene and protein, mutation, notable arrhythmia phenotypes/findings, and reference. Abbreviations: wild type (WT), double knockout (DKO), knockout (KO), homozygous (hom), heterozygous (het), heart rate (HR), monomorphic/polymorphic ventricular tachycardia (MVT, PVT, VT), non-sustained ventricular tachycardia (NSVT), supraventricular tachycardia (SVT), premature ventricular complexes (PVCs), isoproterenol (iso), caffeine (caff), ventricular fibrillation (VF, VF), atrial fibrillation (AF), programmed electrical stimulation (PES), loss of function (LoF), gain of function (GoF), dominant negative (DN), action potential duration (APD), early afterdepolarization (EAD), delayed afterdepolarization (DAD), atrioventricular (AV), action potential duration (AP, APD), ischemia-reperfusion (IR), heart failure (HF), right bundle branch block (RBBB).
| Disease, Type, Animal | Human Ortholog Gene (Protein) | Mutation | Notes | Ref. |
|---|---|---|---|---|
| Arrhythmogenic Right Ventricular Cardiomyopathy | ||||
| Desmosomal | ||||
| Mouse | JUP (Plakoglobin) | +/− | Homs embryonic lethal; hets develop arrhythmia, RV structure change, etc. | 377 |
| Mouse | JUP (Plakoglobin) | c.2037–2038delTG | Truncated form, LoF, mortality, fibrosis | 378 |
| Mouse | JUP (Plakoglobin) | 23654del2 overexpression | Repressed Wnt signaling; fibrosis, dysfunction, death | 379 |
| Zebrafish | JUP (Plakoglobin) | Knockdown | Smaller heart size, blood reflux between chambers, reduced heart rate | 380 |
| Zebrafish | JUP (Plakoglobin) | 2057del2 | Structural change, mortality | 381 |
| Mouse | DSP (Desmoplakin) | +/− | Homs are embryonic lethal; hets have fibrosis, dysfunction, arrhythmias; Wnt signaling implicated | 382 |
| Mouse | DSP (Desmoplakin) | Conduction-specific KO (HCN4) | Migrating atrial pacemakers, sinus rhythm dysfunction, all without cardiac remodeling | 383 |
| Mouse | DSP (Desmoplakin) | R2834H cardiac-specific overexpression | Cardiac fibrosis, other small changes, no dysplasia | 384 |
| Mouse | PKP2 (Plakophilin 2) | +/− | Homs embryonic lethal; hets histologically normal, but electrical remodeling, ultrastructure changes, arrhythmia susceptibility | 82 |
| Mouse | PKP2 (Plakophilin 2) | S329X cardiac-specific overexpression | Histologically normal, but electrical remodeling, ultrastructure changes, arrhythmia susceptibility | 83 |
| Mouse | PKP2 (Plakophilin 2) | inducible cardiac KO | Fibrosis, arrhythmia, remodeling, death, reduced lvEF | 84 |
| Mouse | PKP2 (Plakophilin 2) | R735X AAV9-expression | RV dysfunction with exercise | 385 |
| Zebrafish | PKP2 (Plakophilin 2) | Knockdown | Structural defects, signaling reduced | 386 |
| Zebrafish | DSC2 (Desmocollin 2) | Knockdown | Altered ultrastructure, contractile dysfunction | 387 |
| Mouse | DSC2 (Desmocollin 2) | G790del/G790del, +/G790del | No phenotype | 388 |
| Mouse | DSC2 (Desmocollin 2) | WT overexpression | Necrosis, acute inflammation and patchy cardiac fibrotic remodeling | 80 |
| Mouse | DSG2 (Desmoglein 2) | KO | Embryonic lethal | 389 |
| Mouse | DSG2 (Desmoglein 2) | cardiac-specific KO | Dilation, fibrosis, electrophysiological remodeling | 390 |
| Mouse | DSG2 (Desmoglein 2) | N271S cardiac-specific overexpression | Biventricular dilatation; spontaneous ventricular arrhythmias, cardiac dysfunction, sudden death | 391 |
| Mouse | DSG2 (Desmoglein 2) | Q558X | Fibrosis, decrease in desmosomal size and number, and reduced Wnt signaling | 392 |
| Mouse | PPP1R13L (IASPP) | KO | Inducible Ppp1r13l knockout mouse model, dilation, arrhythmia, sudden death | 81 |
| Mouse | SORBS2 (ArgBP2) | KO | QRS widening, RBBB, spontaneous PVCs, NSVT, VT | 393 |
| Non-Desmosomal | ||||
| Mouse | RYR2 (RyR2) | R176Q+/− | Patients with R176Q also have T250M and present with ARVC; but mouse 176Q alone gives CPVT-like phenotype; no ARVC/D, but slight end-diastolic changes | 348 |
| Mouse | RYR2 (RyR2) | inducible cardiac-specific KO | Sinus bradycardia, block, ventricular tachycardia, sudden death | 394 |
| Mouse | ITGB1 (Integrin β1) | inducible cardiac specific KO | Beta1d isoform is reduced in ARVC patients; KO mice had iso/caff-inducible VT | 395 |
| Mouse | TMEM43 (Luma) | S358L+/− | Fibro-fatty replacement, structural abnormalities, arrhythmia, sudden death | 396 |
| Mouse | ILK (ILK) | cardiac-specific KO | Arrhythmia, sudden death; arrhythmogenic cardiomyopathy in some patients with missense mutations in ILK | 397 |
| Mouse | RPSA (LAMR1) | 1031 bp insertion (spontaneous) | ARVC; conduction abnormalities (QRS widening); no examination of inducible arrhythmias | 398 |
| Unknown | ||||
| Dog | Unknown | autosomal dominant inheritance | Boxers; fatty replacement of RV myocardium, ventricular arrhythmia, syncope, sudden death | 399 |
| Dog | Unknown | Unknown | Weimaraner; syncope, ventricular arrhythmias, and sudden death, with histopathological fatty tissue infiltration | 400 |
| Dog | Unknown | Unknown | English bulldog | 401 |
| Cat | Unknown | Unknown | SVT, VT, PVT, RBBB | 402 |
| Dilated cardiomyopathy (DCM) | ||||
| Mouse | LMNA (Lamin A/C) | +/− | DCM, arrhythmia | 403 |
| Mouse | LMNA (Lamin A/C) | H222P/H222P | Chamber dilation; slowed conduction, AV block, spontaneous PVCs | 404 |
| Mouse | LMNA (Lamin A/C) | N195K/N195K | Bradycardia, exit block, AV block, arrhythmia, sudden death | 405 |
| Mouse | LMNA (Lamin A/C) | G609G/G609G | Truncating splice variant; bradycardia, QRS widening, SA block, LQT | 406 |
| Pig | LMNA (Lamin A/C) | G609G/+ | Bradycardia, SA block, short QTc; spontaneous PVT, 3rd degree block at death | 407 |
| Zebrafish | DES (Desmin) | KO or aggregating mutant | Embryonic tachycardia, “arrhythmia”; however, no reports of electrophysiological changes or arrhythmias in two independent KO mouse models | 408 |
| Mouse | DES (Desmin) | R349P+/− | Human R350P; DCM, ARVC; slowed conduction and AV block; spontaneous and induced atrial fibrillation, PVCs, and VT | 409 |
| Zebrafish | ACTN2 (F-Actin) | knockdown | DCM, bradycardia | 410 |
| Mouse | LDB3 (ZASP) | S196L cardiac-specific overexpression | DCM, arrhythmia | 411 |
| Mouse | CDH2 (Cadherin) | cardiac-specific KO | DCM, arrhythmia, conduction defects | 412 |
| Mouse | LMOD2 (Leiomodin) | KO | DCM; LQT | 413 |
| Rat | RMB20 (RMB20) | KO | DCM; QRS widening, AV block, susceptibility to arrhythmias with PES, sudden death | 414 |
| Mouse | RMB20 (RMB20) | KO | DCM; slowed conduction, LQT; changes in ion channel and calcium-handling proteins; spontaneous calcium release in isolated cardiomyocytes | 415 |
| Mouse | RMB20 (RMB20) | S637A/S637A | DCM; spontaneous AF, spontaneous VT/VF with syncope, sudden death; much more severe cardiomyopathy than KO | 416 |
| Mouse | PLN (Phospholamban) | R14del/R14del | Mice develop severe DCM; no arrhythmias in vivo; explanted hearts have induced ventricular arrhythmias | 417 |
| Mouse | SCN5A (Nav1.5) | cardiac-specific knockdown | Slow conduction, sudden death | 418 |
| Mouse | SCN5A (Nav1.5) | S571E/S571E | Phosphomimetic CaMKII target; LV dilation; LQT, iso-induced PVCs and VT | 77 |
| Mouse | SCN5A (Nav1.5) | D1275N/+ or D1275N/D1275N | Homs have slow conduction, heart block, AF, VT, and DCM without significant fibrosis or myocyte disarray | 88 |
| Zebrafish | SCN5A (Nav1.5) | D1275N | Bradycardia, sinus pause, AV block, sudden death; no AF or VT observed | 89 |
| Pig | DMD (Dystrophin) | KO (exon52del) | Fibrosis, low voltage areas, sudden death | 419 |
| Dog | DMD (Dystrophin) | X-linked DMD | Short PR interval, sinus arrest, spontaneous ventricular arrhythmias | 420 |
| Mouse | DMD (Dystrophin) | KO (mdx strain) | Tachycardia; short PR, QRS, and QTc | 421 |
| Mouse | DMD (Dystrophin) | KO (5cv strain) | Short PR interval, inducible VT | 422 |
| Mouse | VCL (Vinculin) | cardiac-specific KO | Normal sinus rhythm, AV block, spontaneous PVT, sudden death (before onset of DCM phenotype) | 423 |
| Mouse | VASP (VASP) | cardiac-specific overexpression | Bradycardia, AV block, sudden death | 424 |
| Mouse | REST (NRSF) | DN cardiac-specific overexpression | Prolonged PQ, AV block, spontaneous VT, sudden death (observed as VT/VF with asystole) | 425 |
| Dog | Unknown | autosomal dominant | Doberman Pinscher, DCM with age, PVCs on Holter monitor | 426 |
| Dog | Unknown | X-linked recessive inheritance | Great Dane; DCM; AF | 427 |
| Hypertrophic cardiomyopathy (HCM) | ||||
| Mouse | TNNT2 (TnT) | I79N cardiac-specific | Elevated diastolic Ca with elevated HR; iso-inducible ectopy; spontaneous NSVT; no hypertrophy or fibrosis | 96 |
| Mouse | TNNT2 (TnT) | +/ΔK210 and ΔK210/ΔK210 | Cardiac enlargement; HF; TdP, VF, sudden death; homs worse than hets but both had phenotype | 428 |
| Mouse | TNNT2 (TnT) | F110I, R278C, or slow skeletal isoform TG | Iso-induced PVCs and VT in mice with F110I or skeletal isoform; VT inducibility with PES in ex vivo hearts; R278C had no arrhythmias compared to control | 97 |
| Rat | TNNT2 (TnT) | Trunc transgenic overexpression | VT, VF | 429 |
| Mouse | TNNI3 (TnI) | G203S cardiac-specific overexpression | PR prolongation, conduction delay; no arrhythmias, but later shown to have AF | 93 |
| Mouse |
TNNI3 (TnI) x MYH6 (α-MHC) |
G203S x R403Q | Bradycardia, slow conduction (PR and QRS), long QTc, catecholamine-induced VT | 94 |
| Mouse | MYH6 (α-MHC) | R403Q/+ | Right axis deviation, prolonged ventricular repolarization and prolonged sinus node recovery times; programmable VT more in males than females | 91 |
| Mouse | MYH6 (α-MHC) | R403Q overexpression | 430 | |
| Mouse | MYPBC3 (MyBP-C) | trunc/trunc | Arrhythmias with PES (in PMID: 11723028) | 431 |
| Mouse | MYPBC3 (MyBP-C) | KO | Prolonged QTc, spontaneous PVCs and VT | 95 |
| Cat | MYPBC3 (MyBP-C) | A31P | HCM | 432 |
| Cat | MYPBC3 (MyBP-C) | R820W | HCM | 433 |
| Mouse | HRAS (H-Ras) | cardiac-specific overexpression | Sinus arrest, idioventricular rhythm, VT, block, and AF; phenotype stronger and more penetrant in females | 434 |
| Mouse | RYR2 (RyR2) | P1124L/P1124L | Mild HCM; bradycardia, iso/caff-induced VT | 435 |
| Mouse | OBSCN (Obscurin) | R4344Q/R4344Q | Tachycardia, spontaneous PVCs and VT; all without structural remodeling | 436 |
| Kangaroo | Unknown | Unknown | Kangaroos have LV hypertrophy, short QT intervals, and are highly susceptible to VF and sudden death, especially under light anesthesia | 375 |
| Atrial Fibrillation (AF) | ||||
| SR Calcium Release | ||||
| Mouse | RYR2 (RyR2) | L433P+/−, N2386I+/−, R2474S+/− | Mutations from CPVT patients. Also develop AF with atrial PES | 347 |
| Mouse | FKBP1B (FKBP12.6) | KO | No ECG abnormalities or spontaneous arrhythmias; AF with PES | 437 |
| Mouse | CASQ2 (Casq2) | KO | AF with PES | 103 |
| Mouse | JPH2 (Junctophilin 2) | E169K/+ (non AF-associated A399S ctrl) | E169K identified in HCM family with AF; before hypertrophy- AF with PES only in E169K mice | 438 |
| Mouse | CREM (CREM) | IbΔC-X | Atrial dilation; 100% of mice developed paroxysmal and persistent AF with age | 104 |
| Mouse | NLRP3 (NLRP3) | A350V/+ | Constitutively active; normal conduction, spontaneous PACs, pacing-induced AF | 439 |
| Mouse | SLN (Sarcolipin) | KO | Cellular APD prolongation; atrial fibrosis; AF with age | 315 |
| Ion Channel | ||||
| Mouse | SCN5A (Nav1.5) | F1759A+/− | Atrial and ventricular enlargement, myofibril disarray, fibrosis and mitochondrial injury, and electrophysiological dysfunction | 440 |
| Mouse | SCN5A (Nav1.5) | ΔKPQ/+ | Atrial enlargement; increased susceptibility to AF with PES | 441 |
| Mouse | KCNE1 (minK) | KO | Spontaneous AF | 105 |
| Mouse | KCNA1 (Kv1.1) | KO | AF with PES | 68 |
| Mouse | KCNJ2 (Kir2.1) | T75R cardiac-specific overexpression | Long QTc; spontaneous VT; iso-induced PVCs, VT, atrial flutter/fibrillation | 61 |
| Mouse | KCNE5 (MiRP4) | KO | Inducible PVCs, atrial arrhythmia, PVT | 442 |
| Mouse | KCNN2 (KCa2.2) | −/− and +/− | Sinus and AV node dysfunction, AF with PES | 443 |
| Structural | ||||
| Mouse | PITX2 (PITX2) | +/− | Normal cardiac parameters except reduced transpulmonary flow (pulmonary valve narrowing), ex vivo hearts were more susceptible to atrial pacing-induced arrhythmia | 110 |
| Mouse | TBX5 (TBX5) | KO, inducible | Spontaneous AF within 2 weeks post-induction, substantial arrhythmogenesis and cardiac remodeling starting after 3 weeks, calcium-handling protein expression changes | 112 |
| Mouse | GJA1 (Cx43) | G60S/+ | Highly susceptible to inducible AF | 113 |
| Mouse | STK11IP (LKB1) | KO | Spontaneous AF, AV block, atrial flutter, electrical and structural remodeling | 107 |
| Mouse | STK11IP (LKB1) | inducible atrial-specific KO | Spontaneous AF | 106 |
| Mouse | CALCR (Calcitonin Receptor) | KO | Atrial fibrosis, inducible AF | 106 |
| Goat | TGFB1 (TGF-β1) | C33S cardiac-specific overexpression | Constitutively active TGF-beta; atrial fibrosis, prolonged P wave, AF inducible with PES, no spontaneous or persistent AF | 108 |
| Mouse | TGFB1 (TGF-β1) | C33S cardiac-specific overexpression | Constitutively active TGF-beta; atrial fibrosis, atrial inducibility with PES | 444 |
| Mouse | MAP2K4 (MKK4) | atrial-specific KO | Regulator of TGF-beta; reduced P wave amplitude, spontaneous atrial tachycardia, polymorphic atrial beats, AF induced ex vivo with PES | 445 |
| Mouse | ACE (ACE) | cardiac-restricted expression | Atrial enlargement, mild fibrosis; low QRS voltage, spontaneous AF, sudden death (escape rhythm preceded death in observed cases) | 446 |
| Mouse | JDP2 (JDP2) | cardiac-specific overexpression | QRS widening, AV block, spontaneous paroxysmal AF; atrial hypertrophy, fibrosis | 447 |
| Dog | Unknown | Unknown | Great Danes with DCM; AF | 427 |
| Sick Sinus Syndrome | ||||
| Mouse | SCN5A (Nav1.5) | +/− | Bradycardia, slowed conduction, exit block | 115 |
| Mouse | SCN3B (Scn β3) | KO | Bradycardia, sinus conduction slowing, exit block, AV block | 448 |
| Mouse | NOTCH1 (Notch Receptor 1) | Inducible intracellular domain (Notch activation) | Bradycardia, sinus pauses, reduced conduction velocity; atrial arrhythmias with PES; Nkx2–5, Tbx2, Tbx5 expression altered | 449 |
| Zebrafish | SMO (Smoothened) | unreported, homozygous | Bradycardia, reduced spontaneous hyperpolarizing current | 450 |
| Mouse | SLC8A1 (NCX) | atrial-specific KO | Bradycardia, no P waves, junctional escape rhythm (His) | 451 |
| Zebrafish | SLC8A1 (NCX) | Truncation | Embryonic lethal; embryos have atrial fibrillation/bradycardia/tachycardia; some VF but mostly silent ventricle; cardiac morphological defects | 452 |
| Mouse | HCN1 (HCN1) | KO | Bradycardia, sinus dysrhythmia, prolonged SA node recovery time, increased SA conduction time, and recurrent sinus pauses | 119 |
| Mouse | HCN2 (HCN2) | KO | Sinus dysrhythmia | 120 |
| Mouse | HCN4 (HCN4) | KO | Global or cardiac HCN4−/− embryonic lethal, but embryos have sinus bradycardia, isolated cells have no spontaneous pacemaker activity | 117 |
| Mouse | HCN4 (HCN4) | R669Q+/− | Homs embryonic lethal, but hets survive and develop SA exit block during exercise | 453 |
| Mouse | HCN4 (HCN4) | 573X inducible cardiac-specific overexpression | Bradycardia, but no dysrhythmia | 454 |
| Mouse | HCN4 (HCN4) | inducible cardiac-specific KO | Bradycardia, reduced iso response, AV block, sudden death | 118 |
| Mouse | HCN4 (HCN4) | inducible HCN4+ cell ablation | Nodal tissue fibrosis, bradycardia, exit block, SVT, VT, complete block, sudden death | 122 |
| Atrioventricular block (AV block) | ||||
| Mouse | NKX2–5 (NKX2.5) | I183P cardiac-specific overexpression | PR prolongation, worsening AV block with age | 124 |
| Mouse | NKX2–5 (NKX2.5) | R52G+/− | PR prolongation, AV node smaller, AV block | 123 |
| Mouse | DMPK (DMPK) | −/− and +/− | PR prolongation as mice age, +/− mice develop 1st degree block, −/− mice develop 3rd degree block | 455 |
| Mouse | GJA5 (Cx40) | −/− | Conduction delay (first degree block) | 456 |
| Mouse | TRPM4 (TRPM4) | KO | AV block (prolonged PR and QRS widening), Wenckebach | 457 |
| Preexcitation syndrome | ||||
| Mouse | PRKAG2 (AMPK γ2) | R302Q cardiac-specific overexpression | Hypertrophy, preexcitation, accessory pathway, QRS widening, inducible reentrant arrhythmia | 127 |
| Mouse | PRKAG2 (AMPK γ2) | N488I overexpression | Hypertrophy, sinus bradycardia, accessory pathway, preexcitation | 125 |
| Mouse | PRKAG2 (AMPK γ2) | R531G cardiac-specific overexpression | Hypertrophy, impaired contractile function, electrical conduction abnormalities | 126 |
| Mouse | TBX2 (TBX2) | cardiac-specific KO | Accessory pathway, preexcitation | 128 |
| Other Cardiac Conduction Disorders | ||||
| Mouse | GJA1 (Cx43) | D378stop cardiac-specific inducible | Conducting truncation; germline deletion die right after birth; inducible model die 16 days after tamoxifen; 2–3-fold QRS widening, BBB, spontaneous MVT/PVT/VF, sudden cardiac death | 458 |
| Mouse | MAP2K4 (MKK4) | cardiac-specific KO | Reduced Cx43 expression; ~55% QRS widening, long QTc, VT with PES | 459 |
| Zebrafish | KCNJ3 (Kir3.1) | N83H cardiac-specific overexpression | Atrial dilation; sinus arrest, sinus bradycardia, SA block, and AV block; patient mutations associated with AF | 460 |
| Mouse | CACNA1D/G (Cav1.3, Cav3.1) | CACNA1D KO or CACNA1D/CACNA1G DKO | Sinus bradycardia, slow conduction; DKO also had 3rd degree block, escape rhythms, spontaneous VT | 461 |
| Mouse | KCNN3 (KCa2.3) | WT overexpression | Bradyarrhythmias, AV block, abnormal AV node, sudden death | 114 |
| Mouse | IRX3 (IRX-1) | KO | His-Purkinje transcription factor; normal PR, wide QRS, notched R wave, block; spontaneous PVCs and VT; iso- and exercise-induced VT | 462 |
| Developmental | ||||
| Mouse | TBX3 (TBX3) | KO | Ectopic atrial pacemakers | 463 |
| Mouse | TBX5 (TBX5) | +/− | Hypoplasia, arrhythmias, see above in “atrial fibrillation” | 464 |
| Mouse | MECP2 (MeCp2) | KO | X-linked; long QTc, QRS widening, pacing-inducible VT, asystole/sudden death; neuronal KO was similar | 465 |
II.1. Channelopathies
Channelopathies are caused by mutations in ion channel genes or genes that regulate ion channels and generate arrhythmia risk in the structurally normal heart. However, overlap syndromes caused by mutations in channelopathy genes (e.g., SCN5A) can also be associated with alterations in cardiac structure, which are discussed in Section II.2, genetic arrhythmia syndromes associated with structural heart disease.
II.1.1. Catecholaminergic polymorphic ventricular tachycardia (CPVT)
CPVT is characterized by arrhythmogenesis evoked by elevated catecholamines during stress or exercise. CPVT is caused by gain-of-function mutations in proteins that constitute the intracellular Ca2+ release unit of the SR. These mutations result in spontaneous Ca2+ release from RYR2 SR Ca2+ release channels, raising diastolic Ca2+ and generating membrane depolarizations and delayed afterdepolarizations via Ca2+ extrusion through the electrogenic Na+- Ca2+ exchanger. Pathological mutations in RYR2 make up more than half of identified CPVT cases and are inherited in an autosomal dominant fashion. Mutations in regulatory partners of RyR2 are rarer and include calsequestrin, calmodulin, and triadin. Evidence suggests that KCNJ2 mutations may also be involved in CPVT.
Mice have been the primary animal model for studying CPVT and have been valuable not only to establish arrhythmia pathophysiology but also to identify new drug therapy that proved efficacious in humans.29 At least one attempt to generate a rabbit model overexpressing human RYR2-R4497C was unsuccessful, likely due to the selected promotor and size of the transgene.30 The first animal model of CPVT generated was an RYR2-R4496C+/− knock-in mouse31, which had exercise- and catecholamine-inducible ventricular arrhythmias. Since then, several mouse models have been designed that carry RYR2 mutations identified from patients with CPVT (see Table 1). When loss-of-function mutations are combined with gain-of-function mutations, mice are protected from CPVT.32 Identifying the pathogenicity of specific mutations is important because RYR2 mutations are implicated in several other arrhythmia disorders, as detailed below. Interestingly, a RYR2-exon3 deletion was identified in patients with a severe form of CPVT, but the corresponding mouse model failed to reproduce the CPVT phenotype.33
Cardiac calsequestrin (CASQ2) mutations are inherited in an autosomal recessive manner, leading to loss of function. Casq2 serves as a high-capacity Ca2+ buffer in the sarcoplasmic reticulum and regulates RYR2 gating. In the mouse, CASQ2 deletion causes a severe exercise- and/or catecholamine-induced arrhythmia phenotype consistent with patients lacking Casq2 expression. Observations from CASQ2 mouse models carrying mutations identified from patients show these commonly lead to loss of Casq2 expression in the heart. Recent evidence, however, suggests that Casq2 mutations could also be inherited in an autosomal dominant manner.34 A knock-in mouse model expressing Casq2-K180R+/− reported catecholamine-induced arrhythmias validating this inheritance pattern.35
Triadin is another protein in the SR Ca2+ release unit where autosomal recessive inheritance has been reported in patients with CPVT. When triadin was knocked out in mice, they developed substantial ultrastructural changes in the junctional SR and reduced expression of proteins comprising the Ca2+ release unit, leading to catecholamine-inducible arrhythmias.36 Calmodulin genes (CALM1/2/3) encode three identical proteins (CaM) and some mutations are associated with CPVT.37 Zebrafish models have been generated to examine the pathogenesis of overexpressing CALM mutations, and they have successfully demonstrated cardiac arrhythmias (Table 1). Finally, KCNJ2 loss of function mutations, which commonly have been linked to the Anderson-Tawil Syndrome and cause reduced IK1 current (see section II.1.3), have also been identified in patients with CPVT. A knock-in mouse carrying KCNJ2- R67Q+/− had significant evoked arrhythmias without any QT prolongation (Table 1).
The CASQ2 knockout (KO) mouse is an excellent model to investigate CPVT. It has a severe and highly penetrant phenotype, something not always observed with RYR2 mouse models of CPVT (Table 1). The age of onset for CPVT is only a few weeks old and mice have spontaneous arrhythmias under normal housing conditions.38 CASQ2 KO mice were used to establish proof of principle for the efficacy of gene-therapy in CPVT.39 In what may be the only example of its kind for an arrhythmia syndrome, the CASQ2 KO mouse was used to establish the therapeutic efficacy of an existing FDA-approved drug, flecainide, in CPVT.29 CASQ2 KO mice were instrumental to demonstrate that in vivo, RYR2 block is the principal mechanism of flecainide’s antiarrhythmic action.40 Since its discovery in CASQ2 KO mice, flecainide has become the standard of care for preventing arrhythmias in CPVT patients when beta-blockers are insufficient. 41, 42
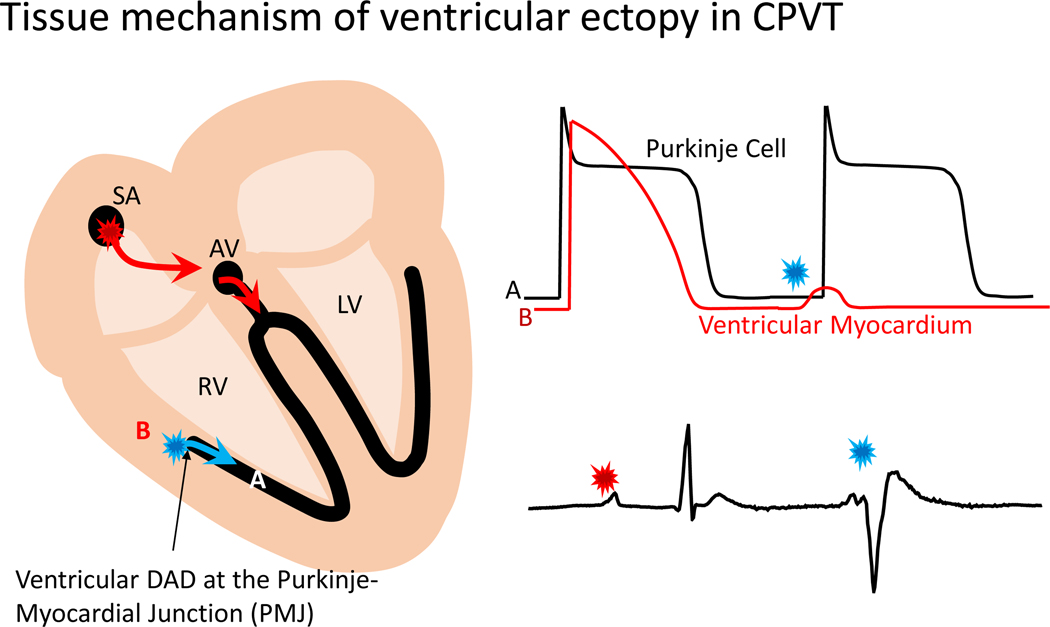
CASQ2 KO mice have also recently been used to establish a novel tissue mechanism responsible for ventricular ectopy in CPVT (Figure 3). To determine the cellular origin of ventricular arrhythmias in CPVT, Blackwell et al. used conditional murine models with Casq2 expression only in ventricular myocardium or in the specialized conduction system, utilizing the contactin-2 promoter to drive Cre expression and control tissue-specific Casq2 expression.43 CPVT occurred when Casq2 was deleted in the ventricular myocardium, but still expressed in the conduction system. Moreover, catecholamine challenge did not elicit any arrhythmias when Casq2 was deleted in the conduction system but still expressed in the ventricular myocardium. Additional experiments determined that the subendocardial ventricular myocardium juxtaposed to Purkinje fibers is the only cellular source for focal ventricular arrhythmias in CPVT. To understand why that was the case, in silico modeling demonstrated an intriguing phenomenon whereby subthreshold DADs in ventricular myocardium elicit full-blown APs in the conduction system to generate arrhythmias, identifying the Purkinje-myocardial junction as the tissue origin of ventricular ectopy in CPVT (Figure 3). This discovery could shape treatment and may be critical to our understanding of arrhythmogenesis in other ventricular arrhythmia syndromes where DADs are the cellular arrhythmia mechanism.
Figure 3: Tissue-targeted CASQ2 knock-out mice help decipher the anatomical origin of ventricular ectopy in CPVT.
Based on a combination of tissue-targeting and in silico modeling, the PMJ was identified as the likely origin of ventricular ectopy in CPVT. DAD – delayed afterdepolarization. (Illustration credit: Ben Smith)
II.1.2. Calcium Release Deficiency Syndrome (CRDS)
Whereas RYR2 gain-of-function mutations have been associated with CPVT, arrhythmogenic cardiomyopathies, and AF, loss-of-function mutations in RYR2 can cause an arrhythmia syndrome recently termed Ca2+ release deficiency syndrome (CRDS).44 In CRDS patients, exercise stress tests do not provoke arrhythmias. Consequently, this syndrome can escape clinical diagnosis and often presents as sudden cardiac death. In CRDS, arrhythmias are thought to develop due to electrophysiological remodeling. Given the substantial number of benign RYR2 mutations observed, predicting pathogenicity without in vitro or in vivo examination is challenging. CRDS has only been recently described, but one knock-in mouse model was generated carrying the patient-specific RYR2-D4646A+/− mutant allele.44 Exercise and catecholamine challenge did not invoke arrhythmias, as predicted. Importantly, this animal model enabled the authors to establish a burst pacing protocol that induced arrhythmias, supporting a possible new clinical diagnostic tool, which was recently confirmed in a clinical study. A previously reported loss-of-function mutation, RYR2-A4860G+/−, was identified in a patient with idiopathic VF and subsequently knocked-in to a mouse, but arrhythmias were not reported in vivo.45 Ex vivo hearts did develop VF in response to isoproterenol but had no arrhythmias in the presence of the RyR2 agonist caffeine. These data hint that this RYR2 loss-of-function mutation may be part of the calcium release deficiency syndrome. Prior work on RYR2 has focused on gain-of-function mutations. CRDS is a relatively new syndrome and animal models provide an opportunity to advance our understanding of CRDS pathophysiology and determine the impact of loss-of-function mutations.
II.1.3. Long QT syndrome (LQTS)
Congenital long QT syndrome (LQTS) often presents as a multi-organ syndrome caused by mutations in proteins responsible for the repolarization of the heart and can cause seizures, syncope, arrhythmia, and sudden death. Alterations in repolarization manifest as prolongation of the AP and, consequently, the QT interval, predisposing the heart to EAD, DAD, and re-entrant circuits. Ca2+, Na+, and many K+ channels play a role in cardiac repolarization; accordingly, the genes involved in this syndrome vary and LQTS can be inherited in autosomal dominant or recessive forms. LQTS is traditionally described as a distinct disease manifestation, however, long QT intervals are observed in many overlap syndromes (see Table 1). Approximately 80% of LQTS cases are caused by mutations in KCNQ1 or KCNH2, with SCN5A constituting 7–10% of cases.46 It should be reiterated that the currents responsible for mouse and human repolarization are quite different (Figure 2) and mice are inadequate to model human disease involving mutations in delayed rectifier K+ channels (e.g. LQT1, LQT2).
An aspect of understanding LQT pathogenesis is the observed sex differences in patients, such as QT interval, the onset of cardiac events, and sudden death.47, 48 Animal models allow for significant insight into these investigations since most in vitro models fail to capture the hormonal factors that influence development and regulation. It is noteworthy that sexual dimorphism in QT is not captured by mouse models and may require other animal models such as rabbits.49, 50 Estradiol treatment in ovariectomized rabbits led to prolongation of the QT interval and changes in proteins responsible for repolarization, whereas dihydrotestosterone did not.51. Another study in rabbits recapitulated findings in patients showing that QT interval was more prolonged in females following treatment with erythromycin.52.
LQT1 is caused by loss-of-function mutations in KCNQ1 (Kv7.1). Knockout of KCNQ1 in mice leads to characteristics of Jervell and Lange-Nielsen syndrome, causing deafness and prolonged QT interval.53 Other models have examined distinct mutations with varying phenotypes, however caution should be used when reviewing the mouse LQT1 literature. Rabbits are a much better model for examining human-like mechanisms of altered repolarization. However, only one transgenic rabbit model is reported, which carries cardiac-specific overexpression of KCNQ1-Y315S, leading to LQT and inducible EADs and VT with sympathetic stimulation.54
LQT2 is caused by loss-of-function mutations in KCNH2 (Kᵥ11.1). In contrast to humans, KCNH2 contributes little to repolarization in mice (Figure 2). As such, mouse models are not realistic for modeling LQT2 and should not be used. A rabbit transgenic model was generated with cardiac-specific overexpression of KCNH2-G628S and developed LQT, spontaneous PVT, and sudden death. Arguably, the most interesting LQT2 model is the work in zebrafish.55 In one study, the endogenous ortholog of hERG (zERG) was knocked down using morpholinos and then various hERG mutants were expressed in its place. The phenotype data (prolonged APD and/or 2:1 AV block) correctly identified 39/39 pathogenic mutants and 9/10 non-pathogenic polymorphisms. Further work using this model could establish the pathogenicity of other variants of uncertain significance.
LQT3 is caused by gain-of-function mutations in SCN5A (Nav1.5). Several mouse models have been generated that recapitulate the LQT phenotype. Both the SCN5A-ΔKPQ/+ and SCN5A-ΔQKP/+ mouse models report many characteristic phenotypes such as prolonged QT interval, a more pronounced T wave, prolonged APD, arrhythmias, and sudden death, which make them suitable for examining LQT3 mechanisms and pathogenesis.56, 57 A successful bench-to-bedside study, conducted in ventricular myocytes isolated from guinea pigs, demonstrated that mexiletine restored the APD in cells treated with the Na+ channel inactivation inhibitor, anthopleurin.58 Mexiletine is now used routinely in the treatment of patients with LQT3. In rare cases, patients have been identified linking LQTS with mutations in the beta accessory proteins for SCN5A. An SCN1B knockout mouse was generated and had long QT, bradycardia, and delayed repolarization59; interestingly, it was found that these mice had increased Na+ channel expression. Nav1.5 channel gating was unaffected, but peak and persistent currents were increased in isolated cardiomyocytes.
Andersen-Tawil syndrome is a rare LQTS associated with physical abnormalities and hypokalemic periodic paralysis and is primarily caused by loss of function mutations in KCNJ2 (Kir2.1), resulting in reduced IK1 current. Neonatal (1 day old) KCNJ2 knockout mice were characterized by long QT and bradycardia, before dying from complete cleft palate and inability to feed.60 A more useful arrhythmia phenotype – LQT and spontaneous VT – was observed in the KCNJ2-T75R cardiac-specific overexpression mouse model.61
The remaining LQTS types result from mutations in K+ channels, Ca2+ channels, and key regulators of ion channel function (Table 1). The ankyrin-B syndrome is characterized as an overlap syndrome, with long QT, sinus node dysfunction, conduction abnormalities, exercise-induced arrhythmia, VF, and VT. The ankyrin-B knockout mouse demonstrated long QT and sinus node dysfunction62; however, a more severe and faithful phenotype was reported in heterozygous mice, capturing many of the same observations from humans.63 KCNE1 knockout mice developed long QT, but only when heart rate deceleration occurred.64 A transgenic rabbit model overexpressing dominant negative KCNE1-G52R developed long QT and increased susceptibility to drug-induced arrhythmia by accelerating IKs and IKr deactivation kinetics65. This model could be useful for examining the proarrhythmic liability of drugs. CACNA1C (Cav1.2) gain-of-function mutations cause LQTS that can be associated with extracardiac manifestations known as Timothy syndrome. In mice, CACNA1C knockout is embryonic lethal, but heterozygous mice survived without any cardiac phenotypes. Cardiac-specific overexpression of the G406R mutation in mice led to long QTc and exercise-induced PVCs and Torsades de Pointes (TdP).66 It was hypothesized that this mutant had altered interaction with AKAP150; in fact, when G406R-overexpressing mice were crossed with AKAP150 KO mice, they were protected against all phenotypes of the G406R mutant.
Investigators have also knocked out many of the potassium channel genes in mice to determine their effects on cardiac electrophysiology. When a dominant-negative fragment of KCNA1 was expressed, mice developed LQT and spontaneous ventricular arrhythmias.67 Interestingly, it was later shown by Glasscock et al. that KCNA1 is preferentially expressed (~10-fold higher) in atria over ventricles.68 Programmed electrical stimulation (PES) induced AF in KCNA1 knockout mice but did not lead to any ventricular arrhythmias and no differences in QT interval were observed. Dominant negative overexpression of KCNA5, KCNB1, or KCND2 all prolonged the QT interval without any other overt electrophysiological changes. Mice do not express KCNE3 in the adult heart, but deletion led to long QTc in aged female mice due to hyperaldosteronism.69
Mouse models of LQTS should be viewed with caution when the repolarizing current of interest does not reflect the human AP. As transgenic rabbit models become more common, they may find strong ground in advancing our understanding LQT pathogenesis. Rabbits accurately capture sex differences and express a similar repolarization ion channel gene profile as humans. An area of value will be evaluating predisposition to drug induced LQT and arrhythmia,70 which rabbit models will be best suited to answer.
II.1.4. Short QT syndrome (SQTS)
Short QT syndrome (SQTS) is an extremely rare disorder; only a few hundred cases have been identified to date. SQTS is caused by the shortening of the cardiac AP. Like long QT syndrome, alterations in cardiac repolarization alter the QT interval. Due to the abbreviated QT interval, the refractory period is also shortened, leaving the heart susceptible to reentrant arrhythmias. Symptoms associated with short QT syndrome include both atrial and ventricular fibrillation, palpitations, and sudden cardiac death. Gain-of-function mutations in KCNH2, KCNQ1, and KCNJ2 or loss-of-function mutations in CACNA1C, CACNB2, CACNA2D1, SCN5A, and SLC4A3 have all been associated with SQTS.
There are few genetic animal models available to examine SQTS in vivo. Zebrafish carrying the KCNH2-L499P mutation have a shortened QT interval.71 Knockdown of SLC4A3 in zebrafish led to a short QT interval that was rescued by expressing WT SLC4A3, but not by SLC4A3-R370H, identified from a patient with SQTS.72 To appreciably capture the human cardiac AP, a rabbit model was engineered to overexpress KCNH2 carrying the N588K mutation.73 Transgenic rabbits had shortened AP and QTc, but a normal T wave height. Ex vivo perfused hearts had inducible VT and VF; this model is arguably the best available and, as discussed above, rabbit models more accurately typify human cardiac repolarization.
II.1.5. Brugada Syndrome
Brugada syndrome (BrS) is a disorder characterized by elevated ST segment, partial bundle branch block, arrhythmia, and sudden cardiac death. It is commonly caused by mutations in SCN5A, however, approximately twenty genes are now associated with BrS. Brugada ECG patterns are sometimes observed in overlap syndromes, such as with long QT syndrome, as SCN5A mutations are associated with several arrhythmia disorders. In mice, SCN5A deletion is embryonic lethal, but heterozygous mice survived and developed slowed conduction, pacing-induced VT, and fibrosis with age.74 Interestingly, the authors observed variability in phenotype penetrance that correlated with NaV1.5 expression levels.75 A mouse model for an overlap syndrome of LQT and Brugada was generated to carry 1795insD in SCN5A.76 Homozygous mice were embryonic lethal, but heterozygous mice developed sinus node dysfunction, slowed conduction, bradycardia, and QT prolongation. Interestingly, CaMKII-dependent phosphorylation of wild type Nav1.5 appears to phenocopy this mouse model, as late current predominates at slower heart rates. Phosphomimetic and phosphoablation mouse models demonstrated that phosphorylation and oxidation modulate Nav1.5 current and susceptibility to arrhythmias.77, 78 A transgenic pig model was generated to better understand BrS disease mechanisms. Pigs were designed to carry the orthologous SCN5A-E558X/+ mutation identified in a patient diagnosed with BrS and developed conduction abnormalities and QRS widening, but had no elevated ST segment, arrhythmias, or sudden death through two years of age.79 However, ex vivo hearts had increased susceptibility to VF with programmed stimulation. One debate surrounding BrS is whether many of the associated genes cause BrS or simply increase susceptibility to developing BrS. SCN5A mutations have variable and incomplete penetrance and differing phenotypes. Moreover, SCN5A mutations are prevalent in the general population and discerning pathogenesis can be difficult. Given the failure of animal BrS models to reproduce the full clinical syndrome, their utility studying BrS pathogenesis and treatment options remains to be determined.
II.2. Genetic arrhythmia syndromes associated with structural heart disease
Animal models have been beneficial for understanding the pathogenesis of arrhythmias caused by mutations in non-ion channel genes that result in structural heart disease. The section below discusses the major arrhythmia syndromes associated with a cardiomyopathy phenotype (ARVC, HCM, DCM) and microscopic structural disease such as AF, sick sinus syndrome, heart block and pre-excitation syndromes.
II.2.1. Arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D)
Arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) is a disease manifesting as fibro-fatty replacement of the right ventricular myocardium and widespread electrophysiological remodeling, predisposing individuals to ventricular arrhythmias and increased risk of sudden death. Approximately half of ARVC cases are caused by mutations in desmosomal proteins, which make up cell-cell mechanical junctions. Arrhythmias often arise during periods of exercise or stress, suggesting that catecholamines contribute to arrhythmogenesis. Accordingly, one of the primary phenotypes in animal models is catecholamine-induced ventricular arrhythmias.
The primary genetic model used to study ARVC has been the mouse. Of note, mice do not get fatty infiltration in the heart, one of the primary phenotypes of ARVC in humans. However, numerous cardiac phenotypes have been found, from remodeling to arrhythmogenesis. The first genetic mouse models to examine the importance of desmosomal proteins were congenital knockouts (Table 2). Mouse models with global knockout of plakoglobin, desmoplakin, plakophilin-2, and desmoglein-2 are all embryonic lethal. However, heterozygotes survived and developed varying degrees of fibrosis and arrhythmia phenotypes (Table 2). Subsequent animal models were based on patient mutations and frequently resulted in haploinsufficiency. This seems to be the primary cause, along with repression of the Wnt signaling pathway. Desmocollin-2 (DSC2) mutations are rare and the link between pathogenesis and this protein is not well understood because data are lacking and conflicting. DSC2 knockout mice are viable but did not develop any cardiac phenotypes. However, DSC2 knockdown reportedly led to altered ultrastructure and contractile dysfunction, while overexpression also led to fibrotic remodeling.80 Germline knockout of desmoglein-2 (DSG2) results in embryonic lethality in mice, but the cardiac-specific knockout led to dilation, fibrosis, and electrophysiological remodeling. Finally, the inhibitor of apoptosis-stimulating protein of p53 (iASPP) has been linked to ARVC; knockout mouse model developed dilation, arrhythmia, and sudden death.81
The plakophilin-2 (PKP2) mouse model is well-suited for understanding ARVC mechanisms and disease progression. Heterozygote PKP2 mice survived and developed arrhythmias in the absence of overt structural remodeling82, which was validated in mice carrying a PKP2 truncation mutant.83 These findings raised an interesting question regarding the cause of arrhythmias: how do arrhythmias arise in the absence of structural changes to the heart? Because PKP2 knockout is embryonic lethal, a cardiac-specific inducible PKP2 knockout mouse was generated, which had many of the characteristic ARVC phenotypes: fibrosis, remodeling, reduced ejection fraction, arrhythmia, and sudden death.84 In this model, PKP2 signaling regulates transcription of many genes involved in Ca2+ homeostasis and proteins of the intracellular Ca2+ release unit are downregulated, causing pro-arrhythmic RyR2 activity. The authors discovered that flecainide, a class Ic antiarrhythmic that inhibits RyR2 channels, effectively prevented arrhythmias in these mice. Exercise exacerbated RyR2 hyperactivity, and it was shown that membrane-permeable beta-blockers had greater efficacy than non-permeable beta-blockers.85
ARVC has been observed in dogs and cats, generally of unknown cause. In these models, fibro-fatty replacement is commonly observed alongside syncope, arrhythmias, and sudden death. Dog models could be considered when disease mechanisms, as they relate to human cardiac (electro)physiology, are essential. Non-desmosomal proteins, such as phospholamban, RyR2, lamin A/C, transmembrane protein 43, and integrin linked kinase have all been associated with ARVC in patients, however, their role is less well understood due to the spectrum of phenotypes in ARVC. For example, some mutations in phospholamban cause DCM, which is a differential diagnosis, but may also present with ARVC. Due to the difficulty of identifying pathogenic mutations, diagnostic criteria rely on interpreting cardiac imaging and electrocardiogram data. An important line of investigation is understanding the molecular mechanisms that drive the development of the disease, as early disease progression escapes detection and the heterogeneity of genes involved makes prognosticating a diagnosis difficult.
II.2.2. Dilated cardiomyopathy (DCM)
Congenital DCM is characterized by ventricular dilation and is commonly caused by mutations in cytoskeletal or myofibrillar proteins leading to remodeling, reduced ejection fraction, conduction abnormalities, arrhythmia, and sudden death. Many animal models have been created to investigate the structural and functional consequences of these mutations. Here we focus on animal models where arrhythmias are a prominent feature of the reported phenotype (Table 2). Of note, there is significant overlap with the clinical diagnosis of arrhythmogenic (right ventricular) cardiomyopathy.
A widely studied DCM mutant is SCN5A-D1275N. A wide spectrum of phenotypes in different families carrying this mutation have been reported, including AF, conduction defects, and sinus dysrhythmia.86, 87 It is speculated that the variation in genetic background between the families contributes to these phenotypes. Homozygous SCN5A-D1275N mice developed many of the characteristic arrhythmia phenotypes observed in humans, while heterozygous mice did not.88 Many changes in the ECG parameters reflected a gene dose-dependent effect and these features were also observed in zebrafish.89
Mutations in LMNA cause severe DCM and sudden death. Several mouse models have been generated that carry mutations identified from patients (Table 2). A variety of symptoms have been reported and spontaneous arrhythmias occur frequently. Mutations in RMB20 also have a severe phenotype in mice, commonly leading to spontaneous ventricular arrhythmias and sudden death. Duchenne muscular dystrophy (DMD) has been studied in pig, dog, and mouse models, each having electrophysiological changes (Table 2).
II.2.3. Hypertrophic Cardiomyopathy (HCM)
Hypertrophic cardiomyopathy (HCM) is characterized by enlargement of the left ventricle primarily caused by mutations in sarcomeric proteins, leading to increased risk of arrhythmia and sudden death.90 HCM is commonly caused by mutations in either the beta myosin heavy chain or myosin binding protein C, together accounting for nearly half of all cases. Mice express beta myosin heavy chain during cardiogenesis, but rapidly switch from the beta to the alpha isoform, postnatal. Numerous HCM animal models have been generated, however, the predominant focus has been the study of underlying changes in sarcomere function, contractility, and hypertrophy, without detailed examination of electrophysiological phenotypes. Many studies have reported on arrhythmia susceptibility in ex vivo hearts, but only reports examining in vivo arrhythmia phenotypes are discussed here.
Alpha myosin heavy chain mutations are commonly associated with HCM, but the only reports of arrhythmias in an animal model come from the R403Q mutant, which was identified in MYH7 from a human patient. The knock-in mouse model was designed to carry R403Q in MYH6.91 Mice developed HCM but only had a modest arrhythmia phenotype. A rabbit model was generated to overexpress the transgene, but did not exhibit an arrhythmia phenotype.92 Troponin mutations are also associated with HCM and a transgenic mouse was generated to carry TNNI3-G203S.93 Mice had conduction defects, but no other arrhythmias. However, when these mice were crossed with MYH6-R403Q mice, they developed a more severe phenotype with conduction defects, LQT, and catecholamine-induced VT.94 Knockout of MYBPC caused long QT and spontaneous VT in mice.95
Several troponin T models have been designed to carry mutations identified in patients. Knollmann et al. showed that TNNT2-I79N mice had tachycardia, isoproterenol-inducible ectopy, and spontaneous non-sustained VT, despite having no hypertrophy or fibrosis.96 Subsequent work in mice demonstrated that Ca2+-sensitizing TNNT2 mutations cause inducible arrhythmias, whereas non-sensitizing mutants (R278C) do not leave the heart susceptible to arrhythmias.97 Myofilament Ca2+ sensitization increases cytosolic Ca2+ binding affinity, alters intracellular Ca2+ homeostasis, and causes pause-dependent Ca2+-triggered arrhythmia.98 In addition, myofilament Ca2+ sensitization causes focal energy deprivation, which further increases arrhythmia susceptibility in mice.99 Hence, myofilament sensitization per se, caused by drugs, mutations, or post-translational modifications after myocardial infarction100, is a novel arrhythmia mechanism.101 These reports illustrate the power of murine HCM models for discovering new arrhythmia mechanisms and identifying therapeutic targets.
II.2.4. Atrial Fibrillation
AF is the most common arrhythmia and is characterized by rapid, abnormal atrial rhythms, with symptoms manifesting as palpitations, syncope, stroke, and heart failure, among others. The etiology of AF is multifactorial, stemming from environmental factors, diet, lifestyle, family history, medication, and surgery. For an in-depth review of acquired AF and various animal models available, the reader is referred to this review.102 Many genetic animal models develop AF alongside their primary disease phenotype (see Tables 1 and 2), but the models discussed in this section are more directly related to AF as a primary pathology.
One trigger for AF is thought to be hypersensitive and leaky RyR2 channels. Thus, animal models of CPVT (both gain-of-function RyR2 and loss-of-function Casq2) have been used to investigate arrhythmogenic mechanisms and screen therapeutic modalities.103 It was demonstrated that RyR2 inhibition can attenuate AF in these models. Other CPVT models are susceptible to AF with PES. A more severe phenotype was seen with CREM mice, which developed atrial dilation and spontaneous paroxysmal and persistent AF that worsened with age.104. A second trigger for AF may be channelopathies that accentuate excitability. KCNE1, SCN5A, KCNQ1, SK2, and SK3 mutations have all been introduced into mice, with varying phenotypes (Table 2). The KCNE1 knockout mouse develops spontaneous AF.105 SCN5A models show overt structural changes, fibrosis, conduction abnormalities, and mitochondrial injury. Proteins associated with development, signaling pathways, and transcription regulation have been found to induce AF. Atrial-specific or complete knockout of LKB1 in mice caused electrical and structural remodeling and mice developed spontaneous AF.106, 107. A goat model overexpressing constitutively active TGF-β1 had atrial fibrosis and AF inducibility with PES.108.
Genome-wide association studies (GWAS) have identified many gene loci associated with increased AF risk.109 AF loci include genes known to affect ion channel function, cardiogenesis, or cell-cell conduction, although in many cases, candidate genes have not been determined. There are several animal models of inherited AF, mostly in mice. A primary challenge with mouse models of AF is that they do not commonly develop spontaneous AF, instead only uncovering the phenotype with PES. Moreover, AF typically lasts for a brief period (on the order of seconds) before resolving. However, some mouse models have more severe and protracted AF that occurs spontaneously (Table 2). AF manifests in a wide range of cardiac diseases and resolving causative vs correlative pathogenesis is ongoing for some genetic models. Despite these shortcomings, investigators have successfully captured AF phenotypes in many different mouse models for genes identified in GWAS studies or laboratory testing.
GWAS studies have identified loss of function (PITX2, TBX5, GJA1) and gain of function (KCNN3) variants associated with patients with AF. PITX2 heterozygous mice had normal cardiac parameters except reduced transpulmonary flow, however, ex vivo hearts were more susceptible to atrial pacing-induced arrhythmia.110 Subsequent work showed AF in vivo with PES and several groups have used this model to study AF and explore treatment111, especially since PITX2 is the most common risk locus identified in patients with AF. Inducible deletion of the TBX5 transcription factor led to a much more severe phenotype of spontaneous AF and electrophysiological remodeling within 2 weeks of tamoxifen treatment.112 Gene profiling identified numerous changes in the expression of Ca2+ handling proteins and ion channels, including PITX2. These data provide a link between TBX5-PITX2 activity and electrophysiological protein regulation in the heart. AF may also be promoted by delayed conduction. Mice carrying the GJA1-G60S/+ mutation are more susceptible to pacing-induced AF.113 Mice overexpressing KCNN3 had bradyarrhythmias, heart block, abnormal AV node morphology, and sudden death.114
Many other models have explored mutations or deletion of other ion channels, transcription factors, developmental pathways, or hormones to examine AF (Table 2). Data from patients have frequently confirmed the downregulation of various proteins, providing yet another link to pathogenesis. AF is a complex multifactorial disease; each animal model is an opportunity to extend our understanding of this disease.
II.2.5. Sick Sinus Syndrome
Sick sinus syndrome occurs when the SA node is not capable of generating a normal rhythm. In heterozygous SCN5A+/− mice (see also Brugada Syndrome), investigators identified sick sinus syndrome phenotypes115 and sex differences in SA node function with age.116 The hyperpolarization-activated cyclic nucleotide-gated channels (HCN) play an essential role in generating spontaneous pacemaker activity. Various models have examined the deletion of HCN1–4. HCN4 is the most abundant isoform, and congenital deletion in mice is embryonic lethal. However, embryonic hearts had sinus bradycardia.117 Inducible cardiac-specific knockout led to severe bradycardia, block, and sudden death within days.118 Mice with germline deletion of HCN1 or HCN2 survived and developed sinus dysrhythmia.119, 120 Deletion of HCN3 did not lead to bradycardia or arrhythmia, but mice had subtle perturbations in their ECG morphology at lower heart rates.121 Perhaps the most elegant and accurate mouse model of sick sinus syndrome is an inducible HCN4-specific cellular ablation mouse.122 This model accurately captured many of the phenotypes observed in patients, such as fibrosis of nodal tissue, SA dysrhythmia, SVT, VT, and sudden death.
II.2.6. Atrioventricular Block
AV block is partial or complete disruption of impulse propagation from the atria to the ventricles. As the single site for collating atrial depolarization and passing it to the ventricle, aberrant AV node function may prevent or delay sinus rhythm from conducting into the ventricle. The AV node makes up part of the conduction system and, as such, expresses many of the same ion channels and gap junctions as the SA node, bundle of His, and Purkinje cells. AV block is observed in many of the models described above. However, some genes appear to specifically alter AV node conduction and effect block without altering sinus node rhythm. The NKX2–5 gene appears to be definitely related to AV block in patients, and mouse models carrying two different mutations independently validated the role of this gene in disease progression.123, 124
II.2.7. Preexcitation Syndrome
Preexcitation occurs when the ventricles are activated prematurely via an accessory pathway. The most common mouse models generated to study preexcitation syndrome have mutations in PRKAG2, a regulatory subunit of 5′ AMP-activated protein kinase.125–127 Mutations in the TBX2 transcription factor also led to the development of an accessory pathway in mice.128
III. ANIMAL MODELS OF ACQUIRED ARRHYTHMIA DISORDERS
Acquired heart disease is the most common etiology of increased arrhythmia risk in humans. Acquired heart disease develops over the course of a person’s life due to structural remodeling associated with hypertension129, coronary artery disease41, non-ischemic cardiomyopathy130, primary and secondary valvular disease131, autoimmune rheumatic diseases130 and myocarditis.132 Acute cardiac stress or injury leads to activation of specific cell signaling pathways 133, 134, mitochondrial dysfunction135, 136, altered Ca2+ handling137–139 and a switch in metabolism140 from fatty acid oxidation to glycolysis.141 This leads to chronic changes in gene expression of trophic and mitotic factors, inflammation, and ultimately to myocyte hypertrophy and fibrosis. Pathological fibrosis is characterized by excessive proliferation of cardiac fibroblasts and extracellular matrix (ECM) protein deposition. Several key pro-fibrotic factors have been identified, including transforming growth factor (TGF)-β, angiotensin II and aldosterone, which contribute to the development of cardiac fibrosis regardless of the underlying pathology.142 However, the relative contribution of a distinct molecular pathway depends on the type and the degree of the initial cardiac injury. Various animal models have been developed to study aspects of these acute and chronic changes leading to arrhythmia risk and are discussed below.
III.1. Transverse aortic constriction
The transverse aortic constriction (TAC) model mimics chronic hypertension or aortic stenosis by causing a stricture in the thoracic aorta.143 Left ventricular and atrial pressure overload increases the wall tension leading to hypertrophy, chamber dilation and fibrosis.144, 145 This can be done in various methods, including sutures143, 146, inflatable cuffs147 or intravascular stents.148 Within hours of injury, myocyte hypertrophy is induced by activation of p38 MAP Kinase149, ERK1/2150, and PI3K/AKT signaling151. Increased TGF-β production due to cytokine152 and adrenergic receptor activation153, 154 leads to fibroblast proliferation and collagen deposition. The renin-angiotensin-aldosterone system plays an important role in developing hypertrophy and arrhythmia, as treatment with ACE inhibitors155 and spironolactone156 reduces fibrosis and improving conduction velocity. This initially leads to ventricular hypertrophy, later followed by chamber dilation.152 While recruitment of Ly6ClowCXCR1+ macrophages has been found in the LV early after TAC157, there is histologically less inflammation in this model than in other cardiac injury models.152 Notable in this model is the upregulation of NCX158 and downregulation of SERCA2a in myocytes over time, which is also seen in explant human hearts with reduced ejection fraction (HFrEF). While restoring SERCA2 has shown promise in improving systolic dysfunction159, 160 and suppression of ventricular arrhythmias161, 162 in animal models, the CUPID2 trial in HFrEF patients showed neutral results.163
In mice with TAC, multiple investigators have shown an increase conduction time, AP duration, and AV nodal refractory period, QT prolongation with inducible atrial (50–60%) and ventricular arrhythmias (40–50%), but spontaneous arrhythmias in vivo are rare.164–167 Redistribution of connexin-43 (Cx43) laterally away from intercalating disk has been purposed to explain, in part, the changes in conduction in this model. 166 Arrhythmia induction can be challenging in this model, which is likely due to variability in the extent of constriction post procedure168, the length of time of injury prior to analysis, sex, and strain.169–173 Care must be taken when comparing results from different injury protocols and strains in this model.
Similarly, rabbits, rats, and guinea swine develop ventricular hypertrophy and dilation after TAC, albeit over a more extended period (8 weeks vs 4 weeks in mice)174 and with a higher incidence of sudden cardiac death. Unlike the mouse model, rabbits175–177 (aortic insufficiency with abdominal aortic constriction), rats161, 165, and guinea pigs178–180 develop spontaneous arrhythmias in response to catecholamine challenge. Similarly, these animals develop ventricular hypertrophy, chamber dilation and fibrosis after TAC, albeit over a more extended period (8 weeks vs 4 weeks in mice)174 and with a higher incidence of sudden cardiac death. AP prolongation is a hallmark in these models, and is linked to increased INaL and INCX with reduced ICaL responsiveness to β-adrenergic stimulus and increased CaMKII activity, which is seen in human heart failure myocytes.181 Overall, these animals appear to represent a better model for arrhythmias than mice, albeit most studies use explanted heart in the Langendorff system.
Unlike small animals, no studies report an increase in arrhythmia in large animals with TAC. Ascending aortic constriction in pig and sheep has been described with polyester band182 or an implanted inflatable cuff.147, 183 The swine model of TAC differs from other species as they tend to develop heart failure with preserved ejection fraction (HFpEF) characterized by LV hypertrophy with diastolic dysfunction.184–187 In contrast to pig, sheep develop cardiac dysfunction at 6–18 weeks post-TAC with elevated markers of ECM remodeling, chemokine production and apoptosis188, but no study reported arrhythmia generation outside procedural effects.
III.2. Myocardial Ischemia
Acute and chronic myocardial ischemia is a major cause of ventricular arrhythmias in humans. During acute ischemic, myocytes are exposed to hypoxia, acidosis, increased extracellular K+ and intracellular Ca2+.189, 190 Under ischemic conditions, cardiomyocyte mitochondria switch to glycolysis from fatty acid oxidation to maximize ATP production with limited oxygen supply.191 Additionally, hypoxia leads to reactive oxygen species (ROS) production that further damages intracellular proteins and organelles.192 Myocardial infarction occurs with myocyte apoptosis and replacement fibrosis if normal blood flow is not restored. This is a highly inflammatory model, with significant infiltration of CD11b+ macrophages and upregulation of inflammatory cytokines (CCL2, TNF-α and IL-10) after injury, leading to pro-inflammatory Ly6chighCCR2+ macrophages early, then pro-wound healing Ly6clowCXCR1+ chronically, which contribute to interstitial fibrosis in the border zone.193 Chronically, myocardial fibrosis leads to conduction heterogeneity, a substrate for reentry arrhythmias.
In mice and rats, myocardial ischemia is induced surgically, either transiently by ischemia/reperfusion (I/R) injury194, 195 or complete occlusion by coronary artery ligation196, 197 or cryoinjury.195, 198, 199 Unlike complete occlusion, I/R injury produces reversible ischemia that leads to significant myocardial dysfunction without widespread necrosis in the area at risk. Spontaneous ventricular arrhythmias are observed during the reperfusion phase.194 However, spontaneous ventricular arrhythmias are rare after complete occlusion aside from isolated pre-ventricular contractions (internal data). In addition, LV dysfunction leads to volume overload in the left atrium, causing fibrosis and susceptibility to AF.200
Atrial and ventricular arrhythmias can be induced universally in isolated explanted post-MI hearts, with the occurrence of VT (inducible in up to 90–100% in mice), VF (inducible in up to 89% of rats), and AF (inducible in 73% of rats and 33% of mice).201–207 In vivo ventricular arrhythmia induction is more difficult, requiring rapid pacing protocols208–210 and a catecholamine challenge. Transvenous, pericardial, and transesophageal protocols have been described for AF and ventricular arrhythmia induction, with the occurrence of VT (20–70% in mice and rats)211 and AF (60–90% in mice).212. Electrophysiological study of isolated myocytes from acute and chronic infarcted hearts has shown both shortening of the myocardial effective refractory period and slowing of condition time in the left atria, infarct, and border zone.201 The proposed mechanism for AF induction is reduced expression of Cx40, dephosphorylation of Cx40 and Cx43, and redistribution from the intercalated disc to the lateral cell membrane.206
Established models for rabbit myocardial ischemia focus extensively on ex vivo studies using Langendorf perfusion systems. After infarction, rabbit myocardium shows prolonged APD and Ca2+ transients ex vivo.213 Unlike mice and rats, infarction in rabbits leads to delayed AV nodal conduction due to fibrosis and reduction in Cx40 expression but did not affect the ventricular effective refractory period.214 No arrhythmia induction protocol was used in these studies.
Dog models focus on atrial ischemia and arrhythmias, with ~40% of animals developing AF.215 Increased INCX current, spontaneous Ca2+ leak and conduction heterogeneity were found in myocytes from the border zone of the atrial infarct, supporting both triggered and reentry as the mechanism in this model.216 The use of beta-blocker (nadolol) and Ca2+ channel inhibitor (nifedipine) were more effective at suppressing atrial arrhythmia in this model than class Ic (flecainide) or class III (dofetilide) antiarrhythmic drugs.217
Pig myocardial ischemia models are well established as they are of similar size and physiology as humans. Ischemia can be induced by either transient intravascular occlusion of the coronary artery or chronic occlusion with an ameroid constructor.218 Acutely, pig are exquisitely sensitive to ischemia due to lack of functional collaterals at baseline, which leads to significant procedural mortality from VF.219 After chronic ischemia, SCD due to spontaneous ventricular arrhythmias occurs in ~60–70% of animals by 3 months.220, 221 Myocytes in the remote zone from have a decreased rapid delayed rectifier K+ current (IKr), altered Na+-Ca2+ exchange current (INCX), and increases of late Na+ current (INaL), Ca2+-activated K+ current [IK(Ca)], and Ca2+-activated Cl− current [ICl(Ca)]. In addition, myocytes in the border zone show the same changes along with a decrease of L-type Ca2+ current (ICaL), a decrease of inward rectifier K+ current (IK1), and arrhythmogenic sarcoplasmic reticulum (SR) Ca2+ release-induced EADs and DADs.222 These changes in the current lead to shortening of the APD in the border zone and prolonged APD in the remote region, setting up a substrate for both triggered and reentrant arrhythmias. Gene therapy with dominant-negative K+ channel (KCNH2-G628S)223 or Cx43224 reduced VT induction by prolonging the ADP and ERP in the border zone.
III.3. CHB and AV node ablation
Complete heart block (CHB) leads to AV dyssynchrony and bradycardia, which acutely causes reduced cardiac output and induces volume overload. Compensatory hemodynamic changes occur to improve cardiac function, including ventricular dilation, hypertrophy, and increased stroke volume but are not able to fully restore cardiac output.225 While genetic models are available for primary CHB, secondary CHB, which is characterized by fibrosis and necrosis of the AV node, is more difficult to reproduce. Outside histological remodeling, little is known about the molecular changes associated with secondary CHB.
Given the size, mouse AV node is challenging to identify without immunostaining.226 Genetic have generated to establish the important transcription factors and ion channels in the AV node (noted in the above section II.2.6). Interestingly, disruption of tissue resident macrophages in the AV node, by either knockout of Cx43 in these cells or by genetic ablation of macrophages using diphtheria toxin receptor (DTR)/diphtheria treatment, lead to progressive AV conduction block.227 While these resident macrophages were noted in human AV nodes, it unclear if the presence or absence in of these cells contribute to human disease.
Electrical needle AV nodal ablation in rats has been described, as it was noted that alcohol injection leads to either transient block or mortality based on the amount used.228, 229 This is a technically challenging model with variable success229 and high early mortality due to bradycardia and ventricular arrhythmias, as only 6 month old female rats survived past 3 days.228 Regardless, the surviving rats recapitulated the cardiac remodeling in humans, with 80% showing spontaneous TdP at baseline which could be induced to sustained VT with programmed electrical stimulation (PES) and isoproterenol challenge.228
The dog model of AV ablation by transvenous catheter injection for formaldehyde has been well established and leads to compensated hypertrophy and QT prolongation.230, 231 This is due to APD prolongation in the setting of bradycardia, with ~50% of animals developing spontaneous and 90% drug-induced TdP.232 Chronically, this reduced IKs current and enhanced Ca2+ influx by NCX, leading to increased SR Ca2+ content,233 which increases the risk for DADs.234 In contrast, goats undergoing AV ablation did not show APD prolongation but led to increased phospholamban (PLB), Troponin-I and myosin light chain kinase by PKA and RyR2 by CaMKII, suggesting increased Ca2+ sensitivity in this model.235
III.4. Chronic tachypacing
Overriding the normal conduction system has many deleterious effects but is often reversible. Rapid atrial HR or pacing can lead to tachycardia-mediated heart failure and long-term RV pacing in humans can lead to ventricular dyssynchrony, reduced cardiac output, and heart failure.225 Long-term pacing has been shown to be detrimental to LV, with significant wall motion abnormalities and perfusion defects in the inferior and apical walls without corresponding coronary disease.236 Conversely, cardiac resynchronization therapy (CRT) improves HF outcomes for patients with HF and left-bundle branch block.
RV pacing has been used as a model of non-ischemic cardiomyopathy, developing significant systolic dysfunction. Long-term RV pacing in AV nodal ablated dogs show the same perfusion mismatches seen in humans and was associated with increased sympathetic innervation of the ventricles.237 Overdrive RV pacing for 4 weeks in dogs can induce spontaneous ventricular arrhythmias and SCD in 25% of dogs.238 Myocytes isolated from paced ventricles showed prolonged ADP with reduced Ito currents239, likely due in part to downregulation of Kv4.3240 and increased INa,L241 in failing heart. In addition, Ca2+ transients showed reduced amplitude, slowed relaxation, and blunted frequency dependence due to reduction in SERCA2a and upregulation of NCX in failing myocytes.242 CRT in this model was showed to normalization of APD, reduce the INa,L current and prevent the negative remodeling associated with heart failure in this model.241, 243, 244 VF can be induced in pig by applying AC current to the RV, but using arrhythmic drugs to improve resuscitation was not seen.245
Ventricular pacing in dogs also leads to secondary atrial fibrosis, dilation and reduced function as the LV fails, inducing AF.246, 247 As with rapid ventricular pacing, rapid atrial pacing leads to a reduction in the Ito, in addition to ICa,L and IKs currents.248 If pacing is stopped and the animal is allowed to recover, the ion currents return to normal, but fibrosis remains, leading to persistent AF. Studying atrial cells from dogs undergoing both rapid atrial and ventricular pacing has shown the atrial ion channel expression in HF, AT and HF with AT can be significantly different.249 Upregulation of pro-fibrotic miRNA has also been described in atrial paced dogs, providing a novel target to prevent atrial fibrosis and AF.250, 251
In rabbits, rapid atrial pacing increases atrial fibrosis and TGF-β signaling, which can be attenuated with losartan.252 AF can be induced in 40% of rabbits in this model. Atrial pacing leads to decrease KCNE1 KCNB2 expression, reduced IKs and shortening the AERP.253 This was thought to be due to microRNA-1 upregulation in the atria.
In sheep, natriuretic peptide release is found immediately after RV pacing, returning to normal after cessation.254 In goats, chronic atrial pacing led to atrial dilation, with reduced PKA phosphorylation of PLB and increased CaMKII phosphorylation of RyR2, leading to reduced SR Ca2+ load.235 While structural changes similar to humans with tachycardia mediated cardiomyopathy are seen, increased arrhythmogenesis has not been reported.
Given their small size, in vivo pacing is difficult in mice. Tethered epicardial pacing has been used to study AV dyssynchrony and synchrony in mice after I/R injury. Dyssynchrony leads to further deterioration of cardiac function and activation of p38, ERK1/2, JNK and MSK1 and inhibition of the GSK3β pathways. This was reversed by resynchrony.255 Recently, the development of fully implantable epicardial micro pacing technology may allow for longitudinal pacing studies.256
In rats, initiation of rapid atrial pacing leads to upregulation of multiple voltage-gated K+ channels (Kv1.5, Kv4.2, and Kv4.3)257, which contribute to repolarization by IKr and ITo. After 2 days of atrial pacing, AF can be induced in ~20% of animals, with upregulation of associated AF genes (CASQ2, KCNJ2 and TGFB) and activation of the TGF-β and IL-6 pathways.258 Rapid transesophageal pacing of the LV has been used to reliably induce VF to study the effects of medication for resuscitation.259
III.5. Inflammation
Myocardial inflammation is a known driver of atrial and ventricular arrhythmias.260, 261 Post-procedural arrhythmias are common after cardiothoracic surgery but, while they are usually self-limiting, they lead to prolonged hospital stays.262 While there is usually a pre-existing arrhythmogenic substrate due to the underlying disease, surgical scarring and inflammation further exacerbate the system, leading to arrhythmia. Large cohort studies have shown elevated pro-inflammatory cytokines associated with persistent AF, including CRP263–265, TNF‐α, IL-1β, IL-6 and IL-10.266, 267 After surgery, there is an increase in both macrophages and neutrophils to the surgical site, with ROS production from myeloperoxidase (MPO) activity.268, 269
Inflammation due to myocarditis is more complex and the course of the acute and chronic phase of the immune response is dependent on the underlying cause (infectious vs rheumatological). The cytokine profiles are found in viral and autoimmune myocarditis includes more pro-inflammatory monocyte infiltration and myocyte necrosis than post-surgical injury.270 This leads to a multitude of ECG changes, including sinus tachycardia, widened QRS patterns, low voltage, prolonged QT, variable AV blocks, and diffuse ST-elevations.271–273
Sterile inflammation has been used to induce AF in dogs274 and sheep.275 Pericardial talc treatment of dog atria to induce sterile inflammation induced AF in ~60% of dogs and was significantly reduced with topical steroids or NSAIDS.274 In sheep, treatment with atorvastatin reduced hs-CRP, IL-6 and TNF-α expression, which improved the atrial ERP at 72-hours.275 While inflammatory myocarditis can be induced in rats276 and guinea pigs277, no current reports on the arrhythmia potential are available.
Currently, there are two models of viral myocarditis due to exposure to coxsackievirus B3 (CVB3)278 and encephalomyocarditis virus A (EMCV).279 There are several issues with the viral myocarditis models in animals. First, of the viruses primarily associated with human myocarditis (parvovirus B19, herpes simplex 9 and coxsackievirus B3), only CVB3 is infectious to animals, with EMCV only rarely causing human disease newborns. Second, the CVB3 mouse model is highly inflammatory and more mimics childhood infections than the milder course in adults.280 Third, only particular stains of mice are susceptible to viral infection. Regardless, C3H/He mice exposed to develop similar arrhythmias to humans (80% sinus arrest, 30% second or third-degree AV block, 30% PACs, 20% PVCs, and 10% VT).281 Ex-vivo electrophysiological studies showed no change to the APD, but mice exposed to CVB3 were hyperpolarized with slightly increased VERP.282 EMCV exposed DBA/2 mice develop AV block in 40% of mice over 2 weeks, with 2/3 of those mice showing mononuclear cell infiltration and edema and another 1/3 showing necrosis of the conduction system.283 No further electrophysiological studies were conducted.
Traditionally, myocarditis can be induced in mice by either immunizing with a cardiac structural peptide (myosin heavy chain (MHC-α) or cardiac troponin I) 284 or delivering primed dendritic cells pulsed with MHC-α.285 Importantly, BALB/c mice are susceptible to peptide immunized myocarditis while C57BL/6 strains are resistant, from which a majority of transgenic lines are created.286 MHC-α peptide-induced myocarditis showed significant immune cell infiltration, increased expression of both TNFα and INFγ, fibrosis and prolongation for the APD in ventricular myocytes, which all could be attenuated with atorvastatin.287 276, 277
Ctla4+/− Pdcd1−/− mice spontaneously develop myocarditis, modeling immune checkpoint inhibitor (ICI) induced myocarditis.288 These mice succumb to progressive ventricular hypertrophy and SCD early. Progressive AV block and sinus arrest occurs in ~30% of transgenic mice, similar to arrhythmia seen in patient with ICI induced myocarditis. Further study using this model would greatly improve treatment for patient with adverse events after ICI therapy.
III.6. Metabolic and Drug-Induced Arrhythmia
Dietary and metabolic considerations also contribute to atrial arrhythmia development as there is a known association with BMI and diabetes in AF289, 290 and incidence of paroxysmal AF is reduced after gastric bypass surgery.291 Off-target drug effects lead to adverse clinical outcomes, particularly arrhythmias induction due to block of delayed rectifier K+ channels, IKr, causing drug-induced long QT syndrome and TdP ventricular arrhythmias.
Streptozotocin-induced diabetic models have been developed for both mice and rats292, and consistently shown prolonged APD, increased sympathetic innervation and inflammation. Studies have shown this is likely due to production of advanced glycation end products (AGEs) in diabetes animals, but they are inconstant in the electrophysiological mechanisms.293 Initial studies showed a reduction in the Ito current as the underlying cause of AF,294–296 while others suggest changes in SA node connexin channel expression,297, 298 atrial myocyte Ca2+ handling proteins (TRPC1/6, RyR3)299 and AV nodal ion channels (TRPC1, CASQ2, RYR2 and RYR3)300 are involved. Diabetic mice and rats also have reduced K+ channel expression, leading to overall reduced K+ currents, that was dependent on glycosylation of CaMKII and activation PKC.301 Further studies showed in the setting of hyperglycemia, mice had more diastolic calcium leak through RyR2, prolonged ADP90, ADP alternans, increased DADs and frequent PVC, which could be suppressed by genetic inhibition of CaMKII.302 Blocking IL-1β activation of CaMKII in this model restores the APD to normal and suppresses VT induction in explanted hearts from diabetic mice.303 These studies all provide a central role for CaMKII in regulating ion channel expression and function in hyperglycemia.
Diet-induced obesity (DIO) has been shown to affect inflammation and gene expression in multiple disease models. Mice fed 3-months of a high-fat diet had a 15% increase in their QTc and increased IKs current than mice on a normal diet and developed a 10-fold increase in PVC burden.304 The increased IKs current was thought to be due to the reduced expression of voltage-gated K+ channels. DIO mice were also found to have reduced NaV1.5 expression and current, leading to reduced ADP and conduction velocity in the atrial and increased incidence of induced AF.305 DIO rats over 8 weeks have increased expression of CaV1.2, HCN4, Kir2.1, RYR2, NCX, and SERCA2a in the LV, which may contribute to DADs and triggered PVCs.306 Rabbits fed a high-fat diet had increased cardiac sympathetic innervation (as seen by increased GAP23 expression), prolonged ADP and increased ICa which led to QTc prolongation and repolarization heterogeneity in the ventricle.307 Isolated hearts were more susceptible to VT induction. While these studies highlight the important changes to ion channel expression animals due to a high-fat Western diet, further study is needed to linking these findings to humans.
A specific line of inbred rats (Fischer F344 at 20–24 months) develop age-dependent adverse cardiac remodeling, with males developing more cardiomyocyte hypertrophy, intestinal fibrosis, and systolic dysfunction and females with more cardiac hypertrophy and diastolic dysfunction.308 Aged female Fischer F344 rats show enlarged atrial, fibrosis, and CD68+ monocyte infiltration, similar to human disease.309 Both male and female Fischer 344 rats are more susceptible to AF induction by atrial pacing, with 80% of animals showing atrial arrhythmia.309, 310 These are the only models of spontaneous AF in aged animals, and could provide important insight to mechanism and future therapies for AF in our aging population.
In rabbits treated with clofilium (K+ channel blocker), 70% of animals develop TdP, which can be increased to 100% with the addition of α1 agonist methoxamine.311 Blockade of calmodulin kinase (CaMK) or PKA in this model reduced pause-dependent VT that was independent of these kinases effect on L-type Ca2+ channel activity.312 Interestingly, the dose-dependent CaMKII blockade did not change the QT interval, unlike the dose-dependent PKA blockade. Further study showed that blocking CaMKII reduced the ratio of the TU interval, which is likely more critical than the QT interval in the induction of pause-dependent VT.313
IV. Emerging etiologies of arrhythmogenesis: opportunity for animal models
Cardiac electrophysiology is regulated not only by the amino acid sequence at the protein level, but also by post-translational modifications, micropeptides, epigenetics, long noncoding RNAs (lncRNA), micro RNAs (miRs), ageing, and environmental factors. Unprecedented advances in sequencing technology, deep mutational scanning, and epigenetic and transcriptome mapping have highlighted several new areas of pathogenesis. A primary challenge with many of the ascribed changes is whether they directly cause disease or, rather, follow disease progression. Animal models have been generated to address this challenge and study new areas of arrhythmia biology, with a select few highlighted below.
In the past decade, micropeptides have been identified that were previously excluded during annotations of open reading frames due to their small size or position in the transcriptome. In the heart, functionally expressed peptides include examples such as sarcolipin and dwarf open reading frame (DWORF), which regulate SR Ca reuptake. Sarcolipin protein levels are decreased in humans with AF or heart failure314 and the sarcolipin knockout mouse developed AF with age.315 In mice with DCM, overexpression of DWORF attenuated the heart failure phenotype, although arrhythmias were not examined.316 The micropeptides apelin and elabela affect cardiogenesis, fibrosis, hypertrophy, and inotropic responses. Reduced apelin levels predicted major myocardial events and MI scar size in patients with a previous MI event.316 Apelin knockout mice developed larger scars and increased mortality following MI, although arrhythmias were not described.317 These findings establish the pathogenic role and therapeutic potential for micropeptides in the heart, but their impact on arrhythmogenesis remains to be examined.
Post-translational modifications, primarily phosphorylation, are documented in many arrhythmias.318 While phosphorylation by PKC, PKA and CaMKII are most studied mediators for changes in Na+ channels77, 78, 319, 320, Ca2+ channels321–323, K+ channels324, NCX325 and RyR2326, conflicting data exist regarding the relative contribution of different phosphorylation sites to ion currents. Phosphomimetic and phosphoablation mouse models have been created to tease out the importance of each site to arrhythmogenesis.77, 321, 327, 328 In addition, post-translational modification of signaling kinases themselves, including PKA329 and CaMKII329, play a role in arrhythmia susceptibility in response to oxidative stress. Other post-translational modifications are less well studied, and it remains to be determined whether these cause disease or are merely epiphenomena.
Epigenetic DNA modifications regulate protein expression. It is now recognized that many cardiovascular diseases are associated with epigenetic changes. For example, cardiac-specific deletion of HDAC1 and HDAC2 in mice led to dilated cardiomyopathy, arrhythmia, and premature death.330 A family with a history of autosomal recessive DCM was later discovered to carry homozygous mutation in GATAD1.331 In AF, epigenetics also contribute to risk as it relates to hypertension, obesity, age, and other factors.332 Patients with persistent AF have genome-wide changes in DNA methylation333 and animal models could help elucidate the pathophysiology of these changes at the whole genome or protein level.
MiRs regulate gene expression by RNA complementation and silencing. In the heart, miRs control cardiogenesis and pathways to affect gene expression during development. Some miRs are muscle specific and may serve as macro-regulators of ion channel expression. In disease, changes in many different miRs have been documented. In dogs with rapid pacing, nicotine administration reduced miR-133 and miR-590 levels and increased AF risk.334 Atrial tachypacing in dogs and development of AF was also associated with changes in many miRs compared to control.335 Dogs with ventricular tachypacing developed CHF and AF and miR-29b levels declined within the first 24 hours of pacing.336 In zebrafish, miR-182 served as a TBX-5 effector and miR-182 upregulation led to block, tachycardia, and arrhythmias.337 In mice, it was shown that miR-1 directly binds to the C-terminus of Kir2.1 and represses channel activity.338 Moreover, a single nucleotide polymorphism in miR-1 identified from patients with AF did not suppress Kir2.1 channel activity and failed to rescue arrhythmia inducibility in miR-1 knockdown mice (whereas WT miR-1 did). Of most interest is the therapeutic potential for RNA regulation to reverse disease progression.
LncRNA regulates gene expression via several mechanisms and changes in lncRNA expression have been documented in a wide range of diseases. Altered expression of lncRNA has been observed in AF339, including altered regulation of PITX2.340 Another study in rabbits with AF induced by atrial tachypacing purported a mechanism whereby lncRNA “sponges” up miR-328 to regulate CACNA1C.341 Yet another report indicated changes in lncRNA in AF reduce expression of JP2 and RYR2.342 These studies demonstrate additional mechanisms of regulation beyond controlling gene expression and open up the field to exciting new opportunities in arrhythmia research.
Conclusions
For decades, animal models have been an essential tool to study arrhythmia pathophysiology and therapeutic approaches. Recent work with genetic mouse models yielded a new diagnostic tool in CRDS, identified lifesaving drug therapies for CPVT, LQT3, and ARVC, identified pathogenic mechanisms, and advanced our understanding of arrhythmogenesis in ways that other models cannot. The broad availability of transgenic mouse models and the option to generate mice with cell-specific and/or time-dependent regulation of gene expression provides a significant advantage of the mouse over other small animal species. A major limitation is that their fast heart rate, small heart size, and differential ionic currents do not fully recapitulate human cardiac electrophysiology. While guinea pigs and rabbits can overcome some of these electrophysiological limitations, genetic manipulation is limited in these species. Large animal electrophysiological and hemodynamic parameters match humans more closely, but can be prohibitively expensive. Regardless, large animals are useful for preclinical studies evaluating new drugs, gene therapy, and devices that necessitate large animal models before moving to humans. Here, we provide an in-depth review of existing animal models to help interpreting published arrhythmia mechanisms as well as planning future experimental studies investigating cardiac arrhythmia diseases.
Table 3. Animal models of acquired arrhythmia disorders.
Representative list of animal models with reference to the method to their development. Abbreviations: Programmed Electrical Stimulation (PES), Sudden Cardiac Death (SCD), Ventricular Tachycardia (VT), Ventricular Fibrillation (VF), Atrial Fibrillation (AF) Torsades de Pointes (TdP), Diet-induced Obesity (DIO).
| Animal | Notes | Ref. |
|---|---|---|
| Transverse Aortic Constriction | ||
| Mouse | Severity of injury dependent on strain (BALB/c > C57BL/6 > 129S1/SvImJ) and substrain (C57BL/6Tac > C57BL/6NCrl > C57BL/6J). Model requires PES to induce in vivo arrhythmias | 143, 146, 168 |
| Rat | Develop spontaneous arrhythmias with catecholamine challenge | 466 |
| Guinea Pig | High mortality, develops catecholamine induced arrhythmias | 467 |
| Rabbit | In vivo injury, but arrhythmia studies completed ex-vivo with Langendorff system | 468 |
| Pig | Model of Heart Failure with Preserved Ejection Fraction, no study of arrhythmias | 148 |
| Ovine | Model of Heart Failure with Reduced Ejection Fraction, no study of arrhythmias | 182 |
| Myocardial Ischemia | ||
| Mouse | Single left coronary artery leads to variation in severity of injury. Model requires PES with catecholamine challenge to induced arrhythmias | 194, 197, 199 |
| Rat | Requires PES and catecholamine challenge to induce ventricular arrhythmias reliably, but rare spontaneous arrhythmias | 469 |
| Rabbit | PES done as ex-vivo in Langendorff system | 470 |
| Dog | Atrial ischemia extensive studied | 471 |
| Pig | High mortality due to poor collateral circulation, early spontaneous VT/VF during injury and late model of SCD | 472 |
| Sheep | High mortality with spontaneous ventricular arrhythmia | 473 |
| AV node ablation | ||
| Rat | High mortality, particularly in male rats. Requires specialized surgical equipment and skill | 228 |
| Rabbit | Studied completed ex-vivo in Langendorff system | 474 |
| Dog | Can be induced minimally invasively with low mortality | 230, 231 |
| Sheep | Can be induced minimally invasively with low mortality, model of TdP | 235 |
| Chronic atrial pacing | ||
| Rat | Model for AF | 257 |
| Rabbit | In-vivo pacing, but PES studied completed ex-vivo with Langendorff system | 252 |
| Dog | AF and spontaneous VT model, recapitulates tachycardia mediated cardiomyopathy | 475 |
| Pig | AF model, recapitulates tachycardia mediated cardiomyopathy | 476 |
| Chronic ventricular pacing | ||
| Mouse | Requires tethered or ex-vivo pacing | 255 |
| Rat | Model tachycardia mediated cardiomyopathy with VF induction with rapid pacing | 477, 478 |
| Dog | Recapitulates tachycardia mediated cardiomyopathy, model of spontaneous AF and VT | 479 |
| Sheep | Recapitulates tachycardia mediated cardiomyopathy with reduced ejection fraction, no studies of arrhythmia | 254 |
| Pig | 480 | |
| Inflammation | ||
| Mouse | Strain specific susceptibility. C3H/He and DBA/2 mice susceptible to viral myocarditits while C57BL/6 are protected. BALB/c susceptible to immunogen induced myocarditis while C57BL/6 more resistive | 284, 285 |
| Rat | Model of AF, but non-physiological induction of inflammation with talc | 276 |
| Guinea Pig | 481 | |
| Dog | 274 | |
| Sheep | 275 | |
| Metabolic/Drug-Induced | ||
| Mouse | Streptozotocin and DIO models well established, increased susceptibility to AF and VT with PES | 482, 483 |
| Rat | Age-dependent fibrosis found in Fisher 344 rat strain, model of AF | 484 |
| Rabbit | Established model of clofilium induced TdP | 485 |
Acknowledgments
Sources of Funding
This work was supported in part by National Institutes of Health (NIH) grant NHLBI R35 HL144980 (BCK), the Leducq Foundation grant 18CVD05 (BCK), the American Heart Association grant 19SFRN34830019 (BCK) and NIH LRP GSNT8763 (JS).
Non-standard Abbreviations and Acronyms
- AF
atrial fibrillation
- APD
action potential duration
- ARVC
arrhythmogenic right ventricular cardiomyopathy
- AT
atrial tachycardia
- BPM
beats per minute
- BrS
Brugada syndrome
- CHB
complete heart block
- CICR
calcium induced calcium release
- CPVT
catecholaminergic polymorphic ventricular tachycardia
- CRDS
calcium release deficiency syndrome
- DCM
dilated cardiomyopathy
- ERP
effective refractory period
- HCM
hypertrophic cardiomyopathy
- HR
heart rate
- LQTS
long QT syndrome
- MAT
multifocal atrial tachycardia
- PAC
premature atrial complex
- PVC
premature ventricular complex
- SQTS
short QT syndrome
- SVT
supraventricular tachycardia
- TAC
transverse aortic constriction
- TdP
Torsades de Pointes
- VF
ventricular fibrillation
- VT
ventricular tachycardia
Footnotes
Disclosures
None.
References
- 1.Khurshid S, Choi SH, Weng LC, Wang EY, Trinquart L, Benjamin EJ, Ellinor PT and Lubitz SA. Frequency of Cardiac Rhythm Abnormalities in a Half Million Adults. Circ Arrhythm Electrophysiol. 2018;11:e006273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, Arensberg D, Baker A, Friedman L, Greene HL, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–8. [DOI] [PubMed] [Google Scholar]
- 3.Cardiac Arrhythmia Suppression Trial III. Effect of the antiarrhythmic agent moricizine on survival after myocardial infarction. N Engl J Med. 1992;327:227–33. [DOI] [PubMed] [Google Scholar]
- 4.Delisle BP, Anson BD, Rajamani S and January CT. Biology of cardiac arrhythmias: ion channel protein trafficking. Circ Res. 2004;94:1418–28. [DOI] [PubMed] [Google Scholar]
- 5.Antzelevitch C and Burashnikov A. Overview of Basic Mechanisms of Cardiac Arrhythmia. Card Electrophysiol Clin. 2011;3:23–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta DK, Giugliano RP, Ruff CT, Claggett B, Murphy S, Antman E, Mercuri MF, Braunwald E, Solomon SD and Effective Anticoagulation with Factor Xa Next Generation in AFTiMIESI. The Prognostic Significance of Cardiac Structure and Function in Atrial Fibrillation: The ENGAGE AF-TIMI 48 Echocardiographic Substudy. J Am Soc Echocardiogr. 2016;29:537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morita N, Mandel WJ, Kobayashi Y and Karagueuzian HS. Cardiac fibrosis as a determinant of ventricular tachyarrhythmias. J Arrhythm. 2014;30:389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao JM, Fishbein MC, Han JB, Lai WW, Lai AC, Wu TJ, Czer L, Wolf PL, Denton TA, Shintaku IP, et al. Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation. 2000;101:1960–9. [DOI] [PubMed] [Google Scholar]
- 9.Brachmann J, Kabell G, Scherlag B, Harrison L and Lazarra R. Analysis of interectopic activation patterns during sustained ventricular tachycardia. Circulation. 1983;67:449–56. [DOI] [PubMed] [Google Scholar]
- 10.Kannankeril PJ, Moore JP, Cerrone M, Priori SG, Kertesz NJ, Ro PS, Batra AS, Kaufman ES, Fairbrother DL, Saarel EV, et al. Efficacy of Flecainide in the Treatment of Catecholaminergic Polymorphic Ventricular Tachycardia: A Randomized Clinical Trial. JAMA Cardiol. 2017;2:759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazzanti A, Maragna R, Faragli A, Monteforte N, Bloise R, Memmi M, Novelli V, Baiardi P, Bagnardi V, Etheridge SP, et al. Gene-Specific Therapy With Mexiletine Reduces Arrhythmic Events in Patients With Long QT Syndrome Type 3. J Am Coll Cardiol. 2016;67:1053–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zipes DP, Jalife J and Stevenson WG. Cardiac electrophysiology : from cell to bedside. Seventh edition. ed. Philadelphia, PA: Elsevier; 2018. [Google Scholar]
- 13.Varro A, Tomek J, Nagy N, Virag L, Passini E, Rodriguez B and Baczko I. Cardiac transmembrane ion channels and action potentials: cellular physiology and arrhythmogenic behavior. Physiol Rev. 2021;101:1083–1176. [DOI] [PubMed] [Google Scholar]
- 14.Varro A, Lathrop DA, Hester SB, Nanasi PP and Papp JG. Ionic currents and action potentials in rabbit, rat, and guinea pig ventricular myocytes. Basic Res Cardiol. 1993;88:93–102. [DOI] [PubMed] [Google Scholar]
- 15.Joukar S. A comparative review on heart ion channels, action potentials and electrocardiogram in rodents and human: extrapolation of experimental insights to clinic. Lab Anim Res. 2021;37:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. [DOI] [PubMed] [Google Scholar]
- 17.Bers DM. Excitation-contraction coupling and cardiac contractile force. 2nd ed. Dordrecht; Boston: Kluwer Academic Publishers; 2001. [Google Scholar]
- 18.Mangoni ME and Nargeot J. Genesis and regulation of the heart automaticity. Physiol Rev. 2008;88:919–82. [DOI] [PubMed] [Google Scholar]
- 19.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P and Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66. [DOI] [PubMed] [Google Scholar]
- 20.Weiss JN, Garfinkel A, Karagueuzian HS, Chen PS and Qu Z. Early afterdepolarizations and cardiac arrhythmias. Heart Rhythm. 2010;7:1891–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bers DM. Calcium and cardiac rhythms: physiological and pathophysiological. Circ Res. 2002;90:14–7. [PubMed] [Google Scholar]
- 22.Tse G. Mechanisms of cardiac arrhythmias. J Arrhythm. 2016;32:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wellens HJ. Contemporary management of atrial flutter. Circulation. 2002;106:649–52. [DOI] [PubMed] [Google Scholar]
- 24.Katritsis DG and Camm AJ. Atrioventricular nodal reentrant tachycardia. Circulation. 2010;122:831–40. [DOI] [PubMed] [Google Scholar]
- 25.Killu AM and Stevenson WG. Ventricular tachycardia in the absence of structural heart disease. Heart. 2019;105:645–656. [DOI] [PubMed] [Google Scholar]
- 26.Wessels A and Sedmera D. Developmental anatomy of the heart: a tale of mice and man. Physiol Genomics. 2003;15:165–76. [DOI] [PubMed] [Google Scholar]
- 27.Kumar D, Hacker TA, Buck J, Whitesell LF, Kaji EH, Douglas PS and Kamp TJ. Distinct mouse coronary anatomy and myocardial infarction consequent to ligation. Coron Artery Dis. 2005;16:41–4. [DOI] [PubMed] [Google Scholar]
- 28.Karakikes I, Ameen M, Termglinchan V and Wu JC. Human induced pluripotent stem cell-derived cardiomyocytes: insights into molecular, cellular, and functional phenotypes. Circ Res. 2015;117:80–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe H, Chopra N, Laver D, Hwang HS, Davies SS, Roach DE, Duff HJ, Roden DM, Wilde AA and Knollmann BC. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nat Med. 2009;15:380–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wakula P, Bisping E, Kockskamper J, Post H, Brauer S, Deuter M, Oehlmann R, Besenfelder U, Lai FA, Brem G and Pieske B. CMV promoter is inadequate for expression of mutant human RyR2 in transgenic rabbits. J Pharmacol Toxicol Methods. 2011;63:180–5. [DOI] [PubMed] [Google Scholar]
- 31.Cerrone M, Colombi B, Santoro M, di Barletta MR, Scelsi M, Villani L, Napolitano C and Priori SG. Bidirectional ventricular tachycardia and fibrillation elicited in a knock-in mouse model carrier of a mutation in the cardiac ryanodine receptor. Circ Res. 2005;96:e77–82. [DOI] [PubMed] [Google Scholar]
- 32.Chen W, Wang R, Chen B, Zhong X, Kong H, Bai Y, Zhou Q, Xie C, Zhang J, Guo A, et al. The ryanodine receptor store-sensing gate controls Ca2+ waves and Ca2+-triggered arrhythmias. Nat Med. 2014;20:184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Wang R, Sun B, Mi T, Zhang J, Mu Y, Chen J, Bround MJ, Johnson JD, Gillis AM and Chen SR. Generation and characterization of a mouse model harboring the exon-3 deletion in the cardiac ryanodine receptor. PLoS One. 2014;9:e95615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ng K, Titus EW, Lieve KV, Roston TM, Mazzanti A, Deiter FH, Denjoy I, Ingles J, Till J, Robyns T, et al. An International Multicenter Evaluation of Inheritance Patterns, Arrhythmic Risks, and Underlying Mechanisms of CASQ2-Catecholaminergic Polymorphic Ventricular Tachycardia. Circulation. 2020;142:932–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wleklinski MJ, Kannankeril PJ and Knollmann BC. Molecular and tissue mechanisms of catecholaminergic polymorphic ventricular tachycardia. J Physiol. 2020;598:2817–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chopra N, Yang T, Asghari P, Moore ED, Huke S, Akin B, Cattolica RA, Perez CF, Hlaing T, Knollmann-Ritschel BE, et al. Ablation of triadin causes loss of cardiac Ca2+ release units, impaired excitation-contraction coupling, and cardiac arrhythmias. Proc Natl Acad Sci U S A. 2009;106:7636–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nyegaard M, Overgaard MT, Sondergaard MT, Vranas M, Behr ER, Hildebrandt LL, Lund J, Hedley PL, Camm AJ, Wettrell G, et al. Mutations in calmodulin cause ventricular tachycardia and sudden cardiac death. Am J Hum Genet. 2012;91:703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knollmann BC, Chopra N, Hlaing T, Akin B, Yang T, Ettensohn K, Knollmann BE, Horton KD, Weissman NJ, Holinstat I, et al. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2006;116:2510–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurtzwald-Josefson E, Yadin D, Harun-Khun S, Waldman M, Aravot D, Shainberg A, Eldar M, Hochhauser E and Arad M. Viral delivered gene therapy to treat catecholaminergic polymorphic ventricular tachycardia (CPVT2) in mouse models. Heart Rhythm. 2017;14:1053–1060. [DOI] [PubMed] [Google Scholar]
- 40.Kryshtal DO, Blackwell DJ, Egly CL, Smith AN, Batiste SM, Johnston JN, Laver DR and Knollmann BC. RYR2 Channel Inhibition Is the Principal Mechanism of Flecainide Action in CPVT. Circ Res. 2021;128:321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2018;138:e272–e391. [DOI] [PubMed] [Google Scholar]
- 42.Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, Blom N, Brugada J, Chiang CE, Huikuri H, et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10:1932–63. [DOI] [PubMed] [Google Scholar]
- 43.Blackwell DJ, Faggioni M, Wleklinski MJ, Gomez-Hurtado N, Venkataraman R, Gibbs CE, Baudenbacher FJ, Gong S, Fishman GI, Boyle PM, et al. The Purkinje-myocardial junction is the anatomical origin of ventricular arrhythmia in CPVT. JCI Insight. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun B, Yao J, Ni M, Wei J, Zhong X, Guo W, Zhang L, Wang R, Belke D, Chen YX, et al. Cardiac ryanodine receptor calcium release deficiency syndrome. Sci Transl Med. 2021;13. [DOI] [PubMed] [Google Scholar]
- 45.Zhao YT, Valdivia CR, Gurrola GB, Powers PP, Willis BC, Moss RL, Jalife J and Valdivia HH. Arrhythmogenesis in a catecholaminergic polymorphic ventricular tachycardia mutation that depresses ryanodine receptor function. Proc Natl Acad Sci U S A. 2015;112:E1669–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shimizu W and Horie M. Phenotypic manifestations of mutations in genes encoding subunits of cardiac potassium channels. Circ Res. 2011;109:97–109. [DOI] [PubMed] [Google Scholar]
- 47.Migdalovich D, Moss AJ, Lopes CM, Costa J, Ouellet G, Barsheshet A, McNitt S, Polonsky S, Robinson JL, Zareba W, et al. Mutation and gender-specific risk in type 2 long QT syndrome: implications for risk stratification for life-threatening cardiac events in patients with long QT syndrome. Heart Rhythm. 2011;8:1537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Locati EH, Zareba W, Moss AJ, Schwartz PJ, Vincent GM, Lehmann MH, Towbin JA, Priori SG, Napolitano C, Robinson JL, et al. Age- and sex-related differences in clinical manifestations in patients with congenital long-QT syndrome: findings from the International LQTS Registry. Circulation. 1998;97:2237–44. [DOI] [PubMed] [Google Scholar]
- 49.Salama G and Bett GC. Sex differences in the mechanisms underlying long QT syndrome. Am J Physiol Heart Circ Physiol. 2014;307:H640–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Odening KE and Koren G. How do sex hormones modify arrhythmogenesis in long QT syndrome? Sex hormone effects on arrhythmogenic substrate and triggered activity. Heart Rhythm. 2014;11:2107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Drici MD, Burklow TR, Haridasse V, Glazer RI and Woosley RL. Sex hormones prolong the QT interval and downregulate potassium channel expression in the rabbit heart. Circulation. 1996;94:1471–4. [DOI] [PubMed] [Google Scholar]
- 52.Drici MD, Knollmann BC, Wang WX and Woosley RL. Cardiac actions of erythromycin: influence of female sex. JAMA. 1998;280:1774–6. [DOI] [PubMed] [Google Scholar]
- 53.Casimiro MC, Knollmann BC, Ebert SN, Vary JC, Jr., Greene AE, Franz MR, Grinberg A, Huang SP and Pfeifer K. Targeted disruption of the Kcnq1 gene produces a mouse model of Jervell and Lange-Nielsen Syndrome. Proc Natl Acad Sci U S A. 2001;98:2526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brunner M, Peng X, Liu GX, Ren XQ, Ziv O, Choi BR, Mathur R, Hajjiri M, Odening KE, Steinberg E, et al. Mechanisms of cardiac arrhythmias and sudden death in transgenic rabbits with long QT syndrome. J Clin Invest. 2008;118:2246–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jou CJ, Barnett SM, Bian JT, Weng HC, Sheng X and Tristani-Firouzi M. An in vivo cardiac assay to determine the functional consequences of putative long QT syndrome mutations. Circ Res. 2013;112:826–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Montnach J, Chizelle FF, Belbachir N, Castro C, Li L, Loussouarn G, Toumaniantz G, Carcouet A, Meinzinger AJ, Shmerling D, et al. Arrhythmias precede cardiomyopathy and remodeling of Ca(2+) handling proteins in a novel model of long QT syndrome. J Mol Cell Cardiol. 2018;123:13–25. [DOI] [PubMed] [Google Scholar]
- 57.Nuyens D, Stengl M, Dugarmaa S, Rossenbacker T, Compernolle V, Rudy Y, Smits JF, Flameng W, Clancy CE, Moons L, et al. Abrupt rate accelerations or premature beats cause life-threatening arrhythmias in mice with long-QT3 syndrome. Nat Med. 2001;7:1021–7. [DOI] [PubMed] [Google Scholar]
- 58.Priori SG, Napolitano C, Cantu F, Brown AM and Schwartz PJ. Differential response to Na+ channel blockade, beta-adrenergic stimulation, and rapid pacing in a cellular model mimicking the SCN5A and HERG defects present in the long-QT syndrome. Circ Res. 1996;78:1009–15. [DOI] [PubMed] [Google Scholar]
- 59.Lopez-Santiago LF, Meadows LS, Ernst SJ, Chen C, Malhotra JD, McEwen DP, Speelman A, Noebels JL, Maier SK, Lopatin AN and Isom LL. Sodium channel Scn1b null mice exhibit prolonged QT and RR intervals. J Mol Cell Cardiol. 2007;43:636–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zaritsky JJ, Redell JB, Tempel BL and Schwarz TL. The consequences of disrupting cardiac inwardly rectifying K(+) current (I(K1)) as revealed by the targeted deletion of the murine Kir2.1 and Kir2.2 genes. J Physiol. 2001;533:697–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu CW, Lin JH, Rajawat YS, Jerng H, Rami TG, Sanchez X, DeFreitas G, Carabello B, DeMayo F, Kearney DL, et al. Functional and clinical characterization of a mutation in KCNJ2 associated with Andersen-Tawil syndrome. J Med Genet. 2006;43:653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chauhan VS, Tuvia S, Buhusi M, Bennett V and Grant AO. Abnormal cardiac Na(+) channel properties and QT heart rate adaptation in neonatal ankyrin(B) knockout mice. Circ Res. 2000;86:441–7. [DOI] [PubMed] [Google Scholar]
- 63.Mohler PJ, Schott JJ, Gramolini AO, Dilly KW, Guatimosim S, duBell WH, Song LS, Haurogne K, Kyndt F, Ali ME, et al. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature. 2003;421:634–9. [DOI] [PubMed] [Google Scholar]
- 64.Drici MD, Arrighi I, Chouabe C, Mann JR, Lazdunski M, Romey G and Barhanin J. Involvement of IsK-associated K+ channel in heart rate control of repolarization in a murine engineered model of Jervell and Lange-Nielsen syndrome. Circ Res. 1998;83:95–102. [DOI] [PubMed] [Google Scholar]
- 65.Major P, Baczko I, Hiripi L, Odening KE, Juhasz V, Kohajda Z, Horvath A, Seprenyi G, Kovacs M, Virag L, et al. A novel transgenic rabbit model with reduced repolarization reserve: long QT syndrome caused by a dominant-negative mutation of the KCNE1 gene. Br J Pharmacol. 2016;173:2046–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheng EP, Yuan C, Navedo MF, Dixon RE, Nieves-Cintron M, Scott JD and Santana LF. Restoration of normal L-type Ca2+ channel function during Timothy syndrome by ablation of an anchoring protein. Circ Res. 2011;109:255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.London B, Jeron A, Zhou J, Buckett P, Han X, Mitchell GF and Koren G. Long QT and ventricular arrhythmias in transgenic mice expressing the N terminus and first transmembrane segment of a voltage-gated potassium channel. Proc Natl Acad Sci U S A. 1998;95:2926–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Glasscock E, Voigt N, McCauley MD, Sun Q, Li N, Chiang DY, Zhou XB, Molina CE, et al. Expression and function of Kv1.1 potassium channels in human atria from patients with atrial fibrillation. Basic Res Cardiol. 2015;110:505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hu Z, Crump SM, Anand M, Kant R, Levi R and Abbott GW. Kcne3 deletion initiates extracardiac arrhythmogenesis in mice. FASEB J. 2014;28:935–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wada Y, Yang T, Shaffer CM, Daniel LL, Glazer AM, Davogustto GE, Lowery BD, Farber-Eger EH, Wells QS and Roden DM. Common Ancestry-Specific Ion Channel Variants Predispose to Drug-Induced Arrhythmias. Circulation. 2022;145:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hassel D, Scholz EP, Trano N, Friedrich O, Just S, Meder B, Weiss DL, Zitron E, Marquart S, Vogel B, et al. Deficient zebrafish ether-a-go-go-related gene channel gating causes short-QT syndrome in zebrafish reggae mutants. Circulation. 2008;117:866–75. [DOI] [PubMed] [Google Scholar]
- 72.Thorsen K, Dam VS, Kjaer-Sorensen K, Pedersen LN, Skeberdis VA, Jurevicius J, Treinys R, Petersen I, Nielsen MS, Oxvig C, et al. Loss-of-activity-mutation in the cardiac chloride-bicarbonate exchanger AE3 causes short QT syndrome. Nat Commun. 2017;8:1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Odening KE, Bodi I, Franke G, Rieke R, Ryan de Medeiros A, Perez-Feliz S, Furniss H, Mettke L, Michaelides K, Lang CN, et al. Transgenic short-QT syndrome 1 rabbits mimic the human disease phenotype with QT/action potential duration shortening in the atria and ventricles and increased ventricular tachycardia/ventricular fibrillation inducibility. Eur Heart J. 2019;40:842–853. [DOI] [PubMed] [Google Scholar]
- 74.Papadatos GA, Wallerstein PM, Head CE, Ratcliff R, Brady PA, Benndorf K, Saumarez RC, Trezise AE, Huang CL, Vandenberg JI, et al. Slowed conduction and ventricular tachycardia after targeted disruption of the cardiac sodium channel gene Scn5a. Proc Natl Acad Sci U S A. 2002;99:6210–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leoni AL, Gavillet B, Rougier JS, Marionneau C, Probst V, Le Scouarnec S, Schott JJ, Demolombe S, Bruneval P, Huang CL, et al. Variable Na(v)1.5 protein expression from the wild-type allele correlates with the penetrance of cardiac conduction disease in the Scn5a(+/−) mouse model. PLoS One. 2010;5:e9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Remme CA, Verkerk AO, Nuyens D, van Ginneken AC, van Brunschot S, Belterman CN, Wilders R, van Roon MA, Tan HL, Wilde AA, et al. Overlap syndrome of cardiac sodium channel disease in mice carrying the equivalent mutation of human SCN5A-1795insD. Circulation. 2006;114:2584–94. [DOI] [PubMed] [Google Scholar]
- 77.Glynn P, Musa H, Wu X, Unudurthi SD, Little S, Qian L, Wright PJ, Radwanski PB, Gyorke S, Mohler PJ,et al. Voltage-Gated Sodium Channel Phosphorylation at Ser571 Regulates Late Current, Arrhythmia, and Cardiac Function In Vivo. Circulation. 2015;132:567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wagner S, Dybkova N, Rasenack EC, Jacobshagen C, Fabritz L, Kirchhof P, Maier SK, Zhang T, Hasenfuss G, Brown JH, et al. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest. 2006;116:3127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park DS, Cerrone M, Morley G, Vasquez C, Fowler S, Liu N, Bernstein SA, Liu FY, Zhang J, Rogers CS, et al. Genetically engineered SCN5A mutant pig hearts exhibit conduction defects and arrhythmias. J Clin Invest. 2015;125:403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brodehl A, Belke DD, Garnett L, Martens K, Abdelfatah N, Rodriguez M, Diao C, Chen YX, Gordon PM, Nygren A, et al. Transgenic mice overexpressing desmocollin-2 (DSC2) develop cardiomyopathy associated with myocardial inflammation and fibrotic remodeling. PLoS One. 2017;12:e0174019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Notari M, Hu Y, Sutendra G, Dedeic Z, Lu M, Dupays L, Yavari A, Carr CA, Zhong S, Opel A, et al. iASPP, a previously unidentified regulator of desmosomes, prevents arrhythmogenic right ventricular cardiomyopathy (ARVC)-induced sudden death. Proc Natl Acad Sci U S A. 2015;112:E973–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cerrone M, Noorman M, Lin X, Chkourko H, Liang FX, van der Nagel R, Hund T, Birchmeier W, Mohler P, van Veen TA, et al. Sodium current deficit and arrhythmogenesis in a murine model of plakophilin-2 haploinsufficiency. Cardiovasc Res. 2012;95:460–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moncayo-Arlandi J, Guasch E, Sanz-de la Garza M, Casado M, Garcia NA, Mont L, Sitges M, Knoll R, Buyandelger B, Campuzano O, et al. Molecular disturbance underlies to arrhythmogenic cardiomyopathy induced by transgene content, age and exercise in a truncated PKP2 mouse model. Hum Mol Genet. 2016;25:3676–3688. [DOI] [PubMed] [Google Scholar]
- 84.Cerrone M, Montnach J, Lin X, Zhao YT, Zhang M, Agullo-Pascual E, Leo-Macias A, Alvarado FJ, Dolgalev I, Karathanos TV, et al. Plakophilin-2 is required for transcription of genes that control calcium cycling and cardiac rhythm. Nat Commun. 2017;8:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van Opbergen C, Bagwan N, Maurya S, Kim J-C, Smith A, Blackwell D, Johnston J, Knollmann B, Cerrone M, Lundby A, et al. Exercise causes arrhythmogenic remodeling of intracellular calcium dynamics in Plakophilin-2 deficient hearts Circulation. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Laitinen-Forsblom PJ, Makynen P, Makynen H, Yli-Mayry S, Virtanen V, Kontula K and Aalto-Setala K. SCN5A mutation associated with cardiac conduction defect and atrial arrhythmias. J Cardiovasc Electrophysiol. 2006;17:480–5. [DOI] [PubMed] [Google Scholar]
- 87.Groenewegen WA, Firouzi M, Bezzina CR, Vliex S, van Langen IM, Sandkuijl L, Smits JP, Hulsbeek M, Rook MB, Jongsma HJ, et al. A cardiac sodium channel mutation cosegregates with a rare connexin40 genotype in familial atrial standstill. Circ Res. 2003;92:14–22. [DOI] [PubMed] [Google Scholar]
- 88.Watanabe H, Yang T, Stroud DM, Lowe JS, Harris L, Atack TC, Wang DW, Hipkens SB, Leake B, Hall L, et al. Striking In vivo phenotype of a disease-associated human SCN5A mutation producing minimal changes in vitro. Circulation. 2011;124:1001–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huttner IG, Trivedi G, Jacoby A, Mann SA, Vandenberg JI and Fatkin D. A transgenic zebrafish model of a human cardiac sodium channel mutation exhibits bradycardia, conduction-system abnormalities and early death. J Mol Cell Cardiol. 2013;61:123–32. [DOI] [PubMed] [Google Scholar]
- 90.Marian AJ and Braunwald E. Hypertrophic Cardiomyopathy: Genetics, Pathogenesis, Clinical Manifestations, Diagnosis, and Therapy. Circ Res. 2017;121:749–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Berul CI, Christe ME, Aronovitz MJ, Maguire CT, Seidman CE, Seidman JG and Mendelsohn ME. Familial hypertrophic cardiomyopathy mice display gender differences in electrophysiological abnormalities. J Interv Card Electrophysiol. 1998;2:7–14. [DOI] [PubMed] [Google Scholar]
- 92.Marian AJ, Wu Y, Lim DS, McCluggage M, Youker K, Yu QT, Brugada R, DeMayo F, Quinones M and Roberts R. A transgenic rabbit model for human hypertrophic cardiomyopathy. J Clin Invest. 1999;104:1683–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tsoutsman T, Chung J, Doolan A, Nguyen L, Williams IA, Tu E, Lam L, Bailey CG, Rasko JE, Allen DG, et al. Molecular insights from a novel cardiac troponin I mouse model of familial hypertrophic cardiomyopathy. J Mol Cell Cardiol. 2006;41:623–32. [DOI] [PubMed] [Google Scholar]
- 94.Tsoutsman T, Kelly M, Ng DC, Tan JE, Tu E, Lam L, Bogoyevitch MA, Seidman CE, Seidman JG and Semsarian C. Severe heart failure and early mortality in a double-mutation mouse model of familial hypertrophic cardiomyopathy. Circulation. 2008;117:1820–31. [DOI] [PubMed] [Google Scholar]
- 95.Toib A, Zhang C, Borghetti G, Zhang X, Wallner M, Yang Y, Troupes CD, Kubo H, Sharp TE, Feldsott E, et al. Remodeling of repolarization and arrhythmia susceptibility in a myosin-binding protein C knockout mouse model. Am J Physiol Heart Circ Physiol. 2017;313:H620–H630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Knollmann BC, Kirchhof P, Sirenko SG, Degen H, Greene AE, Schober T, Mackow JC, Fabritz L, Potter JD and Morad M. Familial hypertrophic cardiomyopathy-linked mutant troponin T causes stress-induced ventricular tachycardia and Ca2+-dependent action potential remodeling. Circ Res. 2003;92:428–36. [DOI] [PubMed] [Google Scholar]
- 97.Baudenbacher F, Schober T, Pinto JR, Sidorov VY, Hilliard F, Solaro RJ, Potter JD and Knollmann BC. Myofilament Ca2+ sensitization causes susceptibility to cardiac arrhythmia in mice. J Clin Invest. 2008;118:3893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schober T, Huke S, Venkataraman R, Gryshchenko O, Kryshtal D, Hwang HS, Baudenbacher FJ and Knollmann BC. Myofilament Ca sensitization increases cytosolic Ca binding affinity, alters intracellular Ca homeostasis, and causes pause-dependent Ca-triggered arrhythmia. Circ Res. 2012;111:170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huke S, Venkataraman R, Faggioni M, Bennuri S, Hwang HS, Baudenbacher F and Knollmann BC. Focal energy deprivation underlies arrhythmia susceptibility in mice with calcium-sensitized myofilaments. Circ Res. 2013;112:1334–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Venkataraman R, Baldo MP, Hwang HS, Veltri T, Pinto JR, Baudenbacher FJ and Knollmann BC. Myofilament calcium de-sensitization and contractile uncoupling prevent pause-triggered ventricular tachycardia in mouse hearts with chronic myocardial infarction. J Mol Cell Cardiol. 2013;60:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huke S and Knollmann BC. Increased myofilament Ca2+-sensitivity and arrhythmia susceptibility. J Mol Cell Cardiol. 2010;48:824–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schuttler D, Bapat A, Kaab S, Lee K, Tomsits P, Clauss S and Hucker WJ. Animal Models of Atrial Fibrillation. Circ Res. 2020;127:91–110. [DOI] [PubMed] [Google Scholar]
- 103.Faggioni M, Savio-Galimberti E, Venkataraman R, Hwang HS, Kannankeril PJ, Darbar D and Knollmann BC. Suppression of spontaneous ca elevations prevents atrial fibrillation in calsequestrin 2-null hearts. Circ Arrhythm Electrophysiol. 2014;7:313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Muller FU, Lewin G, Baba HA, Boknik P, Fabritz L, Kirchhefer U, Kirchhof P, Loser K, Matus M, Neumann J, et al. Heart-directed expression of a human cardiac isoform of cAMP-response element modulator in transgenic mice. J Biol Chem. 2005;280:6906–14. [DOI] [PubMed] [Google Scholar]
- 105.Temple J, Frias P, Rottman J, Yang T, Wu Y, Verheijck EE, Zhang W, Siprachanh C, Kanki H, Atkinson JB, et al. Atrial fibrillation in KCNE1-null mice. Circ Res. 2005;97:62–9. [DOI] [PubMed] [Google Scholar]
- 106.Moreira LM, Takawale A, Hulsurkar M, Menassa DA, Antanaviciute A, Lahiri SK, Mehta N, Evans N, Psarros C, Robinson P, et al. Paracrine signalling by cardiac calcitonin controls atrial fibrogenesis and arrhythmia. Nature. 2020;587:460–465. [DOI] [PubMed] [Google Scholar]
- 107.Ozcan C, Battaglia E, Young R and Suzuki G. LKB1 knockout mouse develops spontaneous atrial fibrillation and provides mechanistic insights into human disease process. J Am Heart Assoc. 2015;4:e001733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Polejaeva IA, Ranjan R, Davies CJ, Regouski M, Hall J, Olsen AL, Meng Q, Rutigliano HM, Dosdall DJ, et al. Increased Susceptibility to Atrial Fibrillation Secondary to Atrial Fibrosis in Transgenic Goats Expressing Transforming Growth Factor-beta1. J Cardiovasc Electrophysiol. 2016;27:1220–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Roselli C, Rienstra M and Ellinor PT. Genetics of Atrial Fibrillation in 2020: GWAS, Genome Sequencing, Polygenic Risk, and Beyond. Circ Res. 2020;127:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kirchhof P, Kahr PC, Kaese S, Piccini I, Vokshi I, Scheld HH, Rotering H, Fortmueller L, Laakmann S, Verheule S, et al. PITX2c is expressed in the adult left atrium, and reducing Pitx2c expression promotes atrial fibrillation inducibility and complex changes in gene expression. Circ Cardiovasc Genet. 2011;4:123–33. [DOI] [PubMed] [Google Scholar]
- 111.Syeda F, Holmes AP, Yu TY, Tull S, Kuhlmann SM, Pavlovic D, Betney D, Riley G, Kucera JP, Jousset F, et al. PITX2 Modulates Atrial Membrane Potential and the Antiarrhythmic Effects of Sodium-Channel Blockers. J Am Coll Cardiol. 2016;68:1881–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nadadur RD, Broman MT, Boukens B, Mazurek SR, Yang X, van den Boogaard M, Bekeny J, Gadek M, Ward T, Zhang M, et al. Pitx2 modulates a Tbx5-dependent gene regulatory network to maintain atrial rhythm. Sci Transl Med. 2016;8:354ra115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tuomi JM, Tyml K and Jones DL. Atrial tachycardia/fibrillation in the connexin 43 G60S mutant (Oculodentodigital dysplasia) mouse. Am J Physiol Heart Circ Physiol. 2011;300:H1402–11. [DOI] [PubMed] [Google Scholar]
- 114.Mahida S, Mills RW, Tucker NR, Simonson B, Macri V, Lemoine MD, Das S, Milan DJ and Ellinor PT. Overexpression of KCNN3 results in sudden cardiac death. Cardiovasc Res. 2014;101:326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lei M, Goddard C, Liu J, Leoni AL, Royer A, Fung SS, Xiao G, Ma A, Zhang H, Charpentier F, et al. Sinus node dysfunction following targeted disruption of the murine cardiac sodium channel gene Scn5a. J Physiol. 2005;567:387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jeevaratnam K, Zhang Y, Guzadhur L, Duehmke RM, Lei M, Grace AA and Huang CL. Differences in sino-atrial and atrio-ventricular function with age and sex attributable to the Scn5a+/− mutation in a murine cardiac model. Acta Physiol (Oxf). 2010;200:23–33. [DOI] [PubMed] [Google Scholar]
- 117.Stieber J, Herrmann S, Feil S, Loster J, Feil R, Biel M, Hofmann F and Ludwig A. The hyperpolarization-activated channel HCN4 is required for the generation of pacemaker action potentials in the embryonic heart. Proc Natl Acad Sci U S A. 2003;100:15235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Baruscotti M, Bucchi A, Viscomi C, Mandelli G, Consalez G, Gnecchi-Rusconi T, Montano N, Casali KR, Micheloni S, Barbuti A, et al. Deep bradycardia and heart block caused by inducible cardiac-specific knockout of the pacemaker channel gene Hcn4. Proc Natl Acad Sci U S A. 2011;108:1705–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fenske S, Krause SC, Hassan SI, Becirovic E, Auer F, Bernard R, Kupatt C, Lange P, Ziegler T, Wotjak CT, et al. Sick sinus syndrome in HCN1-deficient mice. Circulation. 2013;128:2585–94. [DOI] [PubMed] [Google Scholar]
- 120.Ludwig A, Budde T, Stieber J, Moosmang S, Wahl C, Holthoff K, Langebartels A, Wotjak C, Munsch T, Zong X, et al. Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. EMBO J. 2003;22:216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fenske S, Mader R, Scharr A, Paparizos C, Cao-Ehlker X, Michalakis S, Shaltiel L, Weidinger M, Stieber J, Feil S, et al. HCN3 contributes to the ventricular action potential waveform in the murine heart. Circ Res. 2011;109:1015–23. [DOI] [PubMed] [Google Scholar]
- 122.Herrmann S, Fabritz L, Layh B, Kirchhof P and Ludwig A. Insights into sick sinus syndrome from an inducible mouse model. Cardiovasc Res. 2011;90:38–48. [DOI] [PubMed] [Google Scholar]
- 123.Chowdhury R, Ashraf H, Melanson M, Tanada Y, Nguyen M, Silberbach M, Wakimoto H, Benson DW, Anderson RH and Kasahara H. Mouse Model of Human Congenital Heart Disease: Progressive Atrioventricular Block Induced by a Heterozygous Nkx2–5 Homeodomain Missense Mutation. Circ Arrhythm Electrophysiol. 2015;8:1255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kasahara H, Wakimoto H, Liu M, Maguire CT, Converso KL, Shioi T, Huang WY, Manning WJ, Paul D, Lawitts J, et al. Progressive atrioventricular conduction defects and heart failure in mice expressing a mutant Csx/Nkx2.5 homeoprotein. J Clin Invest. 2001;108:189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Arad M, Moskowitz IP, Patel VV, Ahmad F, Perez-Atayde AR, Sawyer DB, Walter M, Li GH, Burgon PG, Maguire CT, et al. Transgenic mice overexpressing mutant PRKAG2 define the cause of Wolff-Parkinson-White syndrome in glycogen storage cardiomyopathy. Circulation. 2003;107:2850–6. [DOI] [PubMed] [Google Scholar]
- 126.Davies JK, Wells DJ, Liu K, Whitrow HR, Daniel TD, Grignani R, Lygate CA, Schneider JE, Noel G, Watkins H, et al. Characterization of the role of gamma2 R531G mutation in AMP-activated protein kinase in cardiac hypertrophy and Wolff-Parkinson-White syndrome. Am J Physiol Heart Circ Physiol. 2006;290:H1942–51. [DOI] [PubMed] [Google Scholar]
- 127.Sidhu JS, Rajawat YS, Rami TG, Gollob MH, Wang Z, Yuan R, Marian AJ, DeMayo FJ, Weilbacher D, Taffet GE, et al. Transgenic mouse model of ventricular preexcitation and atrioventricular reentrant tachycardia induced by an AMP-activated protein kinase loss-of-function mutation responsible for Wolff-Parkinson-White syndrome. Circulation. 2005;111:21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Aanhaanen WT, Boukens BJ, Sizarov A, Wakker V, de Gier-de Vries C, van Ginneken AC, Moorman AF, Coronel R and Christoffels VM. Defective Tbx2-dependent patterning of the atrioventricular canal myocardium causes accessory pathway formation in mice. J Clin Invest. 2011;121:534–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lip GYH, Coca A, Kahan T, Boriani G, Manolis AS, Olsen MH, Oto A, Potpara TS, Steffel J, Marin F, et al. Hypertension and cardiac arrhythmias: a consensus document from the European Heart Rhythm Association (EHRA) and ESC Council on Hypertension, endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS) and Sociedad Latinoamericana de Estimulacion Cardiaca y Electrofisiologia (SOLEACE). Europace. 2017;19:891–911. [DOI] [PubMed] [Google Scholar]
- 130.Narins CR, Aktas MK, Chen AY, McNitt S, Ling FS, Younis A, Zareba W, Daubert JP, Huang DT, Rosero S, et al. Arrhythmic and Mortality Outcomes Among Ischemic Versus Nonischemic Cardiomyopathy Patients Receiving Primary Implantable Cardioverter-Defibrillator Therapy. JACC Clin Electrophysiol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Thomas KL, Jackson LR, 2nd, Shrader P, Ansell J, Fonarow GC, Gersh B, Kowey PR, Mahaffey KW, Singer DE, Thomas L, et al. Prevalence, Characteristics, and Outcomes of Valvular Heart Disease in Patients With Atrial Fibrillation: Insights From the ORBIT-AF (Outcomes Registry for Better Informed Treatment for Atrial Fibrillation). J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Peretto G, Sala S, Rizzo S, De Luca G, Campochiaro C, Sartorelli S, Benedetti G, Palmisano A, Esposito A, Tresoldi M, et al. Arrhythmias in myocarditis: State of the art. Heart Rhythm. 2019;16:793–801. [DOI] [PubMed] [Google Scholar]
- 133.Pinilla-Vera M, Hahn VS and Kass DA. Leveraging Signaling Pathways to Treat Heart Failure With Reduced Ejection Fraction. Circ Res. 2019;124:1618–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Heineke J and Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. [DOI] [PubMed] [Google Scholar]
- 135.Knowlton AA, Chen L and Malik ZA. Heart failure and mitochondrial dysfunction: the role of mitochondrial fission/fusion abnormalities and new therapeutic strategies. J Cardiovasc Pharmacol. 2014;63:196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhou B and Tian R. Mitochondrial dysfunction in pathophysiology of heart failure. J Clin Invest. 2018;128:3716–3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bito V, Heinzel FR, Biesmans L, Antoons G and Sipido KR. Crosstalk between L-type Ca2+ channels and the sarcoplasmic reticulum: alterations during cardiac remodelling. Cardiovasc Res. 2008;77:315–24. [DOI] [PubMed] [Google Scholar]
- 138.Harris DM, Mills GD, Chen X, Kubo H, Berretta RM, Votaw VS, Santana LF and Houser SR. Alterations in early action potential repolarization causes localized failure of sarcoplasmic reticulum Ca2+ release. Circ Res. 2005;96:543–50. [DOI] [PubMed] [Google Scholar]
- 139.Wehrens XH, Lehnart SE, Reiken S, Vest JA, Wronska A and Marks AR. Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proc Natl Acad Sci U S A. 2006;103:511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wende AR, Brahma MK, McGinnis GR and Young ME. Metabolic Origins of Heart Failure. JACC Basic Transl Sci. 2017;2:297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gloschat CR, Koppel AC, Aras KK, Brennan JA, Holzem KM and Efimov IR. Arrhythmogenic and metabolic remodelling of failing human heart. J Physiol. 2016;594:3963–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Koitabashi N, Danner T, Zaiman AL, Pinto YM, Rowell J, Mankowski J, Zhang D, Nakamura T, Takimoto E and Kass DA. Pivotal role of cardiomyocyte TGF-beta signaling in the murine pathological response to sustained pressure overload. J Clin Invest. 2011;121:2301–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rockman HA, Ross RS, Harris AN, Knowlton KU, Steinhelper ME, Field LJ, Ross J, Jr. and Chien KR. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc Natl Acad Sci U S A. 1991;88:8277–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.De Jong AM, Van Gelder IC, Vreeswijk-Baudoin I, Cannon MV, Van Gilst WH and Maass AH. Atrial remodeling is directly related to end-diastolic left ventricular pressure in a mouse model of ventricular pressure overload. PLoS One. 2013;8:e72651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Marionneau C, Brunet S, Flagg TP, Pilgram TK, Demolombe S and Nerbonne JM. Distinct cellular and molecular mechanisms underlie functional remodeling of repolarizing K+ currents with left ventricular hypertrophy. Circ Res. 2008;102:1406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.deAlmeida AC, van Oort RJ and Wehrens XH. Transverse aortic constriction in mice. J Vis Exp. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Moorjani N, Catarino P, El-Sayed R, Al-Ahmed S, Meyer B, Al-Mohanna F and Westaby S. A pressure overload model to track the molecular biology of heart failure. Eur J Cardiothorac Surg. 2003;24:920–5. [DOI] [PubMed] [Google Scholar]
- 148.Gyongyosi M, Pavo N, Lukovic D, Zlabinger K, Spannbauer A, Traxler D, Goliasch G, Mandic L, Bergler-Klein J, Gugerell A, et al. Porcine model of progressive cardiac hypertrophy and fibrosis with secondary postcapillary pulmonary hypertension. J Transl Med. 2017;15:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang Y, Huang S, Sah VP, Ross J, Jr., Brown JH, Han J and Chien KR. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J Biol Chem. 1998;273:2161–8. [DOI] [PubMed] [Google Scholar]
- 150.Li XM, Ma YT, Yang YN, Liu F, Chen BD, Han W, Zhang JF and Gao XM. Downregulation of survival signalling pathways and increased apoptosis in the transition of pressure overload-induced cardiac hypertrophy to heart failure. Clin Exp Pharmacol Physiol. 2009;36:1054–61. [DOI] [PubMed] [Google Scholar]
- 151.Patrucco E, Notte A, Barberis L, Selvetella G, Maffei A, Brancaccio M, Marengo S, Russo G, Azzolino O, Rybalkin SD, et al. PI3Kgamma modulates the cardiac response to chronic pressure overload by distinct kinase-dependent and -independent effects. Cell. 2004;118:375–87. [DOI] [PubMed] [Google Scholar]
- 152.Xia Y, Lee K, Li N, Corbett D, Mendoza L and Frangogiannis NG. Characterization of the inflammatory and fibrotic response in a mouse model of cardiac pressure overload. Histochem Cell Biol. 2009;131:471–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.O’Connell TD, Swigart PM, Rodrigo MC, Ishizaka S, Joho S, Turnbull L, Tecott LH, Baker AJ, Foster E, Grossman W, et al. Alpha1-adrenergic receptors prevent a maladaptive cardiac response to pressure overload. J Clin Invest. 2006;116:1005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhao M, Fajardo G, Urashima T, Spin JM, Poorfarahani S, Rajagopalan V, Huynh D, Connolly A, Quertermous T and Bernstein D. Cardiac pressure overload hypertrophy is differentially regulated by beta-adrenergic receptor subtypes. Am J Physiol Heart Circ Physiol. 2011;301:H1461–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rockman HA, Wachhorst SP, Mao L and Ross J, Jr. ANG II receptor blockade prevents ventricular hypertrophy and ANF gene expression with pressure overload in mice. Am J Physiol. 1994;266:H2468–75. [DOI] [PubMed] [Google Scholar]
- 156.Qu J, Volpicelli FM, Garcia LI, Sandeep N, Zhang J, Marquez-Rosado L, Lampe PD and Fishman GI. Gap junction remodeling and spironolactone-dependent reverse remodeling in the hypertrophied heart. Circ Res. 2009;104:365–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Weisheit C, Zhang Y, Faron A, Kopke O, Weisheit G, Steinstrasser A, Frede S, Meyer R, Boehm O, Hoeft A, et al. Ly6C(low) and not Ly6C(high) macrophages accumulate first in the heart in a model of murine pressure-overload. PLoS One. 2014;9:e112710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kent RL, Rozich JD, McCollam PL, McDermott DE, Thacker UF, Menick DR, McDermott PJ and Cooper Gt. Rapid expression of the Na(+)-Ca2+ exchanger in response to cardiac pressure overload. Am J Physiol. 1993;265:H1024–9. [DOI] [PubMed] [Google Scholar]
- 159.Ujihara Y, Iwasaki K, Takatsu S, Hashimoto K, Naruse K, Mohri S and Katanosaka Y. Induced NCX1 overexpression attenuates pressure overload-induced pathological cardiac remodelling. Cardiovasc Res. 2016;111:348–61. [DOI] [PubMed] [Google Scholar]
- 160.del Monte F, Williams E, Lebeche D, Schmidt U, Rosenzweig A, Gwathmey JK, Lewandowski ED and Hajjar RJ. Improvement in survival and cardiac metabolism after gene transfer of sarcoplasmic reticulum Ca(2+)-ATPase in a rat model of heart failure. Circulation. 2001;104:1424–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lyon AR, Bannister ML, Collins T, Pearce E, Sepehripour AH, Dubb SS, Garcia E, O’Gara P, Liang L, Kohlbrenner E, et al. SERCA2a gene transfer decreases sarcoplasmic reticulum calcium leak and reduces ventricular arrhythmias in a model of chronic heart failure. Circ Arrhythm Electrophysiol. 2011;4:362–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cutler MJ, Wan X, Plummer BN, Liu H, Deschenes I, Laurita KR, Hajjar RJ and Rosenbaum DS. Targeted sarcoplasmic reticulum Ca2+ ATPase 2a gene delivery to restore electrical stability in the failing heart. Circulation. 2012;126:2095–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Greenberg B, Butler J, Felker GM, Ponikowski P, Voors AA, Desai AS, Barnard D, Bouchard A, Jaski B, Lyon AR, et al. Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): a randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet. 2016;387:1178–86. [DOI] [PubMed] [Google Scholar]
- 164.Zhang C, Yasuno S, Kuwahara K, Zankov DP, Kobori A, Makiyama T and Horie M. Blockade of angiotensin II type 1 receptor improves the arrhythmia morbidity in mice with left ventricular hypertrophy. Circ J. 2006;70:335–41. [DOI] [PubMed] [Google Scholar]
- 165.Jin H, Chemaly ER, Lee A, Kho C, Hadri L, Hajjar RJ and Akar FG. Mechanoelectrical remodeling and arrhythmias during progression of hypertrophy. FASEB J. 2010;24:451–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Boulaksil M, Noorman M, Engelen MA, van Veen TA, Vos MA, de Bakker JM and van Rijen HV. Longitudinal arrhythmogenic remodelling in a mouse model of longstanding pressure overload. Neth Heart J. 2010;18:509–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Westphal C, Spallek B, Konkel A, Marko L, Qadri F, DeGraff LM, Schubert C, Bradbury JA, Regitz-Zagrosek V, Falck JR, et al. CYP2J2 overexpression protects against arrhythmia susceptibility in cardiac hypertrophy. PLoS One. 2013;8:e73490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Richards DA, Aronovitz MJ, Calamaras TD, Tam K, Martin GL, Liu P, Bowditch HK, Zhang P, Huggins GS and Blanton RM. Distinct Phenotypes Induced by Three Degrees of Transverse Aortic Constriction in Mice. Sci Rep. 2019;9:5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bosch L, de Haan JJ, Bastemeijer M, van der Burg J, van der Worp E, Wesseling M, Viola M, Odille C, El Azzouzi H, Pasterkamp G, et al. The transverse aortic constriction heart failure animal model: a systematic review and meta-analysis. Heart Fail Rev. 2021;26:1515–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zi M, Stafford N, Prehar S, Baudoin F, Oceandy D, Wang X, Bui T, Shaheen M, Neyses L and Cartwright EJ. Cardiac hypertrophy or failure? - A systematic evaluation of the transverse aortic constriction model in C57BL/6NTac and C57BL/6J substrains. Curr Res Physiol. 2019;1:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Barrick CJ, Rojas M, Schoonhoven R, Smyth SS and Threadgill DW. Cardiac response to pressure overload in 129S1/SvImJ and C57BL/6J mice: temporal- and background-dependent development of concentric left ventricular hypertrophy. Am J Physiol Heart Circ Physiol. 2007;292:H2119–30. [DOI] [PubMed] [Google Scholar]
- 172.Koentges C, Pepin ME, Musse C, Pfeil K, Alvarez SVV, Hoppe N, Hoffmann MM, Odening KE, Sossalla S, Zirlik A, et al. Gene expression analysis to identify mechanisms underlying heart failure susceptibility in mice and humans. Basic Res Cardiol. 2018;113:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nickel AG, von Hardenberg A, Hohl M, Loffler JR, Kohlhaas M, Becker J, Reil JC, Kazakov A, Bonnekoh J, Stadelmaier M, et al. Reversal of Mitochondrial Transhydrogenase Causes Oxidative Stress in Heart Failure. Cell Metab. 2015;22:472–84. [DOI] [PubMed] [Google Scholar]
- 174.Litwin SE, Katz SE, Weinberg EO, Lorell BH, Aurigemma GP and Douglas PS. Serial echocardiographic-Doppler assessment of left ventricular geometry and function in rats with pressure-overload hypertrophy. Chronic angiotensin-converting enzyme inhibition attenuates the transition to heart failure. Circulation. 1995;91:2642–54. [DOI] [PubMed] [Google Scholar]
- 175.Pogwizd SM, Qi M, Yuan W, Samarel AM and Bers DM. Upregulation of Na(+)/Ca(2+) exchanger expression and function in an arrhythmogenic rabbit model of heart failure. Circ Res. 1999;85:1009–19. [DOI] [PubMed] [Google Scholar]
- 176.Desantiago J, Ai X, Islam M, Acuna G, Ziolo MT, Bers DM and Pogwizd SM. Arrhythmogenic effects of beta2-adrenergic stimulation in the failing heart are attributable to enhanced sarcoplasmic reticulum Ca load. Circ Res. 2008;102:1389–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pogwizd SM, Schlotthauer K, Li L, Yuan W and Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: Roles of sodium-calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circ Res. 2001;88:1159–67. [DOI] [PubMed] [Google Scholar]
- 178.Liu T, Takimoto E, Dimaano VL, DeMazumder D, Kettlewell S, Smith G, Sidor A, Abraham TP and O’Rourke B. Inhibiting mitochondrial Na+/Ca2+ exchange prevents sudden death in a Guinea pig model of heart failure. Circ Res. 2014;115:44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang Y, Yuan J, Qian Z, Zhang X, Chen Y, Hou X and Zou J. beta2 adrenergic receptor activation governs cardiac repolarization and arrhythmogenesis in a guinea pig model of heart failure. Sci Rep. 2015;5:7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dey S, DeMazumder D, Sidor A, Foster DB and O’Rourke B. Mitochondrial ROS Drive Sudden Cardiac Death and Chronic Proteome Remodeling in Heart Failure. Circ Res. 2018;123:356–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hegyi B, Morotti S, Liu C, Ginsburg KS, Bossuyt J, Belardinelli L, Izu LT, Chen-Izu Y, Banyasz T, Grandi E, et alEnhanced Depolarization Drive in Failing Rabbit Ventricular Myocytes: Calcium-Dependent and beta-Adrenergic Effects on Late Sodium, L-Type Calcium, and Sodium-Calcium Exchange Currents. Circ Arrhythm Electrophysiol. 2019;12:e007061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Walther T, Falk V, Binner C, Loscher N, Schubert A, Rauch T, von Oppel U, Autschbach R and Mohr FW. Experimental aortic stenosis and corresponding left ventricular hypertrophy in sheep. J Invest Surg. 2000;13:327–31. [DOI] [PubMed] [Google Scholar]
- 183.Aoyagi T, Fujii AM, Flanagan MF, Arnold LW, Brathwaite KW, Colan SD and Mirsky I. Transition from compensated hypertrophy to intrinsic myocardial dysfunction during development of left ventricular pressure-overload hypertrophy in conscious sheep. Systolic dysfunction precedes diastolic dysfunction. Circulation. 1993;88:2415–25. [DOI] [PubMed] [Google Scholar]
- 184.Ye Y, Gong G, Ochiai K, Liu J and Zhang J. High-energy phosphate metabolism and creatine kinase in failing hearts: a new porcine model. Circulation. 2001;103:1570–6. [DOI] [PubMed] [Google Scholar]
- 185.Hiemstra JA, Lee DI, Chakir K, Gutierrez-Aguilar M, Marshall KD, Zgoda PJ, Cruz Rivera N, Dozier DG, Ferguson BS, Heublein DM, et al. Saxagliptin and Tadalafil Differentially Alter Cyclic Guanosine Monophosphate (cGMP) Signaling and Left Ventricular Function in Aortic-Banded Mini-Swine. J Am Heart Assoc. 2016;5:e003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ishikawa K, Aguero J, Oh JG, Hammoudi N, Fish LA, Leonardson L, Picatoste B, Santos-Gallego CG, Fish KM and Hajjar RJ. Increased stiffness is the major early abnormality in a pig model of severe aortic stenosis and predisposes to congestive heart failure in the absence of systolic dysfunction. J Am Heart Assoc. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yarbrough WM, Mukherjee R, Stroud RE, Rivers WT, Oelsen JM, Dixon JA, Eckhouse SR, Ikonomidis JS, Zile MR and Spinale FG. Progressive induction of left ventricular pressure overload in a large animal model elicits myocardial remodeling and a unique matrix signature. J Thorac Cardiovasc Surg. 2012;143:215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Moorjani N, Catarino P, Trabzuni D, Saleh S, Moorji A, Dzimiri N, Al-Mohanna F, Westaby S and Ahmad M. Upregulation of Bcl-2 proteins during the transition to pressure overload-induced heart failure. Int J Cardiol. 2007;116:27–33. [DOI] [PubMed] [Google Scholar]
- 189.Fleet WF, Johnson TA, Graebner CA and Gettes LS. Effect of serial brief ischemic episodes on extracellular K+, pH, and activation in the pig. Circulation. 1985;72:922–32. [DOI] [PubMed] [Google Scholar]
- 190.Johnson TA, Engle CL, Boyd LM, Koch GG, Gwinn M and Gettes LS. Magnitude and time course of extracellular potassium inhomogeneities during acute ischemia in pigs. Effect of verapamil. Circulation. 1991;83:622–34. [DOI] [PubMed] [Google Scholar]
- 191.Huss JM and Kelly DP. Nuclear receptor signaling and cardiac energetics. Circ Res. 2004;95:568–78. [DOI] [PubMed] [Google Scholar]
- 192.Cadenas S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic Biol Med. 2018;117:76–89. [DOI] [PubMed] [Google Scholar]
- 193.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R and Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sakamoto J, Miura T, Tsuchida A, Fukuma T, Hasegawa T and Shimamoto K. Reperfusion arrhythmias in the murine heart: their characteristics and alteration after ischemic preconditioning. Basic Res Cardiol. 1999;94:489–95. [DOI] [PubMed] [Google Scholar]
- 195.Xu Z, Alloush J, Beck E and Weisleder N. A murine model of myocardial ischemia-reperfusion injury through ligation of the left anterior descending artery. J Vis Exp. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lugrin J, Parapanov R, Krueger T and Liaudet L. Murine Myocardial Infarction Model using Permanent Ligation of Left Anterior Descending Coronary Artery. J Vis Exp. 2019. [DOI] [PubMed] [Google Scholar]
- 197.Patten RD, Aronovitz MJ, Deras-Mejia L, Pandian NG, Hanak GG, Smith JJ, Mendelsohn ME and Konstam MA. Ventricular remodeling in a mouse model of myocardial infarction. Am J Physiol. 1998;274:H1812–20. [DOI] [PubMed] [Google Scholar]
- 198.Wang D, Tediashvili G, Hu X, Gravina A, Marcus SG, Zhang H, Olgin JE, Deuse T and Schrepfer S. A Cryoinjury Model to Study Myocardial Infarction in the Mouse. J Vis Exp. 2019. [DOI] [PubMed] [Google Scholar]
- 199.van den Bos EJ, Mees BM, de Waard MC, de Crom R and Duncker DJ. A novel model of cryoinjury-induced myocardial infarction in the mouse: a comparison with coronary artery ligation. Am J Physiol Heart Circ Physiol. 2005;289:H1291–300. [DOI] [PubMed] [Google Scholar]
- 200.Nofi C, Zhang K, Tang YD, Li Y, Migirov A, Ojamaa K, Gerdes AM and Zhang Y. Chronic dantrolene treatment attenuates cardiac dysfunction and reduces atrial fibrillation inducibility in a rat myocardial infarction heart failure model. Heart Rhythm O2. 2020;1:126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Beiert T, Tiyerili V, Knappe V, Effelsberg V, Linhart M, Stockigt F, Klein S, Schierwagen R, Trebicka J, Nickenig G, et al. Relaxin reduces susceptibility to post-infarct atrial fibrillation in mice due to anti-fibrotic and anti-inflammatory properties. Biochem Biophys Res Commun. 2017;490:643–649. [DOI] [PubMed] [Google Scholar]
- 202.Boixel C, Fontaine V, Rucker-Martin C, Milliez P, Louedec L, Michel JB, Jacob MP and Hatem SN. Fibrosis of the left atria during progression of heart failure is associated with increased matrix metalloproteinases in the rat. J Am Coll Cardiol. 2003;42:336–44. [DOI] [PubMed] [Google Scholar]
- 203.Curtis MJ, Macleod BA and Walker MJ. Models for the study of arrhythmias in myocardial ischaemia and infarction: the use of the rat. J Mol Cell Cardiol. 1987;19:399–419. [DOI] [PubMed] [Google Scholar]
- 204.Hundahl LA, Tfelt-Hansen J and Jespersen T. Rat Models of Ventricular Fibrillation Following Acute Myocardial Infarction. J Cardiovasc Pharmacol Ther. 2017;22:514–528. [DOI] [PubMed] [Google Scholar]
- 205.Kolossov E, Bostani T, Roell W, Breitbach M, Pillekamp F, Nygren JM, Sasse P, Rubenchik O, Fries JW, Wenzel D, et al. Engraftment of engineered ES cell-derived cardiomyocytes but not BM cells restores contractile function to the infarcted myocardium. J Exp Med. 2006;203:2315–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Rucker-Martin C, Milliez P, Tan S, Decrouy X, Recouvreur M, Vranckx R, Delcayre C, Renaud JF, Dunia I, Segretain D, et al. Chronic hemodynamic overload of the atria is an important factor for gap junction remodeling in human and rat hearts. Cardiovasc Res. 2006;72:69–79. [DOI] [PubMed] [Google Scholar]
- 207.Zhang Y, Dedkov EI, Lee B 3rd, Li Y, Pun K and Gerdes AM. Thyroid hormone replacement therapy attenuates atrial remodeling and reduces atrial fibrillation inducibility in a rat myocardial infarction-heart failure model. J Card Fail. 2014;20:1012–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Schrickel JW, Bielik H, Yang A, Schimpf R, Shlevkov N, Burkhardt D, Meyer R, Grohe C, Fink K, Tiemann K, et al. Induction of atrial fibrillation in mice by rapid transesophageal atrial pacing. Basic Res Cardiol. 2002;97:452–60. [DOI] [PubMed] [Google Scholar]
- 209.Berul CI, Aronovitz MJ, Wang PJ and Mendelsohn ME. In vivo cardiac electrophysiology studies in the mouse. Circulation. 1996;94:2641–8. [DOI] [PubMed] [Google Scholar]
- 210.Clasen L, Eickholt C, Angendohr S, Jungen C, Shin DI, Donner B, Furnkranz A, Kelm M, Klocker N, Meyer C, et al. A modified approach for programmed electrical stimulation in mice: Inducibility of ventricular arrhythmias. PLoS One. 2018;13:e0201910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gehrmann J, Frantz S, Maguire CT, Vargas M, Ducharme A, Wakimoto H, Lee RT and Berul CI. Electrophysiological characterization of murine myocardial ischemia and infarction. Basic Res Cardiol. 2001;96:237–50. [DOI] [PubMed] [Google Scholar]
- 212.Lubkemeier I, Andrie R, Lickfett L, Bosen F, Stockigt F, Dobrowolski R, Draffehn AM, Fregeac J, Schultze JL, Bukauskas FF, et al. The Connexin40A96S mutation from a patient with atrial fibrillation causes decreased atrial conduction velocities and sustained episodes of induced atrial fibrillation in mice. J Mol Cell Cardiol. 2013;65:19–32. [DOI] [PubMed] [Google Scholar]
- 213.Ng GA, Cobbe SM and Smith GL. Non-uniform prolongation of intracellular Ca2+ transients recorded from the epicardial surface of isolated hearts from rabbits with heart failure. Cardiovasc Res. 1998;37:489–502. [DOI] [PubMed] [Google Scholar]
- 214.Nisbet AM, Camelliti P, Walker NL, Burton FL, Cobbe SM, Kohl P and Smith GL. Prolongation of atrio-ventricular node conduction in a rabbit model of ischaemic cardiomyopathy: Role of fibrosis and connexin remodelling. J Mol Cell Cardiol. 2016;94:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Sinno H, Derakhchan K, Libersan D, Merhi Y, Leung TK and Nattel S. Atrial ischemia promotes atrial fibrillation in dogs. Circulation. 2003;107:1930–6. [DOI] [PubMed] [Google Scholar]
- 216.Nishida K, Qi XY, Wakili R, Comtois P, Chartier D, Harada M, Iwasaki YK, Romeo P, Maguy A, Dobrev D, et al. Mechanisms of atrial tachyarrhythmias associated with coronary artery occlusion in a chronic canine model. Circulation. 2011;123:137–46. [DOI] [PubMed] [Google Scholar]
- 217.Rivard L, Sinno H, Shiroshita-Takeshita A, Schram G, Leung TK and Nattel S. The pharmacological response of ischemia-related atrial fibrillation in dogs: evidence for substrate-specific efficacy. Cardiovasc Res. 2007;74:104–13. [DOI] [PubMed] [Google Scholar]
- 218.Keeran KJ, Jeffries KR, Zetts AD, Taylor J, Kozlov S and Hunt TJ. A Chronic Cardiac Ischemia Model in Swine Using an Ameroid Constrictor. J Vis Exp. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Millard RW. Induction of functional coronary collaterals in the swine heart. Basic Res Cardiol. 1981;76:468–73. [DOI] [PubMed] [Google Scholar]
- 220.Fallavollita JA, Riegel BJ, Suzuki G, Valeti U and Canty JM, Jr. Mechanism of sudden cardiac death in pigs with viable chronically dysfunctional myocardium and ischemic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2005;289:H2688–96. [DOI] [PubMed] [Google Scholar]
- 221.Canty JM, Jr., Suzuki G, Banas MD, Verheyen F, Borgers M and Fallavollita JA. Hibernating myocardium: chronically adapted to ischemia but vulnerable to sudden death. Circ Res. 2004;94:1142–9. [DOI] [PubMed] [Google Scholar]
- 222.Hegyi B, Bossuyt J, Griffiths LG, Shimkunas R, Coulibaly Z, Jian Z, Grimsrud KN, Sondergaard CS, Ginsburg KS, Chiamvimonvat N, et al. Complex electrophysiological remodeling in postinfarction ischemic heart failure. Proc Natl Acad Sci U S A. 2018;115:E3036–E3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sasano T, McDonald AD, Kikuchi K and Donahue JK. Molecular ablation of ventricular tachycardia after myocardial infarction. Nat Med. 2006;12:1256–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Greener ID, Sasano T, Wan X, Igarashi T, Strom M, Rosenbaum DS and Donahue JK. Connexin43 gene transfer reduces ventricular tachycardia susceptibility after myocardial infarction. J Am Coll Cardiol. 2012;60:1103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Syed FF, Hayes DL, Friedman PA and Asirvatham SJ. Hemodynamics of Cardiac Pacing: Optimization and Programming to Enhance Cardiac Function Cardiac Pacing, Defibrillation and Resynchronization; 2013: 41–92. [Google Scholar]
- 226.Xia R, Vlcek J, Bauer J, Kaab S, Ishikawa-Ankerhold H, van den Heuvel DA, Schulz C, Massberg S and Clauss S. Whole-Mount Immunofluorescence Staining, Confocal Imaging and 3D Reconstruction of the Sinoatrial and Atrioventricular Node in the Mouse. J Vis Exp. 2020. [DOI] [PubMed] [Google Scholar]
- 227.Hulsmans M, Clauss S, Xiao L, Aguirre AD, King KR, Hanley A, Hucker WJ, Wulfers EM, Seemann G, Courties G, Iet al. Macrophages Facilitate Electrical Conduction in the Heart. Cell. 2017;169:510–522 e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kim NK, Wolfson D, Fernandez N, Shin M and Cho HC. A rat model of complete atrioventricular block recapitulates clinical indices of bradycardia and provides a platform to test disease-modifying therapies. Sci Rep. 2019;9:6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Gizurarson S, Lorentzon M, Ramunddal T, Waagstein F, Bergfeldt L and Omerovic E. Effects of complete heart block on myocardial function, morphology, and energy metabolism in the rat. Europace. 2007;9:411–6. [DOI] [PubMed] [Google Scholar]
- 230.Oros A, Beekman JD and Vos MA. The canine model with chronic, complete atrio-ventricular block. Pharmacol Ther. 2008;119:168–78. [DOI] [PubMed] [Google Scholar]
- 231.Steiner C and Kovalik AT. A simple technique for production of chronic complete heart block in dogs. J Appl Physiol. 1968;25:631–2. [DOI] [PubMed] [Google Scholar]
- 232.Vos MA, Verduyn SC, Gorgels AP, Lipcsei GC and Wellens HJ. Reproducible induction of early afterdepolarizations and torsade de pointes arrhythmias by d-sotalol and pacing in dogs with chronic atrioventricular block. Circulation. 1995;91:864–72. [DOI] [PubMed] [Google Scholar]
- 233.Sipido KR, Volders PG, de Groot SH, Verdonck F, Van de Werf F, Wellens HJ and Vos MA. Enhanced Ca(2+) release and Na/Ca exchange activity in hypertrophied canine ventricular myocytes: potential link between contractile adaptation and arrhythmogenesis. Circulation. 2000;102:2137–44. [DOI] [PubMed] [Google Scholar]
- 234.de Groot SH, Vos MA, Gorgels AP, Leunissen JD, van der Steld BJ and Wellens HJ. Combining monophasic action potential recordings with pacing to demonstrate delayed afterdepolarizations and triggered arrhythmias in the intact heart. Value of diastolic slope. Circulation. 1995;92:2697–704. [DOI] [PubMed] [Google Scholar]
- 235.Greiser M, Neuberger HR, Harks E, El-Armouche A, Boknik P, de Haan S, Verheyen F, Verheule S, Schmitz W, Ravens U, et al. Distinct contractile and molecular differences between two goat models of atrial dysfunction: AV block-induced atrial dilatation and atrial fibrillation. J Mol Cell Cardiol. 2009;46:385–94. [DOI] [PubMed] [Google Scholar]
- 236.Tse HF and Lau CP. Long-term effect of right ventricular pacing on myocardial perfusion and function. J Am Coll Cardiol. 1997;29:744–9. [DOI] [PubMed] [Google Scholar]
- 237.Lee MA, Dae MW, Langberg JJ, Griffin JC, Chin MC, Finkbeiner WE, O’Connell JW, Botvinick E, Scheinman MM and Rosenqvist M. Effects of long-term right ventricular apical pacing on left ventricular perfusion, innervation, function and histology. J Am Coll Cardiol. 1994;24:225–32. [DOI] [PubMed] [Google Scholar]
- 238.Pak PH, Nuss HB, Tunin RS, Kaab S, Tomaselli GF, Marban E and Kass DA. Repolarization abnormalities, arrhythmia and sudden death in canine tachycardia-induced cardiomyopathy. J Am Coll Cardiol. 1997;30:576–84. [DOI] [PubMed] [Google Scholar]
- 239.Kaab S, Nuss HB, Chiamvimonvat N, O’Rourke B, Pak PH, Kass DA, Marban E and Tomaselli GF. Ionic mechanism of action potential prolongation in ventricular myocytes from dogs with pacing-induced heart failure. Circ Res. 1996;78:262–73. [DOI] [PubMed] [Google Scholar]
- 240.Kaab S, Dixon J, Duc J, Ashen D, Nabauer M, Beuckelmann DJ, Steinbeck G, McKinnon D and Tomaselli GF. Molecular basis of transient outward potassium current downregulation in human heart failure: a decrease in Kv4.3 mRNA correlates with a reduction in current density. Circulation. 1998;98:1383–93. [DOI] [PubMed] [Google Scholar]
- 241.Aiba T, Barth AS, Hesketh GG, Hashambhoy YL, Chakir K, Tunin RS, Greenstein JL, Winslow RL, Kass DA and Tomaselli GF. Cardiac resynchronization therapy improves altered Na channel gating in canine model of dyssynchronous heart failure. Circ Arrhythm Electrophysiol. 2013;6:546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Winslow RL, Rice J, Jafri S, Marban E and O’Rourke B. Mechanisms of altered excitation-contraction coupling in canine tachycardia-induced heart failure, II: model studies. Circ Res. 1999;84:571–86. [DOI] [PubMed] [Google Scholar]
- 243.Li H, Lichter JG, Seidel T, Tomaselli GF, Bridge JH and Sachse FB. Cardiac Resynchronization Therapy Reduces Subcellular Heterogeneity of Ryanodine Receptors, T-Tubules, and Ca2+ Sparks Produced by Dyssynchronous Heart Failure. Circ Heart Fail. 2015;8:1105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.DeMazumder D, Kass DA, O’Rourke B and Tomaselli GF. Cardiac resynchronization therapy restores sympathovagal balance in the failing heart by differential remodeling of cholinergic signaling. Circ Res. 2015;116:1691–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Wiesmann T, Freitag D, Dersch W, Eschbach D, Irqsusi M, Steinfeldt T, Wulf H and Feldmann C. Dantrolene versus amiodarone for cardiopulmonary resuscitation: a randomized, double-blinded experimental study. Sci Rep. 2017;7:40875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Shinagawa K, Shi YF, Tardif JC, Leung TK and Nattel S. Dynamic nature of atrial fibrillation substrate during development and reversal of heart failure in dogs. Circulation. 2002;105:2672–8. [DOI] [PubMed] [Google Scholar]
- 247.Shinagawa K, Li D, Leung TK and Nattel S. Consequences of atrial tachycardia-induced remodeling depend on the preexisting atrial substrate. Circulation. 2002;105:251–7. [DOI] [PubMed] [Google Scholar]
- 248.Cha TJ, Ehrlich JR, Zhang L, Shi YF, Tardif JC, Leung TK and Nattel S. Dissociation between ionic remodeling and ability to sustain atrial fibrillation during recovery from experimental congestive heart failure. Circulation. 2004;109:412–8. [DOI] [PubMed] [Google Scholar]
- 249.Cha TJ, Ehrlich JR, Zhang L and Nattel S. Atrial ionic remodeling induced by atrial tachycardia in the presence of congestive heart failure. Circulation. 2004;110:1520–6. [DOI] [PubMed] [Google Scholar]
- 250.Chen Y, Wakili R, Xiao J, Wu CT, Luo X, Clauss S, Dawson K, Qi X, Naud P, Shi YF, et al. Detailed characterization of microRNA changes in a canine heart failure model: Relationship to arrhythmogenic structural remodeling. J Mol Cell Cardiol. 2014;77:113–24. [DOI] [PubMed] [Google Scholar]
- 251.Luo X, Pan Z, Shan H, Xiao J, Sun X, Wang N, Lin H, Xiao L, Maguy A, Qi XY, et al. MicroRNA-26 governs profibrillatory inward-rectifier potassium current changes in atrial fibrillation. J Clin Invest. 2013;123:1939–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Zhao Y, Gu TX, Zhang GW, Liu HG and Wang C. Losartan affects the substrate for atrial fibrillation maintenance in a rabbit model. Cardiovasc Pathol. 2013;22:383–8. [DOI] [PubMed] [Google Scholar]
- 253.Jia X, Zheng S, Xie X, Zhang Y, Wang W, Wang Z, Zhang Y, Wang J, Gao M and Hou Y. MicroRNA-1 accelerates the shortening of atrial effective refractory period by regulating KCNE1 and KCNB2 expression: an atrial tachypacing rabbit model. PLoS One. 2013;8:e85639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Pemberton CJ, Johnson ML, Yandle TG and Espiner EA. Deconvolution analysis of cardiac natriuretic peptides during acute volume overload. Hypertension. 2000;36:355–9. [DOI] [PubMed] [Google Scholar]
- 255.Stahlberg M, Nakagawa R, Bedja D, Zhu G, Lin BL, Saberi A, Lee DI and Kass DA. Chronic Atrial and Ventricular Pacing in the Mouse. Circ Heart Fail. 2019;12:e005655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Gutruf P, Yin RT, Lee KB, Ausra J, Brennan JA, Qiao Y, Xie Z, Peralta R, Talarico O, Murillo A, et al. Wireless, battery-free, fully implantable multimodal and multisite pacemakers for applications in small animal models. Nat Commun. 2019;10:5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Yamashita T, Murakawa Y, Hayami N, Fukui E, Kasaoka Y, Inoue M and Omata M. Short-term effects of rapid pacing on mRNA level of voltage-dependent K(+) channels in rat atrium: electrical remodeling in paroxysmal atrial tachycardia. Circulation. 2000;101:2007–14. [DOI] [PubMed] [Google Scholar]
- 258.Mulla W, Hajaj B, Elyagon S, Mor M, Gillis R, Murninkas M, Klapper-Goldstein H, Plaschkes I, Chalifa-Caspi V, Etzion S, et al. Rapid Atrial Pacing Promotes Atrial Fibrillation Substrate in Unanesthetized Instrumented Rats. Front Physiol. 2019;10:1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Chen MH, Liu TW, Xie L, Song FQ, He T, Zeng ZY and Mo SR. Ventricular fibrillation induced by transoesophageal cardiac pacing: a new model of cardiac arrest in rats. Resuscitation. 2007;74:546–51. [DOI] [PubMed] [Google Scholar]
- 260.Bruins P, te Velthuis H, Yazdanbakhsh AP, Jansen PG, van Hardevelt FW, de Beaumont EM, Wildevuur CR, Eijsman L, Trouwborst A and Hack CE. Activation of the complement system during and after cardiopulmonary bypass surgery: postsurgery activation involves C-reactive protein and is associated with postoperative arrhythmia. Circulation. 1997;96:3542–8. [DOI] [PubMed] [Google Scholar]
- 261.Chung MK, Martin DO, Sprecher D, Wazni O, Kanderian A, Carnes CA, Bauer JA, Tchou PJ, Niebauer MJ, Natale A, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–91. [DOI] [PubMed] [Google Scholar]
- 262.Dobrev D, Aguilar M, Heijman J, Guichard JB and Nattel S. Postoperative atrial fibrillation: mechanisms, manifestations and management. Nat Rev Cardiol. 2019;16:417–436. [DOI] [PubMed] [Google Scholar]
- 263.Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS and Chung MK. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–10. [DOI] [PubMed] [Google Scholar]
- 264.Marott SC, Nordestgaard BG, Zacho J, Friberg J, Jensen GB, Tybjaerg-Hansen A and Benn M. Does elevated C-reactive protein increase atrial fibrillation risk? A Mendelian randomization of 47,000 individuals from the general population. J Am Coll Cardiol. 2010;56:789–95. [DOI] [PubMed] [Google Scholar]
- 265.Conen D, Ridker PM, Everett BM, Tedrow UB, Rose L, Cook NR, Buring JE and Albert CM. A multimarker approach to assess the influence of inflammation on the incidence of atrial fibrillation in women. Eur Heart J. 2010;31:1730–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Hu YF, Chen YJ, Lin YJ and Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. 2015;12:230–43. [DOI] [PubMed] [Google Scholar]
- 267.Guo Y, Lip GY and Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. 2012;60:2263–70. [DOI] [PubMed] [Google Scholar]
- 268.Reilly SN, Jayaram R, Nahar K, Antoniades C, Verheule S, Channon KM, Alp NJ, Schotten U and Casadei B. Atrial sources of reactive oxygen species vary with the duration and substrate of atrial fibrillation: implications for the antiarrhythmic effect of statins. Circulation. 2011;124:1107–17. [DOI] [PubMed] [Google Scholar]
- 269.Antoniades C, Demosthenous M, Reilly S, Margaritis M, Zhang MH, Antonopoulos A, Marinou K, Nahar K, Jayaram R, Tousoulis D, et al. Myocardial redox state predicts in-hospital clinical outcome after cardiac surgery effects of short-term pre-operative statin treatment. J Am Coll Cardiol. 2012;59:60–70. [DOI] [PubMed] [Google Scholar]
- 270.Tschope C, Ammirati E, Bozkurt B, Caforio ALP, Cooper LT, Felix SB, Hare JM, Heidecker B, Heymans S, Hubner N, et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. 2021;18:169–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Bailey JR, Loftus AR and Allan RJC. Myopericarditis: recognition and impact in the military population. J R Army Med Corps. 2019;165:451–453. [DOI] [PubMed] [Google Scholar]
- 272.Tselios K and Urowitz MB. Cardiovascular and Pulmonary Manifestations of Systemic Lupus Erythematosus. Curr Rheumatol Rev. 2017;13:206–218. [DOI] [PubMed] [Google Scholar]
- 273.Lazaros G, Oikonomou E and Tousoulis D. Established and novel treatment options in acute myocarditis, with or without heart failure. Expert Rev Cardiovasc Ther. 2017;15:25–34. [DOI] [PubMed] [Google Scholar]
- 274.Tselentakis EV, Woodford E, Chandy J, Gaudette GR and Saltman AE. Inflammation effects on the electrical properties of atrial tissue and inducibility of postoperative atrial fibrillation. J Surg Res. 2006;135:68–75. [DOI] [PubMed] [Google Scholar]
- 275.Zhang Y, Wang YT, Shan ZL, Guo HY, Guan Y and Yuan HT. Role of inflammation in the initiation and maintenance of atrial fibrillation and the protective effect of atorvastatin in a goat model of aseptic pericarditis. Mol Med Rep. 2015;11:2615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Izumi T, Kodama M and Shibata A. Experimental giant cell myocarditis induced by cardiac myosin immunization. Eur Heart J. 1991;12 Suppl D:166–8. [DOI] [PubMed] [Google Scholar]
- 277.Radhakrishnan VV. Experimental myocarditis in the guinea-pig. Cardiovasc Res. 1996;31:651–4. [PubMed] [Google Scholar]
- 278.Grodums EI and Dempster G. Myocarditis in experimental Coxsackie B-3 infection. Can J Microbiol. 1959;5:605–15. [DOI] [PubMed] [Google Scholar]
- 279.Kishimoto C, Matsumori A, Ohmae M, Tomioka N and Kawai C. Electrocardiographic findings in experimental myocarditis in DBA/2 mice: complete atrioventricular block in the acute stage, low voltage of the QRS complex in the subacute stage and arrhythmias in the chronic stage. J Am Coll Cardiol. 1984;3:1461–8. [DOI] [PubMed] [Google Scholar]
- 280.Tracy S, Hofling K, Pirruccello S, Lane PH, Reyna SM and Gauntt CJ. Group B coxsackievirus myocarditis and pancreatitis: connection between viral virulence phenotypes in mice. J Med Virol. 2000;62:70–81. [DOI] [PubMed] [Google Scholar]
- 281.Terasaki F, Kitaura Y, Hayashi T, Nakayama Y, Deguchi H and Kawamura K. Arrhythmias in Coxsackie B3 virus myocarditis. Continuous electrocardiography in conscious mice and histopathology of the heart with special reference to the conduction system. Heart Vessels Suppl. 1990;5:45–50. [PubMed] [Google Scholar]
- 282.Kaese S, Larbig R, Rohrbeck M, Frommeyer G, Dechering D, Olligs J, Schonhofer-Merl S, Wessely R, Klingel K, Seebohm G, et al. Electrophysiological alterations in a murine model of chronic coxsackievirus B3 myocarditis. PLoS One. 2017;12:e0180029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Ohmae M, Kishimoto C and Tomioka N. Complete atrioventricular block in experimental murine myocarditis. J Electrocardiol. 2005;38:230–4. [DOI] [PubMed] [Google Scholar]
- 284.Pummerer CL, Luze K, Grassl G, Bachmaier K, Offner F, Burrell SK, Lenz DM, Zamborelli TJ, Penninger JM and Neu N. Identification of cardiac myosin peptides capable of inducing autoimmune myocarditis in BALB/c mice. J Clin Invest. 1996;97:2057–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Machino-Ohtsuka T, Tajiri K, Kimura T, Sakai S, Sato A, Yoshida T, Hiroe M, Yasutomi Y, Aonuma K and Imanaka-Yoshida K. Tenascin-C aggravates autoimmune myocarditis via dendritic cell activation and Th17 cell differentiation. J Am Heart Assoc. 2014;3:e001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Blyszczuk P. Myocarditis in Humans and in Experimental Animal Models. Front Cardiovasc Med. 2019;6:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Tang Q, Huang J, Qian H, Chen L, Wang T, Wang H, Shen D, Wu H and Xiong R. Antiarrhythmic effect of atorvastatin on autoimmune myocarditis is mediated by improving myocardial repolarization. Life Sci. 2007;80:601–8. [DOI] [PubMed] [Google Scholar]
- 288.Wei SC, Meijers WC, Axelrod ML, Anang NAS, Screever EM, Wescott EC, Johnson DB, Whitley E, Lehmann L, Courand PY, et al. A Genetic Mouse Model Recapitulates Immune Checkpoint Inhibitor-Associated Myocarditis and Supports a Mechanism-Based Therapeutic Intervention. Cancer Discov. 2021;11:614–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton-Cheh C, Lubitz SA, Magnani JW, Ellinor PT, Set al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386:154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Joseph LC, Subramanyam P, Radlicz C, Trent CM, Iyer V, Colecraft HM and Morrow JP. Mitochondrial oxidative stress during cardiac lipid overload causes intracellular calcium leak and arrhythmia. Heart Rhythm. 2016;13:1699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Donnellan E, Wazni OM, Elshazly M, Kanj M, Hussein AA, Baranowski B, Kochar A, Trulock K, Aminian A, Schauer P, et al. Impact of Bariatric Surgery on Atrial Fibrillation Type. Circ Arrhythm Electrophysiol. 2020;13:e007626. [DOI] [PubMed] [Google Scholar]
- 292.Furman BL. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr Protoc. 2021;1:e78. [DOI] [PubMed] [Google Scholar]
- 293.Grisanti LA. Diabetes and Arrhythmias: Pathophysiology, Mechanisms and Therapeutic Outcomes. Front Physiol. 2018;9:1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Shimoni Y, Firek L, Severson D and Giles W. Short-term diabetes alters K+ currents in rat ventricular myocytes. Circ Res. 1994;74:620–8. [DOI] [PubMed] [Google Scholar]
- 295.Meo M, Meste O, Signore S, Sorrentino A, Cannata A, Zhou Y, Matsuda A, Luciani M, Kannappan R, Goichberg P, et al. Reduction in Kv Current Enhances the Temporal Dispersion of the Action Potential in Diabetic Myocytes: Insights From a Novel Repolarization Algorithm. J Am Heart Assoc. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Lopez-Izquierdo A, Pereira RO, Wende AR, Punske BB, Abel ED and Tristani-Firouzi M. The absence of insulin signaling in the heart induces changes in potassium channel expression and ventricular repolarization. Am J Physiol Heart Circ Physiol. 2014;306:H747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Mitasikova M, Lin H, Soukup T, Imanaga I and Tribulova N. Diabetes and thyroid hormones affect connexin-43 and PKC-epsilon expression in rat heart atria. Physiol Res. 2009;58:211–217. [DOI] [PubMed] [Google Scholar]
- 298.Hage C, Michaelsson E, Linde C, Donal E, Daubert JC, Gan LM and Lund LH. Inflammatory Biomarkers Predict Heart Failure Severity and Prognosis in Patients With Heart Failure With Preserved Ejection Fraction: A Holistic Proteomic Approach. Circ Cardiovasc Genet. 2017;10. [DOI] [PubMed] [Google Scholar]
- 299.Ferdous Z, Qureshi MA, Jayaprakash P, Parekh K, John A, Oz M, Raza H, Dobrzynski H, Adrian TE and Howarth FC. Different Profile of mRNA Expression in Sinoatrial Node from Streptozotocin-Induced Diabetic Rat. PLoS One. 2016;11:e0153934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Howarth FC, Parekh K, Jayaprakash P, Inbaraj ES, Oz M, Dobrzynski H and Adrian TE. Altered profile of mRNA expression in atrioventricular node of streptozotocininduced diabetic rats. Mol Med Rep. 2017;16:3720–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Hegyi B, Borst JM, Bailey LRJ, Shen EY, Lucena AJ, Navedo MF, Bossuyt J and Bers DM. Hyperglycemia regulates cardiac K(+) channels via O-GlcNAc-CaMKII and NOX2-ROS-PKC pathways. Basic Res Cardiol. 2020;115:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Hegyi B, Fasoli A, Ko CY, Van BW, Alim CC, Shen EY, Ciccozzi MM, Tapa S, Ripplinger CM, Erickson JR, et al. CaMKII Serine 280 O-GlcNAcylation Links Diabetic Hyperglycemia to Proarrhythmia. Circ Res. 2021;129:98–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Monnerat G, Alarcon ML, Vasconcellos LR, Hochman-Mendez C, Brasil G, Bassani RA, Casis O, Malan D, Travassos LH, Sepulveda M, et al. Macrophage-dependent IL-1beta production induces cardiac arrhythmias in diabetic mice. Nat Commun. 2016;7:13344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Huang H, Amin V, Gurin M, Wan E, Thorp E, Homma S and Morrow JP. Diet-induced obesity causes long QT and reduces transcription of voltage-gated potassium channels. J Mol Cell Cardiol. 2013;59:151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.McCauley MD, Hong L, Sridhar A, Menon A, Perike S, Zhang M, da Silva IB, Yan J, Bonini MG, Ai X, et al. Ion Channel and Structural Remodeling in Obesity-Mediated Atrial Fibrillation. Circ Arrhythm Electrophysiol. 2020;13:e008296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Ashrafi R, Yon M, Pickavance L, Yanni Gerges J, Davis G, Wilding J, Jian K, Zhang H, Hart G and Boyett M. Altered Left Ventricular Ion Channel Transcriptome in a High-Fat-Fed Rat Model of Obesity: Insight into Obesity-Induced Arrhythmogenesis. J Obes. 2016;2016:7127898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Liu YB, Wu CC, Lu LS, Su MJ, Lin CW, Lin SF, Chen LS, Fishbein MC, Chen PS and Lee YT. Sympathetic nerve sprouting, electrical remodeling, and increased vulnerability to ventricular fibrillation in hypercholesterolemic rabbits. Circ Res. 2003;92:1145–52. [DOI] [PubMed] [Google Scholar]
- 308.Forman DE, Cittadini A, Azhar G, Douglas PS and Wei JY. Cardiac morphology and function in senescent rats: gender-related differences. J Am Coll Cardiol. 1997;30:1872–7. [DOI] [PubMed] [Google Scholar]
- 309.Mesquita TRR, Zhang R, de Couto G, Valle J, Sanchez L, Rogers RG, Holm K, Liu W, Marban E and Cingolani E. Mechanisms of atrial fibrillation in aged rats with heart failure with preserved ejection fraction. Heart Rhythm. 2020;17:1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Hayashi H, Wang C, Miyauchi Y, Omichi C, Pak HN, Zhou S, Ohara T, Mandel WJ, Lin SF, Fishbein MC, et al. Aging-related increase to inducible atrial fibrillation in the rat model. J Cardiovasc Electrophysiol. 2002;13:801–8. [DOI] [PubMed] [Google Scholar]
- 311.Carlsson L, Almgren O and Duker G. QTU-prolongation and torsades de pointes induced by putative class III antiarrhythmic agents in the rabbit: etiology and interventions. J Cardiovasc Pharmacol. 1990;16:276–85. [DOI] [PubMed] [Google Scholar]
- 312.Mazur A, Roden DM and Anderson ME. Systemic administration of calmodulin antagonist W-7 or protein kinase A inhibitor H-8 prevents torsade de pointes in rabbits. Circulation. 1999;100:2437–42. [DOI] [PubMed] [Google Scholar]
- 313.Gbadebo TD, Trimble RW, Khoo MS, Temple J, Roden DM and Anderson ME. Calmodulin inhibitor W-7 unmasks a novel electrocardiographic parameter that predicts initiation of torsade de pointes. Circulation. 2002;105:770–4. [DOI] [PubMed] [Google Scholar]
- 314.Shanmugam M, Molina CE, Gao S, Severac-Bastide R, Fischmeister R and Babu GJ. Decreased sarcolipin protein expression and enhanced sarco(endo)plasmic reticulum Ca2+ uptake in human atrial fibrillation. Biochem Biophys Res Commun. 2011;410:97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Xie LH, Shanmugam M, Park JY, Zhao Z, Wen H, Tian B, Periasamy M and Babu GJ. Ablation of sarcolipin results in atrial remodeling. Am J Physiol Cell Physiol. 2012;302:C1762–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Makarewich CA, Munir AZ, Schiattarella GG, Bezprozvannaya S, Raguimova ON, Cho EE, Vidal AH, Robia SL, Bassel-Duby R and Olson EN. The DWORF micropeptide enhances contractility and prevents heart failure in a mouse model of dilated cardiomyopathy. Elife. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Wang W, McKinnie SM, Patel VB, Haddad G, Wang Z, Zhabyeyev P, Das SK, Basu R, McLean B, Kandalam V, et al. Loss of Apelin exacerbates myocardial infarction adverse remodeling and ischemia-reperfusion injury: therapeutic potential of synthetic Apelin analogues. J Am Heart Assoc. 2013;2:e000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Marks AR. Calcium cycling proteins and heart failure: mechanisms and therapeutics. J Clin Invest. 2013;123:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Herren AW, Bers DM and Grandi E. Post-translational modifications of the cardiac Na channel: contribution of CaMKII-dependent phosphorylation to acquired arrhythmias. Am J Physiol Heart Circ Physiol. 2013;305:H431–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Galleano I, Harms H, Choudhury K, Khoo K, Delemotte L and Pless SA. Functional cross-talk between phosphorylation and disease-causing mutations in the cardiac sodium channel Nav1.5. Proc Natl Acad Sci U S A. 2021;118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Kamp TJ and Hell JW. Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ Res. 2000;87:1095–102. [DOI] [PubMed] [Google Scholar]
- 322.Hell JW. Beta-adrenergic regulation of the L-type Ca2+ channel Ca(V)1.2 by PKA rekindles excitement. Sci Signal. 2010;3:pe33. [DOI] [PubMed] [Google Scholar]
- 323.Fu Y, Westenbroek RE, Scheuer T and Catterall WA. Phosphorylation sites required for regulation of cardiac calcium channels in the fight-or-flight response. Proc Natl Acad Sci U S A. 2013;110:19621–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Park KS, Yang JW, Seikel E and Trimmer JS. Potassium channel phosphorylation in excitable cells: providing dynamic functional variability to a diverse family of ion channels. Physiology (Bethesda). 2008;23:49–57. [DOI] [PubMed] [Google Scholar]
- 325.Zhang YH and Hancox JC. Regulation of cardiac Na+-Ca2+ exchanger activity by protein kinase phosphorylation--still a paradox? Cell Calcium. 2009;45:1–10. [DOI] [PubMed] [Google Scholar]
- 326.Dobrev D and Wehrens XH. Role of RyR2 phosphorylation in heart failure and arrhythmias: Controversies around ryanodine receptor phosphorylation in cardiac disease. Circ Res. 2014;114:1311–9; discussion 1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.van Oort RJ, McCauley MD, Dixit SS, Pereira L, Yang Y, Respress JL, Wang Q, De Almeida AC, Skapura DG, Anderson ME,et al. Ryanodine receptor phosphorylation by calcium/calmodulin-dependent protein kinase II promotes life-threatening ventricular arrhythmias in mice with heart failure. Circulation. 2010;122:2669–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Campbell HM, Quick AP, Abu-Taha I, Chiang DY, Kramm CF, Word TA, Brandenburg S, Hulsurkar M, Alsina KM, Liu HB, et al. Loss of SPEG Inhibitory Phosphorylation of Ryanodine Receptor Type-2 Promotes Atrial Fibrillation. Circulation. 2020;142:1159–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Trum M, Islam MMT, Lebek S, Baier M, Hegner P, Eaton P, Maier LS and Wagner S. Inhibition of cardiac potassium currents by oxidation-activated protein kinase A contributes to early afterdepolarizations in the heart. Am J Physiol Heart Circ Physiol. 2020;319:H1347–H1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Montgomery RL, Davis CA, Potthoff MJ, Haberland M, Fielitz J, Qi X, Hill JA, Richardson JA and Olson EN. Histone deacetylases 1 and 2 redundantly regulate cardiac morphogenesis, growth, and contractility. Genes Dev. 2007;21:1790–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Theis JL, Sharpe KM, Matsumoto ME, Chai HS, Nair AA, Theis JD, de Andrade M, Wieben ED, Michels VV and Olson TM. Homozygosity mapping and exome sequencing reveal GATAD1 mutation in autosomal recessive dilated cardiomyopathy. Circ Cardiovasc Genet. 2011;4:585–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Zhang W, Song M, Qu J and Liu GH. Epigenetic Modifications in Cardiovascular Aging and Diseases. Circ Res. 2018;123:773–786. [DOI] [PubMed] [Google Scholar]
- 333.Zhao G, Zhou J, Gao J, Liu Y, Gu S, Zhang X and Su P. Genome-wide DNA methylation analysis in permanent atrial fibrillation. Mol Med Rep. 2017;16:5505–5514. [DOI] [PubMed] [Google Scholar]
- 334.Shan H, Zhang Y, Lu Y, Zhang Y, Pan Z, Cai B, Wang N, Li X, Feng T, Hong Y, et al. Downregulation of miR-133 and miR-590 contributes to nicotine-induced atrial remodelling in canines. Cardiovasc Res. 2009;83:465–72. [DOI] [PubMed] [Google Scholar]
- 335.Lu Y, Zhang Y, Wang N, Pan Z, Gao X, Zhang F, Zhang Y, Shan H, Luo X, Bai Y, et al. MicroRNA-328 contributes to adverse electrical remodeling in atrial fibrillation. Circulation. 2010;122:2378–87. [DOI] [PubMed] [Google Scholar]
- 336.Dawson K, Wakili R, Ordog B, Clauss S, Chen Y, Iwasaki Y, Voigt N, Qi XY, Sinner MF, Dobrev D, et al. MicroRNA29: a mechanistic contributor and potential biomarker in atrial fibrillation. Circulation. 2013;127:1466–75, 1475e1–28. [DOI] [PubMed] [Google Scholar]
- 337.Guzzolino E, Pellegrino M, Ahuja N, Garrity D, D’Aurizio R, Groth M, Baumgart M, Hatcher CJ, Mercatanti A, Evangelista M, et al. miR-182–5p is an evolutionarily conserved Tbx5 effector that impacts cardiac development and electrical activity in zebrafish. Cell Mol Life Sci. 2020;77:3215–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Yang D, Wan X, Dennis AT, Bektik E, Wang Z, Costa MGS, Fagnen C, Venien-Bryan C, Xu X, Gratz DH, et al. MicroRNA Biophysically Modulates Cardiac Action Potential by Direct Binding to Ion Channel. Circulation. 2021;143:1597–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Ruan Z, Sun X, Sheng H and Zhu L. Long non-coding RNA expression profile in atrial fibrillation. Int J Clin Exp Pathol. 2015;8:8402–10. [PMC free article] [PubMed] [Google Scholar]
- 340.Gore-Panter SR, Hsu J, Barnard J, Moravec CS, Van Wagoner DR, Chung MK and Smith JD. PANCR, the PITX2 Adjacent Noncoding RNA, Is Expressed in Human Left Atria and Regulates PITX2c Expression. Circ Arrhythm Electrophysiol. 2016;9:e003197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Li Z, Wang X, Wang W, Du J, Wei J, Zhang Y, Wang J and Hou Y. Altered long non-coding RNA expression profile in rabbit atria with atrial fibrillation: TCONS_00075467 modulates atrial electrical remodeling by sponging miR-328 to regulate CACNA1C. J Mol Cell Cardiol. 2017;108:73–85. [DOI] [PubMed] [Google Scholar]
- 342.Wang LY, Shen H, Yang Q, Min J, Wang Q, Xi W, Yin L, Le SG, Zhang YF, Xiao J, et al. LncRNA-LINC00472 contributes to the pathogenesis of atrial fibrillation (Af) by reducing expression of JP2 and RyR2 via miR-24. Biomed Pharmacother. 2019;120:109364. [DOI] [PubMed] [Google Scholar]
- 343.Loaiza R, Benkusky NA, Powers PP, Hacker T, Noujaim S, Ackerman MJ, Jalife J and Valdivia HH. Heterogeneity of ryanodine receptor dysfunction in a mouse model of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2013;112:298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Lehnart SE, Mongillo M, Bellinger A, Lindegger N, Chen BX, Hsueh W, Reiken S, Wronska A, Drew LJ, Ward CW, et al. Leaky Ca2+ release channel/ryanodine receptor 2 causes seizures and sudden cardiac death in mice. J Clin Invest. 2008;118:2230–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Zhang Y, Fraser JA, Jeevaratnam K, Hao X, Hothi SS, Grace AA, Lei M and Huang CL. Acute atrial arrhythmogenicity and altered Ca(2+) homeostasis in murine RyR2-P2328S hearts. Cardiovasc Res. 2011;89:794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Nakamura Y, Yamamoto T, Kobayashi S, Tamitani M, Hamada Y, Fukui G, Xu X, Nishimura S, Kato T, Uchinoumi H, et al. Ryanodine receptor-bound calmodulin is essential to protect against catecholaminergic polymorphic ventricular tachycardia. JCI Insight. 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Shan J, Xie W, Betzenhauser M, Reiken S, Chen BX, Wronska A and Marks AR. Calcium leak through ryanodine receptors leads to atrial fibrillation in 3 mouse models of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2012;111:708–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Kannankeril PJ, Mitchell BM, Goonasekera SA, Chelu MG, Zhang W, Sood S, Kearney DL, Danila CI, De Biasi M, Wehrens XH, et al. Mice with the R176Q cardiac ryanodine receptor mutation exhibit catecholamine-induced ventricular tachycardia and cardiomyopathy. Proc Natl Acad Sci U S A. 2006;103:12179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Fischer E, Gottschalk A and Schuler C. An optogenetic arrhythmia model to study catecholaminergic polymorphic ventricular tachycardia mutations. Sci Rep. 2017;7:17514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Song L, Alcalai R, Arad M, Wolf CM, Toka O, Conner DA, Berul CI, Eldar M, Seidman CE and Seidman JG. Calsequestrin 2 (CASQ2) mutations increase expression of calreticulin and ryanodine receptors, causing catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2007;117:1814–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Flores DJ, Duong T, Brandenberger LO, Mitra A, Shirali A, Johnson JC, Springer D, Noguchi A, Yu ZX, Ebert SN, et al. Conditional ablation and conditional rescue models for Casq2 elucidate the role of development and of cell-type specific expression of Casq2 in the CPVT2 phenotype. Hum Mol Genet. 2018;27:1533–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Blackwell DJ, Faggioni M, Wleklinski MJ, Gomez-Hurtado N, Venkataraman R, Gibbs CE, Baudenbacher FJ, Gong S, Fishman GI, Boyle PM, et al. The Purkinje-myocardial junction is the anatomic origin of ventricular arrhythmia in CPVT. JCI Insight. 2022;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Rizzi N, Liu N, Napolitano C, Nori A, Turcato F, Colombi B, Bicciato S, Arcelli D, Spedito A, Scelsi M, et al. Unexpected structural and functional consequences of the R33Q homozygous mutation in cardiac calsequestrin: a complex arrhythmogenic cascade in a knock in mouse model. Circ Res. 2008;103:298–306. [DOI] [PubMed] [Google Scholar]
- 354.W M, P S, B D and K BC. Autosomal-Dominant CASQ2-K180R Causes CPVT by Altering Intra-SR Calcium Buffering without Reducing Casq2 Protein Levels. Paper presented at: Biophys J; 2021. [Google Scholar]
- 355.Sondergaard MT, Sorensen AB, Skov LL, Kjaer-Sorensen K, Bauer MC, Nyegaard M, Linse S, Oxvig C and Overgaard MT. Calmodulin mutations causing catecholaminergic polymorphic ventricular tachycardia confer opposing functional and biophysical molecular changes. FEBS J. 2015;282:803–16. [DOI] [PubMed] [Google Scholar]
- 356.Da’as SI, Thanassoulas A, Calver BL, Beck K, Salem R, Saleh A, Kontogianni I, Al-Maraghi A, Nasrallah GK, Safieh-Garabedian B, et al. Arrhythmogenic calmodulin E105A mutation alters cardiac RyR2 regulation leading to cardiac dysfunction in zebrafish. Ann N Y Acad Sci. 2019;1448:19–29. [DOI] [PubMed] [Google Scholar]
- 357.Reilly L, Alvarado FJ, Lang D, Abozeid S, Van Ert H, Spellman C, Warden J, Makielski JC, Glukhov AV and Eckhardt LL. Genetic Loss of IK1 Causes Adrenergic-Induced Phase 3 Early Afterdepolariz ations and Polymorphic and Bidirectional Ventricular Tachycardia. Circ Arrhythm Electrophysiol. 2020;13:e008638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Demolombe S, Lande G, Charpentier F, van Roon MA, van den Hoff MJ, Toumaniantz G, Baro I, Guihard G, Le Berre N, Corbier A, et al. Transgenic mice overexpressing human KvLQT1 dominant-negative isoform. Part I: Phenotypic characterisation. Cardiovasc Res. 2001;50:314–27. [DOI] [PubMed] [Google Scholar]
- 359.Lubberding AF, Zhang J, Lundh M, Nielsen TS, Sondergaard MS, Villadsen M, Skovhoj EZ, Boer GA, Hansen JB, Thomsen MB, et al. Age-dependent transition from islet insulin hypersecretion to hyposecretion in mice with the long QT-syndrome loss-of-function mutation Kcnq1-A340V. Sci Rep. 2021;11:12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Arnaout R, Ferrer T, Huisken J, Spitzer K, Stainier DY, Tristani-Firouzi M and Chi NC. Zebrafish model for human long QT syndrome. Proc Natl Acad Sci U S A. 2007;104:11316–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Langheinrich U, Vacun G and Wagner T. Zebrafish embryos express an orthologue of HERG and are sensitive toward a range of QT-prolonging drugs inducing severe arrhythmia. Toxicol Appl Pharmacol. 2003;193:370–82. [DOI] [PubMed] [Google Scholar]
- 362.Tian XL, Yong SL, Wan X, Wu L, Chung MK, Tchou PJ, Rosenbaum DS, Van Wagoner DR, Kirsch GE and Wang Q. Mechanisms by which SCN5A mutation N1325S causes cardiac arrhythmias and sudden death in vivo. Cardiovasc Res. 2004;61:256–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Li H, Guo W, Yamada KA and Nerbonne JM. Selective elimination of I(K,slow1) in mouse ventricular myocytes expressing a dominant negative Kv1.5alpha subunit. Am J Physiol Heart Circ Physiol. 2004;286:H319–28. [DOI] [PubMed] [Google Scholar]
- 364.Xu H, Barry DM, Li H, Brunet S, Guo W and Nerbonne JM. Attenuation of the slow component of delayed rectification, action potential prolongation, and triggered activity in mice expressing a dominant-negative Kv2 alpha subunit. Circ Res. 1999;85:623–33. [DOI] [PubMed] [Google Scholar]
- 365.Barry DM, Xu H, Schuessler RB and Nerbonne JM. Functional knockout of the transient outward current, long-QT syndrome, and cardiac remodeling in mice expressing a dominant-negative Kv4 alpha subunit. Circ Res. 1998;83:560–7. [DOI] [PubMed] [Google Scholar]
- 366.Guo W, Li H, London B and Nerbonne JM. Functional consequences of elimination of i(to,f) and i(to,s): early afterdepolarizations, atrioventricular block, and ventricular arrhythmias in mice lacking Kv1.4 and expressing a dominant-negative Kv4 alpha subunit. Circ Res. 2000;87:73–9. [DOI] [PubMed] [Google Scholar]
- 367.Hu Z, Kant R, Anand M, King EC, Krogh-Madsen T, Christini DJ and Abbott GW. Kcne2 deletion creates a multisystem syndrome predisposing to sudden cardiac death. Circ Cardiovasc Genet. 2014;7:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Kuo HC, Cheng CF, Clark RB, Lin JJ, Lin JL, Hoshijima M, Nguyen-Tran VT, Gu Y, Ikeda Y, Chu PH, et al. A defect in the Kv channel-interacting protein 2 (KChIP2) gene leads to a complete loss of I(to) and confers susceptibility to ventricular tachycardia. Cell. 2001;107:801–13. [DOI] [PubMed] [Google Scholar]
- 369.Seisenberger C, Specht V, Welling A, Platzer J, Pfeifer A, Kuhbandner S, Striessnig J, Klugbauer N, Feil R and Hofmann F. Functional embryonic cardiomyocytes after disruption of the L-type alpha1C (Cav1.2) calcium channel gene in the mouse. J Biol Chem. 2000;275:39193–9. [DOI] [PubMed] [Google Scholar]
- 370.Berchtold MW, Zacharias T, Kulej K, Wang K, Torggler R, Jespersen T, Chen JN, Larsen MR and la Cour JM. The Arrhythmogenic Calmodulin Mutation D129G Dysregulates Cell Growth, Calmodulin-dependent Kinase II Activity, and Cardiac Function in Zebrafish. J Biol Chem. 2016;291:26636–26646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Itokawa K, Sora I, Schindler CW, Itokawa M, Takahashi N and Uhl GR. Heterozygous VMAT2 knockout mice display prolonged QT intervals: possible contributions to sudden death. Brain Res Mol Brain Res. 1999;71:354–7. [DOI] [PubMed] [Google Scholar]
- 372.O’Brien SE, Apkon M, Berul CI, Patel HT, Saupe K, Spindler M, Ingwall JS and Zahler R. Phenotypical features of long Q-T syndrome in transgenic mice expressing human Na-K-ATPase alpha(3)-isoform in hearts. Am J Physiol Heart Circ Physiol. 2000;279:H2133–42. [DOI] [PubMed] [Google Scholar]
- 373.Pott C, Ren X, Tran DX, Yang MJ, Henderson S, Jordan MC, Roos KP, Garfinkel A, Philipson KD and Goldhaber JI. Mechanism of shortened action potential duration in Na+-Ca2+ exchanger knockout mice. Am J Physiol Cell Physiol. 2007;292:C968–73. [DOI] [PubMed] [Google Scholar]
- 374.Schilling JM, Horikawa YT, Zemljic-Harpf AE, Vincent KP, Tyan L, Yu JK, McCulloch AD, Balijepalli RC, Patel HH and Roth DM. Electrophysiology and metabolism of caveolin-3-overexpressing mice. Basic Res Cardiol. 2016;111:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.O’Rourke MF, Avolio AP and Nichols WW. The kangaroo as a model for the study of hypertrophic cardiomyopathy in man. Cardiovasc Res. 1986;20:398–402. [DOI] [PubMed] [Google Scholar]
- 376.Shy D, Gillet L, Ogrodnik J, Albesa M, Verkerk AO, Wolswinkel R, Rougier JS, Barc J, Essers MC, Syam N, Marsman RF, van Mil AM, et al. PDZ domain-binding motif regulates cardiomyocyte compartment-specific NaV1.5 channel expression and function. Circulation. 2014;130:147–60. [DOI] [PubMed] [Google Scholar]
- 377.Kirchhof P, Fabritz L, Zwiener M, Witt H, Schafers M, Zellerhoff S, Paul M, Athai T, Hiller KH, Baba HA, et al. Age- and training-dependent development of arrhythmogenic right ventricular cardiomyopathy in heterozygous plakoglobin-deficient mice. Circulation. 2006;114:1799–806. [DOI] [PubMed] [Google Scholar]
- 378.Zhang Z, Stroud MJ, Zhang J, Fang X, Ouyang K, Kimura K, Mu Y, Dalton ND, Gu Y, Bradford WH, et al. Normalization of Naxos plakoglobin levels restores cardiac function in mice. J Clin Invest. 2015;125:1708–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Lombardi R, da Graca Cabreira-Hansen M, Bell A, Fromm RR, Willerson JT and Marian AJ. Nuclear plakoglobin is essential for differentiation of cardiac progenitor cells to adipocytes in arrhythmogenic right ventricular cardiomyopathy. Circ Res. 2011;109:1342–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Martin ED, Moriarty MA, Byrnes L and Grealy M. Plakoglobin has both structural and signalling roles in zebrafish development. Dev Biol. 2009;327:83–96. [DOI] [PubMed] [Google Scholar]
- 381.Asimaki A, Kapoor S, Plovie E, Karin Arndt A, Adams E, Liu Z, James CA, Judge DP, Calkins H, Churko J, et al. Identification of a new modulator of the intercalated disc in a zebrafish model of arrhythmogenic cardiomyopathy. Sci Transl Med. 2014;6:240ra74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Garcia-Gras E, Lombardi R, Giocondo MJ, Willerson JT, Schneider MD, Khoury DS and Marian AJ. Suppression of canonical Wnt/beta-catenin signaling by nuclear plakoglobin recapitulates phenotype of arrhythmogenic right ventricular cardiomyopathy. J Clin Invest. 2006;116:2012–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Mezzano V, Liang Y, Wright AT, Lyon RC, Pfeiffer E, Song MY, Gu Y, Dalton ND, Scheinman M, Peterson KL, et al. Desmosomal junctions are necessary for adult sinus node function. Cardiovasc Res. 2016;111:274–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Yang Z, Bowles NE, Scherer SE, Taylor MD, Kearney DL, Ge S, Nadvoretskiy VV, DeFreitas G, Carabello B, Brandon LI, et al. Desmosomal dysfunction due to mutations in desmoplakin causes arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Res. 2006;99:646–55. [DOI] [PubMed] [Google Scholar]
- 385.Cruz FM, Sanz-Rosa D, Roche-Molina M, Garcia-Prieto J, Garcia-Ruiz JM, Pizarro G, Jimenez-Borreguero LJ, Torres M, Bernad A, Ruiz-Cabello J, et al. Exercise triggers ARVC phenotype in mice expressing a disease-causing mutated version of human plakophilin-2. J Am Coll Cardiol. 2015;65:1438–50. [DOI] [PubMed] [Google Scholar]
- 386.Moriarty MA, Ryan R, Lalor P, Dockery P, Byrnes L and Grealy M. Loss of plakophilin 2 disrupts heart development in zebrafish. Int J Dev Biol. 2012;56:711–8. [DOI] [PubMed] [Google Scholar]
- 387.Heuser A, Plovie ER, Ellinor PT, Grossmann KS, Shin JT, Wichter T, Basson CT, Lerman BB, Sasse-Klaassen S, Thierfelder L, et al. Mutant desmocollin-2 causes arrhythmogenic right ventricular cardiomyopathy. Am J Hum Genet. 2006;79:1081–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Hamada Y, Yamamoto T, Nakamura Y, Sufu-Shimizu Y, Nanno T, Fukuda M, Ono M, Oda T, Okuda S, Ueyama T, et al. G790del mutation in DSC2 alone is insufficient to develop the pathogenesis of ARVC in a mouse model. Biochem Biophys Rep. 2020;21:100711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Eshkind L, Tian Q, Schmidt A, Franke WW, Windoffer R and Leube RE. Loss of desmoglein 2 suggests essential functions for early embryonic development and proliferation of embryonal stem cells. Eur J Cell Biol. 2002;81:592–8. [DOI] [PubMed] [Google Scholar]
- 390.Kant S, Holthofer B, Magin TM, Krusche CA and Leube RE. Desmoglein 2-Dependent Arrhythmogenic Cardiomyopathy Is Caused by a Loss of Adhesive Function. Circ Cardiovasc Genet. 2015;8:553–63. [DOI] [PubMed] [Google Scholar]
- 391.Pilichou K, Remme CA, Basso C, Campian ME, Rizzo S, Barnett P, Scicluna BP, Bauce B, van den Hoff MJ, de Bakker JM, et al. Myocyte necrosis underlies progressive myocardial dystrophy in mouse dsg2-related arrhythmogenic right ventricular cardiomyopathy. J Exp Med. 2009;206:1787–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Calore M, Lorenzon A, Vitiello L, Poloni G, Khan MAF, Beffagna G, Dazzo E, Sacchetto C, Polishchuk R, Sabatelli P,et al. A novel murine model for arrhythmogenic cardiomyopathy points to a pathogenic role of Wnt signalling and miRNA dysregulation. Cardiovasc Res. 2019;115:739–751. [DOI] [PubMed] [Google Scholar]
- 393.Ding Y, Yang J, Chen P, Lu T, Jiao K, Tester DJ, Giudicessi JR, Jiang K, Ackerman MJ, Li Y, et al. Knockout of SORBS2 Protein Disrupts the Structural Integrity of Intercalated Disc and Manifests Features of Arrhythmogenic Cardiomyopathy . J Am Heart Assoc. 2020;9:e017055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Bround MJ, Asghari P, Wambolt RB, Bohunek L, Smits C, Philit M, Kieffer TJ, Lakatta EG, Boheler KR, Moore ED, et al. Cardiac ryanodine receptors control heart rate and rhythmicity in adult mice. Cardiovasc Res. 2012;96:372–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Wang Y, Li C, Shi L, Chen X, Cui C, Huang J, Chen B, Hall DD, Pan Z, Lu M, et al. Integrin beta1D Deficiency-Mediated RyR2 Dysfunction Contributes to Catecholamine-Sensitive Ventricular Tachycardia in Arrhythmogenic Right Ventricular Cardiomyopathy. Circulation. 2020;141:1477–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Zheng G, Jiang C, Li Y, Yang D, Ma Y, Zhang B, Li X, Zhang P, Hu X, Zhao X, et al. TMEM43-S358L mutation enhances NF-kappaB-TGFbeta signal cascade in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Protein Cell. 2019;10:104–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Quang KL, Maguy A, Qi XY, Naud P, Xiong F, Tadevosyan A, Shi YF, Chartier D, Tardif JC, Dobrev D, et al. Loss of cardiomyocyte integrin-linked kinase produces an arrhythmogenic cardiomyopathy in mice. Circ Arrhythm Electrophysiol. 2015;8:921–32. [DOI] [PubMed] [Google Scholar]
- 398.Asano Y, Takashima S, Asakura M, Shintani Y, Liao Y, Minamino T, Asanuma H, Sanada S, Kim J, Ogai A, et al. Lamr1 functional retroposon causes right ventricular dysplasia in mice. Nat Genet. 2004;36:123–30. [DOI] [PubMed] [Google Scholar]
- 399.Basso C, Fox PR, Meurs KM, Towbin JA, Spier AW, Calabrese F, Maron BJ and Thiene G. Arrhythmogenic right ventricular cardiomyopathy causing sudden cardiac death in boxer dogs: a new animal model of human disease. Circulation. 2004;109:1180–5. [DOI] [PubMed] [Google Scholar]
- 400.Eason BD, Leach SB and Kuroki K. Arrhythmogenic right ventricular cardiomyopathy in a weimaraner. Can Vet J. 2015;56:1035–9. [PMC free article] [PubMed] [Google Scholar]
- 401.Cunningham SM, Sweeney JT, MacGregor J, Barton BA and Rush JE . Clinical Features of English Bulldogs with Presumed Arrhythmogenic Right Ventricular Cardiomyopathy: 31 Cases (2001–2013). J Am Anim Hosp Assoc. 2018;54:95–102. [DOI] [PubMed] [Google Scholar]
- 402.Fox PR, Maron BJ, Basso C, Liu SK and Thiene G. Spontaneously occurring arrhythmogenic right ventricular cardiomyopathy in the domestic cat: A new animal model similar to the human disease. Circulation. 2000;102:1863–70. [DOI] [PubMed] [Google Scholar]
- 403.Wolf CM, Wang L, Alcalai R, Pizard A, Burgon PG, Ahmad F, Sherwood M, Branco DM, Wakimoto H, Fishman GI, et al. Lamin A/C haploinsufficiency causes dilated cardiomyopathy and apoptosis-triggered cardiac conduction system disease. J Mol Cell Cardiol. 2008;44:293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Arimura T, Helbling-Leclerc A, Massart C, Varnous S, Niel F, Lacene E, Fromes Y, Toussaint M, Mura AM, Keller DI, et al. Mouse model carrying H222P-Lmna mutation develops muscular dystrophy and dilated cardiomyopathy similar to human striated muscle laminopathies. Hum Mol Genet. 2005;14:155–69. [DOI] [PubMed] [Google Scholar]
- 405.Mounkes LC, Kozlov SV, Rottman JN and Stewart CL. Expression of an LMNA-N195K variant of A-type lamins results in cardiac conduction defects and death in mice. Hum Mol Genet. 2005;14:2167–80. [DOI] [PubMed] [Google Scholar]
- 406.Osorio FG, Navarro CL, Cadinanos J, Lopez-Mejia IC, Quiros PM, Bartoli C, Rivera J, Tazi J, Guzman G, Varela I, et al. Splicing-directed therapy in a new mouse model of human accelerated aging. Sci Transl Med. 2011;3:106ra107. [DOI] [PubMed] [Google Scholar]
- 407.Dorado B, Ploen GG, Barettino A, Macias A, Gonzalo P, Andres-Manzano MJ, Gonzalez-Gomez C, Galan-Arriola C, Alfonso JM, Lobo M, et al. Generation and characterization of a novel knockin minipig model of Hutchinson-Gilford progeria syndrome. Cell Discov. 2019;5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Ramspacher C, Steed E, Boselli F, Ferreira R, Faggianelli N, Roth S, Spiegelhalter C, Messaddeq N, Trinh L, Liebling M, et al. Developmental Alterations in Heart Biomechanics and Skeletal Muscle Function in Desmin Mutants Suggest an Early Pathological Root for Desminopathies. Cell Rep. 2015;11:1564–76. [DOI] [PubMed] [Google Scholar]
- 409.Clemen CS, Stockigt F, Strucksberg KH, Chevessier F, Winter L, Schutz J, Bauer R, Thorweihe JM, Wenzel D, Schlotzer-Schrehardt U, et al. The toxic effect of R350P mutant desmin in striated muscle of man and mouse. Acta Neuropathol. 2015;129:297–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Gupta V, Discenza M, Guyon JR, Kunkel LM and Beggs AH. alpha-Actinin-2 deficiency results in sarcomeric defects in zebrafish that cannot be rescued by alpha-actinin-3 revealing functional differences between sarcomeric isoforms. FASEB J. 2012;26:1892–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Li Z, Ai T, Samani K, Xi Y, Tzeng HP, Xie M, Wu S, Ge S, Taylor MD, Dong JW, et al. A ZASP missense mutation, S196L, leads to cytoskeletal and electrical abnormalities in a mouse model of cardiomyopathy. Circ Arrhythm Electrophysiol. 2010;3:646–56. [DOI] [PubMed] [Google Scholar]
- 412.Li J, Patel VV, Kostetskii I, Xiong Y, Chu AF, Jacobson JT, Yu C, Morley GE, Molkentin JD and Radice GL. Cardiac-specific loss of N-cadherin leads to alteration in connexins with conduction slowing and arrhythmogenesis. Circ Res. 2005;97:474–81. [DOI] [PubMed] [Google Scholar]
- 413.Li S, Mo K, Tian H, Chu C, Sun S, Tian L, Ding S, Li TR, Wu X, Liu F, et al. Lmod2 piggyBac mutant mice exhibit dilated cardiomyopathy. Cell Biosci. 2016;6:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Guo W, Schafer S, Greaser ML, Radke MH, Liss M, Govindarajan T, Maatz H, Schulz H, Li S, Parrish AM, et al. RBM20, a gene for hereditary cardiomyopathy, regulates titin splicing. Nat Med. 2012;18:766–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.van den Hoogenhof MMG, Beqqali A, Amin AS, van der Made I, Aufiero S, Khan MAF, Schumacher CA, Jansweijer JA, van Spaendonck-Zwarts KY, et al. RBM20 Mutations Induce an Arrhythmogenic Dilated Cardiomyopathy Related to Disturbed Calcium Handling. Circulation. 2018;138:1330–1342. [DOI] [PubMed] [Google Scholar]
- 416.Ihara K, Sasano T, Hiraoka Y, Togo-Ohno M, Soejima Y, Sawabe M, Tsuchiya M, Ogawa H, Furukawa T and Kuroyanagi H. A missense mutation in the RSRSP stretch of Rbm20 causes dilated cardiomyopathy and atrial fibrillation in mice. Sci Rep. 2020;10:17894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Eijgenraam TR, Boukens BJ, Boogerd CJ, Schouten EM, van de Kolk CWA, Stege NM, Te Rijdt WP, Hoorntje ET, van der Zwaag PA, van Rooij E,et al. The phospholamban p.(Arg14del) pathogenic variant leads to cardiomyopathy with heart failure and is unreponsive to standard heart failure therapy. Sci Rep. 2020;10:9819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Hesse M, Kondo CS, Clark RB, Su L, Allen FL, Geary-Joo CT, Kunnathu S, Severson DL, Nygren A, Giles WR, et al. Dilated cardiomyopathy is associated with reduced expression of the cardiac sodium channel Scn5a. Cardiovasc Res. 2007;75:498–509. [DOI] [PubMed] [Google Scholar]
- 419.Moretti A, Fonteyne L, Giesert F, Hoppmann P, Meier AB, Bozoglu T, Baehr A, Schneider CM, Sinnecker D, Klett K, et al. Somatic gene editing ameliorates skeletal and cardiac muscle failure in pig and human models of Duchenne muscular dystrophy. Nat Med. 2020;26:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Moise NS, Valentine BA, Brown CA, Erb HN, Beck KA, Cooper BJ and Gilmour RF. Duchenne’s cardiomyopathy in a canine model: electrocardiographic and echocardiographic studies. J Am Coll Cardiol. 1991;17:812–20. [DOI] [PubMed] [Google Scholar]
- 421.Chu V, Otero JM, Lopez O, Sullivan MF, Morgan JP, Amende I and Hampton TG. Electrocardiographic findings in mdx mice: a cardiac phenotype of Duchenne muscular dystrophy. Muscle Nerve. 2002;26:513–9. [DOI] [PubMed] [Google Scholar]
- 422.Branco DM, Wolf CM, Sherwood M, Hammer PE, Kang PB and Berul CI. Cardiac electrophysiological characteristics of the mdx ( 5cv ) mouse model of Duchenne muscular dystrophy. J Interv Card Electrophysiol. 2007;20:1–7. [DOI] [PubMed] [Google Scholar]
- 423.Zemljic-Harpf AE, Miller JC, Henderson SA, Wright AT, Manso AM, Elsherif L, Dalton ND, Thor AK, Perkins GA, McCulloch AD, et al. Cardiac-myocyte-specific excision of the vinculin gene disrupts cellular junctions, causing sudden death or dilated cardiomyopathy. Mol Cell Biol. 2007;27:7522–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Eigenthaler M, Engelhardt S, Schinke B, Kobsar A, Schmitteckert E, Gambaryan S, Engelhardt CM, Krenn V, Eliava M, Jarchau T, et al. Disruption of cardiac Ena-VASP protein localization in intercalated disks causes dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2003;285:H2471–81. [DOI] [PubMed] [Google Scholar]
- 425.Kuwahara K, Saito Y, Takano M, Arai Y, Yasuno S, Nakagawa Y, Takahashi N, Adachi Y, Takemura G, Horie M, et al. NRSF regulates the fetal cardiac gene program and maintains normal cardiac structure and function. EMBO J. 2003;22:6310–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Wess G, Schulze A, Butz V, Simak J, Killich M, Keller LJ, Maeurer J and Hartmann K. Prevalence of dilated cardiomyopathy in Doberman Pinschers in various age groups. J Vet Intern Med. 2010;24:533–8. [DOI] [PubMed] [Google Scholar]
- 427.Meurs KM, Miller MW and Wright NA. Clinical features of dilated cardiomyopathy in Great Danes and results of a pedigree analysis: 17 cases (1990–2000). J Am Vet Med Assoc. 2001;218:729–32. [DOI] [PubMed] [Google Scholar]
- 428.Du CK, Morimoto S, Nishii K, Minakami R, Ohta M, Tadano N, Lu QW, Wang YY, Zhan DY, Mochizuki M, et al. Knock-in mouse model of dilated cardiomyopathy caused by troponin mutation. Circ Res. 2007;101:185–94. [DOI] [PubMed] [Google Scholar]
- 429.Frey N, Franz WM, Gloeckner K, Degenhardt M, Muller M, Muller O, Merz H and Katus HA. Transgenic rat hearts expressing a human cardiac troponin T deletion reveal diastolic dysfunction and ventricular arrhythmias. Cardiovasc Res. 2000;47:254–64. [DOI] [PubMed] [Google Scholar]
- 430.Vikstrom KL, Factor SM and Leinwand LA. Mice expressing mutant myosin heavy chains are a model for familial hypertrophic cardiomyopathy. Mol Med. 1996;2:556–67. [PMC free article] [PubMed] [Google Scholar]
- 431.McConnell BK, Jones KA, Fatkin D, Arroyo LH, Lee RT, Aristizabal O, Turnbull DH, Georgakopoulos D, Kass D, Bond M, et al. Dilated cardiomyopathy in homozygous myosin-binding protein-C mutant mice. J Clin Invest. 1999;104:1235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Meurs KM, Sanchez X, David RM, Bowles NE, Towbin JA, Reiser PJ, Kittleson JA, Munro MJ, Dryburgh K, Macdonald KA, et al. A cardiac myosin binding protein C mutation in the Maine Coon cat with familial hypertrophic cardiomyopathy. Hum Mol Genet. 2005;14:3587–93. [DOI] [PubMed] [Google Scholar]
- 433.Meurs KM, Norgard MM, Ederer MM, Hendrix KP and Kittleson MD. A substitution mutation in the myosin binding protein C gene in ragdoll hypertrophic cardiomyopathy. Genomics. 2007;90:261–4. [DOI] [PubMed] [Google Scholar]
- 434.Ruan H, Mitchell S, Vainoriene M, Lou Q, Xie LH, Ren S, Goldhaber JI and Wang Y. Gi alpha 1-mediated cardiac electrophysiological remodeling and arrhythmia in hypertrophic cardiomyopathy. Circulation. 2007;116:596–605. [DOI] [PubMed] [Google Scholar]
- 435.Alvarado FJ, Bos JM, Yuchi Z, Valdivia CR, Hernandez JJ, Zhao YT, Henderlong DS, Chen Y, Booher TR, Marcou CA, et al. Cardiac hypertrophy and arrhythmia in mice induced by a mutation in ryanodine receptor 2. JCI Insight. 2019;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Hu LR, Ackermann MA, Hecker PA, Prosser BL, King B, O’Connell KA, Grogan A, Meyer LC, Berndsen CE, Wright NT, et al. Deregulated Ca(2+) cycling underlies the development of arrhythmia and heart disease due to mutant obscurin. Sci Adv. 2017;3:e1603081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Sood S, Chelu MG, van Oort RJ, Skapura D, Santonastasi M, Dobrev D and Wehrens XH. Intracellular calcium leak due to FKBP12.6 deficiency in mice facilitates the inducibility of atrial fibrillation. Heart Rhythm. 2008;5:1047–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Beavers DL, Wang W, Ather S, Voigt N, Garbino A, Dixit SS, Landstrom AP, Li N, Wang Q, Olivotto I, et al. Mutation E169K in junctophilin-2 causes atrial fibrillation due to impaired RyR2 stabilization. J Am Coll Cardiol. 2013;62:2010–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Yao C, Veleva T, Scott L Jr., Cao S, Li L, Chen G, Jeyabal P, Pan X, Alsina KM, Abu-Taha ID, et al. Enhanced Cardiomyocyte NLRP3 Inflammasome Signaling Promotes Atrial Fibrillation. Circulation. 2018;138:2227–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Wan E, Abrams J, Weinberg RL, Katchman AN, Bayne J, Zakharov SI, Yang L, Morrow JP, Garan H and Marx SO. Aberrant sodium influx causes cardiomyopathy and atrial fibrillation in mice. J Clin Invest. 2016;126:112–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Blana A, Kaese S, Fortmuller L, Laakmann S, Damke D, van Bragt K, Eckstein J, Piccini I, Kirchhefer U, Nattel S, et al. Knock-in gain-of-function sodium channel mutation prolongs atrial action potentials and alters atrial vulnerability. Heart Rhythm. 2010;7:1862–9. [DOI] [PubMed] [Google Scholar]
- 442.David JP, Lisewski U, Crump SM, Jepps TA, Bocksteins E, Wilck N, Lossie J, Roepke TK, Schmitt N and Abbott GW. Deletion in mice of X-linked, Brugada syndrome- and atrial fibrillation-associated Kcne5 augments ventricular KV currents and predisposes to ventricular arrhythmia. FASEB J. 2019;33:2537–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Li N, Timofeyev V, Tuteja D, Xu D, Lu L, Zhang Q, Zhang Z, Singapuri A, Albert TR, Rajagopal AV, et al. Ablation of a Ca2+-activated K+ channel (SK2 channel) results in action potential prolongation in atrial myocytes and atrial fibrillation. J Physiol. 2009;587:1087–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Verheule S, Sato T, Everett Tt, Engle SK, Otten D, Rubart-von der Lohe M, Nakajima HO, Nakajima H, Field LJ and Olgin JE. Increased vulnerability to atrial fibrillation in transgenic mice with selective atrial fibrosis caused by overexpression of TGF-beta1. Circ Res. 2004;94:1458–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Davies L, Jin J, Shen W, Tsui H, Shi Y, Wang Y, Zhang Y, Hao G, Wu J, Chen S, et al. Mkk4 is a negative regulator of the transforming growth factor beta 1 signaling associated with atrial remodeling and arrhythmogenesis with age. J Am Heart Assoc. 2014;3:e000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Xiao HD, Fuchs S, Campbell DJ, Lewis W, Dudley SC Jr., Kasi VS, Hoit BD, Keshelava G, Zhao H, Capecchi MR, et al. Mice with cardiac-restricted angiotensin-converting enzyme (ACE) have atrial enlargement, cardiac arrhythmia, and sudden death. Am J Pathol. 2004;165:1019–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Parahuleva MS, Kockskamper J, Heger J, Grimm W, Scherer A, Buhler S, Kreutz J, Schulz R and Euler G. Structural, Pro-Inflammatory and Calcium Handling Remodeling Underlies Spontaneous Onset of Paroxysmal Atrial Fibrillation in JDP2-Overexpressing Mice. Int J Mol Sci. 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Hakim P, Brice N, Thresher R, Lawrence J, Zhang Y, Jackson AP, Grace AA and Huang CL. Scn3b knockout mice exhibit abnormal sino-atrial and cardiac conduction properties. Acta Physiol (Oxf). 2010;198:47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Qiao Y, Lipovsky C, Hicks S, Bhatnagar S, Li G, Khandekar A, Guzy R, Woo KV, Nichols CG, Efimov IR, et al. Transient Notch Activation Induces Long-Term Gene Expression Changes Leading to Sick Sinus Syndrome in Mice. Circ Res. 2017;121:549–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Baker K, Warren KS, Yellen G and Fishman MC. Defective “pacemaker” current (Ih) in a zebrafish mutant with a slow heart rate. Proc Natl Acad Sci U S A. 1997;94:4554–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Groenke S, Larson ED, Alber S, Zhang R, Lamp ST, Ren X, Nakano H, Jordan MC, Karagueuzian HS, Roos KP, et al. Complete atrial-specific knockout of sodium-calcium exchange eliminates sinoatrial node pacemaker activity. PLoS One. 2013;8:e81633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Ebert AM, Hume GL, Warren KS, Cook NP, Burns CG, Mohideen MA, Siegal G, Yelon D, Fishman MC and Garrity DM. Calcium extrusion is critical for cardiac morphogenesis and rhythm in embryonic zebrafish hearts. Proc Natl Acad Sci U S A. 2005;102:17705–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Harzheim D, Pfeiffer KH, Fabritz L, Kremmer E, Buch T, Waisman A, Kirchhof P, Kaupp UB and Seifert R. Cardiac pacemaker function of HCN4 channels in mice is confined to embryonic development and requires cyclic AMP. EMBO J. 2008;27:692–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Alig J, Marger L, Mesirca P, Ehmke H, Mangoni ME and Isbrandt D. Control of heart rate by cAMP sensitivity of HCN channels. Proc Natl Acad Sci U S A. 2009;106:12189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Berul CI, Maguire CT, Gehrmann J and Reddy S. Progressive atrioventricular conduction block in a mouse myotonic dystrophy model. J Interv Card Electrophysiol. 2000;4:351–8. [DOI] [PubMed] [Google Scholar]
- 456.Simon AM, Goodenough DA and Paul DL. Mice lacking connexin40 have cardiac conduction abnormalities characteristic of atrioventricular block and bundle branch block. Curr Biol. 1998;8:295–8. [DOI] [PubMed] [Google Scholar]
- 457.Demion M, Thireau J, Gueffier M, Finan A, Khoueiry Z, Cassan C, Serafini N, Aimond F, Granier M, Pasquie JL, et al. Trpm4 gene invalidation leads to cardiac hypertrophy and electrophysiological alterations. PLoS One. 2014;9:e115256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Lubkemeier I, Requardt RP, Lin X, Sasse P, Andrie R, Schrickel JW, Chkourko H, Bukauskas FF, Kim JS, Frank M, et al. Deletion of the last five C-terminal amino acid residues of connexin43 leads to lethal ventricular arrhythmias in mice without affecting coupling via gap junction channels. Basic Res Cardiol. 2013;108:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Zi M, Kimura TE, Liu W, Jin J, Higham J, Kharche S, Hao G, Shi Y, Shen W, Prehar S, et al. Mitogen-activated protein kinase kinase 4 deficiency in cardiomyocytes causes connexin 43 reduction and couples hypertrophic signals to ventricular arrhythmogenesis. J Biol Chem. 2011;286:17821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Yamada N, Asano Y, Fujita M, Yamazaki S, Inanobe A, Matsuura N, Kobayashi H, Ohno S, Ebana Y, Tsukamoto O, et al. Mutant KCNJ3 and KCNJ5 Potassium Channels as Novel Molecular Targets in Bradyarrhythmias and Atrial Fibrillation. Circulation. 2019;139:2157–2169. [DOI] [PubMed] [Google Scholar]
- 461.Baudot M, Torre E, Bidaud I, Louradour J, Torrente AG, Fossier L, Talssi L, Nargeot J, Barrere-Lemaire S, Mesirca P, et al. Concomitant genetic ablation of L-type Cav1.3 (alpha1D) and T-type Cav3.1 (alpha1G) Ca(2+) channels disrupts heart automaticity. Sci Rep. 2020;10:18906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Zhang SS, Kim KH, Rosen A, Smyth JW, Sakuma R, Delgado-Olguin P, Davis M, Chi NC, Puviindran V, Gaborit N, et al. Iroquois homeobox gene 3 establishes fast conduction in the cardiac His-Purkinje network. Proc Natl Acad Sci U S A. 2011;108:13576–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Hoogaars WM, Engel A, Brons JF, Verkerk AO, de Lange FJ, Wong LY, Bakker ML, Clout DE, Wakker V, Barnett P, et al. Tbx3 controls the sinoatrial node gene program and imposes pacemaker function on the atria. Genes Dev. 2007;21:1098–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Bruneau BG, Nemer G, Schmitt JP, Charron F, Robitaille L, Caron S, Conner DA, Gessler M, Nemer M, Seidman CE, et al. A murine model of Holt-Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell. 2001;106:709–21. [DOI] [PubMed] [Google Scholar]
- 465.McCauley MD, Wang T, Mike E, Herrera J, Beavers DL, Huang TW, Ward CS, Skinner S, Percy AK, Glaze DG, et al. Pathogenesis of lethal cardiac arrhythmias in Mecp2 mutant mice: implication for therapy in Rett syndrome. Sci Transl Med. 2011;3:113ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Del Monte F, Butler K, Boecker W, Gwathmey JK and Hajjar RJ. Novel technique of aortic banding followed by gene transfer during hypertrophy and heart failure. Physiol Genomics. 2002;9:49–56. [DOI] [PubMed] [Google Scholar]
- 467.McAinsh AM, Turner MA, O’Hare D, Nithythyananthan R, Johnston DG, O’Gorman DJ and Sheridan DJ. Cardiac hypertrophy impairs recovery from ischaemia because there is a reduced reactive hyperaemic response. Cardiovasc Res. 1995;30:113–21. [PubMed] [Google Scholar]
- 468.Gilson N, el Houda Bouanani N, Corsin A and Crozatier B. Left ventricular function and beta-adrenoceptors in rabbit failing heart. Am J Physiol. 1990;258:H634–41. [DOI] [PubMed] [Google Scholar]
- 469.Srikanth G, Prakash P, Tripathy N, Dikshit M and Nityanand S. Establishment of a rat model of myocardial infarction with a high survival rate: A suitable model for evaluation of efficacy of stem cell therapy. J Stem Cells Regen Med. 2009;5:30–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Coker SJ. Anesthetized rabbit as a model for ischemia- and reperfusion-induced arrhythmias: effects of quinidine and bretylium. J Pharmacol Methods. 1989;21:263–79. [DOI] [PubMed] [Google Scholar]
- 471.Arimura T, Saku K, Kakino T, Nishikawa T, Tohyama T, Sakamoto T, Sakamoto K, Kishi T, Ide T and Sunagawa K. Intravenous electrical vagal nerve stimulation prior to coronary reperfusion in a canine ischemia-reperfusion model markedly reduces infarct size and prevents subsequent heart failure. Int J Cardiol. 2017;227:704–710. [DOI] [PubMed] [Google Scholar]
- 472.Silvis MJM, van Hout GPJ, Fiolet ATL, Dekker M, Bosch L, van Nieuwburg MMJ, Visser J, Jansen MS, Timmers L and de Kleijn DPV. Experimental parameters and infarct size in closed chest pig LAD ischemia reperfusion models; lessons learned. BMC Cardiovasc Disord. 2021;21:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Charles CJ, Elliott JM, Nicholls MG, Rademaker MT and Richards M. Myocardial infarction with and without reperfusion in sheep: early cardiac and neurohumoral changes. Clin Sci (Lond). 2000;98:703–11. [PubMed] [Google Scholar]
- 474.Suto F, Cahill SA, Wilson GJ, Hamilton RM, Greenwald I and Gross GJ. A novel rabbit model of variably compensated complete heart block. J Appl Physiol (1985). 2002;92:1199–204. [DOI] [PubMed] [Google Scholar]
- 475.Morillo CA, Klein GJ, Jones DL and Guiraudon CM. Chronic rapid atrial pacing. Structural, functional, and electrophysiological characteristics of a new model of sustained atrial fibrillation. Circulation. 1995;91:1588–95. [DOI] [PubMed] [Google Scholar]
- 476.Bauer A, McDonald AD and Donahue JK. Pathophysiological findings in a model of persistent atrial fibrillation and severe congestive heart failure. Cardiovasc Res. 2004;61:764–70. [DOI] [PubMed] [Google Scholar]
- 477.Mulla W, Etzion S, Elyagon S, Gillis R, Murninkas M, Konstantino Y, Mannhardt I, Eschenhagen T, Liel-Cohen N and Etzion Y. Prominent differences in left ventricular performance and myocardial properties between right ventricular and left ventricular-based pacing modes in rats. Sci Rep. 2017;7:5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Lamoureux L, Radhakrishnan J and Gazmuri RJ. A Rat Model of Ventricular Fibrillation and Resuscitation by Conventional Closed-chest Technique. J Vis Exp. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Armstrong PW, Stopps TP, Ford SE and de Bold AJ. Rapid ventricular pacing in the dog: pathophysiologic studies of heart failure. Circulation. 1986;74:1075–84. [DOI] [PubMed] [Google Scholar]
- 480.Amoros-Figueras G, Jorge E, Raga S, Alonso-Martin C, Rodriguez-Font E, Bazan V, Vinolas X, Cinca J and Guerra JM. Comparison between endocardial and epicardial cardiac resynchronization in an experimental model of non-ischaemic cardiomyopathy. Europace. 2018;20:1209–1216. [DOI] [PubMed] [Google Scholar]
- 481.Hosenpud JD, Campbell SM, Hart MV, Paul MS, Rowles JR and Niles NR. Experimental autoimmune myocarditis in the guinea pig. Cardiovasc Res. 1985;19:613–22. [DOI] [PubMed] [Google Scholar]
- 482.Furman BL. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr Protoc Pharmacol. 2015;70:5 47 1–5 47 20. [DOI] [PubMed] [Google Scholar]
- 483.Wang CY and Liao JK. A mouse model of diet-induced obesity and insulin resistance. Methods Mol Biol. 2012;821:421–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Holmes A, Coppey LJ, Davidson EP and Yorek MA. Rat Models of Diet-Induced Obesity and High Fat/Low Dose Streptozotocin Type 2 Diabetes: Effect of Reversal of High Fat Diet Compared to Treatment with Enalapril or Menhaden Oil on Glucose Utilization and Neuropathic Endpoints. J Diabetes Res. 2015;2015:307285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Fenichel RR, Malik M, Antzelevitch C, Sanguinetti M, Roden DM, Priori SG, Ruskin JN, Lipicky RJ, Cantilena LR and Independent Academic Task F. Drug-induced torsades de pointes and implications for drug development. J Cardiovasc Electrophysiol. 2004;15:475–95. [DOI] [PMC free article] [PubMed] [Google Scholar]