Abstract
Background
Psoriasis is a common chronic inflammatory skin disorder that is associated with excess cardiovascular risk. Inflammation is a key mediator in the onset and progression of these cardiometabolic abnormalities; however, the excess cardiovascular risk conferred by psoriatic disease remains understudied. We investigated the prevalence and severity of CMD in patients with psoriasis and determined whether CMD is a result of CV risk factors and atherosclerotic burden.
Methods
This was a consectiuive retrospective cohort study of patients with psoriasis, normal myocardial perfusion and LV ejection fraction (EF) > 50% (n = 62) and matched controls without psoriasis (n = 112). Myocardial perfusion and myocardial flow reserve (MFR) were quantified using PET imaging. Atherosclerotic burden was determined by semi-quantitative computed tomography (CT) coronary calcium assessment.
Results
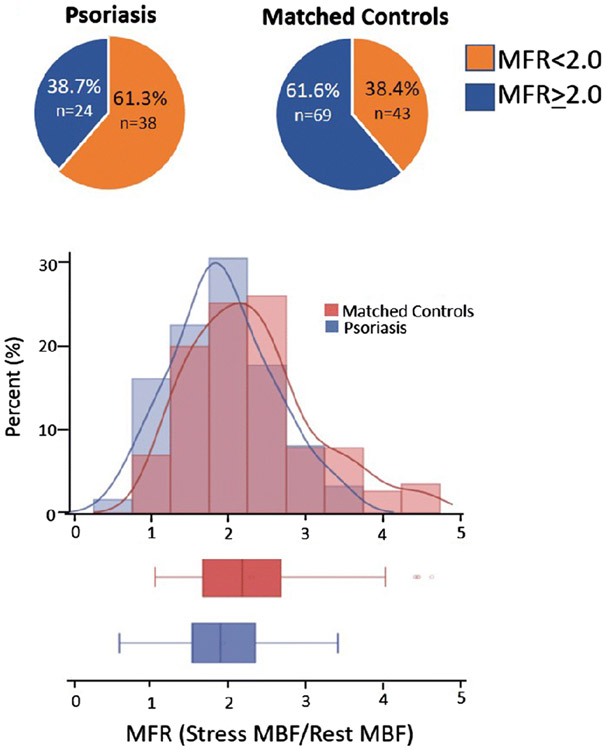
The prevalence of CMD (defined as MFR<2) was 61.3% in patients with psoriatic disease, compared to 38.4% in a matched control population (p=0.004). Furthermore, patients with psoriasis had a more severe reduction in adjusted MFR (2.3±0.81 vs 1.92±0.65, respectively, p=0.001). The degree of atherosclerotic burden, as assessed by qualitative calcium score, was similar between psoriasis and controls.
Conclusions
Patients with psoriasis without overt CAD demonstrated a high prevalence of coronary vasomotor abnormalities that is not entirely accounted for by the commonly associated coronary risk factors or the burden of atherosclerosis.
Introduction
Psoriasis is a chronic inflammatory skin disorder that is associated with increased cardiovascular (CV) risk including myocardial infarction (MI), heart failure and CV mortality.[1-6]. It affects greater than 2% of the population and is associated with joint involvement in up to 30% of patients.[7, 8] Patients with severe psoriasis have approximately seven-fold increased risk of myocardial infarction and 57% increased risk of CV mortality compared to controls matched for age, sex and CV risk factors.[3] The mechanisms underlying these associations are not well understood. Inflammation, a key driver of atherosclerosis and endothelial cell dysfunction affecting the entire coronary vasculature, is thought to play an important role.[9] Myocardial flow reserve (MFR, measured as the ratio of myocardial blood flow at peak stress over that at rest) provides a robust and reproducible clinical measure of the integrated hemodynamic effects of epicardial coronary artery disease (CAD), diffuse atherosclerosis, and coronary microvascular dysfunction (CMD) on myocardial tissue perfusion and is a powerful marker of CV risk. MFR measurements by PET can distinguish patients at high risk for serious adverse events, including heart failure and cardiac death.[9-12] A recent study based on Doppler Echocardiography demonstrated that 15% of asymptomatic moderate-severe psoriatic patients had a reduced coronary flow reserve (CFR) of the left-anterior descending (LAD) artery.[13, 14] However, the lack of control for coronary risk factors and atherosclerotic burden limits the conclusions of whether reduced CFR of the LAD was simply a manifestation of diffuse atherosclerosis in psoriatic patients with coronary risk factors as opposed to microcirculatory dysfunction and a unique feature of psoriatic disease. Consequently, we designed this study to test the hypothesis that CMD is a common and unique feature of psoriatic disease independent of risk factor and atherosclerotic burden. A reduced global MFR (<2) measured by positron emission tomography (PET) in patients with psoriasis without obstructive CAD was used to define CMD.
New Knowledge Gained
Psoriasis is associated with a high prevalence of coronary vasomotor dysfunction and reduced myocardial flow reserve by cardiac PET which is not entirely accounted for by coronary risk factors and atherosclerotic burden.
Methods
Study population
In this retrospective cohort study, we evaluated consectutive men and women undergoing clinically indicated rest/stress myocardial perfusion PET imaging for investigation of chest pain and/or dyspnea between 1/1/2006 and 12/31/2018 who had a diagnosis of psoriasis. Psoriasis was first identified by ICD-9 or ICD-10 codes listed before or at the time of the PET imaging in the electronic medical record (EMR). All records were then independently reviewed by a physician to confirm a diagnosis of psoriasis. Control patients without psoriasis were identified through propensity score matching in a 2:1 fashion using caliper width method based on age, sex, obesity, hyperlipidemia, hypertension, diabetes mellitus and history of CAD (defined as prior myocardial infarction (MI) or PCI). Patients with LVEF < 50%, an abnormal myocardial perfusion study (summed stress score (SSS) ≥ 3), or other systemic inflammatory disorder were excluded. The study was approved by the Partners Healthcare Institutional Review Board and conducted in accordance with institutional guidelines.
PET imaging and myocardial flow quantitation
Myocardial perfusion imaging was performed on a whole-body PET- computed tomography (CT) scanner (Discovery RX or STE Lightspeed 64, GE Healthcare, Milwaukee, WI) in 2- dimensional mode using 82Rubidium or 13N-ammonia as previously described.[15, 16] CT was used for attenuation correction. Coronary vasodilation was achieved using regadenoson or dipyridamole as per standard care. PET images were evaluated semi-quantitatively by a 17-segment visual assessment of gated myocardial perfusion images with a standard five-point scoring system. Rest LVEFs were calculated from gated myocardial perfusion images with commercially available software (Corridor4DM; Ann Arbor, MI). Summed stress score < 3 was considered to be normal and without evidence of obstructive CAD. Rest and stress myocardial blood flow (MBF in mL/min/g) was quantified using a validated tracer kinetic model as previously described.[15, 16] To adjust for differences in baseline cardiac work, rest MBF was normalized by the rest pressure product [(MBF/(rest heart rate x rest systolic blood pressure))x10,000]. Corrected myocardial flow reserve (MFR) was then calculated as stress MBF/corrected rest MBF.[17] Quantitative measures of MBF and MFR were recorded by a single experienced operator blinded to patient data.
Coronary Artery Calcium Assessment
The presence and extent of coronary artery calcium (CAC) was assessed using semi-quantitative visual analysis of the low-dose, non-contrast CT scan obtained for attenuation correction of the PET images.[18] Semi-quantitative assessment of CAC was performed by a cardiologist with advanced cardiovascular imaging training for each of the 174 PET scans in a blinded fashion. The degree of CAC was determined to be none, mild, moderate, or severe as previously described by the National Lung Screening Trial investigators.[19] This approach was previously deemed comparable to Agatston scoring and strongly associated with cardiovascular death.
Statistical analysis
Patient baseline characteristics were reported as frequencies with percent and means with standard deviation (SD) where appropriate. Fisher’s T-test and two-sided t test for continuous variables was used to demonstrate appropriate matching between psoriasis and control patients and to compare the MFR and prevalence of CMD. The prevalence of CMD was defined as MFR<2. For all analyses, α < 0.05 was considered statistically significant. Analyses were performed by using SAS 9.2 (SAS Institute).
Results
Baseline characteristics
We included 62 patients with psoriasis (mean age 69.8, standard deviation (SD) 11.8) and 112 control patients without psoriasis or other inflammatory diseases (mean age 68.6, standard deviation (SD) 11.3). Baseline characteristics were well balanced between the groups and described in Table 1. Patients with psoriasis had a high prevalence of coronary risk factor burden, including 77.4% with hypertension, 62.9% dyslipidemia, 38.7% diabetes mellitus (DM), 45.1% obesity, and 9.1% actively smoking. History of known previous CAD was low and similar in the patient with psoriasis and controls.
Table 1:
Baseline characteristics of the study cohort
| Clinical characteristics | Psoriasis (n=62) | Control (n=112) | P-value |
|---|---|---|---|
| Age, mean (SD) | 69.6 (11.8) | 68.6 (11.3) | 0.6 |
| Female, n (%) | 44 (71) | 76 (68) | 0.86 |
| CV risk factors | |||
| BMI ≥ 30 kg/m, n (%) | 28 (45.1) | 56 (50) | 0.63 |
| Diabetes, n (%) | 24 (38.7) | 43 (38.4) | 1.0 |
| Hypertension, n (%) | 48 (77.4) | 84 (75) | 0.85 |
| Dyslipidemia, n (%) | 39 (62.9) | 71 (63.4) | 1.0 |
| Smoking, n (%) | 6 (9.7) | 7 (6.3) | 0.54 |
| Known CAD, n (%) | 7 (11.3) | 24 (21) | 0.1 |
| Statin, n (%) | 38 (61.3) | 65 (58) | 0.71 |
Values are mean with standard deviation (SD) or n (%).
Known CAD if history of MI or PCI. Smoking indicates active smoking at time of PET scan. CV=cardiovascular. BMI = body mass index. CAD =coronary artery disease.
Myocardial flow reserve is reduced in psoriasis patients compared to matched controls
We first characterized MBF and MFR in psoriasis patients versus controls. At rest, MBF was globally homogeneous and similar across the two groups (Table 2). During hyperemia, MBF was also globally homogeneous in the two groups. However, there was a reduction in the magnitude of flow augmentation and resulting reduced MFR in psoriasis patients compared to controls with a similar risk factor profile [(2.3±0.81 vs 1.92±0.65, respectively, p=0.001] (Figure 1b). Furthermore, patients with psoriasis had a significantly higher prevalence of coronary microvascular dysfunction (MFR<2) (38.7% vs 61.7%, respectively, p=0.004). Hemodynamic parameters, including heart rate and blood pressure, at rest and following vasodilator augmentation was similar between psoriasis and controls. Similarly, there was no difference in the ECG response during vasodilator infusion (Table 1).
Table 2.
PET myocardial blood flow, myocardial flow reserve, and myocardial function in psoriasis and matched controls
| Imaging Findings | Psoriasis (n=62) |
Controls (n=112) |
P value |
|---|---|---|---|
| Rest myocardial blood flow | 1.1 (0.4) | 1.03 (0.38) | 0.1 |
| Stress myocardial blood flow | 2.04 (0.94) | 2.2 (0.69) | 0.26 |
| Myocardial Flow Reserve | 1.92 (0.65) | 2.3 (0.8) | 0.001 |
| Rest LVEF (%) | 63.6 (10.2) | 60.3 (8.2) | 0.03 |
| Stress LVEF (%) | 68.1 (9.9) | 65.7 (8.6) | 0.1 |
| Resting HR | 68.5 (10.6) | 71.9 (13) | 0.08 |
| Peak HR | 90.9 (17.3) | 88.4 (17.6) | 0.4 |
| Resting SBP | 146.2 (26.1) | 145.2 (25.6) | 0.8 |
| Peak SBP | 134.4 (22.4) | 137.9 (25.2) | 0.4 |
| Ischemic ECG response to vasodilator infusion, n (%) | 2 (3.2%) | 2 (1.8%) | 0.6 |
Mean and SD are shown, except where noted. Rest and stress myocardial blood flow in ml/min/g. LVEF =left ventricular ejection fraction. HR=heart rate; SBP= systolic blood pressure. HR in bpm; SBP in mmHg.
Figure 1.
Myocardial flow reserve in psoriatic patients. A. Pie charts demonstrate the prevalence of CMD (defined as MFR<2) in psoriasis and controls (61.3%, vs 38.4%,respectively, p=0.004). B. Histogram and box plot represent the distribution of MFR in psoriasis and controls ((2.3±0.81 vs 1.92±0.65, respectively, p=0.001). MFR=Stress/Rest MBF). MFR was adjusted for differences in resting cardiac work (Adjusted MBF=rest MBF/[rest heart rate *rest systolic blood pressure]multiplied by 10,000).
Relationship between atherosclerotic burden and myocardial flow reserve
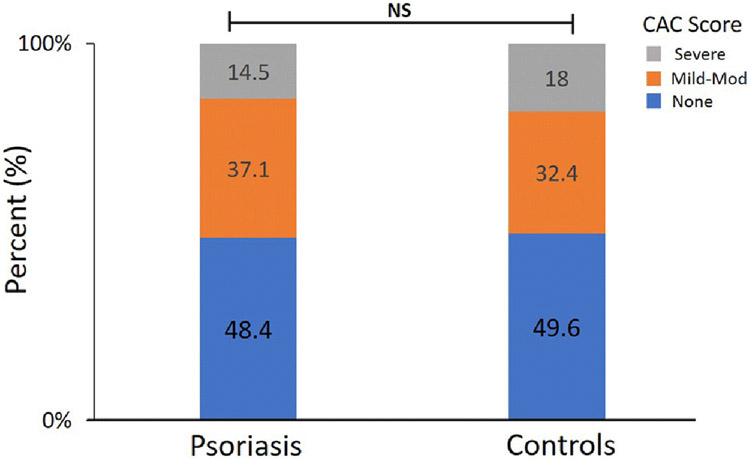
The high prevalence of CV risk factors in psoriatic patients is consistent with prior literature.[20, 21] To determine if the more severe reduction of MFR in psoriatic patients was a manifestation of diffuse atherosclerosis, semi-quantitative analysis of the calcified atheroslcerlotic burden from the non-contrast CT transmission scan was performed. The atherosclerotic burden was similar in psoriasis and controls [p=0.77] (Figure 2). No coronary calcium was detected in 48.4% of psoriatic patients and 49.6% of controls. Mild-moderate calcium burden was identified in 37.1% of psoriatic patients and 32.5% controls. Furthermore, only 14.5% of psoriatic patients had evidence of severe coronary calcium, which was similar to controls (18%). This results suggests that the reduced MFR was not simply a manifestation of diffuse atherosclerosis but represents an abnormal microcirculatory response to stress in psoriatic patients. A representative example of a patient with psoriasis referred for ongoing dyspnea found to have coronary microvacular dysfunction despite normal perfusion and the absence of coronary calcium is shown (Figure 3a).
Figure 2.
Atherosclerotic burden among Psoriasis and controls. The degree of coronary artery calcium (CAC) was semi-quantitatively determined to be none, mild-moderate, or severe as described in methods. Stacked bar plots demonstrate the frequency of each CAC category between psoriasis and controls. Results were not significantly different based on a Fisher’s exact T-test (p=0.77).
Figure 3.
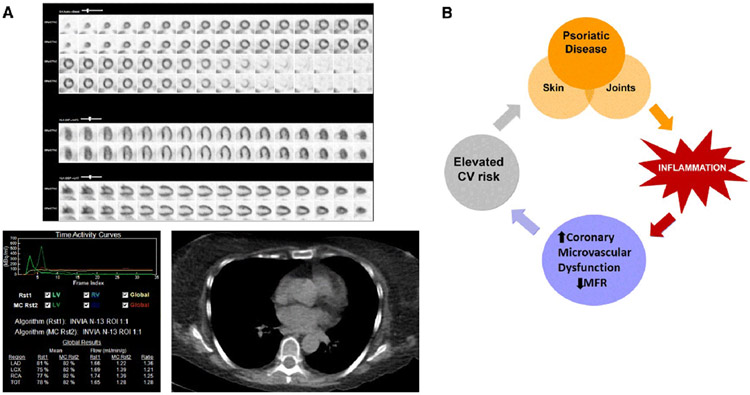
Coronary Microvascular Dysfunction and Psoriasis. A. Representative example of a psoriasis patient in the cohort. 59 year old women with active psoriasis, dyslipidemia, and obesity was referred for cardiac PET for ongoing dyspnea. Top panel represents normal perfusion at rest and with vasodilator infusion. Below left panel demonstrates absolute blood quantification; stress coronary myocardial blood flow was globally reduced with a resultant low MFR (Uncorrected MFR=1.37, Corrected MFR=1.87). Gated CT is shown with no evidence of coronary calcium. B. Proposed pathophysiology of cardiovascular complications in psoriatic disease.
Discussion
Previous studies in psoriasis have shown evidence of large vessel vascular inflammation that is associated with CAD.[14, 22] Our findings are novel and extend the observations of prior studies[13, 14] in two important ways: by showing a high prevalence of coronary vasomotor abnormalities in symptomatic high risk patients with psoriasis without obstructive CAD, and by demonstrating that the severity of these abnormalities represents excess microvascular dysfunction not accounted for by common associated coronary risk factors or the burden of atherosclerosis. The excess microvascular risk in this study supports a potential role for inflammation in driving coronary vasomotor abnormalities that might contribute to excess cardiovascular risk in this population (Figure 3b). We hypothesize a working model where high serum levels of pro-inflammatory cytokines released including interleukin (IL)-17, interferon-alpha and tumor necrosis factor perturb endothelial function and vascular health resulting in coronary vascular dysfunction, subclinical myocardial ischemia and injury, structural and mechanical abnormalities and, ultimately, adverse cardiac events. Th17 cells are increasingly recognized as a driver of psoriasis pathogenesis, and IL-17 is a potential mechanistic link between psoriasis and CMD. Dermal overexpression of IL-17A induces systemic endothelial dysfunction, and prior work has proposed context specific roles of IL-17 in atherogenesis.[23]
Study Limitations
Limitations of the current study include the lack of the validated disease-specific activity index (PASI) to determine psoriasis severity and the treatment history at the time of the cardiovascular assessment. The patients in this cohort were clinically referred for symptoms of dyspnea or chest pain, thus likely representing a higher risk population. However, even after excluding obstructive CAD, LV dysfunction, and matching for similar baseline co-morbitidies, we found that patients with psoriasis have a high prevalence of CMD that is not fully explained by baseline CV risk factors. Low sample size limited the power to ascertain the impact on long-term outcomes although work from our group and others have established the importance of CMD on risk-stratification and long-term outcomes in the general population.[9] Future prospective studies are needed to address CMD across clinical severities of psoriasis and psoriatic arthrits and whether reducing systemic inflammation with the new novel biologics in psoriatic disease (e.g. IL-17 and IL-23 inhibitors) can improve coronary microvascular function and cardiovascular outcomes.
Conclusion
In conclusion, these findings now demonstrate that patients with psoriasis have a higher prevalence and more severe CMD than age-, sex- and CV risk factor-matched controls in the absence of obstructive CAD. Importantly, reduced MFR was not simply a result of diffuse atherosclerosis and suggests an abnormal coronary vasomotor response to stress. The identification of impaired MFR in psoriasis may be a sensitive marker of excess CV risk in this population and could warrant aggressive CV risk reduction.
Supplementary Material
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of Myocardial Infarction in Patients With Psoriasis. JAMA 2006;296:1735. [DOI] [PubMed] [Google Scholar]
- Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch. Dermatol 2007;143:1493–1499. [DOI] [PubMed] [Google Scholar]
- Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur. Heart J 2010;31:1000–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta NN, Yu Y, Pinnelas R, et al. Attributable Risk Estimate of Severe Psoriasis on Major Cardiovascular Events. Am. J. Med 2011;124:775.e1–775.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abuabara K, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Cause-specific mortality in patients with severe psoriasis: A population-based cohort study in the United Kingdom. Br. J. Dermatol 2010;163:586–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid U, Ahlehoff O, Gislason GH, et al. Psoriasis and risk of heart failure: a nationwide cohort study. Eur. J. Heart Fail 2014;16:743–748. [DOI] [PubMed] [Google Scholar]
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J. Am. Acad. Dermatol 2014;70:512–516. [DOI] [PubMed] [Google Scholar]
- Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: A systematic review and meta-analysis of observational and clinical studies. J. Am. Acad. Dermatol 2019;80:251–265.e19. [DOI] [PubMed] [Google Scholar]
- Gupta A, Taqueti VR, van de Hoef TP, et al. Integrated Non-invasive Physiological Assessment of Coronary Circulatory Function and Impact on Cardiovascular Mortality in Patients with Stable Coronary Artery Disease. Circulation 2017;136:2325–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VL, Naya M, Foster CR, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation 2011;124:2215–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne MT, Bajaj NS, Taqueti VR, et al. Coronary Microvascular Dysfunction Identifies Patients at High Risk of Adverse Events Across Cardiometabolic Diseases. J. Am. Coll. Cardiol 2017;70:2835–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taqueti VR, Hachamovitch R, Murthy VL, et al. Global Coronary Flow Reserve Associates with Adverse Cardiovascular Events Independently of Luminal Angiographic Severity, and Modifies the Effect of Early Revascularization. Circulation 2015;131:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piaserico S, Osto E, Famoso G, et al. Long-term prognostic value of coronary flow reserve in psoriasis patients. Atherosclerosis 2019;289:57–63. [DOI] [PubMed] [Google Scholar]
- Joshi AA, Lerman JB, Dey AK, et al. Association Between Aortic Vascular Inflammation and Coronary Artery Plaque Characteristics in Psoriasis. JAMA Cardiol. 2018;3:949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Fakhri G, Kardan A, Sitek A, et al. Reproducibility and accuracy of quantitative myocardial blood flow assessment with (82)Rb PET: comparison with (13)N-ammonia PET. J. Nucl. Med. Off. Publ. Soc. Nucl. Med 2009;50:1062–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lortie M, Beanlands RSB, Yoshinaga K, Klein R, Dasilva JN, DeKemp RA. Quantification of myocardial blood flow with 82Rb dynamic PET imaging. Eur. J. Nucl. Med. Mol. Imaging 2007;34:1765–1774. [DOI] [PubMed] [Google Scholar]
- Camici PG, d’Amati G, Rimoldi O. Coronary microvascular dysfunction: mechanisms and functional assessment. Nat. Rev. Cardiol 2015;12:48–62. [DOI] [PubMed] [Google Scholar]
- Einstein AJ, Johnson LL, Bokhari S, et al. Agreement of Visual Estimation of Coronary Artery Calcium from Low-Dose CT Attenuation Correction Scans in Hybrid PET/CT and SPECT/CT with Standard Agatston Score. J. Am. Coll. Cardiol 2010;56:1914–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiles C, Duan F, Gladish GW, et al. Association of Coronary Artery Calcification and Mortality in the National Lung Screening Trial: A Comparison of Three Scoring Methods. Radiology 2015;276:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azfar RS, Seminara NM, Shin DB, Troxel AB, Margolis DJ, Gelfand JM. Increased Risk of Diabetes and Likelihood of Receiving Diabetes Treatment in Patients with Psoriasis. Arch. Dermatol 2012;148:995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Li M, Wang H, Li Y, Bai B. High prevalence of cardiovascular risk factors in patients with moderate or severe psoriasis in northern China. Arch. Dermatol. Res 2014;306:247–251. [DOI] [PubMed] [Google Scholar]
- Dey AK, Joshi AA, Chaturvedi A, et al. Association Between Skin and Aortic Vascular Inflammation in Patients With Psoriasis: A Case-Cohort Study Using Positron Emission Tomography/Computed Tomography. JAMA Cardiol. 2017;2:1013–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockshin B, Balagula Y, Merola JF. Interleukin 17, inflammation, and cardiovascular risk in patients with psoriasis. J. Am. Acad. Dermatol 2018;79:345–352. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.