Abstract
Background and Objectives
To evaluate whether children born to women who use serotonergic antidepressants during pregnancy have higher risk of neonatal seizures and epilepsy.
Methods
We used Swedish register-based data to examine associations between maternal reported use of selective serotonin reuptake inhibitors (SSRIs) or serotonin–norepinephrine reuptake inhibitors (SNRIs) in pregnancy and diagnosis of neonatal seizures or epilepsy in >1.2 million children. To account for systematic differences between exposed and unexposed children, we adjusted for a wide range of measured confounders. After first evaluating the role of maternal indication for SSRI/SNRI use (i.e., depression or anxiety) and parental epilepsy, we adjusted for remaining parental background factors (e.g., age, comorbidities, education, and family socioeconomic indices) and pregnancy-specific characteristics (e.g., maternal use of other psychotropic medications and tobacco smoking in early pregnancy).
Results
Compared with all other children, children of women who reported use of SSRI/SNRI in pregnancy had an elevated risk of neonatal seizures and epilepsy (risk ratio [RR] 1.41, 95% CI 1.03–1.94; hazard ratio [HR] 1.21, 95% CI 1.03–1.43, respectively). The estimates of association were attenuated by adjustment for maternal indications for SSRI/SNRI use (RR 1.30, 95% CI 0.94–1.80; HR 1.13, 95% CI 0.95–1.33), but not by additional adjustment for parental history of epilepsy. Full adjustment for all measured parental and pregnancy-specific factors resulted in substantial attenuation of the remaining associations (RR 1.10, 95% CI 0.79–1.53; HR 0.96, 95% CI 0.81–1.14).
Discussion
We found no support for the concern that maternal SSRI/SNRI use in pregnancy increases children's risk for neonatal seizures or epilepsy.
Classification of Evidence
This study provides Class II evidence that exposure to SSRIs/SNRIs in the first trimester of pregnancy is not associated with an increased incidence of neonatal seizures/epilepsy.
Several studies have documented an association between serotonergic antidepressant (i.e., selective serotonin reuptake inhibitor [SSRI] or serotonin–norepinephrine reuptake inhibitor [SNRI]) use in pregnancy and neonatal seizures, particularly third trimester use.1-13 Few studies, however, have examined whether these medications are associated with recurrent seizures (i.e., epilepsy) in childhood.14
Given that seizures in neonates can be observed as part of neonatal abstinence syndrome1-13 just after antidepressant supply is cut off, it is possible that observed risks are the result of withdrawal. Prenatal exposure to serotonergic antidepressants may also disrupt synaptogenesis and neuronal growth and differentiation14-17 in such a way that could influence the risk of longer-term, recurrent seizures.
However, before concluding that observed associations are causal, limitations to the extant literature must be addressed. Given the rarity of seizures, most previous studies have been underpowered.1,3,4,6,8,10-13,18 In addition, as noted in a recent review, the existing research provides correlational evidence without adjustment for important confounders, including the maternal indication for antidepressant use, which could be comorbid with seizure disorders (e.g., maternal depression and anxiety).19-22 Thus, it is unclear whether observed risks of seizures in children could be due to prenatal pharmacologic exposure or confounding by maternal background factors.
We aimed to evaluate whether children born to women who use serotonergic antidepressants during pregnancy have higher risk of neonatal seizures and epilepsy in a nationwide sample of children. We considered many confounders not incorporated in prior studies (i.e., parental characteristics, sociodemographics, pregnancy-specific factors). We adjusted for psychiatric and behavioral health problems because there has been well-documented comorbidity between these conditions (particularly depression) and seizures that is likely to be at least partially genetic in origin.20-22,29,30 As such, it stands to reason that parents with psychiatric and behavioral problems may be more likely to have children with seizures. We further sought to expand the previous literature by also examining the potential connection to children's risk of developing epilepsy.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The institutional review board at Indiana University and the regional ethical review board in Stockholm, Sweden, approved this study. According to Swedish law, informed consent was not necessary because the study used data available from national Swedish registries.
Data Availability
The data used in this study are national register information. The authors had no privileges in accessing the data. Dissemination of personal information is regulated by the Swedish Secrecy Act. In accordance with Swedish law, researchers seeking access to individual-level data must apply for permission through a Research Ethics Board and from the primary owners: Statistics Sweden and the National Board of Health and Welfare.
Data Source
Each individual in Sweden is assigned a unique registration number through which records of health and demographics in national registers can be linked to follow individuals over time. The current study centered on information from the Medical Birth Register, which includes data from antenatal visits, delivery, and pediatric examination for approximately 98% of all births since 1973. Since mid-1994, women have reported any use of medications as part of the standardized interview at enrollment to antenatal care, which typically occurs between weeks 8 and 12 of pregnancy.23,24 At this time the midwife also collects information regarding the woman's age, cohabitation status, reproductive history, and use of tobacco. From mid-2005, the maternal self-report of medication use can be evaluated against the filling of prescriptions using the Swedish Prescribed Drug Register (PDR) of all filled prescriptions outside hospital.25 Fathers were identified using the Multi-Generation Register.26 The National Patient Register includes ICD-coded diagnoses made during all inpatient visits since 1987 and at outpatient specialist visits since 2001.27 We used this register to identify parental epilepsy and psychiatric diagnoses (including depression and anxiety) as well as neonatal seizure and epilepsy diagnoses in children. Finally, we used the Integrated Database for Labor Market Research28 and the Education Register to retrieve information on socioeconomic indices.
Sample
With register follow-up available through 2013, we followed all children born from January 1, 1996, to November 30, 2013, with respect to a diagnosis of neonatal seizures (made in the first month of life). In the evaluation of epilepsy, we required a minimum of 2 years of follow-up, thus restricting the sample to children born before December 31, 2011.
Measures
Exposure
We defined SSRI/SNRI exposure from the maternal self-report at enrollment to antenatal care, typically corresponding to the end of the first trimester. However, we used a cohort covered by the PDR (i.e., the 751,887 births from mid-2006 to 2013) to show that of the women who self-reported SSRI/SNRI use during pregnancy, 64% had filled SSRI/SNRI prescriptions in the first trimester, 35% had filled SSRI/SNRI prescriptions in the second trimester, and 34% had filled SSRI/SNRI prescriptions in the third trimester. Using the same cohort, we also found the agreement between self-reported SSRI/SNRI use and second/third trimester SSRI/SNRI filled prescriptions was 98.5% (κ 0.63, 95% CI 0.63–0.64).
SSRIs included medications with an anatomical therapeutic chemical (ATC) code beginning with N06AB (fluoxetine, citalopram, paroxetine, sertraline, fluvoxamine, escitalopram, and unspecified SSRIs). SNRIs included medications with the following ATC codes: N06AX16 (venlafaxine) and N06AX21 (duloxetine). In our cohort, 23,160 (1.49%) children were born to women who reported use of at least 1 SSRI or SNRI (Table 1).
Table 1.
Exposed Offspring in the Complete Analytic Sample

Outcomes
Neonatal seizures were identified via at least 1 diagnosis (ICD-9,779.0 and ICD-10 P90) in the first 30 days of life.1-13,19 Diagnosis of epilepsy (ICD-9 345 and ICD-10 G40-G41) was identified using the same sources at any time after birth.
Covariates
Covariates were identified for their ability to block potential influence from hypothesized common causes of maternal antidepressant use during pregnancy and seizure disorders in children. Pregnancy-specific characteristics included the year of birth, maternal self-report of tobacco smoking, and other psychotropic medication use at the first antenatal visit. Individual parental characteristics included age and highest level of education at the time of birth and diagnosis of epilepsy, mood disorders, anxiety disorders, substance use disorder, schizophrenia, bipolar disorder, suicide attempt, or record of criminal convictions prior to conception. At the family level, we considered sociodemographic factors, including parental cohabitation status, income, and a measure of neighborhood deprivation in the year of the birth. The neighborhood deprivation scores incorporated annual proportions of welfare recipients, unemployed, immigrants, divorced individuals, and individuals with low educational attainment and measures of residential mobility, crime rates, and neighborhood disposable income.31
Data Analytic Plan
We analyzed data for this study using the SAS software system, version 9.4. We first performed descriptive analyses on our identified covariates, exposures, and outcomes. We then estimated associations (unadjusted and adjusted for covariates) between maternal SSRI/SNRI use in pregnancy and children's risk of seizures, using log-binomial regression for the cumulative incidence of seizures in the neonatal period and Cox proportional hazard regression for incident diagnosis of epilepsy during follow-up. We followed children from birth to first diagnosis or censoring at time of emigration, death, or end of follow-up as the underlying time scale.
After estimating the overall associations, we adjusted for maternal indications for antidepressant use (i.e., mood and anxiety disorders) to examine the specific influence of confounding by indication. Next, we further adjusted for maternal and paternal history of epilepsy prior to conception to partially examine the potential influence of genetic confounding, given that research suggests epilepsy is heritable.32 Finally, we estimated associations with full adjustment for all covariates, including measures of other individual parental characteristics, family sociodemographic factors, and pregnancy-specific characteristics.
We conducted a sensitivity analysis to examine whether findings were susceptible to bias related to left censoring of our data, as we were not able to capture outpatient diagnoses prior to 2001. Specifically, we re-estimated associations in a cohort of children born after 2000. We also conducted a sensitivity analysis to re-estimate associations in a cohort that excluded children exposed to multiple antidepressants during pregnancy.
Results
Starting out with all children born from 1996 through 2013 (n = 1,781,353) we sequentially excluded those who were stillborn (n = 6,335), had invalid maternal identifiers (n = 478), were from multiple gestations (n = 52,164), had missing gestational age (n = 1,101), or had missing sex (n = 1), resulting in a cohort of 1,721,274 children. By further exclusion of children with missing information on the covariates of interest (n = 169,368), the complete case sample represented 90% of the target, with 1,551,906 children available for the examination of neonatal seizures and 1,367,087 children for the examination of epilepsy.
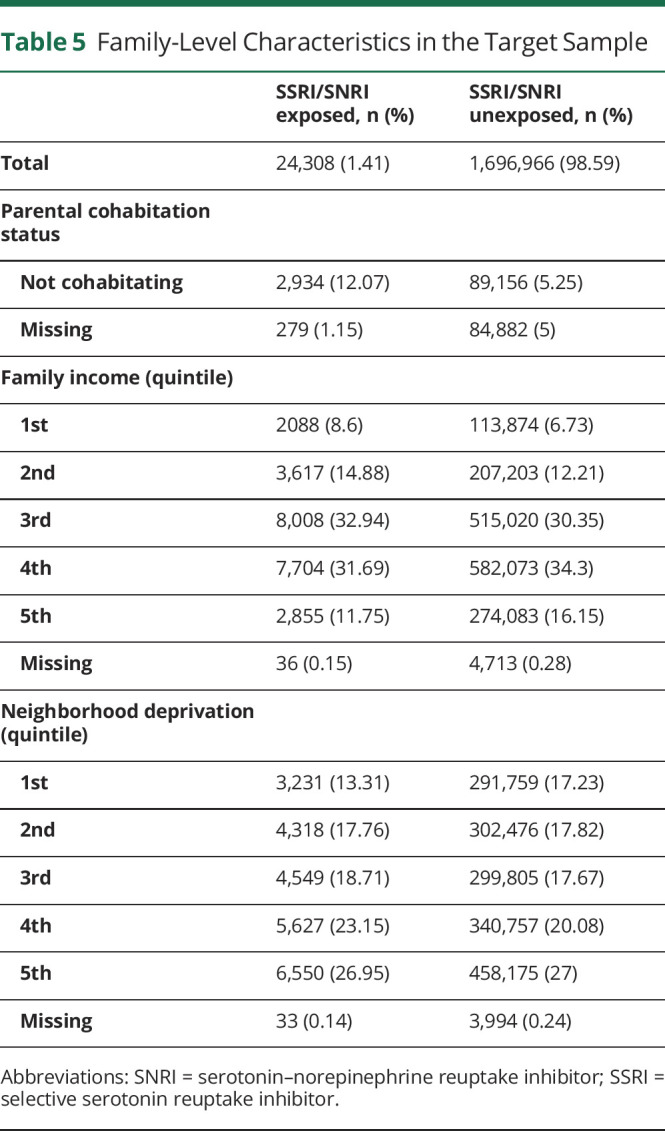
We first examined the distribution of relevant pregnancy (Table 2), maternal (Table 3), paternal (Table 4), and family-level (Table 5) characteristics in relation to maternal self-reports of SSRI/SNRIs. This information is provided for the target sample to also show the distribution of missing data, which ranged from none to approximately 5%. Women who reported use of serotonergic antidepressants in pregnancy were more likely to also report smoking and use of other psychotropic medications. Not only were psychiatric disorders and epilepsy more common among these women, they were also more common in their partners (Tables 3 and 4).
Table 2.
Pregnancy-Specific Characteristics in the Target Sample
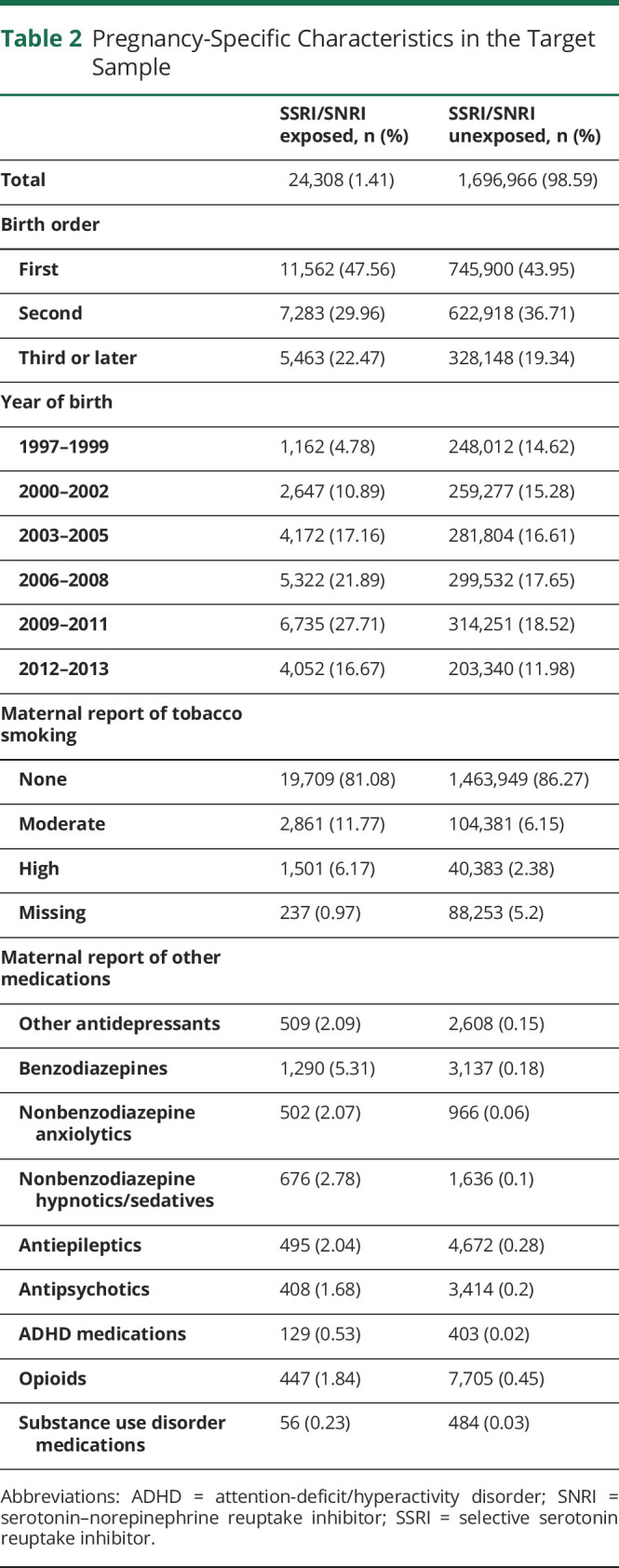
Table 3.
Maternal Characteristics in the Target Sample
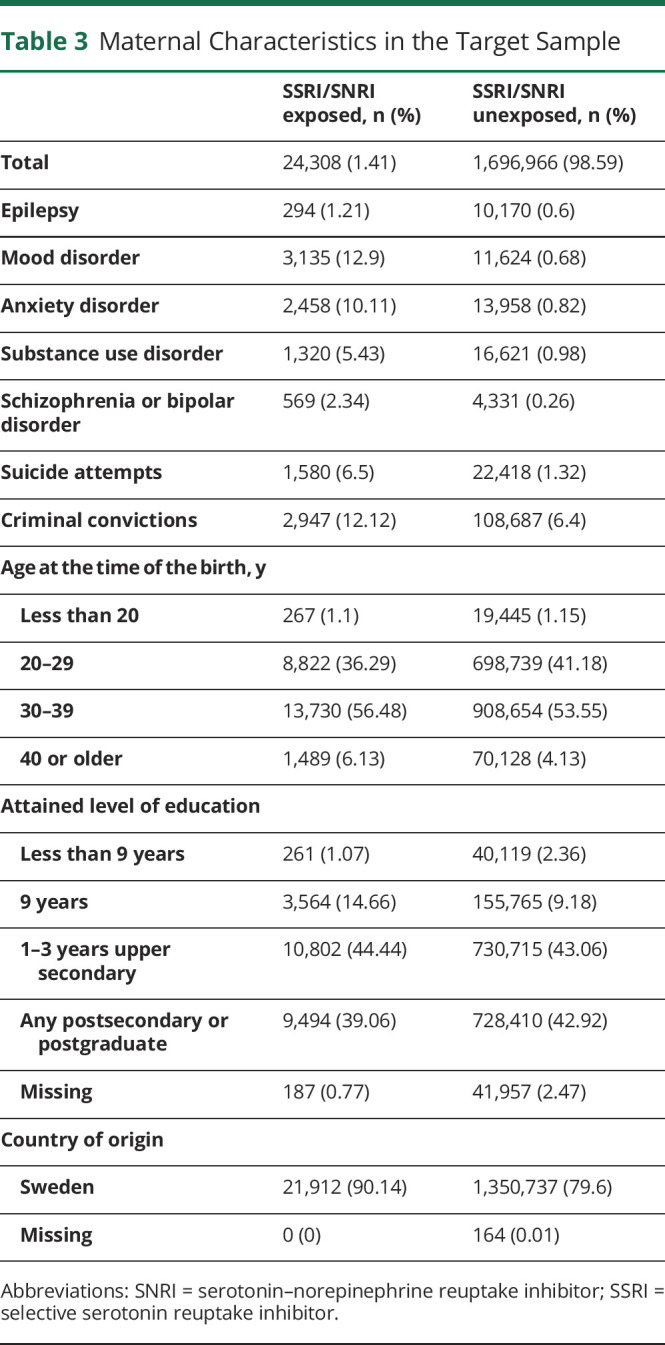
Table 4.
Paternal Characteristics in the Target Sample

Table 5.
Family-Level Characteristics in the Target Sample
In the full analytic sample, 1.36% of women reported use of SSRIs. The most commonly reported medications were sertraline (0.49%), citalopram (0.48%), and fluoxetine (0.20%). Fewer women (0.14%) reported use of SNRIs in pregnancy, with venlafaxine being the most commonly reported medication (0.12%; Table 1).
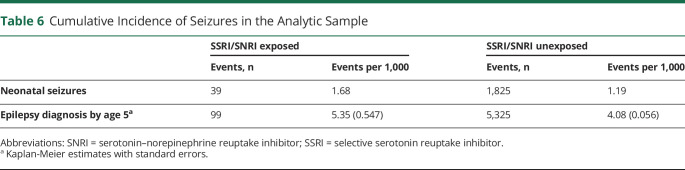
Documented occurrence of seizures in the first month of life were rare, but more common among exposed than unexposed children (1.7 vs 1.2 per 1,000). By age 5 years, 5.4 per 1,000 exposed and 4.1 per 1,000 unexposed children had been diagnosed with epilepsy (Table 6).
Table 6.
Cumulative Incidence of Seizures in the Analytic Sample
Children born to mothers who reported use of SSRIs or SNRIs in pregnancy had 41% greater risk of experiencing seizures in the newborn period (risk ratio [RR] 1.41, 95% CI 1.03–1.94) and 21% greater risk of being diagnosed with epilepsy during follow-up (hazard ratio [HR] 1.21, 95% CI 1.03–1.43) compared with children whose mothers did not report such use (Table 7). Adjustment for maternal indications for SSRI/SNRI use led to some attenuation of the observed associations (RR 1.30, 95% CI 0.94–1.80; HR 1.13, 95% CI 0.95–1.33). Further adjustment for maternal and paternal history of epilepsy had little to no influence on the estimates of association, whereas adjustment for remaining covariates greatly attenuated the remaining associations (RR 1.10, 95% CI 0.79–1.53; HR 0.96, 95% CI 0.81–1.14; Table 7).
Table 7.
Associations Between Maternal-Reported SSRI and SNRI Use in Pregnancy and Seizures in Children
Findings from fully adjusted sensitivity analyses on a restricted subsample of children born after 2000 suggest that our main findings were not influenced by the left censoring of our data (eTable 1, links.lww.com/WNL/B971). Our main findings were not affected by the inclusion of children exposed to >1 antidepressant in pregnancy (eTable 2, links.lww.com/WNL/B972).
This study provides Class II evidence that exposure to SSRIs/SNRIs in the first trimester of pregnancy is not associated with an increased incidence of neonatal seizures and epilepsy.
Discussion
Using a large nationwide sample, and with adjustment for a more extensive set of confounding factors than previous research,19 we examined the risk of seizure disorders in children whose mothers reported use of SSRIs and SNRIs in pregnancy. Our evaluation of 2 seizure outcomes—neonatal seizures and childhood epilepsy—yielded overall reassuring results.
Consistent with prior research,1-13 we observed a higher risk of neonatal seizures among children exposed to SSRIs/SNRIs in pregnancy, with 0.17% of exposed children affected compared with 0.12% of unexposed children. We added to prior research by showing that this association was almost entirely explained by systematic differences between the groups (i.e., maternal indications for SSRIs and SNRIs and a wide range of other pregnancy, parental, and socioeconomic factors).
We further expand on previous literature by examining the risk of epilepsy in children born to mothers reporting SSRI and SNRI use in pregnancy. Our findings are consistent with the only other study that has investigated this association,14 as we observed an elevated risk that was explained by maternal indication for SSRI and SNRI use, parental history of epilepsy, and our other covariates. Thus, we did not find evidence that maternal use of SSRI and SNRI in pregnancy increases children's risk of epilepsy.
These findings are consistent with prior evidence of shared risk for seizures and psychiatric/behavioral health conditions, particularly depression.20-22,29,30 Research has also indicated that these comorbidities are likely to be at least partially genetically driven,30 such that one could expect children of parents with psychiatric and behavioral health problems to have higher risk of seizures due to genetic liability. This may explain why adjustment for these factors led to attenuation of associations in our study. In fact, our findings (1) highlight the importance of adjusting for confounding by indication (i.e., depression and anxiety disorders) and other comorbidities and (2) suggest that past research may have overestimated the risk by not adequately adjusting for confounding factors.2,4,7-13,18
The findings of this study are of clear clinical importance. Pregnancy is a stressful time, and the addition of depression, anxiety, and other mental health conditions add to this burden. As such, these findings may provide respite and reassurance to women (and providers) considering the risks and benefits of medication treatment, especially for those who do not have the time or resources to pursue nonpharmacologic treatment. This is even more pertinent when considering the broader research literature that has observed (1) similar null findings for other adverse outcomes in children (e.g., attention-deficit/hyperactivity disorder, autism spectrum disorder) in relation to antidepressant use in pregnancy33 and (2) that untreated depression and anxiety in pregnancy is related to adverse outcomes in children.34
This study is subject to several limitations. Although much of the existing literature examining neonatal seizures related to antidepressant use has documented strongest associations with exposure towards the end of pregnancy19 and suggests that exposure late in pregnancy may have important implications for longer-term neurodevelopmental outcomes,35-37 our exposure definition was based on maternal self-reports in the first trimester. Whereas use of maternal reports could result in some misclassification of medication use, using maternal reports would also result in less misclassification than prescription records for individuals who fill prescriptions but do not use medications. We also demonstrated high agreement between maternal reports and filled prescriptions for SSRIs/SNRIs in the first as well as later trimesters of pregnancy, which is important for 2 reasons. First, the high concordance demonstrated in this and other studies using Swedish data suggest that maternal self-report of antidepressant use is reliable.38,39 Second, it indicates that women reporting use in the first trimester are likely to continue use throughout pregnancy. Nonetheless, future work should also explore the timing of exposure with comprehensive adjustment for confounding factors. In addition, we did not have detailed clinical information to identify whether a diagnosis of neonatal seizures was confirmed via EEG. Whereas the potential for misclassification is not expected to be differential with respect to exposure (i.e., maternal use of SSRI in pregnancy), random misclassification could bias the estimate of association toward the null, thus hampering our ability to detect a potential small association. Our data also cannot rule out the possibility that prenatal exposure to SSRIs or SNRIs might pose risk in some individuals with specific co-occurring conditions (e.g., mosaic mutations in one's sodium channels). More research is needed to explore and understand any effect modification. Finally, future research is needed to understand the use of additional medications and polypharmacy in pregnancy and its relation to seizure outcomes in children.
Although the current study found that children of women who use SSRIs/SNRIs are at elevated risk of neonatal seizures and epilepsy, this appears to be largely due to background factors rather than to the medication use itself.
Glossary
- ATC
anatomical therapeutic chemical
- HR
hazard ratio
- ICD
International Classification of Diseases
- PDR
Prescribed Drug Register
- RR
relative risk
- SNRI
serotonin–norepinephrine reuptake inhibitor
- SSRI
selective serotonin reuptake inhibitor
Appendix. Authors

Footnotes
Editorial, page 957
Null Hypothesis: NPub.org/Null
Class of Evidence: NPub.org/coe
CME Course: NPub.org/cmelist
Study Funding
This project was supported by the National Institute of Neurologic Disorders and Stroke (F31NS111856), National Institute of Mental Health (T32MH103213), National Institute on Drug Abuse of the NIH (R01DA048042 and R00DA040727), the National Science Foundation (1342962), the Swedish Research Council, and the Swedish Research Council for Health, Working Life and Welfare (FORTE; 50623213). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosure
H. Larsson has served as a speaker for Eli Lilly and Shire and has received research grants from Shire, all outside the submitted work. The other authors have no disclosures to report. Go to Neurology.org/N for full disclosures.
References
- 1.Cantarutti A, Merlino L, Giaquinto C, Corraosafety GD. Use of antidepressant medication in pregnancy and adverse neonatal outcomes: a population-based investigation. Pharmacoepidemiol Drug Saf. 2017;26(9):1100-1108. [DOI] [PubMed] [Google Scholar]
- 2.Leibovitch L, Rymer-Haskel N, Schushan-Eisen I, Kuint J, Strauss T, Maayan-Metzger A. Short-term neonatal outcome among term infants after in utero exposure to serotonin reuptake inhibitors. Neonatology. 2013;104(1):65-70. [DOI] [PubMed] [Google Scholar]
- 3.Hayes RM, Wu P, Shelton RC, et al. Maternal antidepressant use and adverse outcomes: a cohort study of 228,876 pregnancies. Am J Obstet Gynecol. 2012;207(1):49e41-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Källén B, Reis M. Neonatal complications after maternal concomitant use of SSRI and other central nervous system active drugs during the second or third trimester of pregnancy. J Clin Psychopharmacol. 2012;32(5):608-614. [DOI] [PubMed] [Google Scholar]
- 5.Warburton W, Hertzman C, Oberlander TF. A register study of the impact of stopping third trimester selective serotonin reuptake inhibitor exposure on neonatal health. Acta Psychiatr Scand. 2010;121(6):471-479. [DOI] [PubMed] [Google Scholar]
- 6.Davis RL, Rubanowice D, McPhillips H, et al. Risks of congenital malformations and perinatal events among infants exposed to antidepressant medications during pregnancy. Pharmacoepidemiol Drug Saf. 2007;16(10):1086-1094. [DOI] [PubMed] [Google Scholar]
- 7.Maschi S, Clavenna A, Campi R, Schiavetti B, Bernat M, Bonati M. Neonatal outcome following pregnancy exposure to antidepressants: a prospective controlled cohort study. BJOG 2008;115(2):283-289. [DOI] [PubMed] [Google Scholar]
- 8.Lennestål R, Källén B. Delivery outcome in relation to maternal use of some recently introduced antidepressants. J Clin Psychopharmacol. 2007;27(6):607-613. [DOI] [PubMed] [Google Scholar]
- 9.Levinson-Castiel R, Merlob P, Linder N, Sirota L, Klinger G. Neonatal abstinence syndrome after in utero exposure to selective serotonin reuptake inhibitors in term infants. Arch Pediatr Adolesc Med. 2006;160(2):173-176. [DOI] [PubMed] [Google Scholar]
- 10.Wen SW, Yang Q, Garner P, et al. Selective serotonin reuptake inhibitors and adverse pregnancy outcomes. Am J Obstet Gynecol. 2006;194(4):961-966. [DOI] [PubMed] [Google Scholar]
- 11.Sanz EJ, De-las-Cuevas C, Kiuru A, Bate A, Edwards R. Selective serotonin reuptake inhibitors in pregnant women and neonatal withdrawal syndrome: a database analysis. Lancet. 2005;365(9458):482-487. [DOI] [PubMed] [Google Scholar]
- 12.Källén B. Neonate characteristics after maternal use of antidepressants in late pregnancy. Arch Pediatr Adolesc Med. 2004;158(4):312-316. [DOI] [PubMed] [Google Scholar]
- 13.Simon GE, Cunningham ML, Davis RL. Outcomc. Am J Psychiatry. 2002;159(12):2055-2061. [DOI] [PubMed] [Google Scholar]
- 14.Mao Y, Pedersen LH, Christensen J, et al. Prenatal exposure to antidepressants and risk of epilepsy in childhood. Pharmacoepidemiol Drug Saf. 2016;25(11):1320-1330. [DOI] [PubMed] [Google Scholar]
- 15.Zafeiriou DI, Ververi A, Vargiami E. The serotonergic system: its role in pathogenesis and early developmental treatment of autism. Curr Neuropharmacol. 2009;7(2):150-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bozzi Y, Provenzano G, Casarosa S. Neurobiological bases of autism–epilepsy comorbidity: a focus on excitation/inhibition imbalance. Eur J Neurosci. 2018;47(6):534-548. [DOI] [PubMed] [Google Scholar]
- 17.Doshi-Velez F, Ge Y, Kohane IJP. Comorbidity clusters in autism spectrum disorders: an electronic health record time-series analysis. Pediatrics. 2014;133(1):e54-e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oberlander TF, Warburton W, Misri S, Aghajanian J, Hertzman C. Neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitor antidepressants and maternal depression using population-based linked health data. Arch Gen Psychiatry. 2006;63(8):898-906. [DOI] [PubMed] [Google Scholar]
- 19.Uguz F. The use of antidepressant medications during pregnancy and the risk of neonatal seizures: a systematic review. J Clin Psychopharmacol. 2019;39(5):479-484. [DOI] [PubMed] [Google Scholar]
- 20.Hesdorffer DC, Hauser WA, Annegers JF, Cascino G. Major depression is a risk factor for seizures in older adults. Ann Neurol. 2000;47(2):246-249. [PubMed] [Google Scholar]
- 21.Hesdorffer DC, Hauser WA, Olafsson E, Ludvigsson P, Kjartansson O. Depression and suicide attempt as risk factors for incident unprovoked seizures. Ann Neurol. 2006;59(1):35-41. [DOI] [PubMed] [Google Scholar]
- 22.Hesdorffer DC, Ishihara L, Mynepalli L, Webb DJ, Weil J, Hauser WA. Epilepsy, suicidality, and psychiatric disorders: a bidirectional association. Ann Neurol. 2012;72(2):184-191. [DOI] [PubMed] [Google Scholar]
- 23.Källén B, Källén K. The Swedish Medical Birth Register: A Summary of Content and Quality. 2003. lup.lub.lu.se/search/publication/1127699. [Google Scholar]
- 24.Cnattingius S, Ericson A, Gunnarskog J, Källén B. A quality study of a medical birth registry. Scand J Soc Med. 1990;18(2):143-148. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. WHO Collaborating Centre for Drug Statistics Methodology: ATC Classification Index with DDDs and Guidelines for ATC Classification and DDD Assignment. Norwegian Institute of Public Health; 2006. [Google Scholar]
- 26.Statistics Sweden. Multi-Generation Register 2009: A Description of Contents and Quality. Statistics Sweden: 2010. [Google Scholar]
- 27.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11(1):450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ludvigsson JF, Svedberg P, Olén O, Bruze G, Neovius M. The longitudinal integrated database for health insurance and labour market studies (LISA) and its use in medical research. Eur J Epidemiol. 2019;34(4):423-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baldin E, Hauser WA, Pack A, Hesdorffer DC. Stress is associated with an increased risk of recurrent seizures in adults. Epilepsia. 2017;58(6):1037-1046. [DOI] [PubMed] [Google Scholar]
- 30.Hesdorffer DC, Caplan R, Berg AT. Familial clustering of epilepsy and behavioral disorders: evidence for a shared genetic basis. Epilepsia. 2012;53(2):301-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sariaslan A, Långström N, D'Onofrio B, Hallqvist J, Franck J, Lichtenstein P. The impact of neighbourhood deprivation on adolescent violent criminality and substance misuse: a longitudinal, quasi-experimental study of the total Swedish population. Int J Epidemiol. 2013;42(4):1057-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poduri A, Lowenstein D. Epilepsy genetics: past, present, and future. Curr Opin Genet Dev. 2011;21(3):325-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sujan AC, Öberg AS, Quinn PD, D'Onofrio BM. Annual Research Review: maternal antidepressant use during pregnancy and offspring neurodevelopmental problems: a critical review and recommendations for future research. J Child Psychol Psychiatry. 2019;60(4):356-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonari L, Pinto N, Ahn E, Einarson A, Steiner M, Koren G. Perinatal risks of untreated depression during pregnancy. Can J Psychiatry. 2004;49(11):726-735. [DOI] [PubMed] [Google Scholar]
- 35.Mrzljak L, Uylings HB, Van Eden GG, Judáš M. Neuronal development in human prefrontal cortex in prenatal and postnatal stages. Prog Brain Res. 1991;85:185-222. [DOI] [PubMed] [Google Scholar]
- 36.Bouyssi-Kobar M, du Plessis AJ, McCarter R, et al. Third trimester brain growth in preterm infants compared with in utero healthy fetuses. Pediatrics. 2016;138(5):e20161640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flores-Pajot M-C, Ofner M, Do MT, Lavigne E, Villeneuve PJ. Childhood autism spectrum disorders and exposure to nitrogen dioxide, and particulate matter air pollution: a review and meta-analysis. Environ Res. 2016;151:763-776. [DOI] [PubMed] [Google Scholar]
- 38.Sujan AC, Rickert ME, Öberg AS, et al. Associations of maternal antidepressant use during the first trimester of pregnancy with preterm birth, small for gestational age, autism spectrum disorder, and attention-deficit/hyperactivity disorder in offspring. JAMA. 2017;317(15):1553-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stephansson O, Granath F, Svensson T, Haglund B, Ekbom A, Kieler H. Drug use during pregnancy in Sweden: assessed by the prescribed Drug register and the medical birth register. Clin Epidemiol. 2011;3:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this study are national register information. The authors had no privileges in accessing the data. Dissemination of personal information is regulated by the Swedish Secrecy Act. In accordance with Swedish law, researchers seeking access to individual-level data must apply for permission through a Research Ethics Board and from the primary owners: Statistics Sweden and the National Board of Health and Welfare.