Abstract
In livestock, mortality in general, and mortality of the young, is societal worries and is economically relevant for farm efficiency. Genetic change is cumulative; if it exists for survival of the young and genetic merit can be estimated with sufficient accuracy, it can help alleviate the pressure of mortality. Lack of survival is a moving target; livestock production is in continuous change and labor shortage is a given. There is now ample evidence of clear genetic variance and of models able to provide genomic predictions with enough accuracy for selection response. Underlying traits such as birth weight, uniformity in birth weight, gestation length, number of teats, and farrowing duration all show genetic variation and support selection for survival or, alternatively, be selected for on their own merit.
Keywords: farrowing, piglet survival, pre-weaning mortality, selection, teats
Clear genetic variation in piglet survival exists and it follows the infinitesimal model; it is the result of many underlying traits. Geneticists should consult other pig specialists and vice versa to optimize the use of this genetic variation.
Introduction
In livestock, mortality in general, and mortality of the young, is societal worries and is economically relevant for farm efficiency. Mortality is a normal biological process and helps populations to adapt to changing environments. Farming systems should be seen as specific environments, in which animal populations need to adapt to and vice versa; management and nutrition need to be adapted to changing genetics. This review paper focuses on genetics, accompanying other papers in this journal section on environmental influences.
Genetic variation in survival exists and selection takes place; animals unfit to the environment die and will not disseminate their genes to the next generation. Changing livestock practices, genetic selection for other traits, and the desire to reduce mortality faster are reasons to build survival selection into breeding programs. Mortality is a binary trait; an animal is dead or alive. This survival phenotype is subject to many factors, including health, temperature, nutrition, and social interactions (e.g., crushing). Estimation of genetic merit is not easy and requires large datasets.
The piglet survival phenotype is related to other phenotypes such as litter size, birth weight, variation in birthweight, and gestation length, but also to the number of teats and mothering ability of the sow nursing the piglet. Some of these traits such as litter size have a clear economic value by themselves; other traits are mainly relevant because of their correlation with survival, e.g., gestation length. Premature piglets have a high mortality probability and gestation length is quite heritable. Genetics of traits related to survival are therefore relevant.
The total field of piglet survival is quite wide and relations between traits are often non-linear. We chose to visualize several relations using a fairly large dataset of individually recorded piglets. This paper aims to give an overview of approaches to genetically improve early life survival in pigs through direct selection and through understanding of the underlying biology, resulting in related heritable traits as candidates for selection.
Material and Methods
Animal Care and Use Committee approval was not needed because information was obtained from the literature and from a pre-existing database.
Data
The dataset, obtained from Topigs Norsvin, contains 7,351 sows with a total of 16,843 litters and 257,912 piglets. Data were taken from purebred dam line production on three different farms all linked to the same AI station. The sows had on average 2.4 litters between 2019 and 2021, and litter size was 15.3 piglets total born on average. The total number of piglets born per litter smaller than 3 or larger than 25 was left out from analyses to avoid outliers caused by very small group sizes. All piglets, including stillborn, had an individual birth weight record and were scored for farrowing survival (FAS) within 12 h of farrowing, lactation survival (LAS) which was defined from 12 h of life to weaning, and total number of teats. Both FAS and LAS were scored as binary traits, the piglet survived (100) or not (0). Data were analyzed using R software (R Core Team, 2021).
Piglet Survival and Correlated Traits
Genetics of piglet survival
Nielsen et al. (2013) introduced selection for the number of piglets still alive at day 5 after birth (LP5); this trait is an implicit index of litter size and pre-weaning survival, it has a heritability of around 0.10, and its phenotype is assessed at litter level, with the sow as the genotype. Henryon et al. (2022) simulated a situation where next to litter size, individual piglet survival records were available and showed a 30% increased genetic trend for the trait LP5. Guo et al. (2022) concluded from a large existing dataset that an additive maternal model for individual piglet survival records fit the data better, which is a model in which the genotype of the piglet is relevant for survival plus the genotype of the sow farrowing the piglet (Guo et al., 2022). Su et al. (2022) analyzed late survival (from day 5 to slaughter) with both linear and threshold models showing little advantage of one over the other method; this is relevant since interpretation of linear models is easier than of threshold models, where the latter are formally correct. Finally, Sharif-Islam et al. (2022) showed proof of the relevance of proper use of genomic data. The inclusion of the genotypes of dead animals increased genetic trend by 12%–24%. Genotyping dead animals is slightly counterintuitive to animal breeders since these animals stopped being selection candidates, but it is their phenotype that is relevant in this situation.
Leite et al. (2021) engaged most of these complexities of analyzing survival traits. Table 1 offers heritabilities for FAS and LAS both analyzed in additive-maternal models. Heritabilities appear low, but assume LAS to be 90%, then phenotypic variance is 900 (10*90) and, hence, additive genetic variance 45 (heritability* phenotypic variance).
Table 1.
Estimates for direct (ad) and maternal (am) heritability for piglet survival during farrowing (FAS) and during lactation (LAS) using a Bayesian threshold model Leite et al. (2021)
| Trait | h 2 a | h 2 m | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| FAS | 0.02 | 0.01 | 0.09 | 0.01 |
| LAS | 0.05 | 0.01 | 0.08 | 0.01 |
Back to the LP5 approach of Nielsen et al. (2013), they define (pre-weaning) mortality between farrowing and day 5 on litter level and estimate heritabilities of 0.09 and 0.10 in a Landrace and a Yorkshire line. Genetic variances are 30 and 40 and are highly significantly different from zero. This is quite similar to Leite et al. (2021). This litter approach combines the maternal genetic variance with half the additive genetic variance of the piglet.
This indicates that the problem of selection against mortality is not the absence of genetic variance, but more the low accuracy of the genetic evaluation. This low accuracy is compounded by low heritability and the issue that phenotypic variation is irrelevant for choosing selection candidates, since natural selection already took out the negative variants. In situations like this, genomics can help (Leite et al., 2021), especially when stillborn and piglets that die before weaning are genotyped (Sharif-Islam et al., 2022). The genomic relationship matrix then transfers the survival knowledge to other selection candidates, thus increasing accuracy significantly.
Another approach to genetically increase survival is to understand the biology and/or physiology of survival. As an example, the maternal component of LAS in Table 1 is 0.08, indicating considerable genetic variation. This variation in keeping piglets alive can be related to, for example, the number of teats, colostrum, or behavior.
Piglet survival and litter size
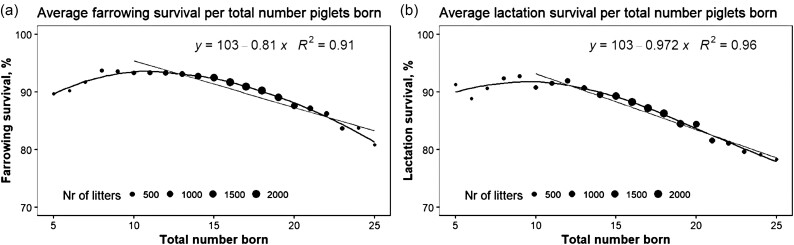
Nielsen et al. (2013) estimated phenotypic correlations between litter size and mortality as 0.14 and 0.09 in Landrace and Yorkshire lines, where the genetic correlations were 0.28 and 0.22. This makes sense since the phenotypic relation is not linear, but curvilinear (see Figure 1a and b); in the test dataset, the genetic correlation is even 0.70. There is little doubt that the relation between litter size and mortality is clearly positive.
Figure 1.
Phenotypic relation between total number of piglets born and farrowing survival (a, left) or lactation survival (b, right). The size of the points reflects the number (Nr) of litters included. Both relationships are curvilinear.
Piglet survival and birth weight
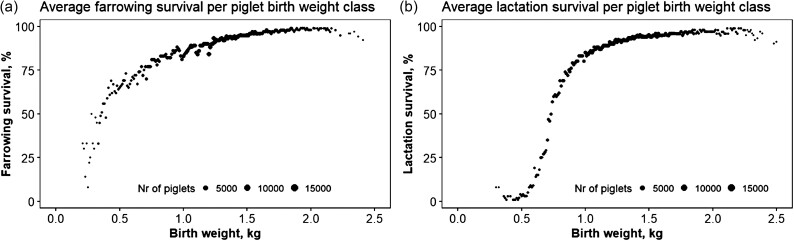
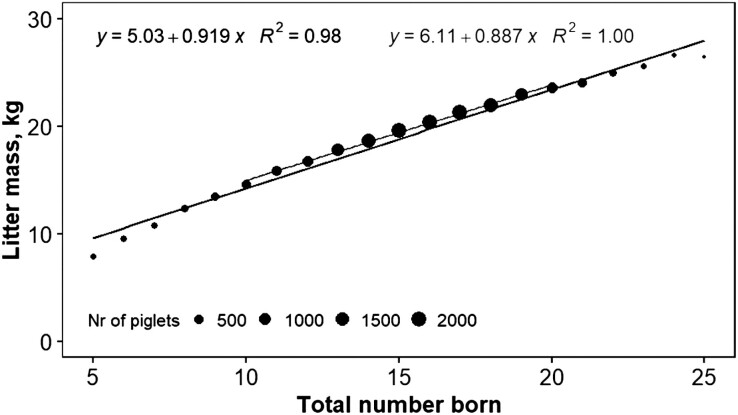
The relation between individual birth weight and piglet survival is non-linear (Figure 2a and b). Birthweight in pigs is mainly a maternal trait; an additive-maternal analysis of individual birth weight yields heritabilities of around 4% for the additive and around 18% for the maternal component (Roehe and Kalm, 2000). This estimate is based on individual weights of piglets. Litter weight, the sum of all piglets, has higher heritabilities, in the range of 0.3–0.4; this is a purely mathematical issue. In animal reproduction and animal breeding, the concept of the maternal trait “uterine capacity,” which is the concept of how many piglets a uterus can provide for, is an important determinant of the litter weight of piglets (Haley and Lee, 1993; Freking et al., 2016; Zak et al., 2017). In Figure 3, the relation between litter mass and litter size is shown. It shows a fairly linear relation between litter sizes with more than 250 observations per litter size with an increment of 0.9 kg per extra piglet. The uterus appears to stretch with increasing litter size. Continued selection for litter size will (infinitesimal) lead to an average birth weight of 0.9 kg in this specific population.
Figure 2.
Relationship between individual birth weight and piglet survival during farrowing (a, left), relationship between individual birth weight and lactation survival including a breakpoint at 50% lactation survival (b, right). The size of the points reflects the number (Nr) of piglets included. The relationship between individual birth weight and survival is curvilinear. Within this population, piglets with birth weights of ±900 g have >80% farrowing survival. Relatively few piglets fall within the category of <50% lactation survival using a breakpoint model set at 50%.
Figure 3.
Relation between litter mass and total number born, with a trendline covering all litter sizes and a trendline covering the most common groups of litter sizes from 10 to 20 piglets born. The size of the points reflects the number (Nr) of litters included. Relation between litter mass and total number born per litter is generally linear, especially for litter sizes from 10 to 20. With continued selection on litter size, the average extra piglet will weigh 900 g at birth in this population.
This relation, at the phenotypic level, strongly suggests that selection for increased birth weight will lead to improved survival. However, this might not necessarily be the case as there are clear differences between breeds; some of the Chinese breeds give birth to piglets with an average weight of around 1 kg and have an excellent survival (Lee and Haley, 1995). The formal within line correlation between survival and birthweight tends to be very low. Management and feeding, however, might increase birth weight with positive consequences.
Piglet survival and uniformity in birth weight
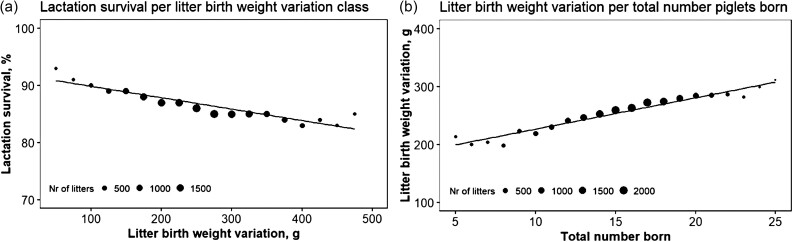
Litter weight is moderately heritable (Knol et al., 2002a; Kapell et al., 2011), and individual phenotypic birth weight is relevant for survival. With increasing litter size, average birth weight decreases, and within litter variation in birth weight increases (Wolf et al., 2008). This causes a litter to have large birth weight piglets at risk of still birth due to dystocia (Grandinson et al., 2002) and low birth weight piglets (Baxter et al., 2008), hence the search for more uniform litters. Uniformity in animal breeding is conceptually difficult, since selection against the lowest and against the highest values of the trait will ultimately bring fixation of the trait at its mean level. This is a good idea if the trait has an optimum; however, simultaneous directional selection and reduction of variation is complex. An alternative approach is to study genetic heterogeneity of residual variance (Mulder et al., 2008). In layman’s terms, phenotypes can be quite accurately predicted by knowledge of breed, parity, genetic make-up, etc. The inevitable residual is, interestingly, under genetic control. Some families stick to the planning; others are more subject to undescribed environmental influences. Since the relation between survival and individual birth weight is clearly non-linear, even suggestive of a breakpoint model (Figure 2b), it is very relevant that as few piglets fall below that breakpoint as possible. Within litter variation in birth weight (LVR, it is heritable with a heritability in the range of 0.04 and 0.10. As a trait, it has a negative phenotypic relation with LAS (Figure 4a), which fits the line of thought (rg = −0.28 ± 0.33; Merour et al., 2010). Selecting against standard deviation in piglet birth weight could, therefore, be a way to improve piglet survival during lactation (Canario et al., 2009). Selection for increased litter size will reduce birth weight and increase LVR (Figure 4b) (Quesnel et al., 2008). These are relevant, but complex relations.
Figure 4.
Relationship between lactation survival and litter birth weight variation (a, left) or total number born (b, right). The size of the points reflects the number (Nr) of litters included. Lactation survival decreases with increasing litter birth weight variation and litter birth weight variation increases with increasing litter sizes. Hence, larger litter sizes result in less uniform litters and lower survival during lactation.
Sell-Kubiak et al. (2015) studied LVR both as a trait of the sow and as a trait of the piglet in the heterogeneity model; they concluded that there was a high level of equivalence between the approaches.
Piglet survival and uniformity in litter size
Sell-Kubiak et al. (2022) extended the approach to uniformity in litter size. This approach is interesting, but not without potential pitfalls. Conceptually, it would be very helpful to farming practices if each farrowing had the same expected litter size. Selection against heterogeneous residuals will make the farrowing outcome more predictable. An important factor for litter size in pigs is parity; 1st parity litters are significantly smaller than second, and third parity litters are, on average, larger again than second parities. Estimating genetic parameters in a model with parity correction will adapt the population to stay close to the parity curve; a model without parity correction will treat parity as an unknown environmental factor and move the population in a direction without parity curve; this is interesting, but against normal biology.
Piglet survival and gestation length (GL)
Piglets should be born at term, and there is a clearly negative relation between gestation length and livability caused by piglets being less physiologically mature at birth (Leenhouwers et al., 1999). Piglets signal their dam that they want to be born; proof of that is the service sire effect on gestation length; and the breed/line and the individual sire within a breed/line are important for gestation length. Genetic variation in the reaction of the sow to this and other signals differs. Uniformity in birth weight is positively related to survival, not so much because of a reduction in small piglets, but mostly through a lack of the heaviest piglets (piglet signal comes later) and the correlated response in increased gestation length. Selection for larger litters, having smaller individual birth weight, might decrease gestation length.
Gestation length is a trait that has genetic variation and is highly heritable (h2 = 0.39) (Lopes et al., 2017), and is affected by the genes of the dam and the litter, as farrowing is caused by an interaction between the dam and its litter (Peltoniemi and Oliviero, 2015) being initiated by the fetuses (Jenkin and Young, 2004). A study by Rydhmer et al. (2008) estimated the genetic variation in gestation length at a nucleus herd attributed to the dam, “when to farrow” to be h2 = 0.2, whereas direct heritability, “when to be born,” was reported to be h2 = 0.3.
Gestation length is not only affected by maternal and additive components at the end of gestation (initiation of the farrowing process), but also by the reproductive traits around the time of insemination (Merks et al., 2000). The time from weaning to first service is a trait that is characterized and has been found to have low to moderate heritability (Hanenberg et al., 2001; Holm et al., 2005). Information on the genetic variation of timing of ovulation for example is not reported to the authors knowledge, although phenotypically it is known that the timing of ovulation is estimated to be during 2/3 way through estrus (Soede and Kemp, 1997). In practical terms, management practices to optimize fertilization within populations of sows having variation in duration of estrus are achieved through multiple breeding.
Supervision of sows during farrowing is one of the most important aspects of pig husbandry which improves survival of the litter and can allow for interventions during the farrowing process. Farrowing induction is used as a tool to increase the predictability in timing of farrowing, so supervision can be provided (Ward et al., 2020). As gestation length can vary between different genetic lines, induction protocols are best applied close to the mature physiological age of the piglets as it is possible. It has recently been reported that piglet quality in terms of birth weight and growth rates during lactation are affected by the gestation day in which farrowing was induced (McGuire et al., 2020).
Piglet survival and farrowing duration
The modern sow’s duration of active farrowing (litter and placentae expulsion) is reported to be 4 h (Van Dijk et al., 2005; Ju et al., 2021). However, there is significant variation in farrowing duration. It has recently been reported that 18% of sows have a farrowing duration of over 300 min (Ju et al., 2021). In terms of animal welfare for both of the sow and piglets, extensive periods of farrowing cause fatigue, affecting the sows ability to successfully complete farrowing and the piglets overall vitality and ability to suckle (Oliviero et al., 2019) and increases the incidence of stillborn (Ju et al., 2021). The farrowing process itself has been characterized (Van Dijk et al., 2005; Canario et al., 2009). It was found that farrowing duration increased with the presence of higher birth weight piglets (Canario et al., 2006a; Canario et al., 2014; Feyera et al., 2018). Independent of farrowing duration, large birth weight piglets are genetically (additive and maternal) correlated with increased incidence of stillbirth (Grandinson et al., 2002), suggesting that there are risks associated with selecting to increase piglet birth weight. Large litters also increase farrowing duration with each piglet increasing it by 12 min (Grandinson et al., 2002), which is similar to the estimates of Fahmy and Friend (Fahmy and Friend, 1981). Management strategies can be put in place to minimize the risks to mother and piglets, including optimizing sows housing environment (Oliviero et al., 2010), and ensuring the proper nutrition of a sow in preparation for farrowing to maintain blood glucose concentration (Feyera et al., 2018; Langendijk et al., 2018) reported that piglets born to sows having an extended duration of farrowing continued to exhibit effects of asphyxia into the nursery phase; those with asphyxia had lower growth rate during lactation and lower weaning weight compared to those having no signs of asphyxia. Sows which were identified as having protracted farrowing durations actually expressed this phenotype in the early farrowing period after the expulsion of the first few piglets, indicating that these at risk sows can be identified soon after the start of farrowing (Langendijk et al., 2018). Variation in the phenotype of this trait, farrowing duration, is present and is reported to have a low heritability (h2 = 0.08–0.10 [Canario et al., 2006b; Merour et al., 2010]). With the advent of camera sensors and computer vision (Oczak et al., 2022), the possibility does exist to quantify this trait and estimate genetic variation which will help in identifying families having extended farrowing duration.
Piglet survival and maternal behavior
Determining how to model the genetics of piglet survival is challenging (see Genetics of piglet survival section). The survival of a piglet can be analyzed at a litter level, i.e., survival within a litter (piglet vitality), which is also the ability of its mother to farrow piglets that survive until weaning. An alternative approach is to envision piglet survival as an interaction between three different genotypes: the sow to develop and farrow piglets (dam effect), its own genes (additive effect) and on the genes of a nurse sow (maternal and foster effects, which is the mothering ability) which may or may not be the same sow which gave birth to it. Coming from this approach, mothering ability is the potential of a sow to raise liveborn piglets entrusted to her until weaning.
There is a high correlation between the observed survival of piglets until weaning and the estimated breeding value for mothering ability (Knol et al., 2002c; Dunkelberger et al., 2019). The biological traits that are affected in relation to EBV mothering ability are lower glucose tolerance in late gestation (Knol et al., 2002c), which could be due to the decrease in insulin sensitivity in late gestation in preparation for lactogenesis (Pere et al., 2000). Sow behavior during parturition was found to not be related to EBV mothering ability (Uitdehaag et al., 2008). It is instead inversely related to the time from first standing to finding a teat and taking its first colostrum, and as sows spend more time lying down in a lateral position with teats available to the piglets (Knol et al., 2002b).
Mothering ability is likely a component of a wider scope of behaviors expressed by the dam which affect piglet survival, such as crushing of piglets under a sow (Hellbrügge et al., 2008a), and aggression in a group setting during gestation (h2 = 0.3) being genetically inversely correlated with piglet survival (Hellbrügge et al., 2008b).
Piglet survival and congenital defects (recessive variants)
Congenital defects are a welfare concern and affect the health and performance of the animals. The most common defects are cryptorchidism and scrotal/inguinal hernias, which have a low incidence occurring at a prevalence of <0.5%–1% (Thaller et al., 1996; Mattsson, 2011). Heritability estimates are reported to be 0.26 and 0.31 for cryptorchidism and hernias, respectively (Sevillano et al., 2015). Advances in knowledge of the genetic architecture of these traits are being achieved with the publishing of QTLs associated with these phenotypes (Sevillano et al., 2015; Grindflek et al., 2018). An interesting approach to identify animals having recessive variants was reported by Derks et al. (2021). They identified a significant candidate region in which haplotypes had a deficit in homozygosity on chromosome 9 in a population of Durocs. The most significant causal variant was a stop gained variant in the MYO7A gene associated with Usher syndrome in humans. The incidence of carrier frequency in the population was 8.2% and carrier by carrier matings had litters with significantly higher preweaning mortality. This genetic/genomic approach is highly interesting to identify recessive deficits within a population having significant effects on animal welfare and productivity, and within a breeding program to reduce the prevalence of these unwanted defects. All of this can be seen as a form of genetic hygiene—selection against different forms of defects.
Piglet survival and number of teats
Preweaning survival is heritable, and there is a clear genetic influence of the sow nursing the piglets. This influence can work through several mechanisms, including number of teats and quantity and quality of colostrum. Wiegert and Knauer (2018) reported that an increase of one functional teat in second parity sows improved piglet survival by 3.25% and also increased total litter weaning weight by 3.6 kg. The number of teats is quite heritable with interesting genetic variation. Heritability estimates most often vary between 0.2 and 0.5 (Borchers et al., 2002; Rohrer and Nonneman, 2017). Differences in heritability estimates between teats positioned posterior and anterior to the navel or on the left and right side have also been reported. Anterior to the naval or on the left side of the belly seemed to be more heritable although the latter was not statistically significant (McKay and Rahnefeld, 1990; Borchers et al., 2002; Rohrer and Nonneman, 2017). Genetic selection for number of teats will also increase the number of functional teats as they have a strong genetic correlation (Lundeheim et al., 2013; Balzani et al., 2016b). Several teat quality traits have a negative genetic correlation with piglet survival such as teat diameter, inter-teat distance, and udder development, whereas number of teats has a positive genetic correlation with piglet survival (Vasdal et al., 2011; Balzani et al., 2016b).
Since litter size continues to increase, either the number of teats has to increase or management measures have to be applied like dedicated nurse sows or artificial rearing. Selecting for increased number of teats is possible and has been applied to make sure each sow can raise her own piglets.
The genetic architecture of number of teats is slowly being unraveled and is fascinating. One QTL for number of teats seem to overlap with QTL for number of vertebrae; on SSC7 the VRTN gene has been found to be important in the variation of teat number and was also found to be associated with variations in the number of vertebrae (Ding et al., 2009; Mikawa et al., 2011; Duijvesteijn et al., 2014). The overlap in QTL for number of teats and number of vertebrae can be explained by the potential of every rib to develop an extra mammary gland (Veltmaat, 2017). Sevillano et al. (2022) found some interesting results looking at QTLs involved in the number of teats. An allelic substitution effect of nearly 0.4 teats in a functional mutation of the VRTN gene on SSC7 in two populations was found. Different QTL can have an effect on the number of teats in different lines; moreover, the same QTL can have opposite trend and allele frequencies in different lines. These results show that there is room for further improvement in the number of teats by adding marker-assisted selection to the breeding program on top of direct selection for the number of teats.
Piglet survival and colostrum
Colostrum intake is clearly relevant for piglet survival as it provides energy, immunity, and warmth (Le Dividich et al., 2005). If a sow is unable to produce enough colostrum, pre-weaning survival is decreased (Quesnel et al., 2012). Piglet survival increases when birth weight increases (Figure 2) but decreases when litter size increases (Figure 1). Also colostrum intake increases with increasing birth weight and decreases with increasing litter size (Le Dividich et al., 2005). The genetic variation of colostrum production by the sow is still unclear as it is the genetic correlation between the number of teats and colostrum production. Colostrum production is difficult to measure in sows; hence, researchers have tried to get around it by measuring, for example, weight gain in piglets over a certain time period. Another approach was performed by Balzani et al. (2016a), who measured colostrum immunoglobulin level. Colostrum immunoglobulin level had a reasonable heritability of 0.35 and a positive genetic correlation was found between colostrum immunoglobulin and the number and weight of piglets born alive (Balzani et al., 2016b).
Piglet survival and environment: G*E
Through domestication sows came to farrow and nurse in crates, mainly for the safety of the piglets. The current trend is towards free farrowing and in a number of countries organic and (even) outdoor farming is not reducing in popularity. The question is then if genetic selection will favor the same sows in these quite different environments. The non-crated systems suffer from increased mortality and genetic parameters have been estimated in these alternative systems.
Discussion
Natural selection adapts populations to their environment. Livestock production systems are such environments and in a crossbred production design, pure line populations will similarly adapt to these environments in several generations. Management, housing, feeding, and health, however, might change faster than the adaptation capacity of these populations. On top of this, selection on production efficiency, e.g., litter size, growth rate, and back fat will have effects on survival. Introduction of survival traits in the selection index is, therefore, a good idea. Genetic variation is large, or even very large, but not easy to exploit because of 1) the low heritability, 2) the complex genetic modeling since the genes of the piglet, the genes of the biological mother and the genes of the sow fostering the piglet play a role, and 3) the statistical issues of the binary character of survival, leading to the use of threshold models; the relatively recent availability of genomics is a positive development. Genetics is a world of big data, statistics, and structural genomics. Results of experiments and analyses are not always easy to grasp; it is a black box, an end of pipeline approach. In the end, an animal is alive or dead. The world of survival is full of biology and physiology, a description of the process leading to the final result: survival.
The black box approach
Selection for LP5 is straightforward; the genetic component of this model combines the maternal side of the contribution of the piglet with the input of the sow. Further development of selection survival includes 1) the separation of LP5 into its components: survival and litter size. Similar to the approach of Johnson et al. (1999), for litter size, it is analyzed as the combination of two traits: ovulation rate and pre-natal survival. This approach is theoretically superior to the single trait approach if the genetic parameters for the population are correct. 2) The change towards an additive-maternal model for survival, allowing for the contribution of the sire. 3) An equivalent approach could be a sire-dam model and a further improvement is the addition of the foster sow as a third genetic component. 4) Binary traits like survival cause statistical issues in analysis. A piglet might have the genes for “80% survival,” the expression of those genes is either “0 dead” or “100 alive.” The error term of this analysis is high. Binary traits with a high frequency can be analyzed as if the trait is normally distributed. Farrowing and lactation mortalities with frequencies between 10% and 20% fall into that category. 5) Classic pedigree worked with relations of 0.5 for full sibs and 0.25 for half sibs. Genotyping (e.g., 50k SNP chip) allows for more accurate estimation of the relation between animals, resulting in more accurate genomic prediction. Even if the cost of genotyping has come down dramatically, large scale genotyping is still costly; the tendency for breeding companies is therefore to genotype mainly selection candidates. In the case of survival, this is not an ideal situation, since the statistical system then never encounters the genetic make-up of weak piglets.
Biology
Whether a piglet lives or dies depends on its intra-uterine development, possibly even on the quality of ovulation prior to fertilization (Zak et al., 1997), on potential congenital defects, birth weight, intrinsic survival, signal to start gestation, etc. It also depends on the mothering ability of the piglet’s foster, which is a combination of, e.g., uterine capacity, gestation length, farrowing duration, number of teats, colostrum, and maternal behavior. Each of these traits can be further broken down into its underlying components, creating a list of dozens of traits as potential candidates for selection. Black box selection for survival is clearly based on the infinitesimal model, since it combines the outcome of a large number of traits. Most of these traits are polygenic, more or less normally distributed and have a moderate heritability. Properly modeled survival selection improves the underlying traits too, but in a suboptimal manner.
Balance
The question is then the balance between large scale simplicity and labor intensive recording of potentially dozens and dozens of traits. Farrowing duration is most probably under genetic control, is influenced by litter size, and reacts on management interventions, like feeding. Should farrowing duration be recorded in purebred or pedigreed crossbred populations or not. If unmonitored, selection for litter size might further increase farrowing duration. Will selection for survival manage to keep extreme farrowing durations under control or will management solve the issue now and in the future?
Breeding
There is clear additive genetic variation in individual piglets, known as intrinsic vitality. Table 2 is copied from Su et al. (2022). It gives a number of interesting insights. Traits are slightly irrelevant for the discussion as M5S is mortality from day five all the way to slaughter. Heritabilities are very low—1%–2%—but quite significant (3*SE), results from the different models are highly consistent; interpretation of the linear model might be slightly biased but is insightful. Estimated additive genetic SD for M5S is 4.4%, and this yields under mass selection 0.5% survival (square root of the heritability multiplied by the genetic SD, assuming selection intensity/generation interval = 1). This is mass phenotypic selection, which is actually natural selection, since dead animals are not selection candidates anymore. Adding pedigree and especially adding genomic information on live and dead animals will greatly improve accuracy of selection and, therefore, directly affect possible genetic trends. Added to this is a somewhat similar amount of genetic variance from the maternal side, which is clearly larger in the case of farrowing and lactation survival. The genetic correlation between maternal and additive effect is very low and will hardly influence possible genetic trends.
Table 2.
Phenotypic variance (σp2), proportion of litter variance (lit2), direct (had2) and maternal (ham2) heritability, correlation between direct and maternal genetic variance (radam), as well as their standard error (±) for mortality from day 5 after birth to slaughter Su et al. (2022)
| Model | σp2 | lit2 | h ad 2 | h am 2 | r adam |
|---|---|---|---|---|---|
| Linear | 0.134 | 0.051 ± 0.0021 | 0.015 ± 0.0026 | 0.005 ± 0.0017 | −0.119 ± 0.1747 |
| Logit | 3.648 | 0.071 ± 0.0038 | 0.017 ± 0.0033 | 0.010 ± 0.0031 | 0.006 ± 0.1884 |
| Probit | 1.107 | 0.069 ± 0.0039 | 0.016 ± 0.0030 | 0.010 ± 0.0031 | 0.032 ± 0.1879 |
Bridging
The text in this discussion states the obvious for geneticists. Breeds and pure line populations harbor considerable genetic variation for survival. The end of pipeline and/or black box approach will definitely work with large numbers of animals and appropriate genotyping, that is, including dead animals. However, the effect of the environment accounts for over 80% of the variation seen for piglet survival, highlighting the importance of research in fields of nutrition, housing, and management to improve piglet survival. Two, similar, research models can help us to bridge the gap between genetics and management/nutrition: 1) genetic high/low sampling for survival, and 2) for the large integrators: top off the highest genetic ranked animals and have them produce in a smaller size production environment. Optimized protocols can then work in the years to come for the majority of the production farms. The high/low approach can show the most limiting traits. If animals clearly differ in gestation length, then predicted farrowing date in sow management systems might be changed. Differences in litter weight might lead to gestation feeding changes. In general, as genetics change so the environment will need to be adapted.
Being pragmatic
Selection programs in livestock species have become very efficient; genetic trends change populations quite fast. Litter sizes have gone up considerably, excess backfat has disappeared, and growth rate is increasing every year. From the traits mentioned in this review, the number of teats appears relevant for day-to-day production since barn labor is more and more difficult to find. Artificial rearing of piglets, or large-scale cross-fostering, is becoming an issue. To have the availability of enough functional teats in the lactation barn reduces the pressure on human labor.
Adequate development of each and every piglet is important for finisher growth rate, but also for survival and to reduce the risk of becoming a victim of damaging behavior. An increase in litter weight and uterine capacity is a must to keep average birth weight constant and to reduce variation in birth weight. At the same time, this increase in uterine capacity will push to increase mature body weight resulting in higher maintenance level and increasing the feed cost per sow. A relatively new trait that needs attention is farrowing duration as the increase in litter size puts pressure on the farrowing process at the cost of more stillborn and somewhat asphyxiated piglets. Gestation length is quite heritable and related to maturity of the piglet at birth. Larger or heavier piglets might signal, in utero, the sow that farrowing should start. Genetic selection for more and heavier sows can, therefore, create a phenotypic effect on gestation length and hence on survival.
Conclusions
Survival traits show considerable genetic variation. Heritability is low, since environmental effects overshadow the genetic make-up. Large scale phenotyping, appropriate statistical modeling, and targeted genotyping can largely overcome this limitation. Understanding the underlying traits is improving and can assist genetic change, where prediction of performance of the next generations can assist management and nutrition protocols.
Glossary
Abbreviations
- EBV
estimated breeding value
- FAS
farrowing survival
- LAS
lactation survival
- LP5
number of piglets alive at day 5 after birth
- LVR
litter birth weight variation
- M5S
mortality from day 5 to slaughter
Contributor Information
Egbert F Knol, Topigs Norsvin Research Center, Beuningen, GE, 6641 SZ, The Netherlands.
Dianne van der Spek, Topigs Norsvin Research Center, Beuningen, GE, 6641 SZ, The Netherlands.
Louisa J Zak, Topigs Norsvin Research Center, Beuningen, GE, 6641 SZ, The Netherlands.
Conflict of Interest Statement
All three authors work for the pig breeding company Topigs Norsvin in the Research Center unit. There is no conflict of interest.
Literature Cited
- Balzani, A., Cordell H., and Edwards S.. . 2016a. Evaluation of an on-farm method to assess colostrum IgG content in sows. Animal 10:643–648. doi: 10.1017/s1751731115002451. [DOI] [PubMed] [Google Scholar]
- Balzani, A., Cordell H., Sutcliffe E., and Edwards S.. . 2016b. Heritability of udder morphology and colostrum quality traits in swine. J. Anim. Sci. 94:3636–3644. [DOI] [PubMed] [Google Scholar]
- Baxter, E., Jarvis S., D’eath R., Ross D., Robson S., Farish M., Nevison I., Lawrence A., and Edwards S.. . 2008. Investigating the behavioural and physiological indicators of neonatal survival in pigs. Theriogenology 69:773–783. doi: 10.1016/j.theriogenology.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Borchers, N., Reinsch N., and Kalm E.. . 2002. Teat number, hairiness and set of ears in a Pietrain cross: variation and effects on performance traits. Arch. Anim. Breed. 45:465–480. doi: 10.5194/aab-45-465-2002. [DOI] [Google Scholar]
- Canario, L., Bidanel J. -P., and Rydhmer L.. . 2014. Genetic trends in maternal and neonatal behaviors and their association with perinatal survival in French Large White swine. Front. Genet. 5:410. doi: 10.3389/fgene.2014.00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canario, L., Billon Y., Caritez J. -C., Bidanel J. P., and Laloë D.. . 2009. Comparison of sow farrowing characteristics between a Chinese breed and three French breeds. Livest. Sci. 125:132–140. [Google Scholar]
- Canario, L., Cantoni E., Le Bihan E., Caritez J., Billon Y., Bidanel J. P., and Foulley J. L.. . 2006a. Between-breed variability of stillbirth and its relationship with sow and piglet characteristics. J. Anim. Sci. 84:3185–3196. [DOI] [PubMed] [Google Scholar]
- Canario, L., Roy N., Gruand J., and Bidanel J. P.. . 2006b. Genetic variation of farrowing kinetics traits and their relationships with litter size and perinatal mortality in French Large White sows. J. Anim. Sci. 84:1053–1058. [DOI] [PubMed] [Google Scholar]
- Derks, M., Megens H. J., Giacomini W., Groenen M., and Lopes M.. . 2021. A natural knockout of the MYO7A gene leads to pre-weaning mortality in pigs. Anim. Genet. 52:514–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, N., Guo Y., Knorr C., Ma J., Mao H., Lan L., Xiao S., Ai H., Haley C. S., and Brenig B.. . 2009. Genome-wide QTL mapping for three traits related to teat number in a White Duroc× Erhualian pig resource population. BMC Genet. 10:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duijvesteijn, N., Veltmaat J. M., Knol E. F., and Harlizius B.. . 2014. High-resolution association mapping of number of teats in pigs reveals regions controlling vertebral development. BMC Genomics 15:542. doi: 10.1186/1471-2164-15-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkelberger, J. R., Vogelzang R., van der Peet-Schwering C., Soede N., and Knol E. F.. . 2019. Genetic selection for better mothers improves piglet survival in both conventional and group-farrowing systems. Poster session presented at Pig Welfare Symposium, Minneapolis, MN.
- Fahmy, M., and Friend D.. . 1981. Factors influencing, and repeatability of the duration of farrowing in Yorkshire sows. Can. J. Anim. Sci. 61:17–22. [Google Scholar]
- Feyera, T., Pedersen T. F., Krogh U., Foldager L., and Theil P. K.. . 2018. Impact of sow energy status during farrowing on farrowing kinetics, frequency of stillborn piglets, and farrowing assistance. J. Anim. Sci. 96:2320–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freking, B., Lents C., and Vallet J.. . 2016. Selection for uterine capacity improves lifetime productivity of sows. Anim. Reprod. Sci. 167:16–21. [DOI] [PubMed] [Google Scholar]
- Grandinson, K., Lund M. S., Rydhmer L., and Strandberg E.. . 2002. Genetic parameters for the piglet mortality traits crushing, stillbirth and total mortality, and their relation to birth weight. Acta Agriculturae Scandinavica, Section A, Animal Sci. 52:167–173. [Google Scholar]
- Grindflek, E., Hansen M. H., Lien S., and van Son M.. . 2018. Genome-wide association study reveals a QTL and strong candidate genes for umbilical hernia in pigs on SSC14. BMC Genomics 19:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, X., Sørensen A. C., Henryon M., and Ostersen T.. . 2022. Piglet Survival is lowly heritable in DanBred Landrace pigs. World Congress on Genetics Applied to Livestock Production, Rotterdam accepted for publication. [Google Scholar]
- Haley, C., and Lee G.. . 1993. Genetic basis of prolificacy in Meishan pigs. J. Reprod. Fertil. Suppl. 247–247. [PubMed] [Google Scholar]
- Hanenberg, E., Knol E., and Merks J.. . 2001. Estimates of genetic parameters for reproduction traits at different parities in Dutch Landrace pigs. Livest. Prod. Sci. 69:179–186. [Google Scholar]
- Hellbrügge, B., Tölle K. -H., Bennewitz J., Henze C., Presuhn U., and Krieter J.. . 2008a. Genetic aspects regarding piglet losses and the maternal behaviour of sows. Part 1. Genetic analysis of piglet mortality and fertility traits in pigs. Animal 2:1273–1280. doi: 10.1017/S1751731108002504. [DOI] [PubMed] [Google Scholar]
- Hellbrügge, B., Tölle K. -H., Bennewitz J., Henze C., Presuhn U., and Krieter J.. . 2008b. Genetic aspects regarding piglet losses and the maternal behaviour of sows. Part 2. Genetic relationship between maternal behaviour in sows and piglet mortality. Animal 2:1281–1288. doi: 10.1017/S1751731108002516. [DOI] [PubMed] [Google Scholar]
- Henryon, M., Ostersen T., Guo X., Su G., and Sørensen A. C.. . 2022. Breeding for component traits of litter size at day 5 increases piglet survival while maintaining litter size at day 5. World Congress on Genetics Applied to Livestock Production, Rotterdam accepted for publication. [Google Scholar]
- Holm, B., Bakken M., Vangen O., and Rekaya R.. . 2005. Genetic analysis of age at first service, return rate, litter size, and weaning-to-first service interval of gilts and sows. J. Anim. Sci. 83:41–48. doi: 10.2527/2005.83141x. [DOI] [PubMed] [Google Scholar]
- Jenkin, G., and Young I. R.. . 2004. Mechanisms responsible for parturition; the use of experimental models. Anim. Reprod. Sci. 82:567–581. [DOI] [PubMed] [Google Scholar]
- Johnson, R. K., Nielsen M. K., and Casey D. S.. . 1999. Responses in ovulation rate, embryonal survival, and litter traits in swine to 14 generations of selection to increase litter size. J. Anim. Sci. 77:541–557. [DOI] [PubMed] [Google Scholar]
- Ju, M., Wang X., Li X., Zhang M., Shi L., Hu P., Zhang B., Han X., Wang K., and Li X.. . 2021. Effects of litter size and parity on farrowing duration of Landrace× Yorkshire sows. Animals 12:94. doi: 10.3390/ani12010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapell, D. N., Ashworth C. J., Knap P. W., and Roehe R.. . 2011. Genetic parameters for piglet survival, litter size and birth weight or its variation within litter in sire and dam lines using Bayesian analysis. Livest. Sci. 135:215–224. [Google Scholar]
- Knol, E., Ducro B., Van Arendonk J., and Van der Lende T.. . 2002a. Direct, maternal and nurse sow genetic effects on farrowing-, pre-weaning-and total piglet survival. Livest. Prod. Sci. 73:153–164. [Google Scholar]
- Knol, E., Ducro B., Van Arendonk J., and Van der Lende T.. . 2002b. Direct, maternal and nurse sow genetic effects on farrowing-, pre-weaning-and total piglet survival. Livest. Prod. Sci. 73:153–164. [Google Scholar]
- Knol, E., Verheijen C., Leenhouwers J., and Van der Lende T.. . 2002c. Genetic and biological aspects of mothering ability in sows. In: Proc. 7th World Congress on Genetics Applied to Livestock Production., Montpellier, France. p. 35–38.
- Langendijk, P., Fleuren M., Van Hees H., and Van Kempen T.. . 2018. The course of parturition affects piglet condition at birth and survival and growth through the nursery phase. Animals 8:60. doi: 10.3390/ani8050060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Dividich, J., Rooke J., and Herpin P.. . 2005. Nutritional and immunological importance of colostrum for the new-born pig. J. Agric. Sci. 143:469–485. [Google Scholar]
- Lee, G., and Haley C.. . 1995. Comparative farrowing to weaning performance in Meishan and Large White pigs and their crosses. Anim. Sci. 60:269–280. [Google Scholar]
- Leenhouwers, J. I., Lende T. V. D., and Knol E. F.. . 1999. Analysis of stillbirth in different lines of pig. Livest. Prod. Sci. 57:243–253. [Google Scholar]
- Leite, N. G., Knol E. F., Garcia A. L. S., Lopes M. S., Zak L., Tsuruta S., Silva F. F. E., and Lourenco D.. . 2021. Investigating pig survival in different production phases using genomic models. J. Anim. Sci. 99:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes, M. S., Bovenhuis H., Hidalgo A. M., Van Arendonk J. A., Knol E. F., and Bastiaansen J. W.. . 2017. Genomic selection for crossbred performance accounting for breed-specific effects. Genet. Sel. Evol. 49:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundeheim, N., Chalkias H., and Rydhmer L.. . 2013. Genetic analysis of teat number and litter traits in pigs. Acta Agric. Scand. Sect A 63:121–125. [Google Scholar]
- Mattsson, P. 2011. Prevalence of congenital defects in Swedish Hampshire, Landrace and Yorkshire pig breeds and opinions on their prevalence in Swedish commercial herds (MSc Thesis). Uppsala: Department of Animal Breeding and Genetics, Swedish University of Agricultural Sciences.http://urn.kb.se/resolve?urn=urn:nbn:se:slu:epsilon-6-474. [Google Scholar]
- McGuire, M. R., Dunkelberger J., Eggert J., Schinckel A. P., and Knol E.. . 2020. PSIII-3 Effect of day of induction on sow and piglet performance. J. Anim. Sci. 98(Supplement 3):232–232. doi: 10.1093/jas/skaa054.406. [DOI] [Google Scholar]
- McKay, R., and Rahnefeld G.. . 1990. Heritability of teat number in swine. Can. J. Anim. Sci. 70:425–430. [Google Scholar]
- Merks, J., Ducro-Steverink D., and Feitsma H.. . 2000. Management and genetic factors affecting fertility in sows. Reprod. Domest. Anim. 35:261–266. [Google Scholar]
- Merour, I., Bernard E., Bidanel J. P., and Canario L.. . 2010. Genetic parameters for litter traits including farrowing duration and piglet survival up to weaning in the French Large White and Landrace sows. In: 61st Annual Meeting of the European Association for Animal Production (EAAP). P. 481.
- Mikawa, S., Sato S., Nii M., Morozumi T., Yoshioka G., Imaeda N., Yamaguchi T., Hayashi T., and Awata T.. . 2011. Identification of a second gene associated with variation in vertebral number in domestic pigs. BMC Genet. 12:51–13. doi: 10.1186/1471-2156-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder, H. A., Bijma P., and Hill W. G.. . 2008. Selection for uniformity in livestock by exploiting genetic heterogeneity of residual variance. Genet. Sel. Evol. 40:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, B., Su G., Lund M. S., and Madsen P.. . 2013. Selection for increased number of piglets at d 5 after farrowing has increased litter size and reduced piglet mortality. J. Anim. Sci. 91:2575–2582. [DOI] [PubMed] [Google Scholar]
- Oczak, M., Bayer F., Vetter S., Maschat K., and Baumgartner J.. . 2022. Comparison of the automated monitoring of the sow activity in farrowing pens using video and accelerometer data. Comput. Electron. Agric. 192:106517. doi: 10.1016/j.compag.2021.106517. [DOI] [Google Scholar]
- Oliviero, C., Heinonen M., Valros A., and Peltoniemi O.. . 2010. Environmental and sow-related factors affecting the duration of farrowing. Anim. Reprod. Sci. 119:85–91. doi: 10.1016/j.anireprosci.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Oliviero, C., Junnikkala S., and Peltoniemi O.. . 2019. The challenge of large litters on the immune system of the sow and the piglets. Reprod. Domest. Anim. 54:12–21. doi: 10.1111/rda.13463. [DOI] [PubMed] [Google Scholar]
- Peltoniemi, O. A. T, and Oliviero C.. . 2015. Housing, management and environment during farrowing and early lactation. In: Farmer, C., editor. The gestating and lactating sow. Wageningen: Academic Publishers. p. 231–252. [Google Scholar]
- Pere, M. -C., Etienne M., and Dourmad J. -Y.. . 2000. Adaptations of glucose metabolism in multiparous sows: effects of pregnancy and feeding level. J. Anim. Sci. 78:2933–2941. [DOI] [PubMed] [Google Scholar]
- Quesnel, H., Brossard L., Valancogne A., and Quiniou N.. . 2008. Influence of some sow characteristics on within-litter variation of piglet birth weight. Animal 2:1842–1849. doi: 10.1017/S175173110800308X. [DOI] [PubMed] [Google Scholar]
- Quesnel, H., Farmer C., and Devillers N.. . 2012. Colostrum intake: influence on piglet performance and factors of variation. Livest. Sci. 146:105–114. doi: 10.1016/j.livsci.2012.03.010. [DOI] [Google Scholar]
- R Core Team. 2021. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. [Google Scholar]
- Roehe, R., and Kalm E.. . 2000. Estimation of genetic and environmental risk factors associated with pre-weaning mortality in piglets using generalized linear mixed models. Anim. Sci. 70:227–240. doi: 10.1017/s1357729800054692. [DOI] [Google Scholar]
- Rohrer, G. A., and Nonneman D. J.. . 2017. Genetic analysis of teat number in pigs reveals some developmental pathways independent of vertebra number and several loci which only affect a specific side. Genet. Sel. Evol. 49:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydhmer, L., Lundeheim N., and Canario L.. . 2008. Genetic correlations between gestation length, piglet survival and early growth. Livest. Sci. 115:287–293. doi: 10.1016/j.livsci.2007.08.014. [DOI] [Google Scholar]
- Sell-Kubiak, E., Bijma P., Knol E., and Mulder H.. . 2015. Comparison of methods to study uniformity of traits: application to birth weight in pigs. J. Anim. Sci. 93:900–911. [DOI] [PubMed] [Google Scholar]
- Sell-Kubiak, E., Knol E. F., and Lopes M.. . 2022. Evaluation of the phenotypic and genomic background of variability based on litter size of Large White pigs. Genet. Sel. Evol. 54:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevillano, C. A., Harlizius B., Derks M. F. L., Lopes M. S., Son M. V., and Knol E. F.. . 2022. Allele frequency differences at epistatic QTL explain different genetic trends in number of teats in two pig lines. World Congress on Genetics Applied to Livestock Production, accepted for publication. [Google Scholar]
- Sevillano, C. A., Lopes M. S., Harlizius B., Hanenberg E. H., Knol E. F., and Bastiaansen J. W.. . 2015. Genome-wide association study using deregressed breeding values for cryptorchidism and scrotal/inguinal hernia in two pig lines. Genet. Sel. Evol. 47:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif-Islam, M., Werf J. H. J. V. D., Henryon M., Chu T. T., Wood B. J., and Hermesch S.. . 2022. Genotyping dead animals improves post-weaning survival of pigs in breeding programs. World Congress on Genetics Applied to Livestock Production accepted for publication. [Google Scholar]
- Soede, N., and Kemp B.. . 1997. Expression of oestrus and timing of ovulation in pigs. J. Reprod. Fertil. Suppl. 52:91–103. [PubMed] [Google Scholar]
- Su, G., Liu T., Christensen O. F., Lund M. S., and Nielsen B.. . 2022. Feasibility of reducing mortality of pigs from birth to slaughter by genetic selection World Congress on Genetics Applied to Livestock Production, accepted for publication. [Google Scholar]
- Thaller, G., Dempfle L., and Hoeschele I.. . 1996. Investigation of the inheritance of birth defects in swine by complex segregation analysis. J. Anim. Breed. Genet. 113:77–92. doi: 10.1111/j.1439-0388.1996.tb00593.x. [DOI] [Google Scholar]
- Uitdehaag, K. A., Ekkel D., Kanis E., and Knol E.. . 2008. Sow behaviour during parturition in relation to the observed and the genetic merit for weaning survival. Appl. Anim. Behav. Sci. 114:86–92. [Google Scholar]
- Van Dijk, A., Van Rens B., Van der Lende T., and Taverne M.. . 2005. Factors affecting duration of the expulsive stage of parturition and piglet birth intervals in sows with uncomplicated, spontaneous farrowings. Theriogenology 64:1573–1590. [DOI] [PubMed] [Google Scholar]
- Vasdal, G., Østensen I., Melišová M., Bozděchová B., Illmann G., and Andersen I. L.. . 2011. Management routines at the time of farrowing—effects on teat success and postnatal piglet mortality from loose housed sows. Livest. Sci. 136:225–231. [Google Scholar]
- Veltmaat, J. M. 2017. Prenatal mammary gland development in the mouse: research models and techniques for its study from past to present. Methods Mol. Biol. 1501:21–76. [DOI] [PubMed] [Google Scholar]
- Ward, S. A., Kirkwood R. N., and Plush K. J.. . 2020. Are larger litters a concern for piglet survival or an effectively manageable trait? Animals 10:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegert, J. G. and Knauer M. T.. . 2018. 98 Sow functional teat number impacts colostrum intake and piglet throughput. J. Anim. Sci. 96(suppl_2):51–52. doi: 10.1093/jas/sky073.096. [DOI] [Google Scholar]
- Wolf, J., Žáková E., and Groeneveld E.. . 2008. Within-litter variation of birth weight in hyperprolific Czech Large White sows and its relation to litter size traits, stillborn piglets and losses until weaning. Livest. Sci. 115:195–205. doi: 10.1016/j.livsci.2007.07.009. [DOI] [Google Scholar]
- Zak, L., Cosgrove J., Aherne F., and Foxcroft G.. . 1997. Pattern of feed intake and associated metabolic and endocrine changes differentially affect postweaning fertility in primiparous lactating sows. J. Anim. Sci. 75:208–216. [DOI] [PubMed] [Google Scholar]
- Zak, L. J., Gaustad A. H., Bolarin A., Broekhuijse M. L., Walling G. A., and Knol E. F.. . 2017. Genetic control of complex traits, with a focus on reproduction in pigs. Mol. Reprod. Dev. 84:1004–1011. [DOI] [PubMed] [Google Scholar]