Abstract
Both molybdate and iron are metals that are required by the obligately aerobic organism Azotobacter vinelandii to survive in the nutrient-limited conditions of its natural soil environment. Previous studies have shown that a high concentration of molybdate (1 mM) affects the formation of A. vinelandii siderophores such that the tricatecholate protochelin is formed to the exclusion of the other catecholate siderophores, azotochelin and aminochelin. It has been shown previously that molybdate combines readily with catecholates and interferes with siderophore function. In this study, we found that the manner in which each catecholate siderophore interacted with molybdate was consistent with the structure and binding potential of the siderophore. The affinity that each siderophore had for molybdate was high enough that stable molybdo-siderophore complexes were formed but low enough that the complexes were readily destabilized by Fe3+. Thus, competition between Fe3+ and molybdate did not appear to be the primary cause of protochelin accumulation; in addition, we determined that protochelin accumulated in the presence of vanadate, tungstate, Zn2+, and Mn2+. We found that all five of these metal ions partially inhibited uptake of 55Fe-protochelin and 55Fe-azotochelin complexes. Also, each of these metal ions partially inhibited the activity of ferric reductase, an enzyme important in the deferration of ferric siderophores. Our results suggest that protochelin accumulates in the presence of molybdate because protochelin uptake and conversion into its component parts, azotochelin and aminochelin, are inhibited by interference with ferric reductase.
Azotobacter vinelandii is a gram-negative obligately aerobic bacterium that is commonly found in soil and aquatic environments. A novel feature of Azotobacter spp. is the ability of these organisms to fix nitrogen nonsymbiotically under aerobic conditions. A. vinelandii forms three nitrogenases that are differentiated on the basis of the metal cofactor (2), and nitrogenase activity is dependent on the acquisition of metals for cofactor synthesis. Both iron and molybdenum are found in the dominant nitrogenase, nitrogenase I; iron and vanadate are found in nitrogenase II; and only iron is present in nitrogenase III. Iron and molybdenum are similar chemically; both are large transition metals which can exist in a number of oxidation states, both are Lewis acids, and both can form six coordinate bonds at physiological pH values (33). In aqueous systems at a neutral pH molybdenum readily interacts with water and forms the highly soluble molybdate (MoO42−) ion. Under these conditions the molybdenum atom has an effective charge of +3.6 and, as a result, interacts with ligands like Fe3+ interacts with ligands (16). Iron, on the other hand, interacts with water to form ferric hydroxides and ferric oxyhydroxides that are very insoluble [Ksp of Fe(OH)3, 2 × 10−39], so the concentration of free iron(III) is on the order of 10−17 M, which is far too low to support bacterial growth (14).
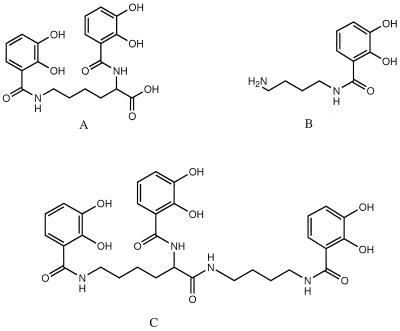
High-affinity uptake systems for both iron and molybdate have been found in Escherichia coli and A. vinelandii (13, 26). High-affinity iron uptake is mediated by small organic molecules called siderophores which have high affinity for iron(III) but lower affinity for all other metal ions (14). A. vinelandii produces the catecholate siderophores azotochelin (5), aminochelin (31), and protochelin (6) (Fig. 1) and the pyoverdin-like siderophore azotobactin (29). In addition to iron, catecholate siderophores bind molybdate to form complexes at a neutral pH. With both iron and molybdenum, this complex formation is the result of the presence of oxygen atoms within the 2,3-dihydroxybenzoic acid (2,3-DHBA) moieties of the catechol molecule, which are very electron dense. These groups displace the less electron-dense oxygen atoms of water and coordinate with either iron or molybdenum to form stable complexes, although the catecholate complexes formed with molybdenum are less stable than the catecholate complexes formed with iron (16). In the presence of a low molybdenum concentration, catecholate-like molybdate-coordinating compounds may be formed by a number of microorganisms. However, it is not clear whether these compounds are dedicated, true mediators of high-affinity molybdate uptake or are simply misidentified members of a high-affinity iron uptake system (20, 32, 35).
FIG. 1.
Catecholate siderophores of A. vinelandii. (A) Azotochelin. (B) Aminochelin. (C) Protochelin.
We have focused on the production of protochelin by A. vinelandii (6, 7). This tricatecholate siderophore has a high affinity for Fe3+ but is normally less abundant in iron-limited culture fluids than azotochelin and aminochelin. Protochelin is composed of one azotochelin molecule and one aminochelin molecule (Fig. 1), and it may be either the product of condensation or the progenitor of the other two catecholates. When A. vinelandii is grown in a medium containing a high concentration of molybdate (1 mM), protochelin is hyperproduced and is the only catecholate produced (6). At this high concentration molybdate interferes with the function of protochelin by competing with iron for binding to the siderophore (6). It has been proposed by Duhme et al. (10) that overproduction of protochelin is due to molybdate binding to azotochelin, which depletes the concentration of this siderophore available for iron transport and leads to hyperproduction of protochelin. These authors also speculated that formation of protochelin compensates for the use of azotochelin in high-affinity molybdate transport. The data presented here suggest that this probably does not occur and that protochelin accumulates in the presence of molybdate as a result of inhibition of ferric siderophore uptake, in which a ferric reductase is the most likely target for inhibition by molybdate and other divalent metal ions that increase protochelin accumulation.
MATERIALS AND METHODS
Strains and growth conditions.
A. vinelandii capsule-negative strain UW (= OP = ATCC 13705) was used in this study. The mutants derived from strain UW included azotobactin-deficient strain UA1 (30), ferredoxin I (fdxA)-negative strain LM100 (25), and strain RP40, which is defective in both high- and low-affinity molybdate uptake (26). Cultures were grown in Burk's medium, which contained 1% (wt/vol) glucose, 15 mM ammonium acetate, 1 μM Na2MoO4 · 2H2O, 0.81 mM MgSO4, and 0.58 mM CaSO4 in 6 mM potassium phosphate buffer (pH 7.6) (37). The iron-sufficient medium used for culture maintenance contained 50 μM ferric citrate, while the iron-limited medium contained 1 μM ferric citrate. The effects of metals other than molybdate on accumulation of protochelin were examined by growing strain UW in iron-limited Burk's medium (20-ml portions in 50-ml Erlenmeyer flasks) at 28°C and 225 rpm. The following divalent metals were used at concentrations ranging from 10 to 1,000 mM: NiCl2 · 6H2O, CoCl2 · 6H2O, MgSO4 · 7H2O, NaVO3 · 2H2O, CaCl2 · 2H2O, ZnSO4 · 7H2O, Na2WO3, MnCl2 · 4H2O, and SrCl2 · 6H2O. All glassware was acid washed with 4 M HCl and then rinsed with 50 mM EDTA (pH 7.0) and Milli-Q deionized water (Millipore) to remove contaminating iron (27).
Spectrophotometric and colorimetric analyses.
Pure preparations of azotochelin, aminochelin, and protochelin were obtained from iron-limited strain LM100 culture supernatant as previously described (7). Siderophores were detected spectrophotometrically by scanning iron-limited culture supernatant fluid which had been acidified to pH 1.8 with HCl. Absorption peaks were measured with a Hitachi model U-2000 recording spectrophotometer at A310 for catechols and at A380 for azotobactin (30). Catecholate siderophore concentrations in stock solutions were quantified by using a molar absorptivity for 2,3-DHBA of 3.26 × 103 A310 M−1 cm−1, corrected for the number of 2,3-DHBA moieties per siderophore (7). Catechol was also quantified by using the colorimetric assay of Barnum (1). The identities of catecholate siderophores were determined by performing thin-layer chromatography (TLC) as described by Cornish and Page (6) and comparing data with data for authentic standards. Total cellular protein contents were determined by the method of Lowry et al. (24).
Molybdo-siderophore molar binding and affinity determination.
The molar binding ratios of each catecholate siderophore and molybdate were determined by the continuous-variation method of Job (4). Each siderophore was mixed with MoO42− (as sodium molybdate) at various ratios in 100 mM MOPS (morpholinepropanesulfonic acid) (pH 7.0) buffer; protochelin was also examined in 100 mM MES (morpholineethanesulfonic acid) (pH 6.0) buffer. The absorbance of each of the mixtures was measured. As the absorbance spectra of all of the molybdo-siderophore complexes exhibited a broad peak at wavelengths from 300 to 500 nm, all measurements were taken at 375 nm. The solution with the highest absorbance at equilibrium was used to represent the correct molar binding ratio of molybdate and the siderophore.
The affinity of each siderophore for molybdate binding was determined by using a modification of the method used by Cornish and Page to determine siderophore-iron affinity (7). To determine the conditional formation constant of a molybdo-siderophore complex, competition between the formation of a ferric siderophore complex and the formation of a molybdo-siderophore complex was analyzed. In this analysis, a ferric siderophore complex (final concentration, 0.1 mM) was allowed to form for 72 h before 0.05 to 2 mM molybdate was added. Each reaction mixture was incubated for 96 h at room temperature in the dark under an nitrogen atmosphere to maintain anaerobic conditions before the equilibrium concentration of the ferric siderophore complex was determined spectrophotometrically (7). Conversely, a molybdo-siderophore complex (final concentration, 0.1 mM) was allowed to form for 72 h before 0.05 to 2 mM ferric nitrate was added. Each reaction mixture was allowed to reach equilibrium (96 h), under the anaerobic conditions described above, before the concentration of the ferric siderophore complex was determined (7). A conditional formation constant for a molybdo-siderophore complex was then calculated as described for the calculation of a ferric siderophore complex (34). All reactions were carried out in 96-well microtiter plates, and absorbance was measured with a Biotek Instruments model EL311 microplate reader; a minimum of two duplicate reaction series, each containing six replicates of each reaction mixture, were used (7).
Competition between molybdate and iron(III) for siderophore binding.
To determine if the presence of molybdate affected the formation of a ferric siderophore complex, ferric nitrate, sodium molybdate, and a purified siderophore were combined, and formation of the metal-siderophore complexes was monitored at A490 for ferric protochelin and ferric aminochelin complexes and at A570 for ferric azotochelin complexes (7). Protochelin was mixed 1:1 with iron and 1:1 with molybdate, and the mixture contained 0.20 μmol of siderophore, 0.20 μmol of molybdate, and 0.20 μmol of iron(III). Alternatively, protochelin was mixed 1:1 with iron and 2:3 with molybdate, and the resulting preparation contained 0.16 μmol of siderophore, 0.24 μmol of molybdate, and 0.16 μmol of iron(III). Azotochelin was mixed 3:2 with iron and 1:1 with molybdate, and the resulting mixture contained 0.20 μmol of siderophore, 0.20 μmol of molybdate, and 0.13 μmol of iron(III). Finally, aminochelin was mixed 3:1 with iron and 2:1 with molybdate, and the resulting preparation contained 0.30 μmol of siderophore, 0.15 μmol of molybdate, and 0.10 μmol of iron(III). In each case, the total volume of the reaction mixture was brought to 2 ml with 6 mM potassium phosphate buffer (pH 7.6).
The stability of a ferric siderophore complex in the presence of equimolar molybdate or excess molybdate was also investigated. A solution containing 12.5 μM ferric protochelin or ferric azotochelin was challenged with either 12.5 μM or 1 mM molybdate, and formation of molybdo-protochelin or molybdo-azotochelin complexes was monitored at 375 nm.
Each reaction mixture was placed in a disposable plastic cuvette (catalog no. 223-9955; Bio-Rad) and was incubated under nitrogen gas using buffers, sparged with nitrogen gas for 10 min, in the dark in an air-tight chromatography tank flushed with nitrogen under high-humidity conditions. The formation of a ferric siderophore complex was monitored for up to 338 h. The percentage of a metal-siderophore complex present was determined by determining the ratio of the concentration of the metal-siderophore complex present to the theoretically maximum possible concentration of the metal-siderophore complex that could be formed, based on the amount of free metal and siderophore added. The concentration of ferric siderophore present was calculated by using the following molar absorptivities: ferric protochelin, 5.45 × 103 A310 M−1 cm−1; ferric azotochelin, 1.01 × 104 A310 M−1 cm−1; and ferric aminochelin, 4.76 × 103 A310 M−1 cm−1. Each reaction was examined three times.
CX preparation.
Strain UW was grown for 24 h in 200 ml of iron-limited Burk's medium containing 1 μM molybdate and 3 μM ferric citrate. Cell extract (CX) was prepared by the method of Page and von Tigerstrom (31), except that lysis in the French press was performed in 50 mM potassium phosphate buffer (pH 7.6) containing 2 mM dithiothreitol. The cell lysate was cleared by centrifugation (40,000 × g, 1 h), and the resulting CX was stored at −20°C.
Ferric reductase assay.
The ferric reductase activity in strain UW CX was measured by monitoring Fe2+ binding to ferrozine [3-(2-pyridyl)-5,6-bis(4-phenylsulfonic acid)-1,2,4-triazine] (catalog no. P-9762; Sigma) as previously described (28). The 1.6 mM iron(III) source used was either ferric citrate, ferric protochelin, ferric azotochelin, or ferric aminochelin complexes formed as described by Cornish and Page (7). Rates of conversion of iron(III) to iron(II) were calculated in the first 2 min of the assay by using a molar absorptivity for the Fe2+-ferrozine complex of 2.79 × 104 A562 M−1 cm−1 (38). Ferric reductase activity was expressed in nanomoles of Fe2+ per minute per milligram of CX protein. Each assay was performed at least twice.
Uptake of 55Fe3+-siderophore complexes.
Uptake of 55Fe-siderophore complexes was examined as described previously (6, 21), with the following modifications. Pure siderophore stock preparations and 55FeCl3 (7 μg of 55FeCl3 ml−1; specific activity, 35 μCi ml−1) were incubated for 72 h in the dark under high-humidity conditions (7) in order to obtain 55Fe-siderophore complexes with a final 55Fe3+ concentration of 12.5 μM. The effect that metal ion addition had on the uptake of a 55Fe-containing complex was expressed as the ratio of the control uptake rate in the absence of the metal ion from 0 to 8 min to the treated uptake rate in the presence of the metal ion from 8 to 16 min, expressed as a percentage. Rates were calculated by using data obtained from a single assay or duplicate assays, as indicated.
RESULTS
Affinities of catecholate siderophores for molybdate.
Each catecholate siderophore bound molybdate in a manner which was consistent with the fact that at a neutral pH molybdate has four sites at which water is coordinated and may be displaced by a more electronegative ligand (9, 16). Although a yellow catecholate siderophore complex was formed immediately after molybdate was added, formation of fully coordinated molybdo-siderophore complexes took time. Azotochelin chelated molybdate immediately at a molar binding ratio of 1:1, forming a complex which was stable for up to 191 h. A 2:1 molybdo-aminochelin complex was not observed until 28 h and was stable only until 98 h, after which it was replaced by other, undefined molybdo-aminochelin complexes. The interaction between protochelin and molybdate was not as easy to define. The predicted stoichiometry of a molybdo-protochelin complex was 2:3. This type of complex was observed at pH 6.0 in MES buffer. However, data obtained at pH 7.0 with MOPS buffer indicated that a complex with a 1:1 ratio of protochelin to molybdate was formed. This implies that at equilibrium protochelin had two empty coordination sites, a condition that would not be expected considering the amount of free molybdate in the test solution. As a result of this ambiguity, which we could not immediately explain, both 1:1 and 2:3 complexes were considered in subsequent calculations of the affinity of protochelin for molybdate. The 1:1 molybdo-protochelin complex was formed when molybdate was added and was stable for 167 h at pH 7.0, while the 2:3 molybdo-protochelin complex formed after 22 h and was stable for 376 h at pH 6.0.
Using the molar binding ratios described above, we determined the proton-dependent formation constant (KForm) for each siderophore with molybdate. Equilibrium was approached from either direction as the ferric siderophore complex was challenged with molybdate and the molybdo-siderophore complex was challenged with iron(III). The KForm values for each mixture were similar (Table 1), which indicated that equilibrium could be approached from either direction. The KForm for molybdo-aminochelin was approached only by competition between the ferric siderophore complex and molybdate due to a lack of pure aminochelin.
TABLE 1.
Log10 KForm for molybdo-siderophore complexes in 100 mM MOPS buffer (pH 7.0)
| Reaction | Log10KForm | Avg log10KForm | pMoO42− (−log10 [MoO42−]) | pFe3+ (−log10[Fe3+])a |
|---|---|---|---|---|
| Protochelin (1:1) complex | ||||
| Molybdo-protochelin + Fe3+ | 24.2 ± 0.2b | 22.7 ± 1.7 | 26 | 27.7 |
| Ferric protochelin + MoO42− | 21.1 ± 0.5 | |||
| Protochelin (2:3) complex | ||||
| Molybdo-protochelin + Fe3+ | 48.0 ± 0.6 | 49.1 ± 1.3 | 16 | NAc |
| Ferric protochelin + MoO42− | 50.2 ± 1.2 | |||
| Azotochelin (1:1) complex | ||||
| Molybdo-azotochelin + Fe3+ | 3.6 ± 0.05 | 3.6 ± 0.06 | 6.1 | 23.1 |
| Ferric azotochelin + MoO42− | 3.5 ± 0.02 | |||
| Aminochelin (2:1) complex | ||||
| Molybdo-aminochelin + Fe3+ | 3.9 ± 0.01 | 3.9 ± 0.01 | 5.6 | 22.0 |
| Ferric aminochelin + MoO42− | NDd |
Data from reference 7.
Average ± standard deviation based on multiple determinations.
NA, not applicable.
ND, not determined.
As a result of the different stoichiometries of the molybdate-binding reactions, it was not possible to directly compare the KForm values generated for each siderophore as the different constants had different units. To directly compare the abilities of different siderophores to bind molybdenum, the amount of free MoO42− in a theoretical molybdo-siderophore system at pH 7.4 was calculated, as described by Harris et al. (15). This required calculation of the proton-independent KForm for each siderophore by using the proton-dependent KForm determined at pH 7.0 (Table 1) and the pKa values for the model compound N,N-dimethyl-2,3-dihydroxybenzamide (23), as described by Reid et al. (34) and Cornish and Page (7). Using this method, we determined the concentration of free molybdate in the system of Harris et al. (15) and expressed it as pMoO42− (−log10[MoO42−]). Thus, the larger the pMoO42− value, the lower the free [MoO42−] at equilibrium and the higher the affinity of a siderophore for molybdenum (Table 1).
Molybdate and iron siderophore complex competition.
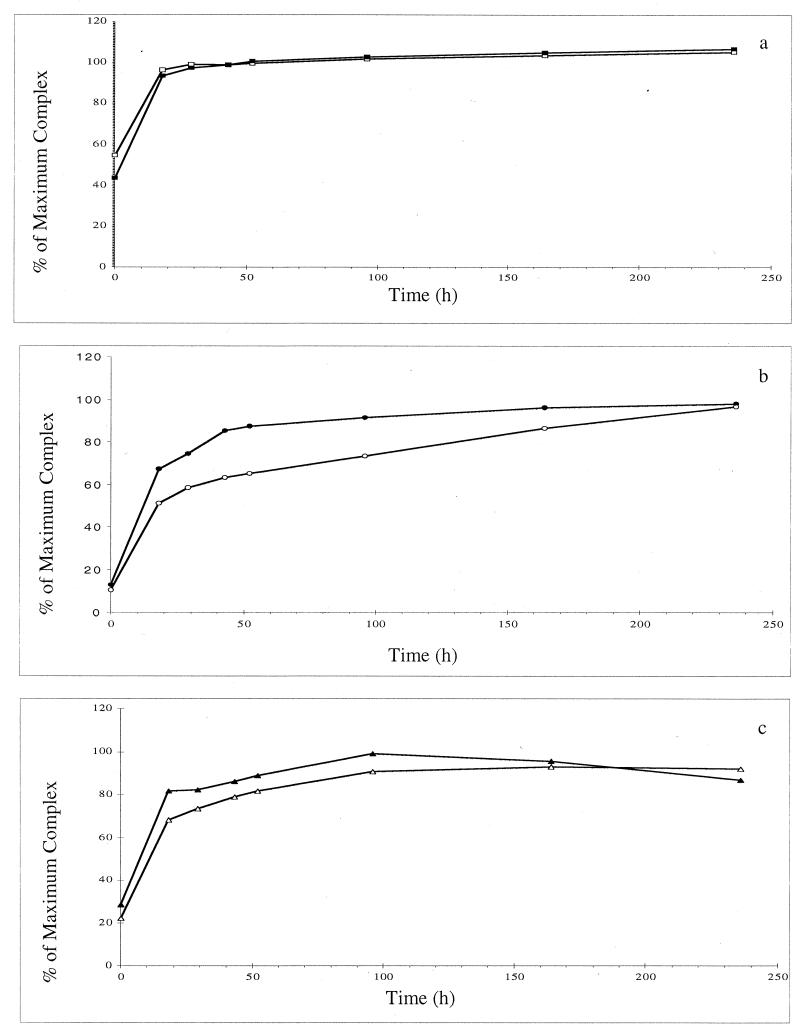
To determine if molybdate could interfere with formation of a ferric siderophore complex, molybdate, Fe3+, and a purified siderophore were incubated together under the anaerobic conditions described above at the correct molar binding ratios, and formation of the ferric siderophore complex was monitored spectrophotometrically. The results of one experiment showed that formation of a ferric protochelin complex was not affected by the presence of molybdate at either a 1:1 ratio (Fig. 2a) or a 3:2 ratio (data not shown) and are representative of the results of multiple experiments in which data were obtained at different times. Formation of a ferric azotochelin complex and formation of a ferric aminochelin complex were both affected to small degrees by the presence of molybdate, and formation of a ferric azotochelin complex was more sensitive to the presence of molybdate (Fig. 2b) than formation of a ferric aminochelin complex (Fig. 2c). The results obtained for formation of a ferric azotochelin complex in the presence of molybdate were consistent with the results of Duhme et al. (9). Ferric siderophore complexes that were already formed were not affected by the presence of 12.5 μM or 1 mM molybdate.
FIG. 2.
Effect of molybdate competition on formation of a ferric siderophore complex. (a) Formation of ferric protochelin in the absence (■) and in the presence (□) of 0.2 mM molybdate. (b) Formation of ferric azotochelin in the absence (●) and in the presence (○) of 0.2 mM molybdate. (c) Formation of ferric protochelin in the absence (▴) and in the presence (▵) of 0.2 mM molybdate. Data were obtained from a single experiment and are representative of the data obtained in multiple assays.
Effects of other metal ions on the accumulation of protochelin.
Divalent metal ions other than molybdate were tested to determine whether they could increase protochelin accumulation. We found that vanadate, tungstate, Mn2+, and Zn2+ increased protochelin accumulation (Table 2), Co2+ and Ni2+ were toxic to A. vinelandii, and Mg2+, Ca2+, and Sr2+ had no effect on protochelin accumulation. The minimum concentrations of the metals required to increase protochelin accumulation were determined by TLC analysis of culture fluids to be 70 μM molybdate, 60 μM vanadate, 30 μM tungstate, 70 μM Mn2+, and 500 μM Zn2+ (data not shown). At these concentrations, the metals were not detrimental to A. vinelandii growth. In addition, strain RP40, which is defective in both high- and low-affinity molybdate transport, was found to form protochelin in response to different molybdate concentrations in a manner which was consistent with formation by wild-type strain UW (data not shown).
TABLE 2.
Effects of metals that increase protochelin accumulation on the uptake of ferric protochelin and ferric azotochelin complexes
| Metal | Concn (μM) | Avg % decrease in 55Fe-protochelin uptake | Avg % decrease in 55Fe-azotochelin uptake |
|---|---|---|---|
| None | NAa | 0 | 0 |
| Molybdate | 70 | 40 ± 7b | 75 ± 8 |
| Vanadate | 100 | 55 ± 2 | 27 ± 15 |
| Tungstate | 30 | 51 ± 6 | 74 ± 6 |
| Mn2+ | 70 | 29 ± 17 | 71 ± 15 |
| Zn2+ | 500 | 56 ± 37 | 90 ± 31 |
NA, not applicable.
Range of values obtained in duplicate assays.
Ferric reductase activity.
It has been shown previously that Mn2+ and Zn2+ are inhibitors of the A. vinelandii ferric reductase, an enzyme important in ferric siderophore uptake (18, 28). A. vinelandii cells grown in medium containing 1 μM molybdate and 3 μM ferric citrate were used to prepare CX. This level of iron repressed azotobactin production (31), and we expected that it would limit our examination of ferric reductases to the enzymes important in catecholate siderophore use. Each metal that increased the accumulation of protochelin was found to be an inhibitor of ferric reductase activity (Table 3). Molybdate, vanadate, and tungstate appeared to have greater inhibitory effects on ferric citrate complex reduction than on ferric siderophore complex reduction. Mn2+ had a greater effect on ferric aminochelin or ferric citrate complex reduction, while Zn2+ appeared to have a greater effect on reduction of high-affinity chelates. It has been shown previously that Ca2+, Sr2+, and Mg2+, which had no effect on protochelin accumulation, activate ferric reductase activity (18).
TABLE 3.
Effects of metals that increase protochelin accumulation on ferric reductase activity
| Iron(III) source | % of control ferric reductase activity in the presence of:
|
||||
|---|---|---|---|---|---|
| Molybdate (1,000 μM) | Vanadate (100 μM) | Tungstate (30 μM) | Mn2+ (70 μM) | Zn2+ (500 μM) | |
| Ferric protochelin | 86 ± 6a | 42b | 64 ± 8 | 77 ± 7 | 32 ± 3 |
| Ferric azotochelin | 71 ± 4 | 66 ± 3 | 75 ± 6 | 87 ± 5 | 61 ± 1 |
| Ferric aminochelin | 60 ± 1 | 44 ± 5 | 83 ± 5 | 46 ± 4 | 94 ± 1 |
| Ferric citrate | 53 ± 5 | 36 ± 6 | 51 ± 3 | 36 ± 2 | 78 ± 5 |
Mean ± standard deviation based on values from at least two replicates.
The standard deviation was not determined.
Inhibition of ferric siderophore complex uptake.
Ferric protochelin was taken up by Fe-limited A. vinelandii at a rate of 6.0 ng of 55Fe3+ 108 cells−1 min−1, and ferric azotochelin was taken up at a rate of 7.0 ng of 55Fe3+ 108 cells−1 min−1. Uptake continued with no decrease in the rate for the full 16 min of the assay. When 1 mM molybdate was added to cells which had been grown in Fe-limited medium containing 1 μM molybdate, the rate of 55Fe-protochelin uptake decreased by 89% and the rate of 55Fe-azotochelin uptake decreased by 68%. When excess molybdate was removed by washing the cells in uptake buffer, the rates of uptake of 55Fe-protochelin and 55Fe-azotochelin increased by 10%. This indicated that the effect of molybdate was in part transient and could be reduced by removing free molybdate.
Inhibition of 55Fe-siderophore uptake was also observed in the presence of 70 μM molybdate, the minimal concentration of molybdate required to increase protochelin accumulation (Table 2). Under these conditions, the uptake rate without molybdate was 4.9 ng of 55Fe3+ 108 cells−1 min−1 (R2 = 0.90), which decreased by 33% to 3.3 ng of 55Fe3+ 108 cells−1 min−1 (R2 = 0.80) after 5 min of incubation with molybdate. The uptake rate in a siderophore-free uptake buffer control was 0.42 ng of 55Fe3+ 108 cells−1 min−1 (R2 = 0.91), indicating that molybdate did not eliminate 55Fe3+ transport. Similarly, 55Fe-azotochelin uptake (4.0 ng of 55Fe3+ 108 cells−1 min−1; R2 = 0.99) decreased by 35% to 2.6 ng of 55Fe3+ 108 cells−1 min−1 (R2 = 0.82) in the presence of 70 μM molybdate.
Other metals that were found to increase protochelin accumulation and to decrease ferric reductase activity were examined to determine whether they had this effect on uptake of 55Fe-protochelin and 55Fe-azotochelin (Table 2). Although the effects of some of the metals, such as Zn2+, on 55Fe-siderophore uptake were quite variable, the results revealed the following trend: the metals that increased protochelin accumulation also decreased the uptake of 55Fe-siderophore complexes.
DISCUSSION
Since it was first observed that high concentrations of molybdate result in accumulation of protochelin in A. vinelandii (6), the manner in which molybdate exerts this effect has been an unresolved question. Molybdate does not spontaneously promote condensation of aminochelin and azotochelin under aqueous conditions (6). Protochelin is a natural product of A. vinelandii; small amounts of it are produced by strain UW (6), and larger amounts are produced by strain LM100 (7) in low-molybdate media. Since higher levels of protochelin are formed in the presence of higher molybdate concentrations, an easy interpretation is that molybdate catalyzes formation of protochelin from its component parts, azotochelin and aminochelin. However, this probably does not occur, since strain RP40, which cannot transport molybdate, continues to form protochelin in the presence of increased molybdate concentrations.
Molybdate appears to impair the function of protochelin as a siderophore. It prevents decolorization of the ferric iron-Chrome Azurol S complex (6) in the universal assay for siderophore activity (36). It also prevents protochelin from promoting the growth of the siderophore-deficient strain A. vinelandii P100 in iron-restricted medium (6). By using a combination of molar binding ratio data, affinity data, and iron-molybdate competition data (this study) it was possible to ascertain, in an indirect manner, how protochelin and molybdate interact. Using affinity data, we calculated that under the hypothetical equilibrium conditions described by Harris et al. (15) the concentrations of free molybdate were 10−26 M for the 1:1 molybdo-protochelin complex and 10−16 M for the 2:3 molybdo-protochelin complex (Table 1). By comparison, the amount of free iron(III) under the same concentration and pH conditions, as determined by using ferric siderophore KForm values, was 10−27.7 M (Table 1) (7). This implies that in a competition assay, protochelin would have approximately equal affinities for molybdate and iron(III) in a 1:1 complex. If this were the case, there should be some inhibition of formation of the ferric protochelin complex in the presence of stoichiometrically balanced amounts of molybdate. This was not observed. Therefore, these data suggest that protochelin and molybdate do not form a 1:1 complex but interact to form a 2:3 complex, as predicted by the structure of protochelin.
Although catecholates and molybdate interact to quickly form a colored complex, it is thought that Fe3+ should displace molybdate bound to protochelin over the course of a 24-h growth period because of the much greater affinity of catecholates for iron (pFe3+ is 27.7 and pMoO42− is 16 in a 2:3 complex). Also, competition assays revealed that molybdate cannot displace iron in a preformed ferric protochelin or ferric azotochelin complex. Thus, interference with ferric siderophore complex formation by molybdate may be minimized over time, suggesting that molybdate affects protochelin accumulation through another site of action.
Molybdate interferes with catecholate siderophore-mediated 55Fe uptake in A. vinelandii. Similarly, vanadate, tungstate, Zn2+, and Mn2+ increase protochelin accumulation and decrease rates of 55Fe uptake (Table 2). While molybdate, vanadate, and tungstate are chemically related, Zn2+ and Mn2+ are not. A common feature of these metal ions is that they all inhibit ferric reductase activity. This has been observed previously in A. vinelandii with Mn2+ and Zn2+ (18, 28) but not with the other ions. In A. vinelandii two ferric reductase enzymes have been localized to either the cytoplasm or the periplasm. It is possible that ferric siderophore reduction, mediated by ferric reductase, takes place at the cell surface and is affected by high concentrations of metal ions (18). In studies performed with A. vinelandii, it was found that ferric reductase activity could not be completely inhibited by high concentrations of metal ions. The pattern of inhibition observed was characteristic of a mixed or partial type of inhibitor, so that Zn2+ and Mn2+ act as both competitive and noncompetitive inhibitors of ferric reductase activity (18, 28). As a result, the cells continue to grow in the presence of these metal ions. Inhibition of ferric reductase may have a direct effect on iron uptake but also may have an indirect effect, as activity of this enzyme affects iron speciation within the cell. It is the ratio of Fe2+ to Fe3+ in the cell that controls siderophore production through the Fur repressor (8). Subtle changes in Fe2+ corepressor availability could result in overproduction of protochelin, as observed previously for azotobactin hyperproduction in the presence of Zn2+ (17).
We believe that protochelin is the true siderophore of A. vinelandii. As a result of the high affinity of protochelin for iron, uptake of this siderophore probably involves ferric reduction, as well as cleavage, like enterobactin uptake in E. coli (3). The cleavage products (azotochelin and aminochelin) are recycled as siderophores and as reducing agents for iron mineral solubilization (30). Preliminary experiments in our laboratory have revealed that a CX of iron-limited A. vinelandii can cleave ferric protochelin into azotochelin and aminochelin (unpublished data). The much greater abundance of the cleavage products than of protochelin (7) suggests that protochelin is used and turned over rapidly, as expected for a very effective, high-affinity siderophore.
The conclusions described above do not preclude the possibility that azotochelin plays a role in high-affinity molybdate transport (10), but they do raise conflicting issues. The cells must be iron limited to form catecholate siderophores, yet high-affinity molybdate transport operates well in iron-sufficient medium (26). Duhme et al. (10) suggested that protochelin replaces azotochelin in medium containing molybdate at a concentration of 70 μM or more, because azotochelin is depleted during high-affinity molybdate transport. However, high-affinity molybdate transport in A. vinelandii is repressed in the presence of 10 μM molybdate (26). In the presence of 1 mM molybdate, ferric protochelin uptake is reduced by 89% and ferric azotochelin uptake is reduced by 68%. Thus, it seems that little is gained by replacing azotochelin with a chelator that functions less well in the presence of high concentrations of molybdate.
Finally, our results suggest that metals like molybdate may be useful in studies of microorganisms that produce small, low-affinity siderophores which appear to be ineffective in iron chelation but at the same time have been shown to be virulence factors. These siderophores include chrysobactin produced by Erwinia chrysanthemi (12), anguibactin produced by Vibrio anguillarum, (19), myxochelin A isolated from Angiococcus disciformis (22), and serratiochelin produced by Serratia marcescens (11). It may be possible to determine if these molecules are the primary siderophores produced by these microorganisms or if there are larger, more effective siderophores that do not accumulate in culture fluid and are not detected. Metal ions like molybdate could be used to “trap” the parent compounds and determine more about their roles in iron acquisition and virulence.
ACKNOWLEDGMENTS
This work was supported by a grant from the Natural Sciences and Engineering Research Council of Canada. Work with strain RP40 was done by Tara Dwinzel, who was supported by a studentship from the Alberta Heritage Foundation for Medical Research.
We thank Robert Jordan for assistance with siderophore affinity calculations.
REFERENCES
- 1.Barnum D W. Spectrophotometric determination of catechol, epinephrine, DOPA, dopamine and other aromatic vic-dols. Anal Chim Acta. 1977;89:157–166. doi: 10.1016/S0003-2670(01)83081-6. [DOI] [PubMed] [Google Scholar]
- 2.Bishop P. Three genetically distinct nitrogenase systems in Azotobacter vinelandii. In: Barton L L, Hemming B C, editors. Iron chelation in plants and soil microorganisms. San Diego, Calif: Academic Press; 1993. pp. 310–324. [Google Scholar]
- 3.Brickman T J, MacIntosh M A. Overexpression and purification of ferric enterobactin esterase from Escherichia coli: demonstration of enzymatic hydrolysis of enterobactin and its iron complex. J Biol Chem. 1992;267:12350–12355. [PubMed] [Google Scholar]
- 4.Chaberek S, Martell A E. Organic sequestering agents. New York, N.Y: John Wiley and Sons, Inc.; 1959. [Google Scholar]
- 5.Corbin J L, Bulen W A. The isolation and identification of 2,3-dihydroxybenzioc acid and 2-N,6-N-di(2,3-dihydroxybenzoyl)-l-lysine formed by iron-deficient Azotobacter vinelandii. Biochemistry. 1969;8:757–762. doi: 10.1021/bi00831a002. [DOI] [PubMed] [Google Scholar]
- 6.Cornish A S, Page W J. Production of the tricatecholate siderophore protochelin by Azotobacter vinelandii. BioMetals. 1995;8:332–338. [Google Scholar]
- 7.Cornish A S, Page W J. The catecholate siderophores of Azotobacter vinelandii: their affinity for iron and role in oxygen stress management. Microbiology. 1998;144:1747–1754. doi: 10.1099/00221287-144-7-1747. [DOI] [PubMed] [Google Scholar]
- 8.Crosa J H. Genetics and molecular biology of siderophore-mediated iron transport in bacteria. Microbiol Rev. 1989;53:517–530. doi: 10.1128/mr.53.4.517-530.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duhme A K, Hider R C, Khodr H H. Spectrophotometric competition study between molybdate and Fe(III) hydroxide on N,N′-bis(2,3-dihydroxylbenzoyl)-l-lysine, a naturally occurring siderophore synthesized by Azotobacter vinelandii. BioMetals. 1996;9:245–248. [Google Scholar]
- 10.Duhme A K, Hider R C, Naldrett M J, Pau R N. The stability of the molybdenum-azotochelin complex and its effect on siderophore production in Azotobacter vinelandii. J Biol Inorg Chem. 1998;3:520–526. [Google Scholar]
- 11.Ehlert G, Taraz K, Budzikiewicz H. Serratiochelin, a new catecholate siderophore from Serratia marcescens. Z Naturforsch. 1994;49:11–17. [Google Scholar]
- 12.Enard C, Franza T, Neema C, Gill P R, Persmark M, Neilands J B, Expert D. The requirement of chrysobactin dependent iron transport for virulence incited by Erwinia chrysanthemi on Saintpaulia ionantha. Plant Soil. 1991;130:263–271. [Google Scholar]
- 13.Grunden A M, Ray R M, Resentel J K, Healy F G, Shammugam K T. Repression of the Escherichia coli modABCD (molybdate transport) operon by ModE. J Bacteriol. 1996;178:735–744. doi: 10.1128/jb.178.3.735-744.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guerinot M L. Microbial iron transport. Annu Rev Microbiol. 1994;48:743–772. doi: 10.1146/annurev.mi.48.100194.003523. [DOI] [PubMed] [Google Scholar]
- 15.Harris W R, Carrani C J, Cooper S R, Sofen S R, Avdeef A E, McArdle J V, Raymond K N. Coordination chemistry of microbial iron transport compounds. 19. Stability constants and electrochemical behavior of ferric enterobactin and model complexes. J Am Chem Soc. 1979;101:6097–6104. [Google Scholar]
- 16.Hider R C. Siderophore mediated absorption of iron. In: Clarke M J, Ibers J A, Mingos D M P, Palmer G A, Sadler P J, Williams R J P, editors. Structure and bonding: siderophores from microorganisms and plants. Vol. 58. Berlin, Germany: Springer-Verlag; 1984. pp. 26–87. [Google Scholar]
- 17.Huyer M, Page W J. Zn2+ increases siderophore production in Azotobacter vinelandii. Appl Environ Microbiol. 1988;54:2625–2631. doi: 10.1128/aem.54.11.2625-2631.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huyer M, Page W J. Ferric reductase activity in Azotobacter vinelandii and its inhibition by Zn2+ J Bacteriol. 1989;171:4031–4037. doi: 10.1128/jb.171.7.4031-4037.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jalal M A F, Hossain M B, van der Helm S, Sanders-Lochr J, Actis L A, Crosa J H. Structure of anguibactin, a unique plasmid-related bacterial siderophore from the fish pathogen Vibrio anguillarum. J Am Chem Soc. 1989;111:292–296. [Google Scholar]
- 20.Ketchum P A, Owens M S. Production of a molybdenum-coordinating compound by Bacillus thuringiensis. J Bacteriol. 1975;122:412–417. doi: 10.1128/jb.122.2.412-417.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knosp O, von Tigerstrom M, Page W J. Siderophore-mediated uptake of iron in Azotobacter vinelandii. J Bacteriol. 1984;159:341–347. doi: 10.1128/jb.159.1.341-347.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunze B, Bedorf N, Kohl W, Hofle G, Reichenbach H. Myxochelin A, a new iron-chelating compound from Angiococcus disciformis (Myxobacterales). Production, isolation, physio-chemical and biological properties. J Antibiot. 1989;42:14–17. doi: 10.7164/antibiotics.42.14. [DOI] [PubMed] [Google Scholar]
- 23.Loomis D L, Raymond K N. Solution equilibria of enterobactin and metal-enterobactin complexes. Inorg Chem. 1991;30:906–911. [Google Scholar]
- 24.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 25.Morgan T V, Lundell D J, Burgess B K. Azotobacter vinelandii ferredoxin I: cloning, sequencing, and mutant analysis. J Biol Chem. 1988;263:1370–1375. [PubMed] [Google Scholar]
- 26.Mouncey N J, Mitchenall L A, Pau R N. Mutational analysis of genes of the mod locus involved in molybdenum transport, homeostasis, and processing in Azotobacter vinelandii. J Bacteriol. 1995;177:5294–5302. doi: 10.1128/jb.177.18.5294-5302.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Page W J. Growth conditions for the demonstration of siderophores and iron-repressible outer membrane proteins in soil bacteria, with an emphasis on free-living diazotrophs. In: Barton L L, Hemmings B C, editors. Iron chelation in plants and soil microorganisms. New York, N.Y: Academic Press; 1993. pp. 75–110. [Google Scholar]
- 28.Page W J. The effect of manganese oxides and manganese ion on growth and siderophore production by Azotobacter vinelandii. BioMetals. 1995;8:30–36. [Google Scholar]
- 29.Page W J, Collinson K, Demange P, Dell A, Abdallah M A. Azotobacter vinelandii strains of disparate origin produce azotobactin siderophores with identical structures. BioMetals. 1991;4:217–222. [Google Scholar]
- 30.Page W J, Huyer M. Derepression of the Azotobacter vinelandii siderophore system using iron-containing minerals to limit iron repletion. J Bacteriol. 1984;158:496–502. doi: 10.1128/jb.158.2.496-502.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Page W J, von Tigerstrom M. Aminochelin, a catecholamine siderophore produced by Azotobacter vinelandii. J Gen Microbiol. 1988;134:453–460. [Google Scholar]
- 32.Patel U, Baxi M D, Modi V V. Evidence for the involvement of iron siderophore in the transport of molybdenum in cowpea Rhizobium. Curr Microbiol. 1988;17:179–182. [Google Scholar]
- 33.Pope M T, Still E R, Williams R J P. A comparison between the chemistry and biochemistry of molybdenum and related elements. In: Coughlan M P, editor. Molybdenum and molybdenum-containing enzymes. New York, N.Y: Pergamon Press; 1980. pp. 3–40. [Google Scholar]
- 34.Reid R T, Live D H, Faulkner D J, Butler A. A siderophore from a marine bacterium with an exceptional ferric ion affinity constant. Nature. 1993;336:455–458. doi: 10.1038/366455a0. [DOI] [PubMed] [Google Scholar]
- 35.Saxena B, Vithlani L, Modi V V. Siderophore-mediated transport of molybdenum in Azospirillum lipoferum strain D-2. Curr Microbiol. 1989;19:291–295. [Google Scholar]
- 36.Schwyn B, Neilands J B. Universal chemical assay for detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 37.Sevinc M S, Page W J. Generation of Azotobacter vinelandii strains defective in siderophore production and characterization of a strain unable to produce known siderophores. J Gen Microbiol. 1992;138:587–596. [Google Scholar]
- 38.Stookey L L. Ferrozine—a new spectrophotometric reagent for iron. Anal Chem. 1970;42:779–781. [Google Scholar]