Abstract
Background
Diffuse large B-cell lymphoma (DLBCL) is a heterogeneous neoplasm and is characterized as the most common subtype of non-Hodgkin lymphoma (NHL). Despite 60–70% of all patients being cured with R-CHOP therapeutic regimen (Cyclophosphamide, doxorubicin, vincristine, and prednisone, combined with rituximab), remaining patients display aggressive disease. Therefore, there is an urgent need to develop novel diagnostic, prognostic, and predictive biomarkers. Recently, exosomal miRNAs have been approved as novel biomarkers in DLBCL due to their potential involvement in lymphomagenesis.
Material and Methods
We conducted an investigation on the potential role of exosomal miRNAs as diagnostic, prognostic, and predictive biomarkers in DLBCL in the PubMed, Scopus, and Web of Science search engines. We searched by using a combination of keywords, such as diffuse large B-cell lymphoma, DLBCL, miRNA, microRNA, miR, exosome, exosomes, exosomal, extracellular vesicles, EVs, and secretome. Then, search results were narrowed based on specific inclusion and exclusion criteria.
Results
Twelve articles were eligible for our systematic reviews. Among them, nine discussed diagnostic biomarkers, three considered prognostic significance, four evaluated therapeutic efficacy, two studies were conducted in vitro, and three assessed molecular pathways associated with these exosomal miRNAs in DLBCL.
Discussion
According to our systematic review, exosomal miRNAs are not only useful for diagnosis and prognosis in DLBCL but are also promising therapeutic tools and predictors of response to therapy. Although promising results so far, more research is required to develop innovative biomarkers.
Keywords: diffuse large B-cell lymphoma (DLBCL), exosome, miRNAs, diagnosis, prognosis, treatment
Introduction
Diffuse large B-cell lymphoma (DLBCL) is a heterogeneous group of lymphoma, accounting for approximately 30% of non-Hodgkin lymphoma (NHL) in the United States, which represents the most prevalent subtype of NHLs (1). Currently, the definitive diagnosis of DLBCL requires histopathology. Despite biopsies-based examination allowing detection of enlarged lymph nodes, it is an invasive procedure (2). Although more than 50% of cases with DLBCL are cured with R-CHOP (Cyclophosphamide, doxorubicin, vincristine, and prednisone, combined with rituximab), DLBCL is an aggressive disease in which relapsed or refractory patients have a poor prognosis (3).
Extracellular vesicles (EVs) can be divided into two categories based on their size: small extracellular vesicles (sEVs) and large extracellular vesicles (lEVs). Exosomes in 40–150 nm diameter are a special group of sEVs (4). Exosomes are secreted by diverse cell types, including cancer cells. Exosomes carry many important bioactive molecules including nucleic acids (DNA, mRNA, miRs), protein, and lipids (5). Tumor cell-derived exosomes communicate between exosome-originated cells within the tumor microenvironment (TME), which potentiates tumorigenesis. Hence the cancer-derived exosome is an applicable biomarker for cancer diagnosis, prognosis, and treatment (6). Exosomes influence different aspects of hematological malignancies (7). In lymphoma, exosomes participate in disease progression via bone marrow microenvironment modulation, enhancing angiogenic ability, suppressing the immune response, and contributing to drug resistance. Moreover, they can serve as drug delivery tools, and be used for therapeutic response prediction and therapeutic targets (8).
microRNAs (miRNAs) are unique content of cancer cell-derived exosomes and have crucial roles in lymphomagenesis. miRNAs impact lymphoma progression by controlling cell growth, proliferation, differentiation, survival, and apoptosis. Therefore, microRNA expression profiles can serve as prognostic, diagnostic, therapeutic, and predictive biomarkers in lymphoma (9). Of note, miRNAs have unique features including stability, detectability in many biological fluids, and relatively resistance to RNAase degradation, making them useful biomarkers (10, 11).
The high prevalence of DLBCL among NHLs and the challenges in diagnosis and treatment of this disease require the development of new approaches to manage this disease. Considering the key roles of exosomal miRNAs in lymphoma-associated molecular mechanisms can direct our attention to using these biomolecules as malignancy-related diagnostic, prognostic, and therapeutic markers. This study aimed to evaluate the potential role of exosomal miRNA as non-invasive biomarkers in DLBCL.
Material and Methods
Literature Search
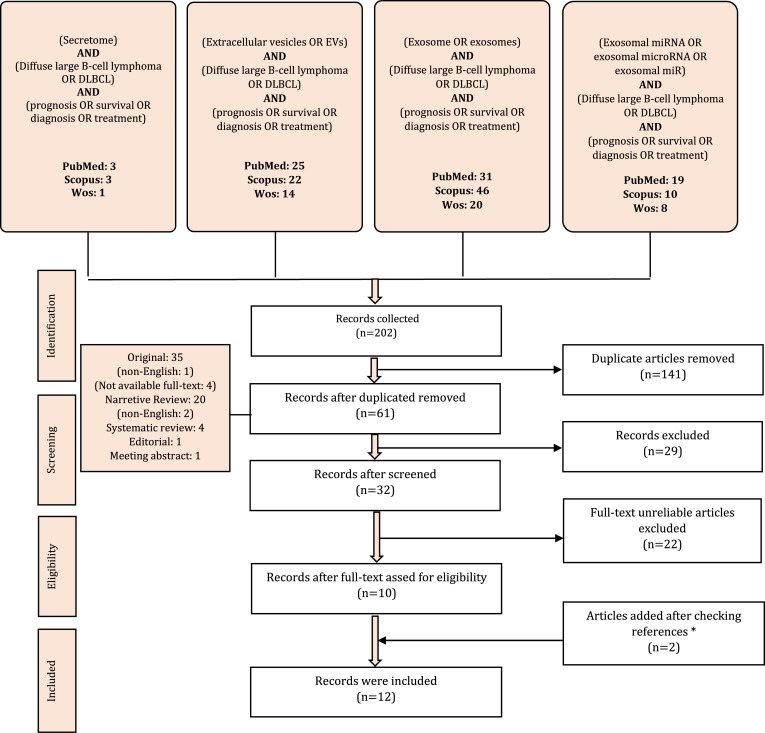
The systematic review was conducted on December 2021 according to the Preferred Reporting Items. To perform this literature review, we searched in the following search engines: PubMed, Scopus, and Web of science to identify all available publications. The search strategy set was adopted utilizing a combination of keywords including: Diffuse large B-cell lymphoma, DLBCL, miRNA, microRNA, miR, exosome, exosomes, exosomal, extracellular vesicles, EVs, and secretome. The full details of the search strategy can be seen in Table 1.
Table 1.
Full details of the search strategy terms.
| Terms | Search strategy terms |
|---|---|
| Term 1 | (Secretome) AND (Diffuse large B-cell lymphoma OR DLBCL) AND (Prognosis OR survival OR diagnosis OR treatment) |
| Term 2 | (Extracellular vesicles OR EVs) AND (Diffuse large B-cell lymphoma OR DLBCL) AND (Prognosis OR survival OR diagnosis OR treatment) |
| Term 3 | (Exosome OR exosomes) AND (Diffuse large B-cell lymphoma OR DLBCL) AND (Prognosis OR survival OR diagnosis OR treatment) |
| Term 4 | (exosomal miRNA OR exosomal microRNA OR exosomal miR) AND (Diffuse large B-cell lymphoma OR DLBCL) AND (Prognosis OR survival OR diagnosis OR treatment) |
Study Selection
Initially, we excluded duplicate articles and articles without original data. Next, the abstracts were downloaded, and the list was narrowed based on inclusion or exclusion criteria. Subsequently, to verify eligibility, the full texts were evaluated. Further, reference lists of the identified publications were searched for additional relevant studies. The inclusion and exclusion criteria utilized are indicated in Table 2. Each eligible criterion was assessed independently by two researchers (S.Y and Z.H) and disagreements were resolved by consensus.
Table 2.
Inclusion and Exclusion Criteria.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
Records included
|
Records excluded
|
Full-text articles included
|
Full-text articles excluded
|
Data Extraction and Quality Assessment
Two researchers independently extracted the following information from articles: first author, publication year, type of sample analyzed (tumor tissue, serum, or plasma), sample size (patient versus controls), exosomal miRNAs assessed, number of miRNAs studied, a chemotherapeutic drug, methodology (exosome isolation and miRNA profiling), the origin of exosome and recipient cell (in the case of in vitro studies), the list of specific exosomal miRNAs and their molecular characteristic which could be used for diagnosis, prognosis, and therapeutic response prediction in DLBCL.
Results
Search Results
The primary search identified 202 articles that may be related to the potential role of exosomal microRNA in DLBCL (PubMed: 78, Scopus: 81, WoS: 43). Among them, 141 duplicate articles were discarded. Next, after removing 29 records including 20 reviews, 4 systematic reviews, 1 editorial, and 4 articles without accessible full text, 32 records remained. Among them, 22 records were discarded after full-text evaluation (2 articles discussed EVs but have not identified them as exosomes). Additionally, 2 articles were further identified by searching through the reference list of relevant reviews. Finally, 12 articles met the inclusion criteria. The flowchart of the literature study and selection process was shown in Figure 1.
Figure 1.
Flowchart of study selection. *We also found two meeting abstracts but did not add them because of the similarity of data with the existing data.
Study Characteristics
Finally, 12 articles investigated the role of exosomal miRNAs in diagnosis, prognosis, and therapeutic response prediction in DLBCL. Of those, 9 articles (75%) discussed miRNAs as diagnostic biomarkers, 4 articles (33.33%) evaluated therapeutic efficacy, 3 articles (25%) provided prognostic biomarkers, 2 articles (16.66%) conducted experiments in vitro, and 3 articles (25%) evaluated molecular pathways associated with these exosomal miRNAs in DLBCL (Tables 3–7).
Table 3.
Exosomal miRNAs as diagnostic biomarkers in DLBCL.
| Study | Exosomal miRNAs | Sample | Source | Exosome isolation | miRNA Profiling | Result |
|---|---|---|---|---|---|---|
| Exosomal miRNAs with significant differential expressed | ||||||
| Rinaldi et al., 2021 (12), | miR-22 | 27 DLBCL vs. 10 controls | serum | DC | – | Upregulation |
| Liu et al., 2021 (13), | miR-485-3p | 42 DLBCL vs. 31 controls | plasma | ExoEasy Maxi | qRT-PCR | Upregulation |
| miR-375-3p miR-107 |
Downregulation | |||||
| Cao et al.,2021 (14),* | miR-135a-3p miR-379-5p miR-4476 |
10 DLBCL vs. 5 controls (Screening stage) 24 DLBCL vs. 24 controls (Training stage) 99 DLBCL vs. 65 controls (Testing stage) 123 DLBCL vs. 89 controls (External testing stage) |
serum | ExoQuick | microarray qRT-PCR |
Upregulation |
| miR-483-3p miR-451a |
Downregulation | |||||
| Caner et al., 2021 (11), | 33 miRNAs miR-3960 miR-6089 miR-939-5p others** |
20 DLBCL vs. 20 controls | plasma | DC | microarray qRT-PCR |
Downregulation |
| Xiao et al., 2019 (15), | miR-451a | 89 DLBCL vs. 48 controls | serum | ExoQuick | qRT-PCR | Downregulation |
| Khare et al., 2017 (16), | miR-124 miR-532-5p |
14 DLBCL vs.20 controls | plasma | DC | RNA seq | Upregulation |
| miR-425 miR-145 others*** |
Downregulation | |||||
| Inada et al., 2015 (17), | miR-21-5p miR-15a-3p |
33 DLBCL vs.22 controls | Serum | DC | qRT-PCR | Upregulation |
| miR-181a-5p miR-210-5p |
Downregulation | |||||
| Exosomal miRNAs with insignificant differential expression | ||||||
| Zare et al., 2019 (18), | miR-146a | 48 DLBCL vs. 6 controls | plasma | Exospin | qRT-PCR | NS |
*Also, a meeting abstract (19) publish these results with this difference; sample size include: 99 DLBCL vs. 94 controls.
**miR-181b-5p, miR-181d-5p, miR-197-5p, miR-432-5p, miR-595, miR-623, miR-937-5p, miR-1207-5p, miR-1268a, miR-1268b, miR-1307-3p, miR-1587, miR-2861, miR-3656, miR-4481, miR-4632-5p, miR-4701-3p, miR-4707-5p, miR-4721, miR-4725-3p, miR-4741, miR-4763-3p, miR-6127, miR-6724-5p, miR-6803-5p, miR-6840-3p, miR-7108-5p, miR-7845-5p, miR-8069, -miR-8071.
***miR-122, miR-128, miR-141, miR-197, miR-345, miR-424, miR-101, let-7e, miR-222, miR-29c, miR-375, miR-324-5P, miR-135a, miR-379, miR-32, let-7i (Sample size in let-7i analysis: 20 patients vs. 20 controls).
DLBCL, Diffuse large B-cell lymphoma; miR, microRNA; DC, Differential centrifugation; qRT-PCR, quantitative-Real time polymerase chain reaction; RNA seq, RNA sequencing; NS, Non-Significant.
“-”: No information is available.
Table 7.
Molecular pathways associated with exosomal miRNAs in DLBCL.
| Study | Exosomal miRNAs | Molecular pathways | Result |
|---|---|---|---|
| Liu et al., 2021 (13), | miR-107 | Targeting 14-3-3 η | Downregulation in DLBCL patients: ↓ PFS |
| Zare et al., 2020 (22), | miR-155-5P | Targeting INPP5D and SOCS-1 | Upregulation in NK cells: ↓ proliferation and cytotoxicity |
| Feng et al., 2019 (20), | 37miRNAs miR-125b-2-3p miR-99a-5p miR-10b-5p miR-125b-5p miR-146b-3p miR-23a-3p miR-1269a let-7c-5p miR-24-3p miR-novel-chr19 26407 |
AMPK signaling* Signaling pathways-regulating pluripotency of stem cells Cellular senescence MicroRNAs in cancer Ras signaling EGFR tyrosine kinase inhibitor resistance mTOR signaling p53 signaling Longevity regulating Autophagy – animal |
Upregulation in DLBCL cell lines |
| Feng et al., 2019 (20), | 17 miRNAs miR-122-5p miR-421 miR-144-5p miR-144-3p miR-98-5p miR-577 miR-novel-chr4 51772 miR-novel-chrX 72977 miR-novel-chr20 33917 miR-novel-chrX 73668 |
Signaling pathways- regulating pluripotency- of stem cells* TGF-beta signaling mTOR signaling Oocyte meiosis p53 signaling Proteoglycans in cancer Pathways in cancer MicroRNAs in cancer Transcriptional- misregulation in cancer cGMP-PKG signaling |
Downregulation in DLBCL cell lines |
*In the first pathway, all five miRNAs are involved and, in another pathway, some of them are involved.
miR, microRNA; PFS, Progression-free survival; ↓, Decreased.
Discussion
DLBCL is a clinically heterogeneous lymphoma comprising the most common NHL in adults (23). Diagnosis requires a pathological review of biopsy, which is invasive and can carry limitations for patients (2). Traditional DLBCL risk assessment relies on the International Prognostic Index (IPI) including age, lactate dehydrogenase (LDH) level in serum, disease stage, number of extranodal sites involved, and Eastern Cooperative Oncology Group (ECOG) performance status (24). Of note, the effectiveness of this index has markedly decreased as a result of the use of rituximab during chemotherapy (25). The R-CHOP immunotherapy is the frontline treatment for DLBCL. Even though this type of treatment works in more than half of the cases, about one-third of patients still relapse after treatment (26). On the other hand, DLBCL is an aggressive disease and the survival time of the untreated patient is less than one year (27).
There are many challenges in the DLBCL diagnosis, assessment of patient outcomes, treatment, and prediction of therapeutic response. Therefore, an alternative approach to address these challenges is needed. Based on the importance of miRNAs-derived exosomes, we investigated the literature to evaluate their effectiveness in this field.
miRNAs dysregulation plays a crucial role in the development of different types of cancers by affecting proliferation, apoptosis, cell cycle, angiogenesis, anticancer immune response, and sponge other non-coding RNAs. Considering their huge role in carcinogenesis, the miRNAs panel has great potential for cancer detection, prognosis, and treatment (28). Circulating miRNAs can be incorporated into membrane-coated vesicles, exosomes, or circulate as non-exosomal miRNAs (29). Two unique features of exosomal miRNA, i.e. the remarkable stability and the special function governed by exosomal miRNA (30, 31) render the miRNAs excellent candidates being effective biomarkers.
Evaluation of the implication of exosomal microRNAs profile in diagnosis in DLBCL has been analyzed by nine studies as can be seen in Table 3. Among the total miRNAs that have been identified, exosomal mir-451a was found in more than one study (14, 15). Based on the concordance finding of these studies, downregulation of exosomal mir-451a can be a useful biomarker. Of note, both of these two studies examined more than 59 DLBCL samples so their results are more reliable than other investigations (14, 15).
Intriguingly, abnormal expression of three isoforms of exosomal mir-181-5p has been reported in these studies. The results highlighted the down-expression of all isoforms (miR-181a-5p and miR-181b-5p and miR-181d-5p), so further investigation could clarify whether any isoform of exosomal mir-1815p is downregulated in DLBCL (11, 17).
Moreover, the search results revealed that exosomal miR-146a does not show significant differential expression between DLBCL patients and controls (18). It has been demonstrated that an increased level of miR-146a in DLBCL patient tissue compared with individuals with reactive hyperplasia lymphoid nodes (10). Also, studies clearly showed miR-146a upregulation in DLBCL patients (32). In the light that circulating miRNAs can be embedded in exosomes, there is a need for additional studies to confirm exosomal miR-146a diagnostic potential in DLBCL (29).
Regarding the suitability of exosomal miRNAs as prognostic indicators, three articles about four miRNAs were identified (Table 4). Among them, elevated levels of exosomal miR-125b-5p and miR-99a-5p were associated with shorter progression-free survival (PFS) while down-expression of exosomal miRNA-107 and miR-451a indicated poor prognosis in DLBCL (13, 14, 20). Despite none of these exosomal miRNAs having been reported in more than one study, analysis on circulating miRNAs can provide evidence that these results are more reliable. For instance, a study identified the prognostic significance of miR-125b-5p in 20 patients with DLBCL (33).
Table 4.
Exosomal miRNAs as prognostic biomarkers in DLBCL.
| Study | Exosomal miRNAs | Sample | Source | Exosome isolation | miRNA Profiling | Result |
|---|---|---|---|---|---|---|
| Liu et al., 2021 (13), | miR-107 | 42 DLBCL vs. 31 controls | plasma | ExoEasy Maxi | qRT-PCR | Downregulation: ↓ PFS |
| Cao et al., 2021 (14), | miR-451a | 220 DLBCL (109 DLBCL analyzed for PFS vs. 111 DLBCL analyzed for OS) |
serum | ExoQuick | microarray qRT-PCR |
Downregulation: ↓ PFS and OS |
| Feng et al., 2019 (20), | miR-125b-5p miR-99a-5p |
116 DLBCL | serum | ExoQuick | RNA seq, qRT-PCR | Upregulation: ↓ PFS |
DLBCL, Diffuse large B-cell lymphoma; miR, microRNA; qRT-PCR, quantitative-Real time polymerase chain reaction; PFS, Progression-free survival; OS, Overall survival; ↓, Decreased.
The utility of exosomal miRNAs as predictors for therapeutic efficacy in DLBCL has been identified in four studies (Table 5). A total of nine exosomal miRNAs were analyzed: miR-10a-5p, miR-10b-5p, miR-let-7i, and miR-146a did not show significant association with therapeutic efficacy (18, 20, 21). With other miRNAs, although upregulation of exosomal miR-125b-5p, miR-99a-5p, and miR-155 contribute to R-CHOP therapy resistance, downregulation of miR-let-7g was related to it (20, 21). A recent study illustrated high levels of exosomal miR-125b-5p as a chemoresistance-related indicator in DLBCL (20). Additionally, regarding the implication of exosomal miRNAs as predictive biomarkers of response to R-CHOP treatment, it is noteworthy that increasing the level of exosomal miR-451a following therapeutic regimens may implicate exosomal miR-451a as a suitable biomarker for predicting treatment response (15).
Table 5.
Exosomal miRNAs as predictor biomarkers for therapeutic efficacy in DLBCL.
| Study | Exosomal miRNAs | Sample | Source | Exosome isolation | miRNA Profiling | Result |
|---|---|---|---|---|---|---|
| Exosomal miRNAs with significant differential expressed | ||||||
| Feng et al., 2019 (20), | miR-125b-5p miR-99a-5p | 116 DLBCL patients (33 chemoresistant group vs. 83 chemosensitive group) | serum | ExoQuick | RNA seq qRT-PCR | Upregulation in chemoresistant group versus chemosensitive group: ↑ R-CHOP resistance |
| Zare et al., 2019 (21), | miR-155 | 48 DLBCL patients (16 refractory/relapsed patients vs. 32 responsive/receiving R-CHOP patients) | plasma | ExoSpin | qRT-PCR | Upregulation in refractory/relapsed patients versus responsive/receiving R-CHOP patients: ↓ response to R-CHOP therapy |
| miR-let-7g | Downregulation in patients receiving R-CHOP versus refractory/relapsed patients: ↓ response to R-CHOP therapy | |||||
| Xiao et al., 2019 (15), | miR-451a | 89 DLBCL patients treated with the R-CHOP regimen vs. 48 controls | serum | ExoQuick | qRT-PCR | Upregulation after treatment: biomarkers for prediction response to R-CHOP regimen |
| Exosomal miRNAs with insignificant differential expressed | ||||||
| Feng et al., 2019 (20), | miR-10a-5p miR-10b-5p | 116 DLBCL patients (33 chemoresistant group vs. 83 chemosensitive group) | serum | ExoQuick | RNA seq qRT-PCR | No significant difference between the two groups |
| Zare et al., 2019 (21), | miR-let-7i | 48 DLBCL patients (16 refractory/relapsed patients vs. 32 responsive/receiving R-CHOP patients) | plasma | ExoSpin | qRT-PCR | No significant difference between the two groups |
| Zare et al., 2019 (18), | miR-146a | 48 DLBCL patients (16 refractory patients vs. 32 responsive/receiving R-CHOP patients) | plasma | Exospin | qRT-PCR | No significant difference between the two groups |
DLBCL, Diffuse large B-cell lymphoma; miR, microRNA; qRT-PCR, quantitative-Real time polymerase chain reaction; RNA seq, RNA sequencing; R-CHOP, Cyclophosphamide, adriamycin, vincristine, and prednisone, combined with rituximab; ↑, Increased; ↓, Decreased.
We also found two other studies which their finding provided more evidence related to the importance of exosomal miRNAs in DLBCL (Table 6). One of these studies evaluated miRNAs expression in DLBCL cell lines (20), while other demonstrated miRNAs transferred through DLBCL-derived exosomes could impacts NK cells function (22).
Table 6.
In vitro study of exosomal miRNA in DLBCL.
| Study | Exosomal miRNAs | Origin of exosome | Recipient cells | Exosome isolation | miRNA Profiling | Result |
|---|---|---|---|---|---|---|
| Zare et al., 2020 (22), | miR-155-5p | DLBCL patients (Responsive and R/R) |
NK cells (Healthy donor) |
Exospin | qRT-PCR | Upregulation in NK cells: ↓ proliferation and cytotoxicity |
| Zare et al., 2020 (22), | let-7g-5p | DLBCL patients (Responsive) |
NK cells (Healthy donor) |
Exospin | qRT-PCR | Upregulation in NK cells |
| Feng et al., 2019 (20), | 37miRNAs miR-125b-2-3p miR-99a-5p miR-10b-5p miR-125b-5p miR-146b-3p miR-23a-3p miR-1269a let-7c-5p miR-24-3p miR-novel-chr19 26407 |
SU-DHL-2 cell SU-DHL-2/R cell |
– | ExoQuick | NGS | Upregulation in DLBCL cell lines |
| Feng et al., 2019 (20), | 17 miRNAs miR-122-5p miR-421 miR-144-5p miR-144-3p miR-98-5p miR-577 miR-novel-chr4 51772 miR-novel-chrX 72977 miR-novel-chr20 33917 miR-novel-chrX 73668 |
SU-DHL-2 cell SU-DHL-2/R cell |
– | ExoQuick | NGS | Downregulation in DLBCL cell lines |
SUD, LY8 and DUL cells, Diffuse large B-cell lymphoma cell lines; A20 cells, Mouse lymphoma cell line; miR, microRNA; R/R, Refractory/Relapsed; Next generation sequencing; qRT-PCR, quantitative-Real time polymerase chain reaction; ↓, Decreased.
FInally, we evaluated the molecular pathways associated with exosomal miRNAs (Table 7). We hope this molecular characteristic will be used for a therapeutic approach. For example, it has been shown that miR-107 plays a role as a tumor suppressor by targeting 14-3-3 η which results in suppressing oncogenes such as FOXO1, PEPCK, CCND1, P27, BAD, and Bcl-2, so its downregulation is associated with shorter PFS in DLBCL (13). According to this pathway, miR-107/14-3-3 η axis may be a promising therapeutic target in DLBCL (13).
Conclusion
Taken together, despite the limited number of articles published on this topic, our systematic review demonstrated that exosomal miRNAs not only can be applied for diagnosis and prognosis in DLBCL but also may serve as promising tools for therapeutic interventions and predicting response to treatment. It is hopeful that future investigations will provide more reliable results regarding the clinical significance of exosomal miRNAs in DLBCL.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author Contributions
SY prepared the manuscript’s backbone and wrote the original draft of the manuscript along with ZH, SK, MI, YL and LB. Z-SC and AG critically revised the manuscript for important intellectual content. All authors have read and approved the final version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
DLBCL, Diffuse large B-cell lymphoma; NLH, non-Hodgkin lymphoma; R-CHOP, Cyclophosphamide, doxorubicin, vincristine, and prednisone, combination with rituximab; EVs, Extracellular vesicles; sEVs, small extracellular vesicles; lEVs, large extracellular vesicles; TME, Tumor microenvironment; miRNAs, microRNAs; IPI, International Prognostic Index; LDH, Lactase dehydrogenase.
References
- 1. Cheson BD, Nowakowski G, Salles G. Diffuse Large B-Cell Lymphoma: New Targets and Novel Therapies. Blood Cancer J (2021) 11(4):68. doi: 10.1038/s41408-021-00456-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu Y, Barta SK. Diffuse Large B-Cell Lymphoma: 2019 Update on Diagnosis, Risk Stratification, and Treatment. Am J Hematol (2019) 94(5):604–16. doi: 10.1002/ajh.25460 [DOI] [PubMed] [Google Scholar]
- 3. He MY, Kridel R. Treatment Resistance in Diffuse Large B-Cell Lymphoma. Leukemia (2021) 35(8):2151–65. doi: 10.1038/s41375-021-01285-3 [DOI] [PubMed] [Google Scholar]
- 4. Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, et al. Reassessment of Exosome Composition. Cell (2019) 177(2):428–45. doi: 10.1016/j.cell.2019.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xu R, Rai A, Chen M, Suwakulsiri W, Greening DW, Simpson RJ. Extracellular Vesicles in Cancer — Implications for Future Improvements in Cancer Care. Nat Rev Clin Oncol (2018) 15(10):617–38. doi: 10.1038/s41571-018-0036-9 [DOI] [PubMed] [Google Scholar]
- 6. Yang E, Wang X, Gong Z, Yu M, Wu H, Zhang D. Exosome-Mediated Metabolic Reprogramming: The Emerging Role in Tumor Microenvironment Remodeling and its Influence on Cancer Progression. Signal Transduction Targeting Ther (2020) 5(1):1–13. doi: 10.1038/s41392-020-00359-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Litwińska Z, Łuczkowska K, Machaliński B. Extracellular Vesicles in Hematological Malignancies. Leuk Lymphoma (2019) 60(1):29–36. doi: 10.1080/10428194.2018.1459606 [DOI] [PubMed] [Google Scholar]
- 8. Liu J, Wang X. Focus on Exosomes—From Pathogenic Mechanisms to the Potential Clinical Application Value in Lymphoma. J Cell Biochem (2019) 120(12):19220–8. doi: 10.1002/jcb.29241 [DOI] [PubMed] [Google Scholar]
- 9. Bradshaw G, Sutherland HG, Haupt LM, Griffiths LR. Dysregulated MicroRNA Expression Profiles and Potential Cellular, Circulating and Polymorphic Biomarkers in Non-Hodgkin Lymphoma. Genes (Basel) (2016) 7(12):130. doi: 10.3390/genes7120130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhong H, Xu L, Zhong JH, Xiao F, Liu Q, Huang HH, et al. Clinical and Prognostic Significance of miR-155 and miR-146a Expression Levels in Formalin-Fixed/Paraffin-Embedded Tissue of Patients With Diffuse Large B-Cell Lymphoma. Exp Ther Med (2012) 3(5):763–70. doi: 10.3892/etm.2012.502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Caner V, Cetin GO, Hacioglu S, Baris IC, Tepeli E, Turk NS, et al. The miRNA Content of Circulating Exosomes in DLBCL Patients and In Vitro Influence of DLBCL-Derived Exosomes on miRNA Expression of Healthy B-Cells From Peripheral Blood. Cancer Biomarkers (2021) 32(4):519–29. doi: 10.3233/cbm-210110 [DOI] [PubMed] [Google Scholar]
- 12. Rinaldi F, Marchesi F, Palombi F, Pelosi A, Di Pace AL, Sacconi A, et al. MiR-22, a Serum Predictor of Poor Outcome and Therapy Response in Diffuse Large B-Cell Lymphoma Patients. Br J Haematol (2021) 195(3):399–404. doi: 10.1111/bjh.17734 [DOI] [PubMed] [Google Scholar]
- 13. Liu J, Han Y, Hu S, Cai Y, Yang J, Ren S, et al. Circulating Exosomal MiR-107 Restrains Tumorigenesis in Diffuse Large B-Cell Lymphoma by Targeting 14-3-3η. Front Cell Dev Biol (2021) 9:667800. doi: 10.3389/fcell.2021.667800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cao D, Cao X, Jiang Y, Xu J, Zheng Y, Kang D, et al. Circulating Exosomal microRNAs as Diagnostic and Prognostic Biomarkers in Patients With Diffuse Large B-Cell Lymphoma. Hematol Oncol (2022) 40(2):172–80. doi: 10.1002/hon.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xiao XB, Gu Y, Sun DL, Ding LY, Yuan XG, Jiang HW, et al. Effect of Rituximab Combined With Chemotherapy on the Expression of Serum Exosome miR-451a in Patients With Diffuse Large B-Cell Lymphoma. Eur Rev Med Pharmacol Sci (2019) 23(4):1620–5. doi: 10.26355/EURREV_201902_17121 [DOI] [PubMed] [Google Scholar]
- 16. Khare D, Goldschmidt N, Bardugo A, Gur-Wahnon D, Ben-Dov IZ, Avni B. Plasma microRNA Profiling: Exploring Better Biomarkers for Lymphoma Surveillance. PloS One (2017) 12(11):e0187722. doi: 10.1371/JOURNAL.PONE.0187722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Inada K, Okoshi Y, Cho Y, Saito H, Iijima T, Hori M, et al. Availability of Circulating MicroRNAs as a Biomarker for Early Diagnosis of Diffuse Large B-Cell Lymphoma. Open J Blood Dis (2015) 5(4):48–58. doi: 10.4236/OJBD.2015.54008 [DOI] [Google Scholar]
- 18. Zare N, Eskandari N, Mehrzad V, Javanmard SH. The Expression Level of Hsa − miR − 146a − 5p in Plasma − Derived Exosomes of Patients With Diffuse Large B − Cell Lymphoma. J Res Med Sci (2019) 24:10. doi: 10.4103/jrms.JRMS [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Di C. Circulating Exosomal microRNA Signature As a Noninvasive Biomarker for Diagnosis of Diffuse Large B-Cell Lymphoma. Blood (2018) 132:5406. doi: 10.1182/blood-2018-99-115940 [DOI] [Google Scholar]
- 20. Feng Y, Zhong M, Zeng S, Wang L, Liu P, Xiao X, et al. Exosome-Derived miRNAs as Predictive Biomarkers for Diffuse Large B-Cell Lymphoma Chemotherapy Resistance. Epigenomics (2019) 11(1):35–51. doi: 10.2217/EPI-2018-0123 [DOI] [PubMed] [Google Scholar]
- 21. Zare N, Javanmard SH, Mehrzad V, Kefayat A. Evaluation of Exosomal miR-155 , Let-7g and Let-7i Levels as a Potential Noninvasive Biomarker Among Refractory / Relapsed Patients , Responsive Patients and Patients Receiving R-CHOP. Leuk Lymphoma (2019) 0(0):1–13. doi: 10.1080/10428194.2018.1563692 [DOI] [PubMed] [Google Scholar]
- 22. Zare N, Haghjooy Javanmard SH, Mehrzad V, Eskandari N, Andalib AR. Effect of Plasma-Derived Exosomes of Refractory / Relapsed or Responsive Patients With Diffuse Large B-Cell Lymphoma on Natural Killer Cells Functions. Cell J (2020) 22(1):40–54. doi: 10.22074/cellj.2020.6550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hematol J, Wang L, Li L, Young KH. New Agents and Regimens for Diffuse Large B Cell Lymphoma. J Hematol Oncol (2020) 13(1):175. doi: 10.1186/s13045-020-01011-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li S, Young KH, Medeiros LJ. Diffuse large B-cell lymphoma. Pathology (2018) 50(1):74–87. doi: 10.1016/j.pathol.2017.09.006 [DOI] [PubMed] [Google Scholar]
- 25. Ollila TA, Olszewski AJ. Extranodal Diffuse Large B Cell Lymphoma: Molecular Features, Prognosis, and Risk of Central Nervous System Recurrence. Curr Treat Options Oncol (2018) 19(8):38 doi: 10.1007/s11864-018-0555-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Autio M, Leivonen S, Brück O, Mustjoki S, Mészáros Jørgensen J, Karjalainen-Lindsberg ML, et al. Immune Cell Constitution in the Tumor Microenvironment Predicts the Outcome in Diffuse Large B-Cell Lymphoma. Haematologica (2021) 106(3). doi: 10.3324/haematol.2019.243626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mey U, Hitz F, Lohri A, Pederiva S, Taverna C, Tzankov A, et al. Diagnosis and Treatment of Diffuse Large B-Cell Lymphoma. Swiss Med Wkly (2012) 142:w13511. doi: 10.4414/smw.2012.13511 [DOI] [PubMed] [Google Scholar]
- 28. He B, Zhao Z, Cai Q, Zhang Y, Zhang P, Shi S, et al. miRNA-Based Biomarkers, Therapies, and Resistance in Cancer. Int J Biol Sci (2020) 16(14):2628–47. doi: 10.7150/IJBS.47203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nik MohamedKamal NNSB, Shahidan WNS. Non-Exosomal and Exosomal Circulatory MicroRNAs: Which Are More Valid as Biomarkers? Front Pharmacol (2020) 10:1500. doi: 10.3389/fphar.2019.01500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ge Q, Zhou Y, Lu J, Bai Y, Xie X, Lu Z. miRNA in Plasma Exosome is Stable Under Different Storage Conditions. Molecules (2014) 19(2):1568–75. doi: 10.3390/molecules19021568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang J, Li S, Li L, Li M, Guo C, Yao J. Exosome and Exosomal MicroRNA : Trafficking , Sorting , and Function. Genomics Proteomics Bioinformatics (2015) 13(1):17–24. doi: 10.1016/j.gpb.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhuang H, Shen J, Zheng Z, Luo X, Gao R, Zhuang X. MicroRNA-146a Rs2910164 Polymorphism and the Risk of Diffuse Large B Cell Lymphoma in the Chinese Han Population. Med Oncol (2014) 31(12):306. doi: 10.1007/s12032-014-0306-z [DOI] [PubMed] [Google Scholar]
- 33. Yuan WXIN, Gui YXI, Na WNA, Chao J, Yang X. Circulating microRNA-125b and microRNA-130a Expression Profiles Predict Chemoresistance to R-CHOP in Diffuse Large B-Cell Lymphoma Patients. Oncol Lett (2016) 11(1):423–32. doi: 10.3892/ol.2015.3866 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.