Abstract
Context
Primary hyperparathyroidism (PHPT) is associated with an increased risk of kidney stones. Few studies account for PHPT severity or stone risk when comparing stone events after parathyroidectomy vs nonoperative management.
Objective
Compare the incidence of kidney stone events in PHPT patients treated with parathyroidectomy vs nonoperative management.
Design
Longitudinal cohort study with propensity score inverse probability weighting and multivariable Cox proportional hazards regression.
Setting
Veterans Health Administration integrated health care system.
Patients
A total of 44 978 patients with > 2 years follow-up after PHPT diagnosis (2000-2018); 5244 patients (11.7%) were treated with parathyroidectomy.
Main outcomes measure
Clinically significant kidney stone event.
Results
The cohort had a mean age of 66.0 years, was 87.8% male, and 66.4% White. Patients treated with parathyroidectomy had higher mean serum calcium (11.2 vs 10.8mg/dL) and were more likely to have a history of kidney stone events. Among patients with baseline history of kidney stones, the unadjusted incidence of ≥ 1 kidney stone event was 30.5% in patients managed with parathyroidectomy (mean follow-up, 5.6 years) compared with 18.0% in those managed nonoperatively (mean follow-up, 5.0 years). Patients treated with parathyroidectomy had a higher adjusted hazard of recurrent kidney stone events (hazard ratio [HR], 1.98; 95% CI, 1.56-2.51); however, this association declined over time (parathyroidectomy × time: HR, 0.80; 95% CI, 0.73-0.87).
Conclusion
In this predominantly male cohort with PHPT, patients treated with parathyroidectomy continued to be at higher risk of kidney stone events in the immediate years after treatment than patients managed nonoperatively, although the adjusted risk of stone events declined with time, suggesting a benefit to surgical treatment.
Keywords: primary hyperparathyroidism, parathyroidectomy, kidney stones, nephrolithiasis
Kidney stones are common sequelae of primary hyperparathyroidism (PHPT) and contribute to chronic kidney disease (CKD) and poor quality of life (1, 2). Parathyroidectomy is the only definitive cure for PHPT and is recommended in patients with a history of symptomatic kidney stones, asymptomatic kidney stones on imaging, or hypercalciuria (3). However, the benefits of parathyroidectomy related to kidney stone incidence and recurrence have not been well studied. This knowledge gap limits the ability of clinicians to appropriately counsel patients with PHPT about the risks and benefits of parathyroidectomy.
Parathyroidectomy is associated with improvements in kidney stone risk factors, including a reduction in urinary calcium excretion (4). Conventional wisdom, based on uncontrolled observational studies, holds that surgical treatment reduces kidney stone recurrence in patients with PHPT and a history of stone disease (5-7). However, 2 recent studies have called into question whether parathyroidectomy reduces stone events compared with nonoperative management. Our prior work used contemporary approaches to account for treatment selection bias in an administrative claims database and demonstrated no difference in the odds of 5-year kidney stone events in privately insured patients with PHPT who were treated with parathyroidectomy vs nonoperative management (8). Huang et al recently found that parathyroidectomy mitigated the risk of recurrent kidney stones, but that the risk of stone recurrence after surgery was not different from patients managed nonoperatively after adjusting for demographics and biochemical severity of disease (7). These 2 studies, which included appropriate comparator groups, had long-term follow-up, and adjusted for relevant confounders, produced similar findings that challenge the consensus opinion driving guideline recommendations for parathyroidectomy in all patients with PHPT and a history of urinary stone disease. Given that more than one-half of patients with PHPT and kidney stones are managed nonoperatively (9), it is critical to clarify the association of parathyroidectomy with the risk of recurrent or new stone events to assess the appropriateness of this practice.
The aim of this study was to determine if parathyroidectomy is associated with a reduction in clinically significant kidney stone events in patients with PHPT. In this analysis, we leveraged the rich clinical information available in Veterans Affairs (VA) data to adjust for the severity of PHPT and established risk factors for stone disease. We also evaluated the association of parathyroidectomy with surrogate endpoints indicative of kidney stone risk. We hypothesized that parathyroidectomy would be associated with a reduced adjusted risk of kidney stone events after accounting for differences in kidney stone risk between operatively and nonoperatively managed groups.
Materials and Methods
Data Source
We conducted a longitudinal cohort study of patients with a biochemical diagnosis of PHPT using the VA Corporate Data Warehouse (CDW), accessed through the VA Informatics and Computing Infrastructure. The CDW includes data from patients treated at VA facilities nationally. We identified patients and their demographic and clinical information with the Observational Medical Outcomes Partnership Common Data Model, which aims to standardize data across time and treatment centers to facilitate research analysis (10, 11). This study was approved by the Stanford University institutional review board and the VA Palo Alto Research and Development Committee. This study was performed in accordance with Strengthening the Reporting of Observational Studies in Epidemiology guidelines for observational research (12).
Study Cohort
We identified patients with an incident biochemical diagnosis of PHPT from 2000 to 2018, defined as elevated PTH laboratory result (between 66 and 999 pg/mL), elevated serum calcium (10.3-18 mg/dL) within 3 months of PTH result, and estimated glomerular filtration rate (eGFR) above 30 mL/min/1.73 m2, calculated using the Chronic Kidney Disease Epidemiology Collaboration equation, within 6 months of PTH result (13). We excluded patients with: (1) any eGFR result below 30 mL/min/1.73 m2 within 6 months of PTH laboratory result; (2) parathyroidectomy before diagnosis; or (3) possible secondary or tertiary hyperparathyroidism, defined based on having claim codes indicating stage 5 CKD, receipt of hemodialysis, or having a kidney transplant before PHPT diagnosis.
We divided patients into 2 PHPT treatment groups: patients treated with parathyroidectomy within 2 years of diagnosis and patients observed nonoperatively. Patients treated with parathyroidectomy were identified based on: International Classification of Diseases (ICD), 9th Revision codes 06.81, 06.89, and 06.99; ICD 10th Revision codes 0GBLx, 0GBMx, 0GBNx, 0GBPx, 0GBQx, and 0GBRx; and Current Procedural Terminology codes 60500, 60502, and 60505. The treatment date was the date of surgery in the parathyroidectomy group. Patients in the nonoperative group were randomly assigned a “treatment date” with the same overall distribution of time from diagnosis to treatment as the parathyroidectomy group to ensure similar time to follow-up.
Outcome
Our primary outcome was a clinically significant kidney stone event, defined as an emergency department or inpatient admission with a kidney stone diagnosis code or a urinary stone procedure performed in any setting. Our secondary outcomes were rate of kidney stone development, for which kidney stones at least 120 days apart were counted as separate events, percentage of patients considered surgically cured, and change in 24-hour urinary calcium. Surgical cure was defined as serum calcium less than 10.3 mg/dL at earliest laboratory test from 6 months through 2 years after surgery. Patients were followed from treatment date until their last known date of care within the VA health care system up until December 31, 2020.
Covariates
Covariates were determined a priori based on clinical significance and included patient demographic and clinical characteristics. Demographic covariates included age at diagnosis (< 35, 35-49, 50-64, 65-74, ≥ 75 years), sex (male, female), race (American Indian or Alaska Native, Asian, Black, Native Hawaiian or other Pacific Islander, White, unknown), and ethnicity (Hispanic or Latino, not Hispanic or Latino, unknown). Patients with a history of kidney stones were identified based on kidney stone claim diagnosis codes at any time before PHPT diagnosis. Patients were further separated into those with a diagnosis code only or at least 1 prior clinically significant kidney stone event (as described previously). Other clinical covariates included body mass index (categorized to underweight < 18.5, normal weight 18.5-<25, over weight 25-<30, obese 30-<40, and severely obese ≥ 40); Charlson-Deyo Comorbidity Index (14) (categorized to 0, 1, or ≥ 2); indicators of PHPT severity, including a serum calcium measurement ≥ 11.3 mg/dL (1 mg/dL greater than the upper limit of normal), history of osteoporosis, and history of stage 3 CKD (assessed by any eGFR < 60 mL/min/1.73 m2); kidney stone risk factors, including a 24-hour urinary calcium measurement above 400 mg/d, hypertension, diabetes, or gout; and specialist care, including endocrinologist or either urologist or nephrologist care. Specialty care was defined as any visit with a relevant provider taxonomy code (endocrinology: 207RE0101X; urology: 208800000X, 2088F00F0X, 2088P0231X; nephrology: 207RN0300X) or VA clinic code (endocrinology: 305; urology: 414, 430; nephrology: 313). Clinical covariates were assessed in the 2 years before treatment start, except having a history of osteoporosis and kidney stones, which was assessed with all available claims before PHPT diagnosis.
Statistical Analysis
Analyses were stratified by history of kidney stones at time of PHPT diagnosis. We incorporated inverse probability weighting in our outcome analysis to reduce treatment selection bias. To calculate the weights, we calculated a propensity model using generalized boosted regression. The outcome of the model was treatment with parathyroidectomy within 2 years of diagnosis. Covariates in the model included all variables described in the covariate section (ie, characteristics associated with either treatment selection [parathyroidectomy] or development of the outcome [kidney stone]). We compared covariate balance between patients treated with parathyroidectomy to patients treated with medical management using standardized mean difference, the mean difference divided by pooled SD, and considered standardized mean difference < 10% to indicate adequate balance (15).
We assessed the relationship between treatment and our primary outcome, clinically significant kidney stone event, with inverse probability–weighted (IPW) Cox regression models. Patient follow-up started at the time of treatment and was censored at the date of death or last recorded visit date. We found that the proportional hazards assumption for our main predictor, treatment type, was violated, and therefore included a treatment by time (per year) interaction term in our models (parathyroidectomy × time). We performed a sensitivity analysis excluding patients with delayed (2 or more years after diagnosis) parathyroidectomy. All models were adjusted for all covariates of interest.
Propensity score models and inverse probability weights were calculated using the TWANG package (16) in R (R Foundation for Statistical Computing, Vienna, Austria). All other data cleaning and analyses were conducted using Stata MP v. 15 (StataCorp LLC, College Station, TX, USA). Significance was assessed at the .05 level and all tests were 2-tailed.
Results
Cohort Characteristics and Profile of Patients Treated With Parathyroidectomy
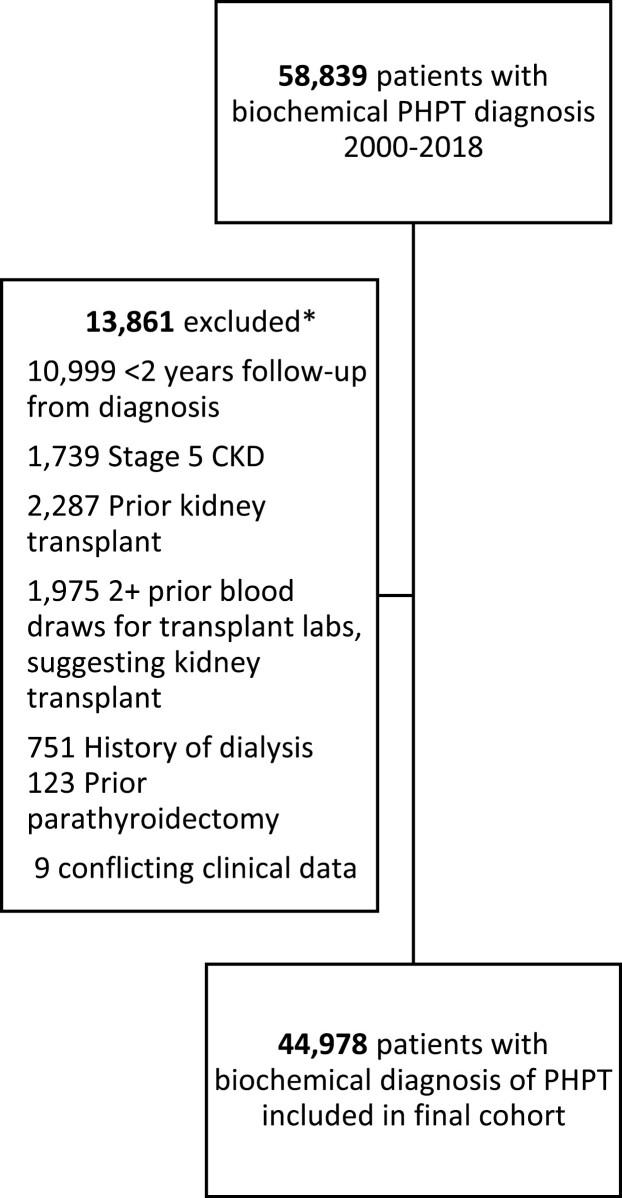
We identified 44 978 patients with a new biochemical diagnosis of PHPT from 2000 to 2018 within the VA Health Care System, after applying cohort selection criteria and minimum follow-up requirements (Fig. 1). Patients with PHPT had a mean age of 66.0 (SD, 11.8) years, were 87.8% male, 66.4% White, and 91.1% non-Hispanic (Table 1). A total of 5627 (12.5%) had a history of kidney stones at the time of PHPT diagnosis, with 2099 (37.3%) of those patients having a history of at least 1 clinically significant kidney stone event, defined as an emergency department visit or inpatient admission for stone disease or receipt of a kidney stone procedure.
Figure 1.
Consort diagram. *Some patients met multiple exclusion criteria.
Table 1.
Baseline characteristics of patients with a biochemical diagnosis of PHPT (2000-2017) according to treatment
| Unadjusted | IPW-adjusteda | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall cohort (n = 44 978) | Parathyroidectomy (n = 5244) | Nonoperatively managed (n = 39 734) | SMD | Parathyroidectomy | Nonoperatively managed | SMD | ||||
| N | % | N | % | N | % | % | % | |||
| Sex | 0.083 | 0.019 | ||||||||
| F | 5485 | 12.2 | 770 | 14.7 | 4715 | 11.9 | 12.8 | 12.2 | ||
| M | 39493 | 87.8 | 4474 | 85.3 | 35019 | 88.1 | 87.2 | 87.8 | ||
| Age, y | 0.541 | 0.051 | ||||||||
| <35 | 603 | 1.3 | 134 | 2.6 | 469 | 1.2 | 1.1 | 1.3 | ||
| 35-49 | 3050 | 6.8 | 632 | 12.1 | 2418 | 6.1 | 6.8 | 6.7 | ||
| 50-64 | 15835 | 35.2 | 2510 | 47.9 | 13325 | 33.5 | 35.8 | 35.2 | ||
| 65-74 | 14545 | 32.3 | 1488 | 28.4 | 13057 | 32.9 | 33.8 | 32.4 | ||
| 75+ | 10 945 | 24.3 | 480 | 9.2 | 10 465 | 26.3 | 22.5 | 24.4 | ||
| Race | 0.163 | 0.077 | ||||||||
| White | 29 883 | 66.4 | 3787 | 72.2 | 26 096 | 65.7 | 70.1 | 66.5 | ||
| Black or African American | 10 476 | 23.3 | 991 | 18.9 | 9485 | 23.9 | 20.9 | 23.3 | ||
| Asian | 129 | 0.3 | 26 | 0.5 | 103 | 0.3 | 0.3 | 0.3 | ||
| Native Hawaiian or Other Pacific Islander | 279 | 0.6 | 34 | 0.7 | 245 | 0.6 | 0.5 | 0.6 | ||
| American Indian or Alaska Native | 179 | 0.4 | 34 | 0.7 | 145 | 0.4 | 0.4 | 0.4 | ||
| Unknown | 4032 | 9.0 | 372 | 7.1 | 3660 | 9.2 | 7.9 | 9.0 | ||
| Ethnicity | 0.077 | 0.082 | ||||||||
| Hispanic or Latino | 1790 | 4.0 | 248 | 4.7 | 1542 | 3.9 | 2.6 | 4.0 | ||
| Not Hispanic or Latino | 40 963 | 91.1 | 4800 | 91.5 | 36163 | 91.0 | 93.1 | 91.1 | ||
| Unknown | 2225 | 5.0 | 196 | 3.7 | 2029 | 5.1 | 4.3 | 4.9 | ||
| Charlson Comorbidity Index | 0.25 | 0.024 | ||||||||
| 0 | 11 926 | 26.5 | 1863 | 35.5 | 10 063 | 25.3 | 27.5 | 26.5 | ||
| 1 | 7746 | 17.2 | 970 | 18.5 | 6776 | 17.1 | 16.8 | 17.2 | ||
| 2+ | 25 306 | 56.3 | 2411 | 46.0 | 22 895 | 57.6 | 55.7 | 56.3 | ||
| Mean serum calcium, mg/dL, N (SD) | 10.8 (0.5) | 11.2 (0.7) | 10.7 (0.5) | 0.690 | 10.9 (0.6) | 10.8 (0.5) | 0.300 | |||
| Mean PTH, pg/mL (SD) | 114.0 (62.4) | 152.7 (97.0) | 108.9 (54.2) | 0.557 | 146.9 (88.9) | 110.2 (55.7) | 0.495 | |||
| Mean eGFR, mL/min/1.73 m2, (SD) | 70.8 (21.2) | 79.2 (20.2) | 69.7 (21.1) | 0.458 | 72.6 (20.7) | 70.7 (21.2) | 0.092 | |||
| Mean creatinine, mg/dL (SD) | 1.2 (0.3) | 1.1 (0.3) | 1.2 (0.3) | 0.397 | 1.1 (0.3) | 1.2 (0.3) | 0.111 | |||
| Stone history | 0.299 | 0.020 | ||||||||
| None | 39 351 | 87.5 | 4090 | 78.0 | 35 261 | 88.7 | 87.9 | 87.5 | ||
| Diagnosis | 3528 | 7.8 | 642 | 12.2 | 2886 | 7.3 | 7.3 | 7.8 | ||
| Clinically significant event | 2099 | 4.7 | 512 | 9.8 | 1587 | 4.0 | 4.8 | 4.7 | ||
| Osteoporosis | 5117 | 11.4 | 840 | 16.0 | 4277 | 10.8 | 0.155 | 11.1 | 11.4 | 0.008 |
| Stage 3 CKD, eGFR < 60 mL/min/1.73 m2 | 22 650 | 50.4 | 1930 | 36.8 | 20 720 | 52.2 | 0.312 | 48.1 | 50.4 | 0.046 |
| Calcium ≥ 11.3 mg/dL | 11 571 | 25.7 | 3255 | 62.1 | 8316 | 20.9 | 0.919 | 28.3 | 25.6 | 0.059 |
| Urinary calcium, mg | 0.815 | 0.047 | ||||||||
| < 400 | 7936 | 17.6 | 1848 | 35.2 | 6088 | 15.3 | 19.1 | 17.6 | ||
| ≥ 400 | 1619 | 3.6 | 864 | 16.5 | 755 | 1.9 | 4.0 | 3.5 | ||
| Missing | 35 423 | 78.8 | 2532 | 48.3 | 32 891 | 82.8 | 77.0 | 78.9 | ||
| Endocrinologist visit | 20 056 | 44.6 | 4349 | 82.9 | 15 707 | 39.5 | 0.995 | 48.5 | 44.5 | 0.080 |
| Nephrologist or urologist visit | 16 016 | 35.6 | 1787 | 34.1 | 14 229 | 35.8 | 0.036 | 33.6 | 35.6 | 0.042 |
| Hypertension | 36 088 | 80.2 | 3731 | 71.2 | 32 357 | 81.4 | 0.244 | 80.6 | 80.3 | 0.008 |
| Diabetes | 4493 | 10.0 | 307 | 5.9 | 4186 | 10.5 | 0.171 | 9.8 | 10.0 | 0.006 |
| Gout | 4756 | 10.6 | 353 | 6.7 | 4403 | 11.1 | 0.153 | 11.4 | 10.6 | 0.026 |
| BMI | 0.054 | 0.061 | ||||||||
| Underweight | 331 | 0.7 | 34 | 0.7 | 297 | 0.8 | 0.5 | 0.7 | ||
| Normal weight | 8141 | 18.1 | 897 | 17.1 | 7244 | 18.2 | 18.5 | 18.1 | ||
| Overweight | 15 638 | 34.8 | 1793 | 34.2 | 13 845 | 34.8 | 33.6 | 34.8 | ||
| Obese | 17 232 | 38.3 | 2112 | 40.3 | 15 120 | 38.1 | 40.3 | 38.4 | ||
| Severely obese | 2952 | 6.6 | 342 | 6.5 | 2610 | 6.6 | 5.6 | 6.5 | ||
| Unknown | 684 | 1.5 | 66 | 1.3 | 618 | 1.6 | 1.5 | 1.5 | ||
Abbreviations: BMI, body mass index; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; IPW, inverse probability weighted; SMD, standardized mean difference.
a The propensity score used for IPW in this table was based on all categorical patient characteristics listed in this table.
There were 5244 (11.7%) patients treated with parathyroidectomy within 2 years of PHPT diagnosis. Patients treated with parathyroidectomy had more severe PHPT biochemical profiles when compared with those managed nonoperatively (mean serum calcium 11.2 vs 10.8 mg/dL, mean PTH 152.7 vs 108.9 pg/mL; both P < 0.001). Operatively managed patients also had higher baseline risk of kidney stones according to 24-hour urinary calcium (≥400 mg in 16.5% vs 1.9% of patients, respectively) and a higher prevalence of prior kidney stone events; however, comorbidities associated with stone risk (ie, hypertension, diabetes, gout) were not different between treatment groups. Among 4943 patients who were treated with parathyroidectomy and had postoperative laboratory testing 6 months after surgery, 4536 (91.8%) were cured of their PHPT.
Kidney Stone Events and Surrogate Markers of Stone Risk Among PHPT Patients With a History of Kidney Stones
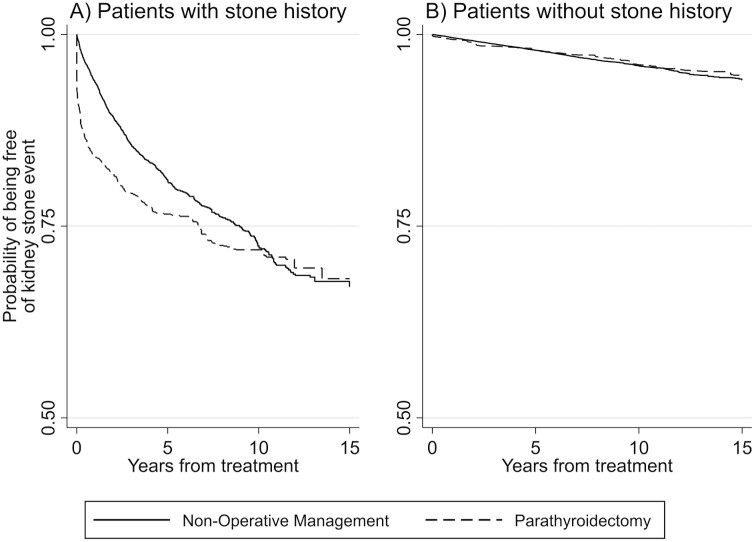
Over a mean follow-up time of 5.1 years, 1156 (20.5%) patients with a history of kidney stones experienced a recurrent stone event. The unadjusted cumulative incidence of ≥ 1 kidney stone event was 30.5% in patients managed with parathyroidectomy (mean follow-up, 5.6 years) compared with 18.0% in those managed nonoperatively (mean follow-up, 5.0 years). Figure 2A shows Kaplan-Meier curves indicating survival free from kidney stone events in IPW groups who underwent parathyroidectomy vs nonoperative management. Patients treated with parathyroidectomy had an average reduction in 24-hour urinary calcium of 105 mg, compared with an increase of 6.1 mg among those managed nonoperatively (P < 0.001, Table 2).
Figure 2.
Kaplan-Meier estimates of kidney stone events among inverse probability–weighted patients with PHPT treated with parathyroidectomy vs nonoperative management within 2 years, stratified according to baseline history of kidney stones.
Table 2.
Postoperative outcomes following parathyroidectomy vs nonoperative management, stratified by baseline history of kidney stones
| Patients with kidney stone history at PHPT diagnosis | Patients without kidney stone history at PHPT diagnosis | |||
|---|---|---|---|---|
| Parathyroidectomy within 2 y (n = 1154) | Nonoperatively managed (n = 4473) | Parathyroidectomy within 2 y (n = 4090) | Nonoperatively managed (n = 35 261) | |
| Mean preoperative serum calcium, mg/dL (SD) | 11.0 (0.6) | 10.7 (0.5) | 11.2 (0.8) | 10.7 (0.5) |
| Mean postoperative serum calcium, mg/dL (SD) | 9.4 (0.7) | 10.2 (0.7) | 9.4 (0.6) | 10.2(0.7) |
| Mean preoperative 24-h urinary calcium, mg (SD) | 303.4 (176.9) | 220.7 (142.4) | 297.9 (169.5) | 201.4 (135.6) |
| Mean postoperative 24-h urinary calcium, mg (SD) | 225.7 (143.5) | 227.0(140.5) | 212.0 (148.4) | 206.1 (138.0) |
| Mean change in 24-h urinary calcium after treatment, mg (SD)a | -105.0 (183.9) | 6.1 (140.5) | -104.5 (195.9) | 0.5 (142.9) |
| Mean change in serum calcium after treatment, mg/dL (SD) | -1.6 (0.9) | -0.6 (0.7) | -1.8 (0.9) | -0.5 (0.7) |
| Clinical setting of first kidney stone event, no. (%)b | ||||
| Emergency department visit | 107 (9.3%) | 352 (7.9%) | 90 (2.2%) | 384 (1.1%) |
| Inpatient stay | 177 (15.3%) | 300 (6.7%) | 68 (1.7%) | 335 (0.9%) |
| Stone-related procedure | 76 (6.6%) | 167 (3.7%) | 39 (0.9%) | 213 (0.6%) |
Abbreviations: PHPT, primary hyperparathyroidism.
a Mean change in 24-h urinary calcium calculated based on patients with both pre- and postoperative testing available.
b Clinical settings are not mutually exclusive for a given stone event.
After IPW adjustment using propensity scores, patients treated with parathyroidectomy had a higher adjusted rate of kidney stone events when compared with those managed nonoperatively (hazard ratio [HR], 1.98; 95% CI, 1.56-2.51); however, this association declined on average by 20% per year (parathyroidectomy × time HR, 0.80; 95% CI, 0.73-0.87)(Table 3). Patient characteristics associated with a greater adjusted rate of kidney stone events, included serum calcium ≥ 11.3 mg/dL at PHPT diagnosis (HR, 1.33; 95% CI, 1.11-1.59), history of a clinically significant kidney stone event (HR, 2.05; 95% CI, 1.68-2.48), and receipt of specialist care by a nephrologist or urologist (HR, 1.92; 95% CI, 1.53-2.41). Older age was associated with a lower adjusted rate of kidney stone events (HR age 65-74 years, 0.42 [95% CI, 0.25-0.70]; HR age ≥ 75 years, 0.33 [95% CI, 0.18-0.59] compared with patients younger than 35 years).
Table 3.
Patient characteristics associated with kidney stone events among patients with PHPT based on IPW-adjusteda multivariable Cox proportional hazards regression, stratified by baseline history of kidney stones
| Patients with kidney stone history (n = 5627) | Patients without kidney stone history (39 351) | |||
|---|---|---|---|---|
| Adjusted HR | 95% CI | Adjusted HR | 95% CI | |
| Parathyroidectomy | 1.98 | 1.56-2.51 | 1.14 | 0.76-1.71 |
| Parathyroidectomy time, y | 0.80 | 0.73-0.87 | 0.94 | 0.88-1.01 |
| Age, y | ||||
| < 35 | Referent | Referent | ||
| 35-49 | 0.84 | 0.51-1.40 | 0.88 | 0.53-1.48 |
| 50-64 | 0.68 | 0.42-1.10 | 0.97 | 0.59-1.61 |
| 65-74 | 0.42 | 0.25-0.70 | 0.59 | 0.35-0.99 |
| 75+ | 0.33 | 0.18-0.59 | 0.41 | 0.23-0.73 |
| Male | 0.99 | 0.77-1.28 | 1.47 | 1.04-2.07 |
| Race | ||||
| White | Referent | Referent | ||
| American Indian or Alaska Native | 1.15 | 0.53-2.48 | 0.95 | 0.41-2.19 |
| Asian | 1.06 | 0.36-3.16 | 1.04 | 0.40-2.70 |
| Black or African American | 0.98 | 0.77-1.26 | 0.78 | 0.56-1.08 |
| Native Hawaiian or Other Pacific Islander | 1.07 | 0.49-2.33 | 1.00 | 0.45-2.21 |
| Unknown | 0.93 | 0.57-1.53 | 0.66 | 0.47-0.93 |
| Ethnicity | ||||
| Not Hispanic or Latino | Referent | Referent | ||
| Hispanic or Latino | 1.13 | 0.82-1.57 | 0.93 | 0.63-1.36 |
| Unknown | 0.98 | 0.51-1.86 | 0.88 | 0.46-1.70 |
| Charlson Comorbidity Index | ||||
| 0 | Referent | Referent | ||
| 1 | 0.97 | 0.74-1.26 | 0.91 | 0.62-1.35 |
| 2+ | 0.97 | 0.76-1.22 | 0.90 | 0.67-1.22 |
| Calcium ≥ 11.3 mg/dL | 1.33 | 1.11-1.59 | 1.41 | 1.11-1.79 |
| Osteoporosis | 0.95 | 0.71-1.26 | 0.98 | 0.71-1.35 |
| eGFR < 60 mL/min/1.73 m2 | 1.01 | 0.82-1.26 | 0.88 | 0.69-1.12 |
| Diabetes | 0.95 | 0.67-1.36 | 0.72 | 0.48-1.08 |
| Hypertension | 0.88 | 0.70-1.10 | 0.99 | 0.73-1.34 |
| Gout | 0.87 | 0.61-1.24 | 0.96 | 0.58-1.61 |
| Urinary calcium, mg | ||||
| <400 | Referent | Referent | ||
| ≥ 400 | 0.86 | 0.67-1.11 | 1.09 | 0.79-1.51 |
| Missing | 0.93 | 0.78-1.11 | 1.01 | 0.77-1.31 |
| BMI | ||||
| Underweight | Referent | Referent | ||
| Normal weight | 1.85 | 0.63-5.47 | 1.13 | 0.47-2.72 |
| Overweight | 1.69 | 0.58-4.94 | 0.93 | 0.40-2.19 |
| Obese | 1.79 | 0.61-5.21 | 1.17 | 0.49-2.80 |
| Severely obese | 1.62 | 0.53-4.90 | 1.66 | 0.63-4.38 |
| Unknown | 1.68 | 0.44-6.50 | 1.33 | 0.48-3.68 |
| Stone history | ||||
| Diagnosis | Referent | - | ||
| Clinically significant event | 2.05 | 1.68-2.48 | - | |
| Nephrologist/urologist visit | 1.92 | 1.53-2.41 | 1.40 | 1.05-1.87 |
| Endocrinologist visit | 1.06 | 0.85-1.32 | 1.63 | 1.23-2.17 |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; HR, hazard ratio; IPW, inverse probability weighting; PHPT, primary hyperparathyroidism.
a The propensity score used for IPW in this table was based on all variables in this table (excluding interaction terms).
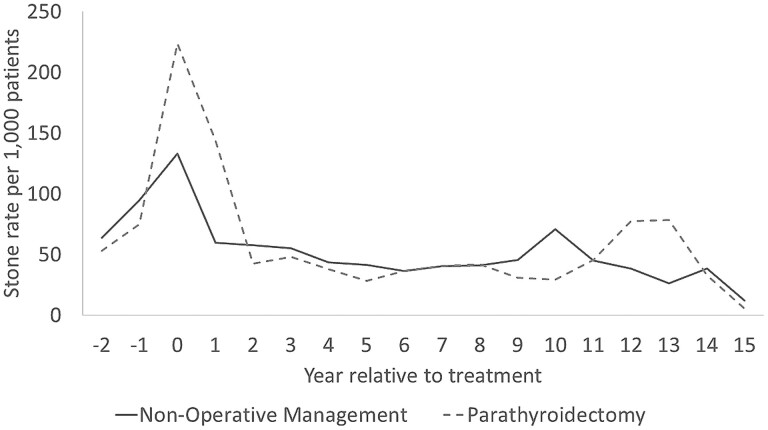
The IPW-adjusted yearly rate of stone events was higher in the year before and after treatment for patients managed with parathyroidectomy when compared with those managed nonoperatively, but this annual rate of stone events became similar with long-term follow-up (Fig. 3). Among the parathyroidectomy group, the rate of stone events per 1000 patients was 224 in the year before treatment, 144 in the year after treatment, and 28 five years posttreatment. Among the observation group, the rate of stone events per 1000 patients was 133 in the year before treatment, 60 in the year after treatment, and 41 five years posttreatment.
Figure 3.
Annual rate of kidney stone events before and after treatment among inverse probability–weighted (IPW) groups of patients with PHPT and a baseline history of kidney stones treated with parathyroidectomy within 2 years vs nonoperative management.a,b. aThe propensity score used for IPW in this table was based on sex, age group, race, ethnicity, Charlson Comorbidity Index, endocrinologist or nephrologist/urologist care within 6 months of PHPT diagnosis, operative indications for parathyroidectomy (history of kidney stones, osteoporosis, or stage 3 chronic kidney disease at the time of PHPT diagnosis), and risk factors for kidney stones (hypertension, diabetes, gout, body mass index). bRate of stone events at time 0 represents rate in year before treatment date.
Kidney Stone Events and Surrogate Markers of Stone Risk Among Patients Without a History of Kidney Stones
Among all 39 351 patients without a history of kidney stones at PHPT diagnosis, 1100 (2.8%) developed a kidney stone event on follow-up (mean follow-up time, 6.4 years). The unadjusted incidence of ≥ 1 kidney stone event was 4.7% in patients managed with parathyroidectomy (mean follow-up, 8.1 years) compared with 2.6% in those managed nonoperatively (mean follow-up, 6.2 years). Figure 2B shows Kaplan-Meier curves with stone-free survival in IPW-weighted groups who underwent parathyroidectomy vs nonoperative management. Patients treated with parathyroidectomy had an average reduction in 24-hour urinary calcium of 104.5 mg compared with an increase of 0.5 mg among those managed nonoperatively (P < 0.001).
After IPW adjustment using propensity scores for patients without a history of kidney stone disease, there was no difference in incident kidney stone events between patients who were treated with parathyroidectomy vs nonoperative management (HR, 1.14; 95% CI, 0.76-1.71), and this association did not change over time (HR parathyroidectomy × time 0.94; 95% CI, 0.88-1.01) (Table 3). The same patient characteristics had statistically significant associations with the adjusted rate of kidney stone events as in the analysis of patients with a history of stone events at PHPT diagnosis.
Sensitivity Analysis
The association between parathyroidectomy and stone events was similar in sensitivity analyses excluding 2327 patients who underwent delayed parathyroidectomy more than 2 years after PHPT diagnosis (Table 4).
Table 4.
Patient characteristics associated with kidney stone events among those with PHPT based on IPW-adjusteda multivariable Cox proportional hazards regression after excluding patients who underwent delayed parathyroidectomy (> 2 y after PHPT diagnosis)
| Patients with kidney stone history (n = 5315) | Patients without kidney stone history (n = 37 336) | |||
|---|---|---|---|---|
| Adjusted HR | 95% CI | Adjusted HR | 95% CI | |
| Parathyroidectomy | 2.14 | 1.67-2.74 | 1.31 | 0.87-1.98 |
| Parathyroidectomy time, y | 0.81 | 0.75-0.88 | 0.95 | 0.89-1.02 |
| Age, y | ||||
| < 35 | Referent | Referent | ||
| 35-49 | 0.77 | 0.45-1.30 | 0.76 | 0.43-1.34 |
| 50-64 | 0.64 | 0.39-1.06 | 0.94 | 0.54-1.63 |
| 65-74 | 0.42 | 0.25-0.70 | 0.56 | 0.31-1.01 |
| 75+ | 0.31 | 0.17-0.58 | 0.41 | 0.22-0.77 |
| Male | 0.96 | 0.73-1.26 | 1.46 | 1.00-2.11 |
| Race | ||||
| White | Referent | Referent | ||
| American Indian or Alaska Native | 1.37 | 0.63-2.98 | 0.94 | 0.39-2.26 |
| Asian | 1.24 | 0.39-3.95 | 0.94 | 0.32-2.80 |
| Black or African American | 1 | 0.77-1.29 | 0.79 | 0.55-1.13 |
| Native Hawaiian or Other Pacific Islander | 1.15 | 0.53-2.52 | 0.97 | 0.40-2.35 |
| Unknown | 1.01 | 0.60-1.69 | 0.71 | 0.50-1.03 |
| Ethnicity | ||||
| Not Hispanic or Latino | Referent | Referent | ||
| Hispanic or Latino | 1.11 | 0.79-1.56 | 0.99 | 0.66-1.48 |
| Unknown | 0.95 | 0.47-1.94 | 0.91 | 0.47-1.77 |
| Charlson Comorbidity Index | ||||
| 0 | Referent | Referent | ||
| 1 | 0.97 | 0.73-1.29 | 0.99 | 0.65-1.51 |
| 2+ | 0.97 | 0.75-1.25 | 0.97 | 0.69-1.35 |
| Calcium ≥ 11.3 mg/dL | 1.27 | 1.05-1.55 | 1.32 | 1.02-1.70 |
| Osteoporosis | 0.99 | 0.72-1.36 | 0.98 | 0.69-1.39 |
| eGFR < 60 mL/min/1.73 m2 | 0.99 | 0.78-1.24 | 0.89 | 0.68-1.15 |
| Diabetes | 0.97 | 0.68-1.40 | 0.72 | 0.47-1.10 |
| Hypertension | 0.9 | 0.70-1.15 | 1.05 | 0.75-1.47 |
| Gout | 0.87 | 0.60-1.24 | 1 | 0.57-1.75 |
| Urinary calcium, mg | ||||
| <400 | Referent | Referent | ||
| ≥ 400 | 0.81 | 0.61-1.06 | 1.08 | 0.76-1.54 |
| Missing | 0.9 | 0.75-1.09 | 0.98 | 0.73-1.30 |
| BMI | ||||
| Underweight | Referent | Referent | ||
| Normal weight | 1.64 | 0.55-4.88 | 1.14 | 0.45-2.88 |
| Overweight | 1.52 | 0.52-4.49 | 0.94 | 0.38-2.31 |
| Obese | 1.64 | 0.56-4.80 | 1.14 | 0.45-2.86 |
| Severely obese | 1.52 | 0.50-4.63 | 1.75 | 0.62-4.91 |
| Unknown | 1.65 | 0.44-6.20 | 1.39 | 0.47-4.08 |
| Stone history | ||||
| Diagnosis | Referent | |||
| Clinically significant event | 2.1 | 1.70-2.58 | ||
| Nephrologist/urologist visit | 1.97 | 1.55-2.50 | 1.4 | 1.03-1.89 |
| Endocrinologist visit | 1.07 | 0.85-1.36 | 1.7 | 1.25-2.33 |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; HR, hazard ratio; IPW, inverse probability weighting; PHPT, primary hyperparathyroidism.
a The propensity score used for IPW in this table was based on all variables in this table (excluding interaction terms).
Discussion
In this large observational cohort with biochemical diagnosis of PHPT, patients with a history of kidney stones had twice the adjusted hazard of a clinically significant recurrent kidney stone event after parathyroidectomy compared with patients managed nonoperatively. However, kidney stone events after parathyroidectomy waned over time. More severe hypercalcemia at PHPT diagnosis, a history of clinically significant kidney stone events, and receipt of specialty care by a nephrologist or urologist were all associated with a higher adjusted rate of recurrent stone events, whereas older age was associated with a significantly lower adjusted rate of stone events. There was no difference in the risk of new stone events according to treatment strategy among patients with PHPT without a history of kidney stones, suggesting that there is no significant benefit to parathyroidectomy for primary prevention of kidney stones. Taken together, these findings suggest parathyroidectomy mitigates the long-term risk of recurrent kidney stone disease, but that patients managed operatively remain at higher risk for stone recurrence than those managed nonoperatively, especially in the immediate years after surgery, likely because of a higher severity and burden of preexisting stone disease.
Our finding that parathyroidectomy was not associated with reduced rates of kidney stone events is consistent with prior studies. Vestergard et al found almost 2-fold higher odds of any kidney stone event among 3213 Danish patients treated with parathyroidectomy compared with nonoperative management after adjusting for age, sex, and baseline history of stone events (17). The authors attributed the higher risk of stone recurrence in surgically treated patients to more severe PHPT and higher preexisting stone risk. Our research group published a longitudinal cohort study of privately insured patients with PHPT and found that there was no difference in the odds of 5-year clinically significant stone events in patients treated with parathyroidectomy compared with nonoperative management after accounting for patient demographics, prior stone events, and relevant comorbidities (8). In addition, Huang et al recently showed that among a diverse population of 1252 patients with PHPT, approximately 1 in 3 stone formers with PHPT who were managed with parathyroidectomy had a kidney stone recurrence; despite a reduction in the rate of stone events after treatment, there remained a higher risk of recurrence among those managed with surgery compared with those managed nonoperatively (HR, 1.89; 95% CI, 1.44-2.47) (7). We suggest that it is unlikely that parathyroidectomy increases the rate of kidney stone events because many studies, including this one, have demonstrated a reduction in surrogates of stone risk, such as urinary calcium excretion, after parathyroidectomy (4). In fact, our data show that patients who receive parathyroidectomy experienced a reduction in the rate of stone formation with each year after surgery. Taken together, the data suggest that the risk of stone recurrence is high in patients with PHPT managed with parathyroidectomy, and this risk is mitigated but still high after operative management. As a result, patients with PHPT and a history of kidney stones may benefit from referral to a nephrologist or urologist to discuss diet modification and pharmacologic therapy to prevent recurrent kidney stones, regardless of whether they undergo parathyroidectomy.
The high rate of early kidney stone events following parathyroidectomy may be due to a large preexisting stone burden in patients treated with parathyroidectomy, who we found to have more severe kidney stone disease at PHPT diagnosis and worse PHPT biochemical profiles than those managed nonoperatively. In addition, given specialty care by a urologist or nephrologist is associated with a higher likelihood that PHPT is managed with parathyroidectomy (18), the increased adjusted rate of recurrent clinically significant stone events in operatively managed patients may be due to increased detection or proactive intervention for kidney stones in a population that is more connected to the health care system. Future studies accounting for stone burden at the time of PHPT diagnosis and indications for procedural stone interventions would improve our ability to assess the comparative effectiveness of parathyroidectomy to reduce morbidity related to kidney stones and tailor strategies to prevent stone-related complications in this population.
Our findings have implications for the management of older adults with PHPT and kidney stone disease. Current guidelines recommend all patients with symptomatic or asymptomatic kidney stones be treated with parathyroidectomy (3). However, our data suggest the benefits of operative management for this indication (ie, a reduction in the rate of stone formation) will take years to be realized and should be weighed against the operative risks and life expectancy for an individual patient. Given that older adults tend to have a lower incidence of kidney stone formation than younger patients (19), the benefits of parathyroidectomy related to stone disease are likely to be lower in this group overall. In addition, when parathyroidectomy is performed in stone formers, patients should concurrently receive treatment for secondary prevention of kidney stones with diet modification and pharmacologic interventions, because the risk of recurrent stones is highest in the first 2 years after surgery. One consideration for parathyroidectomy in patients with PHPT and recurrent kidney stones is that parathyroidectomy may allow prescription of thiazide diuretics to reduce urine calcium excretion and stone risk. Without parathyroidectomy, many patients with PHPT develop hypercalcemia when treated with thiazide diuretics; after parathyroidectomy, patients are often able to tolerate thiazide diuretics and realize the benefits of these medications, including a decrease in stone risk and an increase in bone mineral density. Given there are other documented benefits of parathyroidectomy for the treatment of PHPT related to fracture risk reduction and quality of life (20, 21), these data related to the mitigation of kidney stone risk should be incorporated into patient counseling to contribute to individualized treatment decisions. It is important that clinicians and patients are informed about the expected benefits of surgical cure for PHPT and the time horizon to benefit.
The strengths of our study include the availability of granular clinical information and laboratory data in a large, national cohort of patients newly diagnosed with PHPT and accounting for the severity of hypercalcemia and hypercalciuria in our analyses. Our study has limitations related to its observational nature and the use of administrative claim codes to identify kidney stone events and comorbid conditions relevant to stone risk. As discussed previously, the absence of granular data about stone risk factors may result in residual confounding of the association between parathyroidectomy and stone events. It is also important to note that our cohort of patients who receive care within the VA health care system was 88% male, which is not representative of the predominately female population with PHPT, and VA patients in general have a higher rate of comorbidity than nonveteran populations (22). Although our multivariable analyses adjusted for sex and comorbidity, it is possible that the association of parathyroidectomy with kidney stone events would be different in a nonveteran cohort with a larger proportion of women. Our analysis did not account for kidney stones that form but are asymptomatic, which are included in operative guidelines for PHPT. In addition, we chose to focus on kidney stone events that were clinically significant based on the requirement for a procedure or emergency department/inpatient admission, which does not account for the patient discomfort or inconvenience associated with kidney stones that are symptomatic but managed in an outpatient setting. A larger benefit of parathyroidectomy may have been seen if kidney stone events not requiring a procedure or hospitalization were considered. Last, our inclusion of patients who underwent delayed parathyroidectomy in the nonoperatively managed group was meant to mitigate treatment selection bias and mimic an intention-to-treat analysis but may have also reduced the observed treatment benefit of parathyroidectomy.
Conclusion
In this longitudinal study, patients with PHPT and a history of kidney stone disease who were treated with parathyroidectomy continued to be at higher risk of kidney stone events after surgery compared to patients managed nonoperatively. However, the adjusted rate of stone events declined with time after surgery. Despite current guideline recommendations, our results suggest that any long-term benefits of parathyroidectomy related to kidney stone formation should be weighed against the risks of surgery and life expectancy for an individual patient, especially among older adults who have a lower risk of recurrent stones.
Acknowledgments
C.D.S. and K.D.A. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Glossary
Abbreviations
- CKD
chronic kidney disease
- eGFR
estimated glomerular filtration rate
- HR
hazard ratio
- ICD
International Classification of Diseases
- IPW
inverse probability weighting
- PHPT
primary hyperparathyroidism
- VA
Veterans Administration
Contributor Information
Carolyn D Seib, Stanford–Surgery Policy Improvement Research and Education Center, Department of Surgery, Stanford University School of Medicine, Palo Alto, CA 94304, USA; Department of Surgery, Stanford University School of Medicine, Palo Alto, CA 94305, USA; Division of General Surgery, Palo Alto Veterans Affairs Health Care System, Palo Alto, CA 94304, USA; Geriatric Research, Education and Clinical Center, Veterans Affairs Palo Alto, Palo Alto, CA 94304, USA.
Calyani Ganesan, Division of Nephrology, Stanford University School of Medicine, Palo Alto, CA 94305, USA.
Katherine D Arnow, Stanford–Surgery Policy Improvement Research and Education Center, Department of Surgery, Stanford University School of Medicine, Palo Alto, CA 94304, USA.
Alan C Pao, Division of Nephrology, Stanford University School of Medicine, Palo Alto, CA 94305, USA; Department of Urology, Stanford University School of Medicine, Palo Alto, CA 94305, USA.
John T Leppert, Division of Nephrology, Stanford University School of Medicine, Palo Alto, CA 94305, USA; Department of Urology, Stanford University School of Medicine, Palo Alto, CA 94305, USA.
Nicolas B Barreto, Stanford–Surgery Policy Improvement Research and Education Center, Department of Surgery, Stanford University School of Medicine, Palo Alto, CA 94304, USA.
Electron Kebebew, Department of Surgery, Stanford University School of Medicine, Palo Alto, CA 94305, USA.
Manjula Kurella Tamura, Geriatric Research, Education and Clinical Center, Veterans Affairs Palo Alto, Palo Alto, CA 94304, USA; Division of Nephrology, Stanford University School of Medicine, Palo Alto, CA 94305, USA.
Funding
The authors acknowledge funding support from the National Institutes of Health, National Institute on Aging by awards R03AG060097 and K76AG068526 (C.D.S.), a Department of Veterans Affairs Health Services Research and Development Service Locally Initiated Project LIP 21-CS-1 (C.D.S.), and a Veterans Affairs Merit Grant I01HX003091 (C.G., A.C.P., J.T.L.). Funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. The contents do not represent the views of the US Department of Veterans Affairs or the US Government.
Disclosures
The authors have no conflicts of interest relevant to this project. C.D.S reported prior consulting for Virtual Incision Corporation. No other disclosures were reported.
Data Availability
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will, on request, detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Rule AD, Bergstralh EJ, Melton LJ, Li X, Weaver AL, Lieske JC. Kidney stones and the risk for chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(4):804-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bryant M, Angell J, Tu H, Goodman M, Pattaras J, Ogan K. Health related quality of life for stone formers. J. Urol., Balt. 2012;188(2):436-440. [DOI] [PubMed] [Google Scholar]
- 3. Bilezikian JP, Brandi ML, Eastell R, et al. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the fourth international workshop. J Clin Endocrinol Metab. 2014;99(10):3561-3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shariq OA, Strajina V, Lyden ML, et al. Parathyroidectomy improves hypercalciuria in patients with primary hyperparathyroidism. Surgery 2020;168(4):594-600. [DOI] [PubMed] [Google Scholar]
- 5. Mollerup CL, Vestergaard P, Frøkjær VG, Mosekilde L, Christiansen P, Blichert-Toft M. Risk of renal stone events in primary hyperparathyroidism before and after parathyroid surgery: controlled retrospective follow up study. BMJ 2002;325(7368):807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parks JH, Coe FL, Evan AP, Worcester EM. Clinical and laboratory characteristics of calcium stone-formers with and without primary hyperparathyroidism. BJU international 2009;103(5):670-678. [DOI] [PubMed] [Google Scholar]
- 7. Huang S-Y, Burchette R, Chung J, Haigh PI. Parathyroidectomy for nephrolithiasis in primary hyperparathyroidism: Beneficial but not a panacea. Surgery 2022;171(1):29-34. [DOI] [PubMed] [Google Scholar]
- 8. Seib CD, Ganesan C, Arnow KD, et al. Association of parathyroidectomy with 5-year clinically significant kidney stone events in patients with primary hyperparathyroidism. Endocr Pract. 2021;27(9):948-955. [DOI] [PubMed] [Google Scholar]
- 9. Seib CD, Meng T, Suh I, et al. Undertreatment of primary hyperparathyroidism in a privately insured US population: decreasing utilization of parathyroidectomy despite expanding surgical guidelines. Surgery 2021;169(1):87-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. FitzHenry F, Brannen J, Denton JN, et al. Transforming the National Department of Veterans Affairs Data Warehouse to the OMOP common data model. Paper presented at: American Medical Informatics Association Annual Meeting 2015. [Google Scholar]
- 11. Voss EA, Makadia R, Matcho A, et al. Feasibility and utility of applications of the common data model to multiple, disparate observational health databases. J Am Med Inform Assoc 2015;22(3):553-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335(7624):806-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. [DOI] [PubMed] [Google Scholar]
- 15. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. twang. Toolkit for Weighting and Analysis of Nonequivalent Groups [computer program]. Version R package version 1.62020. [Google Scholar]
- 17. Vestergaard P, Mosekilde L. Cohort study on effects of parathyroid surgery on multiple outcomes in primary hyperparathyroidism. BMJ 2003;327(7414):530-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ganesan C, Weia B, Thomas IC, et al. Analysis of primary hyperparathyroidism screening among US Veterans with kidney stones. JAMA Surgery 2020;155(9):861-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Romero V, Akpinar H, Assimos DG. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol 2010;12(2-3):e86-e96. [PMC free article] [PubMed] [Google Scholar]
- 20. Yeh MW, Zhou H, Adams AL, et al. The relationship of parathyroidectomy and bisphosphonates with fracture risk in primary hyperparathyroidism: an observational study. Ann Intern Med 2016;164(11):715-723. [DOI] [PubMed] [Google Scholar]
- 21. Ambrogini E, Cetani F, Cianferotti L, et al. Surgery or surveillance for mild asymptomatic primary hyperparathyroidism: a prospective, randomized clinical trial. J Clin Endocrinol 2007;92(8):3114-3121. [DOI] [PubMed] [Google Scholar]
- 22. Kazis LE, Ren XS, Lee A, et al. Health status in VA patients: results from the Veterans Health Study. Am J Med Qual 1999;14(1):28-38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will, on request, detail the restrictions and any conditions under which access to some data may be provided.