Abstract
Purpose
The objectives of the ongoing, Phase 3, open-label extension trial enliGHten are to assess the long-term safety and efficacy of weekly administered long-acting growth hormone lonapegsomatropin in children with growth hormone deficiency.
Methods
Eligible subjects completing a prior Phase 3 lonapegsomatropin parent trial (heiGHt or fliGHt) were invited to participate. All subjects were treated with lonapegsomatropin. Subjects in the United States switched to the TransCon hGH Auto-Injector when available. Endpoints were long-term safety, annualized height velocity, pharmacodynamics [insulin-like growth factor-1 SD score (SDS) values], and patient- and caregiver-reported assessments of convenience and tolerability.
Results
Lonapegsomatropin treatment during enliGHten was associated with continued improvements in height SDS through week 104 in treatment-naïve subjects from the heiGHt trial (−2.89 to −1.37 for the lonapegsomatropin group; −3.0 to −1.52 for the daily somatropin group). Height SDS also continued to improve among switch subjects from the fliGHt trial (−1.42 at fliGHt baseline to −0.69 at week 78). After 104 weeks, the average bone age/chronological age ratio for each treatment group was 0.8 (0.1), showing only minimal advancement of bone age relative to chronological age with continued lonapegsomatropin treatment among heiGHt subjects. Fewer local tolerability reactions were reported with the TransCon hGH Auto-Injector compared with syringe/needle.
Conclusions
Treatment with lonapegsomatropin continued to be safe and well-tolerated, with no new safety signals identified. Children treated with once-weekly lonapegsomatropin showed continued improvement of height SDS through the second year of therapy without excess advancement of bone age.
Keywords: growth hormone, growth hormone deficiency, growth hormone replacement therapy, long-acting growth hormone, TransCon hGH, lonapegsomatropin
Growth hormone deficiency (GHD), characterized by insufficient levels of GH to sustain normal growth and metabolism, has been treated for decades with recombinant human GH (rhGH) (1). For more than 35 years after its advent, rhGH therapy for children with GHD had remained available only as a daily injection formulation. The necessary regimen of daily injections, which may be painful as well as stressful and cumbersome for children and caregivers, is a probable cause of nonadherence and decreased compliance, both of which have been linked to suboptimal height outcomes (2, 3). Up to 77% of adolescents with GHD may be noncompliant with daily injections (4). There is an unmet need for a less burdensome GH therapeutic that offers a combination of efficacy and safety comparable to daily-administered hGH.
Long-acting GH (LAGH) analogs have been under development to allow for reduced injection frequency; these therapies can potentially increase compliance and persistence by improving the convenience of hGH therapy. Lonapegsomatropin is the first approved commercially available, weekly LAGH preparation in the United States for children with GHD (5). Elsewhere, 3 LAGH therapeutics have been approved for use in children: 1 in South Korea, 1 in China, and 1 approved in Canada, Australia, Japan and the EU (6-8). However, LAGHs for children are still under evaluation to determine whether they have equivalent efficacy and safety outcomes compared to daily therapies over long-term treatment.
Lonapegsomatropin (TransCon hGH; Skytrofa™) is a long-acting prodrug approved for treatment of pediatric patients ≥1 year, weighing ≥11.5 kg, and who have growth failure due to inadequate secretion of endogenous GH. It consists of 3 components: unmodified somatropin (hGH), an inert methoxypolyethylene glycol carrier, and a TransCon linker that transiently binds the other 2 components (9). The inert methoxypolyethylene glycol acts as a carrier, extending hGH circulation time in the body through a shielding effect that minimizes GH receptor binding and renal excretion. At physiologic pH and temperature, lonapegsomatropin releases fully active, unmodified hGH via autocleavage of the TransCon linker in a controlled manner that follows first-order kinetics (10, 11). The released hGH is designed to maintain the same mode of action as daily-administered hGH, with the same weekly exposure as 7 daily injections of hGH, by allowing the sustained release of unmodified rhGH. Somatropin released from lonapegsomatropin and resultant insulin-like growth factor 1 (IGF-1) levels return to baseline levels by 5 and 7 days after injection, respectively, ensuring that there is no accumulation of somatropin or IGF-1 from week to week (12)
The primary objective of the open-label extensions trial (enliGHten) was to assess the long-term safety of weekly lonapegsomatropin in children with GHD previously treated in the Phase 3 lonapegsomatropin trials (either heiGHt or fliGHt). The secondary objectives included efficacy, assessing IGF-1 SD score (SDS), patient-reported outcomes, and immunogenicity. The following is a report of subjects who continued into the Phase 3 enliGHten open-label extension trial from heiGHt or fliGHt, with outcomes reported for up to 2 years of treatment with lonapegsomatropin from the start of the enliGHten trial.
Methods
Study Oversight
The study protocol was approved by the institutional review board of each participating site, and informed consent of the parent or legal guardian of the subject (and written assent of the subject) was required for inclusion into the trial. An independent safety committee provides trial oversight.
Trial Design
The enliGHten trial is an ongoing multicenter, Phase 3, open-label, long-term extension trial of weekly lonapegsomatropin administration in children with GHD who previously participated in a Phase 3 lonapegsomatropin trial (heiGHt or fliGHt). This trial is being conducted at 63 international clinical sites that specialize in the management of pediatric GHD.
Trial Subjects
All subjects completing a prior Phase 3 lonapegsomatropin parent trial (either heiGHt or fliGHt), who did not permanently discontinue trial medication, were without evidence of closed epiphyses (defined as bone age >14.0 years for females or >16.0 years for males), and met all other entry criteria were invited to participate in the long-term extension trial. Key exclusion criteria included poorly controlled diabetes mellitus or diabetic complications and pregnancy.
heiGHt was a 52-week, open-label, active-controlled, pivotal Phase 3 trial in which treatment-naive, prepubertal subjects (males 3-12 years old; females 311 years old) with GHD were randomized 2:1 to receive once-weekly lonapegsomatropin 0.24 mg hGH/kg/week via vial/syringe or an equivalent weekly dose of daily somatropin via vial/syringe (9).
fliGHt was a 26-week, open-label Phase 3 trial in which treatment-experienced subjects (6 months-17 years old; subjects <3 years old could be treatment-naive) with GHD switched from their previous daily somatropin to lonapegsomatropin 0.24 mg hGH/kg/week via vial/syringe.
After informed consent was provided, subjects entered the long-term extension trial and began or continued lonapegsomatropin (depending on trial medication from parent trial heiGHt or fliGHt). To continue uninterrupted treatment with GH, Day 1 (the first weekly dose) of lonapegsomatropin occurred on the day of Visit 1 in the long-term extension, or as soon as possible thereafter.
Interventions
All subjects who enrolled into the long-term extension trial received lonapegsomatropin at their previous dose via vial/syringe. Daily somatropin subjects from heiGHt started lonapegsomatropin at 0.24 mg hGH/kg/week. Lonapegsomatropin was provided in single-use vials and administered with syringe and needle, initially at a concentration after reconstitution of 11 ng/mL hGH and subsequently at either 11 ng/mL hGH or 22 ng/mL hGH.
When the TransCon hGH Auto-Injector became available in the enliGHten trial (in the United States only), lonapegsomatropin was also supplied in dual-chamber cartridges for administration. The cartridges each delivered single fixed doses of lonapegsomatropin. Dosing and dose adjustments were made based on bracketed dosing, choosing among cartridges delivering lonapegsomatropin doses varying in 20% increments. The available cartridges delivered 3, 3.6, 4.3, 5.2, 6.3, 7.6, 9.1, 11, or 13.3 mg hGH. When administered using vials, lonapegsomatropin doses were chosen to match those of the cartridges.
The lonapegsomatropin dose (in mg) was adjusted according to the subject’s weight at each study visit. Additionally, the lonapegsomatropin dose could be adjusted according to the IGF-1 SDS measured at each visit. The goal range for IGF-1 SDS was 0 to +2. Thus, if the IGF-1 SDS measured at a visit was <0 or >2, the dose may have been increased or decreased, respectively, by approximately 20% to the dose of the next higher or lower cartridge dose strength.
A designation of subject completion reflected that (based on investigator judgment) the subject had reached satisfactory height, and it was no longer necessary for the subject to continue in the trial and receive treatment for childhood GHD. Additionally, trial completion was required when there was evidence of closed epiphyses (bone age >14.0 years for females and >16.0 years for males).
Endpoints and Assessments
Safety
The primary endpoint of interest was long-term safety of weekly lonapegsomatropin in children with GHD previously treated in a Phase 3 lonapegsomatropin trial. Safety assessments included adverse events (AEs), physical examinations, chemistry and hematology parameters, hormone levels (including thyroid status and morning cortisol), parameters of glucose and lipid metabolism, immunogenicity assessments, fundoscopy, pubertal status, bone age, and vital sign measurements. Incidence of antibodies against lonapegsomatropin anti-hGH was evaluated.
Efficacy
Efficacy assessments included the endpoints of annualized height velocity (AHV) and height SDS. Growth outcomes were evaluated approximately every 13 weeks, and 3 groups were analyzed: (1) treatment-naive subjects treated with lonapegsomatropin in heiGHt, followed by continuation of lonapegsomatropin in enliGHten; (2) treatment-naive subjects treated with daily somatropin in heiGHt, followed by lonapegsomatropin in enliGHten; and (3) subjects previously treated with daily somatropin, who switched to lonapegsomatropin in fliGHt, followed by continuation of lonapegsomatropin in enliGHten
Pharmacodynamics
The proportion of subjects with average IGF-1 SDS of 0 to +2.0 was evaluated as a secondary endpoint. Serum IGF-1 was obtained on postdose day 5 (±1) in fliGHt and enliGHten, corresponding to weekly average levels; in the parent trial heiGHt, weekly average IGF-1 values for lonapegsomatropin were estimated based on a population pharmacodynamic (PD) model.
Patient-reported outcome assessments
Patient-reported outcomes for use of the TransCon hGH Auto-Injector were assessed through the Treatment Satisfaction Questionnaire for Medication-Version 9 (TSQM-9) (13) and the Device Usability Questionnaire (DUQ). The convenience and overall satisfaction domains of the TSQM-9 were assessed among caregivers of lonapegsomatropin-treated children who transitioned from vial/syringe administration to the TransCon hGH Auto-Injector device. Additionally, the DUQ was administered to assess the TransCon hGH Auto-Injector comfort, ease-of-use, and safety among the 160 subjects who switched from vial/syringe administration. It consisted of 8 statements that the caregiver or subject was asked to rank on a scale indicating level of agreement. The scale used for the questions was strongly agree, somewhat agree, neither agree nor disagree, somewhat disagree, and strongly disagree.
Tolerability assessments
Local tolerability was defined based on whether an injection site reaction was deemed abnormal from those ordinarily observed in subcutaneous injections (including pain, intensity, or duration). Between visits, local tolerability was evaluated and documented by the subject or caregiver in the subject diary. At clinic visits, assessment of local tolerability was performed by injection site examination by trial staff (documented as part of the physical exam), in conjunction with subject diary review.
Statistical Analyses
Baseline and demographic data were summarized to characterize the study population. Data from clinical assessments were summarized using descriptive statistics. Numerical variables were summarized by mean, median, SD, minimum, and maximum, while categorical variables are summarized by counts and proportions.
For most endpoints, the extension trial baseline values were utilized for the analyses except for antibodies, which were analyzed for lonapegsomatropin treatment across parent trials and long-term extension. Safety was evaluated throughout the trial periods and was summarized by parent trial and long-term extension. For immunogenicity analysis, the antibody status before the first dose of lonapegsomatropin was considered as the baseline.
For AHV, a rolling baseline was used to ensure there was a 1-year span in the calculation. Calculation of AHV and Δ height SDS were based on predetermined rules, outlined a priori. Comparisons between the 2 heiGHt treatment groups allowed for the evaluation of safety and efficacy outcomes as they had similar baseline demographics and comparable treatment histories. A by-visit analysis of covariance (ANCOVA) model was applied on AHV and Δ height SDS for heiGHt subjects, which included the heiGHt treatment group (lonapegsomatropin vs daily somatropin) as a factor. In addition, the AHV model also included baseline age, peak stimulated GH levels (log-transformed), and Δ average parental height SDS (baseline height SDS − average parental height SDS) as covariates, and sex as a factor; the Δ height SDS model included baseline age, peak stimulated GH levels (log-transformed), and baseline height SDS as covariates and sex as a factor. AHV and Δ height SDS for fliGHt subjects were summarized by descriptive statistics.
For PD post-baseline assessments, the frequency and percentages of subjects at each visit by each group and overall were presented, and the absolute values and change from baseline at each visit for IGF-1 and IGF-1 SDS were presented with descriptive statistics.
A by-visit ANCOVA model was applied on average IGF-1 SDS for heiGHt subjects, which included the heiGHt treatment group (lonapegsomatropin vs daily somatropin) as a factor. The average IGF-1 SDS values during the heiGHt period were derived from a population PD model for lonapegsomatropin group since IGF-1 was sampled at peak or trough levels throughout the trial; the average IGF-1 SDS values for the daily somatropin group were represented by observed values since samples drawn at any time can reflect the average IGF-1 levels. During the enliGHten period, samples were collected around the average IGF-1 time, and the observed values were used to represent average IGF-1 levels. The ANCOVA model also included baseline age, peak stimulated GH levels (log-transformed) at diagnosis, and baseline IGF-1 SDS as covariates and sex as a factor. For fliGHt subjects, observed IGF-1 values were used to represent average IGF-1 levels and were summarized by descriptive statistics.
For patient-reported outcomes, descriptive analyses were conducted for the TSQM-9 and DUQ.
Analyses were conducted using SAS version 9.4.
Results
Nearly all subjects who completed the heiGHt (158/159) and fliGHt (140/144) parent trials continued into the enliGHten long-term extension trial (Table 1). Overall, subject retention has remained high with 92.2% of parent trial subjects currently active in enliGHten.
Table 1.
Subject disposition
| heiGHt | fliGHt | Total | ||
|---|---|---|---|---|
| Lonapegsomatropin, n (%) | Daily somatropin, n (%) | Lonapegsomatropin, n (%) | ||
| Enrolled and dosed in parent trial | 105 | 56 | 146 | 307 |
| Completed parent trial | 104 (99.0) | 55 (98.2) | 144 (98.6) | 303 (98.7) |
| Enrolled and dosed in enliGHtena | 103 (98.1) | 55 (98.2) | 140 (95.9) | 298 (97.0) |
| Active subjectsa | 100 (97.1) | 54 (98.2) | 129 (92.1) | 283 (95.0) |
| Withdrew from enliGHtenb | 3 (2.9) | 1 (1.8) | 4 (2.9) | 8 (2.7) |
| Completed enliGHtenb,c | 0 | 0 | 7 (5.0) | 7 (2.3) |
aDenominator based on subjects who were enrolled and dosed in parent trial.
bDenominator based on subjects enrolled and dosed in the enliGHten trial.
cA designation of enliGHten trial “completer” reflects that, based on investigator judgement, these subjects have reached satisfactory height and that it is no longer necessary for the subject to continue in the trial and receive treatment with pediatric doses of growth hormone therapy. Trial completion was required when there is evidence of closed epiphyses (bone age >14.0 years for females and >16.0 years for males).
Demographics and Baseline Characteristics
Most subjects enrolled into the enliGHten trial were male [n = 235 (78.9%)], White [n = 270 (90.6%)], and from the United States [n = 174 (58.4%)]. The mean (SD) age of subjects was 10.3 (3.4) years (range: 1.7-17.8 years) with a mean bone age of 8.4 years (range: 1.5-14.5 years) at the start of the enliGHten trial. Most subjects [n = 214 (71.8%)] were assessed as Tanner stage 1. At the start of the enliGHten trial, all subjects had been previously treated with daily somatropin, lonapegsomatropin, or a combination of daily somatropin and lonapegsomatropin. Mean (SD) for height SDS was−1.6 (0.9). The overall trial baseline value for IGF-1 SDS was 0.97 (1.3; including subjects who were treated with daily somatropin in the heiGHt trial). Upon entry into the enliGHten trial, subjects from the fliGHt trial were generally older and more advanced in Tanner stage compared to those entering from the heiGHt trial (Table 2).
Table 2.
Demographics and baseline characteristics at start of the enliGHten trial
| Subjects treated with lonapegsomatropin during the heiGHt trial (n = 103) | Subjects treated with daily somatropin during the heiGHt trial (n = 55) | Subjects treated with lonapegsomatropin during the fliGHt trial (n = 140) | Total (n = 298) | |
|---|---|---|---|---|
| Male, n (%) | 84 (81.6) | 45 (81.8) | 106 (75.7) | 235 (78.9) |
| Age | ||||
| Mean age, years (SD) | 9.5 (2.7) | 9.5 (2.8) | 11.1 (3.9) | 10.3 (3.4) |
| Min, max | 4.4, 14.1 | 4.2, 13.9 | 1.7, 17.8 | 1.7, 17.8 |
| Race, n (%) | ||||
| Asian | 1 (1.0) | 0 (0) | 5 (3.6) | 6 (2.0) |
| Black or African American | 2 (1.9) | 0 (0) | 3 (2.1) | 5 (1.7) |
| Native Hawaiian or other Pacific Islander | 0 (0) | 0 (0) | 2 (1.4) | 2 (0.7) |
| White | 98 (95.1) | 52 (94.5) | 120 (85.7) | 270 (90.6) |
| Multiple/other | 2 (1.9) | 3 (5.5) | 2 (1.4) | 7 (2.3) |
| Unknown | 0 (0) | 0 (0) | 8 (5.7) | 8 (2.7) |
| Country | ||||
| United States | 27 (26.2) | 14 (25.5) | 133 (95.0) | 174 (58.4) |
| Outside of United States | 76 (73.8) | 41 (74.5) | 7 (5.0) | 124 (41.6) |
| Height SDS, mean (SD) | −1.9 (0.7) | −2.1 (0.8) | −1.1 (0.8) | −1.6 (0.9) |
| BMI SDS, mean (SD) | −0.04 (0.9) | −0.41 (1.0) | 0.12 (1.0) | −0.04 (1.0) |
| Average IGF-1 SDSa, mean (SD) | 0.6 (0.9) | −0.05 (1.2) | 1.6 (1.3) | 0.97 (1.3) |
| IGF-1,a ng/mL, mean (SD) | 263.8 (92.0) | 222.6 (115.6) | 410.0 (188.4) | 324.9 (169.0) |
| Tanner stage, n (%) | ||||
| Stage I | 92 (89.3) | 45 (81.8) | 77 (55.0) | 214 (71.8) |
| Stage II | 11 (10.7) | 8 (14.5) | 21 (15.0) | 40 (13.4) |
| Stage III | 0 | 2 (3.6) | 22 (15.7) | 24 (8.1) |
| Stage IV | 0 | 0 | 17 (12.1) | 17 (5.7) |
| Stage V | 0 | 0 | 3 (2.1) | 3 (1.0) |
| Bone age/chronological age ratio, mean (SD) | 0.75 (0.15) | 0.75 (0.14) | 0.87 (0.12) | 0.81 (0.15) |
| Peak stimulated GH prior to hGH therapy, ng/mL, mean (SD) | 5.9 (2.8) | 5.5 (3.0) | 5.9 (2.5) | 5.8 (2.7) |
Abbreviations: BMI, body mass index; GH, growth hormone; hGH, human growth hormone; IGF-1, insulin-like growth factor-1; SDS, SD score.
aIGF-1 values for the heiGHt lonapegsomatropin group were model-derived and represented the average IGF-1 level at the end of the heiGHt trial; IGF-1 samples for the heiGHt trial daily somatropin group were collected at approximately 12 hours postdose of injection at the end of the heiGHt trial; IGF-1 samples for the fliGHt group were collected approximately 96 to 144 hours postdose of lonapegsomatropin injection and represent average weekly levels.
All heiGHt subjects were Tanner stage 1 at heiGHt trial baseline. At week 104, 116 (75.8%) remained in Tanner stage 1, while 18 (11.8%) were Tanner stage 2, 15 (9.8%) were Tanner stage 3, and 4 (2.6%) were Tanner stage 4.
At fliGHt trial baseline, 95 (65.1%) were Tanner stage 1, 14 (9.6%) were Tanner stage 2, 30 (20.5%) were Tanner stage 3, and 7 (4.8%) were Tanner stage 4. At week 78, 55 (42.6%) were Tanner stage 1, 18 (14.0%) were Tanner stage 2, 18 (14.0%) were Tanner stage 3, 26 (20.2%) were Tanner stage 4, and 12 (9.3%) were Tanner stage 5.
Lonapegsomatropin Exposure and Dosing
Overall, the mean (SD) duration of lonapegsomatropin treatment was 1.4 (0.4) years in enliGHten, with a maximum of 2.3 years. As specified by the parent trial (heiGHt and fliGHt) protocols, the nominal starting dose was 0.24 mg hGH/kg/week (regardless of prior hGH therapy). If children were on a reduced dose from the fliGHt trial, this dose was continued into the enliGHten trial. At the time of report, the most recent mean (SD) weekly dose was 0.22 (0.04) mg hGH/kg/week, suggesting that most subjects maintained the planned dosage during the long-term extension trial. Dose reductions occurred in 89 subjects (29.9%). Thirty subjects (10.1%) had their doses increased during the trial. Analyses of lonapegsomatropin dose and IGF-1 response have shown that, on average, changing to the next higher dose increased IGF-1 SDS by approximately 0.3 SDS. Likewise, on average, changing to the next lower dose decreased IGF-1 SDS by approximately 0.3 SDS.
Height Outcomes
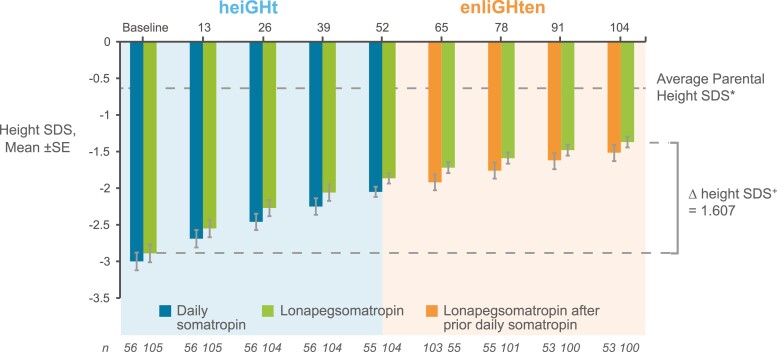
Subjects from heiGHt who started lonapegsomatropin in the parent trial and continued to approach their average parental height SDS (average of mother’s and father’s height SDS), with height SDS improving from −2.89 at heiGHt trial baseline to −1.37 at week 104. Subjects who were on daily somatropin during heiGHt and switched to lonapegsomatropin also continued to approach their average parental height, with height SDS improving from −3.0 at heiGHt trial baseline to −1.52 at week 104 (Fig. 1). The treatment difference in least squares mean ∆ height SDS (lonapegsomatropin vs daily somatropin) at the end of heiGHt (week 52) was 1.10 vs 0.96 (P = 0.015). At week 104, the treatment difference was no longer statistically significant (P = 0.158). The change in observed mean height SDS from baseline to week 104 was 1.52 in the group that continued lonapegsomatropin and 1.48 in the group that switched from daily somatropin to lonapegsomatropin (data not shown).
Figure 1.
Sustained improvement in height SD score (SDS) for heiGHt subjects. Changes in height SDS over 104 weeks in treatment-naïve subjects who enrolled in the heiGHt trial and continued into the enliGHten trial. Subjects who were treated with daily somatropin in heiGHt (blue bars) switched to weekly lonapegsomatropin in enliGHten (orange bars). Subjects who were on weekly lonapegsomatropin in heiGHt continued weekly lonapegsomatropin in enliGHten (green bars). *Based on n = 159 at heiGHt trial baseline. +ΔHeight SDS value is the least squares means from the analysis of covariance model.
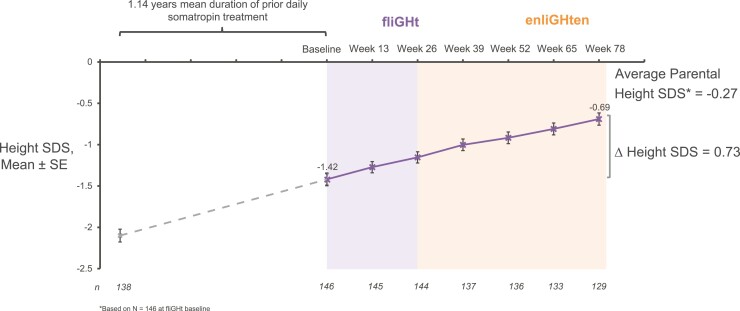
fliGHt subjects also continued to approach their average parental height SDS, with height SDS improving from −1.42 at fliGHt baseline to −0.69 at week 78 (Fig. 2). Mean AHV at week 78 was 8.4 cm/year and was consistent with clinical expectations given the characteristics of the enrolled subjects (14). Among heiGHt subjects who switched from daily somatropin to lonapegsomatropin, a lower-than-expected attenuation in the second-year AHV was observed. The mean (SE) AHV for subjects previously treated with daily somatropin was 10.2 (0.32) cm/year at week 52 and 8.9 (0.25) cm/year at week 104. For subjects treated with lonapegsomatropin, the mean (SE) AHV was 10.9 (0.23) cm/year at week 52, and 8.5 (0.16) cm/year at week 104.
Figure 2.
Sustained improvement in height SD score (SDS) for fliGHt subjects. Height SDS over 78 weeks in subjects who had been previously treated with daily somatropin before enrolling in the fliGHt trial (weekly lonapegsomatropin, light purple background) and then continuing in the enliGHten trial (weekly lonapegsomatropin, light orange background). *Based on n= 146 at fliGHt baseline.
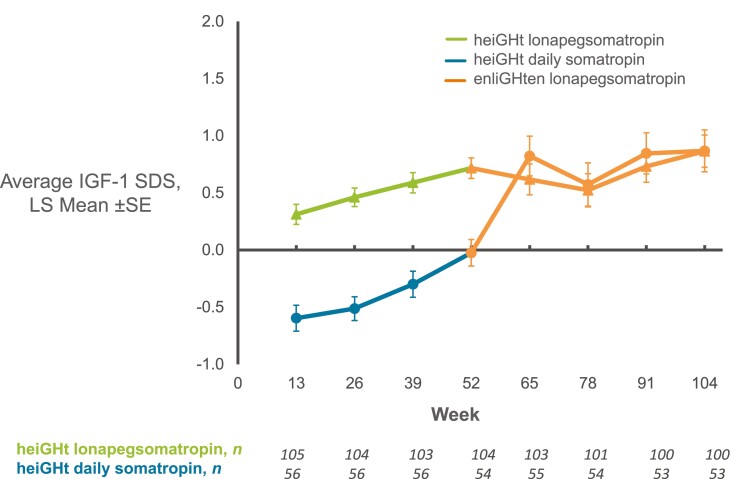
Pharmacodynamic Outcomes
Beyond 52 weeks, average IGF-1 SDS for heiGHt subjects who started on lonapegsomatropin generally remained stable without further increase; for heiGHt subjects who switched from daily somatropin to lonapegsomatropin, an initial increase in average IGF-1 SDS with subsequent stabilization was observed (Fig. 3). In heiGHt, average IGF-1 SDS values were higher for lonapegsomatropin-treated subjects compared with daily somatropin-treated subjects, paralleling the observed improved growth outcomes.
Figure 3.
Average insulin-like growth factor-1 (IGF-1) SD score (SDS) over 104 weeks for heiGHt subjects. Average IGF-1 SDS over 104 weeks for patients who were treated with lonapegsomatropin (green line, triangles) or daily somatropin (blue line, circles) in the heiGHt trial and were treated with lonapegsomatropin in the enliGHten trial (orange lines, triangles or circles).
For fliGHt subjects, observed mean (SD) average IGF-1 SDS increased from 0.85 (1.3) at fliGHt baseline to 1.62 (1.3) at week 26 and 1.81 (1.1) at week 78 (data not shown).
Safety Outcomes
Longer-term treatment with lonapegsomatropin was safe and well-tolerated in this long-term extension trial. With continued lonapegsomatropin treatment in the long-term extension, the AE profile remained consistent with what was observed in the parent trials and across the Phase 3 studies, with no new safety signals. There were no remarkable changes in laboratory test results, vital sign measurements, or fundoscopy findings observed. Hemoglobin A1c, cortisol, and free thyroxine were stable and generally remained within the normal range throughout the trials (Table 3).
Table 3.
Laboratory parameters in enliGHten trial
| Baseline | Week 26 | Week 52 | Week 78 | |
|---|---|---|---|---|
| Cortisol, ug/dL | n = 298 8.8 (4.3) |
n = 287 8.5 (4.1) |
n = 277 8.6 (5.7) |
n = 147 9.0 (3.8) |
| Hemoglobin A1c, % | n = 290 5.2 (0.3) |
n = 281 5.2 (0.3) |
n = 272 5.2 (0.3) |
n = 144 5.3 (0.4) |
| Free thyroxine, ng/dL | n = 293 1.1 (0.2) |
n = 288 1.3 (0.2) |
n = 278 1.2 (0.2) |
n = 147 1.2 (0.2) |
Data are given as mean (SD).
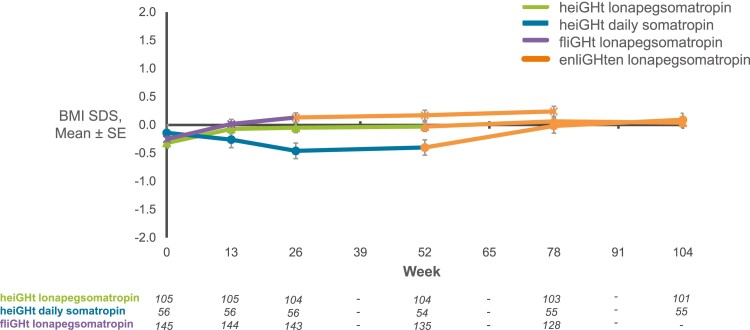
The most common AEs to date were respiratory tract infection (21.1%), nasopharyngitis (11.1%), and cough (8.7%) (Table 4). No serious AEs were considered to be related to the study drug. Overall, AEs were assessed by investigators as mild (41.3%) or moderate (21.5%). Out of events assessed by investigators as related to lonapegsomatropin, most have been related to injection site reactions (data not shown). Transient, low-titer nonneutralizing anti-lonapegsomatropin–binding antibodies were detected in 6.3% of lonapegsomatropin-treated subjects across the heiGHt, flight, and enliGHten trials, and no neutralizing antidrug antibodies were identified. No slipped capital femoral epiphyses or intracranial hypertension has been reported to date. Over time, treatment with lonapegsomatropin was associated with an increase in mean body mass index (BMI) SDS that stabilized toward 0 (Fig. 4).
Table 4.
Common adverse events in enliGHten trial
| Preferred term | Total, n (%) (n = 298) |
|---|---|
| Any TEAE | 195 (65.4) |
| Upper respiratory infection | 63 (21.1) |
| Nasopharyngitis | 33 (11.1) |
| Cough | 26 (8.7) |
| Pyrexia | 25 (8.4) |
| Influenza | 23 (7.7) |
| Headache | 21 (7.0) |
| Viral upper respiratory tract infection | 21 (7.0) |
| Pharyngitis streptococcal | 19 (6.4) |
| Gastroenteritis | 15 (5.0) |
Proportions are based on subject-level counts.
Abbreviation: TEAE, treatment-emergent adverse event.
Figure 4.
Body mass index (BMI) SD score (SDS) across all trials. BMI SDS for subjects from the fliGHt trial who were treated with lonapegsomatropin (purple line, stars) and continued lonapegsomatropin in the enliGHten trial (orange line, stars), subjects from the heiGHt trial who were treated with daily somatropin (blue line, circles) and were treated with lonapegsomatropin in the enliGHten trial (orange line, circles), and subjects from the heiGHt trial who were treated with lonapegsomatropin (green line, triangles) and were treated with lonapegsomatropin in the enlighten trial (orange line, triangles).
Among children from the heiGHt trial treated with lonapegsomatropin or switched from daily somatropin to lonapegsomatropin, there was a similar change in bone age at week 104. For subjects in the heiGHt lonapegsomatropin group, the mean (SD) bone age/chronological age ratio was 0.8 (0.2) at baseline. For subjects in the heiGHt daily somatropin group, the mean (SD) bone age/chronological age ratio at baseline was similar at 0.8 (0.1). After 104 weeks, the average ratio for each treatment group was 0.8 (0.1), showing that there was, at most, only minimal advancement of bone age relative to chronological age with continued lonapegsomatropin treatment.
Compliance and Patient-reported Outcomes for the TransCon hGH Auto-Injector
Subcutaneous injection with lonapegsomatropin was well-tolerated in this pediatric population, and treatment compliance during the trial was high, with a mean compliance of 98.8%.
Once available, subjects in enliGHten at select sites in the United States were switched from vial and syringe to the TransCon hGH Auto-Injector; currently, 160 subjects have used the TransCon hGH Auto-Injector in enliGHten. While using lonapegsomatropin cartridges and the TransCon hGH Auto-Injector, mean duration of treatment was 30.9 weeks (range: 1-42 weeks), and 141 subjects had received lonapegsomatropin using the TransCon hGH Auto-Injector for ≥26 weeks. Based on exposure-adjusted rates of local tolerability at the injection site (assessing swelling, redness, bruising, or itching), 7.7% experienced a reaction with the syringe/needle vs 2.7% with the TransCon hGH Auto-Injector in these 160 subjects (P < 0.0001), suggesting an improved tolerability with the TransCon hGH Auto-Injector. Overall, fewer local tolerability reactions were reported with the TransCon hGH Auto-Injector (2.7% of 5098 total injections) compared with syringe/needle (7.7% of 6815 total injections).
Subjects and caregivers indicated overall ease of use of the device. Based on the DUQ, as assessed by subjects who switched to the TransCon hGH Auto-Injector, most subjects strongly agreed that the device was comfortable, easy to use, and safe. Responses were available for 115 subjects who completed the DUQ after 13 weeks of TransCon hGH Auto-Injector use. The subjects reported that the GH Auto-Injector did not cause a lot of pain or discomfort (82.6%), the medicine could be injected without difficulty or making a mistake (94.0%) in a short amount of time (94.0%), without touching blood (95.7%), and left few to no marks on the skin (95.7%). No subject experienced an injury caused by the TransCon hGH Auto-Injector that required care from a clinician.
While global satisfaction scores remained high across timepoints, the convenience score increased notably upon transition to the TransCon hGH Auto-Injector from either lonapegsomatropin administered via syringe/needle or daily somatropin administered via a pen in the heiGHt and fliGHt trials (Table 5). These results suggest that both frequency and mode of administration factor heavily into caregiver’s assessment of convenience. Once-weekly lonapegsomatropin as administered with TransCon hGH Auto-Injector was associated with relatively high convenience and satisfaction scores as assessed by caregivers.
Table 5.
Convenience and overall satisfaction domains of TSQM-9 for subjects who transitioned to the TransCon hGH Auto-Injector (completed by caregiver)
| Summary score | Baseline (reflects lonapegsomatropin vial/syringe or Genotropin pen) |
Transition week 6a (reflects lonapegsomatropin via TransCon hGH Auto-Injector) |
Transition week 13a (reflects lonapegsomatropin via TransCon hGH Auto-Injector) |
|---|---|---|---|
| Convenience | (n = 158) 73.8 (15.6) |
(n = 142) 86.1 (13.7) |
(n = 111) 87.0 (14.8) |
| Global satisfaction | (n = 157) 86.3 (14.5) |
(n = 142) 89.5 (12.6) |
(n = 111) 90.8 (12.5) |
Data are given as mean (SD).
aTransition week × means approximately × weeks after transition from lonapegsomatropin via syringe/needle to lonapegsomatropin via TransCon hGH Auto-Injector.
Discussion
The current study reports outcomes of up to 2 years in children treated with weekly doses of lonapegsomatropin. Across the broad population of the Phase 3 program for lonapegsomatropin, children treated with lonapegsomatropin continued to grow well, with a safety profile comparable to daily injections of somatropin (9). Persistence of effect was observed beyond the first year among children from the heiGHt parent trial with an approximately 15% increase with lonapegsomatropin in height SDS maintained through week 78 compared to somatropin. Children switching from daily somatropin to lonapegsomatropin continued to follow the same growth speed of those originally randomized to lonapegsomatropin, with an increase of approximately 0.5 height SDS from week 52 to week 104 (least squares mean height SDS 1.10-1.61 for continuous lonapegsomatropin and 0.96-1.49 for switch to lonapegsomatropin). The observed mean height SDS improvements at week 104 (1.52 and 1.48 for the group that continued lonapegsomatropin and the group that switched from daily somatropin to lonapegsomatropin, respectively) exceeded height SDS improvements reported in another trial that evaluated daily somatropin treatment in prepubertal, treatment-naive subjects (1.16-1.25 height SDS at 24 months) (15).
When children switched from daily injections of somatropin (in the heiGHt trial) to once-weekly lonapegsomatropin, they experienced an increase in IGF-1 accompanied by a less pronounced decline in second-year AHV (relative to first-year AHV), suggesting an improved treatment effect of lonapegsomatropin relative to the previous daily somatropin. The longer-term efficacy data for lonapegsomatropin showed continued effectiveness in the cohort of lonapegsomatropin-treated children from heiGHt. In the treatment-experienced children originally enrolled in fliGHt, growth continued as expected for children from a broader set of demographics.
The safety profile of lonapegsomatropin was consistent across the Phase 3 studies, including in this long-term extension trial. AEs to date were generally mild. The most common AEs in enliGHten represented common childhood ailments and were consistent with AEs reported in other clinical trials evaluating daily injections of somatropin in children with GHD (16, 17). Throughout treatment, hemoglobin A1c, cortisol, free thyroxine, and BMI remained stable, and no serious AEs were assessed as causally related to lonapegsomatropin.
GH affects body composition via opposing GH (lipolytic) and IGF-1 (adipogenic) effects. Additionally, GH increases relative lean body mass by decreasing protein oxidation and increasing protein synthesis in skeletal muscle. A key concern in the development of LAGHs has been that modified GH formulations may result in variable tissue distribution due to molecular weight (18). Nonphysiological tissue distribution can result in increased serum IGF-1 levels due to GH activity in the liver but a lack of GH effects in size-restricted target tissues such as fat. Therefore, elevated IGF-1 levels in adipose tissue in the absence of GH’s lipolytic effects may result in an adipogenic effect, which can result in net fat accumulation and weight gain. Somatropin released from the lonapegsomatropin prodrug is unmodified and is expected to exhibit a pattern of tissue distribution and affinity for the GH receptor identical to that of endogenous GH and daily somatropin therapies. Indeed, treatment with lonapegsomatropin in this long-term extension was associated with mean BMI SDS that stabilized toward 0.
There was no increase in bone age advancement with lonapegsomatropin therapy, indicating that the longer-term effects of lonapegsomatropin (up to 104 weeks) did not occur at the expense of accelerated skeletal maturation. It follows then that improvements in near-final height could be anticipated with lonapegsomatropin treatment.
In enliGHten, where doses could be adjusted based on investigator judgment, there was no evidence that higher doses were required over time. Somatropin released from lonapegsomatropin produces a dose-linear IGF-1 response (10). Based on findings from a dose-ranging Phase 2 trial conducted in prepubertal, treatment-naive children with GHD, a dose change of 20% (corresponding to the next lower or higher cartridge) would result, on average, in an IGF-1 change of approximately 0.3 SDS (10). Analyses from the Phase 3 clinical trials (heiGHt, fliGHt, and enliGHten) showed a similar relationship of dose to IGF-1 change. This relationship was consistent across a wide range of ages and pubertal stages, giving clinicians an important tool for dose titration and individualization based on clinical response and treatment goals. Furthermore, modeling and simulation show that IGF-1 response can be checked after 2 weeks have elapsed.
Across the Phase 3 program, administration of lonapegsomatropin by both needle/syringe and the TransCon hGH Auto-Injector were well-tolerated in children with GHD. A good tolerability profile is particularly important for conditions requiring long-term therapy, and this has not always been achieved by other LAGH injections (19). Although adherence and compliance are complex and multifactorial, complexity of treatment is 1 of the key factors to poor compliance (20). Since GHD in pediatric patients necessitates regular treatment over many years to reach desired outcomes, LAGHs that are safe and effective and with improved tolerance may improve patient adherence and alleviate the burden of chronic daily injections, resulting in more consistent use and potentially improved growth outcomes. The emergence of LAGH therapies shows promising utility for long-term treatment of GHD.
Limitations
The current study has limitations. The authors acknowledge that mostly boys were enrolled into the trial, which is consistent with literature describing the sex imbalance in short-stature referrals in clinical practice and thought to be influenced by social and cultural pressures (18, 21, 22). That said, no differences in clinical outcomes have been observed across sex for lonapegsomatropin, and the lonapegsomatropin population pharmacokinetic/PD modeling evaluations for pediatric GHD showed no clinically meaningful effect of sex on pharmacokinetic or PD parameters.
Additional studies are needed to better understand the effect of weekly lonapegsomatropin on dosing adherence in a noninterventional setting. Because GHD is uncommon, the study population size for clinical trials is expectedly modest and may be unable to detect small to moderate effects that may be clinically important. Furthermore, surveillance periods of longer than 2 years are needed to better understand outcomes and the long-term safety profile of weekly lonapegsomatropin.
Conclusions
Children treated with weekly doses of lonapegsomatropin showed continued improvement of height SDS through their second year of therapy without excess advancement of bone age. Lonapegsomatropin continued to demonstrate a safety and tolerability profile comparable to that of daily somatropin therapy, with treatment compliance of over 98%.
Acknowledgments
The authors would like to thank the study participants and their families, the enliGHten trial investigators, and study site staff. We also wish to acknowledge members of the trial’s independent safety committee and Kathleen P. Hopf and Cindy J. Gode for medical writing assistance.
Contributor Information
Aristides K Maniatis, Rocky Mountain Pediatric Endocrinology, Centennial, CO, USA.
Samuel J Casella, Children’s Hospital at Dartmouth-Hitchcock, Lebanon, NH, USA.
Ulhas M Nadgir, Center of Excellence in Diabetes and Endocrinology, Sacramento, CA, USA.
Paul L Hofman, Liggins Institute, University of Auckland, Auckland, New Zealand.
Paul Saenger, NYU Langone Health, New York, NY, USA.
Elena D Chertock, Voronezh State Medical University, Voronezh, Russia.
Elena M Aghajanova, Yerevan State Medical University, Yerevan, Armenia.
Maria Korpal-Szczyrska, Klinika Pediatrii, Diabetologii i Endokrynologii Uniwersyteckie Centrum Kliniczne, Gdansk, Poland.
Elpis Vlachopapadopoulou, Children’s Hospital. P. A. Kyriakou, Athens, Greece.
Oleg Malievskiy, Bashkir State Medical University, Ufa, Russia.
Tetyana Chaychenko, MHI Regional Child Clinical Hospital, Child Endocrinology Center, Kharkiv National Medical University, Kharkiv, Ukraine.
Marco Cappa, UOC di Endocrinologia, Ospedale Pediatrico Bambino Gesù, IRCCS, Rome, Italy.
Wenjie Song, Ascendis Pharma, Palo Alto, CA, USA.
Meng Mao, Ascendis Pharma, Palo Alto, CA, USA.
Per Holse Mygind, Ascendis Pharma, Palo Alto, CA, USA.
Alden R Smith, Ascendis Pharma, Palo Alto, CA, USA.
Steven D Chessler, Ascendis Pharma, Palo Alto, CA, USA.
Allison S Komirenko, Ascendis Pharma, Palo Alto, CA, USA.
Michael Beckert, Ascendis Pharma A/S, Hellerup, Denmark.
Aimee D Shu, Ascendis Pharma, Palo Alto, CA, USA.
Paul S Thornton, Cook Children’s Health Care System, Fort Worth, TX, USA.
Funding
This study was sponsored by Ascendis Pharma Endocrinology Division A/S.
Clinical Trial Information
ClinicalTrials.gov number: NCT03344458.
Disclosures
A.K.M. has received research funding and is an advisory board consultant for Ascendis Pharma, Novo Nordisk, OPKO, and Pfizer. S.J.C has received research funding from Ascendis Pharma. U.M.N, E.A., T.K., E.G., P.S., and P.L.H. are research investigators for Ascendis Pharma. E.V. is a research investigator for Ascendis Pharma, OPKO, and Janssen and has received honoraria for lectures from Sandoz, Eli Lilly, and Novo Nordisk. W.S., M.M., P.H.M, A.R.S, S.D.C., A.S.K., and A.D.S. are employees of Ascendis Pharma, Inc. M.B. is an employee of Ascendis Pharma A/S. P.S.T. has received research funding from Ascendis Pharma, Novo Nordisk, Pfizer, and OPKO.
Data Availability
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Tidblad A. The history, physiology and treatment safety of growth hormone. Acta Paediatr. 2022;111(2):215-224. [DOI] [PubMed] [Google Scholar]
- 2. Yuen KCJ, Miller BS, Boguszewski CL, Hoffman AR. Usefulness and potential pitfalls of long-acting growth hormone analogs. Front Endocrinol (Lausanne). 2021;12:637209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cutfield WS, Derraik JGB, Gunn AJ, et al. . Non-compliance with growth hormone treatment in children is common and impairs linear growth. PLoS One. 2011;6(1):e16223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rosenfeld RG, Bakker B. Compliance and persistence in pediatric and adult patients receiving growth hormone therapy. Endocr Pract. 2008;14(2):143-154. [DOI] [PubMed] [Google Scholar]
- 5. SKYTROFA (lonapegsomatropin-tcgd). Hellerup, Denmark. Ascendis Pharma Endocrinology Division A/S. 2022. [Google Scholar]
- 6. Khadilkar V, Radjuk KA, Bolshova E, et al. . 24-month use of once-weekly GH, LB03002, in prepubertal children with GH deficiency. J Clin Endocrinol Metab. 2014;99(1):126-132. [DOI] [PubMed] [Google Scholar]
- 7. Luo X, Hou L, Liang L, et al. . Long-acting PEGylated recombinant human growth hormone (Jintrolong) for children with growth hormone deficiency: Phase II and Phase III multicenter, randomized studies. Eur J Endocrinol. 2017;177(2):195-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lamb YN. Somatrogon: first approval. Drugs. 2022;82(2):227-234. [DOI] [PubMed] [Google Scholar]
- 9. Thornton PS, Maniatis AK, Aghajanova E, et al. . Weekly lonapegsomatropin in treatment-naïve children with growth hormone deficiency: the Phase 3 heiGHt trial. J Clin Endocrinol Metab. 2021;106(11):3184-3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chatelain P, Malievskiy O, Radziuk K, et al. . Randomized Phase 2 study of long-acting TransCon GH vs daily GH in childhood GH deficiency. J Clin Endocrinol Metab. 2017;102(5):1673-1682. [DOI] [PubMed] [Google Scholar]
- 11. Sprogøe K, Mortensen E, Karpf DB, Leff JA. The rationale and design of TransCon Growth Hormone for the treatment of growth hormone deficiency. Endocr Connect. 2017;6(8):R171-R181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lin Z, Shu AD, Bach M, Miller BS, Rogol AD. Average IGF-1 prediction for once-weekly lonapegsomatropin in children with growth hormone deficiency. Pediatric Endocrine Society. 2021;6(1):bvab168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bharmal M, Payne K, Atkinson MJ, Desrosiers M-P, Morisky DE, Gemmen E. Validation of an abbreviated Treatment Satisfaction Questionnaire for Medication (TSQM-9) among patients on antihypertensive medications. Health Qual Life Outcomes. 2009;7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bakker B, Frane J, Anhalt H, Lippe B, Rosenfeld RG. Height velocity targets from the national cooperative growth study for first-year growth hormone responses in short children. J Clin Endocrinol Metab. 2008;93(2):352-357. [DOI] [PubMed] [Google Scholar]
- 15. Romer T, Peter F, Saenger P, et al. . Efficacy and safety of a new ready-to-use recombinant human growth hormone solution. J Endocrinol Invest. 2007;30(7):578-589. [DOI] [PubMed] [Google Scholar]
- 16. Cohen P, Bright GM, Rogol AD, Kappelgaard A-M, Rosenfeld RG. Effects of dose and gender on the growth and growth factor response to GH in GH-deficient children: implications for efficacy and safety. J Clin Endocrinol Metab. 2002;87(1): 90-98. [DOI] [PubMed] [Google Scholar]
- 17. Peterkova V, Arslanoglu I, Bolshova-Zubkovskaya E, et al. . Randomized, double-blind study to assess the efficacy and safety of valtropin, a biosimilar growth hormone, in children with growth hormone deficiency. Horm Res Paediatr. 2007;68(6):288-293. [DOI] [PubMed] [Google Scholar]
- 18. Christiansen JS, Backeljauw PF, Bidlingmaier M, et al. . Growth Hormone Research Society perspective on the development of long-acting growth hormone preparations. Eur J Endocrinol. 2016;174(6):C1-C8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Silverman BL, Blethen SL, Reiter EO, Attie KM, Neuwirth RB, Ford KM. A long-acting human growth hormone (Nutropin Depot®): efficacy and safety following two years of treatment in children with growth hormone deficiency. J Pediatr Endocrinol Metab. 2002;15(suppl 2):715-722. [DOI] [PubMed] [Google Scholar]
- 20. Brod M, Rousculp M, Cameron A. Understanding compliance issues for daily self-injectable treatment in ambulatory care settings. Patient Prefer Adherence. 2008;2:129-136. [PMC free article] [PubMed] [Google Scholar]
- 21. Grimberg A, Kutikov JK, Cucchiara AJ. Sex differences in patients referred for evaluation of poor growth. J Pediatr. 2005;146(2):212-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grimberg A, Ramos M, Grundmeier R, et al. . Sex-based prevalence of growth faltering in an urban pediatric population. J Pediatr. 2009;154(4):567-572.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.