Abstract
Context
It is unclear to what extent the plasma proteome of abdominal fat distribution differs from that of body mass index, and whether the differences have clinical implications.
Objective
To evaluate the difference between the plasma proteomic profiles of body mass index (BMI) and waist-to-hip ratio (WHR), and then examine the identified BMI- or WHR-specific proteins in relation to incidence of diabetes.
Methods
Data were obtained from the Malmö Diet and Cancer-Cardiovascular Cohort study in the general community. Participants (n = 4203) with no previous diabetes (aged 57.2 ± 6.0 years, 37.8% men) were included. Plasma proteins (n = 136) were measured by the Proseek proximity extension method. BMI- and WHR-specific proteins were identified at baseline using a 2-step iterative resampling approach to optimize internal replicability followed by β coefficient comparisons. The identified proteins were considered internally replicated and were then studied in relation to incident diabetes by Cox proportional hazards regression analysis. The main outcome measure was incident diabetes over a mean follow-up of 20.3 ± 5.9 years.
Results
After excluding 21 overlapping proteins and proteins that did not show significantly different associations with BMI vs WHR, 10 internally replicated proteins were found to be specific to BMI, and 22 were found to be specific to WHR (false discovery rate-adjusted P < .05). Of the WHR-specific proteins, 18 remained associated with diabetes risk after multivariate adjustments, whereas none of the BMI-specific proteins showed associations with diabetes risk.
Conclusion
Abdominal fat distribution was associated with some unique characteristics of the plasma proteome that potentially could be related to its additional risk of diabetes beyond general obesity.
Keywords: body mass index, cohort, diabetes, proteomics, waist-to-hip ratio
The prevalence of obesity is high and rapidly increasing worldwide (1). Obesity carries a remarkable risk of cardiometabolic diseases, such as type 2 diabetes, which subsequently leads to high health and economic burden (1). Body mass index (BMI) has been traditionally used to measure obesity. However, it mainly reflects overall adiposity and fails to distinguish different fat depots. It has been argued that BMI is a “very poor proxy of health” since metabolic health can vary significantly for the same BMI (2, 3).
Fatness located at different body sites may exert paradoxical impacts on health. The abdominal (upper-body) fat is known to be detrimental (3, 4). In contrast, the protective role of gluteofemoral (lower-body) fat has been supported by its associations with improved lipid and glucose homeostasis and a decreased tendency to develop metabolic abnormalities (3, 5). Whereas the exact mechanisms remain to be analyzed, the variations of upper- and lower-body fat in lipolysis, fatty acid uptake, and adipokine profiles have been proposed as possible explanations for their divergent effects (3-5). As a ratio of waist-to-hip circumference (waist-to-hip ratio, WHR), WHR considers both the detrimental effects of abdominal fat and the beneficial effects of gluteofemoral fat, and it is widely used as a proxy of body fat distribution (6, 7).
WHR significantly improves the prediction of diabetes risk on top of BMI (8, 9). The genetic basis of WHR only marginally overlaps with that of BMI (7, 10), and the genetic predisposition to WHR adjusted for BMI is predictive of diabetes (11). All these results indicate that WHR may differ from BMI in biology. Recently, a Swedish cohort study reported that changes in BMI and WHR over a 10-year period were related to changes in different plasma proteins (6). Nevertheless, it remains unclear to what extent the plasma proteome of WHR differs from that of BMI, and more importantly, whether these differences have clinical implications, for instance could confer differential risk of diabetes.
In the present study, we aimed to investigate differences in protein profiles between BMI and WHR based on a targeted proteomics approach, and, of particular interest, to see if the identified BMI- and WHR-specific proteins are prospectively related to risk of diabetes, a common consequence of obesity. Our results will provide some insight into plausible mechanisms underlying the different roles of general obesity and abdominal fat distribution in health.
Materials and Methods
Study Participants
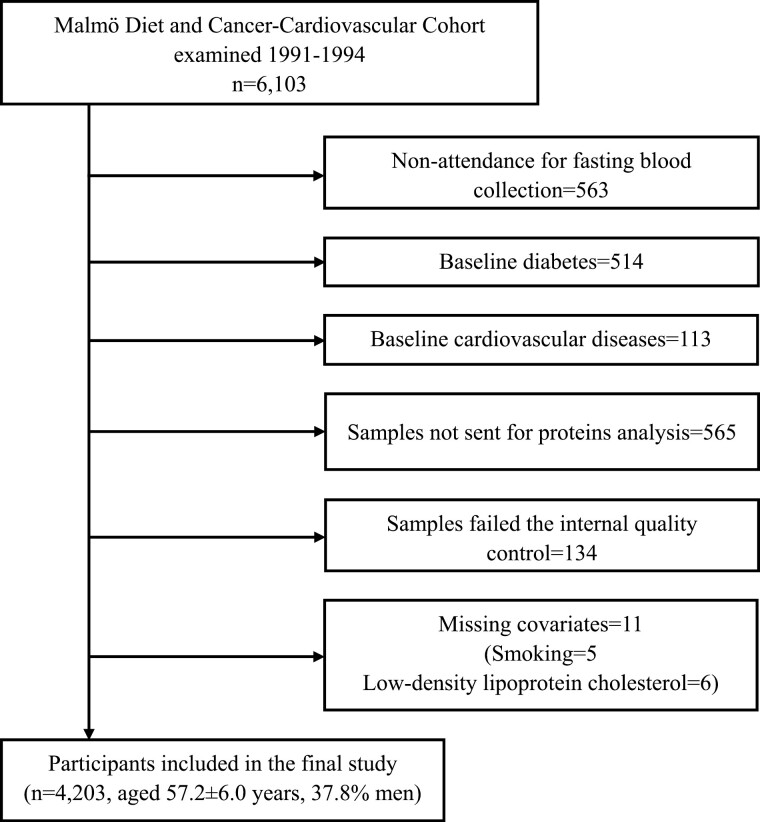
The Malmö Diet and Cancer (MDC) study is a large prospective cohort study, with participants enrolled from the general population of Malmö, Sweden (12). From 1991 to 1994, a random sample (n = 6103) from the MDC population was recruited to a subcohort named the Malmö Diet and Cancer-Cardiovascular Cohort (MDC-CC). A total of 4913 participants had fasting blood samples obtained and were free of diabetes and cardiovascular diseases (CVDs) at baseline. Among them, 4348 had blood samples sent for plasma protein analysis, out of which 4214 passed the internal quality control. Of the remaining participants, 11 with missing data on smoking (n = 5) or low-density lipoprotein cholesterol (LDL, n = 6) were excluded. Therefore, 4203 participants were left for the final analyses (Fig. 1). All participants signed written informed consent. The study was approved by the Regional Ethical Review Board in Lund, Sweden (LU 51/90) (LU 2012/762) and was in accordance with the Declaration of Helsinki.
Figure 1.
Study population flow chart.
Proteomic Analyses
Fasting plasma samples were stored at –80°C immediately after collection until protein analysis in 2015. Fifty-seven plasma proteins were measured using Olink Proseek® Multiplex Oncology I reagent kit and 92 were measured using Olink Proseek® Multiplex CVD I kit, from the SciLifeLab analysis service (Uppsala, Sweden). There was no overlap in proteins between these 2 panels. The reagents were based on proximity extension assay (PEA) technology (13) where a pair of DNA oligonucleotides linked to a set of matched antibodies was used to detect the target protein in a homogeneous assay. Upon binding of the 2 oligonucleotides, a DNA duplex was formed, which then was extended by a DNA polymerase to generate a real-time PCR amplicon. Since only correctly matched DNA string pairs could generate detectable and quantifiable signals, the PEA technology exhibited high specificity and excellent sensitivity (13, 14). Values were presented as normalized protein expression (NPX) units, which were real-time qPCR cycle values on a log2 scale (15). Quality control over the technical performance of the assays and the samples was performed using the Olink® NPX Manager software, the basis of which depends on 4 internal controls that are spiked into all samples and external controls in every Olink analysis. Values below the lower limits of detection as determined from a negative control were imputed as limit of detection/2. Proteins with a call rate less than 75% were removed (n = 13), resulting in 136 proteins available for proteomic analyses (see Table S1 for a full list of the included proteins (16)). Due to different call rates for proteins, samples used for different proteins varied between 3478 and 4198 subjects. Also see the Olink webpage (http://www.olink.com) for detailed information regarding proteomic panels, PEA technology, assay performance, quality control and validation.
Anthropometric Measurements
Baseline anthropometric measurements were conducted by trained nurses following standard procedures. Standing height (m) was measured using a fixed calibrated stadiometer. Weight (kg) was measured using balance-beam scales while subjects wore light clothing without shoes, and the values were recorded to the nearest 0.1 kg. Waist circumference (cm) was measured midway between the lowest rib margin and iliac crest, and hip circumference (cm) was determined as the largest circumference between waist and thighs. BMI was calculated as weight in kilograms divided by the square of height in meters (kg/m2), and WHR was calculated as waist circumference divided by hip circumference.
Other Measurements
Blood pressure (BP, mmHg) was measured with a mercury column sphygmomanometers after a 10-minute rest in a supine position. Medical history, medications, alcohol consumption, and smoking habits were assessed in self-administered questionnaires. Smoking status was categorized as nonsmokers or current smokers. High alcohol consumption was defined as >40 g of alcohol per day for men and >30 g per day for women. After an overnight fast, fresh blood samples were drawn to measure fasting blood glucose (mmol/L) and plasma lipids according to standard procedures at the Department of Clinical Chemistry, University Hospital, Malmö. LDL (mmol/L) was determined based on the Friedewald’s formula.
Ascertainment of Endpoints
All participants were free of diabetes at baseline and were followed until incident diabetes, emigration from Sweden, death, or the end of follow-up on December 31, 2016, whichever came first. Information on cases of new-onset diabetes was retrieved from validated national and local registers (17), including the Swedish National Diabetes Register, the regional Diabetes 2000 Register of the Scania region, the Malmö HbA1c Register, the Swedish Inpatient register, the Swedish Outpatient register (for hospital-based policlinics), and the nationwide Swedish Drug Prescription Register. In the Swedish National Diabetes Register and the Diabetes 2000 Register, new cases of diabetes were diagnosed according to established criteria (fasting plasma glucose concentration ≥7.0 mmol/L with 2 repeated tests on separate occasions). In the Malmö HbA1c Register, if an individual had at least 2 HbA1c records ≥6.0% (42 mmol/mol) with the Swedish Mono-S standardization system (corresponding to 7.0% [53 mmol/mol] according to the US National Glycohemoglobin Standardization Program) during the follow-up, he or she would be considered to have incident diabetes. In the Swedish Inpatient and Outpatient registers, diabetes was diagnosed by a senior physician. In the nationwide Drug Prescription Register, a filled prescription of insulin or glucose lowering medications (anatomical–therapeutic–chemical classification code A10) was required for diagnosis of diabetes.
Statistical Analyses
Correlation between obesity measures was estimated by Pearson’s correlation analysis. Baseline characteristics were assessed across quartiles of BMI and WHR, respectively. Sex-specific quartile limits were used for BMI or WHR to ensure equal sex ratios in each quartile. Categorical variables are presented as numbers (percentages) while continuous variables are presented as mean ± SD or median (interquartile range). Differences in continuous or categorical variables across quartiles were examined by linear or logistic regression, respectively.
First, the association of BMI (both in quartiles and per 1 SD change) with incidence of diabetes were determined using Cox proportional hazards regression with time-on-study as the timescale. WHR was adjusted for as a covariate. Other potential confounders were taken into account: age, sex, smoking, high alcohol consumption, systolic BP, LDL, and antihypertensive medication. The association of WHR with incidence of diabetes was similarly assessed after adjusting for BMI and other potential confounders.
Second, proteins associated with BMI or WHR were discovered with a 2-step iterative resampling (TSIR) approach to optimize internal replicability. This method was primarily used for loci discovery in genome-wide association studies where the power and efficiency have been proven (18). A 70:30 split into discovery and replication sets and a cutoff of 20 out of 100 replicated pairs showed excellent performance to determine proteins to be internally replicated while providing conservative type I error control (18). It has also been used for protein discovery in a recent study from MDC-CC (19). Similarly, in the present study, all participants were randomly divided into the discovery (2/3 of the population) and replication (1/3 of the population) sets. Random splits were repeated 100 times. In the first step, multivariate linear regressions were separately conducted for every single protein (as the dependent variable) to explore its association with BMI and WHR (as the independent variables). BMI and WHR were adjusted for each other to examine the associations of proteins with BMI adjusted for WHR and with WHR adjusted for BMI, respectively. Covariates taken into account were age, sex, smoking, high alcohol consumption, systolic BP, LDL, and antihypertensive medication. Afterward, the proteins with significant associations discovered in the first step were similarly studied in relation to BMI and WHR in the replication set. Both BMI and WHR were used as continuous variables (per 1 SD change) in the TSIR approach. P-values adjusted for false discovery rate (FDR) of .05 by the Benjamini–Hochberg method were always used to identify proteins with significant associations with BMI or WHR. Proteins were considered internally replicated if they were significantly (FDR-adjusted at .05) associated with BMI (or WHR) in the same direction in both the discovery and replication sets in at least 20 out of 100 of the randomly generated pairs.
Third, for each of the single internally replicated protein that was associated with BMI, but not with WHR (or vice versa), multivariate linear regression was similarly performed using the whole study sample. BMI and WHR were included simultaneously in the same model, and β coefficients were generated for BMI and WHR, respectively, regarding their associations with a specific protein. These 2 β coefficients were compared using an F-test. A protein was considered to be specific to BMI if it was associated with BMI but not WHR in the TSIR procedure, and was significantly more closely associated with BMI than with WHR in β coefficient comparisons. Likewise, WHR-specific proteins were identified. These proteins were then filtered by an orthogonality criterion (20). Proteins with large Spearman correlations (|r|>0.8) with others were removed by taking the protein from each cluster with more associations with BMI or WHR in the TSIR approach as its representative. After orthogonal filtering, Spearman correlations coefficients between each pair of BMI- or WHR-specific proteins were then visualized using heat-map graphs. To facilitate the interpretation of the findings, protein–protein interaction (PPI) network analysis was performed using STRING database (http://www.string-db.org). The interactions among the BMI- or WHR-specific proteins after the filtering were illustrated using Cytoscape 3.5 (http://www.cytoscape.org/) with MCODE (Cytoscape plug-in) used to carry out the module analysis. Gene Ontology functional and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were further conducted using DAVID 6.8 (https://david.ncifcrf.gov/) to show the critical biological implications associated with the identified proteins. Data from the Genotype-Tissue Expression (GTEx) project (https://gtexportal.org/home/) were used to show expression levels of genes corresponding to BMI- and WHR-specific proteins in tissues that are highly relevant to obesity and insulin resistance, such as subcutaneous and visceral adipose tissue, liver, skeletal muscle, and whole blood.
Longitudinal associations of BMI- or WHR-specific proteins with incidence of diabetes were investigated using Cox proportional hazards regression with Z-score standardized proteins and with time-on-study as the timescale. Hazard ratios (HRs) with 95% CIs were estimated after adjusting for age, sex, smoking, high alcohol consumption, systolic BP, LDL, antihypertensive medication, and BMI (for WHR-specific proteins) or WHR (for BMI-specific proteins). In a sensitivity analysis, the P values were corrected using a more strict FDR correction by taking all the protein-diabetes associations (multiple testing = 136 times) into consideration. Potential interactions between protein levels and sex were examined using multiplicative interaction terms in the Cox models. Volcano plots were drawn with Graphpad Prism 9 to illustrate the associations of 136 plasma proteins with BMI and WHR, respectively. All the other analyses were performed using the Statistical Analysis System version 9.3 for Windows (SAS Institute, Cary, NC, USA).
Results
Study Population Characteristics
A total of 4203 participants were included, with a mean age of 57.2 ± 6.0 years, 37.8 % of whom were men. The mean BMI was 25.8 ± 3.2 kg/m2 for men and 25.2 ± 4.0 kg/m2 for women, while the mean WHR was 0.94 ± 0.06 for men and 0.78 ± 0.05 for women. A moderate correlation was found between BMI and WHR (r = 0.58, P < .0001 for men and r = 0.35, P < .0001 for women). Baseline characteristics of participants across quartiles of BMI or WHR are shown elsewhere (Tables S2 and S3 (16)). WHR, but not BMI, increased with high alcohol consumption, while BMI, but not WHR, increased with age and decreased with smoking. Other cardiometabolic risk factors showed a similar increasing trend across BMI or WHR quartiles (all P for trend < .0001).
Predictive Values of BMI and WHR for Incidence of Diabetes
After a mean follow-up of 20.3 ± 5.9 years, 619 participants developed incident diabetes. As presented in Table 1, the association between WHR and diabetes was relatively stronger than the association between BMI and diabetes. The adjusted HR was 1.31 (95% CI 1.22-1.42, P < .0001) and 1.52 (95% CI 1.33-1.72, P < .0001) for per 1 SD change in BMI and WHR, respectively.
Table 1.
Incidence of diabetes in relation to sex-specific quartiles of BMI or WHR (n = 4203)
| Q1 | Q2 | Q3 | Q4 | P for trenda | Per 1 SD | P | |
|---|---|---|---|---|---|---|---|
| BMI | |||||||
| Incidence of diabetes | 108 | 115 | 137 | 259 | — | 619 | — |
| Incidence per 1000 person years | 4.94 | 5.23 | 6.36 | 13.1 | — | 7.27 | — |
| Model 1b | Reference | 1.02 (0.78, 1.32) | 1.19 (0.92, 1.53) | 2.30 (1.82, 2.91) | <.0001 | 1.43 (1.33, 1.53) | <.0001 |
| Model 2c | Reference | 0.95 (0.73, 1.24) | 1.03 (0.80, 1.34) | 1.77 (1.39, 2.26) | <.0001 | 1.31 (1.22, 1.42) | <.0001 |
| WHR | |||||||
| Incidence of diabetes | 96 | 160 | 152 | 211 | — | 619 | — |
| Incidence per 1000 person years | 3.81 | 6.7 | 8.23 | 12 | — | 7.27 | — |
| Model 1b | Reference | 1.31 (0.99, 1.74) | 1.69 (1.29, 2.22) | 2.48 (1.92, 3.19) | <.0001 | 1.71 (1.54, 1.89) | <.0001 |
| Model 2c | Reference | 1.24 (0.93, 1.64) | 1.47 (1.12, 1.93) | 1.90 (1.45, 2.47) | <.0001 | 1.52 (1.33, 1.72) | <.0001 |
Abbreviations: BMI, body mass index; SD, standard deviation; WHR, waist-to-hip ratio.
a Analysis by Cox proportional hazards model.
b Hazard ratios (95% CI) adjusted for age, sex, smoking, high alcohol consumption, systolic blood pressure, low density lipoprotein, and antihypertensive medication.
c Adjusted for covariates in Model 1 plus WHR or BMI.
Plasma Proteins Specific to BMI or WHR
The association between plasma proteins and BMI and WHR, before applying the TSIR approach and without mutual adjustment for BMI and WHR, is shown in Figure S1 (16).
The TSIR approach identified 59 and 45 plasma proteins to be internally replicated to associate with BMI or WHR, respectively, including 21 overlapping proteins (Tables S4 and S5 (16)). Therefore, 38 proteins were associated with BMI but not WHR, while 24 were associated with WHR but not BMI. As shown in Table 2, out of these proteins, F-tests confirmed that 10 were specific to BMI, including 3 with positive associations. In addition, 22 proteins were identified to be specific to WHR; all of which except for proto-oncogene tyrosine protein kinase Src showed positive associations.
Table 2.
Internally replicated proteins that are specific to BMI or WHR (n = 4203)a
| BMI | WHR | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Proteins | N | β (SE) | FDR-adjusted P1b | FDR-adjusted P2c | Proteins | N | β (SE) | FDR-adjusted P1b | FDR-adjusted P2c |
| AM | 4131 | 0.27 (0.02) | 1.00 × 10–30 | 6.11 × 10–07 | PRSS8 | 4198 | 0.10 (0.01) | 2.75 × 10–12 | .00017 |
| IL6 | 4131 | 0.13 (0.02) | 2.43 × 10–14 | .47 | IL18 | 4131 | 0.16 (0.03) | 4.27 × 10–07 | .0069 |
| TNFR1 | 4131 | 0.17 (0.02) | 3.70 × 10–22 | .05 | CCL20 | 4131 | 0.16 (0.03) | 5.07 × 10–07 | .0074 |
| CSF1 | 4131 | 0.15 (0.02) | 9.26 × 10–17 | .1 | CCL4 | 4131 | 0.16 (0.03) | 5.07 × 10–07 | .0029 |
| IL16 | 4131 | 0.14 (0.02) | 1.42 × 10–14 | .068 | IL8 | 4131 | 0.16 (0.03) | 8.40 × 10–07 | 9.44 × 10–05 |
| RAGE | 4131 | –0.16 (0.02) | 2.94 × 10–18 | .05 | CDKN1A | 4091 | 0.13 (0.03) | 2.34 × 10–06 | .0019 |
| GAL | 4131 | –0.15 (0.02) | 8.48 × 10–17 | .0051 | CXCL6 | 4131 | 0.15 (0.03) | 2.95 × 10–06 | .003 |
| VEGFD | 4131 | –0.16 (0.02) | 1.14 × 10–18 | .083 | CXCL11 | 4194 | 0.14 (0.03) | 6.88 × 10–06 | .0019 |
| EMMPRIN | 4198 | 0.04 (0.01) | 6.03 × 10–15 | 2.22 × 10–06 | GDF15 | 4131 | 0.13 (0.03) | 2.95 × 10–06 | .0024 |
| ILT3 | 4179 | 0.05 (0.01) | 1.41 × 10–09 | .71 | CHI3L1 | 4131 | 0.13 (0.03) | 5.48 × 10–06 | .0031 |
| IL12 | 4198 | 0.08 (0.01) | 3.17 × 10–11 | .27 | CA125 | 4131 | 0.14 (0.03) | 1.09 × 10–05 | .0031 |
| TRANCE | 4131 | 0.12 (0.02) | 3.57 × 10–11 | .21 | LAPTGFbeta1 | 4198 | 0.08 (0.02) | 5.27 × 10–06 | .003 |
| EZR | 4121 | 0.04 (0.01) | 9.87 × 10–11 | .05 | CTSL1 | 4131 | 0.13 (0.03) | 1.59 × 10–05 | .039 |
| TNFR2 | 4131 | 0.11 (0.02) | 8.66 × 10–10 | .55 | TNFSF14 | 4131 | 0.12 (0.03) | .00016 | .075 |
| ESM1 | 4131 | –0.11 (0.02) | 8.66 × 10–10 | .18 | CASP3 | 4131 | 0.12 (0.03) | 7.42 × 10–05 | .003 |
| MB | 4131 | 0.09 (0.02) | 6.22 × 10–08 | .068 | CXCL5 | 4198 | 0.11 (0.03) | .00013 | .0046 |
| TRAIL | 4131 | 0.10 (0.02) | 6.54 × 10–08 | .52 | HBEGF | 4131 | 0.12 (0.03) | .00013 | .0079 |
| FRalpha | 4198 | –0.05 (0.01) | 5.19 × 10–08 | .21 | CXCL13 | 4128 | 0.08 (0.02) | 8.34 × 10–05 | .012 |
| KLK6 | 4131 | –0.10 (0.02) | 5.19 × 10–08 | .024 | IL7 | 4149 | 0.07 (0.02) | .00024 | .0074 |
| Gal3 | 4131 | 0.10 (0.02) | 4.11 × 10–08 | .016 | Dkk1 | 4131 | 0.11 (0.03) | .00045 | .003 |
| NTRK3 | 4198 | –0.04 (0.01) | 3.10 × 10–08 | .67 | MMP7 | 4131 | 0.11 (0.03) | .00032 | .0029 |
| hK11 | 4131 | –0.10 (0.02) | 5.33 × 10–08 | .0021 | MICA | 3726 | 0.07 (0.02) | .00056 | .22 |
| TRAILR2 | 4131 | 0.09 (0.02) | 5.19 × 10–08 | .83 | OPG | 4131 | 0.10 (0.03) | .0006 | .0023 |
| MMP10 | 4131 | –0.10 (0.02) | 2.47 × 10–08 | .0051 | SRC | 4131 | -0.11 (0.03) | .00032 | .003 |
| HE4 | 4198 | –0.04 (0.01) | 6.16 × 10–08 | .00047 | |||||
| REG4 | 4198 | –0.04 (0.01) | 4.88 × 10–07 | .016 | |||||
| PTX3 | 4131 | –0.08 (0.02) | 6.73 × 10–06 | .13 | |||||
| mAmP | 4131 | –0.09 (0.02) | 2.41 × 10–06 | .00075 | |||||
| ITGA1 | 4198 | –0.03 (0.01) | 6.99 × 10–05 | .73 | |||||
| ECP | 4131 | 0.07 (0.02) | 5.15 × 10–05 | .73 | |||||
| ErbB4HER4 | 4198 | –0.03 (0.01) | 4.61 × 10–05 | .22 | |||||
| MIA | 4198 | –0.03 (0.01) | 8.10 × 10–05 | .068 | |||||
| TM | 4131 | 0.07 (0.02) | 0.0001 | .55 | |||||
| AGRP | 4131 | –0.07 (0.02) | 8.10 × 10–05 | .18 | |||||
| IL6RA | 4131 | 0.07 (0.02) | 0.00023 | .21 | |||||
| Flt3L | 4198 | 0.03 (0.01) | 0.00028 | .55 | |||||
| SCF | 4131 | –0.06 (0.02) | 0.00042 | .68 | |||||
| MPO | 4131 | 0.06 (0.02) | 0.00093 | .99 | |||||
AGRP, Agouti-related protein; AM, Adrenomedullin; BMI, body mass index; CA125, Ovarian cancer–related tumor marker CA 125; CASP3, Caspase-3; CCL20, C-C motif chemokine 20; CCL4, C-C motif chemokine 4; CDKN1A, Cyclin-dependent kinase inhibitor 1; CHI3L1, Chitinase-3-like protein 1; CSF1, Macrophage colony-stimulating factor 1; CTSL1, Cathepsin L1; CXCL11, C-X-C motif chemokine 11; CXCL13, C-X-C motif chemokine 13; CXCL5, C-X-C motif chemokine 5; CXCL6, C-X-C motif chemokine 6; Dkk1, Dickkopf-related protein 1; ECP, Eosinophil cationic protein; EMMPRIN, Extracellular matrix metalloproteinase inducer; ErbB4HER4, Receptor tyrosine-protein kinase erbB-4; ESM1, Endothelial cell-specific molecule 1; EZR, Ezrin; FDR, false discovery rate; Flt3L, Fms-related tyrosine kinase 3 ligand; FRalpha, Folate receptor alpha; GAL, Galanin peptides; Gal3, Galectin-3; GDF15, Growth/differentiation factor 15; HBEGF, heparin binding epidermal growth factor like growth factor; HE4, Epididymal secretory protein E4; hK11, Kallikrein-11; IL12, Interleukin-12; IL16, Interleukin-16; IL18, Interleukin-18; IL6, Interleukin-6; IL6RA, Interleukin-6 receptor subunit alpha; IL7, Interleukin-7; IL8, Interleukin-8; ILT3, Immunoglobulin-like transcript 3; ITGA1, Integrin alpha-1; KLK6, Kallikrein-6; LAPTGFbeta1, Latency-associated peptide transforming growth factor beta-1; mAmP, Membrane-bound aminopeptidase P; MB, Myoglobin; MIA, Melanoma-derived growth regulatory protein; MICA, MHC class I polypeptide-related sequence A; MMP10, matrix metalloproteinase-10; MMP7, matrix metalloproteinase-7; MPO, Myeloperoxidase; NTRK3, NT-3 growth factor receptor; OPG, Osteoprotegerin; PRSS8, Prostasin; PTX3, Pentraxin-related protein PTX3; RAGE, Receptor for advanced glycosylation end products; REG4, Regenerating islet-derived protein 4; SCF, Stem cell factor; SE, standard error; SRC, Proto-oncogene tyrosine-protein kinase Src; TM, Thrombomodulin; TNFR1, Tumor necrosis factor receptor 1; TNFR2, Tumor necrosis factor receptor 2; TNFSF14, Tumor necrosis factor ligand superfamily member 14; TRAIL, TNF-related apoptosis-inducing ligand; TRAILR2, TNF-related apoptosis-inducing ligand receptor 2; TRANCE, TNF-related activation-induced cytokine; VEGFD, Vascular endothelial growth factor D; WHR, waist-to-hip ratio.
a A protein was considered to be specific to BMI if (1) it was internally replicated to associate with BMI but not WHR and (2) the β coefficient for its association with BMI statistically differed from that for its association with WHR in multivariate linear regressions (and vice versa for proteins specific to WHR). Proteins specific to BMI or WHR were highlighted in bold font.
b Linear regressions with BMI and WHR adjusted for each other in the same model and adjusted for age, sex, smoking, high alcohol consumption, systolic blood pressure, low density lipoprotein, and antihypertensive medication. FDR-adjusted P1 < .05 was used as the cutoff.
c F-tests were conducted to compare if the absolute values for weighs of BMI and WHR, as reflected by β coefficients, differed significantly in multivariate linear regressions. FDR-adjusted P2 < .05 was used as the cutoff.
With the orthogonal screening, all BMI-specific proteins showed weak to moderate correlations with each other (largest Spearman correlation |r|=0.61). As for WHR-specific proteins, heparin-binding endothelial growth factor-like growth factor and cyclin-dependent kinase inhibitor 1 showed high correlation with dickkopf-related protein 1 (Dkk1, r = 0.82) and caspase-3 (r = 0.83), respectively, and had more associations with WHR in the TSIR approach (see Table S5 (16)). After excluding Dkk1 and caspase-3, correlations between pairs of the remaining proteins are shown in Figure S2 (16). According to the PPI analysis (Figure S3 (16)), the most important module for WHR-specific proteins includes 9 nodes and 68 edges (Module 1). All the genes included are those encoding chemokines, chemokine receptors, or interleukins. Relatively loose interaction was observed among genes corresponding to the BMI-specific proteins. Figure S4 (16) shows the top Gene Ontology terms. Genes corresponding to WHR-specific proteins are primarily enriched in extracellular space, chemokine activity, cell–cell signaling, chemokine-mediated signaling pathway, inflammatory response, and neutrophil chemotaxis. Genes corresponding to BMI-specific proteins are primarily enriched in extracellular space, serine-type endopeptidase activity, and proteolysis. The KEGG pathway enrichment analysis (Figure S5 (16)) indicates that the genes corresponding to WHR-specific proteins are mainly involved in cytokine–cytokine receptor interaction. However, no significantly enriched KEGG pathways were found for BMI-specific proteins. According to Genotype-Tissue Expression (Figure S6 (16)), among the examined tissues that are highly relevant to obesity and insulin resistance, adipose tissue is a major production site for most of the BMI- or WHR-specific proteins. The expression levels of different transcripts can vary widely between subcutaneous and visceral fat. Those encoding commonly known inflammatory proteins are more likely to be expressed in visceral fat than in subcutaneous fat (eg, CCL4, CCL20, CXCL8, IL18, and CXCL6), and the encoded proteins (eg, C-C motif chemokines 4 and 20 [CCL4, CCL20], interleukins 8 and 18 [IL8, IL18], and C-X-C motif chemokine 6 [CXCL6], respectively) tend to be WHR-specific proteins.
Associations of BMI- and WHR-specific Proteins With Incidence of Diabetes
As presented in Table 3, only 2 (ie, adrenomedullin and galectin-3) of the 10 BMI-specific proteins were associated with increased risk of diabetes after adjusting for potential confounders (Model 1). None of the associations remained significant after additionally adjusting for WHR. Notably, 19 of the 22 WHR-specific proteins were positively associated with diabetes in Model 1. Adding BMI as a covariate only had minor impact on the associations. Significant HRs were still observed for 18 proteins (Model 2). In a sensitivity analysis, after performing a more strict FDR correction including 136 times of multiple testing, the significance for the protein–diabetes associations was generally consistent with those in Model 2. Proteins that remained significant in Model 2 were marked in red in the volcano plots (Figure S7 (16)). No statistically significant gender differences were noted in the associations between proteins levels and risk of diabetes (all P for interaction > .05).
Table 3.
Associations with diabetes incidence for internally replicated proteins that are specific to BMI or WHR (n = 4203)
| Proteins | Model 1a | Model 2b | Sensitivity analysisc | |||
|---|---|---|---|---|---|---|
| N | HR (95% CI) | FDR-adjusted P | HR (95% CI) | FDR-adjusted P | Adjusted P | |
| BMI | ||||||
| AM | 4131 | 1.16 (1.06, 1.27) | .0092 | 1.11 (1.01, 1.21) | .13 | .055 |
| GAL | 4131 | 0.93 (0.85, 1.01) | .27 | 0.95 (0.87, 1.03) | .36 | .32 |
| EMMPRIN | 4198 | 1.07 (0.98, 1.16) | .27 | 1.07 (0.99, 1.16) | .31 | .15 |
| KLK6 | 4131 | 0.95 (0.88, 1.04) | .34 | 0.96 (0.89, 1.05) | .44 | .45 |
| Gal3 | 4131 | 1.12 (1.03, 1.22) | .039 | 1.12 (1.03, 1.22) | .073 | .02 |
| hK11 | 4131 | 0.94 (0.87, 1.02) | .27 | 0.94 (0.87, 1.02) | .31 | .23 |
| MMP10 | 4131 | 0.95 (0.88, 1.04) | .34 | 0.95 (0.88, 1.04) | .36 | .35 |
| HE4 | 4198 | 1.03 (0.94, 1.12) | .52 | 1.04 (0.95, 1.13) | .50 | .55 |
| REG4 | 4198 | 0.97 (0.89, 1.05) | .45 | 0.98 (0.90, 1.06) | .54 | .62 |
| mAmP | 4131 | 1.06 (0.98, 1.15) | .28 | 1.06 (0.98, 1.15) | .31 | .24 |
| WHR | ||||||
| PRSS8 | 4198 | 1.29 (1.18, 1.42) | 3.09 × 10 –07 | 1.25 (1.15, 1.37) | 1.38 × 10 –05 | 1.22 × 10 –05 |
| IL18 | 4131 | 1.18 (1.09, 1.28) | .00034 | 1.14 (1.05, 1.24) | .0038 | .0073 |
| CCL20 | 4131 | 1.23 (1.14, 1.32) | 3.63 × 10 –07 | 1.20 (1.12, 1.30) | 1.38 × 10 –05 | 1.71 × 10 –05 |
| CCL4 | 4131 | 1.15 (1.07, 1.24) | .00053 | 1.13 (1.05, 1.22) | .0036 | .0071 |
| IL8 | 4131 | 1.15 (1.06, 1.24) | .00082 | 1.15 (1.06, 1.24) | .0016 | .0027 |
| CDKN1A | 4091 | 1.14 (1.05, 1.23) | .0023 | 1.11 (1.03, 1.21) | .011 | .023 |
| CXCL6 | 4131 | 1.19 (1.10, 1.29) | .00012 | 1.17 (1.08, 1.26) | .00093 | .0013 |
| CXCL11 | 4194 | 1.01 (0.93, 1.10) | .78 | 1.00 (0.92, 1.08) | .91 | .95 |
| GDF15 | 4131 | 1.20 (1.09, 1.31) | .00037 | 1.18 (1.08, 1.30) | .0016 | .0027 |
| CHI3L1 | 4131 | 1.14 (1.05, 1.24) | .0037 | 1.11 (1.02, 1.21) | .016 | .034 |
| CA125 | 4131 | 1.07 (0.98, 1.16) | .12 | 1.05 (0.97, 1.14) | .26 | .36 |
| LAPTGFbeta1 | 4198 | 1.17 (1.08, 1.27) | .00028 | 1.15 (1.06, 1.24) | .0016 | .0027 |
| CTSL1 | 4131 | 1.18 (1.08, 1.28) | .00037 | 1.14 (1.05, 1.24) | .0042 | .0090 |
| CASP3 | 4131 | 1.11 (1.02, 1.20) | .017 | 1.10 (1.01, 1.19) | .035 | .066 |
| CXCL5 | 4198 | 1.13 (1.04, 1.23) | .0044 | 1.12 (1.03, 1.22) | .012 | .025 |
| HBEGF | 4131 | 1.14 (1.06, 1.24) | .0016 | 1.13 (1.04, 1.22) | .0042 | .0090 |
| CXCL13 | 4128 | 1.10 (1.02, 1.19) | .022 | 1.08 (1.00, 1.17) | .064 | .11 |
| IL7 | 4149 | 1.15 (1.06, 1.24) | .00082 | 1.14 (1.05, 1.23) | .0031 | .0054 |
| Dkk1 | 4131 | 1.14 (1.05, 1.24) | .0021 | 1.14 (1.05, 1.24) | .0036 | .0071 |
| MMP7 | 4131 | 1.20 (1.10, 1.31) | .00016 | 1.21 (1.11, 1.32) | .00014 | .00022 |
| OPG | 4131 | 1.12 (1.02, 1.22) | .018 | 1.12 (1.03, 1.22) | .015 | .031 |
| SRC | 4131 | 0.94 (0.87, 1.02) | .13 | 0.94 (0.87, 1.02) | .13 | .19 |
Plasma proteins are standardized. Significant P values were highlighted in bold font.
Abbreviations: AM, Adrenomedullin; BMI, body mass index; CA125, Ovarian cancer-related tumor marker CA 125; CASP3, Caspase-3; CCL20, C-C motif chemokine 20; CCL4, C-C motif chemokine 4; CDKN1A, Cyclin-dependent kinase inhibitor 1; CHI3L1, Chitinase-3-like protein 1; CI, confidence interval; CTSL1, Cathepsin L1; CXCL11, C-X-C motif chemokine 11; CXCL13, C-X-C motif chemokine 13; CXCL5, C-X-C motif chemokine 5; CXCL6, C-X-C motif chemokine 6; Dkk1, Dickkopf-related protein 1; EMMPRIN, Extracellular matrix metalloproteinase inducer; FDR, false discovery rate; GAL, Galanin peptides; Gal3, Galectin-3; GDF15, Growth/differentiation factor 15; HBEGF, heparin binding epidermal growth factor like growth factor; HE4, Epididymal secretory protein E4; hK11, Kallikrein-11; HR, hazard ratio; IL18, Interleukin-18; IL7, Interleukin-7; IL8, Interleukin-8; KLK6, Kallikrein-6; LAPTGFbeta1, Latency-associated peptide transforming growth factor beta-1; mAmP, Membrane-bound aminopeptidase P; MMP10, Matrix metalloproteinase-10; MMP7, Matrix metalloproteinase-7; OPG, Osteoprotegerin; PRSS8, Prostasin; REG4, Regenerating islet-derived protein 4; SRC, Proto-oncogene tyrosine-protein kinase Src; WHR, waist-to-hip ratio. FDR-adjusted P < 0.05 was used as the cutoff.
a Cox proportional hazards regressions adjusted for age, sex, smoking, high alcohol consumption, systolic blood pressure, low density lipoprotein, and antihypertensive medication.
b Adjusted for covariates in Model 1 plus WHR or BMI.
c P values generated in multivariate analysis (Model 2) were corrected using a more strict FDR correction by taking all the protein–diabetes associations (multiple testing = 136 times) into consideration.
Discussion
The present observational study identified plasma proteins that were specifically associated with BMI or WHR, respectively. WHR-specific proteins, but not BMI-specific proteins, were positively associated with increased risk of diabetes. From a proteomic perspective, we demonstrated for the first time that compared with general obesity, abdominal fat distribution may have some unique pathological features, which could potentially explain its ability to confer additional risk of diabetes.
Previously, several other studies have linked Olink proteomics to obesity. Ferreira et al (21) identified discriminant protein biomarkers for obesity (BMI ≥ 30 kg/m2) using a biostatistical method. Folkersen et al (22) found causal evidence for the associations of several proteins with BMI or WHR using a Mendelian randomization analysis. In addition, Pang et al (23) reported that only some of the BMI-associated proteins were predictive of CVD risk. However, none of those studies focused on the unique proteomic features of different obesity measures and their role in diabetes. WHR was only moderately correlated with BMI in our study. Meanwhile, in line with previous observations (8, 9), WHR was predictive of diabetes independent of BMI, clearly indicating that WHR identified different and unique aspects of obesity-related risk from that of BMI. Previous genome-wide association studies have revealed different genetic bases for BMI and WHR (7, 10). In addition, several studies indicate that BMI and body fat distribution are differently linked to circulating proteins (6, 24, 25). Typically, only a few well-studied proteins have been investigated (24, 25), such as adiponectin, CRP, IL6, and tumor necrosis factor α. Unlike prior studies, we focused mainly on the proteins that were specific for BMI or WHR. We also included a broader spectrum of proteins and adopted a TSIR approach (18, 19) to ensure internal replicability for identified proteins. More importantly, we took a further step to investigate whether the identified proteins could drive differential metabolic risks, which has not been explored earlier.
In our TSIR approach, a significant overlap was found between proteins associated with BMI and WHR. Most of the overlapping proteins are among the top proteins that show positive associations with BMI (eg, leptin [LEP], fatty acid-binding protein, adipocyte [FABP4] and IL1 receptor antagonist protein [IL1ra]) or WHR (eg, cathepsin D [CTSD], CCL3, tissue-type plasminogen activator [tPA]). LEP and FABP4 are adipokines with well-established roles in adipocyte biology (6). IL1ra and CCL3 are known to be involved obesity-associated inflammation (26). CTSD is a lysosomal aspartyl protease that could be activated by weight gain and contributes to adipocyte death (27). TPA, a serine protease, is also increased in obesity, possibly due to a compensatory response to impaired fibrinolysis caused by obesity-associated metabolic stress (28). BMI and WHR still share some proteomic features, which is not surprising since they both measure obesity. However, obesity is also a heterogeneous disorder. In this study, both BMI and WHR have their unique proteins. Most of these proteins are predominantly expressed in adipose tissue but levels of expression vary between subcutaneous and visceral fat. This suggests that the different protein profiles of BMI and WHR may at least partly result from discrepancy between protein expression in visceral and subcutaneous fat depots.
BMI- and WHR-specific proteins appear to be involved in different biological processes. Our results indicate that proteolysis is the primary biological process associated with the BMI-specific proteins. Consistently, a major proportion of the BMI-specific proteins are proteases (eg, kallikrein-6, kallikrein-11, matrix metalloproteinase [MMP] 10, and membrane-bound aminopeptidase P) or could importantly regulate proteolytic activity (eg, extracellular matrix metalloproteinase inducer and epididymal secretory protein E4 (29)). In line with this finding, previous data showed that proteolytic systems (eg, the ubiquitin–proteasome system and autophagy) are altered in obesity (30) and increased proteolysis in obesity may be related to consequences such as muscle catabolism (31). In contrast, WHR-specific proteins are primarily involved in cell–cell signaling, chemokine activity, and inflammatory and immune responses. Unlike a loose connection observed among BMI-specific proteins in the PPI network, WHR-specific proteins seem tightly connected, with chemokines, chemokine receptors, and interleukins forming the most important module. Together, these results support that heterogeneity exists in obesity. Compared with BMI, high WHR is more likely to occur together with signs of inflammation.
Most of the WHR-specific proteins remained predictive of diabetes risk even after multivariate adjustment. It is speculated that WHR-related inflammatory signals may at least partly explain or mirror why WHR contributes to metabolic risk in addition to BMI. Inflammation is a key link between obesity and diabetes (32). Among WHR-specific proteins that are predictive of diabetes, several have already been shown to be involved in adipose inflammation and diabetes-related pathogenesis (IL18, IL8, CCL20, CCL4, CXCL6 (26, 32, 33)). In contrast, the increased expression of growth/differentiation factor 15 might be a compensatory mechanism against inflammation but inadequate to reduce inflammation-related diabetes risk (15, 34). Some proteins might be involved in pathways related to adipogenesis or inflammation, for instance the canonical Wnt-β–catenin pathway (eg, Dkk1 (35) and MMP7 (36)) or its downstream adipogenic transcription factor, peroxisome proliferator–activated receptor gamma (eg, cathepsin L1 (37) and osteoprotegerin (38)), which has a pivotal role in regulating inflammation, adipogenesis, insulin synthesis and secretion, and pancreatic beta cell proliferation (39). Nevertheless, the observed associations could be causal or the elevated protein may simply reflect a pre-existing inflammatory state and diabetes risk.
In contrast, hardly any of the BMI-specific proteins are predictive of diabetes risk. One possible explanation might be that some of the BMI-associated proteins that could confer diabetes risk are proteins shared by BMI and WHR (eg, E-selectin (22)). In addition, even though some diabetes risk–related proteins showed association with BMI but not WHR, their association with BMI vs WHR did not significantly differ. These proteins (eg, receptor for advanced glycosylation end products (22)) were still excluded because we used relatively strict filter out strategies aiming at focusing on the specific proteins.
Strengths of the present study included the prospective design, the long-term follow-up with a high follow-up rate, and a large number of proteins measured at baseline. In addition, the adoption of the TSIR approach significantly increased internal validity. However, the absence of an external validation cohort was a major limitation which made it inappropriate to directly translate any specific protein into the clinical setting. Our study has several other limitations that need to be considered. First, targeted proteomic analysis was conducted on proteins known or suspected to be involved in tumors or CVDs. Thus other proteins of significance could have been missed. Second, both BMI and WHR could be subject to measurement errors. Even though both usually change slowly over time, it is possible that these anthropometric measures changed over the long follow-up period. However, if anything, this should be similar for both measures and reduce the relationships with diabetes. Third, although we excluded all individuals with prevalent diabetes at baseline, and a large majority of incident diabetes can be assumed to be type 2 diabetes, it is still possible that a few were latent autoimmune diabetes in the adult patients. This number can be assumed to be small though. Fourth, even though the diabetes registers have been validated previously (17), different parameters were used in different registries and the definition of incident diabetes was not standardized. Fifth, sample sizes for analyzing different proteins varied between 3478 and 4198 subjects, which might potentially restrict the direct comparison between the proteins. Last, due to the nature of an epidemiological design, residual confounding cannot be ruled out even after extensive adjustment for potential confounders.
Taken together, the present study indicates that, compared with general obesity, abdominal fat distribution carries a unique proteomic signature that can contribute to diabetes risk. Once better understood, the findings may help to characterize specific metabolic phenotypes of obesity or risk mechanisms, and suggest better strategies to prevent metabolic disorders related to abdominal fat distribution.
Acknowledgements
The authors acknowledge the information provided by the National Diabetes Register of Sweden, the Malmö HbA1c Register, the Diabetes 2000 register, and the registers provided by the Swedish Board of Health and Welfare.
Glossary
Abbreviations
- BMI
body mass index
- BP
blood pressure
- CCL
C-C motif chemokine
- CTSD
cathepsin D
- CVD
cardiovascular disease
- CXCL
C-X-C motif chemokine
- Dkk1
dickkopf-related protein 1
- FABP
fatty acid–binding protein, adipocyte
- FDR
false discovery rate
- HR
hazard ratio
- IL
interleukin
- IL1ra
IL1 receptor antagonist protein
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- LDL
low-density lipoprotein cholesterol
- LEP
leptin
- MDC
Malmö Diet and Cancer
- MDC-CC
Malmö Diet and Cancer-Cardiovascular Cohort
- MMP
matrix metalloproteinase
- NPX
normalized protein expression
- PEA
proximity extension assay
- PPI
protein-protein interaction
- SD
standard deviation
- tPA
tissue-type plasminogen activator
- TSIR
2-step iterative resampling
- WHR
waist-to-hip ratio
Contributor Information
Xue Bao, Department of Cardiology, Nanjing Drum Tower Hospital, the Affiliated Hospital of Nanjing University Medical School, Nanjing, China; Department of Clinical Sciences, Malmö, Lund University, Malmö, Sweden.
Biao Xu, Department of Cardiology, Nanjing Drum Tower Hospital, the Affiliated Hospital of Nanjing University Medical School, Nanjing, China.
Songjiang Yin, The First Clinical Medical College, Nanjing University of Chinese Medicine, Nanjing, China.
Jingxue Pan, Department of Clinical Sciences, Malmö, Lund University, Malmö, Sweden.
Peter M Nilsson, Department of Clinical Sciences, Malmö, Lund University, Malmö, Sweden.
Jan Nilsson, Department of Clinical Sciences, Malmö, Lund University, Malmö, Sweden.
Olle Melander, Department of Clinical Sciences, Malmö, Lund University, Malmö, Sweden.
Marju Orho-Melander, Department of Clinical Sciences, Malmö, Lund University, Malmö, Sweden.
Gunnar Engström, Department of Clinical Sciences, Malmö, Lund University, Malmö, Sweden.
Author contributions
X.B., B.X., and G.E. designed the study. X.B. performed the statistical analysis. X.B., B.X., S.Y., J.P., P.M.N., J.N., O.M., M.O.-M., and G.E. contributed to the analysis and interpretation of the data. X.B. wrote the first draft of the manuscript. X.B., B.X., S.Y., J.P., P.M.N., J.N., O.M., M.O.-M., and G.E. reviewed, edited, and critically revised the manuscript, and contributed to the discussion. All authors gave final approval of the manuscript prior to submission.
Data Availability
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
Funding
This work was supported by the Swedish Heart Lung foundation (grant number 20200173); the National Natural Science Foundation of China (grant number 82100478); and the Natural Science Foundation of Jiangsu Province (grant number BK20200128). The Malmö Diet and Cancer study was funded by grants from the Swedish Cancer Society, the Swedish Medical Research Council, AFA insurance, the Albert Påhlsson and Gunnar Nilsson Foundations, the Malmö city council and Lund University Infrastructure grant “Malmö population-based cohorts” [STYR 2019/2046].
Conflict of Interest
The authors declare that there is no conflict of interests regarding the publication of this article.
References
- 1. Bluher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288-298. [DOI] [PubMed] [Google Scholar]
- 2. Mahase E. Stop using body mass index as measure of health, say MPs. BMJ 2021;373:n941. doi: 10.1136/bmj.n941 [DOI] [PubMed] [Google Scholar]
- 3. Piche ME, Vasan SK, Hodson L, et al. . Relevance of human fat distribution on lipid and lipoprotein metabolism and cardiovascular disease risk. Curr Opin Lipidol. 2018;29(4):285-292. [DOI] [PubMed] [Google Scholar]
- 4. Karpe F, Pinnick KE. Biology of upper-body and lower-body adipose tissue--link to whole-body phenotypes. Nat Rev Endocrinol. 2015;11(2):90-100. [DOI] [PubMed] [Google Scholar]
- 5. Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes (Lond). 2010;34(6):949-959. [DOI] [PubMed] [Google Scholar]
- 6. Lind L, Figarska S, Sundstrom J, et al. . Changes in proteomic profiles are related to changes in BMI and fat distribution during 10 years of aging. Obesity (Silver Spring) 2020;28(1):178-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shungin D, Winkler TW, Croteau-Chonka DC, et al. . New genetic loci link adipose and insulin biology to body fat distribution. Nature 2015;518(7538):187-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qin L, Corpeleijn E, Jiang C, et al. . Physical activity, adiposity, and diabetes risk in middle-aged and older Chinese population: the Guangzhou Biobank Cohort Study. Diabetes Care. 2010;33(11):2342-2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Koning L, Gerstein HC, Bosch J, et al. . Anthropometric measures and glucose levels in a large multi-ethnic cohort of individuals at risk of developing type 2 diabetes. Diabetologia. 2010;53(7):1322-1330. [DOI] [PubMed] [Google Scholar]
- 10. Locke AE, Kahali B, Berndt SI, et al. . Genetic studies of body mass index yield new insights for obesity biology. Nature 2015;518(7538):197-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Emdin CA, Khera AV, Natarajan P, et al. . Genetic association of waist-to-hip ratio with cardiometabolic traits, type 2 diabetes, and coronary heart disease. JAMA 2017;317(6):626-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berglund G, Elmstahl S, Janzon L, et al. . The Malmo Diet and Cancer Study. Design and feasibility. J Intern Med. 1993;233(1):45-51. [DOI] [PubMed] [Google Scholar]
- 13. Hjelm F, Tran B, Fredriksson S. Sensitive detection of cytokines in 1-μl serum samples using Proseek®. Nat Methods. 2011;8:iii-iiv. doi: 10.1038/nmeth.f.348 [DOI] [Google Scholar]
- 14. Lundberg M, Eriksson A, Tran B, et al. . Homogeneous antibody-based proximity extension assays provide sensitive and specific detection of low-abundant proteins in human blood. Nucleic Acids Res. 2011;39(15):e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bao X, Borne Y, Muhammad IF, et al. . Growth differentiation factor 15 is positively associated with incidence of diabetes mellitus: the Malmo Diet and Cancer-Cardiovascular Cohort. Diabetologia. 2019;62(1):78-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bao X, Xu B, Yin SJ, et al. . Data from: Proteomic profiles of body mass index and abdominal fat distribution and their role in incidence of diabetes. Figshare. Deposited 4 March 2022. Doi: 10.6084/m9.figshare.19304438 [DOI] [Google Scholar]
- 17. Enhorning S, Sjogren M, Hedblad B, et al. . Genetic vasopressin 1b receptor variance in overweight and diabetes mellitus. Eur J Endocrinol. 2016;174(1):69-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kang G, Liu W, Cheng C, et al. . Evaluation of a two-step iterative resampling procedure for internal validation of genome-wide association studies. J Hum Genet. 2015;60(12):729-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ramne S, Drake I, Ericson U, et al. . Identification of inflammatory and disease-associated plasma proteins that associate with intake of added sugar and sugar-sweetened beverages and their role in type 2 diabetes risk. Nutrients. 2020;12(10):3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yan Y, Drew DA, Markowitz A, et al. . Structure of the mucosal and stool microbiome in lynch syndrome. Cell Host Microbe. 2020;27(4):585-600 e584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ferreira JP, Pizard A, Machu JL, et al. . Plasma protein biomarkers and their association with mutually exclusive cardiovascular phenotypes: the FIBRO-TARGETS case-control analyses. Clin Res Cardiol. 2020;109(1):22-33. [DOI] [PubMed] [Google Scholar]
- 22. Folkersen L, Gustafsson S, Wang Q, et al. . Genomic and drug target evaluation of 90 cardiovascular proteins in 30,931 individuals. Nat Metab. 2020;2(10):1135-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pang Y, Kartsonaki C, Lv J, et al. . Associations of adiposity, circulating protein biomarkers, and risk of major vascular diseases. JAMA Cardiol 2021;6(3):276-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schlecht I, Fischer B, Behrens G, et al. . Relations of visceral and abdominal subcutaneous adipose tissue, body mass index, and waist circumference to serum concentrations of parameters of chronic inflammation. Obes Facts 2016;9(3):144-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Panagiotakos DB, Pitsavos C, Yannakoulia M, et al. . The implication of obesity and central fat on markers of chronic inflammation: the ATTICA study. Atherosclerosis 2005;183(2):308-315. [DOI] [PubMed] [Google Scholar]
- 26. Xu L, Kitade H, Ni Y, et al. . Roles of chemokines and chemokine receptors in obesity-associated insulin resistance and nonalcoholic fatty liver disease. Biomolecules 2015;5(3):1563-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eguchi A, Feldstein AE. Lysosomal cathepsin D contributes to cell death during adipocyte hypertrophy. Adipocyte 2013;2(3): 170-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zheng Z, Nakamura K, Gershbaum S, et al. . Interacting hepatic PAI-1/tPA gene regulatory pathways influence impaired fibrinolysis severity in obesity. J Clin Invest. 2020;130(8):4348-4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim KK, Turner R, Khazan N, et al. . Role of trypsin and protease-activated receptor-2 in ovarian cancer. PLoS One. 2020;15(5):e0232253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Homma T, Fujii J. Emerging connections between oxidative stress, defective proteolysis, and metabolic diseases. Free Radic Res. 2020;54(11-12):931-946. [DOI] [PubMed] [Google Scholar]
- 31. Zhou Q, Du J, Hu Z, et al. . Evidence for adipose-muscle cross talk: opposing regulation of muscle proteolysis by adiponectin and Fatty acids. Endocrinology 2007;148(12):5696-5705. [DOI] [PubMed] [Google Scholar]
- 32. Sharma BR, Kanneganti TD. NLRP3 inflammasome in cancer and metabolic diseases. Nat Immunol. 2021;22(5):550-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gustafson B. Adipose tissue, inflammation and atherosclerosis. J Atheroscler Thromb. 2010;17(4):332-341. [DOI] [PubMed] [Google Scholar]
- 34. Unsicker K, Spittau B, Krieglstein K. The multiple facets of the TGF-beta family cytokine growth/differentiation factor-15/macrophage inhibitory cytokine-1. Cytokine Growth Factor Rev. 2013;24(4):373-384. [DOI] [PubMed] [Google Scholar]
- 35. Lu H, Li X, Mu P, et al. . Dickkopf-1 promotes the differentiation and adipocytokines secretion via canonical Wnt signaling pathway in primary cultured human preadipocytes. Obes Res Clin Pract 2016;10(4):454-464. [DOI] [PubMed] [Google Scholar]
- 36. Dey N, Young B, Abramovitz M, et al. . Differential activation of Wnt-beta-catenin pathway in triple negative breast cancer increases MMP7 in a PTEN dependent manner. PLoS One. 2013;8(10):e77425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang M, Zhang Y, Pan J, et al. . Cathepsin L activity controls adipogenesis and glucose tolerance. Nat Cell Biol. 2007;9(8):970-977. [DOI] [PubMed] [Google Scholar]
- 38. Zhang C, Luo X, Chen J, et al. . Osteoprotegerin promotes liver steatosis by targeting the ERK-PPAR-gamma-CD36 pathway. Diabetes. 2019;68(10):1902-1914. [DOI] [PubMed] [Google Scholar]
- 39. Chen J, Ning C, Mu J, et al. . Role of Wnt signaling pathways in type 2 diabetes mellitus. Mol Cell Biochem. 2021;476(5):2219-2232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.