Abstract
Background
Few studies to date have attempted to measure serum anti-Müllerian hormone (AMH) levels in adult men, and solid references ranges have not yet been defined in a large cohort.
Objective
In this study, we aimed, first, to establish the reference ranges for serum AMH and AMH-to-total testosterone ratio (AMH/tT) in adult males. Second, we investigated the relationship between serum AMH and both reproductive hormones and semen parameters.
Methods
This single-center retrospective study included 578 normozoospermic adult men. Serum AMH concentrations were determined with an automated sandwich chemiluminescent immunoassay.
Results
The median serum AMH was 43.5 pmol/L. The 2.5th and 97.5th percentile values for serum AMH and AMH/tT were 16.4 and 90.3 pmol/L and 0.45 and 3.43, respectively. AMH was positively correlated with inhibin B and sperm concentration and negatively correlated with age, follicle-stimulating hormone (FSH), and progressive sperm motility. Interestingly, using immunofluorescence, we documented for the first time that AMH type II receptor (AMH-R2) is expressed in ejaculated human spermatozoa and gonadotrophic cells in the postmortem pituitary gland.
Conclusions
We establish a new age-specific reference range for serum AMH and AMH/tT. Moreover, AMH-R2 expression in human spermatozoa and gonadotrophic cells, together with the relationship between serum AMH levels and sperm motility or mean FSH levels, highlight new potential functions of AMH in regulating sperm motility or FSH secretion in adult men.
Keywords: anti-Müllerian hormone, reference ranges, adult men, AMH-R2, FSH secretion, spermatozoa motility
Anti-Müllerian hormone (AMH) is a dimeric glycoprotein that belongs to the transforming growth factor-beta superfamily. It binds to its exclusive AMH type II receptor (AMH-R2), which has been shown to interact with the type 1 receptor serine/threonine kinases ACVR1 (activin A receptor, type 1), BMPR1A (bone morphogenetic protein receptor, type 1A), and BMPR1B (bone morphogenetic protein receptor, type 1B) (1-3). Upon AMH binding, this complex leads to the phosphorylation of SMAD1/5/8 proteins, which are translocated to the nucleus and are involved in the regulation of gene expression (4, 5).
AMH is the first hormone secreted by immature Sertoli cells at approximately 8 weeks of gestation and is responsible for regression of the Müllerian ducts in the male fetus during the sexual differentiation process (6). AMH is secreted at high levels in boys from early fetal life until the onset of puberty. However, increased production of testicular testosterone (T) from the Leydig cells at puberty reduces AMH secretion, in parallel with Sertoli cell maturation and the appearance of meiotic germ cells in the seminiferous tubules (7, 8). As a result, AMH serum levels in males start declining compared with prepubertal levels and continue to decrease progressively throughout adulthood. Hence, AMH is considered a good marker for testicular endocrine function.
In men with impaired T synthesis or in cases of androgen insensitivity syndrome related to androgen receptor mutations, serum AMH levels are abnormally elevated after the onset of puberty. Intermediate serum AMH values are also observed in men with gonadotropin-releasing hormone deficiency (hypogonadotropic hypogonadism) (9). Conversely, AMH is low in men with gonadal dysgenesis, such as in cryptorchidism, Klinefelter syndrome, disorders of sex development, and acquired primary testicular hypogonadism (10). Indeed, at puberty and then in adult men, blood-circulating AMH is an important marker of testicular function.
Based on the previously reported clinical data, it can be inferred that multiple developmentally regulated mechanisms control AMH secretion in men in various physiological and pathological conditions, thereby increasing the complexity of interpreting serum AMH values and AMH function in different clinical conditions. Among other factors, consideration of serum T levels and the functional status of the Sertoli cells are required. Consequently, using the ratio of AMH to total T (AMH/tT) may provide a potentially effective biomarker for predicting the severity of testicular failure, as previously suggested (11, 12). However, the current lack of serum AMH reference ranges in normozoospermic adult men obscures the interpretation of such analyses. Before 2014, commercial assays for the measurement of blood-circulating AMH levels were manual and had several drawbacks (ie, low sensitivity, interference with complement). The 2 most commonly used were enzyme-linked immunosorbent assays: EIA AMH/MIS from Immunotech, and Gen II from Beckman Coulter (13). Since 2014, Roche and Beckman Coulter have distributed automated methods for AMH measurement on their analyzers (Cobas or DxI Access, respectively). These methods have good analytical performance (higher sensitivity and better reproducibility) and are robust tools for clinical applications (14, 15).
Our study aimed to direct the use of serum AMH and AMH/tT in adult male patients by determining robust reference ranges in a large cohort of normozoospermic adult men. Second, to explore new potential physiological functions, we investigated the relationship between serum AMH and both reproductive hormones and semen parameters.
Methods
Study Design
The study population included normozoospermic adult men followed in the Departments of Andrology and Reproductive Medicine at Lille University Hospital. Included individuals were the male members of subfertile couples referred for andrological phenotyping. Our study was conducted between January 2016 and June 2021. This study followed the tenants of the Declaration of Helsinki and was approved by the Ethics Committee of Lille University Hospital (no. DEC21-213). All patients provided their written informed consent for participation.
Written informed consent was also obtained from each adult man prior to the inclusion of any sperm sample in the Germetheque biobank. The use of sperm samples was approved by the Germetheque Scientific Committee (DE-S1-030-11). The biobank registered declaration DC-2014–2202 and authorization AC-2015–2350.
Human brain tissue was obtained in accordance with French bylaws (Good Practice Concerning the Conservation, Transformation, and Transportation of Human Tissue to be Used Therapeutically, published on December 29, 1998). Permission to use human tissues was obtained from the French Agency for Biomedical Research (Agence de la Biomedecine, Saint-Denis la Plaine, France, protocol no. PFS16-002) and the Lille Neurobiobank.
Patients and Study Outcomes
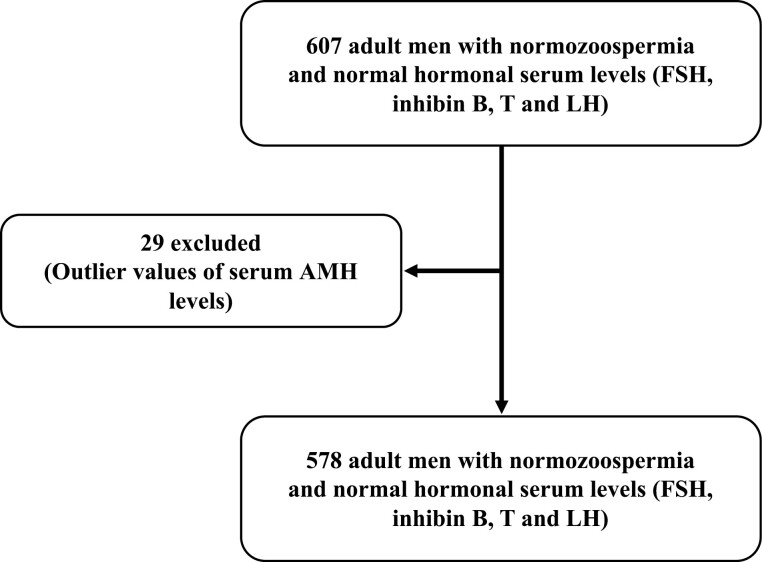
The flow chart of the study is presented in Figure 1. Initially, 607 normozoospermic adult men were selected based on normal semen analysis and normal serum values of inhibin B (92-316 pg/mL), follicle-stimulating hormone (FSH; 1.2-7.8 IU/L), luteinizing hormone (LH; 0.6-12 IU/L), and total T [2.40-8.7 ng/mL (8.32-30.18 nmol/L)] (16, 17). Using the robust regression followed by the outlier identification method (ROUT), 29 individuals with outlier AMH serum values were identified and excluded. A total of 578 adult men were finally included in this study. Semen and blood samples were provided on the same day. No patients included in our study had received hormonal stimulation (such as clomiphene, human chorionic gonadotropin, or recombinant FSH).
Figure 1.
Flow chart of the study.
Hormone Analysis
All blood samples for hormone assays were collected from the cubital vein and then centrifuged at 4°C to recover the serum. Since serum hormone concentrations decrease during the daytime (18), we collected a single serum sample per patient in the morning (between 8 and 10 am) and in nonfasting state.
A fully automated sandwich chemiluminescent immunoassay using the DxI Access system (Beckman Coulter, USA) was used to measure serum AMH levels. Its limit of quantification was 0.47 pmol/L. Below this threshold, AMH was defined as undetectable. Intra- and interassay coefficients of variation were <2 and <3%, respectively.
FSH, LH, and total T were measured by immunoassay using an automatic analyzer (Architect, Abbott Laboratories, USA). The limit of quantification of each assay was as follows: FSH = 0.2 IU/L, LH = 0.5 IU/L, and total T = 0.04 ng/ml. Intra- and interassay coefficients of variation were 3.1% to 5.6% for FSH, 4.3% to 6.4% for LH, and 4.7% to 5.2% for total T (19). Reference ranges were from 1.2 to 7.8 IU/L for FSH, 0.60 to12 IU/L for LH, and 2.40 to 8.70 ng/mL (8.3-30.2 nmol/L) for total T.
The serum inhibin B assay was determined using an Inhibin B Gen II ELISA kit (Beckman Coulter, Villepinte, France). The assay’s limit of quantification was 8 pg/mL. Below this threshold, inhibin B was defined as undetectable. The reference range for serum inhibin B was from 92 to 316 pg/mL (16).
AMH/tT values were calculated using serum AMH and serum total T concentration values as nanograms per milliliter.
Semen Collection and Analysis
Semen samples were provided in the laboratory by masturbation into a sterile plastic specimen cup, following a requested period of 2 to 7 days of sexual abstinence. After 30 minutes of liquefaction at room temperature, a manual semen analysis was performed as previously described (16). The semen volume, total sperm count, sperm concentration, motility, and vitality were interpreted according to the 2010 World Health Organization guidelines (20). Normal forms were analyzed and interpreted according to the standardized David’s modified classification method (21).
Immunofluorescence of Semen Samples
Human semen samples (n = 4) were used. After the sperm had been prepared, 100 μL of homogenate was fixed with 2% paraformaldehyde in phosphate-buffered saline (PBS) 1× and incubated for 15 minutes at room temperature. Spermatozoa were isolated by centrifugation (10 minutes, 350 × g), and the supernatant was removed. The sperm pellet was resuspended in PBS 1×, washed twice by centrifugation (10 minutes, 350 × g) and then resuspended in 100 μL of PBS 1×. A thick drop was placed on a SuperFrost slide (Menzel-Gläser, Braunschweig, Germany) and stored at 4°C.
Sperm smears were placed in acetone for 5 minutes and then rinsed twice in PBS 1× (5 minutes). Samples then were treated with PBS-10% normal goat serum at room temperature for 1 hour. For double immunostaining, samples were simultaneously incubated with polyclonal rabbit anti-AMH-R2 antibody (custom-made immunogenic peptide: CELWALAVEERKRPNIPS-NH2, intracellular serine/threonine kinase domain, CASLO, Denmark, diluted 1:2000 in PBS 1×-0.3% Triton) or rabbit polyclonal anti-human AMH-R2 antibody (recombinant fragment corresponding to Human AMHR2 aa 200-450, ab197148, Abcam, diluted 1:500 in PBS 1×-0.3% Triton) and with mouse monoclonal anti-α-tubulin antibody [RRID: AB_477593, http://antibodyregistry.org/AB_477593 (Sigma-Aldrich), diluted 1:2000 in PBS 1×-0.3% Triton] or a mouse monoclonal anti-A-kinase anchor protein 4 (AKAP4) antibody [RRID: AB_2909420, http://antibodyregistry.org/AB_2909420, 4BDX-1602, clone 7E10 (4BioDx), diluted 1:1000 in PBS 1×-0.3% Triton] overnight at 4°C. Negative control slides were performed by omitting the primary antibody.
After two 5-minute washes in PBS 1×, slides were incubated for 90 minutes at room temperature with Alexa Fluor 488 Goat Anti-Rabbit IgG (H + L) (RRID: AB_2630356, http://antibodyregistry.org/AB_2630356, Invitrogen, Eugene, OR, USA) and Alexa Fluor 568 Goat Anti-Mouse IgG (H + L) (RRID: AB_2534072, http://antibodyregistry.org/AB_2534072, Invitrogen) diluted 1:600 in PBS 1×. The slides were then washed twice for 5 minutes in PBS 1×. Nuclear counterstaining was performed using diaminopyrolylindole 4,6-diamino, 2-pyrolylindole (Invitrogen). Lastly, slides were washed twice for 5 minutes in PBS 1× and mounted using Dako Fluorescence Mounting Medium (Agilent Technologies, CA, USA). Immunofluorescence-stained slides were imaged using a Zeiss Spinning disk confocal microscope with a 100× oil-immersion lens (NA 1.4). For each subject, ≥100 spermatozoa were evaluated. Images were processed with ZEN software (Carl Zeiss, version 14.0.0.201, Germany).
Immunofluorescence of the Postmortem Adult Human Pituitary Gland
An adult human pituitary was obtained between 24 and 36 hours postmortem from 1 autopsied individual: an adult man, aged 65 years, who had donated his body for scientific research in accordance with French bioethics laws. A review of his medical records indicated that he had no known neurological or neuroendocrinological disorders. A dissected block of the adult brain containing the pituitary gland was fixed by immersion in 4% paraformaldehyde in 0.1M phosphate buffer, pH 7.4, at 4°C for 2 weeks. The tissues were cryoprotected in 30% sucrose/PBS at 4°C overnight, embedded in Tissue-Tek OCT compound (Sakura Finetek), frozen in dry ice, and stored at −80°C until sectioning.
A citrate-buffer antigen retrieval step, 10 mM citrate in PBS-0.3% Triton, pH 6, for 30 minutes at 70°C, was performed on 20-μm sections before immunolabeling. After three 5-minute washes with PBS-0.3% Triton, the sections were blocked in incubation solution (10% normal goat serum, 1 mg/mL bovine serum albumin in PBS-0.3% Triton, pH 7.4) for 1 hour.
For double immunostaining, samples were simultaneously incubated with a previously validated in AMH-R2 knockout animals (22) polyclonal rabbit anti-AMH-R2 [RRID:AB_2909430, http://antibodyregistry.org/AB_2909430, custom-made immunogenic peptide: CELWALAVEERKRPNIPS-NH2, intracellular serine/threonine kinase domain, CASLO, Denmark, diluted 1:2000 in an incubation solution] or with rabbit polyclonal anti-human AMH-R2 antibody [RRID:AB_2909403, http://antibodyregistry.org/AB_2909403, recombinant fragment corresponding to Human AMHR2 aa 200-450, ab197148, Abcam, diluted 1:500 in an incubation solution] and with mouse monoclonal anti-β-FSH antibody [RRID: AB_10895714, http://antibodyregistry.org/AB_10895714, 411 (Biocare Medical), diluted 1:200 in incubation solution]. Blocking was followed by primary antibody incubation in an incubation solution for 48 hours at 4°C. Primary antibodies were then rinsed out before incubation in fluorophore-coupled secondary antibodies [Alexa Fluor 488 Goat anti-Rabbit IgG (H + L) and Alexa Fluor 568 Goat anti-Mouse IgG (H + L); RRID: AB_2630356 and AB_2534072, respectively, Invitrogen; diluted 1:600 in PBS 1× for 2 hours in PBS-0.3% Triton at room temperature].
Secondary antibodies were washed, and sections were counterstained with diaminopyrolylindole 4,6-diamino, 2-pyrolylindole (Invitrogen). Finally, the sections were quenched for autofluorescence using Autofluorescence Eliminator Reagent (2160, Millipore) following the manufacturer’s instructions and then mounted using Dako Fluorescence Mounting Medium (Agilent Technologies). Immunofluorescence-stained slides were imaged using a Zeiss Spinning disk confocal microscope with a 40× oil-immersion lens (NA 1.3 with an optical resolution of 176 nm). Images were processed with ZEN software (Carl Zeiss, version 14.0.0.201, Germany).
Statistical Analysis
In all analyses, the normality of distribution of the studied variables was assessed using the Shapiro-Wilk test.
Data were analyzed according to the Clinical and Laboratory Standards Institute guideline EP28-A3 (23). Current guidelines define a reference interval as 2 limiting values that 95% of the population fall within; ideally, it is determined using a minimum of 120 individuals to have statistical significance (24, 25). As previously described, the robust regression followed by outlier identification method was used to identify outliers of AMH serum values in individuals of the reference group (26).
As the semen and hormone variables did not follow a normal distribution or log-normal distribution, nonparametric tests were performed, and no transformation was applied.
Relationships between quantitative variables were studied by the Spearman’s rank correlation coefficient. Possible confounding factors were selected based on biological relevance. The partial correlation coefficient was used to analyze the correlation between variables while adjusting for age, serum FSH, and inhibin B levels.
All tests were 2-tailed, with a significance level set at 0.05. Statistical analyses were performed using GraphPad Prism 7.0 software (La Jolla, CA, USA), and for the partial correlation coefficient analysis, SPSS Version 23 (IBM SPSS Inc., Chicago, IL, USA) was used.
Results
Demographic Characteristics, Hormonal Profiles, and Semen Characteristics
Semen characteristics and hormone level distributions are summarized in Table 1.
Table 1.
Semen characteristics and hormone level distributions
| Median | Interquartile range (first and third quartiles) | 2.5th and 97.5th percentiles | |
|---|---|---|---|
| Age, years | 35 | 31-39 | 24.5-51 |
| FSH, IU/l | 3.2 | 2.4-4.3 | 1.3-6.6 |
| LH, IU/L | 2.7 | 2.0-3.5 | 1.2-5.5 |
| Total T, nmol/L | 17.5 | 13.9-21.4 | 8.9-28.7 |
| Inhibin B, pg/mL | 175 | 144-215 | 99-289 |
| AMH, pmol/L | |||
| Overall population | 43.5 | 31.5-58.3 | 16.4-90.3 |
| Adults 20-39 years | 43.8 | 32.5-59.4 | 18.1-90.9 |
| Adults >39 years | 38.3 | 29.4-53.1 | 12.3-87.6 |
| AMH/tTa | |||
| Overall population | 1.21 | 0.83-1.74 | 0.45-3.43 |
| Adults 20-39 years | 1.22 | 0.82-1.72 | 0.45-3.44 |
| Adults >39 years | 1.16 | 0.80-1.76 | 0.36-3.36 |
| Semen parameters | |||
| Semen volume, mL | 3.3 | 2.4-4.5 | 1.5-7.4 |
| Total sperm count, million/ejaculate | 229.5 | 138.8-394.8 | 55-890.5 |
| Sperm concentration, million/mL | 70.2 | 43.2-114.9 | 18.8-248.3 |
| Progressive motility, % | 60 | 48.7-65 | 35-75 |
| Normal forms, % | 37 | 29-47 | 23-62.9 |
Abbreviations: AMH, anti-Müllerian hormone; FSH, follicle-stimulating hormone; LH, luteinizing hormone; T, testosterone; tT, total testosterone.
aAMH/tT values were calculated using serum AMH and serum total T concentration values in ng/mL.
The median, first quartile, and third quartile for serum AMH concentrations were 43.5, 31.5, and 58.3 pmol/L, respectively. The 2.5th and 97.5th percentile values for serum AMH were 16.4 and 90.3 pmol/L, respectively.
Reference Ranges of Serum Anti-Müllerian Hormone Concentrations and AMH/tT Values
Because serum AMH levels had a significant negative correlation with age (see following discussion), we first compared serum AMH levels between different quartile age groups [Supplementary Table 1, Supplementary Figure 1 (27)]. Adults aged between 39 (third quartile) and 62 (maximum age value) years had significantly lower serum AMH values (P = 0.02) compared with those between 31 and 35 years old (first and second quartiles, respectively) or under 31 years (P = 0.02). As a result, we established the reference ranges in terms of age groups. The first group includes adult men aged between 20 and 39 years (minimum age value and the third quartile, respectively), and the second group includes those over 39 years.
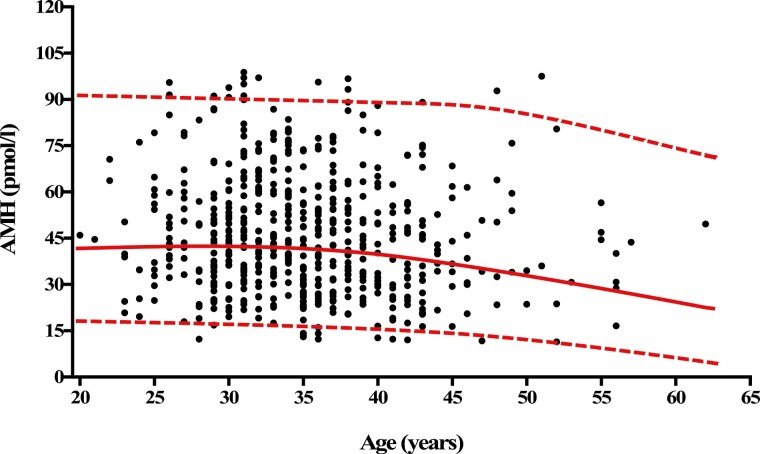
The median (interquartile range) of serum AMH in individuals <39 years was 43.8 (32.5-59.4) pmol/L; the 2.5th and 97.5th percentile values for serum AMH were 18.1 and 91 pmol/L, respectively. In the group of individuals >39 years, the median (interquartile range) of serum AMH was 38.3 (29.4-53.1) pmol/L; the 2.5th and 97.5th percentile values for serum AMH were 12.3 and 87.6 pmol/L, respectively (Table 1, Fig. 2). The median (interquartile range) of AMH/tT in adult men <39 years was 1.22 (0.82-1.72; the 2.5th and 97.5th percentile values for AMH/tT were 0.44 and 3.44, respectively. The median (interquartile range) of AMH/tT in normozoospermic adult men >39 years was 1.16 (0.80-1.76); 2.5th and 97.5th percentile values for AMH/tT were 0.36 and 3.36, respectively (Table 1, Fig. 2).
Figure 2.
Age distribution of serum anti-Müllerian hormone (AMH) concentrations in normozoospermic adult men. The curves represent the median (red medial curve), the 2.5th percentile (bottom red dashed curve), and the 97.5th percentile (top red dashed curve). The normal range for AMH serum concentrations was 18.1 to 90.9 pmol/L in individuals between 20 and 39 years of age. For individuals aged >39 years, the normal range for AMH serum concentrations was 12.3 to 87.6 pmol/L.
Correlations Between Serum AMH Concentrations and Hormone Levels or Semen Parameters
Correlations between serum AMH, inhibin B, FSH, LH, total T levels, and age are summarized in Table 2. Interestingly, serum AMH concentrations were negatively correlated with age (r = −0.11; P = 0.009), FSH (r = −0.20; P < 0.0001), and progressive sperm motility (r = −0.10; P = 0.01) and positively correlated with serum inhibin B levels (r = 0.31; P < 0.0001), sperm concentration (r = 0.10; P = 0.01), and total sperm count (r = 0.12; P = 0.004). Also, AMH remained negatively correlated with serum FSH concentrations after adjustment for age and serum inhibin B levels (r = −0.09; P = 0.02) [Supplementary Table 2 (27)].
Table 2.
Relationship between hormone levels and semen parameters
| Hormone levels | AMH, pmol/L | AMH/tT, ng.mL-1/ng.mL−1 | FSH, IU/L | LH, IU/L | T, nmol/L | Inhibin B, pg/mL |
|---|---|---|---|---|---|---|
| FSH, IU/L |
−0.20
(<0.0001) |
−0.3 (<0.0001) |
— | — | — | — |
| LH, IU/L | −0.07 (0.09) |
−0.23
(<0.0001) |
0.31
(<0.0001) |
— | — | — |
| Total T, nmol/L | 0.02 (0.57) |
−0.54
(<0.0001) |
0.09
(0.03) |
0.30
(<0.000) |
— | — |
| Inhibin B, pg/mL |
0.31
(<0.0001) |
0.22
(<0.0001) |
−0.40
(<0.0001) |
−0.18(<0.0001) | 0.07 (0.08) | — |
| Age, years |
−0.11
(0.009) |
−0.03 (0.50) |
0.06 (0.14) | −0.01 (0.77) |
−0.12
(0.005) |
−0.02 (0.63) |
| Semen volume, mL | 0.03 (0.42) |
0.005 (0.89) |
−0.01 (0.80) |
0.01 (0.74) |
0.04 (0.34) |
0.10
(0.01) |
| Sperm concentration, million/mL |
0.10
(0.01) |
0.12
(0.002) |
−0.12
(0.002) |
−0.09
(0.02) |
−0.05 (0.20) |
0.20
(<0.0001) |
| Total sperm count, million/ejaculate |
0.12
(0.004) |
0.12
(0.002) |
−0.12
(0.004) |
−0.07 (0.09) |
−0.08 (0.05) |
0.24
(<0.0001) |
| Progressive motility, % |
−0.10
(0.01) |
−0.04 (0.30) |
−0.07 (0.06) |
−0.05 (0.20) |
−0.03 (0.39) |
0.01 (0.69) |
Data are reported as r (P-value). The relationship between different variables was assessed by Spearman’s rank correlation. AMH/tT values were calculated using serum AMH and serum total T concentration values in ng/mL. P values greater than 0.05 are shown in bold.
Abbreviations: AMH, anti-Müllerian hormone; FSH, follicle-stimulating hormone; LH, luteinizing hormone; T, testosterone; tT, total testosterone.
A significant positive relationship was observed between AMH and serum inhibin B levels after adjustment for age and serum FSH concentrations (r = 0.22; P = 0.0001) [Supplementary Table 3 (27)]. Also, serum AMH concentrations were negatively correlated with progressive sperm motility after adjustment for age and serum FSH or inhibin B concentrations [Supplementary Tables 2 and 3 (27)]. When adjusting for serum FSH levels and age, we observed that the correlations of serum AMH and inhibin B levels with sperm concentration and total sperm count were similar [Supplementary Table 3 (27)].
Correlations between AMH/tT values and hormone levels or semen parameters are summarized in Table 2 and Supplementary Tables 2 and 3 (27).
Expression of AMH-R2 in Human Spermatozoa and the Human Anterior Lobe of the Pituitary Gland
In seeking a possible functional explanation for the negative correlation that we observed between circulating AMH levels in adult men and sperm motility as well as FSH levels, we decided to explore the expression of the exclusive AMH binding receptor (AMH-R2) in human spermatozoa and in β-FSH gonadotrophs, respectively.
The same results were obtained with the custom-made anti-AMH-R2 antibody and the commercial anti-human AMH-R2 antibody (Abcam).
Immunofluorescence of AMH-R2 in human ejaculated spermatozoa
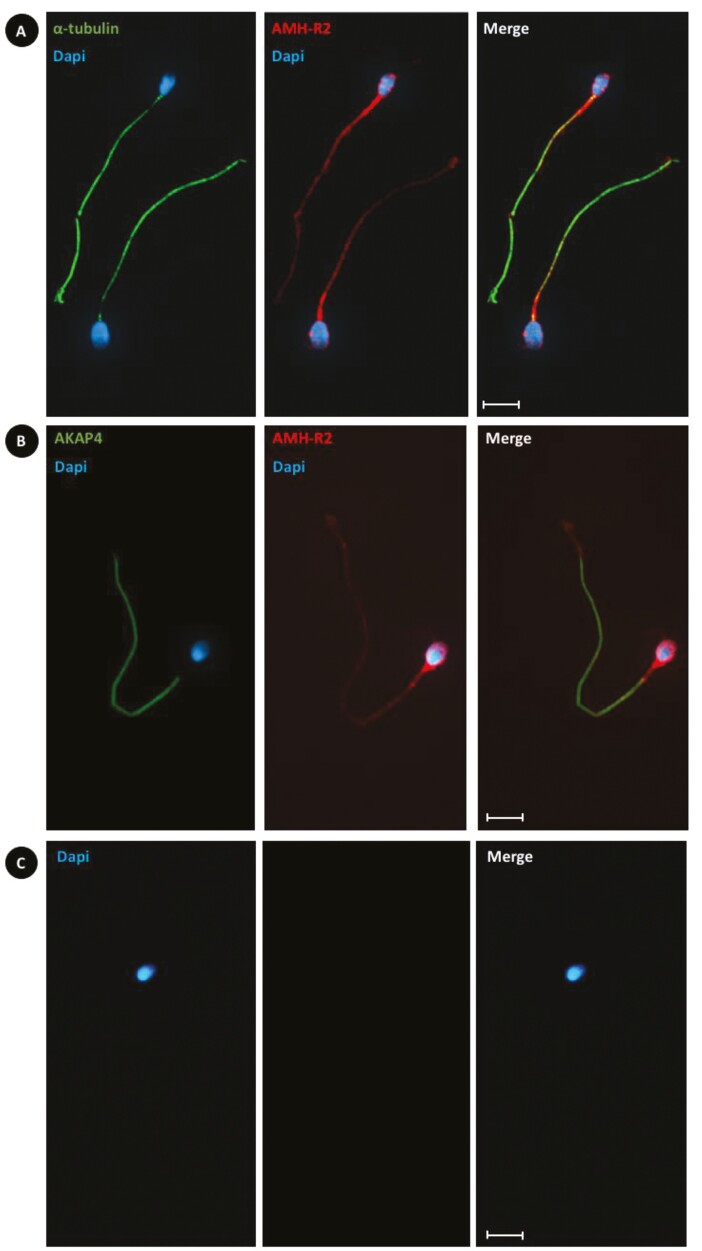
Immunocytochemistry in ejaculated sperm demonstrated AMH-R2 immunoreactivity in the spermatozoa head and all along the tail, with the highest AMH-R2 expression detected at the level of the head and midpiece of the tail [Fig. 3A and 3B; also see Supplementary Figure 2A and 2B) (27)]. α-Tubulin is localized in the tail (midpiece, principal piece, and the end piece). Conversely, AKAP4 is localized only in the principal piece.
Figure 3.
Immunolocalization of anti-Müllerian hormone type II receptor (AMH-R2; using the custom-made antibody) with α-tubulin or A-kinase anchor protein 4 (AKAP4) in human ejaculated spermatozoa. In human ejaculated spermatozoa, AMH-R2 (red; anti-AMH-R2 antibody) is expressed in the head, middle piece, and tail. (A) α-Tubulin (green) is expressed in the middle piece, and tail. (B) AKAP4 (green) is expressed only in the tail. (C) Absence of staining without primary antibodies. Only secondary antibodies and diaminopyrolylindole 4,6-diamino, 2-pyrolylindole nuclear counterstaining (blue) were used. Scale bars = 5 μ>m.
Immunofluorescence of AMH-R2 in the anterior lobe of the pituitary gland
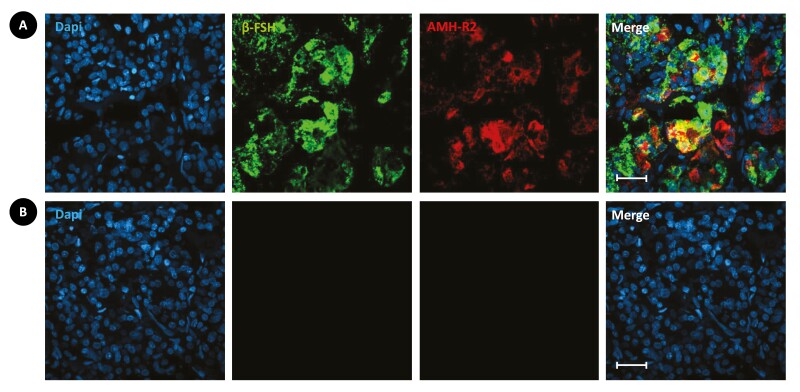
Immunofluorescence experiments were then performed in the adult human pituitary gland. Simultaneous double-labeling using antibodies against AMH-R2 and β-FSH, respectively, showed that AMH-R2 is expressed by β-FSH immunoreactive gonadotropic cells of the anterior lobe [Fig. 4; also see Supplementary Figure 3 (27)].
Figure 4.
Immunolabeling for anti-Müllerian hormone type II receptor (AMH-R2; using the custom-made antibody) and β-follicle-stimulating hormone (β-FSH) in longitudinal sections of adult human pituitary gland. (A) In the control adult human pituitary gland, AMH-R2 (red; anti-AMH-R2 antibody) is expressed abundantly with β-FSH (green, anti-human β-FSH antibody) in the gonadotropic cells of the anterior lobe. (B) Absence of staining without primary antibodies. Only secondary antibodies and diaminopyrolylindole 4,6-diamino, 2-pyrolylindole nuclear counterstaining (blue) were used. Scale bars = 25 μ>m.
Discussion
AMH measurement is currently used in pediatric endocrinology for diagnostic purposes. However, poor knowledge of AMH function in adult men, together with the lack of reliable reference ranges of circulating AMH levels in normozoospermic adult men, have prevented the use of AMH measurement in adulthood for clinical purposes. To fill this gap in knowledge, we analyzed blood levels of AMH and AMH/tT values in a large cohort of normozoospermic adult men.
We first reported the reference range for serum AMH concentrations and AMH/tT values in normozoospermic adult men. Second, we studied the relationship between serum AMH and both reproductive hormones and semen parameters.
The Reference Range for Serum AMH Concentrations and AMH/tT Values
Interestingly, serum AMH concentrations were negatively correlated with age in normozoospermic adult men. In our study, the normal range for AMH serum concentrations was from 18.1 to 90.9 pmol/L for individuals between 20 and 39 years and from 12.3 to 87.6 pmol/L for those over 39 years. In 2010 Aksglaede et al reported a reference range for serum AMH levels (13-98 pmol/L) in a cohort of 150 adult men from the general population aged between 21.6 and 64.4 years (7). Indeed, in the latter study, exhaustive andrological phenotyping including inhibin B measurement and the evaluation of spermatic parameters were not performed. Their AMH assay was also less sensitive than the one used in our study.
We have also reported for the first time the reference range for blood AMH/tT (20-39 years of age: 0.45-3.44 pmol/L; >39 years of age: 0.36-3.36 pmol/L) in normozoospermic adult men.
The reference ranges of circulating AMH levels established by our study will help in the use and interpretation of serum AMH measurements in adult endocrinology, for example, in cases of partial androgen resistance syndrome found in adulthood (28) or in adults with suspicious mutations of either AMH or AMH-R2 genes (29). Also, serum AMH measurements could be a helpful tool for better assessing the end of puberty period while transitioning from pediatric to adult endocrinology. This situation is often observed in cases of obesity, energy deficit, or history of chemotherapy or radiotherapy for pediatric cancer.
Relationship Between Serum AMH and Both Reproductive Hormones and Semen Parameters
A significant positive relationship was observed in our study between blood-circulating AMH and inhibin B levels. A close correlation was also observed after adjustment for serum FSH concentrations and age. Furthermore, serum inhibin B and serum AMH levels were positively correlated with total sperm count and sperm concentration. This relationship remained even after adjustment for age and serum FSH levels. Accordingly, serum AMH values are a reliable marker for both Sertoli cells and exocrine testicular functions and can thus be a good marker of spermatogenesis in adult men. The association between circulating AMH levels and semen quality has been assessed previously, but the results are divergent. Similar to our finding, a significant positive relationship between serum AMH and total sperm count was described previously (30, 31). However, others have reported no significant association between serum AMH levels and semen characteristics (32-35).
The New Potential Function of AMH on the Regulation of FSH Secretion
We also found an interesting significant negative correlation between serum FSH and both serum AMH and inhibin B, which conforms with data from other studies (30, 34-36). This significant relationship remained after adjusting for age and serum inhibin B levels. In seeking a possible functional explanation for the negative correlation that we observed between circulating AMH levels and serum FSH concentrations, we explored the expression of the AMH-R2 in human gonadotroph cells. Interestingly, to the best of our knowledge, we are the first to document AMH-R2 expression in gonadotropic cells of an adult man’s pituitary gland. The role of AMH in the regulation of gonadotropin secretion has been previously investigated in many preclinical studies. AMH has been reported to activate β-LH and β-FSH gene expression in LβT2 cells, a murine gonadotroph-derived cell line (37). Similarly, AMH was found to stimulate FSH secretion in rats in vivo; however, such stimulation was restricted to prepubertal female rats (38). In both male and female mice, AMH increases gonadotropin-releasing hormone–dependent LH pulsatility and secretion (22). In adult men, like inhibin B but to a lesser degree, AMH may contribute to the classical feedback loop of the pituitary-gonadal axis to decrease FSH secretion by modulating gonadotropic cell activity in the pituitary gland. Nevertheless, further in vitro experiments using isolated human gonadotropic cells are needed to elucidate whether AMH can regulate FSH secretion.
The New Potential Function of AMH on Regulating Sperm Motility
In the present study, we observed an interesting negative correlation between serum AMH levels and progressive sperm motility. In looking for a possible functional explanation for this relationship, we found that AMH-R2 is expressed mainly in the middle piece, the structure that controls flagellum motility (39). To our knowledge, the expression of AMH-R2 in human spermatozoa was not been reported before.
The mechanisms involving AMH in the regulation of sperm motility are not known. After puberty, AMH is secreted bidirectionally by the Sertoli cells: basally to the circulation and apically into the lumen of the seminiferous tubules. The concentration of AMH may be up to 10× higher in seminal fluid compared with serum (40). Data on the relationship between circulating-blood AMH or seminal concentrations of AMH and sperm motility had contrasting results.
Unlike our findings, an association between serum AMH concentrations and progressive sperm motility was not found previously (30, 32, 35). Study evaluating seminal plasma AMH described a significant positive association between seminal plasma AMH levels and progressive sperm motility (32, 41).
Interestingly, some authors have reported that AMH can improve sperm motility (42, 43). The expression of AMH-R2 by human spermatozoa documented in our study may lend support to this hypothesis. Siow et al studied the effects of recombinant human AMH on fresh and cryopreserved spermatozoa and found significantly higher motility in both fresh and cryopreserved spermatozoa after 5 and 22 hours of incubation. However, the effects of AMH were suppressed by coincubation with anti-AMH antibodies (42). In another study, AMH in seminal plasma was suggested as a predictive marker for recovery of sperm motility after cryopreservation in asthenozoospermic men (43). These 2 studies, however, had some limitations, such as the lower number of individuals included and the use of visual evaluation to study sperm motility.
An important strength of our study is that we included a large cohort of normozoospermic adult men with complete andrological phenotyping and normal hormonal serum levels. We are also the first to document AMH-R2 expression in gonadotropic cells of an adult man’s pituitary gland and human ejaculated spermatozoa.
Our study also has some limitations. The participants were patients in the andrology department and may not represent all individuals from the general population. Furthermore, it is difficult to compare our results with those of some previous studies that used older and less sensitive AMH assays. Since T levels exhibit significant diurnal variations and may be suppressed by food intake or glucose, current guidelines recommend measuring T in the morning in fasting state (44). However, a recent study found no significant differences between fasting and nonfasting serum T levels (45). For all individuals of our study, blood samples were collected in the morning (between 8 and 10 am) to limit diurnal variation of testosterone. However, blood samples were collected in a nonfasting state, which may indeed introduce a limiting factor for the interpretation of the reference range of AMH/tT.
In conclusion, the present study established the reference range values for both circulating AMH and AMH/tT depending on age in a large cohort of normozoospermic adult men and reported a new potential physiological role of blood-circulating AMH in the negative feedback regulation of FSH secretion, probably through modulation of gonadotropic cell activity in the pituitary gland. AMH could also regulate sperm motility. Further studies are nevertheless needed to confirm these hypotheses.
Acknowledgments
The authors would like to thank Drs. Carole Marchetti, Catherine Guittard, and Samira Bouzaib-Benderradji, who participated in patient recruitment for the study. Our gratitude is also extended to Blandine Delebecq, Cécile Catelle, Aurélie Cousin, Marine Coudrais, Laura Ringeval, Virginie Cloet, Marc Verstraete, and DevGen’s team for their help during data collection. We would also like to thank the patients who participated in this study.
Glossary
Abbreviations
- AMH
anti-Müllerian hormone
- AMH-R2
anti-Müllerian hormone type II receptor
- AMH/tT
anti-Müllerian hormone-to-total testosterone ratio
- AKAP4
A-kinase anchor protein 4
- FSH
follicle-stimulating hormone
- LH
luteinizing hormone
- PBS
phosphate-buffered saline
- T
testosterone
Contributor Information
Hamza Benderradji, Department of Andrology, Urology and Renal Transplantation, University of Lille, CHU Lille, Lille, France; Lille Neuroscience & Cognition (UMR-S1172), CHU Lille, Inserm, University of Lille, Lille, France.
Anne-Laure Barbotin, Lille Neuroscience & Cognition (UMR-S1172), CHU Lille, Inserm, University of Lille, Lille, France; Department of Reproductive Biology-Spermiology-CECOS, University of Lille, CHU Lille, Lille, France.
Maryse Leroy-Billiard, Department of Endocrine Gynecology and Reproductive Medicine, University of Lille, CHU Lille, Lille, France.
Julie Prasivoravong, Department of Andrology, Urology and Renal Transplantation, University of Lille, CHU Lille, Lille, France.
François Marcelli, Department of Andrology, Urology and Renal Transplantation, University of Lille, CHU Lille, Lille, France.
Christine Decanter, Department of Endocrine Gynecology and Reproductive Medicine, University of Lille, CHU Lille, Lille, France.
Geoffroy Robin, Department of Andrology, Urology and Renal Transplantation, University of Lille, CHU Lille, Lille, France; Department of Endocrine Gynecology and Reproductive Medicine, University of Lille, CHU Lille, Lille, France.
Valérie Mitchell, Lille Neuroscience & Cognition (UMR-S1172), CHU Lille, Inserm, University of Lille, Lille, France; Department of Reproductive Biology-Spermiology-CECOS, University of Lille, CHU Lille, Lille, France.
Jean-Marc Rigot, Department of Andrology, Urology and Renal Transplantation, University of Lille, CHU Lille, Lille, France.
Antonino Bongiovanni, University of Lille, Institut Pasteur de Lille, BioImaging Center Lille, Lille, France.
Florent Sauve, Lille Neuroscience & Cognition (UMR-S1172), CHU Lille, Inserm, University of Lille, Lille, France.
Luc Buée, Lille Neuroscience & Cognition (UMR-S1172), CHU Lille, Inserm, University of Lille, Lille, France.
Claude-Alain Maurage, Lille Neuroscience & Cognition (UMR-S1172), CHU Lille, Inserm, University of Lille, Lille, France; University of Lille, CHU Lille, Department of Pathological Anatomy, Lille, France.
Maryse Cartigny, Department of Pediatric Endocrinology, DevGen, Reference Centre for Genital Development Abnormalities, University of Lille, CHU Lille, Lille, France.
Arnauld Villers, Department of Andrology, Urology and Renal Transplantation, University of Lille, CHU Lille, Lille, France.
Vincent Prevot, Lille Neuroscience & Cognition (UMR-S1172), CHU Lille, Inserm, University of Lille, Lille, France.
Sophie Catteau-Jonard, Lille Neuroscience & Cognition (UMR-S1172), CHU Lille, Inserm, University of Lille, Lille, France; Department of Endocrine Gynecology and Reproductive Medicine, University of Lille, CHU Lille, Lille, France.
Nicolas Sergeant, Lille Neuroscience & Cognition (UMR-S1172), CHU Lille, Inserm, University of Lille, Lille, France.
Paolo Giacobini, Lille Neuroscience & Cognition (UMR-S1172), CHU Lille, Inserm, University of Lille, Lille, France.
Pascal Pigny, Department of Biochemistry & Hormonology, CHU Lille, Lille, France; University of Lille, Inserm, UMR-S 1277, Lille, France.
Clara Leroy, Department of Andrology, Urology and Renal Transplantation, University of Lille, CHU Lille, Lille, France; Department of Pediatric Endocrinology, DevGen, Reference Centre for Genital Development Abnormalities, University of Lille, CHU Lille, Lille, France.
Funding
This research did not receive any specific grants from any funding agency in the public, commercial, or nonprofit sector.
Author Contributions
H.B. contributed to experimental design, performed immunofluorescence for both human spermatozoa and pituitary gland, prepared the figures, the inclusion of patients, study design, execution, statistical analysis, interpretation of data, manuscript drafting, and critical discussion. A-L.B. contributed to the study design, execution, interpretation of data, manuscript drafting, and critical discussion. M.L-B., J.P., F.M., C.D., G.R., J-M.R., M.C., and S.C-J. contributed to the inclusion of patients and revised the manuscript for critical content. F.S. processed human tissues. A.B. contributed to confocal microscopy settings adjust. V.M., A.V., L.B., V.P., and C-A.M. revised the manuscript for critical content. N.S. and P.G. contributed to experimental design, interpretation of data, manuscript drafting, and critical discussion. P.P. contributed to the study design, execution, analysis and interpretation of data, manuscript drafting, and critical discussion. C.L. contributed to the inclusion of patients, study design, execution, analysis and interpretation of data, manuscript drafting, and critical discussion. All authors had access to the data and participated in the writing of the manuscript and have seen and approved the submitted version.
Disclosures
The authors have nothing to disclose, and they declare that there is no conflict of interest that could be perceived as influencing the impartiality of the research reported.
Data Availability
Because of restrictions based on privacy regulations and informed consent of the participants, data cannot be made freely available in a public repository. Anonymized data will be shared by the corresponding author on reasonable request.
References
- 1. Mishina Y, Rey R, Finegold MJ, et al. . Genetic analysis of the Müllerian-inhibiting substance signal transduction pathway in mammalian sexual differentiation. Genes Dev. 1996;10(20):2577-2587. [DOI] [PubMed] [Google Scholar]
- 2. Baarends WM, Hoogerbrugge JW, Post M, et al. . Anti-Müllerian hormone and anti-Müllerian hormone type II receptor messenger ribonucleic acid expression during postnatal testis development and in the adult testis of the rat. Endocrinology. 1995;136(12):5614-5622. [DOI] [PubMed] [Google Scholar]
- 3. di Clemente N, Wilson C, Faure E., et al. . Cloning, expression, and alternative splicing of the receptor for anti-Müllerian hormone. Mol Endocrinol. 1994;8(8):1006-1020. [DOI] [PubMed] [Google Scholar]
- 4. Josso N, di Clemente N. Transduction pathway of anti-Müllerian hormone, a sex-specific member of the TGF-β family. Trends Endocrinol Metab. 2003;14(2):91-97. [DOI] [PubMed] [Google Scholar]
- 5. Shi Y, Massagué J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell. 2003;113(6):685-700. [DOI] [PubMed] [Google Scholar]
- 6. Barbotin A-L, Peigné M, Malone SA, Giacobini P. Emerging roles of anti-Müllerian hormone in hypothalamic-pituitary function. Neuroendocrinology. 2019;109(3):218-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aksglaede L, Sørensen K, Boas M, et al. . Changes in anti-Müllerian hormone (AMH) throughout the life span: a population-based study of 1027 healthy males from birth (cord blood) to the age of 69 years. J Clin Endocrinol Metab. 2010;95(12):5357-5364. [DOI] [PubMed] [Google Scholar]
- 8. Boukari K, Meduri G, Brailly-Tabard S, et al. . Lack of androgen receptor expression in sertoli cells accounts for the absence of anti-Mullerian hormone repression during early human testis development. J Clin Endocrinol Metab. 2009;94(5):1818-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Young J, Xu C, Papadakis GE, et al. . Clinical management of congenital hypogonadotropic hypogonadism. Endocr Rev. 2019;40(2):669-710. [DOI] [PubMed] [Google Scholar]
- 10. Xu H, Zhang M, Zhang H, et al. . Clinical applications of serum anti-Müllerian hormone measurements in both males and females: an update. Innovation (N Y). 2021;2(1):100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alfano M, Ventimiglia E, Locatelli I, et al. . Anti-Mullerian hormone-to-testosterone ratio is predictive of positive sperm retrieval in men with idiopathic non-obstructive azoospermia. Sci Rep. 2017;7(1):17638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Benderradji H, Prasivoravong J, Marcelli F, et al. . Contribution of serum anti-Müllerian hormone in the management of azoospermia and the prediction of testicular sperm retrieval outcomes: a study of 155 adult men. Basic Clin Androl. 2021;31(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kumar A, Kalra B, Patel A, McDavid L, Roudebush WE. Development of a second generation anti-Müllerian hormone (AMH) ELISA. J Immunol Methods. 2010;362(1-2):51-59. [DOI] [PubMed] [Google Scholar]
- 14. Bonifacio M, Bradley CK, Karia S, Livingstone M, Bowman MC, McArthur SJ. The original Beckman Coulter Generation II assay significantly underestimates AMH levels compared with the revised protocol. J Assist Reprod Genet. 2015;32(11):1691-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rustamov O, Smith A, Roberts SA, et al. . Anti-Mullerian hormone: poor assay reproducibility in a large cohort of subjects suggests sample instability. Hum Reprod. 2012;27(10):3085-3091. [DOI] [PubMed] [Google Scholar]
- 16. Barbotin A-L, Ballot C, Sigala J, et al. . The serum inhibin B concentration and reference ranges in normozoospermia. Eur J Endocrinol. 2015;172(6):669-676. [DOI] [PubMed] [Google Scholar]
- 17. Storring PL, Das REG. The Second International Standard for Human Pituitary LH: its collaborative study by bioassays and immunoassays. J Endocrinol. 1993;138(2):345-359. [DOI] [PubMed] [Google Scholar]
- 18. Chong YH, Pankhurst MW, McLennan IS. The daily profiles of circulating AMH and INSL3 in men are distinct from the other testicular hormones, inhibin B and testosterone. PLoS One. 2015;10(7):e0133637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rahman SA, Grant LK, Gooley JJ, Rajaratnam SMW, Czeisler CA, Lockley SW. Endogenous circadian regulation of female reproductive hormones. J Clin Endocrinol Metab. 2019;104(12):6049-6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. World Health Organization; 2010. [Google Scholar]
- 21. Auger J, Eustache F, David G. Standardisation de la classification morphologique des spermatozoïdes humains selon la méthode de David modifiée. Andrologie. 2000;10(4):358-373. [Google Scholar]
- 22. Cimino I, Casoni F, Liu X, et al. . Novel role for anti-Müllerian hormone in the regulation of GnRH neuron excitability and hormone secretion. Nat Commun. 2016;7(1):10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Clinical and Laboratory Standards Institute. Defining, establishing and verifying reference intervals in the clinical laboratory: approved guideline. 3th ed. Clinical and Laboratory Standards Institute; 2010. [Google Scholar]
- 24. Horowitz GL. Reference intervals: practical aspects. EJIFCC. 2008;19(2):95-105. [PMC free article] [PubMed] [Google Scholar]
- 25. Martinez-Sanchez L, Marques-Garcia F, Ozarda Y, et al. . Big data and reference intervals: rationale, current practices, harmonization and standardization prerequisites and future perspectives of indirect determination of reference intervals using routine data. Adv Lab Med/Av En Med Lab 2021;2(1):9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Motulsky HJ, Brown RE. Detecting outliers when fitting data with nonlinear regression—a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinf. 2006; 7(1):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Benderradji H, Barbotin AL, Leroy-Billiard et al. Supplementary data for : Defining reference ranges for serum anti-Müllerian hormone (AMH) on a large cohort of normozoospermic adult men highlights new potential physiological functions of AMH on FSH secretion and sperm motility. Zenodo, Dataset. April 2022. 10.5281/zenodo.6471697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Werner R, Grötsch H, Hiort O. 46,XY disorders of sex development—the undermasculinised male with disorders of androgen action. Best Pract Res Clin Endocrinol Metab. 2010;24(2):263-277. [DOI] [PubMed] [Google Scholar]
- 29. Picard J-Y, Cate RL, Racine C, Josso N. The persistent Müllerian duct syndrome: an update based upon a personal experience of 157 cases. Sex. Dev. 2017;11(3):109-125. [DOI] [PubMed] [Google Scholar]
- 30. Appasamy M, Muttukrishna S, Pizzey A, et al. . Relationship between male reproductive hormones, sperm DNA damage and markers of oxidative stress in infertility. Reprod Biomed Online. 2007;14(2):159-165. [DOI] [PubMed] [Google Scholar]
- 31. Al-Qahtani A, Muttukrishna S, Appasamy M, et al. . Development of a sensitive enzyme immunoassay for anti-Mullerian hormone and the evaluation of potential clinical applications in males and females. Clin Endocrinol (Oxf). 2005;63(3):267-273. [DOI] [PubMed] [Google Scholar]
- 32. Andersen JM, Herning H, Witczak O, Haugen TB. Anti-Müllerian hormone in seminal plasma and serum: association with sperm count and sperm motility. Hum Reprod. 2016;31(8):1662-1667. [DOI] [PubMed] [Google Scholar]
- 33. El-Halawaty S, Azab H, Said T, et al. . Assessment of male serum anti-Mullerian hormone as a marker of spermatogenesis and ICSI outcome. Gynecol Endocrinol. 2011;27(6):401-405. [DOI] [PubMed] [Google Scholar]
- 34. Tüttelmann F, Dykstra N, Themmen APN, Visser JA, Nieschlag E, Simoni M. Anti-Müllerian hormone in men with normal and reduced sperm concentration and men with maldescended testes. Fertil Steril. 2009;91(5):1812-1819. [DOI] [PubMed] [Google Scholar]
- 35. Aksglaede L, Olesen IA, Carlsen E, Petersen JH, Juul A, Jørgensen N. Serum concentration of anti-Müllerian hormone is not associated with semen quality. Andrology. 2018;6(2):286-292. [DOI] [PubMed] [Google Scholar]
- 36. Muttukrishna S, Yussoff H, Naidu M, et al. . Serum anti-Müllerian hormone and inhibin B in disorders of spermatogenesis. Fertil Steril. 2007;88(2):516-518. [DOI] [PubMed] [Google Scholar]
- 37. Bedecarrats GY, O’Neill FH, Norwitz ER, Kaiser UB, Teixeira J. Regulation of gonadotropin gene expression by Mullerian inhibiting substance. Proc Natl Acad Sci U S A. 2003;100(16):9348-9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Garrel G, Racine C, L’Hôte D, et al. . Anti-Müllerian hormone: a new actor of sexual dimorphism in pituitary gonadotrope activity before puberty. Sci Rep. 2016;6(1):23790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lehti MS, Sironen A. Formation and function of sperm tail structures in association with sperm motility defects. Biol Reprod. 2017;97(4):522-536. [DOI] [PubMed] [Google Scholar]
- 40. Fénichel P, Rey R, Poggioli S, Donzeau M, Chevallier D, Pointis G. Anti-Müllerian hormone as a seminal marker for spermatogenesis in non-obstructive azoospermia. Hum Reprod. 1999;14(8):2020-2024. [DOI] [PubMed] [Google Scholar]
- 41. Mostafa T, Amer MK, Abdel-Malak G, et al. . Seminal plasma anti-Müllerian hormone level correlates with semen parameters but does not predict success of testicular sperm extraction (TESE). Asian J Androl. 2007;9(2):265-270. [DOI] [PubMed] [Google Scholar]
- 42. Siow Y, Fallat ME, Amin FA, Belker AM. Müllerian inhibiting substance improves longevity of motility and viability of fresh and cryopreserved sperm. J Androl. 1998;19(5):568-572. [PubMed] [Google Scholar]
- 43. Nery SF, Vieira MAF, Dela Cruz C, et al. . Seminal plasma concentrations of anti-Müllerian hormone and inhibin B predict motile sperm recovery from cryopreserved semen in asthenozoospermic men: a prospective cohort study. Andrology. 2014;2(6):918-923. [DOI] [PubMed] [Google Scholar]
- 44. Bhasin S, Brito JP, Cunningham GR, et al. . Testosterone therapy in men with hypogonadism: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2018;103(5):1715-1744. [DOI] [PubMed] [Google Scholar]
- 45. Livingston M, Hackett G, Ramachandran S, Heald A. Is a fasting testosterone level really necessary for the determination of androgen status in men? Clin Chim Acta. 2021;521:64-69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Because of restrictions based on privacy regulations and informed consent of the participants, data cannot be made freely available in a public repository. Anonymized data will be shared by the corresponding author on reasonable request.