Abstract
Context
Cardiovascular benefits of empagliflozin in patients with type 2 diabetes mellitus (T2DM) have been reported; however, the underlying mechanism remains unknown.
Objective
We hypothesized that the cardiovascular benefits of empagliflozin are associated with altered gut microbiota and plasma metabolites, and that empagliflozin may be used as an initial treatment for patients with T2DM at risk of cardiovascular diseases (CVDs).
Methods
This randomized, open-label, 3-month, 2-arm clinical trial included 76 treatment-naïve patients with T2DM and risk factors for CVD who were treated with either empagliflozin (10 mg/d, n = 40) or metformin (1700 mg/d, n = 36). We investigated changes in clinical parameters related to glucose metabolism and CVD risk factors, gut microbiota using 16S rRNA gene sequencing, and plasma metabolites using LC-MS.
Results
We found significant and similar reduction in HbA1c levels and alleviation of glucose metabolism in both groups. However, only empagliflozin improved CVD risk factors. Empagliflozin significantly reshaped the gut microbiota after 1 month of treatment; this alteration was maintained until the end of the trial. Empagliflozin increased the levels of plasma metabolites such as sphingomyelin, but reduced glycochenodeoxycholate, cis-aconitate, and uric acid levels. Concurrently, empagliflozin elevated levels of short-chain fatty acid-producing bacteria such as species from Roseburia, Eubacterium, and Faecalibacterium, and reduced those of several harmful bacteria including Escherichia-Shigella, Bilophila, and Hungatella.
Conclusion
Empagliflozin may be a superior initial therapy for patients with T2DM at risk of CVDs; its cardiovascular benefits may be associated with shifts in gut microbiota and plasma metabolites.
Keywords: cardiovascular disease, empagliflozin, gut microbiota, metabonomics, type 2 diabetes mellitus
Empagliflozin, a selective inhibitor of sodium-glucose cotransporter 2 (SGLT2i), reduces hyperglycemia in patients with type 2 diabetes mellitus by decreasing renal glucose reabsorption and elevating urinary glucose excretion (1). Furthermore, treatment with empagliflozin not only improves hyperglycemia, but also results in body-weight loss, a reduction in blood pressure, and a decrease of cardiovascular events and mortality (2). Therefore, the Food and Drug Administration has added an indication for empagliflozin, as the first antihyperglycemic medication to reduce the risk of major adverse cardiovascular death in adults with type 2 diabetes mellitus and cardiovascular diseases (CVDs) (3). However, almost all guidelines from the American Diabetes Association, International Diabetes Federation, and World Health Organization (WHO) on the management of type 2 diabetes recommend metformin as an initial therapy for patients with no contraindications (4-6). Given the cardiovascular benefit of empagliflozin, we hypothesized that it may be a better initial therapy for patients with type 2 diabetes at risk of CVDs.
Moreover, the mechanisms underlying the effects of empagliflozin on the cardiovascular system remain unclear. A previous study in animal models suggested that, in addition to empagliflozin-related diuresis, the antioxidant, anti-inflammatory, and anti-apoptotic effects of empagliflozin may also contribute to its cardiovascular benefits (7). Besides, emerging evidence indicates that the gut microbiota modulates metabolism as well as oxidative and inflammatory activities in the host, thereby significantly influencing the pathogenesis of type 2 diabetes and CVDs (8-10). Recently, an animal study suggested that another SGLT2i, dapagliflozin, subtly alters the composition of the gut microbiota in mice with type 2 diabetes (11). It has also been reported that several antidiabetic drugs such as metformin, acarbose, and liraglutide partly achieve their glucose-lowering effects and additional metabolic improvements by modulating the gut microbiota and its metabolites (12-15). However, it remains unknown whether treatment with empagliflozin alters the gut microbiota in patients with type 2 diabetes; furthermore, the relationship between the gut microbiota and CVDs-related beneficial effects of empagliflozin remains elusive.
Herein, we investigate the clinical benefits of empagliflozin and possible associations between its cardiovascular benefits and alterations in plasma metabolites and the gut microbiota in patients with type 2 diabetes and at risk of CVDs. To this end, we conducted a randomized, open-label, two-arm clinical trial on treatment-naïve patients with type 2 diabetes and risk factors for CVDs treated with either empagliflozin or metformin for 3 months.
Methods
Clinical Study Design
This study was a randomized, open-label, two-arm clinical trial approved by the Ethics Committee and the Committee for Clinical Investigation of Henan Provincial People’s Hospital (Henan, China) and registered with the Chinese Clinical Trial Registry (ChiCTR1800018825). Written informed consent was obtained from all individuals, and the study was performed according to the principles of the Declaration of Helsinki.
Seventy-six individuals with treatment-naïve type 2 diabetes and risk factors for CVDs were recruited from the Henan Provincial People’s Hospital and randomized to receive treatment with empagliflozin (10 mg/d, n = 40, Boehringer Ingelheim Pharma, Ingelheim am Rhrin, Germany) or metformin (1700 mg/d, n = 36, Merck Serono, Darmstadt, Germany) for 3 months. Randomization codes were generated by a biostatistician who did not participate in the enrollment of the participants in the study. We recommended a hypocaloric diet containing 20 to 25 kcal/kg and lifestyle changes including regular physical activity (150 min/week).
Inclusion criteria were: 1) age from 18 to 70 years; 2) treatment-naïve type 2 diabetes as defined by the 1999 WHO Criteria (13); 3) glycated hemoglobin (HbA1c) level from 6.5% to 13%; and 4) at least one of the following risk factors for CVDs: (a) systolic blood pressure (SBP) ≥ 140 mmHg or diastolic blood pressure (DBP) ≥ 90 mmHg; (b) older than 50 years if male or 60 years if female; (c) low-density lipoprotein cholesterol ≥ 2.6 mmol/L or total triglycerides ≥ 2.3 mmol/L or high-density lipoprotein cholesterol ≤ 0.88 mmol/L; (d) body mass index ≥ 28 kg/m2 (16); (e) urine albumin creatinine ratio ≥ 30; (f) ankle brachial index of either side ≤ 0.9.
Exclusion criteria were as follows: 1) type 1 diabetes mellitus diagnosis; 2) severe diabetic complications, including diabetic foot; 3) systolic blood pressure ≥ 180/110 mmHg or total cholesterol ≥ 6.2 mmol/L; 4) antibiotic use for more than 3 days within the last 3 months; 5) pregnancy or lactation period; 6) alcohol consumption of 5 times a week or more; 7) severe mental disease within 6 months before enrollment; 8) receiving drug therapy for cholecystitis, peptic ulcers, urinary tract infection, acute pyelonephritis, urocystitis, or hyperthyreosis; 9) gastrointestinal surgery except for appendicitis or hernia surgery; 10) severe hepatic diseases, including chronic hepatitis, liver cirrhosis, or the co-occurrence of positive hepatitis B virus surface antigen and abnormal alanine transaminase or aspartate transaminase levels (> 2.5 times the upper normal limit); 11) inflammatory bowel disease or Cushing syndrome; 12) pituitary dysfunction; 13) severe systemic disease, including cancer, coronary heart disease, and stroke; 14) infectious diseases, including acquired immune deficiency syndrome and pulmonary tuberculosis; 15) inability to understand the nature, scope, and possible consequences of the study; and 16) hemoglobinuria < 10 g/dL.
All participants received education on diabetes control. Standard questionnaires were administered, and anthropometric and metabolic assessments were performed at baseline and during monthly visits. Blood, urine, and fecal samples were collected at baseline and at monthly visits.
Enzyme-Linked Immunosorbent Assay
Samples were collected from the patients after 8 hours of overnight fasting. To obtain serum, blood samples were stored at 25 °C for 30 minutes and then centrifuged at 3000g for 20 minutes. Plasma and serum samples were kept on dry ice before storage at −80 °C. Fecal and urine samples were frozen in dry ice immediately after collection and then stored at −80 °C.
The serum inflammatory cytokine concentrations were measured using the following enzyme-linked immunosorbent assay (ELISA) kits, in accordance with the manufacturers’ instructions: lipopolysaccharide-binding protein (HBT-HK315-02, RRID: AB_10989485, Hycult Biotech, the Netherlands), interleukin (IL)-6 (HS600C, RRID: AB_2893335, QuantikineHS, R&D Systems, USA), and tumor necrosis factor-alpha (TNF-α) (HSTA00E, RRID: AB_2893336, QuantikineHS, R&D Systems). All ELISA kits used were highly sensitive, with a detection limit was 4.4 ng/mL for lipopolysaccharide-binding protein, 0.031 pg/mL for IL-6, and 0.022 pg/mL for TNF-α. The manufacturers’ instructions in each of the kits were followed for analyses.
Statistical Analysis
Statistical analysis was performed using STATA 15.0 (STATA Corp., College Station, TX, USA). The loss-to-follow-up rate was 20%; therefore, 40 participants per group could provide 90% power to detect a 0.5% difference in HbA1c. Continuous variables are presented as the mean ± SD. Categorical variables are presented as numbers (proportions). Wilcoxon rank-sum tests were used to estimate differences in clinical parameters between the empagliflozin group and the metformin group. The Wilcoxon matched-pairs signed-rank test was used to estimate the difference in continuous variables before and after intervention. Chi-square tests (two-tailed) were used to analyze differences in the proportion of subjects. P values < 0.05 were considered statistically significant.
Untargeted Metabolomics Analysis
An ultra-high-performance liquid chromatograph (UHPLC) (1290 Infinity LC, Agilent Technologies, Santa Clara, CA, USA) coupled to a quadrupole time-of-flight (AB Sciex TripleTOF 6600) was used by Shanghai Applied Protein Technology Co., Ltd. (Shanghai, China) to conduct liquid chromatography–tandem mass spectrometry (LC-MS/MS) analysis.
Differential metabolites were identified based on their variable importance in the projection (VIP) threshold of 1 from the 10-fold cross-validated orthogonal partial least-squares discriminant analysis (OPLS-DA) model, which was validated at a false discovery rate (FDR) value < 0.1 via the Wilcoxon matched-pairs signed-rank test using the original FDR method of Benjamini and Hochberg. Significantly differential metabolites were defined based on VIP > 1 and FDR value of < 0.05. A heatmap of the differential metabolites between week 0 and weeks 4, 8, and 12 in the empagliflozin and metformin groups was generated using MATLAB 2019b. The description of the metabolites was defined referenced their annotated KEGG (https://www.kegg.jp/), BRITE, and HMDB (https://hmdb.ca/) Subclass results.
DNA Extraction and 16S rRNA Gene Sequencing
Genomic DNA was extracted from fecal samples using the QIAamp PowerFecal Pro DNA Kit (51804, QIAGEN, Hilden, Germany). 16S rRNA gene amplicon sequencing was used to profile the composition of the bacterial community in the fecal samples. PCR was performed targeting the V3-V4 region of the 16S rRNA gene using forward [5′-CCTACGGGNGGCWGCAG-3′] and Reverse [5′-GACTACHVGGGTATCTAATCC-3′] primers (17). The subsequent amplicon sequencing was performed on a MiSeq platform to generate paired-end reads of 300 base pairs (bp) in length (Illumina, San Diego, CA, USA). Sequencing data were demultiplexed with the QIIME2 pipeline version 2019.7. For all the sequencing data, forward reads were trimmed at 279 bp and reverse reads were trimmed at 230 bp. An average of 51 543 reads were used as input, with an average of 31 106 reads recovered after filtering, denoising, merging forward and reverse reads, and removing chimeras. Amplicon sequence variants (ASVs) were defined using the DADA2 plugin (18). All samples were randomly subsampled to equal depths of 12 272 reads before the subsequent fecal microbiome analysis using QIIME2 diversity plugins. The raw Illumina sequence data in this study are available in the sequence read archive at NCBI under accession no. SRP278004.
Sequencing Data Analysis
Representative sequences for ASVs were built into a phylogenetic tree using core-metrics-phylogenetic pipeline in QIIME2 and were assigned into taxonomy with Silva database (release 132) (19). For the calculation of α-diversity, the Shannon index and observed ASVs were evaluated in QIIME2 and visualized using GraphPad Prism 8.0 (GraphPad Software Inc., La Jolla, CA, USA). Unweighted UniFrac distances between samples were evaluated in QIIME2 to test the variation of the gut microbiota. Permutational multivariate analysis of variance (PerMANOVA) and subject-adjusted principal coordinate analysis (aPCoA) were performed by R to test if the gut microbiota of the patients were altered significantly at 4, 8, or 12 weeks following treatment with empagliflozin or metformin based on between-sample unweighted UniFrac distances. PerMANOVA was performed using the Vegan package, and aPCoA was performed using the aPCoA package and plotted using package ggplot2. Differential ASVs between week 0 and weeks 4, 8, and 12 in the empagliflozin- and metformin-treated groups were identified using LEfSe analysis (http://huttenhower.sph.harvard.edu/galaxy/) respectively. Spearman correlation coefficients and a heatmap of all the differential ASVs were calculated and performed using MATLAB 2019b.
The Shannon index and observed ASVs at weeks 4, 8, and 12 were compared with those at week 0 in the empagliflozin and metformin groups using mixed-effects model analysis with Dunnett’s multiple tests in Prism version 8.0.1 (GraphPad Software Inc.). The principal component (PC) scores of samples on each PC (PC1 and PC3 for the empagliflozin group; PC2 and PC5 for the metformin group) at weeks 4, 8, and 12 were compared with those at week 0 using paired one-way ANOVA tests with Geisser-Greenhouse correction. A P value of < 0.05 was considered statistically significant.
Multi-omics Correlation Analysis
The correlation coefficients of the differential ASVs, differential serum metabolites, and clinical parameters at week 0 and week 12 in the empagliflozin and metformin groups were calculated using the method described by Bland and Altman, respectively (20). The P values of the correlations were adjusted to FDR values with the original FDR method of Benjamini and Hochberg. And the correlations were defined as significant when the FDR values were < 0.05. The visual presentation of multiple omics correlations was conducted with the Cytoscape version 3.7.2.
Results
Empagliflozin Improves Glucose Metabolism and CVD-Related Risks
A total of 116 patients with treatment-naïve type 2 diabetes and risk factors for CVDs were screened. We enrolled 76 participants and randomly assigned them to receive either empagliflozin or metformin for 3 months. Ninety-one percent of the enrolled participants completed the study and were included in the final analysis (Supplementary Fig. 1 (21)). There was no significant difference between the groups in terms of glucose metabolism, anthropomorphic and most biochemical parameters, and counts of risk factors for CVDs at baseline (Supplementary Table 1 (21)).
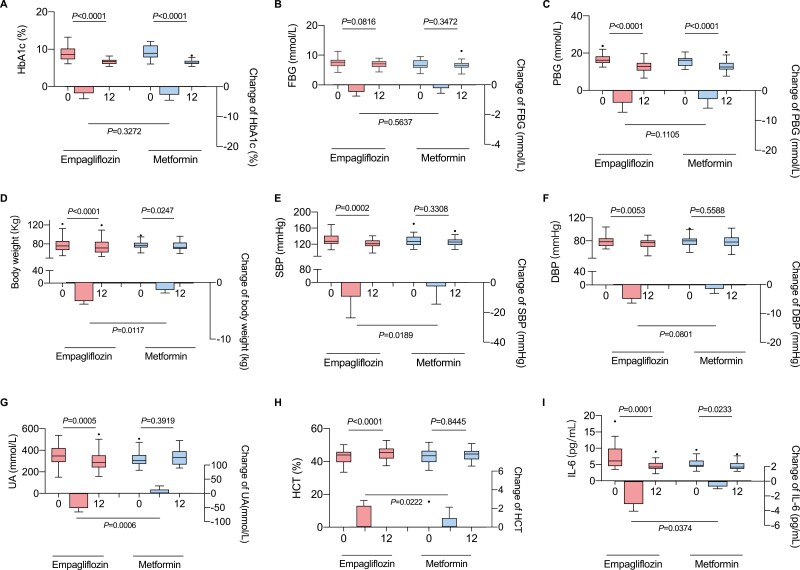
After a 3-month intervention period, the HbA1c and postprandial plasma glucose levels were significantly reduced in both the empagliflozin and metformin groups, suggesting that empagliflozin showed the equivalent improvement in glycemic control as metformin (Fig. 1). In addition, the patients in both groups had significantly decreased central obesity-related parameters such as body weight, waist circumference, and waist–hip circumference ratio (WHR) and significantly improved systemic inflammatory markers (Fig. 1 and Supplementary Table 1 (21)). Notably, the decrease in body weight and IL-6 levels were more pronounced in the empagliflozin group (Fig. 1). Moreover, the decrease in blood pressure and uric acid levels, and the increase in hematocrit and adipokine were only observed in patients treated with empagliflozin (Fig. 1, Supplementary Table 1 (21)). This finding suggests that empagliflozin is more beneficial to the cardiovascular system, as indicated by the significant improvement in clinical parameters that are risk factors for CVDs (Supplementary Table 1 (21)).
Figure 1.
Main clinical parameters in patients after treatment with empagliflozin or metformin. Abbreviations: DBP, diastolic blood pressure; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HCT, hematocrit; IL-6, interleukin-6; PPG, postprandial plasma glucose; SBP, systolic blood pressure; UA, uric acid. In the box plots, the line in the middle of the box represents the median, and the inferior and superior limits of the box refer to the 25th and 75th percentiles, respectively. The whiskers represent the 10th and 90th percentiles, and outliers are denoted.
Empagliflozin Changes Plasma Metabolites
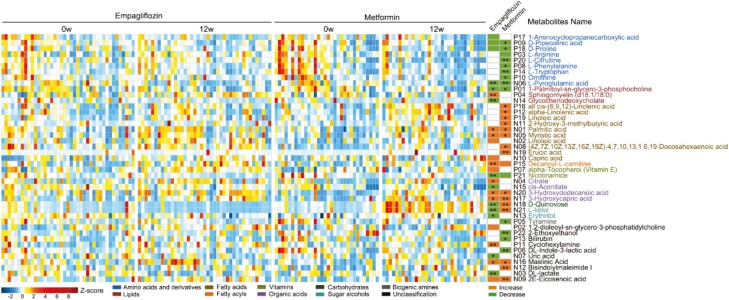
We next analyzed the plasma metabolite profiles before and after treatment and identified 27 and 30 metabolites that were altered in the empagliflozin and the metformin groups, respectively, using the 10-fold cross-validated OPLS-DA model (Fig. 2 and Supplementary Fig. 2 (21)). After the 3-month treatment period, empagliflozin increased the levels of fatty acids, fatty acyls, organic acids, phosphosphingolipids, and other metabolites such as maslinic acid but reduced the levels of amino acids and derivatives, lipids, vitamins, sugar alcohols, and other metabolites such as uric acid (Fig. 2). Metformin increased the serum levels of fatty acids and organic acids and reduced those of several amino acids and derivatives as well as other metabolites such as bilirubin and tyramine (Figure 2). We found a significant increase in the sphingomyelin and capric acid levels and a decrease in glycochenodeoxycholate, cis-aconitate, erythritol, and uric acid in the empagliflozin group but not in the metformin group (Fig. 2). Otherwise, we found metabolites, like all cis- (6, 9, 12)-linolenic acid, alpha-linolenic acid, 2-hydroxy-3-methylbutyric acid, linolenic acid, (4Z, 7Z, 10Z, 13Z, 16Z, 19Z)-4, 7, 10, 13, 16, 19-docosahexaenoic acid, erucic acid, D-quinovose, L-iditol, and bisindolylmaleimide I increased significantly with metformin, which was not found during empagliflozin use (Fig. 2).
Figure 2.
Changes in plasma metabolites during empagliflozin or metformin treatment. The abundance profiles of metabolites were transformed into Z scores. The Z score was negative (shown in blue) when the row abundance was lower than the mean. The differential metabolites were defined as the variable importance for the projection (VIP) in OPLS-DA model above 1 and FDR value < 0.1 tested via Wilcoxon matched-pairs signed-rank test using the original FDR method of Benjamini and Hochberg. The significantly different metabolites were defined as those with VIP > 1 and FDR value of < 0.05 as tested using the Wilcoxon matched-pairs signed-rank test with the original FDR method of Benjamini and Hochberg. ** represents FDR value < 0.01, * represents FDR value < 0.05.
Empagliflozin Alters the Composition of the Gut Microbiota
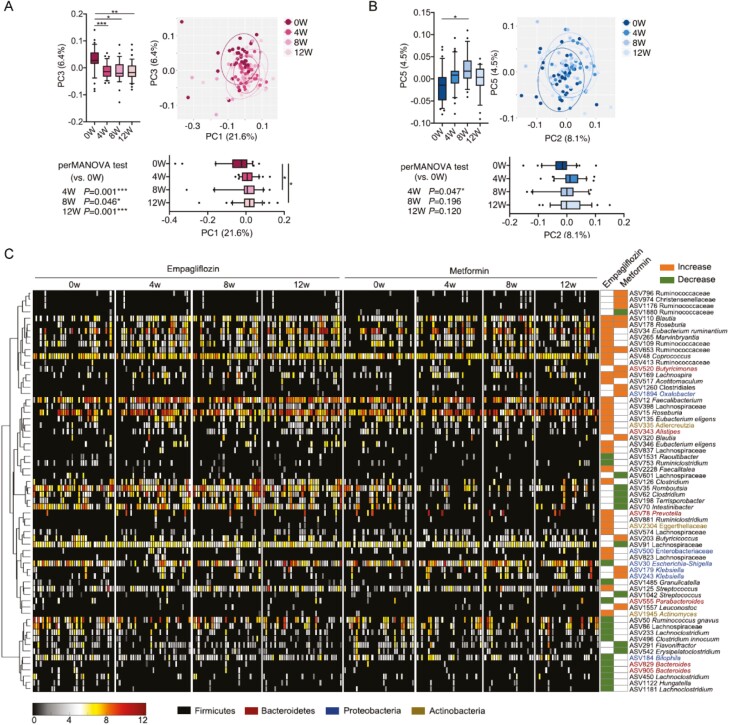
To compare the effects of empagliflozin and metformin on the gut microbiota, we conducted V3-V4 region sequencing of the 16S rRNA gene in 245 fecal samples at baseline and 4, 8, and 12 weeks. We found that the richness (observed ASVs) and diversity (Shannon index) of the gut microbiota significantly increased in the empagliflozin group but not in the metformin group (Supplementary Fig. 3 (21)). The aPCoA of Bray-Curtis distance demonstrated that the gut microbiome of the patients showed a significant shift after 4 weeks of empagliflozin treatment and then remained relatively stable (Fig. 3A). However, a significant alteration relative to the baseline was only observed in week 4 of metformin treatment (Fig. 3B). Next, we used linear discriminant analysis effect size (LEfSe) analysis to identify the bacteria responding to the empagliflozin or metformin treatment. In total, 43 ASVs in the empagliflozin group and 25 ASVs in the metformin group were significantly altered (Fig. 3C). ASVs that responded to the empagliflozin or metformin treatment were different (Fig. 3C and Supplementary Fig. 4 (21)). Only 4 ASVs were identified to belong to Firmicutes, namely ASV110 in Blautia, ASV178 in Roseburia, ASV653 in Ruminococcaceae, and ASV169 in Lachnospira; these exhibited the same effect in response to empagliflozin or metformin and were enriched after treatment. Furthermore, we found a significant increase in ASVs in Eubacterium, Faecalibacterium, Lachnospiraceae, and Eggerthellaceae, and a decrease in Escherichia-Shigella, Bilophila, and Hungatella in the empagliflozin group; however, this was not found in the metformin group.
Figure 3.
Changes in the gut microbiota during empagliflozin or metformin treatment. Subject-adjusted principal coordinate analysis (aPCoA) based on unweighted UniFrac distance for the (A) empagliflozin and the (B) metformin groups. The P values of the PerM ANOVA test based on unweighted UniFrac distance (strata in subject, 999 permutations) are shown. *P < 0.05 and ***P < 0.001. Marginal box plots show the changes in the gut microbiota at different time points on PC (the line in the middle of the box is plotted at the median, the inferior and superior limits of the box correspond to the 25th and 75th percentiles, the whiskers correspond to the 10th and 90th percentiles, and outliers are denoted). Paired one-way ANOVA tests with Geisser-Greenhouse correction were used to analyze the difference at 4, 8, or 12 weeks vs 0 weeks. *P < 0.05, **P < 0.01, and ***P < 0.001. (c) Amplicon sequence variants (ASVs) that were significantly altered after empagliflozin or metformin treatment; the cluster of the ASVs based on Spearman correlation coefficients is shown on the left. The heat map in the middle shows the relative abundance (log2-transformed) of each ASV in individual patient samples at different time points during treatment. The changes in the ASVs are shown on the right columns of the heatmap; columns colored in orange represent the right ASVs that increased in the corresponding groups; the columns colored in green represent the right ASVs that decreased in the corresponding groups. The differential ASVs were identified using the LEfSe model.
Clinical Benefits of Empagliflozin Associated With Gut Microbiota and Metabolites
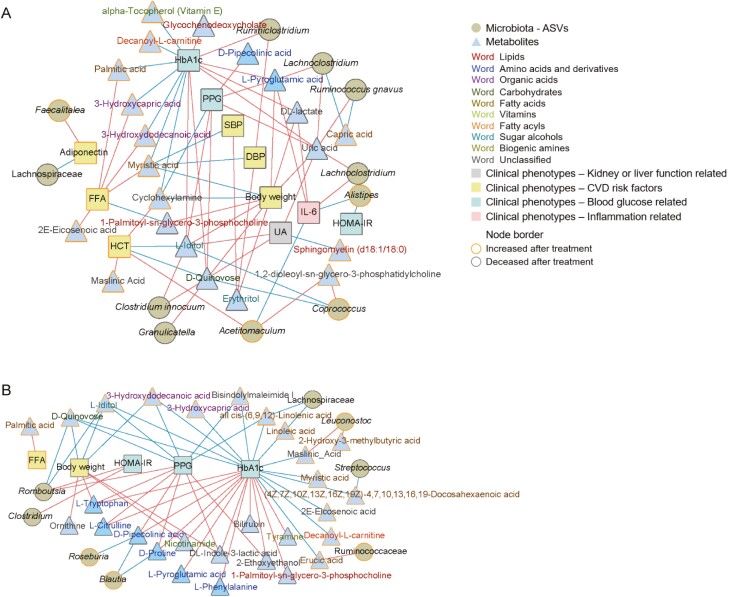
Finally, we integrated these 3 datasets to determine the correlation between the alterations in the gut microbiota, metabolites, and clinical phenotypes in patients treated with empagliflozin or metformin (Fig. 4). The clinical benefits of both empagliflozin and metformin are related to altered plasma metabolites; plasma metabolite alterations, in turn, are associated with changes in the gut microbiota of patients. Empagliflozin modified plasma metabolites and gut bacteria related to clinical parameters, including blood glucose levels, inflammatory factors, and CVD-related factors, whereas metformin treatment was associated only with changes in blood glucose levels and body weight-related modifications in plasma metabolites and gut bacteria.
Figure 4.
Correlations in the alterations of the microbiota, metabolites, and clinical phenotypes in patients treated with empagliflozin or metformin. (A) Correlations in the changes in the microbiota, metabolites, and clinical phenotypes in patients treated with empagliflozin. (B) Correlations in the changes in the microbiota, metabolites, and clinical phenotypes in patients treated with metformin. Correlation coefficients were calculated using the method described by Bland and Altman. Red connections indicate the positive correlation (FDR < 0.05), whereas blue connections show correlations that were negative (FDR < 0.05). Abbreviations: DBP, diastolic blood pressure; FFA, free fatty acid; HbA1c, glycated hemoglobin; HOMA-IR, homeostasis model assessment of insulin resistance; IL-6, interleukin-6; PPG, postprandial plasma glucose; SBP, systolic blood pressure; UA, uric acid.
Discussion
In this randomized, open-label, 2-arm clinical trial, we found that although both empagliflozin and metformin reduced HbA1c levels, empagliflozin was more likely to ameliorate risk factors for CVDs. Empagliflozin altered the plasma metabolites and gut microbiota through mechanisms that differed from those of metformin.
In our study, we found that empagliflozin had similar hypoglycemic effects, but with additional cardiovascular benefits, in patients with type 2 diabetes who had at least one risk factor for CVDs after the 3-month intervention period compared with metformin, which is a first-line antidiabetic medicine. As the CVD continuum is initiated by a myriad of risk factors, leading to the development of end-stage CVDs (22), early management of risk factors for CVD contributes to a delay in the development of CVDs. Therefore, empagliflozin may be a better drug for patients with type 2 diabetes with at least one risk factor for CVDs than metformin; however, large-sample multicenter clinical trials are warranted to confirm this.
We used untargeted metabolomics analysis to systematically investigate the effect of empagliflozin on plasma metabolites. For example, sphingomyelin, the second most common sphingolipid in mammalian cells, may prevent the translocation of gut bacteria–derived lipopolysaccharide and inhibit its proinflammatory effects (23). The levels of these beneficial metabolites increased only in the empagliflozin treatment group. The levels of glycochenodeoxycholate acid, a conjugated bile acid and farnesoid X receptor agonist that is correlated with obesity-induced insulin resistance and hepatic steatosis (24, 25); and cis-aconitate, which produces the anti-inflammatory molecule itaconate that regulates macrophage function (26, 27); were decreased in the empagliflozin treatment group. Moreover, the levels of uric acid, which is associated with the risk of congestive heart failure, arterial hypertension, atrial fibrillation, and all-cause and cardiovascular mortality (28), were also decreased in the empagliflozin treatment group. These differences in plasma metabolites induced by empagliflozin relative to metformin might contribute to glycemic and cardiovascular benefits in type 2 diabetes.
Herein, empagliflozin significantly altered the structure and composition of the gut microbiota in patients with type 2 diabetes and risk factors for CVDs after 3 months of treatment. Only 1 study was found to investigate the SGLT2i effect on microbiota. Bommel et al conducted a double-blind randomized trial in patients with type 2 diabetes to investigate the effect of another SGLT2i (dapagliflozin) on fecal microbiota and they observed that the composition of gut microbiota was not altered by dapagliflozin (29). In our study, we found empagliflozin elevated levels of short-chain fatty acid-producing bacteria, such as species from Roseburia, Eubacterium, and Faecalibacterium, and reduced those of several harmful bacteria including Escherichia-Shigella, Bilophila, and Hungatella. The inconsistent may be attributable to limitations related to the analysis of the gut microbiota and selection of the intervention and cohort. To our knowledge, this is the first study to systematically investigate the effect of empagliflozin on the gut microbiota in treatment-naïve type 2 diabetes patients with risk factors for CVDs.
Empagliflozin significantly increased the richness and diversity of the gut microbiota, which has been associated with an improved lifetime CVDs risk (30). We observed that the most important change in the gut microbiota in response to empagliflozin was the increase in short-chain fatty acid (SCFA)-producing bacteria such as the ASVs in Roseburia, Eubacterium, Ruminococcaceae, and Faecalibacterium (31-34). Emerging evidence demonstrated that SCFAs can modulate glycemic control, exhibit anti-inflammatory and antitumorigenic activity, and decrease oxidative stress (35, 36). Furthermore, SCFAs can induce AMP-activated protein kinase activation and glucose transporter 4 (GLUT4) expression in adipose tissue and ameliorate CVD-related metabolic disorders in diabetic mice; SCFAs are therefore regarded as a novel potential strategy for preventing CVDs (37). Moreover, another characteristic of the gut microbiota in the present empagliflozin-treated patients was a reduction in the abundance of several harmful bacteria such as the ASVs in Escherichia-Shigella, Bilophila, and Hungatella, which are all gram-negative; many members are opportunistic pathogens that induce inflammation and disrupt gut barrier function (38-40), which may contribute to insulin resistance, hyperglycemia, and CVDs. Thus, the modulation of the gut microbiota by empagliflozin may improve glycemic control and provide additional benefits to the cardiovascular system.
A series of studies were done to identify the alteration of gut microbiota caused by metformin, which also contribute to improve hyperglycemia. A common finding of previous studies was that participants with diabetes taking metformin had higher relative abundance of Akkermansia muciniphila, a microbiota known for mucin degradation, and several SCFA-producing microbiota such as Butyrivibrio, Bifidobacterium bifidum, Megasphaera, Shewanella and Blautia, and decreased Intestinibacter bartlettii, Roseburia, Intestinibacter, and Ruminococcaceae (12, 41-44). That is consistent with our study to some extent, as we found Blautia, Klebsiella, Ruminococcaceae increased and Clostridium and Intestinibacter decreased after 12 months’ metformin use. But we also found metformin decreased Streptococcus and increased Christensenellaceae, which was different from previous studies (43).
Among those increased metabolites altered in metformin treatment versus empagliflozin, plasma linoleic acid, docosahexaenoic acid, and erucic acid were reported to be beneficial metabolites. Plasma linoleic acid is the predominant type of dietary polyunsaturated fatty acids was inversely related to type 2 diabetes, metabolic syndrome, or cardiovascular diseases risk (45-48). Previous studies suggested that docosahexaenoic acid may play a protective role against diabetes and may restore insulin sensitivity by preventing lipotoxicity and inflammation (49). It also is anti-inflammatory in the brain and protected against lipopolysaccharide-induced neuronal loss (49). And erucic acid is a monounsaturated omega-9 fatty acid which has an ameliorative effect in mice with scopolamine-induced memory deficits (50).
One strength of our study is that it is the first randomized clinical trial with a carefully controlled study procedure for treatment-naïve type 2 diabetes mellitus patients with risk factors for CVDs, systematically evaluating the effects of empagliflozin on gut microbiota and plasma metabolites compared to those of metformin. However, this study had some limitations. First, the study had an open-label design. Second, the sample size was relatively small. Third, the study follow-up period was too short to evaluate the long-term effects of empagliflozin and metformin on cardiovascular events in patients with type 2 diabetes mellitus.
The multiple beneficial effects of empagliflozin cannot be explained solely by its inhibition of sodium-glucose cotransporter 2, and our work suggests that modulation of the gut microbiota and plasma metabolites may be one of the potential mechanisms involved. To systematically elucidate the mechanism of action of a drug, it is necessary to investigate its effect on the metabonomic profile and gut microbiome of the patient. The mechanism by which empagliflozin affects plasma metabolites and the gut microbiota of the patient requires further investigation.
Conclusion
In conclusion, our study suggests that empagliflozin improves hyperglycemia as well as risk factors for CVDs in patients with treatment-naïve type 2 diabetes with risk factors for CVDs compared with metformin, and that empagliflozin may represent a superior therapeutic option for these patients. Additionally, the cardiovascular benefits of empagliflozin may be associated with a shift in the gut microbiota and plasma metabolites in patients. However, additional studies on fecal metabolomics are essential to investigate the interaction between gut-derived metabolites and the gut bacteria involved in improving the clinical parameters in this patient group.
Acknowledgments
The authors would like to thank all participants in the study and Adfontes Bio-technology Co., Ltd. (Shanghai, China) for technical assistance.
Glossary
Abbreviations
- aPCoA
subject-adjusted principal coordinate analysis
- ASV
amplicon sequence variant
- bp
base pairs
- CVD
cardiovascular disease
- ELISA
enzyme-linked immunosorbent assay
- FDR
false discovery rate
- HbA1c
glycated hemoglobin A1c
- IL-6
interleukin-6
- OPLS-DA
orthogonal partial least-squares discriminant analysis
- PC
principal component
- SCFA
short-chain fatty acid
- SGLT2i
sodium glucose cotransporter 2 inhibitor
- VIP
variable importance in the projection
- WHO
World Health Organization
Financial Support
The work was supported by the National Natural Science Foundation of China under Grant 81970705; and Central Plains Thousand Talents Plan under Grant 204200510026.
Disclosures
The authors have nothing to disclose.
Clinical Trial Information
The trial was registered with the Chinese Clinical Trial Registry (http://www.chictr.org.cn/index.asp) on October 11, 2018, no. ChiCTR1800018825.
Data Availability
The raw Illumina sequence data generated during and/or analyzed during the current study are available in the sequence read archive at NCBI under accession No. SRP278004. The other data generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
- 1. Rieg T, Vallon V. Development of SGLT1 and SGLT2 inhibitors. Diabetologia. 2018;61(10):2079-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zinman B, Wanner C, Lachin JM, et al. ; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. [DOI] [PubMed] [Google Scholar]
- 3. American Diabetes Association. 10. Cardiovascular disease and risk management: standards of medical care in diabetes–2020. Diabetes Care. 2020;43(Suppl 1):S111-S134. [DOI] [PubMed] [Google Scholar]
- 4. American Diabetes Association. 9. Pharmacologic approaches to glycaemic treatment: standards of medical care in diabetes–2020. Diabetes Care. 2020;43(Suppl 1):S98-S110. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization. Diagnosis and management of type 2 diabetes (HEARTS–D). 2020. [Google Scholar]
- 6. International Diabetes Federation. Recommendations For Managing Type 2 Diabetes In Primary Care. 2017. www.idf.org/managing-type2-diabetes [Google Scholar]
- 7. Perrone-Filardi P, Avogaro A, Bonora E, et al. . Mechanisms linking empagliflozin to cardiovascular and renal protection. Int J Cardiol. 2017;241:450-456. [DOI] [PubMed] [Google Scholar]
- 8. Gill SR, Pop M, Deboy RT, et al. . Metagenomic analysis of the human distal gut microbiome. Science. 2006;312(5778):1355-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao L. The gut microbiota and obesity: from correlation to causality. Nat Rev Microbiol. 2013;11(9):639-647. [DOI] [PubMed] [Google Scholar]
- 10. Moran-Ramos S, Lopez-Contreras BE, Canizales-Quinteros S. Gut microbiota in obesity and metabolic abnormalities: a matter of composition or functionality? Arch Med Res. 2017;48(8):735-753. [DOI] [PubMed] [Google Scholar]
- 11. Lee DM, Battson ML, Jarrell DK, et al. . SGLT2 inhibition via dapagliflozin improves generalized vascular dysfunction and alters the gut microbiota in type 2 diabetic mice. Cardiovasc Diabetol. 2018;17(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu H, Esteve E, Tremaroli V, et al. . Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23(7):850-858. [DOI] [PubMed] [Google Scholar]
- 13. Zhang X, Fang Z, Zhang C, et al. . Effects of acarbose on the gut microbiota of prediabetic patients: a randomized, double-blind, controlled crossover trial. Diabetes Ther. 2017;8(2):293-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang L, Li P, Tang Z, Yan X, Feng B. Structural modulation of the gut microbiota and the relationship with body weight: compared evaluation of liraglutide and saxagliptin treatment. Sci Rep. 2016;6:33251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Montandon SA, Jornayvaz FR. Effects of antidiabetic drugs on gut microbiota composition. Genes (Basel) 2017;8(10):250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou BF. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults––study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15(1):83-96. [PubMed] [Google Scholar]
- 17. Klindworth A, Pruesse E, Schweer T, et al. . Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013;41(1):e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13(7):581-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pruesse E, Peplies J, Glockner FO. SINA: accurate high-throughput multiple sequence alignment of ribosomal RNA genes. Bioinformatics. 2012;28(14):1823-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: Part 1––Correlation within subjects. BMJ. 1995;310(6977):446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Deng X, Zhang C, Wang P, et al. (2022), Cardiovascular benefits of empagliflozin are associated with gut microbiota and plasma metabolites in type 2 Diabetes. Zenodo. Deposited 14 July 2022. 10.5281/zenodo.6833149. [DOI]
- 22. Dzau VJ, Antman EM, Black HR, et al. . The cardiovascular disease continuum validated: clinical evidence of improved patient outcomes: Part I: Pathophysiology and clinical trial evidence (risk factors through stable coronary artery disease). Circulation. 2006;114(25):2850-2870. [DOI] [PubMed] [Google Scholar]
- 23. Norris GH, Blesso CN. Dietary sphingolipids: potential for management of dyslipidemia and nonalcoholic fatty liver disease. Nutr Rev. 2017;75(4):274-285. [DOI] [PubMed] [Google Scholar]
- 24. Sun L, Pang Y, Wang X, et al. . Ablation of gut microbiota alleviates obesity-induced hepatic steatosis and glucose intolerance by modulating bile acid metabolism in hamsters. Acta Pharmaceutica Sinica B. 2019;9(4):702-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wahlstrom A, Sayin SI, Marschall HU, Backhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016;24(1):41-50. [DOI] [PubMed] [Google Scholar]
- 26. Sugimoto T, Kato T, Park EY. Functional analysis of cis-aconitate decarboxylase and trans-aconitate metabolism in riboflavin-producing filamentous Ashbya gossypii. J Biosci Bioeng. 2014;117(5):563-568. [DOI] [PubMed] [Google Scholar]
- 27. Mills EL, Ryan DG, Prag HA, et al. . Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature. 2018;556(7699):113-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ndrepepa G. Uric acid and cardiovascular disease. Clin Chim Acta. 2018;484:150-163. [DOI] [PubMed] [Google Scholar]
- 29. van Bommel EJM, Herrema H, Davids M, Kramer MHH, Nieuwdorp M, van Raalte DH. Effects of 12-week treatment with dapagliflozin and gliclazide on faecal microbiome: results of a double-blind randomized trial in patients with type 2 diabetes. Diabetes Metab. 2020;46(2):164-168. [DOI] [PubMed] [Google Scholar]
- 30. Kelly TN, Bazzano LA, Ajami NJ, et al. . Gut microbiome associates with lifetime cardiovascular disease risk profile among Bogalusa heart study participants. Circ Res. 2016;119(8):956-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kasahara K, Krautkramer KA, Org E, et al. . Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat Microbiol. 2018;3(12):1461-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xu J, Liang R, Zhang W, et al. . Faecalibacterium prausnitzii-derived microbial anti-inflammatory molecule regulates intestinal integrity in diabetes mellitus mice via modulating tight junction protein expression. J Diabetes. 2020;12(3):224-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kang JD, Myers CJ, Harris SC, et al. . Bile acid 7α-dehydroxylating gut bacteria secrete antibiotics that inhibit Clostridium difficile: role of secondary bile acids. Cell Chem Biol. 2019;26(1):27-34.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Boesmans L, Valles-Colomer M, Wang J, et al. . Butyrate producers as potential next–generation probiotics: Safety assessment of the administration of Butyricicoccus pullicaecorum to healthy volunteers. mSystems. 2018;3(6):e00094-e00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27(2):104-119. [DOI] [PubMed] [Google Scholar]
- 36. Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91-119. [DOI] [PubMed] [Google Scholar]
- 37. Gao F, Lv YW, Long J, et al. . Butyrate improves the metabolic disorder and gut microbiome dysbiosis in mice induced by a high-fat diet. Front Pharmacol. 2019;10:1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pinaud L, Sansonetti PJ, Phalipon A. Host cell targeting by enteropathogenic bacteria T3SS effectors. Trends Microbiol. 2018;26(4):266-283. [DOI] [PubMed] [Google Scholar]
- 39. David LA, Maurice CF, Carmody RN, et al. . Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Genoni A, Christophersen CT, Lo J, et al. . Long-term Paleolithic diet is associated with lower resistant starch intake, different gut microbiota composition and increased serum TMAO concentrations. Eur J Nutr. 2020;559(5):1845-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. de la Cuesta-Zuluaga J, Mueller NT, Corrales-Agudelo V, et al. . Metformin is associated with higher relative abundance of mucin-degrading Akkermansia muciniphila and several short-chain fatty acid-producing microbiota in the gut. Diabetes Care. 2017;40(1):54-62. [DOI] [PubMed] [Google Scholar]
- 42. McCreight LJ, Bailey CJ, Pearson ER. Metformin and the gastrointestinal tract. Diabetologia. 2016;59(3):426-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Weersma RK, Zhernakova A, Fu J. Interaction between drugs and the gut microbiome. Gut. 2020;69(8):1510-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mueller NT, Differding MK, Zhang M, et al. . Metformin Affects Gut Microbiome Composition and Function and Circulating Short-Chain Fatty Acids: A Randomized Trial. Diabetes Care. 2021;44(7):1462-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Henderson G, Crofts C, Schofield G. Linoleic acid and diabetes prevention. Lancet Diabetes Endocrinol. 2018;6(1):12-13. [DOI] [PubMed] [Google Scholar]
- 46. Marangoni F, Agostoni C, Borghi C, et al. . Dietary linoleic acid and human health: focus on cardiovascular and cardiometabolic effects. Atherosclerosis. 2020;292:90-98. [DOI] [PubMed] [Google Scholar]
- 47. Pertiwi K, Wanders AJ, Harbers MC, et al. . Plasma and dietary linoleic acid and 3-year risk of type 2 diabetes after myocardial infarction: a prospective analysis in the alpha omega cohort. Diabetes Care. 2020;43(2):358-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Riccardi G. Linoleic acid and risk of type 2 diabetes. Lancet Diabetes Endocrinol. 2017;5(12):929-930. [DOI] [PubMed] [Google Scholar]
- 49. Huang JP, Cheng ML, Hung CY. Docosapentaenoic acid and docosahexaenoic acid positively associate with insulin sensitivity in rats fed high-fat and high-fructose diets. J Diabetes. 2017;9(10):936-946. [DOI] [PubMed] [Google Scholar]
- 50. Kim E, Ko HJ, Jeon SJ, et al. . The memory-enhancing effect of erucic acid on scopolamine-induced cognitive impairment in mice. Pharmacol Biochem Behav. 2016;142:85-90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw Illumina sequence data generated during and/or analyzed during the current study are available in the sequence read archive at NCBI under accession No. SRP278004. The other data generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.