Abstract
Patients with obesity have a high prevalence of nonalcoholic fatty liver disease (NAFLD), representing a spectrum of simple steatosis to nonalcoholic steatohepatitis (NASH), without and with fibrosis. Understanding the etiology of NAFLD is clinically relevant since NAFLD is an independent risk factor for diabetes and cardiovascular disease. In addition, NASH predisposes patients to the development of cirrhosis and hepatocellular carcinoma, and NASH cirrhosis represents the fastest growing indication for liver transplantation in the United States. It is appreciated that multiple factors are involved in the development and progression of NAFLD. Growth hormone (GH) and insulin-like growth factor 1 (IGF1) regulate metabolic, immune, and hepatic stellate cell function, and alterations in the production and function of GH is associated with obesity and NAFLD/NASH. Therefore, this review will focus on the potential role of GH and IGF1 in the regulation of hepatic steatosis, inflammation, and fibrosis.
Keywords: growth hormone, GH, insulin-like growth factor 1, IGF1, nonalcoholic fatty liver disease, NAFLD, nonalcoholic steatohepatitis, NASH
Growth hormone (GH) signals through its type I cytokine receptor, GH receptor (GHR), to activate multiple intracellular signal transduction pathways (1). Although the GHR is expressed throughout the body, the most understood action is GH/GHR-mediated phosphorylation (activation) of signal transducer and activator of transcription 5 (STAT5) that is required to stimulate the production of hepatic insulin-like growth factor 1 (IGF1) and its binding proteins (2). Hepatic IGF1 contributes to the majority of IGF1 found in the circulation (3). IGF1 is also produced locally by a variety of tissues, with variable tissue-specific expression of the IGF1 receptor (IGF1R) (4). Although the mature hepatocyte does not express the IGF1R, it is expressed in a wide variety of hepatic supporting cells and immune cells (5). Both GH and IGF1 are essential for normal growth and development. In addition, both GH and IGF1 regulate the production and action of insulin, where each work in a context- and tissue-specific fashion to regulate metabolic function (6).
As clearly articulated and reviewed in detail by Moller and Jorgensen in 2009 (7), “the effects of GH on substrate metabolism in humans are simple: during conditions of energy surplus (feast), GH, in concert with IGF1 and insulin, promotes nitrogen retention, and when food is sparse (famine/fasting), GH alters fuel consumption from the use of carbohydrates and protein to the use of lipids, thereby allowing conservation of vital protein stores.” In lean healthy subjects, circulating GH is elevated prior to a meal and then declines as circulating nutrients are increased, leading to a rise in insulin that acts to promote the storage of dietary nutrients (8). With fasting, circulating insulin and IGF1 decline, while GH rises to directly stimulate white adipose tissue (WAT) lipolysis, providing glycerol for gluconeogenesis and nonesterified fatty acids (NEFA) to be used as an energy source (to spare carbohydrates and protein) (7). However, in the Western world, periods of feast are not often offset by famine (or relevant fasting), and food intake exceeds energy demands, leading to obesity and insulin resistance. Hyperinsulinemia, hyperglycemia, and hyperlipidemia are observed in both the fed and fasted state, while circulating GH levels decline, with variable effects on IGF1 (see Fig. 1). In this context, nonalcoholic fatty liver disease (NAFLD) develops in many, but not all, subjects.
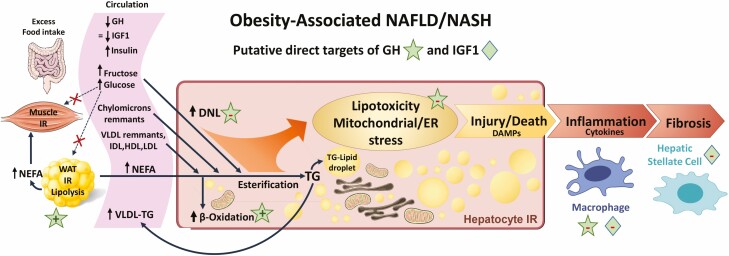
Figure 1.
Representation of the changes in circulating hormones and nutrients and liver phenotype observed in patients with obesity-associated nonalcoholic fatty liver disease/nonalcoholic steatohepatitis (NAFLD/NASH). In these patients circulating growth hormone (GH) is reduced with normal or reduced insulin-like growth factor 1 (IGF1), associated with hyperinsulinemia, hyperglycemia, and hyperlipidemia. Increased substrate availability from excess dietary intake and white adipose tissue and muscle insulin resistance (IR), elevates circulating glucose and nonesterified fatty acids, thereby fueling steatosis. Although the steatotic liver exhibits enhanced lipid (β) oxidation and very low density lipoprotein triglyceride assembly/release, due to substrate availability and hepatocyte IR, this is not sufficient to counteract excess lipid accumulation (lipotoxicity). Lipotoxicity promotes mitochondrial and endoplasmic reticulum stress and leads to hepatocyte injury and release of damage associated molecular patterns (DAMPs) that promote activation of Kupffer cells (resident macrophages) and infiltration of immune cells that release cytokines. Cytokines, in conjunction with DAMPs, activate hepatic stellate cells, leading to fibrosis. As reviewed in the main text, low levels of GH/IGF1 may promote NAFLD/NASH progression, where sustaining GH/IGF1 in a physiologic range prevents or reverses disease progression. Clinical and animal studies indicate that the therapeutic effect of GH/IGF1 is multifactorial and includes enhancing whole body nutrient utilization and reducing inflammation, which would serve to ultimately improve insulin sensitivity and shift nutrients away from the liver. In addition, GH may act directly on the hepatocyte to reduce steatosis by suppressing de novo lipogenesis and increasing β oxidation. In addition, GH via STAT5 may prevent hepatocyte damage by regulating detoxification programs. Finally, sustained GH/IGF1 may directly reduce inflammation, while IGF1 may prevent fibrosis by suppressing hepatic stellate cell activation.
This review will briefly summarize our current understanding of the etiology of NAFLD, provide an overview of the clinical data that implicates the GH/IGF1 axis in the development and progression of NAFLD, and discuss the indirect and direct mechanisms by which GH and IGF1 may regulate steatosis and liver injury. The clinical literature reviewed will be confined to the associations and actions of GH and IGF1 in adults, with an emphasis on interventional trials. References to animal studies are provided to lend insight in the potential tissue-specific mechanisms of action of GH and IGF1, as these investigations are often not possible in human subjects.
Etiology of NAFLD
Hepatocytes are the main cell type of the liver, accounting for ~60% of total cells and ~80% of liver volume. Accumulation of fat in ≥5% of the hepatocyte area (hepatic steatosis) is considered early-stage NAFLD. NAFLD advances to nonalcoholic steatohepatitis (NASH) with the onset of hepatocyte ballooning and inflammation, with or without fibrosis. As illustrated in Figure 1, the ballooned hepatocytes exhibit derangement of endoplasmic reticulum and mitochondrial morphology and function that increases the production of reactive oxygen species (9). It is believed that excess accumulation of free fatty acids, sphingolipids, and lipid peroxidation (lipotoxicity) drive hepatocyte damage, leading to the production of damage-associated molecular patterns that activate liver endothelial sinusoidal cells and resident macrophages (Kupffer cells) to promote inflammation and attract immune cells. In addition, damage-associated molecular patterns and inflammatory cytokines activate hepatic stellate cells (HSC), which remodel local extracellular matrix and deposit excess collagen and other proteins (fibrosis).
Approximately 25% of the general population has early-stage NAFLD, with the percentage rising to >50% of patients with obesity or type 2 diabetes mellitus (10). Up to 25% of these patients progress to NASH, while a subset of patients with NASH can progress to irreversible stages of cirrhosis and hepatocellular carcinoma (11). Whereas liver steatosis may be the consequence of obesity and diabetes, a combination of severity of metabolic disease, genetic background, environmental toxins, dietary factors (such as saturated fats, fructose, cholesterol), medications, microbiota alterations, sex, and age are now known to contribute to the progression from steatosis to NASH (12). Certainly, progression to NASH is a multifactorial process, where the pathophysiology of disease is not “one size fits all.”
Overview of the Potential Sources of Excess Hepatic Lipid Accumulation in Obesity-associated NAFLD
Liver steatosis is the result of an imbalance between uptake, utilization, and export of lipids by the hepatocytes. As illustrated in Figure 1, in the obese insulin-resistant state, hepatic fatty acids are derived from two main sources: (1) excess carbohydrates that provides substrate for de novo lipogenesis (DNL) and (2) excess dietary fat delivered to the liver as chylomicron remnants, recycled lipoproteins, and NEFA that are produced by lipolysis in the WAT, a process enhanced by insulin resistance. These fatty acids can be used as a source of energy in mitochondrial β-oxidation or re-esterified with glycerol in the membranes of the mitochondria and endoplasmic reticulum to generate phospholipids and neutral triglycerides (TG). Newly formed TG are exported out of the hepatocytes by very low-density lipoproteins (VLDL-TG) or stored as lipid droplets within the hepatocytes. An imbalance of these processes leads to the accumulation of neutral TG, free fatty acids, and complex lipids that induce lipotoxicity in hepatocytes and contribute to the progression of steatosis to NASH.
Elegant labeling experiments in humans with NAFLD measured the contribution of NEFA, recycled chylomicrons, and DNL to the development of steatosis (13-15). Remarkably, in patients with obesity and NAFLD, the contribution of hepatic DNL to the hepatic TG pool was 38%, as compared to 19% in patients with obesity without NAFLD (15). This high rate of DNL in patients with obesity and NAFLD was not suppressed between meals, in contrast to the meal-associated decrease in the contribution of WAT-derived NEFA and postprandial chylomicron remnants (13). This chronic elevation in the rate of DNL in patients with obesity and NAFLD may be fueled by the availability of glucose and fructose to hepatocytes, as well as the high levels of insulin. Briefly, after a meal, newly absorbed carbohydrates and pancreas-derived insulin are delivered to the liver via the portal vein. Although glucose uptake in the hepatocytes is insulin-independent (in contrast to muscle and adipose tissue), the high concentration of insulin in the sinusoids reaches up to 3× that of peripheral circulation. This leads to increased activation of insulin receptors on hepatocytes, resulting in the increased activity and expression of enzymes involved in re-esterification of NEFA, glycogenesis, glycolysis, and DNL (16). Although VLDL-TG assembly/release and β-oxidation that promote lipid disposal from the liver are suppressed by insulin, in patients with insulin resistance and steatosis, these processes are increased due to hepatic insulin resistance and substrate (NEFA) availability (16, 17). Nonetheless, these changes are not sufficient to prevent excess fat accumulation.
Are There Targetable Mechanisms to Prevent/Reverse NAFLD?
Diet modification and exercise can reverse steatosis, inflammation, and fibrosis associated with NAFLD and NASH. Diet and exercise improve systemic insulin sensitivity and reduce substrates that fuel steatosis (18). In fact, at least 5% weight loss through lifestyle measures can lead to significant improvement in hepatic steatosis, while at least 7% to 10% weight loss has been associated with improvements in NASH and fibrosis (19, 20). In addition, exercise has been shown to reduce hepatic steatosis, liver stiffness, alanine aminotransferase (ALT), and aspartate aminotransferase (AST), even in the absence of significant weight loss (21, 22). These data form the basis of lifestyle recommendations for patients with NAFLD and NASH (23). However, since lifestyle interventions have proven to be difficult to sustain and to date there are no Food and Drug Administration–approved drugs to treat NAFLD, many clinical trials are underway to test existing insulin sensitizing drugs or new drugs that modulate hepatocyte metabolism, as well as those that target nonparenchymal cells to reduce inflammation and fibrosis (24, 25). However, it should be noted that many drugs from this pipeline have thus far failed to show clinical efficacy and/or have been abandoned due to deleterious side effects (26). Given that progression of NAFLD and NASH is a multistep process involving multiple organs and cell types, it is likely that a patient-centered approach with combination therapy will be necessary for effective treatment. Therefore, further research is required to understand the myriad of cell-dependent mechanisms, factors and hormones that regulate the integrative pathophysiology in NAFLD patients. As summarized in the following discussion, clinical evidence is accumulating that alterations in the GH/IGF1 axis contribute to the development and progression of NAFLD, and therefore, targeting the GH/IGF1 axis may represent a potential novel therapeutic approach for this highly prevalent and morbid disease.
Challenges in Studying the Association Between the GH/IGF1-Axis and NAFLD
Studying the relationship between the GH/IGF1 axis and NAFLD/NASH in human subjects is challenging, as it requires precise hepatic and hormone phenotyping. Random GH levels are not informative due to the pulsatile nature of the hormone, and GH stimulation testing is costly and time consuming for subjects. IGF1 is generally reflective of GH activity in patients with pituitary disorders; however, IGF1 levels can be relatively preserved in obesity, despite the reduction in GH (27-29). Additionally, GH and IGF1 may each have distinct beneficial effects in NAFLD/NASH, which will be discussed in subsequent sections. However, it is difficult to differentiate between the independent effects of GH and IGF1 in human studies, and IGF1 is often used as a proxy for GH axis activity. Regarding hepatic phenotyping, gold standard radiologic quantification of intrahepatic lipid (IHL) content by magnetic resonance spectroscopy (1H-MRS) is costly and not widely available, and full assessment of hepatocyte ballooning and inflammation for the diagnosis of NASH requires liver biopsy, which is both costly and invasive. Despite these challenges, there is literature in certain conditions, such as hypopituitarism with frank GH deficiency (GHD), acromegaly, overweight/obesity with relative GHD, and HIV, that implicate the GH/IGF1 axis in the pathophysiology and progression of NAFLD. These studies are discussed in more detail next, with interventional trials summarized in Table 1.
Table 1.
Summary intervention trials that altered growth hormone and studied the impact on nonalcoholic fatty liver/nonalcoholic steatohepatitis endpoints
| Author | Condition | NAFLD/NASH status | Design/intervention | Number of Subjects | Duration | NAFLD-related endpointsa | Glucose-related exclusion criteria | Glucose/insulin-related endpoints and discontinuations |
|---|---|---|---|---|---|---|---|---|
| Hypopituitary studies | ||||||||
| Nishizawa et al (30) | Hypopituitary GHD | Unselected for NAFLD | Open label daily GH | 19 | 6 months | ALT improved | ||
| Nishizawa et al (30) | Hypopituitary GHD | NASH (subset of above) | Open label daily GH | 5 | 6-12 months | Improved histologic steatosis and fibrosis | ||
| Meienberg et al (31) | Hypopituitary GHD | Unselected for NAFLD | Daily GH vs no treatment | 18 (9 GH, 9 untreated) | 6 months | No difference in absolute % ∆IHL | No difference in Δ FPG, fasting insulin, HOMA-IR or HbA1c | |
| Acromegaly studies | ||||||||
| Bredella, et al (32) | Active acromegaly | Unselected for NAFLD | Biochemical control | 16 | mean 8 months (3-22 months) | Increase in IHL by lipid/water ratio | Improved HOMA-IR, fasting insulin & OGTT insulin AUC; no ∆ in FPG or OGTT glucose AUC | |
| Reyes-Vidal et al (33) | Active acromegaly | Unselected for NAFLD | Biochemical control s/p TSS | 23 (12 with 1H-MRS) | 0.5-2 years | Increase in IHL | Improved FPG and HOMA-IR | |
| Winhofer et al (34) | Active acromegaly | Unselected for NAFLD | Biochemical control s/p TSS | 7 | 6 months | No change in absolute % IHL | T2DM | Improved fasting insulin; no change in FPG or mean OGTT glucose |
| Madsen et al (35) | Acromegaly controlled on SSA | Unselected for NAFLD | Randomized: continue SSA vs PEG + reduced dose SSA | 18 (6 SSA, 12 PEG + SSA) | 6 months | Increase in % IHL and ALT in PEG + SSA vs SSA group | No difference in ΔHOMA-IR | |
| Kuker et al (36) | Active acromegaly | Unselected for NAFLD | Initiation of PEG therapy | 21 | mean 5.7 years (1-13.4 years) | Increase in % IHL with PEG: 1.8% to 3.0%, P = 0.04 | Improved HOMA-IR, QUICKI and HbA1c with PEG | |
| Obesity studies | ||||||||
| Pan et al (37) | Young adults (18-29 years) with obesity and NAFLD | NAFLD (≥5% IHL by 1H-MRS) | Randomized, open-label daily GH vs no treatment | 24 (13 GH) | 6 months | No difference in ∆ % IHL or Δ ALT; NAFLD resolution (<5% IHL): 5/9 GH vs 1/9 no treatment | T2DM or any antidiabetic medications | No difference in FPG or OGTT 2hPG. Discontinuations: no subject met drop criteria (FPG > 126 mg/dL or OGTT 2hPG > 200 mg/dL). |
| Bredella et al (38) | Adult men (18-45 years), BMI ≥ 25 kg/m2 and abdominal fat | Unselected for NAFLD | Randomized, double-blind: daily GH vs placebo | 62 (32 GH) | 6 months | Decrease in IHL by ∆ in lipid/water ratio when controlled for ∆ weight | T2DM, FPG ≥ 126 mg/dL, OGTT 2hPG ≥200 mg/dL | Increase in OGTT 2hPG in GH vs pbo; no difference in FPG, HbA1c or HOMA-IR. Discontinuations: 5 subjects met drop criteria, including 2 GH with FPG ≥ 126 mg/dL, 1 GH and 1 placebo with OGTT 2h PG ≥ 200 mg/dL, and 1 GH with HbA1c ≥ 6.5%. |
| HIV and NAFLD studies | ||||||||
| Stanley et al (39) | Adults (18-65 years) with HIV and abdominal fat | Unselected for NAFLD | Randomized, double-blind: daily tesamorelin vs placebo | 54 (28 tesamorelin) | 6 months | Decrease in IHL in tesamorelin group; absolute net treatment effect -2.9%, P = 0.003 | FPG ≥ 126 mg/dL or use of antidiabetic meds | Increased HbA1c; no effect on FPG, OGTT 2hPG, fasting insulin or HOMA-IR; euglycemic hyperinsulinemic clamp (n = 24): IS decreased at 3 months, no longer significant at 6 months. Four subjects met hyperglycemia criteria: n = 1 per group with FPG ≥ 126 mg/dL and n =1 per group with OGTT 2hPG ≥ 200 mg/dL. No subjects met discontinuation criteria of FPG ≥ 150 mg/dL. |
| Stanley et al (40) | Adults (18-70 years) with HIV and NAFLD | NAFLD (≥5% IHL by 1H-MRS) | Randomized, double-blind: daily tesamorelin vs placebo | 61 (31 tesamorelin) | 12 months | Decrease in IHL in tesamorelin vs placebo group: absolute net treatment effect −4.1% (−7.6% to 0.7%), P < 0.02; no treatment effect on ALT | T2DM unless HbA1c ≤ 7% on stable antidiabetic meds (≥6 months) and no insulin or TZDs. Tesamorelin group had 12.9% with T2DM and 9.7% on antidiabetic meds at baseline; similar rates in placebo group. | No treatment effect on FPG or HbA1c. Number meeting hyperglycemia criteria (FPG ≥ 126 mg/dL) were similar in tesamorelin (12/31) vs placebo (11/30) groups. Two subjects in tesamorelin met discontinuation criteria: 1 FPG > 150 mg/dL (non-T2DM drop) and 1 FPG > 180 mg/dL (T2DM drop). |
Abbreviations: 1H-MRS, proton magnetic resonance spectroscopy; 2hPG, 2-hour plasma glucose; ALT, alanine aminotransferase; AUC, area under the curve; BMI, body mass index; FPG, fasting plasma glucose; GHD, growth hormone deficiency; HbA1c, glycated hemoglobin A1c; HIV, human immunodeficiency virus; HOMA-IR, homeostatic model assessment for insulin resistance; IHL, intrahepatic lipid; IS, insulin sensitivity; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; OGTT, oral glucose tolerance test; PEG, pegvisomant; QUICKI, Quantitative Insulin Sensitivity Check Index; SSA, somatostatin analog; s/p TSS, status post transsphenoidal surgery; T2DM, type 2 diabetes mellitus; TZD, thiazolidinediones.
aSteatosis endpoints by 1H-MRS unless otherwise noted.
Adults With Severe GHD
Adult hypopituitary patients with severe GHD are known to have a higher rate of metabolic syndrome than their counterparts without GHD, and investigations also support a direct role for GHD in the development and progression of NAFLD. A small early study (41) demonstrated that hypopituitary adults with GHD had a higher rate of NAFLD by computed tomography (7 of 13 patients), as compared to similar controls with hypopituitarism and comparable body mass index (BMI) but no GHD (0 of 5 patients). A relatively larger study of hypopituitary adults (n = 66) with GHD compared to sex-, age-, and BMI-matched healthy controls (n = 83) also demonstrated a higher rate of NAFLD by ultrasound (77% vs 12%) and higher mean ALT and AST (30) in the GHD subjects. A study of hypopituitary individuals with a history of craniopharyngioma also demonstrated a significant risk of severe or progressive NASH with cirrhosis (42). However, in this study, only 7 out of 21 patients had confirmed GHD and craniopharyngioma treatment was complicated by rapid gains in BMI (average 2.2 kg/m2 per year), which could also contribute to NAFLD/NASH (42).
Not only is GHD associated with NAFLD/NASH, but data suggest that physiologic GH administration in hypopituitary adults with GHD and NAFLD improves hepatic endpoints. An initial case report demonstrated that 6 months of GH administration in an adult with NASH associated with childhood onset GHD significantly improved histologic steatosis with resolution of NASH (including histologic inflammation and hepatocyte ballooning). There were concurrent improvements in histologic markers of oxidative stress, aminotransferases, and TG (43). A subsequent study confirmed similar improvements in liver enzymes with physiologic GH replacement in adults with GHD, despite the fact that only 11 of 19 subjects had NAFLD at baseline assessment (30). Additionally, in that same study (30), a subset of 5 treated subjects with histologically confirmed NASH at baseline had a repeat biopsy after 6 to 12 months of physiologic GH replacement, with significant improvement in histologic steatosis and inflammation when all other replacement hormones and drugs to treat dyslipidemia were held constant. One randomized study of adults with GHD unselected for NAFLD at baseline did not show improvement in hepatic steatosis by 1H-MRS with GH replacement (n = 9) vs those without GH replacement (n = 9). However, IHL levels were <5% in the cohort at baseline, potentially limiting the impact of GH therapy and power of the study to detect a change in NAFLD-related endpoints. The study may have been generally underpowered to detect effects of GH as well, as they were unable to demonstrate a significant reduction in visceral adipose tissue (31), which is an expected effect of adequate GH treatment (44).
Adults With Acromegaly
Patients with acromegaly offer an additional model to study the effects of GH/IGF1 in NAFLD. Patients with active acromegaly have lower levels of hepatic steatosis by 1H-MRS as compared to age-, sex-, and BMI-matched controls, despite higher levels of insulin resistance (32, 34). Several studies (32-34) have demonstrated an increase in IHL by 1H-MRS after biochemical control of acromegaly, associated with improvements in insulin sensitivity despite no change in BMI. GH receptor antagonism by pegvisomant has also been shown to increase IHL in patients with acromegaly. Specifically, a randomized study of somatostatin analog treatment vs somatostatin analog plus pegvisomant cotreatment demonstrated that the cotreatment group had a significant increase in IHL content by 1H-MRS that was positively correlated with pegvisomant dose but independent of changes in insulin sensitivity (35). A subsequent study of adults with active acromegaly confirmed that IHL content, which was significantly lower than matched controls pre-treatment, increased with pegvisomant monotherapy to a level similar to controls (36). Given the disconnect between hepatic lipid content with insulin sensitivity and visceral fat in acromegaly pre- and posttherapy, these studies suggest that high GH plays a significant and direct role in the reduction of hepatic steatosis.
Adults With Overweight/Obesity Without Pituitary Disorders
Obesity Is a State of Relative GHD
Obesity is a well-known state of relative GHD (29), with demonstrated reductions in GH burst amplitude, half-life, and daily secretion rate (45). Both peak-stimulated GH and mean 24-hour GH production decrease in a stepwise fashion across lean to overweight to obese populations (28), and visceral fat is particularly associated with decreases in GH secretion (46). It should be noted that even short-term (2 weeks) overeating in lean subjects suppresses GH levels (47). In patients with obesity, calorie restriction prior to significant weight loss can improve peak response of GH to GH-releasing hormone (GHRH), but weight loss appears to be required for normalization of spontaneous GH secretion (29). It remains to be determined the exact mechanisms leading to reduced GH in the obese state, however it was reported that spontaneous GH secretion was partially restored in adults with obesity by administration of acipimox, an inhibitor of lipolysis that lowers systemic free fatty acids, as well as insulin levels (48). Nonetheless, it is important to keep in mind that to diagnose clinical GHD, lower cutoffs for GH stimulation testing must be applied to patients with overweight/obesity, as compared to their lean peers (49). Despite a reduction in GH, IGF1 levels, and particularly free IGF1 levels, are relatively preserved in obesity (27-29).
Association Between GH and IGF1 and NAFLD in Overweight/Obesity
Several studies have demonstrated that mean random GH levels are lower in NAFLD vs non-NAFLD controls with overweight/obesity (50-52). However, the utility and interpretation of unstimulated GH levels is limited due to its pulsatility, short half-life, and diurnal variation. However, there are a few studies that demonstrate inverse associations between peak-stimulated GH and some measure of NAFLD. Specifically, peak-stimulated GH levels by GHRH-arginine testing were shown to be significantly lower in subjects with sonographic NAFLD vs non-NAFLD controls of a similar BMI (53). Peak-stimulated GH by GHRH-arginine testing was also inversely correlated with IHL content quantified by 1H-MRS in women with overweight/obesity unselected for NAFLD (54).
Given the challenges related to the assessment of GH levels, IGF1 levels have been used as a surrogate marker of GH axis activity with the previously noted caveats. A number of studies have demonstrated that lower IGF1 levels are associated with both radiographic (53, 55) and histologic (56) NAFLD as compared to non-NAFLD controls. Studies of histologic NAFLD have primarily found associations between lower IGF1 and lobular inflammation, hepatocyte ballooning, NASH, and fibrosis (50, 56-59), with less evidence that IGF1 is as strongly correlated with steatosis itself (57, 59). While IGF1 is used as a marker to reflect GH activity, it is also possible that lower IGF1 is more reflective of hepatocyte injury, resulting in impaired hepatic synthetic capacity. Indeed, sustaining IGF1 may be important in controlling inflammation and fibrosis directly through effects on hepatic supporting cells, beyond its known role in improving insulin sensitivity (60), as discussed in later sections of this review.
Impact of GH Augmentation on NAFLD in Overweight/Obesity
To date, there is a limited number of studies that have examined the impact of GH administration on NAFLD-related endpoints in overweight/obesity. One study randomized 24 young adults (ages 18-29 years) with obesity and NAFLD (≥5% steatosis by 1H-MRS) to low-dose GH vs no treatment for 6 months with a primary endpoint of reduction in IHL content. The effect size of GH treatment on reduction in percentage steatosis was −3.3% (95% CI −7.8% to 1.2%) but did not reach significance (P = 0.14). Investigators did note that a greater proportion of the GH group had resolution of NAFLD (<5% steatosis by 1H-MRS) as compared to the placebo group (5/9 vs 1/9, P = 0.04), suggesting a potential effect of GH on reduction of hepatic steatosis in this population (37). A study of adult men with overweight/obesity unselected for NAFLD at baseline demonstrated an improvement in IHL content by 1H-MRS after 6 months of low-dose daily subcutaneous GH vs placebo (38). There is a study of daily, subcutaneous GH administration in adults with overweight/obesity and NAFLD that has recently completed with results pending (NCT02217345). There is also an ongoing study of GH augmentation through administration of the GHRH analog, tesamorelin, in subjects with overweight/obesity and NAFLD (NCT03375788).
Impact of GH Augmentation on Liver Fat in HIV-associated NAFLD
Patients with HIV and NAFLD also exhibit relative GHD (61), and raising endogenous GH and IGF1 by treatment with, tesamorelin, improved hepatic steatosis by 1H-MRS and prevented development or progression of histologic fibrosis (39, 40). In addition, recent reports show the therapeutic actions of tesamorelin in patients with HIV are associated with a reduction in circulating markers of inflammation (62, 63), as well as changes in hepatic proteomic and transcriptomic profile indicative of reduced proinflammatory and fibrotic molecular programs (64, 65).
Implication of GH/IGF1 Axis Activity on Insulin Resistance and Glycemic Status
GH can promote insulin resistance directly through insulin antagonism and indirectly through lipolysis-induced release of free fatty acids, leading to hyperinsulinemia and hyperglycemia. However, low-dose GH replacement has been shown to have a relatively neutral impact on insulin resistance and glycemic profiles in adults with GHD unselected for NAFLD (66). Evidence in adults with GHD suggests that there is an initial but temporary increase in insulin resistance with GH therapy that is subsequently offset by improvements in body composition (67). Preserving glycemic status is of particular interest in studies of GH administration in subjects with overweight/obesity and NAFLD. These studies generally include subjects with prediabetes or well-controlled diabetes only and have strict drop criteria if glycemic status worsens (Table 1). In this setting, GH augmentation has been shown to be well tolerated, with few dropouts due to glycemic disturbance and clinically insignificant effects on glycemic measures, despite a significant reduction in liver fat (38-40). Therefore, evidence is emerging that augmenting GH levels within a physiologic range can reduce NAFLD without deleterious effects on glucose control in appropriately selected populations with overweight/obesity.
Potential Mechanisms by Which GH Protects Against Steatosis and NAFLD Progression
As reviewed in the previous discussion, there is a clear association between lower GH/IGF1 activity and the development and progression of NAFLD. The following section discusses some of the mechanisms that may contribute to the protective and therapeutic actions of GH and IGF1.
GH Enhances Resting Energy Expenditure
GH may help to prevent steatosis by increasing nutrient utilization. As reviewed in detail by Moller and Jorgensen (7) and supported by additional studies (68-70), acute GH treatment in normal subjects, acute or prolonged GH treatment in GHD, or elevations in endogenous GH due to acromegaly are all consistently associated with an increase in resting energy expenditure (REE). In converse, in subjects with acromegaly, pegvisomant reduces REE (71). The energy used at rest is predominantly derived from fat (72). In healthy lean subjects, GH infusion under hyperinsulinemic/euglycemic clamp conditions leads to increases in REE that are associated with an increase in circulating NEFA and whole-body fatty acid oxidation (FAO) (73). Of note, GH-mediated IGF1 may contribute to the rise in REE, based on a study showing IGF1 treatment of healthy adults enhanced REE and FAO (74). Also, REE and FAO were increased in patients with GHD after short-term IGF1 or GH treatment, while the impact of combination therapy was additive (75). The positive effect of IGF1 on REE and FAO is believed to be indirect via reduction in insulin and enhanced muscle insulin sensitivity (75).
In some, but not all, long-term studies, the GH-mediated increase in REE is associated with an increase in whole-body FAO, with or without a shift in body composition toward an increase in fat-free mass (FFM). The variable impact of GH may be due to differences in treatment duration, dose, severity of associated GHD, and the relative contribution of variable activity levels and diet that are not typically controlled for in long-term clinical studies. Certainly, a GH-associated increase in FFM could contribute to the increase in REE observed after long term GH treatment, since FFM accounts for the majority of calories burned at rest [estimated to be 79% = brain (20%) + liver (21%) + heart (8%) + kidney (8%) + muscle (22%)] (72). As previously reviewed (76), an increase in muscle mass can be observed in GHD subjects (but not in healthy lean subjects), after more than 12 months of GH therapy, which could contribute to its energetic effects. However, the fact that GH acutely increases REE and that an increase in REE is observed prior to any change in FFM in long-term studies suggest that additional mechanisms are involved.
The Role of GH in Substrate Mobilization and Utilization
It is now understood that the rise in GH that occurs in response to fasting in healthy lean subjects contributes to circulating NEFA by directly stimulating WAT lipolysis (77-79). The NEFA derived from WAT lipolysis is used by the liver as a substrate for ketogenesis, as well as fuel for gluconeogenesis, while the corresponding rise in glycerol provides substrate for gluconeogenesis. In the fasted muscle, nutrient utilization switches from glucose to NEFA. Although GH is commonly considered to promote insulin resistance, evidence is accumulating that the acute anti-insulin effects of GH on skeletal muscle are not due to impairment of canonical insulin receptor signaling. Instead, evidence supports a GH-mediated increase in the supply of NEFA to the skeletal muscle leading to substrate competition (NEFA over glucose) (80).
Although GH was shown to directly enhance FAO in human fibroblasts in vitro (81), there is no clear evidence for a direct role of GH in enhancing molecular programs critical for FAO in human muscle. Specifically, skeletal muscle biopsies were taken from patients with hypopituitarism and severe GHD before and after 2 weeks of treatment with GH sufficient to raise IGF1 levels, REE, and whole-body lipid utilization. These GH-treated muscle biopsy samples exhibited suppressed expression of skeletal muscle genes related to FAO (82). Also, in a mouse model of postnatal GHR knockout in skeletal and cardiac muscle, although skeletal muscle expression of FAO-related genes was reduced, this did not impact whole-body FAO in response to exercise or fasting (83). Taken together, it appears that at least within the skeletal muscle, GH indirectly enhances whole-body FAO due to substrate availability and not by enhancing the molecular pathways required for β-oxidation.
Alternatively, GH has been proposed to act on skeletal muscle to enhance local mobilization and uptake of NEFA. Specifically, when GH replacement was combined with short-term exercise in adults with GHD, the expression of muscle hormone sensitive lipase and fatty acid binding protein 3 increased, suggesting that GH may enhance intramyocellular lipid mobilization and uptake, respectively (84). It has also been suggested that IGF1 directly improves FAO in the muscle by enhancing NEFA uptake (60). However, at least in mice, the IGF1 receptor is only expressed early in muscle development, while the insulin receptor is preferentially expressed in mature myocytes. Consistent with these findings, knockout of the IGF1 receptor in differentiated myocytes of mice had minimal effect on muscle morphology or metabolic endpoints (85).
GH May Alter REE Secondary to Promoting Thyroxine to Triiodothyronine Conversion
Triiodothyronine (T3) maintains REE by regulating obligatory thermogenesis, with fatty acids as the primary fuel source (86-88). GH replacement therapy with or without thyroxine (T4) replacement enhances free T3 levels in a dose-dependent fashion (89-93). In addition, T3 levels are reduced in subjects with acromegaly after transsphenoidal surgery (93). The GH-mediated changes in T3 are associated with a reciprocal change in T4, indicating that GH plays an important role in the conversion of T4 to T3. This conversion is mediated by tissue-specific expression of deiodinases (Dio1 and Dio2), and a recent report demonstrated that GH increased Dio2 expression (messenger RNA, protein) and Dio2 activity in a human thyroid carcinoma cell line (93). It should be noted that when exogenous T3 was provided to healthy subjects to match circulating T3 levels observed after GH treatment, it did not significantly increase REE but did increase heart rate, suggesting GH-mediated rise in T3 is not a major contributor to increased REE (89). However, it is possible that exogenous T3 delivery may not mimic the tissue-specific local concentrations and actions of T3 achieved after GH treatment. In fact, the GH-mediated conversion of T4 to T3 was shown to be tissue-specific (liver and kidney, not brown adipose tissue) and dependent on T4 levels in a hypophysectomized rat model (94). GH regulation of T4 to T3 conversion may also be tissue-specific in humans, as GH treatment of patients with hypopituitarism caused the expected reciprocal shift in peripheral T4 to T3 but reduced deiodinase expression in subcutaneous fat biopsies (95). Additionally, a liver-specific increase in Dio1 expression was shown to reduce diet-induced fatty liver, supporting an important role for local hepatic conversion of T4 to T3 in NAFLD (96).
GH Acts Centrally to Modify Systemic Metabolic Function
GH receptors are expressed throughout the brain. Genetically engineered mouse models that allow neuronal specific deactivation or activation of GHR signaling demonstrate that GH acts centrally to control systemic metabolic function (97). The specific actions of GH are dependent on the neuronal target and physiologic setting. It was reported that selective activation of steroidogenic factor 1 (SF1) neurons in the ventromedial nucleus increases energy expenditure and FAO (98), while knockout of SF1 in the ventromedial nucleus prevents an exercise-induced rise in energy expenditure and impairs weight loss (99). GH appears to be important in activating the SF1 neuronal population, based on a recent study showing knockout of the GHR in SF1 neurons impaired adaptation to aerobic exercise and increased fat mass (100). Additionally, knockout of the GHR in corticotropin-releasing hormone neurons reduced energy expenditure in female but not male mice, without altering body composition or FAO (101). Of note, selective knockout of the GHR in agouti-related peptide neurons within the arcuate nucleus increased energy expenditure and led to weight loss (102). However, collective activation of GHR positive neurons in the arcuate nucleus increased energy expenditure and heat production. These changes were associated with an increase in carbohydrate utilization and improved muscle insulin sensitivity (103). Although this area of investigation is in its infancy, the GH-mediated central regulation of energy expenditure needs to be considered as a potential mechanism of the impact of GH on NAFLD/NASH progression.
GH Regulates Immune Cell Function
Clinically, raising endogenous GH or GH administration has been shown to reduce markers of inflammation (62-65, 104, 105). This action may be in part due to the direct actions of GH/IGF1 on immune cell function. Specifically, congenital knockout of the GHR in macrophage precursor cells increased systemic insulin resistance in diet-induced obese mice that was associated with an increase in the number of M1 (inflammatory) macrophages in adipose tissue (106). Conversely, mice with elevated GH levels are protected from autoimmune diabetes (107) and recover faster after experimental colitis (108), attributed to the repolarization of macrophages to an anti-inflammatory/reparative (M2) profile. A switch from inflammatory (M1) to anti-inflammatory/reparative (M2) program was also observed after treatment with GH in both mouse and human primary mononuclear cell cultures (108). Local production and action of IGF1 may also contribute to shifting the macrophage phenotype. For example, M2 macrophages express more IGF1, and knockout of the IGF1 receptor in macrophage precursor cells increases M1 macrophages and worsens metabolic dysfunction in diet-induced obese mice (109). In the context of liver injury, evidence is accumulating that a shift in the macrophage phenotype from M1 to M2 may be protective against early progression of NAFLD but may promote fibrosis at later stages (110).
GH/IGF1 Regulation of Hepatic Stellate Cell Function
When a mouse model of inflammatory cholestasis and liver fibrosis was crossbred to mice with whole-body GHR knockout (Laron mice), liver injury was exacerbated (111). An antifibrotic effect of GH may be mediated in part by IGF1, since IGF1 has been shown to protect against the development of fibrosis in multiple rodent models (112-116). The protective role of IGF1 is thought to be due to IGF1 directly inducing HSC senescence (116). Although a direct action of GH on HSC function has not been investigated to date, the direct effects of IGF1 on HSC, coupled with the direct actions of GH and IGF1 on macrophage function (addressed in the previous discussion), would be predicted to reduce inflammatory signals that activate HSC and slow fibrosis progression.
GH Acts Directly on the Hepatocyte to Suppress Steatosis and Liver Injury
To date, our understanding of how GH directly regulates hepatocyte function to reduce steatosis and prevent liver injury is largely based on genetically engineered mouse models that allow for liver (hepatocyte)-specific knockout of GHR or its downstream effectors [Janus kinase 2 (JAK2) or STAT5]. The first models reported were of knockout of hepatocyte GHR signaling early in development (congenital knockouts). In these congenital mouse models, steatosis develops in adults associated with systemic insulin resistance and glucose intolerance (117-121). Experimental loss of any component of the hepatic GHR/JAK2/STAT5 signal cascade dramatically reduces circulating IGF1 levels, which in turn alters systemic metabolic function, since IGF1 has been shown to promote the actions of insulin (60). Also, since IGF1 negatively feeds back to suppress GH secretion, the experimental reduction in IGF1 leads to a rise in circulating GH. Chronically elevated GH drives systemic insulin resistance and WAT lipolysis leading to a flux of NEFA to the liver (118, 121). In addition, low IGF1 paired with high GH levels during early maturation alters the development of other metabolically relevant tissues (118, 122), which could indirectly contribute to the liver phenotype reported in adult mice. It should be noted that these changes in circulating IGF1 are not thought to directly regulate hepatocyte function since the mature hepatocyte does not express the IGF1R (5).
However, a direct action of GH on hepatocyte steatosis and injury is supported by the observation that expression of an IGF1 transgene in hepatocytes of mice with congenital knockout of hepatocyte GHR normalized circulating IGF1 and GH level as well as systemic metabolic function, but did not completely protect the liver against steatosis and injury (123). In addition, in a mouse model of adult-onset, hepatocyte-specific GHR knockdown (aHepGHRkd), steatosis rapidly develops independent of alterations in systemic insulin sensitivity, glucose tolerance, WAT lipolysis, and VLDL-TG release (124, 125). Steatosis persists in aHepGHRkd mice and early signs of NASH develop with age, independent of alterations in circulating IGF1 levels (126, 127). It should be noted that steatosis rapidly develops in aHepGHRkd male mice and ovariectomized female mice, while ovary-intact female mice are protected (124). These sex differences are consistent with clinical data demonstrating that the prevalence of NAFLD is lower in premenopausal women compared to men but is greater in women compared to men after menopause (128).
Despite differences in steatosis development, both male and female aHepGHRkd mice exhibit an increase in hepatic DNL (124). Studies to date suggest that this increase in aHepGHRkd mice cannot be attributed to enhanced hepatic insulin signaling but is associated with an increase in endpoints indicative of enhanced glycolysis. Specifically, these mice demonstrate an increase in the hepatic expression of glucokinase gene expression and cytoplasmic (active) protein content, as well as an increase in fructose 2,6 bisphosphate, the most potent activator of glycolysis and inhibitor of gluconeogenesis. These findings may have translational relevance since hepatic glucokinase expression (129) and enhanced hepatic DNL (13-15) are highly correlated with hepatic TG content in human subjects with obesity. Of note, the enhanced DNL observed in the aHepGHRkd model is also associated with a reduction in hepatic carnitine palmitoyltransferase 1a messenger RNA and β-hydroxybutyrate coenzyme A levels, indicative of reduced β-oxidation. In addition, the expression of phosphoenolpyruvate carboxykinase is reduced, a key enzyme in gluconeogenesis (124). As previously reviewed (6), there are number of studies suggesting GH may play a direct role in enhancing gluconeogenesis, β-oxidation, and ketogenesis. In fact, it was recently reported that hepatic adenosine 5′-triphosphate levels are elevated in acromegalic subjects, indicative of increased mitochondrial activity that may prevent steatosis despite systemic insulin resistance (130). Therefore, it remains to be determined whether GH directly inhibits glycolysis-mediated DNL or whether the primary action of GH on hepatocyte metabolism is to shift nutrients away from DNL to favor β-oxidation and glucose production.
Potential Role of Hepatocyte GHR-mediated STAT5 Activation
In livers of obese and diabetic mice, the pattern of STAT5 target gene expression was shown to be indicative of suppressed STAT5 activity (131). It remains to be determined whether this is solely due to a reduction in production of GH observed in the obese/diabetic state (132) or due to the development of hepatic GH resistance. Nonetheless, a reduction in hepatic STAT5 activity may contribute to fatty liver since congenital, liver-specific loss of STAT5 (LivStat5KO) increases hepatic lipid accumulation and predisposes the liver to secondary injury, associated with fibrosis and the development of hepatocellular carcinoma (117, 133-136). Based on these reports, it may be assumed that GHR signals through STAT5 to regulate hepatic lipid accumulation and prevent liver injury. However, it is now recognized that the compensatory increase in STAT3 activity that occurs in livers of LivStat5KO mice, plays a major role in disease progression (117, 133-135). Therefore, it remains to be directly tested whether GHR-mediated STAT5 activity is the primary signal transduction pathway by which GH suppresses steatosis and injury. However, it is recognized that in rodent models, STAT5 regulates a large subset of hepatic genes through classic binding to proximal promoters and epigenetic alterations in a sex-dependent fashion (137, 138). Since many of these genes are important in sex-steroid and xenobiotic processing, it has been hypothesized that GH-mediated STAT5 may contribute to the sex bias in the development NAFLD and hepatocellular carcinoma. Hepatocyte GH signaling has also been shown to promote liver regeneration after partial hepatectomy in a STAT5-dependent (117) and STAT5-independent fashion (139), and it is now recognized that maladaptive regeneration of hepatocytes is an important contributor to NASH progression (140). However, any positive effects of GH/STAT5 on hepatocyte regeneration in the context of liver injury should be viewed with caution, since NASH is associated with an increased risk of hepatocellular carcinoma, characterized by an increase in IGF1R expression (141).
Conclusion
GH and IGF1 levels are reduced in obesity and are associated with NAFLD/NASH. Although limited, clinical trials suggest that the fall in GH and IGF1 may contribute to NAFLD/NASH progression since enhancing GH axis activity can reduce hepatic steatosis, inflammation, and fibrosis (Table 1). GH augmentation has been studied mainly in adults with prediabetes or well-controlled diabetes, and careful monitoring of glycemic status has demonstrated excellent tolerability in these populations. Both clinical and animal studies suggest that GH and IGF1 mediate these therapeutic effects by enhancing whole-body nutrient utilization and reducing inflammation, which would serve to ultimately improve insulin sensitivity and shift nutrients away from the liver, particularly if GH levels are maintained in a physiologic range. In addition, there are multiple liver-specific actions of GH and IGF1, primarily identified using preclinical models (highlighted in Figure 1; stars and diamonds, respectively), that would favor prevention and protection against NAFLD progression. Given the fact that NAFLD/NASH is a significant clinical burden and there are no Food and Drug Administration–approved drugs to treat it, the emerging evidence that GH and IGF1 can act at multiple levels to prevent and slow the progression of NAFLD/NASH warrants additional clinical and basic investigations that may lead to the development of unique targeted therapeutics to improve hepatic steatosis, inflammation, and fibrosis in NAFLD.
Acknowledgments
We would like to thank Drs. Karen K. Miller, Mercedes del Rio-Moreno, and Mari C. Vázquez-Borrego for their careful review of this manuscript and Dr. del Rio-Moreno for designing Figure 1.
Contributor Information
Laura E Dichtel, Neuroendocrine Unit, Department of Medicine, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Jose Cordoba-Chacon, Department of Medicine, Division of Endocrinology, Diabetes and Metabolism, University of Illinois at Chicago, Chicago, IL, USA.
Rhonda D Kineman, Department of Medicine, Division of Endocrinology, Diabetes and Metabolism, University of Illinois at Chicago, Chicago, IL, USA; Jesse Brown VA Medical Center, Research and Development Division, Chicago, IL, USA.
Financial Support
National Institutes of Health K23 DK113220 (L.E.D.), K01DK115525 (J.C.C.), R01DK116878 (R.D.K.), Early Career Development Award of the Central Society for Clinical and Translational Research (J.C.C.), and Veterans Administration Merit Award I01BX004448 and Research Career Scientist Award 5IK6BX005382 (R.D.K.).
Disclosures
L.E.D. has received drug donation (GH and identical placebo) from Pfizer by investigator-initiated request for an National Institutes of Health–funded trial. The other authors have no conflicts of interest to report.
Data Availability
This is a review article of previous published work, and therefore no original data was generated.
References
- 1. Chhabra Y, Lee CMM, Muller AF, Brooks AJ. GHR signalling: receptor activation and degradation mechanisms. Mol Cell Endocrinol. 2021;520:111075. [DOI] [PubMed] [Google Scholar]
- 2. Domene S, Domene HM. The role of acid-labile subunit (ALS) in the modulation of GH-IGF-I action. Mol Cell Endocrinol. 2020;518:111006. [DOI] [PubMed] [Google Scholar]
- 3. Liu JL, Yakar S, LeRoith D. Conditional knockout of mouse insulin-like growth factor-1 gene using the Cre/loxP system. Proc Soc Exp Biol Med. 2000;223(4):344-351. [DOI] [PubMed] [Google Scholar]
- 4. Kineman RD, Del Rio-Moreno M, Sarmento-Cabral A. 40 YEARS of IGF1: understanding the tissue-specific roles of IGF1/IGF1R in regulating metabolism using the Cre/loxP system. J Mol Endocrinol. 2018;61(1):T187-T198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Waraky A, Aleem E, Larsson O. Downregulation of IGF-1 receptor occurs after hepatic linage commitment during hepatocyte differentiation from human embryonic stem cells. Biochem Biophys Res Commun. 2016;478(4):1575-1581. [DOI] [PubMed] [Google Scholar]
- 6. Vazquez-Borrego MC, Del Rio-Moreno M, Kineman RD. Towards understanding the direct and indirect actions of growth hormone in controlling hepatocyte carbohydrate and lipid metabolism. Cells. 2021;10(10):2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moller N, Jorgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev. 2009;30(2):152-177. [DOI] [PubMed] [Google Scholar]
- 8. Birketvedt GS, Geliebter A, Kristiansen I, Firgenschau Y, Goll R, Florholmen JR. Diurnal secretion of ghrelin, growth hormone, insulin binding proteins, and prolactin in normal weight and overweight subjects with and without the night eating syndrome. Appetite. 2012;59(3):688-692. [DOI] [PubMed] [Google Scholar]
- 9. Caldwell S, Ikura Y, Dias D, et al. . Hepatocellular ballooning in NASH. J Hepatol. 2010;53(4):719-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kanwal F, Shubrook JH, Younossi Z, et al. . Preparing for the NASH epidemic: a call to action. Diabetes Care. 2021;44(9):2162-2172. [DOI] [PubMed] [Google Scholar]
- 11. Younossi Z, Tacke F, Arrese M, et al. . Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2019;69(6):2672-2682. [DOI] [PubMed] [Google Scholar]
- 12. Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65(8):1038-1048. [DOI] [PubMed] [Google Scholar]
- 13. Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115(5):1343-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lambert JE, Ramos-Roman MA, Browning JD, Parks EJ. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 2014;146(3):726-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smith GI, Shankaran M, Yoshino M, et al. . Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J Clin Invest. 2020;130(3):1453-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rui L. Energy metabolism in the liver. Compr Physiol. 2014;4(1):177-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Choi SH, Ginsberg HN. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends Endocrinol Metab. 2011;22(9):353-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Younossi ZM, Corey KE, Lim JK. AGA clinical practice update on lifestyle modification using diet and exercise to achieve weight loss in the management of nonalcoholic fatty liver disease: expert review. Gastroenterology. 2021;160(3):912-918. [DOI] [PubMed] [Google Scholar]
- 19. Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. . Weight loss through lifestyle modification significantly reduces features of nonalcoholic Steatohepatitis. Gastroenterology. 2015;149(2):367-378, e365; quiz e314-e365. [DOI] [PubMed] [Google Scholar]
- 20. Promrat K, Kleiner DE, Niemeier HM, et al. . Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51(1):121-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oh S, Tsujimoto T, Kim B, et al. . Weight-loss-independent benefits of exercise on liver steatosis and stiffness in Japanese men with NAFLD. JHEP Rep. 2021;3(3):100253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Babu AF, Csader S, Lok J, et al. . Positive effects of exercise intervention without weight loss and dietary changes in NAFLD-related clinical parameters: a systematic review and meta-analysis. Nutrients. 2021;13(9):3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chalasani N, Younossi Z, Lavine JE, et al. . The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328-357. [DOI] [PubMed] [Google Scholar]
- 24. Vuppalanchi R, Noureddin M, Alkhouri N, Sanyal AJ. Therapeutic pipeline in nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol. 2021;18(6):373-392. [DOI] [PubMed] [Google Scholar]
- 25. Ferguson D, Finck BN. Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat Rev Endocrinol. 2021;17(8):484-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Drenth JPH, Schattenberg JM. The nonalcoholic steatohepatitis (NASH) drug development graveyard: established hurdles and planning for future success. Expert Opin Investig Drugs. 2020;29(12):1365-1375. [DOI] [PubMed] [Google Scholar]
- 27. Frystyk J, Brick DJ, Gerweck AV, Utz AL, Miller KK. Bioactive insulin-like growth factor-I in obesity. J Clin Endocrinol Metab. 2009;94(8):3093-3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Utz AL, Yamamoto A, Sluss P, Breu J, Miller KK. Androgens may mediate a relative preservation of IGF-I levels in overweight and obese women despite reduced growth hormone secretion. J Clin Endocrinol Metab. 2008;93(10):4033-4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scacchi M, Pincelli AI, Cavagnini F. Growth hormone in obesity. Int J Obes Relat Metab Disord. 1999;23(3):260-271. [DOI] [PubMed] [Google Scholar]
- 30. Nishizawa H, Iguchi G, Murawaki A, et al. . Nonalcoholic fatty liver disease in adult hypopituitary patients with GH deficiency and the impact of GH replacement therapy. Eur J Endocrinol. 2012;167(1):67-74. [DOI] [PubMed] [Google Scholar]
- 31. Meienberg F, Yee M, Johnston D, et al. . Liver fat in adults with GH deficiency: comparison to matched controls and the effect of GH replacement. Clin Endocrinol (Oxf). 2016;85(1):76-84. [DOI] [PubMed] [Google Scholar]
- 32. Bredella MA, Schorr M, Dichtel LE, et al. . Body composition and ectopic lipid changes with biochemical control of acromegaly. J Clin Endocrinol Metab. 2017;102(11):4218-4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reyes-Vidal CM, Mojahed H, Shen W, et al. . Adipose tissue redistribution and ectopic lipid deposition in active acromegaly and effects of surgical treatment. J Clin Endocrinol Metab. 2015;100(8):2946-2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Winhofer Y, Wolf P, Krššák M, et al. . No evidence of ectopic lipid accumulation in the pathophysiology of the acromegalic cardiomyopathy. J Clin Endocrinol Metab. 2014;99(11):4299-4306. [DOI] [PubMed] [Google Scholar]
- 35. Madsen M, Krusenstjerna-Hafstrom T, Moller L, et al. . Fat content in liver and skeletal muscle changes in a reciprocal manner in patients with acromegaly during combination therapy with a somatostatin analog and a GH receptor antagonist: a randomized clinical trial. J Clin Endocrinol Metab. 2012;97(4):1227-1235. [DOI] [PubMed] [Google Scholar]
- 36. Kuker AP, Shen W, Jin Z, et al. . Body composition changes with long-term pegvisomant therapy of acromegaly. J Endocr Soc. 2021;5(3):bvab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pan CS, Weiss JJ, Fourman LT, et al. . Effect of recombinant human growth hormone on liver fat content in young adults with nonalcoholic fatty liver disease. Clin Endocrinol. 2021;94(2):183-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bredella MA, Gerweck AV, Lin E, et al. . Effects of GH on body composition and cardiovascular risk markers in young men with abdominal obesity. J Clin Endocrinol Metab. 2013;98(9):3864-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stanley TL, Feldpausch MN, Oh J, et al. . Effect of tesamorelin on visceral fat and liver fat in HIV-infected patients with abdominal fat accumulation: a randomized clinical trial. JAMA. 2014;312(4):380-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stanley TL, Fourman LT, Feldpausch MN, et al. . Effects of tesamorelin on non-alcoholic fatty liver disease in HIV: a randomised, double-blind, multicentre trial. Lancet HIV. 2019;6(12):e821-e830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ichikawa T, Hamasaki K, Ishikawa H, Ejima E, Eguchi K, Nakao K. Non-alcoholic steatohepatitis and hepatic steatosis in patients with adult onset growth hormone deficiency. Gut. 2003;52(6):914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Adams LA, Feldstein A, Lindor KD, Angulo P. Nonalcoholic fatty liver disease among patients with hypothalamic and pituitary dysfunction. Hepatology. 2004;39(4):909-914. [DOI] [PubMed] [Google Scholar]
- 43. Takahashi Y, Iida K, Takahashi K, et al. . Growth hormone reverses nonalcoholic steatohepatitis in a patient with adult growth hormone deficiency. Gastroenterology. 2007;132(3):938-943. [DOI] [PubMed] [Google Scholar]
- 44. Brummer RJ. Effects of growth hormone treatment on visceral adipose tissue. Growth Horm IGF Res. 1998;8(suppl B):19-23. [DOI] [PubMed] [Google Scholar]
- 45. Iranmanesh A, Lizarralde G, Veldhuis JD. Age and relative adiposity are specific negative determinants of the frequency and amplitude of growth hormone (GH) secretory bursts and the half-life of endogenous GH in healthy men. J Clin Endocrinol Metab. 1991;73(5):1081-1088. [DOI] [PubMed] [Google Scholar]
- 46. Pijl H, Langendonk JG, Burggraaf J, et al. . Altered neuroregulation of GH secretion in viscerally obese premenopausal women. J Clin Endocrinol Metab. 2001;86(11):5509-5515. [DOI] [PubMed] [Google Scholar]
- 47. Cornford AS, Barkan AL, Horowitz JF. Rapid suppression of growth hormone concentration by overeating: potential mediation by hyperinsulinemia. J Clin Endocrinol Metab. 2011;96(3):824-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kok P, Buijs MM, Kok SW, et al. . Acipimox enhances spontaneous growth hormone secretion in obese women. Am J Physiol Regul Integr Comp Physiol. 2004;286(4):R693-R698. [DOI] [PubMed] [Google Scholar]
- 49. Tritos NA, Biller BMK. Current concepts of the diagnosis of adult growth hormone deficiency. Rev Endocr Metab Disord. 2021;22(1):109-116. [DOI] [PubMed] [Google Scholar]
- 50. Ichikawa T, Nakao K, Hamasaki K, et al. . Role of growth hormone, insulin-like growth factor 1 and insulin-like growth factor-binding protein 3 in development of non-alcoholic fatty liver disease. Hepatol Int. 2007;1(2):287-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xu L, Xu C, Yu C, et al. . Association between serum growth hormone levels and nonalcoholic fatty liver disease: a cross-sectional study. PLoS One. 2012;7(8):e44136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lonardo A, Loria P, Leonardi F, Ganazzi D, Carulli N. Growth hormone plasma levels in nonalcoholic fatty liver disease. Am J Gastroenterol. 2002;97(4):1071-1072. [DOI] [PubMed] [Google Scholar]
- 53. Fusco A, Miele L, D’Uonnolo A, et al. . Nonalcoholic fatty liver disease is associated with increased GHBP and reduced GH/IGF-I levels. Clin Endocrinol (Oxf). 2012;77(4):531-536. [DOI] [PubMed] [Google Scholar]
- 54. Bredella MA, Torriani M, Thomas BJ, et al. . Peak growth hormone-releasing hormone-arginine-stimulated growth hormone is inversely associated with intramyocellular and intrahepatic lipid content in premenopausal women with obesity. J Clin Endocrinol Metab. 2009;94(10):3995-4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Arturi F, Succurro E, Procopio C, et al. . Nonalcoholic fatty liver disease is associated with low circulating levels of insulin-like growth factor-I. J Clin Endocrinol Metab. 1644;96(10):E1640. [DOI] [PubMed] [Google Scholar]
- 56. Sumida Y, Yonei Y, Tanaka S, et al. . Lower levels of insulin-like growth factor-1 standard deviation score are associated with histological severity of non-alcoholic fatty liver disease. Hepatol Res. 2014. [DOI] [PubMed] [Google Scholar]
- 57. Garcia-Galiano D, Sanchez-Garrido MA, Espejo I, et al. . IL-6 and IGF-1 are independent prognostic factors of liver steatosis and non-alcoholic steatohepatitis in morbidly obese patients. Obes Surg. 2007;17(4):493-503. [DOI] [PubMed] [Google Scholar]
- 58. Colak Y, Senates E, Ozturk O, et al. . Serum concentrations of human insulin-like growth factor-1 and levels of insulin-like growth factor-binding protein-5 in patients with nonalcoholic fatty liver disease: association with liver histology. Eur J Gastroenterol Hepatol. 2012;24(3):255-261. [DOI] [PubMed] [Google Scholar]
- 59. Dichtel LE, Corey KE, Misdraji J, et al. . The association between IGF-1 levels and the histologic severity of nonalcoholic fatty liver disease. Clin Transl Gastroenterol. 2017;8(1):e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Clemmons DR. Metabolic actions of insulin-like growth factor-I in normal physiology and diabetes. Endocrinol Metab Clin North Am. 2012;41(2):425-443, vii-viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rochira V, Guaraldi G. Growth hormone deficiency and human immunodeficiency virus. Best Pract Res Clin Endocrinol Metab. 2017;31(1):91-111. [DOI] [PubMed] [Google Scholar]
- 62. Stanley TL, Fourman LT, Zheng I, et al. . Relationship of IGF-1 and IGF-binding proteins to disease severity and glycemia in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2021;106(2): e520-e533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stanley TL, Fourman LT, Wong LP, et al. . Growth hormone releasing hormone reduces circulating markers of immune activation in parallel with effects on hepatic immune pathways in individuals with HIV-infection and nonalcoholic fatty liver disease. Clin Infect Dis. 2021;73(4):621-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fourman LT, Billingsley JM, Agyapong G, et al. . Effects of tesamorelin on hepatic transcriptomic signatures in HIV-associated NAFLD. JCI Insight. 2020;5(16):e140134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fourman LT, Stanley TL, Billingsley JM, et al. . Delineating tesamorelin response pathways in HIV-associated NAFLD using a targeted proteomic and transcriptomic approach. Sci Rep. 2021;11(1):10485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kim S-H, Park M-J. Effects of growth hormone on glucose metabolism and insulin resistance in human. Ann Pediatr Endocrinol Metab. 2017;22(3):145-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jorgensen JO, Vestergaard E, Gormsen L, et al. . Metabolic consequences of GH deficiency. J Endocrinol Invest. 2005;28(5 suppl):47-51. [PubMed] [Google Scholar]
- 68. Stenlof K, Johansson JO, Lonn L, Sjostrom L, Bengtsson BA. Diurnal variations in twenty-four-hour energy expenditure during growth hormone treatment of adults with pituitary deficiency. J Clin Endocrinol Metab. 1997;82(4):1255-1260. [DOI] [PubMed] [Google Scholar]
- 69. Momozono A, Hayashi A, Takano K, Shichiri M. The effectiveness of growth hormone replacement on energy expenditure and body composition in patients with adult growth hormone deficiency. Endocr J. 2021;68(4):469-475. [DOI] [PubMed] [Google Scholar]
- 70. O’Sullivan AJ, Kelly JJ, Hoffman DM, Freund J, Ho KK. Body composition and energy expenditure in acromegaly. J Clin Endocrinol Metab. 1994;78(2):381-386. [DOI] [PubMed] [Google Scholar]
- 71. Lindberg-Larsen R, Moller N, Schmitz O, et al. . The impact of pegvisomant treatment on substrate metabolism and insulin sensitivity in patients with acromegaly. J Clin Endocrinol Metab. 2007;92(5):1724-1728. [DOI] [PubMed] [Google Scholar]
- 72. Melzer K. Carbohydrate and fat utilization during rest and physical activiity. E-SPEN. 2011;6:e45-e52. [Google Scholar]
- 73. Bak JF, Moller N, Schmitz O. Effects of growth hormone on fuel utilization and muscle glycogen synthase activity in normal humans. Am J Physiol. 1991;260(5 Pt 1):E736-E742. [DOI] [PubMed] [Google Scholar]
- 74. Hussain MA, Schmitz O, Mengel A, et al. . Insulin-like growth factor I stimulates lipid oxidation, reduces protein oxidation, and enhances insulin sensitivity in humans. J Clin Invest. 1993;92(5):2249-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hussain MA, Schmitz O, Mengel A, et al. . Comparison of the effects of growth hormone and insulin-like growth factor I on substrate oxidation and on insulin sensitivity in growth hormone-deficient humans. J Clin Invest. 1994;94(3):1126-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chikani V, Ho KK. Action of GH on skeletal muscle function: molecular and metabolic mechanisms. J Mol Endocrinol. 2014;52(1):R107-R123. [DOI] [PubMed] [Google Scholar]
- 77. Hoyer KL, Hogild ML, List EO, et al. . The acute effects of growth hormone in adipose tissue is associated with suppression of antilipolytic signals. Physiol Rep. 2020;8(3):e14373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sharma VM, Vestergaard ET, Jessen N, et al. . Growth hormone acts along the PPARgamma-FSP27 axis to stimulate lipolysis in human adipocytes. Am J Physiol Endocrinol Metab. 2019;316(1):E34-E42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kopchick JJ, Berryman DE, Puri V, Lee KY, Jorgensen JOL. The effects of growth hormone on adipose tissue: old observations, new mechanisms. Nat Rev Endocrinol. 2020;16(3):135-146. doi:10.1038/s41574-019-0280-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hjelholt AJ, Charidemou E, Griffin JL, et al. . Insulin resistance induced by growth hormone is linked to lipolysis and associated with suppressed pyruvate dehydrogenase activity in skeletal muscle: a 2 × 2 factorial, randomised, crossover study in human individuals. Diabetologia. 2020;63(12):2641-2653. [DOI] [PubMed] [Google Scholar]
- 81. Leung KC, Ho KK. Stimulation of mitochondrial fatty acid oxidation by growth hormone in human fibroblasts. J Clin Endocrinol Metab. 1997;82(12):4208-4213. [DOI] [PubMed] [Google Scholar]
- 82. Sjogren K, Leung KC, Kaplan W, Gardiner-Garden M, Gibney J, Ho KK. Growth hormone regulation of metabolic gene expression in muscle: a microarray study in hypopituitary men. Am J Physiol Endocrinol Metab. 2007;293(1):E364-E371. [DOI] [PubMed] [Google Scholar]
- 83. Vijayakumar A, Wu Y, Buffin NJ, et al. . Skeletal muscle growth hormone receptor signaling regulates basal, but not fasting-induced, lipid oxidation. PLoS One. 2012;7(9):e44777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Trepp R, Fluck M, Stettler C, et al. . Effect of GH on human skeletal muscle lipid metabolism in GH deficiency. Am J Physiol Endocrinol Metab. 2008;294(6):E1127-E1134. [DOI] [PubMed] [Google Scholar]
- 85. O’Neill BT, Lauritzen HP, Hirshman MF, Smyth G, Goodyear LJ, Kahn CR. Differential role of insulin/IGF-1 receptor signaling in muscle growth and glucose homeostasis. Cell Rep. 2015;11(8):1220-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yavuz S, Salgado Nunez DPS, Celi FS. Thyroid hormone action and energy expenditure. J Endocr Soc. 2019;3(7):1345-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94(2):355-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Solmonson A, Mills EM. Uncoupling proteins and the molecular mechanisms of thyroid thermogenesis. Endocrinology. 2016;157(2):455-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wolthers T, Groftne T, Moller N, et al. . Calorigenic effects of growth hormone: the role of thyroid hormones. J Clin Endocrinol Metab. 1996;81(4):1416-1419. [DOI] [PubMed] [Google Scholar]
- 90. Jorgensen JO, Pedersen SA, Laurberg P, Weeke J, Skakkebaek NE, Christiansen JS. Effects of growth hormone therapy on thyroid function of growth hormone-deficient adults with and without concomitant thyroxine-substituted central hypothyroidism. J Clin Endocrinol Metab. 1989;69(6):1127-1132. [DOI] [PubMed] [Google Scholar]
- 91. Jorgensen JO, Moller J, Laursen T, Orskov H, Christiansen JS, Weeke J. Growth hormone administration stimulates energy expenditure and extrathyroidal conversion of thyroxine to triiodothyronine in a dose-dependent manner and suppresses circadian thyrotrophin levels: studies in GH-deficient adults. Clin Endocrinol (Oxf). 1994;41(5):609-614. [DOI] [PubMed] [Google Scholar]
- 92. Glynn N, Kenny H, Salim T, et al. . Alterations in thyroid hormone levels following growth hormone replacement exert complex biological effects. Endocr Pract. 2018;24(4):342-350. [DOI] [PubMed] [Google Scholar]
- 93. Yamauchi I, Sakane Y, Yamashita T, et al. . Effects of growth hormone on thyroid function are mediated by type 2 iodothyronine deiodinase in humans. Endocrine. 2018;59(2):353-363. [DOI] [PubMed] [Google Scholar]
- 94. Gotzsche LS, Flyvbjerg A, Marshall S, Jorgensen KD, Weeke J. The influence of growth hormone and thyroxine on iodothyronine deiodinase activity in the liver, kidney and brown adipose tissue in hypophysectomized rats. Acta Endocrinol (Copenh). 1991;125(2):219-226. [DOI] [PubMed] [Google Scholar]
- 95. Glynn N, Kenny H, Quisenberry L, et al. . The effect of growth hormone replacement on the thyroid axis in patients with hypopituitarism: in vivo and ex vivo studies. Clin Endocrinol (Oxf). 2017;86(5):747-754. [DOI] [PubMed] [Google Scholar]
- 96. Bruinstroop E, Zhou J, Tripathi M, et al. . Early induction of hepatic deiodinase type 1 inhibits hepatosteatosis during NAFLD progression. Mol Metab. 2021;53:101266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Donato J Jr., Wasinski F, Furigo IC, Metzger M, Frazao R. Central regulation of metabolism by growth hormone. Cells. 2021;10(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Coutinho EA, Okamoto S, Ishikawa AW, et al. . Activation of SF1 neurons in the ventromedial hypothalamus by DREADD technology increases insulin sensitivity in peripheral tissues. Diabetes. 2017;66(9):2372-2386. [DOI] [PubMed] [Google Scholar]
- 99. Fujikawa T, Castorena CM, Pearson M, et al. . SF-1 expression in the hypothalamus is required for beneficial metabolic effects of exercise. Elife. 2016;5:e18206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Pedroso JAB, Dos Santos LBP, Furigo IC, et al. . Deletion of growth hormone receptor in hypothalamic neurons affects the adaptation capacity to aerobic exercise. Peptides. 2021;135:170426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Dos Santos WO, Gusmao DO, Wasinski F, List EO, Kopchick JJ, Donato J Jr. Effects of growth hormone receptor ablation in corticotropin-releasing hormone cells. Int J Mol Sci. 2021;22(18):9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Furigo IC, Teixeira PDS, de Souza GO, et al. . Growth hormone regulates neuroendocrine responses to weight loss via AgRP neurons. Nat Commun. 2019;10(1):662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. de Lima JBM, Debarba LK, Rupp AC, et al. . ARC(GHR) neurons regulate muscle glucose uptake. Cells. 2021;10(5):1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sesmilo G, Biller BM, Llevadot J, et al. . Effects of growth hormone administration on inflammatory and other cardiovascular risk markers in men with growth hormone deficiency: a randomized, controlled clinical trial. Ann Intern Med. 2000;133(2):111-122. [DOI] [PubMed] [Google Scholar]
- 105. Sesmilo G, Biller BM, Llevadot J, et al. . Effects of growth hormone (GH) administration on homocyst(e)ine levels in men with GH deficiency: a randomized controlled trial. J Clin Endocrinol Metab. 2001;86(4):1518-1524. [DOI] [PubMed] [Google Scholar]
- 106. Lu C, Kumar PA, Sun J, et al. . Targeted deletion of growth hormone (GH) receptor in macrophage reveals novel osteopontin-mediated effects of GH on glucose homeostasis and insulin sensitivity in diet-induced obesity. J Biol Chem. 2013;288(22):15725-15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Villares R, Kakabadse D, Juarranz Y, Gomariz RP, Martinez AC, Mellado M. Growth hormone prevents the development of autoimmune diabetes. Proc Natl Acad Sci U S A. 2013;110(48): E4619-E4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Soler Palacios B, Nieto C, Fajardo P, et al. . Growth hormone reprograms macrophages toward an anti-inflammatory and reparative profile in an MAFB-dependent manner. J Immunol. 2020;205(3):776-788. [DOI] [PubMed] [Google Scholar]
- 109. Spadaro O, Camell CD, Bosurgi L, et al. . IGF1 shapes macrophage activation in response to immunometabolic challenge. Cell Rep. 2017;19(2):225-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Thibaut R, Gage MC, Pineda-Torra I, Chabrier G, Venteclef N, Alzaid F. Liver macrophages and inflammation in physiology and physiopathology of non-alcoholic fatty liver disease. FEBS J. Published online April 15, 2021. Doi: 10.1111/febs.15877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Stiedl P, McMahon R, Blaas L, et al. . Growth hormone resistance exacerbates cholestasis-induced murine liver fibrosis. Hepatology. 2015;61(2):613-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Fiore EJ, Bayo JM, Garcia MG, et al. . Mesenchymal stromal cells engineered to produce IGF-I by recombinant adenovirus ameliorate liver fibrosis in mice. Stem Cells Dev. 2015;24(6):791-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Garcia-Fernandez M, Castilla-Cortazar I, Diaz-Sanchez M, et al. . Antioxidant effects of insulin-like growth factor-I (IGF-I) in rats with advanced liver cirrhosis. BMC Gastroenterol. 2005;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Mirpuri E, Garcia-Trevijano ER, Castilla-Cortazar I, et al. . Altered liver gene expression in CCl4-cirrhotic rats is partially normalized by insulin-like growth factor-I. Int J Biochem Cell Biol. 2002;34(3):242-252. [DOI] [PubMed] [Google Scholar]
- 115. Sobrevals L, Rodriguez C, Romero-Trevejo JL, et al. . Insulin-like growth factor I gene transfer to cirrhotic liver induces fibrolysis and reduces fibrogenesis leading to cirrhosis reversion in rats. Hepatology. 2010;51(3):912-921. [DOI] [PubMed] [Google Scholar]
- 116. Nishizawa H, Iguchi G, Fukuoka H, et al. . IGF-I induces senescence of hepatic stellate cells and limits fibrosis in a p53-dependent manner. Sci Rep. 2016;6:34605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Cui Y, Hosui A, Sun R, et al. . Loss of signal transducer and activator of transcription 5 leads to hepatosteatosis and impaired liver regeneration. Hepatology. 2007;46(2):504-513. [DOI] [PubMed] [Google Scholar]
- 118. Fan Y, Menon RK, Cohen P, et al. . Liver-specific deletion of the growth hormone receptor reveals essential role of growth hormone signaling in hepatic lipid metabolism. J Biol Chem. 2009;284(30):19937-19944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. List EO, Berryman DE, Funk K, et al. . Liver-specific GH receptor gene-disrupted (LiGHRKO) mice have decreased endocrine IGF-I, increased local IGF-I, and altered body size, body composition, and adipokine profiles. Endocrinology. 2014;155(5):1793-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Mueller KM, Kornfeld JW, Friedbichler K, et al. . Impairment of hepatic growth hormone and glucocorticoid receptor signaling causes steatosis and hepatocellular carcinoma in mice. Hepatology. 2011;54(4):1398-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Sos BC, Harris C, Nordstrom SM, et al. . Abrogation of growth hormone secretion rescues fatty liver in mice with hepatocyte-specific deletion of JAK2. J Clin Invest. 2011;121(4):1412-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Yakar S, Setser J, Zhao H, et al. . Inhibition of growth hormone action improves insulin sensitivity in liver IGF-1-deficient mice. J Clin Invest. 2004;113(1):96-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Liu Z, Cordoba-Chacon J, Kineman RD, et al. . Growth hormone control of hepatic lipid metabolism. Diabetes. 2016;65(12):3598-3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Cordoba-Chacon J, Majumdar N, List EO, et al. . Growth hormone inhibits hepatic de novo lipogenesis in adult mice. Diabetes. 2015;64(9):3093-3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wolf Greenstein A, Majumdar N, Yang P, Subbaiah PV, Kineman RD, Cordoba-Chacon J. Hepatocyte-specific, PPARgamma-regulated mechanisms to promote steatosis in adult mice. J Endocrinol. 2017;231(1):107-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Sarmento-Cabral A, Del Rio-Moreno M, Vazquez-Borrego MC, et al. . GH directly inhibits steatosis and liver injury in a sex-dependent and IGF1-independent manner. J Endocrinol. 2021;248(1):31-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Cordoba-Chacon J, Sarmento-Cabral A, Del Rio-Moreno M, Diaz-Ruiz A, Subbaiah PV, Kineman RD. Adult-onset hepatocyte GH resistance promotes NASH in male mice, without severe systemic metabolic dysfunction. Endocrinology. 2018;159(11):3761-3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Della Torre S. Beyond the X factor: relevance of sex hormones in NAFLD pathophysiology. Cells. 2021;10(9):2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Peter A, Stefan N, Cegan A, et al. . Hepatic glucokinase expression is associated with lipogenesis and fatty liver in humans. J Clin Endocrinol Metab. 2011;96(7):E1126-E1130. [DOI] [PubMed] [Google Scholar]
- 130. Fellinger P, Wolf P, Pfleger L, et al. . Increased ATP synthesis might counteract hepatic lipid accumulation in acromegaly. JCI Insight. 2020;5(5):e134638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Oshida K, Waxman DJ, Corton JC. Chemical and hormonal effects on STAT5b-dependent sexual dimorphism of the liver transcriptome. PLoS One. 2016;11(3):e0150284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Luque RM, Kineman RD. Impact of obesity on the growth hormone axis: evidence for a direct inhibitory effect of hyperinsulinemia on pituitary function. Endocrinology. 2006;147(6):2754-2763. [DOI] [PubMed] [Google Scholar]
- 133. Baik M, Yu JH, Hennighausen L. Growth hormone-STAT5 regulation of growth, hepatocellular carcinoma, and liver metabolism. Ann N Y Acad Sci. 2011;1229:29-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Hennighausen L, Robinson GW. Interpretation of cytokine signaling through the transcription factors STAT5A and STAT5B. Genes Dev. 2008;22(6):711-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Hosui A, Kimura A, Yamaji D, Zhu BM, Na R, Hennighausen L. Loss of STAT5 causes liver fibrosis and cancer development through increased TGF-{beta} and STAT3 activation. J Exp Med. 2009;206(4):819-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Kaltenecker D, Themanns M, Mueller KM, et al. . Hepatic growth hormone—JAK2—STAT5 signalling: Metabolic function, non-alcoholic fatty liver disease and hepatocellular carcinoma progression. Cytokine. 2019;124:154569. [DOI] [PubMed] [Google Scholar]
- 137. Hao P, Waxman DJ. STAT5 regulation of sex-dependent hepatic CpG methylation at distal regulatory elements mapping to sex-biased genes. Mol Cell Biol. 2021;41(2):e00166-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Lau-Corona D, Bae WK, Hennighausen L, Waxman DJ. Sex-biased genetic programs in liver metabolism and liver fibrosis are controlled by EZH1 and EZH2. PLoS Genet. 2020;16(5):e1008796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Ishikawa M, Brooks AJ, Fernandez-Rojo MA, et al. . Growth hormone stops excessive inflammation after partial hepatectomy, allowing liver regeneration and survival through induction of H2-Bl/HLA-G. Hepatology. 2021;73(2):759-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Zhu C, Tabas I, Schwabe RF, Pajvani UB. Maladaptive regeneration—the reawakening of developmental pathways in NASH and fibrosis. Nat Rev Gastroenterol Hepatol. 2021;18(2):131-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Pivonello C, De Martino MC, Negri M, et al. . The GH-IGF-SST system in hepatocellular carcinoma: biological and molecular pathogenetic mechanisms and therapeutic targets. Infect Agent Cancer. 2014;9:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is a review article of previous published work, and therefore no original data was generated.