Abstract
Introduction:
The effects on offspring craniofacial bone morphology and accretion because of altered maternal exposure to dietary components such as calcium (Ca) and phosphorus (P) are unclear. The objective of this study was to investigate the changes in offspring skull morphology and tissue mineral density (TMD), including sex-specific changes, with exposure to a maternal diet high in Ca-to-P levels during gestation and lactation in mice.
Methods:
Time-mated FVB wild-type mice were fed a normal or experimental diet during gestation until weaning. The experimental diet contained a 3-fold increase in Ca and a 3-fold decrease in P (Ca:P molar ratio, 10.5) compared with normal mouse chow (Ca:P molar ratio, 1.5). The heads of 6-week-old control and experimental offspring mice were collected and scanned using microcomputed tomography. Three-dimensional geometric morphometric analysis was performed to analyze changes in craniofacial morphology. TMD measurements were also analyzed.
Results:
We observed subtle changes and no significant differences between offspring control and experimental skulls when we compared all samples. However, when we separated skulls by sex, we discovered significant differences in craniofacial morphology and TMD. Experimental female offspring possessed skulls that were smaller, narrower transversely, taller vertically, and decreased in TMD. Experimental male offspring possessed skulls that were larger, wider transversely, shorter vertically, and increased in TMD.
Conclusions:
Maternal exposure to diet and increased Ca:P molar ratio during gestation and lactation led to significant, sex-specific morphologic and TMD changes in 6-week-old mouse skulls.
Calcium (Ca) and phosphorus (P) are the 2 most abundant minerals in our body and essential for bone growth and health, largely in the form of hydroxyapatite in mineralized tissues. Dietary Ca and P are important for proper bone formation, and to date, a 1:1 molar ratio of Ca:P is believed to be the optimal ratio for proper bone development.1 Alterations of maternal diet during pregnancy, particularly vitamin D levels, stimulate Ca and P absorption, Ca mobilization from bone, and Ca reabsorption,2 which led to craniofacial malformations in offspring of rodents.3,4 However, the effects of increasing Ca:P ratio exposure during pregnancy on offspring craniofacial form (size and shape) and mineralization, including potential sex-specific changes, are unclear. We believe that generating a tractable, dietary mouse model is required to advance the cellular and molecular mechanism linking Ca:P ratios to skull form.
Under adequate Ca and P intake, the skeleton of a late-term fetus accumulates Ca at a rate of ~150 mg/kg/d, which drops to 30–40 mg/kg/d in the normal neonate.5 Skeletal accumulation of P during development is less clear; however, serum mineral concentrations in fetal circulation are significantly higher than maternal and adult values—serum calcium is usually increased 0.30–0.50 mmol/L and serum P increased by 0.5 mmol/L.5 The high levels of fetal serum Ca and P are unclear but may protect against hypocalcemia and hypophosphatemia as the neonate becomes dependent on dietary Ca and P from the lactating mother to continue to meet the daily demands of the developing musculoskeletal system.5 In general, increased Ca intake leads to increased mineral accretion and stronger bones,1 whereas increased P intake negatively affects bone health by affecting Ca availability and bone metabolism,6,7 leading to hyperphosphatemia and hypocalcemia.8 In many countries, P intake is 2–3 fold higher than the dietary reference intake for P (700 mg/d), whereas Ca intake is often below recommended levels.7 Zhu et al9 showed that a combination of high Ca and low P induced the greatest growth suppression and bone mineralization loss in Pekin ducklings. Therefore, in the hopes of inducing the greatest changes, we generated an experimental mouse diet that maximized the Ca:P ratio (experimental diet Ca:P molar ratio, 10.5; control diet Ca:P molar ratio, 1.5) and minimized any other changes to the remaining dietary components.
Long-term Ca supplementation in rural Gambian females during pregnancy resulted in sex-specific skeletal effects (skulls were not analyzed) in preadolescent female offspring (aged 8–12 years) such as shorter stature, smaller bone size, and decreased mineral accrual.10 The opposite was observed in preadolescent male offspring with taller stature, large bone size, and increased mineral accrual.10 No significant changes in offspring birth weight, length, growth, and bone mineral accretion were observed in the first year of life.11,12
To our knowledge, no study has explored the effects of maternal exposure to an increase in dietary Ca:P ratio during gestation and lactation on offspring craniofacial morphology. Our prior study showed significant morphologic changes in the offspring mandible with more robust effects observed when sexes were separated.13 Thus, we hypothesize that a higher dietary Ca:P ratio during gestation and lactation would also affect the morphology of the cranial and midface portion of the skull (skull minus the mandible) in a sex-dependent manner. To investigate this hypothesis, we exposed pregnant and lactating mice to an experimental diet containing a 3-fold increase in Ca and a 3-fold decrease in P levels (Ca:P molar ratio, 10.5) compared with the control diet (Ca:P molar ratio, 1.5). We collected offspring mice at 6 weeks of age to analyze craniofacial form (size and shape) using linear measurements, 3-dimensional (3D) geometric morphometrics, and tissue mineral density (TMD). Here, we report significant sex-specific changes—experimental female offspring exhibited skulls that were smaller, narrower, and taller with decreased TMD relative to controls. Conversely, experimental male offspring possessed skulls that were larger, wider, and shorter with increased TMD. Our findings suggest increasing dietary Ca and decreasing dietary P levels are associated with sex-specific form and accretion changes in the skull.
MATERIAL AND METHODS
Pregnant FVB/NJ mice (Jackson laboratory) from embryonic day (E) 0.5 and newborn mice until weaning (3 weeks of age) were exposed to control diet (Supplementary Table I; AIN93M Maintenance Purified Diet; LabDiet, St Louis, MO; n = 10 [6 females, 4 males]) or experimental diet (Supplementary Table II; Modified AIN93M; LabDiet; n = 10 [6 females, 4 males] as previously described (Fig 1, A).13 The presence of a vaginal plug 12–16 hours on mating was deemed E 0.5. The control and experimental diets were identical except that the control diet contained 0.5% Ca, 0.33% P, and Ca:P molar ratio of 1.5, whereas the experimental diet contained 1.5% Ca, 0.11% P, and Ca:P molar ratio of 10.5 (3× greater than Ca; 3× lower than P). At 3 weeks of age, control and experimental offspring were all exposed to the control diet until analysis. All mice were fed the control diet at weaning (3–6 weeks old). Food and water were available ad libitum. Mice were housed under a 12-hour light and dark cycle, at a constant temperature of 22° ± 1°C, and humidity of 50 ± 5%.
Fig 1.
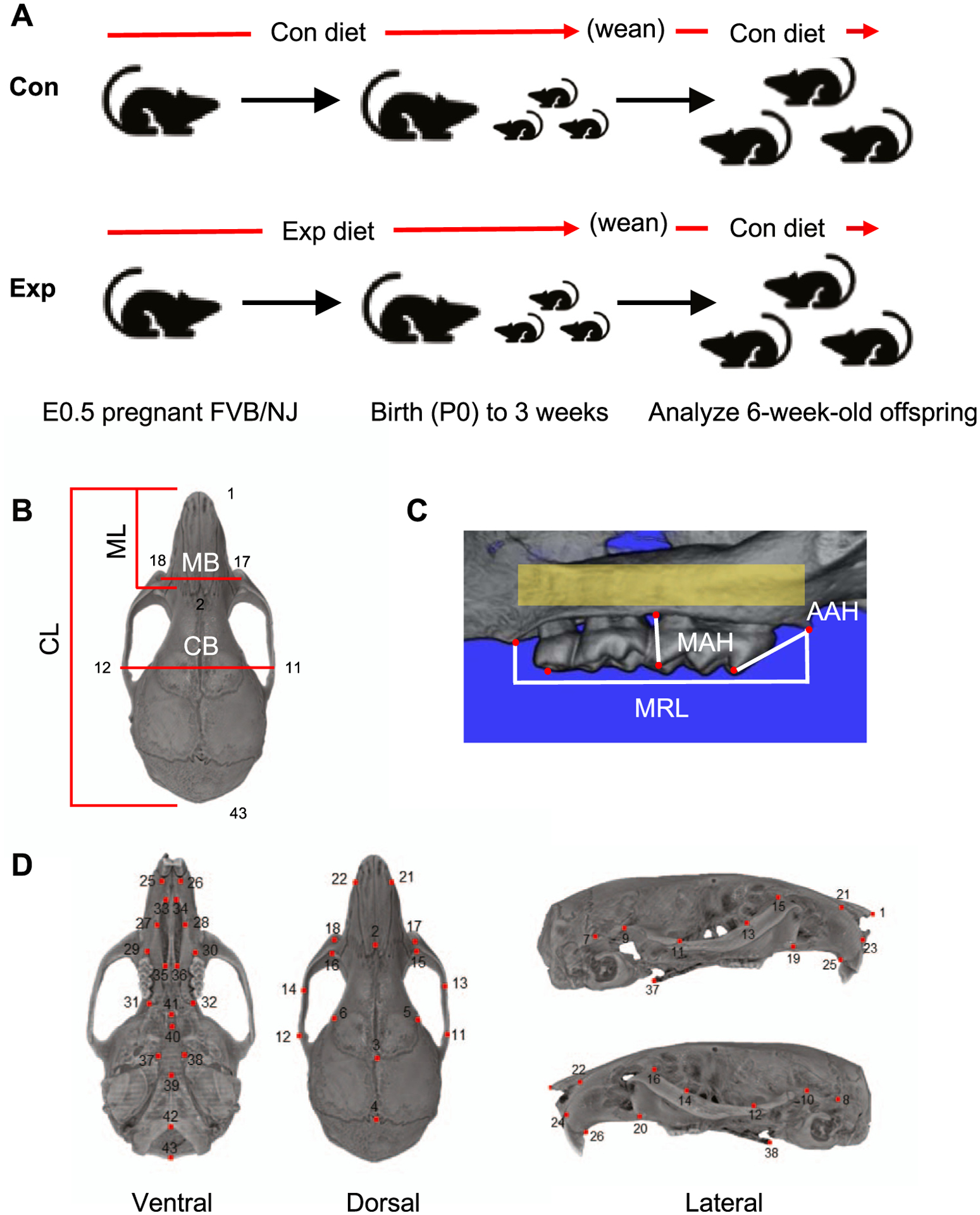
Experimental design and landmarks: A, Pregnant FVB/NJ mice at E 0.5 were exposed to a control (Con) or experimental (Exp) diet containing 3-fold increased Ca and 3-fold decreased P (Ca:P molar ratio, 10.5 vs 1.5 in control diet) through gestation and lactation (3-week-old). All mice were fed the control diet from 3 to 6 weeks. All mice were analyzed at 6 weeks of age; B, Linear measurements of cranial length (CL), cranial breadth (CB), midface length (ML), and midface breadth (MB) were collected at the landmark points indicated (D) (Supplementary Table I); C, Linear measurements of MRL, AAH, and MAH were collected; D, Skull landmarks are indicated (Supplementary Table I).
Each animal was considered an experimental unit, and animal numbers were derived from power analysis that 11 animals per group would be sufficient to detect a 0.1 mm difference in shape at 90% power. Six-week-old offspring mice were weighed (Supplementary Fig 1), killed using carbon dioxide inhalation and cervical dislocation, and mouse heads were fixed in 4% paraformaldehyde for 48 hours. All aspects of animal care and experiments were approved by the Institutional Animal Care and Use Committee at the University of California San Francisco and performed under animal research protocol no. AN164201. The National Institutes of Health guide for the care and use of laboratory animals (National Institutes of Health Publications No. 8023, revised 1978) was followed.
Fixed mouse heads were subjected to microcomputed tomography (microCT) using a SkyScan 1076 microCT at the Small Animal Tomographic Analysis Facility located at Seattle Children’s Research Institute. Specimens were scanned at 17.2-μm resolution (55 kV, 150 mA, 0.5-mm Al filter). Reconstructions were generated using NRecon (version 1.6.9.4) with consistent thresholding parameters and then converted to 3D volumes. Skull segmentations from microCT data were performed with Avizo Lite (version 9.1.1).14–16
Traditional morphometrics or standard 2-dimensional (2D) measurements were employed in the initial analyses of mouse skull variation. The following measurements were acquired: cranial length, midfacial length, cranial breadth, and midface breadth (Fig 1, B). All measurements were obtained using Landmark software.17 Three alveolar-dental measurements (molar row length [MRL]; anterior alveolar height [AAH]; middle alveolar height [MAH]) were collected using Avizo Lite (version 9.1.1) (Fig 1, C). Bilateral measurements of MRL, AAH, and MAH were averaged for each mouse.14–16
TMD analysis was performed with Avizo Lite (version 9.1.1) using gray-scale intensity to measure relative bone density. First, all samples were oriented in the same position and cropped to exclude excess blank spaces. The same area of interest along the alveolar bone surrounding the molars was captured using the brush tool set to 120 and creating 2 circles at the most anterior point of the first molar and most posterior point of the third molar. The interpolate function was applied to capture all 3 molars, including the roots and surrounding tissues, in 3D. Finally, we used the wand tool to capture any of the maxilla that was within the volume and was added to the created material. After selecting the region of interest (yellow), we used the Material Statistics tab to extract raw data (Fig 1, C). Measurements were performed on both sides and averaged for each mouse.18
Three-dimensional coordinate locations of 43 skull landmarks of control (n = 10; 4 males, 6 females) and experimental (n = 10; 4 males, 6 females) from 6-week old offspring were marked using Landmark software (Fig 1, D; Supplementary Table III).17 The observer (M.G.H) was blinded to each mouse during data collection. To determine the accuracy and reproducibility of landmark identification, an initial random subset of 7 samples was landmarked twice using the 43 skulls (cranium and midface) landmarks. We calculated the difference in the matching landmark coordinates from the 2 measurements (ie, intraobserver error) and removed those that consistently exceeded an arbitrary difference of 7 voxels (0.125 mm) between measurements.19 Furthermore, we used centroid sizes, the square root of the sum of squared Euclidean distances from each landmark to their centroid, as a proxy for cranial and midface size.20 To determine interobserver landmark reproducibility, 2 observers (M.G.H and C.C) independently located the landmarks on 10 randomly selected samples (Supplementary Fig 2). A 10,000 round permutation test was performed on the Procrustes distance between the observers’ landmarked samples, testing for mean overall shape differences between them.
Variations in skull shape were assessed using principal components analysis (PCA). Two types of PCA were carried out separately for the analysis of skull morphology, (1) a PCA based on variation in form (size and shape together) followed by, and (2) PCA using the residuals of multivariate regression of Procrustes coordinates on centroid size to investigate the shape variation independent of size (shape only).21,22 PCA of Procrustes coordinates is based on the eigenvalue decomposition of a covariance matrix, which transforms the Procrustes coordinates into scores along with principal components (PCs). In most patients, the first few PCs described most of the variance in the dataset.23 Each observation was scored for each principal axis, and the score of observation along the principal axes map in the morphospace was defined by the principal component axes using MorphoJ software.24
Statistical analysis
For centroid sizes, 2D measurements, and TMD measurements, the normal distribution of data was tested using the Kolmogorov-Smirnov nonparametric test. Independent, pairwise t tests were employed to verify the existence of any significant differences between control and experiment groups, in addition to male and female skull measurements.
RESULTS
Skull (cranial and midface) centroid sizes and 2D measurements showed little or no differences between control and experimental offspring (Fig 2, top; Supplementary Table IV). The only exception was in midface breadth—experimental offspring showed a significant decrease, but notably, the data points were in 2 clusters (Fig 2; Supplementary Table IV). When offspring were separated by sex, significant sex-specific changes in all measurements were observed (Fig 2; Supplementary Table IV). Cranial and midface centroid sizes of experimental male and female offspring were significantly larger and smaller than controls, respectively. Cranial length, midface length, cranial breadth, and midface breadth were significantly smaller in experimental female offspring. Experimental male offspring showed significantly larger measurements except for midface breadth. These differences indicate changes in size and, potentially, shape. Measurements of dentition and alveolar bone (MRL, AAH, MAH) were significantly smaller in experimental females, whereas experimental males were significantly larger (Fig 1, C; Supplementary Table V).
Fig 2.
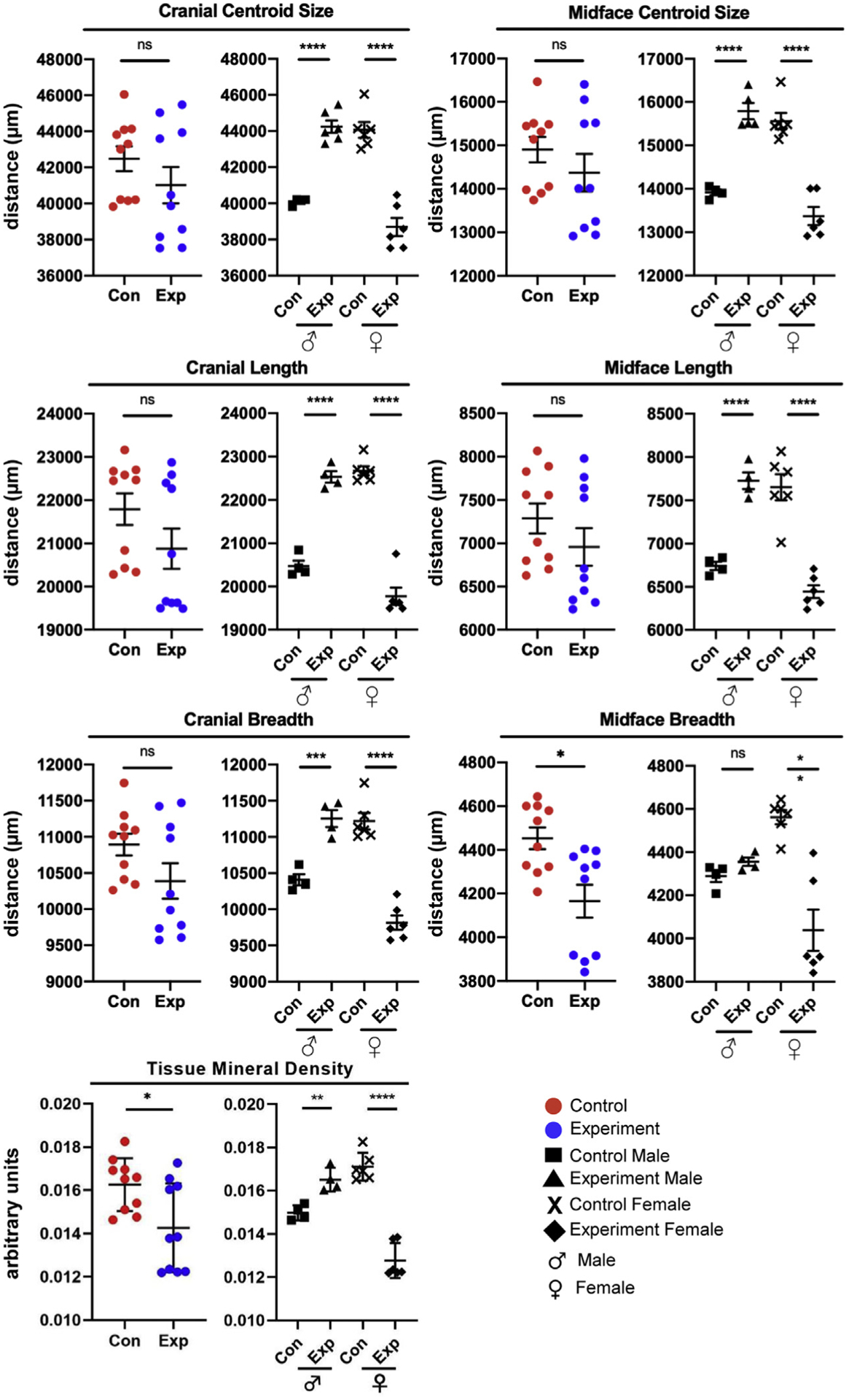
Measurements of the 2D skull and TMD. Data are presented as mean ± standard deviation (n = 20). Differences between groups were analyzed by an unpaired t test. A 2-tailed P<0.05 was considered statistically significant. Con, Control; Exp, experimental.
Experimental offspring exhibited significantly lower TMD than controls (Fig 2, bottom; Supplementary Table IV). Analysis based on sex revealed an increase in TMD in experimental male offspring and a decrease in TMD in experimental female offspring.
Using 3D geometric morphometric analysis, we analyzed both the relative amounts of variation because of skull form (ie, size and shape) and skull shape alone (ie, data normalized for size) (Figs 3 and 4). When skull form was considered, ~68% of the morphologic variation was attributed to PC1 (~54%) and PC2 (~14%) (Fig 3, A). Control and experimental offspring showed clusters showing potential small differences in PC2; however, many of the data points were scattered (Fig 3, B). When offspring were separated by sex, significant and large differences in form were observed (Fig 3, C). Experimental female offspring showed positive, increased PC1 values compared with control, whereas experimental male offspring showed negative, decreased PC1 values, again demonstrating opposing changes in sex-specific size and potentially shape. Higher PC1 values were correlated with decreased cranial lengths (sagittal dimension), increased transverse widths, and taller crania (from cranial base to top of the skull) (Figs 3, B and C; blue). Lower PC1 values were associated with opposite changes, namely increased cranial lengths (sagittal dimension), decreased transverse widths, and shorter crania (from cranial base to top of the skull) (Figs 3, B and C; red).
Fig 3.
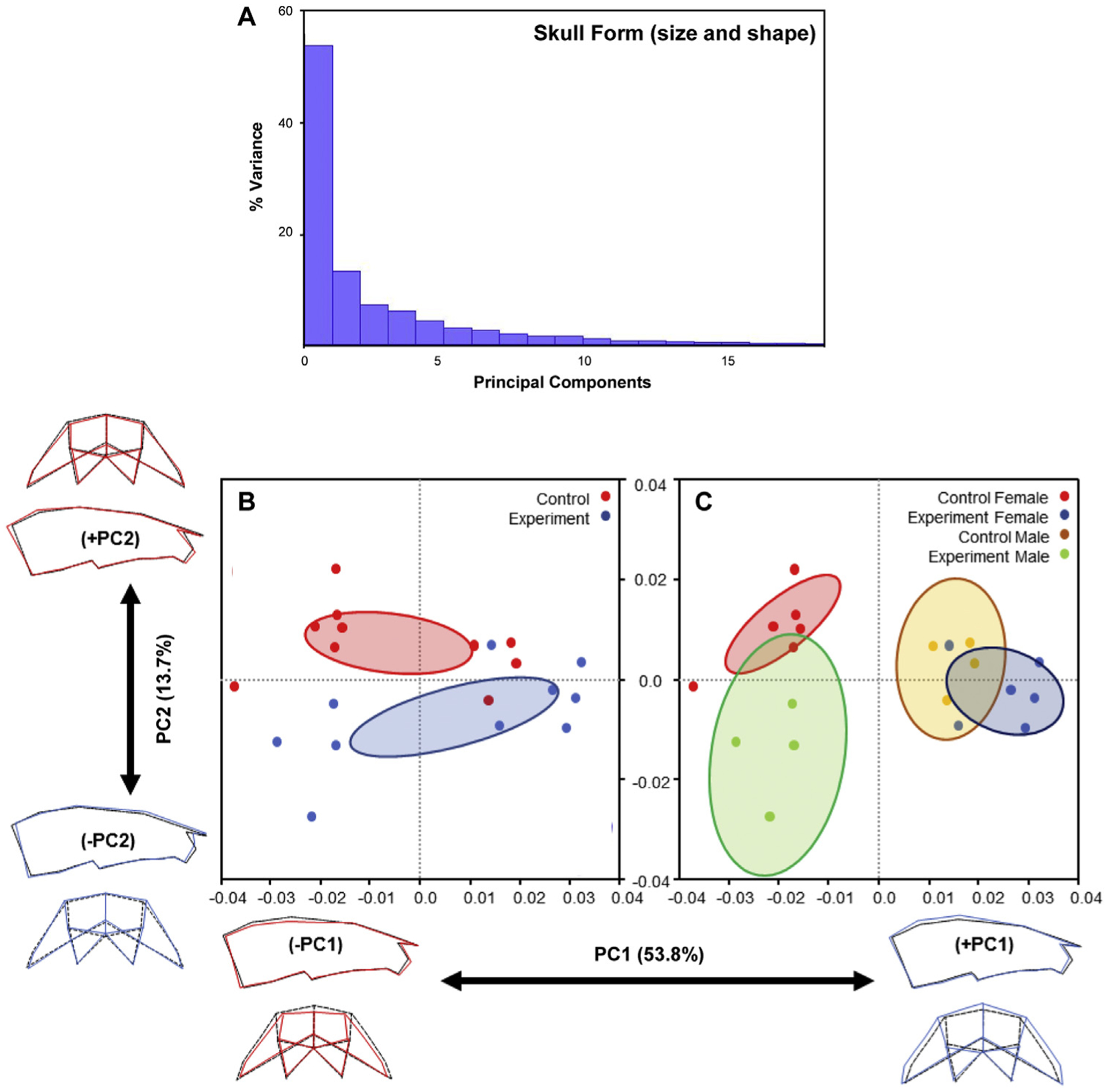
Variations in skull form (size and shape): A, The plot of principal component scores of skull landmarks for all mice (n = 20); B and C, Scatter plots of individual scores based on PCA of skull form. Distribution of control and experimental mice along PC1 and PC2 (B). Sex-specific distribution of control and experimental mice along PC1 and PC2 (C). The ellipses represent the confidence range of the means of each group. Skull outlines show extreme shapes for each axis—lowest to highest PC1 values (left to right) and lowest to highest PC2 values (bottom to top).
Fig 4.
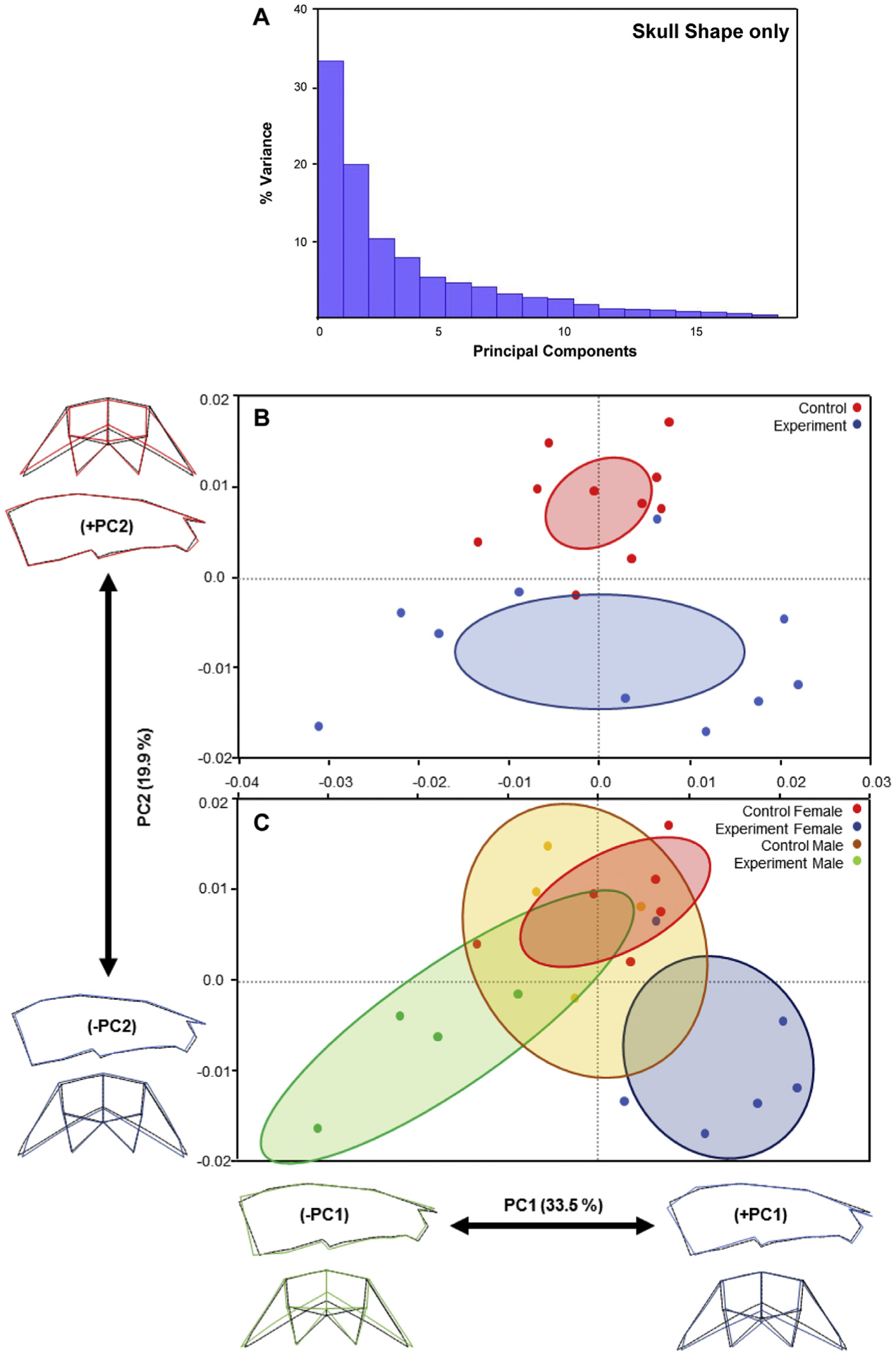
Variations in skull shape only: A, The plot of principal component scores of skull landmarks for all mice (n = 20); B and C, Scatter plots of individual scores based on PCA of skull shape only. Distribution of control and experimental mice along PC1 and PC2 (B). Sex-specific distribution of control and experimental mice along PC1 and PC2 (C). The ellipses represent the confidence range of the means of each group. Skull outlines show extreme shapes for each axis—lowest to highest PC1 values (left to right) and lowest to highest PC2 values (bottom to top).
We next eliminated the influence of size changes and analyzed skulls because of their shape alone. This led to ~53% of morphologic variation attributed to PC1 (34%) and PC2 (20%) (Fig 4, A). Similar to comparisons of skull form, control and experimental offspring shape showed clustering, but many of the data points were scattered (Figs 3 and 4, B). When specimens were separated by sex, significant and large differences in shape were revealed (Fig 4, C). Control and experimental female offspring showed significant shape differences, largely in PC2. Lower PC2 values, as observed in experimental female offspring, were correlated with decreased transverse widths and taller skulls (Fig 4, C; blue). Control and experimental male offspring showed fewer shape differences, as demonstrated by their overlapping clusters (Fig 4, C).
Interestingly, control male and female clusters overlapped, indicating similar skull shapes between wild-type male and female offspring. Experimental male and female offspring showed opposite PC1 values—experimental male offspring possessed increased transverse widths and shorter crania (green), whereas experimental females possessed decreased transverse widths and taller crania (Fig 4, C; blue). Thus, sex-specific differences between control and experimental offspring in form and shape only indicate significant variations in craniofacial size, as well as shape (Figs 3 and 4).
DISCUSSION
Our study revealed that exposing mice to an increased Ca:P ratio-containing diet during gestation and lactation was associated with sex-specific changes in offspring craniofacial size, shape, and TMD. Six-week-old experimental female offspring (whose mothers were exposed to an experimental diet containing a Ca:P molar ratio of 10.5) were smaller, narrower transversely, and taller vertically with decreased TMD than female control offspring (Figs 2–4). Conversely, 6-week-old experimental male offspring possessed skulls that were larger, wider transversely, and shorter vertically with increased TMD relative to control male offspring (Figs 2–4). TMDs were measured around the alveolar bone and were consistent with prior measurements in the mandibular rami (Fig 1, C).13 Consistent with skull changes, molar tooth rows, and alveolar bone heights were increased and decreased in male and female offspring, respectively (Supplementary Table V). To our knowledge, our study is the first to report opposing, sex-specific responses to changes in maternal diet during gestation and lactation on offspring craniofacial form and mineralization.
Our study supports the prior longitudinal study of sex-specific, skeletal changes in preadolescent Gambian children.10 Maternal Ca supplementation in females from 20 weeks to birth resulted in sex-specific skeletal effects in 8 to 12-year-old offspring such as shorter stature, smaller bone size, and decreased mineral accrual in females with the opposite effects in males. These sex-specific differences may be due to decreased and accelerated growth trajectories in females and males, respectively.10 The whole body, less the head, was scanned using dual-energy x-ray absorptiometry and peripheral quantitative computed tomography.10 Sex-specific differences with higher calcium carbonate supplementation in Gambian women were strikingly similar to our craniofacial findings in mice (ie, opposite effects in female and male offspring; female skulls were smaller, narrower transversely, and taller vertically with decreased TMD, whereas male skulls were bigger, wider transversely, and shorter vertically with increased TMD). We must be cautious when comparing human vs mouse studies because of species-specific differences in genetics, gestational period, and litter size. Notably, unlike in the Gambian study, our mice were exposed to the experimental diet for the entire gestation period and the 3-week lactation period (newborn to weaning).
Diet studies are notoriously difficult. It is virtually impossible to control how much each mouse will ingest. Considerations were made at the initiation of our study to measure the amount of food ingested by weighing the remaining food at the end of each day. However, we realized that we could not control for individual variations in appetite, metabolism, energy expenditure, and the presence of uningested food at the bottom of each cage. These factors convinced us to forgo measurements of the ingested food. We did weigh the mice before collection and determined that weights were very similar within sexes, suggesting no gross discrepancies in food consumption (Supplementary Material). Beyond the potential to uncover sex-specific cellular and molecular mechanisms of action, our experimental model system may be modified to determine the dynamics and outcome of sex-specific changes (ie, analyze at various time points), distinguish the importance of gestational vs lactational periods (ie, vary the timing of exposure to diet), and allow testing of different levels of Ca and P (ie, Ca:P ratios) on skull form and mineral accretion. For these future experiments, we will revisit and improve on our experimental controls.25,26
Sex-specific differences in offspring have previously been reported for developmental origins of health and disease, such as maternal obesity, famine, and intake of micronutrient supplements.27–29 Endocrine hormones that regulate Ca and P metabolism include calcitonin, fibroblast growth factor 23, parathyroid hormone, and vitamin D. Ca and P are transferred from maternal circulation to the fetus, and vitamin D is required for optimal absorption. During pregnancy, maternal parathyroid hormone, vitamin D, and Ca absorption and mobilization from bones increase to fulfill maternal minerals needs. However, fetal circulation is not affected by these changes because these hormones are found not to cross the placental barrier except for vitamin D.5 Regulation of P levels likely requires PTH, calcitriol, and peptides known as the “phosphatonins,” of which FGF23 has been shown to reduce the activity of both sodium-phosphate cotransporter 2A and sodium-dependent phosphate cotransporter 2C to increase urinary phosphate excretion.30,31
Sexual dimorphism may be attributed to circulating levels of insulin growth factor 1 (IGF1) and related factors such as insulin growth factor binding protein 1, which are inversely related to growth hormone (GH) levels.32 Thus, smaller females possessed higher levels of IGF1 and, in turn, lower levels of GH. Higher and lower GH levels generally correlate well with males and females, respectively.32–34 IGF1 was found to be increased in male offspring (14%) compared with females (−18%) during middle childhood from Gambian mothers given Ca supplementation.35 However, no differences were detected at birth or early childhood growth but only became evident at late childhood and adolescence.10,11 Sex-specific differences are not clearly understood but likely involve the aforementioned pathways.
The empirical results reported in this study should be considered in light of some limitations. The first limitation is the relatively small sample size, although the numbers were evenly balanced across the 4 subgroups. Although our experimental design was relatively new in orthodontic research, we did not expect that recruitment might not meet targets. Second, although the findings of this study offer new, potentially useful information for the possible etiology of some dentofacial discrepancies, future longitudinal studies should be conducted to illustrate the cellular and epigenetic pathways responsible for the detected sex-specific and long-term effects of the maternal Ca and P intake on offspring craniofacial growth.
CONCLUSIONS
Our study suggests that altering dietary Ca and P levels during gestation and lactation leads to sex-specific differences in offspring craniofacial size, shape, and TMD. Exposure of mice during gestation and lactation to our modified experimental diet containing a Ca:P molar ratio of 10.5 was associated with smaller, narrower transversely, and taller vertically with decreased TMD in female offspring skulls relative to control female offspring. The opposite effects were observed in male offspring skulls, which were larger, wider transversely, and shorter vertically with increased TMD than male control offspring. Together with our prior sex-specific changes in the mandible,13 we believe that our modified mouse diet may be used by us and others as a tool in future studies to uncover sex-specific pathways involved in the regulation of internal Ca and P levels and their ultimate effects on skull form and mineral accretion.
Supplementary Material
Acknowledgments
Mohamed G. Hassan was supported by the Fulbright Program and AMIDEAST and the Rita Levi-Montalcini Postdoctoral Fellowship award from the Washington University in St Louis Center of Regenerative Medicine. Andrew H. Jheon was supported in part by the University of California San Francisco Clinical and Translational Science Institute Pilot Fund program (RAS no. A127552).
Footnotes
AUTHOR CREDIT STATEMENT
Mohamed G. Hassan contributed to conceptualization, data curation, formal analysis, investigation, methodology, supervision, validation, and manuscript review and editing; Christopher Chen contributed to formal analysis, methodology, manuscript review and editing; Hanan A. Ismail contributed to supervision and manuscript review and editing; Abbas R. Zaher contributed to supervision and manuscript review and editing; Timothy C. Cox contributed to resources and manuscript review and editing; Alice F. Goodwin contributed to supervision and manuscript review and editing; Andrew H. Jheon contributed to conceptualization, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, original draft preparation, and manuscript review and editing.
SUPPLEMENTARY DATA
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.ajodo.2021.12.015.
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest, and none were reported.
REFERENCES
- 1.Ciosek Ż, Kot K, Kosik-Bogacka D, Łanocha-Arendarczyk N, Rotter I. The effects of calcium, magnesium, phosphorus, fluoride, and lead on bone tissue. Biomolecules 2021;11:506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeLuca HF. The metabolism and functions of vitamin D. Adv Exp Med Biol 1986;196:361–75. [DOI] [PubMed] [Google Scholar]
- 3.Engström C, Linde A, Thilander B. Craniofacial morphology and growth in the rat. Cephalometric analysis of the effects of a low calcium and vitamin D-deficient diet. J Anat 1982;134:299–314. [PMC free article] [PubMed] [Google Scholar]
- 4.Kwon HJ. Vitamin D receptor signaling regulates craniofacial cartilage development in zebrafish. J Dev Biol 2019;7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kovacs CS. Calcium, phosphorus, and bone metabolism in the fetus and newborn. Early Hum Dev 2015;91:623–8. [DOI] [PubMed] [Google Scholar]
- 6.Kemi VE, Kärkkäinen MUM, Lamberg-Allardt CJE. High phosphorus intakes acutely and negatively affect Ca and bone metabolism in a dose-dependent manner in healthy young females. Br J Nutr 2006;96:545–52. [PubMed] [Google Scholar]
- 7.Kemi VE, Rita HJ, Kärkkäinen MUM, Viljakainen HT, Laaksonen MM, Outila TA, et al. Habitual high phosphorus intakes and foods with phosphate additives negatively affect serum parathyroid hormone concentration: a cross-sectional study on healthy premenopausal women. Public Health Nutr 2009;12:1885–92. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe A, Koizumi T, Horikawa T, Sano Y, Uki H, Miyajima K, et al. Impact of altered dietary calcium-phosphorus ratio caused by high-phosphorus diets in a rat chronic kidney disease (CKD) model created by partial ligation of the renal arteries. J Toxicol Pathol 2020;33:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu YW, Wen J, Jiang XX, Wang WC, Yang L. High calcium to phosphorus ratio impairs growth and bone mineralization in Pekin ducklings. Poult Sci 2018;97:1163–9. [DOI] [PubMed] [Google Scholar]
- 10.Ward KA, Jarjou L, Prentice A. Long-term effects of maternal calcium supplementation on childhood growth differ between males and females in a population accustomed to a low calcium intake. Bone 2017;103:31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldberg GR, Jarjou LMA, Cole TJ, Prentice A. Randomized, placebo-controlled, calcium supplementation trial in pregnant Gambian women accustomed to a low calcium intake: effects on maternal blood pressure and infant growth. Am J Clin Nutr 2013;98:972–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarjou LMA, Prentice A, Sawo Y, Laskey MA, Bennett J, Goldberg GR, et al. Randomized, placebo-controlled, calcium supplementation study in pregnant Gambian women: effects on breast-milk calcium concentrations and infant birth weight, growth, and bone mineral accretion in the first year of life. Am J Clin Nutr 2006;83:657–66. [DOI] [PubMed] [Google Scholar]
- 13.Hassan MG, Vargas R, Zaher AR, Ismail HA, Lee C, Cox TC, et al. Altering calcium and phosphorus levels in utero affects adult mouse mandibular morphology. Orthod Craniofac Res 2019; 22(Suppl 1):113–9. [DOI] [PubMed] [Google Scholar]
- 14.An JY, Kerns KA, Ouellette A, Robinson L, Morris HD, Kaczorowski C, et al. Rapamycin rejuvenates oral health in aging mice. Elife 2020;9:e54318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen L, Ai H, Liang Y, Ren X, Anthony CB, Goodlett CR, et al. Effect of prenatal alcohol exposure on bony craniofacial development: a mouse MicroCT study. Alcohol 2013;47:405–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hassan MG, Kaler H, Zhang B, Cox TC, Young N, Jheon AH. Effects of multi-generational soft diet consumption on mouse craniofacial morphology. Front Physiol 2020;11:783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiley DF, Amenta N, Alcantara DA, Ghosh D, Kil YJ, Delson E, et al. Evolutionary morphing. Minneapolis: IEEE; 2005. p. 431–8. [Google Scholar]
- 18.Tu SJ, Wang SP, Cheng FC, Chen YJ. Extraction of gray-scale intensity distributions from micro computed tomography imaging for femoral cortical bone differentiation between low-magnesium and normal diets in a laboratory mouse model. Sci Rep 2019;9:8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maga AM, Navarro N, Cunningham ML, Cox TC. Quantitative trait loci affecting the 3D skull shape and size in mouse and prioritization of candidate genes in-silico. Front Physiol 2015;6:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dryden IL, Mardia KV. Statistical shape analysis. 1st ed. Chichester, United Kingdom: Wiley; 1998. p. 376. [Google Scholar]
- 21.Falsetti AB, Jungers WL, Colle TM. Morphometrics of the callitrichid forelimb: a case study in size and shape. Int J Primatol 1993;14:551–72. [Google Scholar]
- 22.Darroch JN, Mosimann JE. Canonical and principal components of shape. Biometrika 1985;72:241–52. [Google Scholar]
- 23.Spassov A, Toro-Ibacache V, Krautwald M, Brinkmeier H, Kupczik K. Congenital muscle dystrophy and diet consistency affect mouse skull shape differently. J Anat 2017;231: 736–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klingenberg CP. MorphoJ: an integrated software package for geometric morphometrics. Mol Ecol Resour 2011;11:353–7. [DOI] [PubMed] [Google Scholar]
- 25.Martin B, Ji S, Maudsley S, Mattson MP. “Control” laboratory rodents are metabolically morbid: why it matters. Proc Natl Acad Sci U S A 2010;107:6127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Research Council. Nutrient Requirements of Laboratory Animals. 4th ed. Washington, DC: National Academies Press; 1995. [PubMed] [Google Scholar]
- 27.Tarrade A, Panchenko P, Junien C, Gabory A. Placental contribution to nutritional programming of health and diseases: epigenetics and sexual dimorphism. J Exp Biol 2015; 218:50–8. [DOI] [PubMed] [Google Scholar]
- 28.Tobi EW, Lumey LH, Talens RP, Kremer D, Putter H, Stein AD, et al. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum Mol Genet 2009;18: 4046–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberfroid D, Huybregts L, Lanou H, Henry MC, Meda N, Kolsteren FP, et al. Effect of maternal multiple micronutrient supplements on cord blood hormones: a randomized controlled trial. Am J Clin Nutr 2010;91:1649–58. [DOI] [PubMed] [Google Scholar]
- 30.Chang AR, Anderson C. Dietary phosphorus intake and the kidney. Annu Rev Nutr 2017;37:321–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goretti Penido M, Alon US. Phosphate homeostasis and its role in bone health. Pediatr Nephrol 2012;27:2039–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geary MPP, Pringle PJ, Rodeck CH, Kingdom JCP, Hindmarsh PC. Sexual dimorphism in the growth hormone and insulin-like growth factor axis at birth. J Clin Endocrinol Metab 2003;88: 3708–14. [DOI] [PubMed] [Google Scholar]
- 33.Holt RIG. Fetal programming of the growth hormone-insulin-like growth factor axis. Trends Endocrinol Metab 2002;13:392–7. [DOI] [PubMed] [Google Scholar]
- 34.Holt RIG, Simpson HL, Sönksen PH. The role of the growth hormone-insulin-like growth factor axis in glucose homeostasis. Diabet Med 2003;20:3–15. [DOI] [PubMed] [Google Scholar]
- 35.Prentice A, Ward KA, Nigdikar S, Hawkesworth S, Moore SE. Pregnancy supplementation of Gambian mothers with calcium carbonate alters mid-childhood IGF1 in a sex-specific manner. Bone 2019;120:314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


