Abstract
Many Canadians use cannabis for medicinal and recreational purposes. We describe the current understandings of how cannabis is metabolized in the liver and its potential interactions with other common drugs. We also summarize how cannabis may exert various effects in chronic liver diseases (CLDs), especially in chronic hepatitis C virus (HCV) and fatty liver disease.
Keywords: cannabidiol, cannabis, chronic liver disease, pharmacokinetics, tetrahydrocannabinol
Introduction
Cannabis, which encompasses cannabis and its derivatives, has been consumed globally. In Canada, 10%–15% of adults and 25%–30% of youths reported having used cannabis in the past 1 year (1). Recreational cannabis use became legal in Canada on October 17, 2018. There are, however, many unanswered questions with regards to the health outcomes of long-term cannabis users. For example, the impact of cannabis on many areas of the public health burden is under-studied. In the past few decades, research gained traction on the effect of cannabis on cognition and psychomotor function, finding higher incidence of fatal motor vehicle accidents among cannabis users (2), while others examined the influence of smoking cannabis on respiratory and cardiovascular systems and pregnancy, suggesting various harmful outcomes in these areas (1,3).
However, the general public and health care providers are not aware or certain about the benefits and harms of cannabis, especially in those individuals with liver disease. We scanned the literature and summarized what is known and what needs to be studied in the near future.
Pharmacology of cannabis
Pharmacokinetic studies of cannabis unveiled its chemistry on the molecular level and helped to make health recommendations. We know one of the most abundant constituents, tetrahydrocannabinol (THC), is also the main psychotropic ingredient (1). Cannabidiol to THC ratio was used to thwart the unwanted side effects on the central nervous system. Vaporized and edible forms were preferred to minimize the deleterious effect on the respiratory system (1). The bioavailability and peak concentration of cannabis likely differ between the routes of administration (ie, smoking versus oral). Oral administration is associated with a slower absorption by the gastrointestinal system, reduced bioavailability and delayed peak concentration (4). Results from public health studies cautioned users to not drive while under the influence of cannabis (1) or not drive for at least 6 hours after use, knowing that the concentration of THC peaks at 5–30 min and the blood concentration of THC would linger for at least 3 to 6 hours (1). In Canada, driving while impaired to any degree by drugs or alcohol is a criminal offence. Since 2018 driving under the influence of cannabis falls under drug-impaired driving, according to the new national guidelines published online by the Government of Canada. Three new offences were added for having a prohibited concentration of drugs in the blood within 2 hours of driving, where detection of THC more than 2 ng/mL of blood is subject to penalty.
Cannabis has played many roles in digestive disorders such as inflammatory bowel disease, irritable bowel syndrome and other gastrointestinal motility disorders (5). Cannabis hyperemesis syndrome is a well-recognized gastrointestinal (GI) side effect among long-term users.
One of the earliest studies investigating the effect of cannabis on the liver was published in The Lancet in 1971; abnormal liver enzymes and hepatic dysfunction were found in young consumers who were chronic cannabis users, amplified by co-ingestion of alcohol (6). From there, knowledge in this area continued to grow. Figure 1 outlines the principal hepatic metabolism of cannabis by the cytochrome 450 (CYP450) system, a critical step followed by glucuronidation, which facilitates the final fecal and urinary excretions (7,8,9,10).
Figure 1:
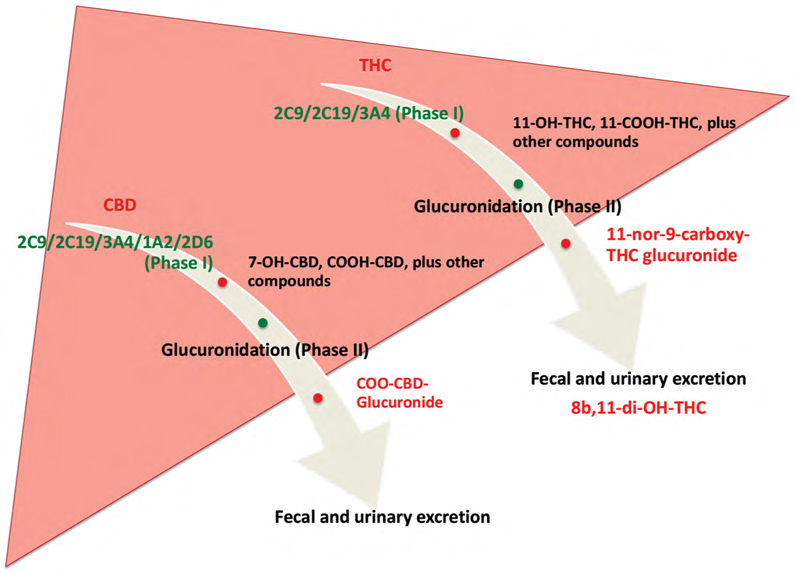
Metabolism of THC and CBD by the liver.
THC and CBD undergo two phases of metabolism in the liver. Phase I is conducted by the cytochrome P450 system. Phase II is glucuronidation of the phase I metabolites, facilitating fecal and urinary excretion by the kidneys.
THC = tetrahydrocannabinol; CBD = cannabidiol
Cannabis metabolism in patients with advanced liver disease
The expressions of the cannabinoid receptors, CB1 and CB2, which make up the endocannabinoid system, are up-regulated in the course of progressive liver disease (11), particularly in myofibroblasts and vascular endothelial cells, allowing more significant interaction between cannabis and the abnormal liver in comparison to the healthier liver (12). Many studies have shown the effect of advanced liver disease on CYP enzymes. In cirrhosis, expression of 1A2 and 3A4 isoenzymes is decreased. There are reports of alteration of clearance of drugs metabolized by 3A4 in patients of cirrhosis. Activity of 2C19 is also reported to be decreased in patients with liver disease. Levels of 2C subfamily have been reported to be up-regulated in patients with hepatic carcinoma. Detailed knowledge of these isoenzymes affected in disease states would be used to enhance the design of rational drug therapy.
There are potential drug–drug interactions (DDIs) (13), since many drugs either up-regulate or down-regulate the CYP450 system which may result in a higher or lower level of these drugs (Table 1). Unfortunately, few data are available regarding the potential drug interactions associated with cannabis. Nevertheless, we can make some predictions of potential interactions based on the known pharmacology of cannabis (4).
Table 1:
CYP450 system, cannabis and drug–drug interactions (DDIs)
| CYP450 isoenzymes | Involvement in hepatic metabolism of drugs (%) | Inducers potentially lowering cannabis effect | Inhibitors potentially increasing cannabis effect |
|---|---|---|---|
| Involved in CBD and THC metabolism | |||
| 2C9 | Rifampicin | Ketoconazole Fluconazole Amiodarone, benzbromarone Cimetidine |
|
| 2C19 | Artemisinin | Fluvoxamine Fluoxetine Omeprazole Ticlopidine Cimetidine Ketoconazole |
|
| 3A4 | 30%–40% | Azole antifungals, (eg, ketoconazole, fluconazole) Macrolide antimicrobials, (eg, erythromycin, clarithromycin) Selective serotonin re-uptake inhibitors (SSRIs) (eg, fluoxetine, paroxetine) Calcium channel blockers (eg, verapamil, diltiazem) Protease inhibitors Grapefruit juice Ciprofloxacin Cimetidine Propofol |
Rifampicin Rifabutin Carbamazepine phenytoin, phenobarbitone (13) |
| Involved only in CBD metabolism | |||
| 1A2 | Tobacco | Fluvoxamine Fluoxetine Ciprofloxacin Grapefruit juice Cimetidine Verapamil, diltiazem Estradiol, levonorgestrel Omeprazole |
|
| 2D6 | SSRIs (eg, fluoxetine) Propafenone Cimetidine Quinidine Terbinafine Amiodarone |
||
CYP450 = cytochrome P450; CBD = cannabidiol; THC = tetrahydrocannabinol
Tetrahydrocannabinol (THC)
THC is metabolized by CYP2C9 and CYP3A4 and has an inhibitory effect in them. Patients who are poor metabolizers of CYP2C9 have been shown to have THC concentrations that are about 3-fold higher than those of extensive metabolizers of CYP2C9 We are unaware of any studies examining the effect of CYP2C9 inhibitors on the elimination of THC. Based on genetic studies, inhibitors of CYP2C9 would be expected to increase the plasma concentration of THC. CYP2C9 inhibitors that would be expected to inhibit THC elimination include amiodarone, cimetidine, cotrimoxazole, metronidazole, fluoxetine, fluvoxamine, fluconazole, and voriconazole (4).
Ketoconazole, an inhibitor of CYP3A4, has been reported to increase the peak concentration and area under the concentration-time curve of THC by 1.2-fold and 1.8- fold, respectively, with greater increases in the concentration of THC metabolites. Other CYP3A4 inhibitors, including clarithromycin, erythromycin, cyclosporine, verapamil, itraconazole, voriconazole, and boceprevir, would be expected to produce similar increases in THC concentrations. Conversely, rifampin, a CYP3A4 inducer, has been reported to reduce THC levels by 20% to 40% (4).
Cannabidiol
Cannabidiol (also known as CBD) is a substrate of CYP3A4 and CYP2C19 Similar to THC, ketoconazole was noted to increase the plasma concentration of CBD by about 2-fold, while rifampin reduced CBD levels by 50% to 60%. Other CYP3A4 inhibitors and inducers should be expected to have a similar effect on CBD plasma concentrations if co-administered. Omeprazole, a modest inhibitor of CYP2C19, did not alter the plasma concentration of CBD in one study (14).
Cannabis and specific liver disorders
Chronic HCV
In chronic hepatitis C virus (HCV), cannabis can worsen liver fibrosis and steatosis in animal studies and cellular cultures (12,15). Cannabis was linked to immunosuppression and profibrogenic response in HCV patients versus healthy controls, by the mechanism of CB1 and CB2 up-regulation, resulting in the suppression of anti-viral immunity (16,17). Daily cannabis smoking was an independent risk predictor in the grading of steatosis on biopsy in chronic HCV patients (18); a similar trend was seen in a prospective HCV cohort study (19). However, other studies reported a positive influence on HCV outcome. In September 2018, a retrospective cohort study reported a lower percentage of HCV-related cirrhosis and lower total health costs among cannabis users compared with nonusers (20). Cannabis use improved HCV virologic outcome in the era of interferon and ribavirin by bettering patient adherence to the anti-viral treatment (21). Another study reported no negative impact of cannabis smoking on liver fibrosis in a subpopulation of HCV with HIV co-infection (22).
Alcoholic and non-alcoholic fatty liver diseases
Cannabis may also be a potential therapeutic target in alcoholic and non-alcoholic fatty liver disease. In animal models and cell cultures, CB1 and CB2 played different roles in promoting fibrosis, whereas CB2 activation was pro-inflammatory and led to insulin resistance (23). The CB1 antagonist has been shown to decrease fibrosis in human cell cultures (24,25).
Hepatic encephalopathy
There is little evidence on the safety of cannabis use in hepatic encephalopathy (HE). whether to make a recommendation against using cannabis in HE requires further validation (26). Surprisingly, animal model evidence suggested HE improved with cannabis in fulminant failure (27). The proposed mechanisms were anti-inflammatory properties of cannabis that activated 5-hydroxytryptamine receptor 5-HT1A, and the partial restoration of brain and liver functions. However, if an agent such as cannabis can cross the blood–brain barrier to influence the central nervous system, it could theoretically adversely worsen HE. There have been no human studies to date.
Liver transplant recipients
Due to the significant lack of data on the safety and efficacy of cannabis use in liver transplant recipients and their outcomes, a general recommendation cannot be made at this time. In the pre– and post–liver transplant periods, patient survival appeared to be the same in users and non-users, based on a small group of data (28,29). The effect on renal function post liver transplant can be extrapolated from the kidney transplant study. A study examined the safety of cannabis in 1,225 renal transplant recipients and reported similar kidney graft survival and graft function at 1 year post-transplant (30). The safety of cannabis in a liver transplant patient was affirmed by a case report (31). Another study found no negative impact on hepatic function (32).
One should use cannabis with caution in this special population for several reasons. When used by an immune-compromised patient, there were reported health concerns, specifically pertaining to smoking unsterilized cannabis which resulted in rare Aspergillus infection of the lung (33). Potential DDI with tacrolimus may occur, especially in individuals with certain allelic variability in CYP3A, and as a CYP3A inhibitor, cannabis may heighten tacrolimus toxicity (34). Tacrolimus toxicity that reached clinical significance was reported in a bone marrow transplant patient, who used edible cannabis (35). This was a cautionary tale to users in the post-transplant period and that frequent drug monitoring is required. Beyond anecdotal reports, there are no systematic studies published to date on the topic of DDI between cannabis and various immunosuppressive medications in the liver transplant population.
Potential future of cannabis and hepatology Studies
There are both controversies and advances in using the endocannabinoid system as a potential therapeutic target in treating chronic liver diseases (CLDs) (26,36,37). A few pathways to explore the endocannabinoid system are in the making, aimed at changing the progression of fibrosis and control of portal hypertension. Reducing ischemic reperfusion injury in organ transplants is another potential therapeutic interest (38).
Conclusions
In summary, cannabis is widely used by Canadians for recreational and medicinal purposes. The impact of cannabis on liver health and CLD is gaining attention, making it an attractive area of future research. The high frequency and increasing use of cannabis invites the need for health care providers to familiarize themselves with potential DDIs in persons receiving select psychotropic agents, and additionally consuming medical cannabis and/or recreational cannabis. Although no striking contraindications, until more data on its safety and DDIs, there remain unanswered questions of cannabis use in CLD and long-term data are required to elucidate the future of cannabis in CLD. The endocannabinoid system is also being investigated as the new therapeutic target in treating sub-populations of liver disease and liver fibrosis.
Funding:
No funding was received for this work.
Disclosures:
The authors have nothing to disclose.
References
- Fischer B, Russell C, Sabioni P, van den Brink W, et al. Lower-risk cannabis use guidelines: a comprehensive update of evidence and recommendations. Am J Public Health. 2017;107(8):e1–e12. Epub 2017 Jun 23. 10.2105/AJPH.2017.303818. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asbridge M, Hayden JA, Cartwright JL. Acute cannabis consumption and motor vehicle collision risk: systematic review of observational studies and meta-analysis. BMJ. 2012;344:e536. 10.1136/bmj.e536. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost L, Mostofsky E, Rosenbloom JI, Mukamal KJ, Mittleman MA. Marijuana use and long-term mortality among survivors of acute myocardial infarction. Am Heart J. 2013;165(2):170–5. Epub 2012 Dec 29. 10.1016/j.ahj.2012.11.007. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong C, Carmona NE, Lee YL, et al. Drug-drug interactions as a result of co-administering ∆9-THC and CBD with other psychotropic agents. Expert Opin Drug Saf. 2018;17(1):51–4. Epub 2017 Oct 31. 10.1080/14740338.2017.1397128. Medline: [DOI] [PubMed] [Google Scholar]
- Goyal H, Singla U, Gupta U, May E. Role of cannabis in digestive disorders. Eur J Gastroenterol Hepatol. 2017;29(2):135–43. 10.1097/MEG.0000000000000779. Medline: [DOI] [PubMed] [Google Scholar]
- Hochman JS, Brill NQ. Chronic marihuana usage and liver function. Lancet. 1971;2(7728):818–9. 10.1016/s0140-6736(71)92771-1. Medline: [DOI] [PubMed] [Google Scholar]
- Sharma P, Murthy P, Bharath MM. Chemistry, metabolism, and toxicology of cannabis: clinical implications. Iran J Psychiatry. 2012;7(4):149–56. Medline: [PMC free article] [PubMed] [Google Scholar]
- Lucas CJ, Galettis P, Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br J Clin Pharmacol. 2018;84(11):2477–82. Epub 2018 Aug 7. 10.1111/bcp.13710. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4(8):1770–804. 10.1002/cbdv.200790152. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. 2003;42(4):327–60. 10.2165/00003088-200342040-00003. Medline: [DOI] [PubMed] [Google Scholar]
- Patsenker E, Stoll M, Millonig G, et al. Cannabinoid receptor type I modulates alcohol-induced liver fibrosis. Mol Med. 2011;17(11–12):1285–94. Epub 2011 Aug 19. 10.2119/molmed.2011.00149. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfieniuk A, Flisiak R. Role of cannabinoids in chronic liver diseases. World J Gastroenterol. 2008;14(40):6109–14. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston TE, Bebin EM, Cutter GR, Liu Y, Szaflarski JP; UAB CBD Program. Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia. 2017;58(9):1586–92. Epub 2017 Aug 6. 10.1111/epi.13852. Medline: [DOI] [PubMed] [Google Scholar]
- Stout SM, Cimino NM. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2014;46(1):86–95. Epub 2013 Oct 25. 10.3109/03602532.2013.849268. Medline: [DOI] [PubMed] [Google Scholar]
- Tarantino G, Citro V, Finelli C. Recreational drugs: a new health hazard for patients with concomitant chronic liver diseases. J Gastrointestin Liver Dis. 2014;23(1):79–84. Medline: [PubMed] [Google Scholar]
- Patsenker E, Sachse P, Chicca A, et al. Elevated levels of endocannabinoids in chronic hepatitis C may modulate cellular immune response and hepatic stellate cell activation. Int J Mol Sci. 2015;16(4):7057–76. 10.3390/ijms16047057. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe RF, Siegmund SV. Potential role of CB2 receptors in cannabis smokers with chronic hepatitis C. Hepatology. 2005;42(4): 975–6. 10.1002/hep.20895. Medline: [DOI] [PubMed] [Google Scholar]
- Hezode C, Zafrani ES, Roudot-Thoraval F, et al. Daily cannabis use: a novel risk factor of steatosis severity in patients with chronic hepatitis C. Gastroenterology. 2008;134(2):432–9. Epub 2007 Nov 28. 10.1053/j.gastro.2007.11.039. Medline: [DOI] [PubMed] [Google Scholar]
- Ishida JH, Peters MG, Jin C, et al. Influence of cannabis use on severity of hepatitis C disease. Clin Gastroenterol Hepatol. 2008;6(1):69–75. 10.1016/j.cgh.2007.10.021. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adejumo AC, Adegbala OM, Adejumo KL, Bukong TN. Reduced incidence and better liver disease outcomes among chronic HCV infected patients who consume cannabis. Can J Gastroenterol Hepatol. 2018;2018:Article ID 9430953 [9 p.]. 10.1155/2018/9430953. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvestre DL, Clements BJ, Malibu Y. Cannabis use improves retention and virological outcomes in patients treated for hepatitis C. Eur J Gastroenterol Hepatol. 2006;18(10):1057–63. 10.1097/01.meg.0000216934.22114.51. Medline: [DOI] [PubMed] [Google Scholar]
- Brunet L, Moodie EE, Rollet K, et al; Canadian Co-infection Cohort Investigators. Marijuana smoking does not accelerate progression of liver disease in HIV-hepatitis C coinfection: a longitudinal cohort analysis. Clin Infect Dis. 2013;57(5):663–70. Epub 2013 Jun 28. 10.1093/cid/cit378. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallat A, Lotersztajn S. Endocannabinoids and their role in fatty liver disease. Dig Dis. 2010;28(1):261–6. Epub 2010 May 7. 10.1159/000282100. Medline: [DOI] [PubMed] [Google Scholar]
- Teixeira-Clerc F, Julien B, Grenard P, et al. CB1 cannabinoid receptor antagonism: a new strategy for the treatment of liver fibrosis. Nat Med. 2006;12(6):671–6. Epub 2006 May 21. 10.1038/nm1421. Medline: [DOI] [PubMed] [Google Scholar]
- Wasmuth HE, Trautwein C. CB1 cannabinoid receptor antagonism: a new strategy for the treatment of liver fibrosis. Hepatology. 2007;45(2):543–4. 10.1002/hep.21527. Medline: [DOI] [PubMed] [Google Scholar]
- Basu PP, Aloysius MM, Shah NJ, Brown RS. Review article: the endocannabinoid system in liver disease, a potential therapeutic target. Aliment Pharmacol Ther. 2014;39(8):790–801. Epub 2014 Feb 24. 10.1111/apt.12673. Medline: [DOI] [PubMed] [Google Scholar]
- Avraham Y, Grigoriadis N, Poutahidis T, et al. Cannabidiol improves brain and liver function in a fulminant hepatic failure-induced model of hepatic encephalopathy in mice. Br J Pharmacol. 2011;162(7):1650–8. 10.1111/j.1476-5381.2010.01179.x. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranney DN, Acker WB, Al-Holou SN, et al. Marijuana use in potential liver transplant candidates. Am J Transplant. 2009;9(2):280–5. Epub 2008 Nov 27. 10.1111/j.1600-6143.2008.02468.x. Medline: [DOI] [PubMed] [Google Scholar]
- Nickels M, Jain A, Sharma R, et al. Polysubstance abuse in liver transplant patients and its impact on survival outcome. Exp Clin Transplant. 2007;5(2):680–5. Medline: [PubMed] [Google Scholar]
- Greenan G, Ahmad SB, Anders MG, Leeser A, Bromberg JS, Niederhaus SV. Recreational marijuana use is not associated with worse outcomes after renal transplantation. Clin Transplant. 2016;30(10):1340–6. Epub 2016 Sep 5. 10.1111/ctr.12828. Medline: [DOI] [PubMed] [Google Scholar]
- Meng H, Hanlon JG, Katznelson R, Ghanekar A, McGilvray I, Clarke H. The prescription of medical cannabis by a transitional pain service to wean a patient with complex pain from opioid use following liver transplantation: a case report. Can J Anaesth. 2016;63(3):307–10. Epub 2015 Oct 27. 10.1007/s12630-015-0525-6. Medline: [DOI] [PubMed] [Google Scholar]
- Bonnet U, Canbay A, Specka M, Scherbaum N. Long-term heavy recreational cannabis use and serum delta-9-tetrahydrocannabinol levels are not associated with an impaired liver function in cannabis dependents. J Psychoactive Drugs. 2018;50(4):355–60. Epub 2018 Jul 27. 10.1080/02791072.2018.1482031. Medline: [DOI] [PubMed] [Google Scholar]
- McPartland JM, Pruitt PL. Medical marijuana and its use by the immunocompromised. Altern Ther Health Med. 1997;3(3):39–45. Medline: [PubMed] [Google Scholar]
- Rai HS, Winder GS. Marijuana use and organ transplantation: a review and implications for clinical practice. Curr Psychiatry Rep. 2017;19(11):91. 10.1007/s11920-017-0843-1. Medline: [DOI] [PubMed] [Google Scholar]
- Hauser N, Sahai T, Richards R, Roberts T. High on cannabis and calcineurin inhibitors: a word of warning in an era of legalized marijuana. Case Rep Transplant. 2016;2016:Article ID 4028492 [3 p.]. Epub 2016 Aug 9. 10.1155/2016/4028492. Medline:. Erratum in: Case Rep Transplant. 2018;2018: Article ID 7095846 [1 p.]. Corrected at: https://www.hindawi.com/journals/crit/2018/7095846/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal H, Rahman MR, Perisetti A, Shah N, Chhabra R. Cannabis in liver disorders: a friend or a foe? Eur J Gastroenterol Hepatol. 2018;30(11):1283–90. 10.1097/MEG.0000000000001256. Medline: [DOI] [PubMed] [Google Scholar]
- Tam J, Liu J, Mukhopadhyay B, Cinar R, Godlewski G, Kunos G. Endocannabinoids in liver disease. Hepatology. 2011;53(1):346–55. 10.1002/hep.24077. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay P, Rajesh M, Horvath B, et al. Cannabidiol protects against hepatic ischemia/reperfusion injury by attenuating inflammatory signaling and response, oxidative/nitrative stress, and cell death. Free Radic Biol Med. 2011;50(10):1368–81. Epub 2011 Mar 11. 10.1016/j.freeradbiomed.2011.02.021. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]


