Abstract
Background
Hepatocellular carcinoma (HCC) is a global health problem, accounting for 4.7% of all new cancer cases and 8.2% of all cancer deaths worldwide in 2018. Resection and transplantation are the only modalities that offer a cure for HCC; however, most patients are diagnosed at an advanced stage, precluding these curative treatments. A number of local (ie, ablative therapies) and/or local-regional therapies (ie, chemo-embolization) are used and followed by systemic therapy for advanced or progressive disease. Other treatments are available, but their efficacy compared with these standards is not well known.
Methods
Literature searches (1/2000 to 1/2020 or 1/2005 to 1/2020, depending on the specific systematic review question) were conducted, including MEDLINE, Embase and the Cochrane Database of Systematic Reviews.
Results
Over 30,000 articles were identified. In total, 49 studies were included in the systematic review.
Conclusions
There is no evidence to support the addition of sorafenib to any local or regional therapy. First-line systemic therapy options for unresectable or metastatic HCC include sorafenib, lenvatinib, and atezolizumab + bevacizumab. Regorafenib or cabozantinib provide survival benefits when given as second-line treatment.
Keywords: hepatocellular carcinoma, non-surgical treatment, systematic review, systemic therapy, tyrosine kinase inhibitor
Glossary
Local therapies
RFA – radiofrequency ablation
SBRT – stereotactic body radiation therapy
TEA – transarterial ethanol ablation
Regional therapies
cTACE – conventional transarterial chemoem- bolization
DEB-TACE – drug eluting bead transarterial chemoembolization
SIRT – selective internal radiation therapy (same as TARE)
TAE – bland transarterial embolization
TARE – transarterial radioembolization
Other therapies
BSC – best supportive care
Outcomes
ORR – objective response rate
OS – overall survival
PFS – progression free survival
TTP – time to progression
Other terms
CI – confidence interval
HR – hazard ratio
NE – not estimable
NR – not reported
ns – not significant
Definitions
(http://www.cancer.ca/en/cancer-information/cancer-type/liver/staging/?region=qc)
- Barcelona Clinic Liver Cancer (BCLC) Stage B (Intermediate stage)
- Child-Pugh A or B
- Multifocal disease but tumours are not causing symptoms
- ECOG = 0
- Barcelona Clinic Liver Cancer (BCLC) Stage C (Advanced stage)
- Child-Pugh A or B
- Tumour(s) have grown into blood vessels or there has been spread to other body sites. Tumour(s) are causing symptoms.
- ECOG = 1 or 2
Background
The incidence of liver cancer steadily increased in Canadian men and women between 1989 and 2011 (1). Specifically, the incidence has increased by 3.8% and 2.7% per year in males and females, respectively. This rising incidence may partially be attributed to immigration from regions where exposure to liver cancer risk factors such as hepatitis B, hepatitis C, and aflatoxin are much more common (1). The mortality from liver cancer has also been steadily increasing. Since the mid-1990s, mortality has increased by 3.1% per year in males and 2.2% per year in females in Canada (1). Hepatocellular carcinoma (HCC) accounts for approximately 72% of all liver cancers in Canada. This disease is a global health problem, accounting for 4.7% of all new cancer cases and 8.2% of all cancer deaths worldwide in 2018 (2). In the province of Ontario in 2019, there will be an estimated 1,170 new-incident cases of liver cancer (39.3 % of the estimated new-incident liver cancer cases in Canada) and 550 deaths from liver cancer (39.9% of the estimated liver cancer deaths in Canada) (1). The predicted net observed survival for 2012 to 2014 for liver cancer was 19% (95% CI 18%–20%) for males and females combined (1).
Resection and transplantation are the foundations for a cure for HCC; however, most patients are diagnosed at an advanced stage, precluding these curative treatments. Non-curative treatments are usually transarterial chemoembolization (TACE) and, in the case of advanced disease, sorafenib. Other treatments are available, but their efficacy compared with TACE and sorafenib is not well known. The purpose of this systematic review is to evaluate the current evidence for treatment options for advanced, unresectable HCC.
Research Questions
This systematic review examined the evidence to answer the following questions in those with locally advanced or advanced HCC (Barcelona Clinic Liver Cancer [BCLC] Stage B or higher):
What are the benefits of other local therapies (transarterial ethanol ablation [TEA], bland transarterial embolization [TAE], radiofrequency ablation [RFA], transarterial radioembolization [TARE], stereotactic body radiation therapy [SBRT], and drug-eluting bead transarterial chemoembolization [DEB-TACE]), versus transarterial chemoembolization (TACE)?
What is the benefit of other systemic treatment regimens versus sorafenib?
What is the benefit of second-line systemic therapy following sorafenib?
Methods
Search strategy and selection criteria
Clinical practice guidelines
A search was conducted for existing clinical practice guidelines. Only guidelines based on a systematic review and covering a question of interest were retained. All retained guidelines were evaluated for quality using the AGREE II framework (3).
Systematic reviews
A search was conducted for existing systematic reviews in the databases MEDLINE, Embase, and the Cochrane Database of Systematic Reviews, from 2005 to January 2020. English language systematic reviews that covered any of the current questions of interest were included.
Primary literature
A search for primary studies was undertaken for all questions. If more than one publication was available for a given trial, only the most recent publication was included. The search strategy for guidelines, systematic reviews, and primary studies is available upon request.
Study selection criteria and process
Selected studies had to be English language studies addressing the question of interest in adult participants (N = 30 minimally) with locally advanced or advanced HCC (BCLC Stage B or intermediate stage or higher) who were not suitable for transplant or surgery, included a comparison of interest, and included at least one outcome of interest. Randomized controlled trials were preferred. If none were available for a particular comparison, other comparative studies were included.
A review of the titles and abstracts that resulted from the search was independently conducted by one reviewer (author RC). A full-text review was conducted by one reviewer (RC). If there was any question regarding the eligibility of a given study, then two reviewers (RC and BMM) reviewed each item in collaboration to determine eligibility.
Data extraction and assessment of study quality and potential for bias
Data from all included studies were extracted by one member of the working group (RC). All extracted data were subsequently audited by an independent auditor.
RCTs were assessed for quality and potential bias using the Cochrane Risk of Bias tool (section 8 of the Cochrane’s and Handbook for Systematic Review of Interventions, available at http://handbook.cochrane.org/). All non-RCTs were assessed using the ROBINS-I tool from Cochrane Risk of Bias in Non-Randomized Studies – of Interventions (available at https://sites.google.com/site/riskofbiastool/). Systematic reviews were evaluated using the AMSTAR tool (4).
Results
Search for existing clinical practice guidelines
A search for systematic reviews uncovered 11,279 documents. Of these, 398 underwent full-text review, and none were retained.
Search for existing systematic reviews
A search for systematic reviews uncovered 6,783 documents. Of these, 394 underwent full-text review and none were retained.
Search for primary literature
A search for primary studies uncovered 13,166 documents. Of these, 461 underwent a full-text review and 49 were retained, including one relevant pooled analysis. For a summary of the full literature search results (including guidelines and systematic reviews), please refer to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) study selection flow diagram in Figure 1.
Figure 1:
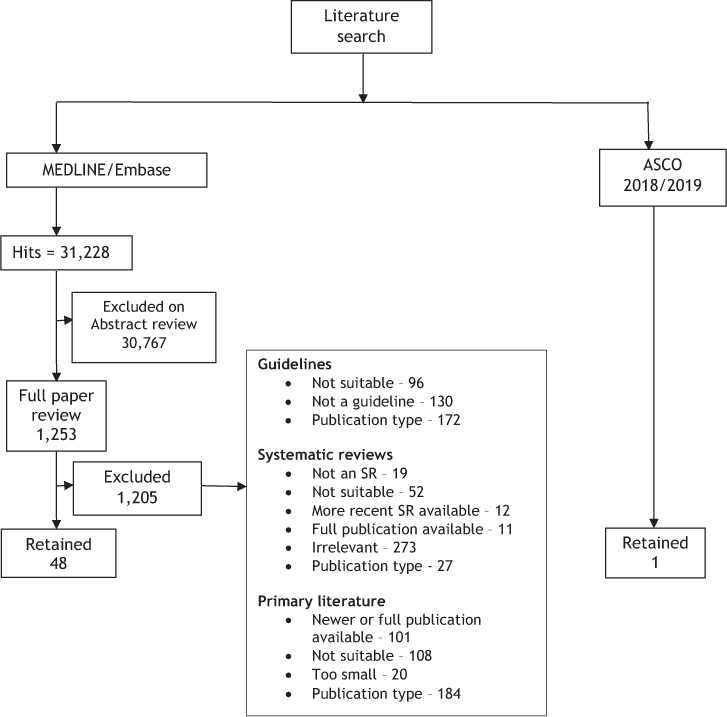
PRISMA flow diagram for literature search
PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses (http://www.prisma-statement.org/); ASCO = ASCO Publications (https://ascopubs.org/); SR = Systematic review
Study design and quality
Randomized controlled trials
Forty RCTs published in 47 manuscripts (5–51) were included in this guidance document and were assessed using Cochrane’s Risk of Bias tool. Many of the included RCTs could not be assessed on at least one element of the risk of bias tool. This was particularly evident in abstracts, which report very limited information. These items were therefore rated as “unclear.” Overall, there were only 6 RCTs that had a low risk of bias (38,41,45,47,48,51). Eighteen RCTs (5,7,8,10,11,19,21–23,29,30,33,35,40,44,46,49,50) were considered to have an unclear risk of bias as at least one of the domains was rated as “unclear.” Sixteen RCTs (6,9,12,13,18,20,24–28,31,32,34,36,37) were considered to have a serious risk of bias (risk of bias evaluations are available upon request).
Non-randomized controlled studies
This guidance document includes two non-RCTs (52,53) that were each assessed using the ROBINS-I tool. This tool assesses each trial on seven domains of bias as well as an overall assessment of risk of bias. These were only available in abstract form and therefore were assessed as “no information,” as there was not enough information in the abstracts to evaluate risk of bias (risk of bias evaluations are available upon request from the corresponding author).
Outcomes
Question 1: What is the benefit of the addition of sorafenib to local therapies (TEA, TAE, RFA, TARE, SBRT, DEB-TACE, and TACE)?
TEA + sorafenib versus TEA
No studies were found.
TAE + sorafenib versus TAE
No studies were found.
RFA + sorafenib versus RFA
One trial of 62 participants, with lesions ranging from 3.1 to 5.0 cm, was retained (5). One-, 2-, and 3-year recurrence rates were significantly higher in the RFA-alone arm (87.5% versus 56.7%, p < 0.01). Median time to progression (TTP) was significantly longer in the RFA + sorafenib arm (17.0 months versus 6.1 months, p < 0.05). There were no serious toxicities in the RFA arm. However, 8.1% and 6.5% of participants in the combination arm experienced a Grade 3 increase in alanine aminotransferase (ALT) and aspartate transaminase (AST), respectively. No subgroup analysis for tumour size was reported.
TARE + sorafenib versus TARE
No RCTs were found. However, 2 abstracts (one retrospective study and one case-control study) were retained. Ma et al (52) conducted a retrospective study of 55 participants in one centre. Median survival in the combined arm was significantly higher than in the TARE-only arm (21.0 months versus 7.0 months; p = 0.003). Adverse effects were reported in 1 participant in the combined treatment arm and 6 participants in the TARE-only arm. However, severities of the toxicities were not reported. Maccauro et al (53) conducted a case-control study of 15 cases and 30 controls. There were no significant differences between the groups on any reported outcome, including median PFS, median overall survival (OS), and ORR.
SBRT + sorafenib versus SBRT
No studies were found.
DEB-TACE + sorafenib versus DEB-TACE
Two trials were retained (10,11). The SPACE trial (10) included 307 participants with intermediate-stage HCC. TTP was not significantly different in the two study arms (HR 0.797; 95% CI 0.588–1.080; p = 0.072). OS was also not significantly different in the two study arms (HR 0.898, 95% CI 0.606–1.330; p = 0.295). ORR was 35.7% in the DEB-TACE/sorafenib arm and 28.1% in the DEB-TACE/placebo arm (p = NR). The TACE 2 trial (11) included 399 participants and was terminated early for futility. Median PFS (HR 0.99; 95% CI 0.77–1.27; p = 0.94) and median OS (HR 0.91; 95% CI 0.67–1.24; p = 0.57) were not significantly different in the two trial arms.
DEB-TACE + sorafenib versus DEB-TACE
Two trials were retained (10,11). The SPACE trial (10) included 307 participants with intermediate-stage HCC. TTP was not significantly different in the two study arms (HR 0.797; 95% CI 0.588–1.080; p = 0.072). OS was also not significantly different in the two study arms (HR 0.898, 95% CI 0.606–1.330; p = 0.295). ORR was 35.7% in the DEB-TACE/sorafenib arm and 28.1% in the DEB-TACE/ placebo arm (p = NR). The TACE 2 trial (11) included 399 participants and was terminated early for futility. Median PFS (HR 0.99; 95% CI 0.77–1.27; p = 0.94) and median OS (HR 0.91; 95% CI 0.67–1.24; p = 0.57) were not significantly different in the two trial arms.
TACE + sorafenib versus TACE
Four trials were retained (6–9). Kudo et al (6) conducted a phase III trial of 458 participants with unresectable HCC. Median TTP was not significantly different in the two arms of the trial (HR 0.87; 95% CI 0.70–10.9; p = 0.252). Median OS was also not significantly different in the two arms of the study (p = 0.790). The incidence of drug-related adverse events (AEs) was higher in the TACE/sorafenib arm (18%) compared with the TACE/placebo arm (9%), but no p value is reported. Sansonno et al (7) conducted a smaller trial of 80 intermediate-stage HCC participants. There was a significantly longer TTP in the TACE/sorafenib arm compared with the TACE/placebo arm (9.2 months versus 4.9 months, p < 0.001). There were more drug-related AEs in the TACE/sorafenib arm; however, no p values are reported. Kudo et al (8) conducted a trial of 256 participants with unresectable HCC in 33 centres. Median PFS was significantly longer in the TACE/sorafenib arm compared with the TACE-alone arm (25.2 months versus 13.5 months [HR 0.56; 95% CI 0.38–0.83; p = 0.004]). Park et al (9) conducted a phase III trial of 330 participants with advanced HCC. Median OS was not significantly different in the two study arms (HR 0.91; 95% CI 0.69–1.21; p = 0.290). However, both median TTP (HR 0.67; 95% CI 0.53–0.85; p = 0.003) and median PFS (HR 0.73; 95% CI 0.59–0.91; p = 0.01) significantly favoured the TACE/sorafenib arm.
Question 2: What is the benefit of other systemic treatment regimens versus sorafenib?
Single drugs versus sorafenib alone
Lenvatinib versus sorafenib
A phase III non-inferiority trial of lenvatinib versus sorafenib was reported in one full publication (13), and 4 abstracts were retained (14–17). This trial enrolled 954 participants. The data indicate that lenvatinib is non-inferior to sorafenib with respect to median OS (HR 0.92; 95% CI 0.79–1.06). Median PFS (HR 0.66; 95% CI 0.57–0.77, p <0.0001), median TTP (HR 0.63; 95% CI 0.53–0.73, p <0.0001), and ORR (24.1% versus 9.2%, p <0.0001) were all significantly better in the lenvatinib arm (15) (Table 1). This trial had very strict inclusion criteria. Specifically, only those with ECOG PS 0–1 were included, and those with main portal vein thrombosis were excluded. This limits the generalizability of the results. Subgroup analysis demonstrated that median OS was similar in the 2 study arms in HBV-positive participants in general (HR 0.83; 95% CI 0.68–1.02) and HBV-positive participants from the Asia-Pacific (HR 0.82; 95% CI 0.66–1.02) (14). Health-related QOL was reported in 3 abstracts (15–17). Lenvatinib was significantly better with respect to role function (p = 0.0098), pain (p = 0.006), diarrhea (p <0.0001), body image (p = 0.0041), and nutrition (p = 0.006).
Table 1:
Outcomes from included studies on other systemic treatments versus sorafenib
| Study | Treatment allocation | N (evaluated) | Median OS (months) | Median TTP (months) | Median PFS (months) | ORR No. (%) | Terminated early? |
|---|---|---|---|---|---|---|---|
| Single drugs vs. sorafenib alone | |||||||
| Linifanib vs. sorafenib | |||||||
| Cainap, 2015 (12) | Linifanib | 514 (510) | 9.1 | 5.4 | 4.2 | 10.1% | Yes, for futility |
| Sorafenib | 521 (519) | 9.8 | 4.0 | 2.9 | 6.1% | ||
| HR 1.046; 95% CI 0.896–1.221; p = ns | HR 0.759; 95% CI 0.643–0.895; p = 0.001 | HR 0.813; 95% CI 0.697–0.948; p = 0.008 | p = 0.018 | ||||
| Lenvatinib vs. sorafenib | |||||||
| Kudo, 2018 (13) | Lenvatinib | 478 | 13.6 | 8.9 | 7.4 | 115 (24) | No |
| Sorafenib | 476 | 12.3 | 3.7 | 3.7 | 44 (9) | ||
| HR 0.92; 95% CI 0.79–1.06; p = NR | HR 0.63; 95% CI 0.53–0.73; p <0.0001 | HR 0.66; 95% CI 0.57–0.77; p <0.0001 | p <0.0001 | ||||
| Han, 2017 (14), abstract | HBV-positive participants | ||||||
| Lenvatinib | 259 | 13.4 | |||||
| Sorafenib | 244 | 10.2 | |||||
| HR 0.83; 95% CI 0.68–1.02; p = NR | |||||||
| HBV-positive Asia-Pacific participants | |||||||
| Lenvatinib | 218 | 13.1 | |||||
| Sorafenib | 208 | 9.4 | |||||
| HR 0.82; 95% CI 0.66–1.02; p = NR | |||||||
| Sunitinib vs. sorafenib | |||||||
| Cheng, 2013 (18) | Sunitinib | 530 | 7.9 | 4.1 | 3.6 | NR | Yes, for futility and safety |
| Sorafenib | 544 | 10.2 | 3.8 | 3.0 | |||
| HR 1.30; 95% CI 1.13–1.50; p = 0.9990 | HR 1.13; 95% CI 0.98–1.31; p = 0.8312 | HR 1.13; 95% CI 0.99–1.30; p = 0.8785 | |||||
| Nintedanib vs. sorafenib | |||||||
| Yen, 2018 (19) | Nintedanib | 63 | 10.2 | 2.8 | 2.7 | NR | No |
| Sorafenib | 32 | 10.7 | 3.7 | 3.7 | |||
| HR 0.94; 95% CI 0.59–1.49; p = NR | HR 1.21; 95% CI 0.73–2.01; p = NR | HR 1.19; 95% CI 0.73–1.93; p = NR | |||||
| Palmer, 2015 (20), abstract | Nintedanib | 62 | 11.9 | 5.5 (investigator assessed) | NR | NR | No |
| Sorafenib | 31 | 11.4 | 3.8 (investigator assessed) | ||||
| HR 0.88; 95% CI 0.52–1.47; p = NR | HR 1.05; 95% CI 0.63–1.76; p = NR | ||||||
| Brivanib vs. sorafenib | |||||||
| BRISK-FL, 2013 (21) | Brivanib | 577 (575) | 9.5 | 4.2 | NR | 12% | No |
| Sorafenib | 578 (575) | 9.9 | 4.1 | 9% | |||
| HR 1.07; 95% CI 0.94–1.23; p = 0.3116 | HR 1.01; 95% CI 0.88–1.16; p = 0.8532 | p = 0.569 | |||||
| Capecitabine vs. sorafenib | |||||||
| Wahab, 2012 (22), abstract | Capecitabine | N total | 5.07 | NR | 4 | 3.0% | No |
| Sorafenib | 52 | 7.05 | 6 | 14.5% | |||
| p <0.016 | p <0.005 | p = NR | |||||
| Nivolumab vs. sorafenib | |||||||
| Yau, 2019 (23), abstract | Nivolumab | 371 | 16.4 | NR | 3.7 | 57 (15) | No |
| Sorafenib | 372 | 14.7 | 3.8 | 26 (7) | |||
| HR 0.85; 95% CI 0.72–1.02; p = 0.0752 | p = NR | p = NR | |||||
| Drug combinations vs. sorafenib alone | |||||||
| Atezolizumab + bevacizumab vs. sorafenib | |||||||
| Nivolumab vs. sorafenib | |||||||
| Finn, 2020 (24) | Atezolizumab + bevacizumab | 336 | NE | 6.8 | 27% | No | |
| Sorafenib | 165 | 13.2 | 4.3 | 12% | |||
| HR 0.58; 95% CI 0.42–0.79; p <0.001 | HR 0.059; 95% CI 0.47–0.76; p <0.001 | p <0.001 | |||||
| Doxorubicin + sorafenib vs. sorafenib | |||||||
| Soradox trial, 2015 (25), abstract | Doxorubicin + sorafenib | 15 (11) | 6.97 | 7.11 | NR | NR | No |
| Sorafenib | 15 (12) | 19.8 | 8.45 | ||||
| p = 0.14 | p = 0.96 | ||||||
| CALGB 80802, 2019 (26) | Doxorubicin + sorafenib | 180 | 9.3 | 4.7 | 4.0 | 15 (10) | Yes, for futility |
| Sorafenib | 176 | 9.4 | 4.2 | 3.7 | 8 (5.4) | ||
| HR 1.03; 95% CI 0.82–1.29; p = 0.83 | HR 0.92; 95% CI 0.71–1.18; p = 0.49 | HR 0.93; 95% CI 0.75–1.16; p = 0.54 | p = ns | ||||
| GEMOX + sorafenib vs. sorafenib | |||||||
| GONEXT trial, 2019 (27) | GEMOX + sorafenib | 48 (40) | 13.5 | 6.2 | 6.2 | 6 (15) | No |
| Sorafenib | 46 (38) | 14.8 | 4.6 | 4.6 | 4 (9) | ||
| p = NR | p = NR | p = NR | p = NR | ||||
| Tigatuzumab + sorafenib vs. sorafenib | |||||||
| Cheng, 2015 (28) | Tigatuzumab (6/2mg/kg) + sorafenib | 53 (53) | 8.2 | 3.0 | NR | 5.7% | No |
| Tigatuzumab (6/6mg/kg) + sorafenib | 55 (54) | 12.2 | 3.9 | 14.8% | |||
| Sorafenib | 55 (55) | 8.2 | 2.8 | 10.9% | |||
| All pairwise comparisons; p = ns | All pairwise comparisons; p = ns | ||||||
| Mapatumumab + sorafenib vs. sorafenib + placebo | |||||||
| Ciuleanu, 2016 (29) | Mapatumumab + sorafenib | 50 | 10.0 | 4.1 | 3.2 | NR | No |
| Sorafenib + placebo | 51 | 10.1 | 5.6 | 4.2 | |||
| HR 1.195; 90% CI 0–1.651*; p = 0.7823 | HR 1.192; 95% CI 0–1.737; p = 0.7382 | HR 1.066; 90% CI 0–1.43*; p = NR | |||||
| Everolimus + sorafenib vs. sorafenib | |||||||
| Koeberle, 2016 (30) | Everolimus + sorafenib | 60 (50) | 12 | NR | 5.7 | 6 (10) | No |
| Sorafenib | 46 (43) | 10 | 6.6 | 0 (0) | |||
| p = NR | p = NR | p = NR | |||||
| AEG35256 + sorafenib vs. sorafenib | |||||||
| Lee, 2016 (31) | AEG35256 + sorafenib | 31 | 6.5 | NR | 4.0 | 3 (9.7) | No |
| Sorafenib | 17 | 5.4 | 2.6 | 0 (0.0) | |||
| Bevacizumab + erlotinib vs. sorafenib | |||||||
| Thomas, 2018 (32) | Bevacizumab + erlotinib | 47 | 8.6 | NR | NR | 15% | No |
| Sorafenib | 43 | 8.6 | 9% | ||||
| HR 0.92; 95% CI 0.57–1.47; p = NR | p = NR | ||||||
| Erlotinib + sorafenib vs. sorafenib + placebo | |||||||
| SEARCH, 2015 (33) | Erlotinib + sorafenib | 362 (362) | 9.5 | 3.2 | NR | 6.6% | No |
| Sorafenib + placebo | 358 (355) | 8.5 | 4.0 | 3.9% | |||
| HR 0.929; 95% CI 0.78–1.11; p = 0.408 | HR 1.135; 95% CI 0.94–1.37; p = 0.18 | p = 0.102 | |||||
| Pravastatin+sorafenib vs. sorafenib | |||||||
| Blanc, 2018 (34), abstract | Pravastatin+ sorafenib | 40 | 4.0 | NR | 3.4 | NR | No |
| Sorafenib | 41 | 3.8 | 3.2 | ||||
| p = NR | |||||||
| Resminostat + sorafenib vs. sorafenib | |||||||
| Tak, 2018 (35) | Resminostat + sorafenib | 86 (84) | 11.8 | 2.8 | NR | 3 (3.6) | No |
| Sorafenib | 84 (84) | 14.1 | 2.8 | 8 (9.5) | |||
| HR 1.046; 95% CI 0.70–1.55; p = 0.824 | HR 0.984; 95% CI 0.68–1.41; p = 0.925 | p = NR | |||||
| Tegafur–uracil (UFT) + sorafenib vs. sorafenib | |||||||
| Azim, 2018 (36) | UFT + sorafenib | 36 | 8.2 | 7.5 | 6 | NR | Yes, for futility |
| Sorafenib | 38 | 10.5 | 8.2 | 6 | |||
| HR 1.58; 95% CI 0.90– 2.76; p = 0.112 | HR 1.07; 95% CI 0.52–2.22; p = 0.855 | HR 1.19; 95% CI 0.71–2.01; p = 0.508 | |||||
Note this is a 90% confidence interval
OS = Overall survival; TTP = Time to progression; PFS = Progression-free survival; ORR = Objective response rate; HR = Hazard ratio; CI = Confidence interval; HBV = Hepatitis B virus; NR = Not reported; GEMOX = Gemcitabine/oxaliplatin; NE = Not estimable; ns = Not significant
Other single drugs versus sorafenib
All other comparisons of single drugs to sorafenib, including linifanib (12), sunitinib (18), nintedanib (19,20), brivanib (21), capecitabine (22), and nivolumab (23) were non-significant, not non- inferior, or too small to make any conclusions about (Table 1).
Drug combinations versus sorafenib alone
Atezolizumab + bevacizumab versus sorafenib
The phase III IMbrave150 trial comparing atezolizumab/bevacizumab versus sorafenib was retained (24). This trial is currently only available in abstract form and is technically an interim analysis, which would normally result in it not being included in the systematic review. However, since the results of the interim analysis have met the stated primary end points, they were considered final. This trial of 501 participants demonstrated significantly better median OS (HR 0.58; 95% CI 0.42–0.79; p <0.001) and median PFS (HR 0.059; 95% CI 0.47–0.76; p <0.001) for the combination arm compared with the sorafenib alone arm (Table1). These results are intriguing, but a final recommendation would only be made once the final publication is available.
Other drug combinations versus sorafenib
All comparisons of drug combination to sorafenib including doxorubicin/sorafenib (25,26), gemcitabine/oxaliplatin/sorafenib (27), tigatuzumab/sorafenib (28), mapatumumab/sorafenib (29), everolimus/sorafenib (30), AEG35256/sorafenib (31), becvacizumab/erlotinib (32), erlotinib/sorafenib (33), pravastatin/sorafenib (34), resminostat/sorafenib (35), and UFT/sorafenib (36) were non-significant (Table 1).
Question 3: What is the benefit of second-line systemic therapy following sorafenib?
Regorafenib + best supportive care (BSC) versus placebo + BSC
One full publication (41) and two abstracts (42,43) of the RESORCE trial were retained. This was a phase III RCT of regorafenib/BSC versus placebo/BSC. The authors (41) reported significantly better median OS (HR 0.63; 95% CI 0.50–0.79, p <0.0001), median PFS (HR 0.46; 95% CI 0.37–0.56, p <0.0001) and median TTP (HR 0.44; 95% CI 0.36–0.55, p <0.0001) in the regorafenib arm of the trial. ORR was also significantly better in the regorafenib arm (11% versus 4%; p = 0.0047 (Table 2). Updated OS results are very similar to the primary analysis (HR 0.62; 95% CI 0.50–0.75; p<0.0001) (42). Grade 3/4 toxicity was greater in the regorafenib arm overall (67% versus 39%) including hand-foot skin reaction (13% versus 1%), diarrhea (3% versus 0%), fatigue (9% versus 5%), and hypertension (15% versus 5%). No p values are reported (41). All measures of QOL were similar in the two treatment arms (43).
Table 2:
Outcomes from included studies on the benefit of second-line systemic therapy following sorafenib
| Study | Treatment allocation | N (evaluated) | Median OS (months) | Median TTP (months) | Median PFS (months) | ORR No. (%) | Terminated early? |
|---|---|---|---|---|---|---|---|
| Regorafenib + BSC vs. placebo + BSC | |||||||
| Bruix, 2017 (41) | Regorafenib + BSC | 379 | 10.6 | 3.2 | 3.1 | 40 (11) | No |
| Placebo + BSC | 194 | 7.8 | 1.5 | 1.5 | 8 (4) | ||
| HR 0.63; 95% CI 0.50–0.79; p <0.0001 | HR 0.44; 95% CI 0.36–0.55; p <0.0001 | HR 0.46; 95% CI 0.37–0.56; p <0.0001 | p = 0.0047 | ||||
| Cabozantinib vs. placebo | |||||||
| Abou-Alfa, 2018 (44) | Cabozantinib | 470 | 10.2 | NR | 5.2 | 18 (4) | Yes, for efficacy |
| Placebo | 237 | 8.0 | 1.9 | 1 (<1) | |||
| HR 0.76; 95% CI 0.63–0.92; p = 0.005 | HR 0.44; 95% CI 0.36–0.52; p <0.001 | p = 0.009 | |||||
| Ramucirumab + BSC vs. placebo + BSC | |||||||
| REACH – Zhu, 2015 (38) | Ramucirumab + BSC | 283 (277) | 9.2 | 3.5 | 2.8 | 20 (7) | No |
| Placebo + BSC | 282 (276) | 7.6 | 2.6 | 2.1 | 2 (<1.0) | No | |
| HR 0.87; 95% CI 0.72–1.05; p = 0.14 | HR 0.59; 95% CI 0.49–0.72; p <0.0001 | HR 0.63; 95% CI 0.52–0.75; p <0.0001 | p <0.0001 | ||||
| REACH-2, 2019 (40) | Ramucirumab | 197 | 8.5 | 2.8 | 9 (5) | ||
| Placebo + BSC | 95 | 7.3 | 1.6 | 1 (1) | |||
| HR 0.710; 95% CI 0.53–0.95; p = 0.0199 | HR 0.452; 95% CI 0.34–0.60; p <0.0001 | p = 0.1697 | |||||
| ADI-peg 20 + BSC vs. placebo + BSC | |||||||
| Abou-Alfa, 2018 (37) | ADI-peg 20 + BSC | 424 | 7.8 | NR | 2.6 | 2 (<1.0) | No |
| Placebo + BSC | 211 | 7.4 | 2.6 | 6 (2.8) | |||
| HR 1.022; 95% CI 0.847–1.233; p = 0.884 | HR 1.175; 95% CI 0.964–1.432; p = 0.075 | p = NR | |||||
| S-1 vs. placebo | |||||||
| S-CUBE, 2017 (45) | S-1 | 222 | 11.1 | 2.6 | 2.6 | 12 (5) | No |
| Placebo | 111 | 11.2 | 1.4 | 1.4 | 1 (1) | ||
| HR 0.86; 95% CI 0.067–1.10; p = 0.220 | HR 0.59; 95% CI 0.46–0.76; p <0.0001 | HR 0.60; 95% CI 0.46–0.77; p <0.0001 | p = 0.068 | ||||
| Brivanib + BSC vs. placebo + BSC | |||||||
| BRISK-PS – Llovet, 2013 (46) | Brivanib + BSC | 263 (261) | 9.4 | 4.2 | NR | 10 | No |
| Placebo + BSC | 132 (131) | 8.2 | 2.7 | 2 | |||
| HR 0.89; 95% CI 0.69–1.15; p = 0.3307 | HR 0.56; 95% CI 0.42–0.76; p <0.001 | p = 0.0030 | |||||
| Tivantinib vs. placebo | |||||||
| Santoro, 2013 (47) | Tivantinib | 71 | 6.6 | 1.6 | 1.5 | 3 | No |
| Placebo | 36 | 6.2 | 1.4 | 1.4 | 0 | ||
| HR 0.90; 95% CI 0.57–1.40; p = 0.63 | HR 0.64; 90% CI†, 0.43–0.94; p = 0.04 | HR 0.67; 95% CI 0.44–1.04; p = 0.06 | |||||
| Rimassa, 2018 (48) | Tivantinib | 226 | 8.4 | 2.4 | 2.1 | NR | No |
| Placebo | 114 | 9.1 | 3.0 | 2.0 | |||
| HR 0.97; 95% CI 0.75–1.25; p = 0.81 | HR 0.96; 95% CI 0.74–1.25; p = 0.76 | HR 0.96; 95% CI 0.75–1.22; p = 0.72 | |||||
| RO5137382/GC33 vs. placebo | |||||||
| Yen, 2014 (49), abstract | RO5137382/GC33 | 121 | 6.8 | 2.9 | 2.6 | NR | No |
| Placebo | 64 | 6.7 | 1.7 | 1.5 | |||
| p = 0.99 | p = 0.85 | p = 0.87 | |||||
| Everolimus + BSC vs. placebo + BSC | |||||||
| Zhu, 2014 (50) | Everolimus + BSC | 362 | 7.6 | NR | NR | 2.2 | No |
| Placebo + BSC | 184 | 7.3 | NR | 1.6 | |||
| HR 1.05; 95% CI 0.86–1.27; p = 0.68 | HR 0.93; 95% CI 0.75–1.15; p = ns | p = NR | |||||
| Pembrolizumab + BSC vs. placebo + BSC | |||||||
| Finn, 2019 (51) | Pembrolizumab + BSC | 278 | 13.9 | 3.8 | 3.0 | 51 (18.3) | No |
| Placebo + BSC | 135 | 10.6 | 2.8 | 2.8 | 6 (4.4) | ||
| HR 0.78; 95% CI 0.61–1.00; p = 0.0238‡ | HR 0.69; 95% CI 0.54–0.88; p = 0.0011 | HR 0.71; 95% CI 0.57–0.90; p = 0.0022‡ | p = 0.00007 | ||||
Note this is a 90% confidence interval
Did not meet pre-specified boundaries for statistical significance set prior to the start of the trial
OS = Overall survival; TTP = Time to progression; PFS = Progression-free survival; ORR = Objective response rate; BSC = Best supportive care; HR = Hazard ratio; CI = Confidence interval; NR = Not reported; ns = Not significant
Cabozantinib versus placebo
One full publication of the phase III CELESTIAL trial of second- or third-line cabozntinib versus placebo was retained (44). Median OS (HR 0.76; 95% CI 0.63–0.92; p = 0.005), median PFS (HR 0.44; 95% CI 0.36–0.52, p <0.001) and ORR (4% versus < 1%; p = 0.009) were significantly better in the cabozantinib arm (Table 2). Grade 3/4 toxicity was greater in the cabozantinib arm compared with the placebo arm (68% versus 36%), including for hand-foot skin reaction (17% versus 0%), hypertension (16% versus 2%), fatigue (10% versus 4%), and diarrhea (10% versus 2%). No p values are reported.
Ramucirumab/BSC versus placebo/BSC
Two full publications (38,39) of the REACH trial were retained as well as one full publication (40) of the REACH-2 trial (Table 2). REACH is a phase III trial that compared second-line ramucirumab + BSC to placebo + BSC. Each of these REACH trial publications reports different outcomes. Zhu et al (38) report the main findings of the REACH trial. There was no significant difference between the groups with respect to median OS (HR 0.87; 95% CI 0.72–1.05; p = 0.14). The ramucirumab arm was significantly better than the placebo arm with respect to median PFS (HR 0.63; 95% CI 0.52–0.75; p <0.0001), median TTP (HR 0.59; 95% CI 0.49–0.72; p <0.0001), and ORR (7% versus <1%, p <0.0001). Although no p values are reported, ascites, hypertension, asthenia, and thrombocytopenia occurred more often in the ramucirumab arm. In contrast, increased AST, hyperbilirubinemia, and increased blood bilirubin occurred more often in the placebo group. Chau et al (39) reported participant-focused outcomes from the REACH trial using the FACT Hepatobiliary Symptom Indexes. There were no significant differences between the two arms of the trial. Therefore, treatment with ramucirumab did not lead to any improvement or impairment with respect to symptoms or participant functioning.
REACH-2 (40) was a phase III RCT of ramucirumab versus placebo/BSC in participants with elevated alpha-fetoprotein (AFP) at ≥400 ng/mL following first-line sorafenib. Median OS (HR 0.710; 95% CI 0.53–0.95; p = 0.0199) and median PFS (HR 0.452; 95% CI 0.34–0.60, p <0.0001) were both significantly better in the ramucirumab arm compared with placebo. There was no significant difference in ORR (Table 2).
All other second-line systemic therapy
All other second-line systemic therapy regimens including ADI-peg 20/BSC (37), S-1 (45), brivanib/BSC (46), tivantinib (47,48), RO5137382/GC33 (49), everlimus/BSC (50), and pembrolizumab (51) were non-significant or too small to make any conclusions about (Table 2).
Discussion
The majority of those with newly diagnosed HCC are not eligible for curative therapies, including local or regional ablative therapies, hepatic resection, or transplant. Previous guidelines have reviewed the evidence for local or regional ablative therapies (54,55). In this systematic review, we reviewed the current evidence for treatment options for advanced, unresectable HCC. We focused on two areas: TACE and systemic therapies.
TACE
Following the treatment of local or regional therapies, there is no evidence to support the addition of sorafenib following this. The majority of these studies were small and of moderate to poor quality. Following the failure of local or regional therapies, those suitable for systemic therapy should be considered for treatment.
Even though some of the studies demonstrated an advantage for TACE/sorafenib over TACE alone with respect to TTP and PFS, this did not always ultimately translate into a survival advantage. It is not known that better TTP/PFS always translates to OS. It is possible that subsequent treatments will confound these endpoints. It is also likely that the timing of treatment (ex sorafenib pre-, post-, or concurrently with TACE) is an important factor. Finally, TACE protocols are heterogeneous and it becomes difficult to compare different studies.
Systemic therapies
For those who are either ineligible for local or regional therapies or have progressed following them, the number of systemic therapies now available has increased since earlier in the decade. First-line systemic therapy options for unresectable or metastatic HCC include sorafenib, lenvatinib, and atezolizumab + bevacizumab. In addition, the PD-L1 nivolumab is being compared with sorafenib in an active clinical trial (NCT0257650).
In the second-line setting, both regorafenib and cabozantinib have received approval by the US Food and Drug Administration (FDA) (the latter based on abstract publication only). In addition, nivolumab has received provisional approval (FDA/Health Canada) based on the response rates seen.
Gaps in knowledge
There are definite gaps in knowledge in the existing literature. Studies typically categorize patients by BCLC staging. For example, BCLC B with a large or multifocal HCC may have a worse prognosis than a small volume disease with a single metastatic lung or nodal metastasis. However, this prognostication is not well captured. Moreover, trials do not evaluate real-world experiences in treating patients; however, the concept of multi-modality therapy has been proposed by several groups (56,57). Nuanced sequencing of appropriate local and systemic therapy has not been addressed in any studies, but this approach likely more accurately portrays real-world, multidisciplinary teams.
Conclusions
There is no evidence to support the addition of sorafenib to any local or regional therapy. Single-agent sorafenib or lenvatinib, or a combination of atezolizumab + bevacizumab, are recommended for first-line systemic treatment of intermediate-stage HCC. Regorafenib or cabozantinib provide survival benefits when given as second-line treatment after progression on sorafenib. With immunotherapy combinations becoming a new standard of care, the assumptions regarding localized and sequencing lines of therapies will need further study. More active systemic regimens should move earlier in the treatment course for HCC patients. Lessons learned from the many studies reviewed systemically in this paper will help guide the next important questions.
Acknowledgements:
The authors would like to thank the following individuals for their assistance in developing this report: Melissa Brouwers, Laurie Elit, Ted Hong, Donna Maziak, Sheila McNair, Morris Sherman, and Emily Vella for providing feedback on draft versions; Jillian Sing for conducting a data audit; and Sara Miller for copy editing.
Ethics Approval:
N/A
Informed Consent:
N/A
Registry and Registration No. of the Study/Trial:
N/A
Funding:
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The Program in Evidence-Based Care (PEBC) is a provincial initiative of Ontario Health (Cancer Care Ontario) supported by the Ontario Ministry of Health. All work produced by the PEBC is editorially independent of the Ontario Ministry of Health.
Disclosures:
Dr Meyers reports other compensation from Amgen, grants and other compensation from Astra-Zeneca, other compensation from Bayer, other compensation from BMS, grants and other compensation from Eisai, other compensation from Ipsen, other compensation from Merck, other compensation from Roche, other compensation from Sanofi Genzyme, other compensation from Taiho, grants from Sillajen, grants from Galera, grants from GSK, grants from Exelixis, outside the submitted work; Dr Knox reports research support for investigator initiated trials from Merck, Ibsen and Astra Zeneca, consulting fees from Roche, Pfizer, Merck, Eisai and Insen outside the submitted work; Dr Feld reports grants and personal fees from AbbVie, personal fees from Arbutus, personal fees from Entanta, grants and personal fees from Gilead, personal fees from GSK, personal fees from Roche, grants from Alexion, grants from Eiger, grants from Janssen, grants from Waka/Fujifilm, outside the submitted work. The remaining authors have nothing to disclose.
Peer Review:
This article has been peer reviewed.
References
- 1.Canadian Cancer Society's Steering Committee on Cancer Statistics. Canadian Cancer Statistics 2019 [Internet]. Toronto: Canadian Cancer Society; 2019. [cited 2019 Sep]. Available from: https://www.cancer.ca/~/media/cancer.ca/CW/cancer%20information/cancer%20101/Canadian%20cancer%20statistics/Canadian-Cancer-Statistics-2019-EN.pdf?la=en. [Google Scholar]
- 2.Cancer Today [Internet]. Cancer fact sheet: Liver and intrahepatic bile ducts. Lyon, FR: International Agency for Research in Cancer; 2018. [cited 2019 Sep; updated 2020 Dec]. Available from: http://gco.iarc.fr/today/fact-sheets-cancers. [Google Scholar]
- 3.Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182(18):E839–42. 10.1503/cmaj.090449. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10. 10.1186/1471-2288-7-10. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kan X, Jing Y, Wan QY, et al. Sorafenib combined with percutaneous radiofrequency ablation for the treatment of medium-sized hepatocellular carcinoma. Eur Rev Med Pharmacol Sci. 2015;19(2):247–55. https://www.europeanreview.org/article/8409. Medline: [PubMed] [Google Scholar]
- 6.Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47(14):2117–27. 10.1016/j.ejca.2011.05.007. Medline: [DOI] [PubMed] [Google Scholar]
- 7.Sansonno D, Lauletta G, Russi S, Conteduca V, Sansonno L, Dammacco F. Transarterial chemoembolization plus sorafenib: a sequential therapeutic scheme for HCV-related intermediate-stage hepatocellular carcinoma: a randomized clinical trial. Oncologist. 2012;17(3):359–66. 10.1634/theoncologist.2011-0313. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kudo M, Ueshima K, Torimura T, et al. Randomized, open label, multicenter, phase II trial of transcatheter arterial chemoembolization (TACE) therapy in combination with sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. J Clin Oncol. 2018;36(15_suppl):206. 10.1200/JCO.2018.36.15_suppl.4017. [DOI] [Google Scholar]
- 9.Park JW, Kim YJ, Kim DY, et al. Sorafenib with versus without concurrent conventional transarterial chemoembolization (cTACE) in patients with advanced hepatocellular carcinoma (HCC): results from a multicenter, open-label, randomized, controlled phase III STAH trial. J Hepatol. 2018;68 (Supplement 1): S2. 10.1016/S0168-8278(18)30222-8. [DOI] [Google Scholar]
- 10.Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: the SPACE trial. J Hepatol. 2016;64(5):1090–8. 10.1016/j.jhep.2016.01.012. Medline: [DOI] [PubMed] [Google Scholar]
- 11.Meyer T, Fox R, Ma YT, et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2(8):565–75. 10.1016/S2468-1253(17)30156-5. Medline:. [DOI] [PubMed] [Google Scholar]
- 12.Cainap C, Qin S, Huang WT, et al. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol. 2015;33(2):172–9. 10.1200/JCO.2013.54.3298. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–73. 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 14.Han KH, Qin S, Piscaglia F, et al. Efficacy and safety of lenvatinib for unresectable hepatocellular carcinoma in patients with baseline hepatitis B virus (HBV) [abstract]. Hepatology. 2017;66(S1):740A-1A. https://journals.scholarsportal.info/browse/02709139/v66is1. [Google Scholar]
- 15.Vogel A, Qin S, Kudo M, et al. Health-related quality of life (HRQOL) and disease symptoms in patients with unresectable hepatocellular carcinoma (HCC) treated with lenvatinib (LEN) or sorafenib (SOR). Value Health. 2017;20(9):A454-A5. 10.1016/j.jval.2017.08.318. [DOI] [Google Scholar]
- 16.Vogel A, Qin S, Kudo M, et al. Health-related quality of life (HRQOL) and disease symptoms in patients with unresectable hepatocellular carcinoma (HCC) treated with lenvatinib (LEN) or sorafenib (SOR). Hepatology. 2017;66(Supplement 1):734A. 10.1093/annonc/mdx369.002. [DOI] [Google Scholar]
- 17.Vogel A, Qin S, Kudo M, et al. Health-related quality of Life (HRQOL) and disease symptoms in patients with unresectable hepatocellular carcinoma (HCC) treated with lenvatinib (LEN) or sorafenib (SOR). Ann Oncol. 2017;28(Supplement 5):v210. https://www.sciencedirect.com/science/article/pii/S0923753420378923. [Google Scholar]
- 18.Cheng AL, Kang YK, Lin DY, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31(32):4067–75. 10.1200/JCO.2012.45.8372. Medline: [DOI] [PubMed] [Google Scholar]
- 19.Yen CJ, Kim TY, Feng YH, et al. A phase I/randomized phase II study to evaluate the safety, pharmacokinetics, and efficacy of nintedanib versus sorafenib in Asian patients with advanced hepatocellular carcinoma. Liver Cancer. 2018;7(2):165–78. 10.1159/000486460. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmer DH, Ma YT, Peck-Radosavljevic M, et al. Randomized phase II trial comparing the efficacy and safety of nintedanib versus sorafenib in patients with advanced hepatocellular carcinoma (HCC). J Clin Oncol. 2015;33(3_Suppl). 10.1200/jco.2015.33.3_suppl.238. [DOI] [Google Scholar]
- 21.Johnson PJ, Qin S, Park JW, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31(28):3517–24. https://ascopubs.org/doi/10.1200/JCO.2012.48.4410. [DOI] [PubMed] [Google Scholar]
- 22.Wahab MA, Shaker M, Wahab SA, Elbassiouny M, Ellithy M, Abdelrahman O. Sorafenib versus oral fluoropyrimidines in the management of advanced hepatocellular carcinoma [abstract]. Ann Oncol. 2012;23(Suppl_4):iv52. 10.1016/S0923-7534(20)30278-7. [DOI] [Google Scholar]
- 23.Yau T, Park JW, Finn RS, et al. CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC) [abstract]. Ann Oncol. 2019;30(5_suppl):v874. 10.1093/annonc/mdz394.029. [DOI] [Google Scholar]
- 24.Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Eng J Med. 2020;382(20):1894–905. 10.1056/NEJMoa1915745. Medline: [DOI] [PubMed] [Google Scholar]
- 25.Ettrich T, Perkofer L, Berger AW, et al. Sorafenib plus doxorubicin versus sorafenib alone for advanced hepatocellular carcinoma (Soradox trial): Final results of a prospective, randomized, open-label, multicenter phase IIb trial. Eur J Cancer. 2015;51(Suppl_3):S457. 10.1016/S0959-8049(16)31279-5. [DOI] [Google Scholar]
- 26.Abou-Alfa GK, Shi Q, Knox JJ, et al. Assessment of treatment with sorafenib plus doxorubicin vs sorafenib alone in patients with advanced hepatocellular carcinoma: phase 3 CALGB 80802 randomized clinical trial. JAMA Oncol. 2019;5(11):1582–8. 10.1001/jamaoncol.2019.2792. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Assenat E, Pageaux GP, Thezenas S, et al. Sorafenib alone vs. sorafenib plus GEMOX as 1st-line treatment for advanced HCC: the randomized PRODIGE 10 trial. Br J Cancer. 2019;120(9):896–902. 10.1038/s41416-019-0443-4. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng AL, Kang YK, He AR, et al. Safety and efficacy of tigatuzumab plus sorafenib as first-line therapy in subjects with advanced hepatocellular carcinoma: a phase 2 randomized study. J Hepatol. 2015;63(4):896–904. 10.1016/j.jhep.2015.06.001. Medline: [DOI] [PubMed] [Google Scholar]
- 29.Ciuleanu T, Bazin I, Lungulescu D, et al. A randomized, double-blind, placebo-controlled phase II study to assess the efficacy and safety of mapatumumab with sorafenib in patients with advanced hepatocellular carcinoma. Ann Oncol. 2016;27(4):680–7. 10.1093/annonc/mdw004. Medline: [DOI] [PubMed] [Google Scholar]
- 30.Koeberle D, Dufour JF, Demeter G, et al. Sorafenib with or without everolimus in patients with advanced hepatocellular carcinoma (HCC): a randomized multicenter, multinational phase II trial (SAKK 77/08 and SASL 29). Ann Oncol. 2016;27(5):856–61. 10.1093/annonc/mdw054. Medline: [DOI] [PubMed] [Google Scholar]
- 31.Lee FA, Zee BC, Cheung FY, et al. Randomized phase II study of the X-linked inhibitor of apoptosis (XIAP) antisense AEG35156 in combination with sorafenib in patients with advanced hepatocellular carcinoma (HCC). Am J Clin Oncol. 2016;39(6): 609–13. 10.1097/COC.0000000000000099. Medline: [DOI] [PubMed] [Google Scholar]
- 32.Thomas MB, Garrett-Mayer E, Anis M, et al. A randomized phase II open-label multi-institution study of the combination of bevacizumab and erlotinib compared to sorafenib in the first-line treatment of patients with advanced hepatocellular carcinoma. Oncology. 2018;94(6):329–39. 10.1159/000485384. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu AX, Rosmorduc O, Evans TR, et al. SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015;33(6):559–66. 10.1200/JCO.2013.53.7746. Medline: [DOI] [PubMed] [Google Scholar]
- 34.Blanc JF, Khemissa F, Bronowicki JP, et al. Results of the phase II randomized French trial PRODIGE 21 comparing sorafenib vs pravastatin vs sorafenib and pravastatin vs best supportive care for the palliative treatment of HCC in CHILD B cirrhotic patients. J Hepatol. 2018;68(Suppl_1):S195. 10.1016/S0168-8278(18)30601-9. [DOI] [Google Scholar]
- 35.Tak WY, Ryoo BY, Lim HY, et al. Phase I/II study of first-line combination therapy with sorafenib plus resminostat, on oral HDAC inhibitor, versus sorafenib monotherapy for advanced hepatocellular carcinoma in east Asian patients. Invest New Drugs. 2018;36(6):1072–84. 10.1007/s10637-018-0658-x. Medline: [DOI] [PubMed] [Google Scholar]
- 36.Azim HA, Omar A, Atef H, et al. Sorafenib plus tegafur–uracil (UFT) versus sorafenib as first line systemic treatment for patients with advanced stage HCC: a phase II trial (ESLC01 study). J Hepatocell Carcinoma. 2018;5: 109–19. 10.2147/JHC.S169285. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abou-Alfa GK, Qin S, Ryoo BY, et al. Phase III randomized study of second line ADI-PEG 20 plus best supportive care versus placebo plus best supportive care in patients with advanced hepatocellular carcinoma. Ann Oncol. 2018;29(6):1402–8. 10.1093/annonc/mdy101. Medline: [DOI] [PubMed] [Google Scholar]
- 38.Zhu AX, Park JO, Ryoo BY, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16(7):859–70. 10.1016/S1470-2045(15)00050-9. [DOI] [PubMed] [Google Scholar]
- 39.Chau I, Peck-Radosavljevic M, Borg C, et al. Ramucirumab as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib: Patient-focused outcome results from the randomised phase III REACH study. Eur J Cancer. 2017;81:17–25. 10.1016/j.ejca.2017.05.001. Medline:. Erratum in: Eur J Cancer. 2018; 100:135–36. Corrected at: . [DOI] [PubMed] [Google Scholar]
- 40.Zhu AX, Kang Y-K, Yen C-J, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282–96. 10.1016/S1470-2045(18)30937-9. Medline: [DOI] [PubMed] [Google Scholar]
- 41.Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. 10.1016/S0140-6736(16)32453-9. Medline: [DOI] [PubMed] [Google Scholar]
- 42.Waldschmidt D, Granito A, Merle P, et al. Overall survival (OS) update: 2-year follow-up from the phase-3 RESORCE trial of regorafenib for patients with hepatocellular carcinoma (HCC) progressing on sorafenib. Z Gastroenterol. 2019;57(9):e265. 10.1055/s-0039-1695314. [DOI] [Google Scholar]
- 43.Bruix J, Merle P, Granito A, et al. Efficacy, safety, and health-related quality of life (HRQoL) of regorafenib in patients with hepatocellular carcinoma (HCC) progressing on sorafenib: results of the international, double-blind phase 3 RESORCE trial. Ann Oncol. 2016;27(Suppl_6):VI564. 10.1093/annonc/mdw435.19. [DOI] [Google Scholar]
- 44.Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54–63. 10.1056/NEJMoa1717002. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kudo M, Moriguchi M, Numata K, et al. S-1 versus placebo in patients with sorafenib-refractory advanced hepatocellular carcinoma (S-CUBE): a randomised, double-blind, multicentre, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2(6):407–17. 10.1016/S2468-1253(17)30072-9. [DOI] [PubMed] [Google Scholar]
- 46.Llovet JM, Decaens T, Raoul JL, et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol. 2013;31(28):3509–16. https://ascopubs.org/doi/10.1200/JCO.2012.47.3009. [DOI] [PubMed] [Google Scholar]
- 47.Santoro A, Rimassa L, Borbath I, et al. Tivantinib for second-line treatment of advanced hepatocellular carcinoma: a randomised, placebo-controlled phase 2 study. Lancet Oncol. 2013;14(1):55–63. 10.1016/S1470-2045(12)70490-4. Medline: [DOI] [PubMed] [Google Scholar]
- 48.Rimassa L, Assenat E, Peck-Radosavljevic M, et al. Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): a final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol. 2018;19(5):682–93. 10.1016/S1470-2045(18)30146-3. Medline: [DOI] [PubMed] [Google Scholar]
- 49.Yen CJ, Daniele B, Kudo M, et al. Randomized phase II trial of intravenous RO5137382/GC33 at 1600 mg every other week and placebo in previously treated patients with unresectable advanced hepatocellular carcinoma. J Clin Oncol. 2014;32(15_suppl):4102. 10.1200/jco.2014.32.15_suppl.4102.25403208 [DOI] [Google Scholar]
- 50.Zhu AX, Kudo M, Assenat E, et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA. 2014;312(1):57–67. 10.1001/jama.2014.7189. Medline: [DOI] [PubMed] [Google Scholar]
- 51.Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-bline, phase III trial. J Clin Oncol. 2019;38(3):193–202. 10.1200/JCO.19.01307. Medline: [DOI] [PubMed] [Google Scholar]
- 52.Ma MX, Adams L, Garas G, et al. Selective internal radiation therapy for hepatocellular carcinoma: combination with sorafenib is associated with improved survival outcomes. J Hepatol. 2014;60(1):S211. 10.1016/S0168-8278(14)60592-4. [DOI] [Google Scholar]
- 53.Maccauro M, Sposito C, Chiesa C, et al. Trans-arterial radioembolization (TARE) with Y90 glass microspheres plus sorafenib versus tare alone for the treatment of unresectable hepatocellular carcinoma (HCC): a matched case-control study. In: Proceedings of EANM'14, Annual Congress of the European Association of Nuclear Medicine; 2014 Oct 18-22, Gothenburg, Sweden. In: Eur J Nucl Med Mol Imaging. 2014;41(2):S291. https://link.springer.com/journal/259/volumes-and-issues/41-2/supplement. [Google Scholar]
- 54.Baldassarre FG, Baerlocher M, Beecroft R, Dawson LA. Focal tumor ablation: thermal ablation of hepatocellular carcinoma and metastases from colorectal carcinoma [Internet]. Toronto, ON: Cancer Care Ontario; 2014. Available from: https://www.cancercareontario.ca/en/guidelines-advice/types-of-cancer/941. [Google Scholar]
- 55.Menard A, Baldassare FG, Martel G, Kachura J. Focal tumour ablation: Transarterial chemoembolization for hepatocellular carcinoma [Internet]. Toronto, ON: Cancer Care Ontario; 2015. Available from: https://www.cancercareontario.ca/en/guidelines-advice/types-of-cancer/901. [Google Scholar]
- 56.Cardarelli-Leite L, Hadjivassiliou A, Klass D, et al. Current locoregional therapies and treatment strategies in hepatocellular carcinoma. Curr Oncol. 2020;27(Suppl_3):S144-51. Epub 2020 Nov 1. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kudo M, Han K-H, Ye S-L, et al. A changing paradigm for the treatment of intermediate-stage hepatocellular carcinoma: Asia-Pacific primary liver cancer expert consensus statements. Liver Cancer. 2020;9(3):245–60. Epub 2020 May 13. 10.1159/000507370. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]


