Abstract
Liver transplantation has been historically recommended for patients with congenital absence of the portal vein associated with extrahepatic congenital portosystemic shunts. Here, based on a case report of a 2-year-old girl and a thorough review of all published cases from 1974 to 2020, we show that such a diagnosis most often conceals a hypoplastic portal vein, which can be successfully re-permeabilized through the closure of the shunt in order to re-establish a physiological vascular anatomy. This highlights the importance of achieving a detailed anatomical description of extrahepatic congenital portosystemic shunts with a balloon occlusion test in order to plan the best surgical approach and avoid unnecessary liver transplantation.
Keywords: Abernethy malformation, portal vein, portosystemic shunts, vascular malformations
Introduction
Congenital portosystemic shunts (CPSS) are rare vascular malformations causing the venous blood from the mesenteric and splenic circulations to bypass the liver and drain directly into the systemic circulation. CPSS are silent in most cases. However, patients may present hypoglycemia, hyperammonemia, and jaundice in the neonatal period, and are at risk of pulmonary hypertension, hepatic encephalopathy, and liver tumours later in life (1,2). The age of diagnosis is variable, ranging from prenatal life to adulthood (3,4). There are two categories of shunts: intrahepatic, which may resolve spontaneously; and extrahepatic, which usually require surgical correction and, in some cases, liver transplantation (2,5). Extrahepatic CPSS, also known as Abernethy malformations, are classified into two types: type I, exhibiting no visible portal flow in the liver (end-to-side portocaval fistula); and type II, consisting of a partial deviation of the portal flow (side-to-side portocaval fistula) (6,7). Type I shunts, which are often referred to as congenital absence of the portal vein (CAPV), can also be divided into two subtypes according to the anatomy of the shunt (1). The most frequent is subtype b, which is characterized by the superior mesenteric vein and splenic vein joining to form a common vessel that drains into the inferior vena cava. In contrast, in subtype a, the superior mesenteric vein and splenic vein drain separately into the inferior vena cava (1,7). A different classification, suggested by Blanc et al, differentiates extrahepatic shunts originating from any root of the portal vein (corresponding to Abernethy type Ia shunts) from portocaval shunts (8). The latter are further divided in end-to-side portocaval shunts (ESPC, grossly corresponding to Abernethy type Ib), and side-to-side and H-type portocaval shunts (mostly falling within Abernethy type II shunts). Although Blanc’s classification is more anatomically accurate and directly correlates anatomy to a proposed surgical approach, here we decided to use Abernethy’s classification because of its widespread use allowing the analysis and comparison of previously published literature.
Surgical or percutaneous closure is usually feasible only for type II extrahepatic CPSS (1). Liver transplantation has been considered as the standard therapeutic procedure for type I shunts (so-called CAPV). More recently, the therapeutic approach was suggested to be tailored not only on the anatomy of the shunt but on the presence of a patent intrahepatic portal tract (7). Here, based on a case report of a 2-year-old girl and a thorough review of all published cases since 1974, we show that CAPV diagnosis often conceals a hypoplastic portal vein, which can be successfully repermeabilized through the closure of the shunt in order to re-establish a physiological vascular anatomy.
Case presentation
A 2-year-old girl was referred to our tertiary pediatric hepatology centre for liver transplantation, subsequent to a recent diagnosis of CAPV. Her medical history reported type 1 Arnold–Chiari malformation with a ventriculoperitoneal shunt. A diagnosis of CAPV was made after finding no portal flow and identifying the presence of an extrahepatic portocaval shunt at a Doppler ultrasound of the abdomen performed to assess her ventriculoperitoneal shunt.
When first evaluated at our facility, the patient was asymptomatic and presented a hypertrophic left liver lobe, without any signs of chronic liver disease. The results of her blood tests were in the normal range except for a moderately elevated serum ammonia level (70 µM). Contrast-enhanced abdominal magnetic resonance imaging (MRI) confirmed the presence of a large Abernethy malformation type Ib, with superior mesenteric and splenic veins joining to form a short portal vessel connected to the inferior vena cava through a large (14 mm) end-to-side shunt (an end-to-side portocaval shunt according to Blanc’s classification). There were no other associated malformations, nor pulmonary hypertension or liver tumour detected. A percutaneous venogram confirmed the absence of the portal vein (Figure 1A–B). We performed a direct catheterization of the shunt through the inferior vena cava and carried out a temporary balloon occlusion, which allowed the visualization of a hypoplastic portal vein originating from the posterior face of the shunt (Figure 1C). A two-step closure procedure was chosen because of the high post-closure portal pressure (36 mmHg), in order to avoid hemodynamic complications and portal hypertension (5).
Figure 1:
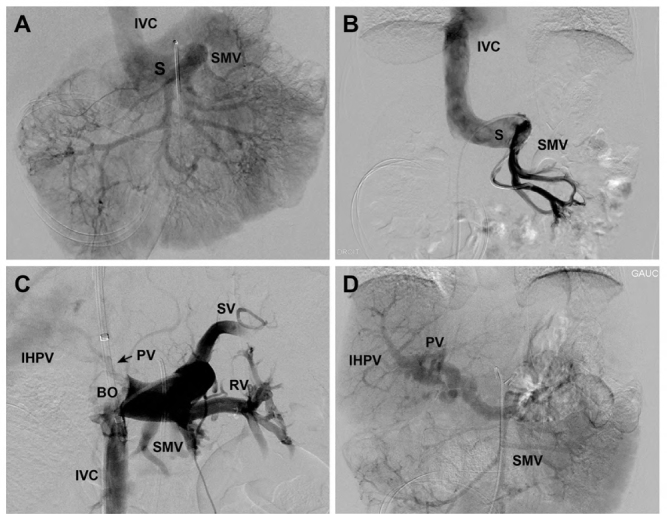
Venogram images of the extrahepatic portosystemic shunt
(A) Angiography performed through the right femoral artery. Selective catheterism of the superior mesenteric artery with an injection of contrast medium shows a direct communication (S) of the SMV and the IVC without opacification of the intrahepatic portal venous system. (B) Cavography performed by femoral venous approach. Selective catheterism of the superior mesenteric vein via the shunt (S) showed no opacification of the portal vein and the IHPV branches. (C) Temporary balloon occlusion. A long 10 Fr introducer placed in the right jugular vein with the extremity in the IVC. At the junction of the shunt in the IVC, we installed a BO (20 mm) via the introducer. The proximal shunt was catheterized with a 4 Fr glide catheter introduced via a femoral vein approach. Opacification of the portovenous system via the 4 Fr catheter during the inflation of the balloon, showed the SV, the IVC, the SMV, and the RV. Also, the temporary balloon occlusion of the shunt allowed the identification of a very thin PV with a “puff of smoke”-like appearance (arrow). (D) Assessing shunt 8 months post-surgery. Superior mesenteric artery contrast injection demonstrated the complete closure of the extrahepatic portosystemic shunt with a tortuous but patent PV and IHPV system.
SMV = Superior mesenteric vein; IVC = Inferior vena cava; IHPV = Intrahepatic portal venous; BO =Balloon occlusion; SV = Splenic vein; RV = Renal vein; PV = Portal vein
A partial banding (about 50% of the shunt diameter) was carried out without significant complications (normal liver tests, minimal transient ascites). Intravenous heparin was administered after surgery to prevent thrombosis of the banded shunt. After the procedure, the portal flow was re-established and showed a gradual increase at follow-up Doppler ultrasounds. One week after the procedure, heparin was replaced by subcutaneous low molecular weight heparin for a period of 3 months. Ten weeks after the procedure, mild ascites, as well as a moderate elevation of liver enzymes (3 times the upper limit of the normal), were detected. The shunt was no longer visible at ultrasound, probably due to thrombotic occlusion. Ascites resolved spontaneously within a few weeks, and transaminase levels normalized. A control ultrasound showed a normal portal flow and no sign of the shunt.
Six months after the surgical procedure, a new percutaneous venogram confirmed the complete closure of the shunt and full permeability of the portal vein (Figure 1D). The child was still asymptomatic 4 years after the procedure, and all the lab tests remained normal.
Literature Review and Discussion
We identified 385 reported cases of extrahepatic CPSS published from 1974 to 2019 (including the case just described), 204 of which were classified as type I portosystemic shunts, and 159 as type II. In 22 cases, the type of shunt could not be determined. Type I subtype b was predominant (124/204, 60.8%), while subtype a shunts represented 14.2% (29/204) of cases. Lack of anatomical details prevented us from classifying 51 cases.
The only recognized treatment for type I shunts was historically considered to be liver transplantation (1,9–13). Over the last 20 years, some teams have started practicing percutaneous venograms with temporary shunt occlusions, not only in type II but also in type I shunts (5,7,13–23). Balloon occlusion allows the detection of the intrahepatic portal vein when present, which is otherwise not perfused because of the shunt. It is interesting to note that this technique was performed in only 26% (53/204) of the reports, including our case. Nevertheless, when occlusion was performed, a portal vein was detected in 81.1% (43/53) of such cases. The newly identified portal vein was described in most cases (31/43) as hypoplastic (5,7,13–23). This description varied from mild (portal vein well visualized after balloon occlusion), to severe (little or no visualization of the portal vein, “puff of smoke”-like or thread-like appearance) (8,24). When the portal vein was identified, the shunt could be successfully closed in 81.4% (35/43) of the cases, with very few complications.
CAPV was diagnosed in 152 patients without performing any occlusion test. Thirty-eight of them (25%) ended up receiving a liver transplant (which is supposedly an underestimation, as 50% of the published reports do not describe patient outcome). On the contrary, among all the patients who underwent an occlusion test, only 5 (9.4%) were transplanted, and only one of them had the intrahepatic portal vein visible upon balloon occlusion.
The aim of this work was to highlight the importance of precise anatomic assessment of extrahepatic CPSS. We described a child with suspected CAPV referred for liver transplantation who was instead successfully treated by surgical banding of her portocaval shunt, a relatively minor procedure. When confronted with a suspected diagnosis of CAPV, practitioners should always question the true absence of the portal vein (which is rarer than initially believed in children) by performing a percutaneous venogram with temporary shunt occlusion. A clear anatomical description of extrahepatic CPSS with balloon occlusion test is always warranted to plan the best surgical approach. A single- or two-step surgical/percutaneous closure should be performed if the portal vein is identified, to re-establish the portal flow. Closure during early childhood can potentially prevent the progressive atresia of the portal vein, and eventually the need for liver transplantation.
Funding Statement
N Laverdure was supported by scholarships from “Réunion des Pédiatres de Rhone Alpes Auvergne” and CHU Sainte-Justine Foundation. M Paganelli is a Junior 1 Research Scholar of the Fonds de Recherche du Québec – Santé (FRQS).
Ethics Approval:
N/A
Informed Consent:
Informed consent was obtained from the patient.
Registry and the Registration No. of the Study/Trial:
N/A
Funding:
N Laverdure was supported by scholarships from “Réunion des Pédiatres de Rhone Alpes Auvergne” and CHU Sainte-Justine Foundation. M Paganelli is a Junior 1 Research Scholar of the Fonds de Recherche du Québec – Santé (FRQS).
Disclosures:
The authors have no potential conflicts of interest to disclose related to this manuscript. M Paganelli is co-founder, shareholder, and interim CEO of the cell therapy company Morphocell Technologies Inc., but no conflict of interest exists regarding this article.
Peer Review:
This article has been peer reviewed.
References
- 1.Ghuman SS, Gupta S, Buxi TB, et al. The Abernethy malformation-myriad imaging manifestations of a single entity. Indian J Radiol Imaging. 2016;26(3):364–72. 10.4103/0971-3026.190420. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paganelli M, Lipsich JE, Sciveres M, Alvarez F. Predisposing factors for spontaneous closure of congenital portosystemic shunts. J Pediatr. 2015;167(4):931–5.e12. 10.1016/j.jpeds.2015.06.073. Medline: [DOI] [PubMed] [Google Scholar]
- 3.Pohl A, Jung A, Vielhaber H, et al. Congenital atresia of the portal vein and extrahepatic portocaval shunt associated with benign neonatal hemangiomatosis, congenital adrenal hyperplasia, and atrial septal defect. J Pediatr Surg. 2003;38(4):633–4. 10.1053/jpsu.2003.50140. Medline: [DOI] [PubMed] [Google Scholar]
- 4.Takeuchi H, Takeda Y, Takahashi M, Hayashi S, Fukuzawa Y, Nakano T. A rare congenital extrahepatic portosystemic shunt affecting the inferior mesenteric vein, inferior vena cava, and left ovarian vein. Surg Radiol Anat. 2014;36(7):729–32. 10.1007/s00276-013-1230-1. Medline: [DOI] [PubMed] [Google Scholar]
- 5.Bernard O, Franchi-Abella S, Branchereau S, Pariente D, Gauthier F, Jacquemin E. Congenital portosystemic shunts in children: recognition, evaluation, and management. Semin Liver Dis. 2012;32(4):274–87. 10.1055/s-0032-1329896. Medline: [DOI] [PubMed] [Google Scholar]
- 6.Abernethy J, Banks J. 1997 IX. Account of two instances of uncommon formation, in the viscera of the human body. Philos Trans R Soc. 1793;83:59–66. 10.1098/rstl.1793.0010. [DOI] [Google Scholar]
- 7.Morgan G, Superina R. Congenital absence of the portal vein: two cases and a proposed classification system for portasystemic vascular anomalies. J Pediatr Surg. 1994(9);29:1239–41. 10.1016/0022-3468(94)90812-5. Medline: [DOI] [PubMed] [Google Scholar]
- 8.Blanc T, Guerin F, Franchi-Abella S, et al. Congenital portosystemic shunts in children: a new anatomical classification correlated with surgical strategy. Ann Surg. 2014; 260(1):188–98. 10.1097/SLA.0000000000000266. Medline: [DOI] [PubMed] [Google Scholar]
- 9.Wojcicki M, Post M, Pakosz-Golanowska M, et al. Vascular complications following adult piggyback liver transplantation with end-to- side cavo-cavostomy: a single-center experience. Transplant Proc. 2009;41(8):3131–4. 10.1016/j.transproceed.2009.07.092. Medline: [DOI] [PubMed] [Google Scholar]
- 10.Charre L, Roggen F, Lemaire J, et al. Hematochezia and congenital extrahepatic portocaval shunt with absent portal vein: successful treatment by liver transplantation. Transplantation. 2004;78(9):1404–6. 10.1097/01.TP.0000137931.51504.F7. Medline: [DOI] [PubMed] [Google Scholar]
- 11.Shinkai M, Ohhama Y, Nishi T, et al. Congenital absence of the portal vein and role of liver transplantation in children. J Pediatr Surg. 2001;36(7):1026–31. 10.1053/jpsu.2001.24731. Medline: [DOI] [PubMed] [Google Scholar]
- 12.Elias N, Scirica CV, Hertl M. Liver transplantation for the Abernethy malformation. N Engl J Med. 2008; 358(8):858. 10.1056/NEJMc0707762. Medline: [DOI] [PubMed] [Google Scholar]
- 13.Emre S, Arnon R, Cohen E, Morotti RA, Vaysman D, Shneider BL. Resolution of hepatopulmonary syndrome after auxiliary partial orthotopic liver transplantation in Abernethy malformation. A case report. Liver Transpl. 2007;13(12):1662–8. 10.1002/lt.21349. Medline: [DOI] [PubMed] [Google Scholar]
- 14.Franchi-Abella S, Branchereau S, Lambert V, et al. Complications of congenital portosystemic shunts in children: therapeutic options and outcomes. J Pediatr Gastroenterol Nutr. 2010; 51(3):322–30. 10.1097/MPG.0b013e3181d9cb92. Medline: [DOI] [PubMed] [Google Scholar]
- 15.Yonemitsu H, Mori H, Kimura T, et al. Congenital extrahepatic portocaval shunt associated with hepatic hyperplasic nodules in a patient with Dubin-Johnson syndrome. Abdom Imaging. 2000;25(6):572–5. 10.1007/s002610000044. Medline: [DOI] [PubMed] [Google Scholar]
- 16.Kimura T, Soh H, Hasegawa T, et al. Laparoscopic correction of congenital portosystemic shunt in children. Surg Laparosc Endosc Percutan Tech. 2004;14(5):285–8. 10.1097/00129689-200410000-00012. Medline: [DOI] [PubMed] [Google Scholar]
- 17.Sanada Y, Mizuta K, Kawano Y, et al. Living donor liver transplantation for congenital absence of the portal vein. Transplant Proc. 2009;41(10):4214–9. 10.1016/j.transproceed.2009.08.080. Medline: [DOI] [PubMed] [Google Scholar]
- 18.Law YM, Mack CL, Sokol RJ, Rice M, Parsley L, Ivy D. Cardiopulmonary manifestations of portovenous shunts from congenital absence of the portal vein: pulmonary hypertension and pulmonary vascular dilatation. Pediatr Transplant 2011;15(8):E162–8. 10.1111/j.1399-3046.2010.01355.x. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruckheimer E, Dagan T, Atar E, et al. Staged transcatheter treatment of portal hypoplasia and congenital portosystemic shunts in children. Cardiovasc Intervent Radiol. 2013; 36(6):1580–5. 10.1007/s00270-013-0581-7. Medline: [DOI] [PubMed] [Google Scholar]
- 20.Stewart JK, Kuo WT, Hovsepian DM, Hofmann LV, Bonham CA, Sze DY. Portal venous remodeling after endovascular reduction of pediatric autogenous portosystemic shunts. J Vasc Interv Radiol. 2011;22(8):1199–205. 10.1016/j.jvir.2011.01.438. Medline: [DOI] [PubMed] [Google Scholar]
- 21.Kanazawa H, Nosaka S, Miyazaki O, et al. The classification based on intrahepatic portal system for congenital portosystemic shunts. J Pediatr Surg. 2015;50(4):688–95. 10.1016/j.jpedsurg.2015.01.009. Medline: [DOI] [PubMed] [Google Scholar]
- 22.Matsuura T, Takahashi Y, Yanagi Y, et al. Surgical strategy according to the anatomical types of congenital portosystemic shunts in children. J Pediatr Surg. 2016;51(12):2099–104. 10.1016/j.jpedsurg.2016.09.046. Medline: [DOI] [PubMed] [Google Scholar]
- 23.Mori T, Yamada Y, Abe K, et al. Laparoscopic partial closure for congenital portosystemic shunt-indications, postoperative management, and subsequent complete closure. J Laparoendosc Adv Surg Tech A. 2019;29(4): 573–8. 10.1089/lap.2018.0581. Medline: [DOI] [PubMed] [Google Scholar]
- 24.Kanazawa H, Nosaka S, Miyazaki O, et al. The classification based on intrahepatic portal system for congenital portosystemic shunts. J Pediatr Surg. 2015(4);50:688–95. 10.1016/j.jpedsurg.2015.01.009. Medline: [DOI] [PubMed] [Google Scholar]


