9TH CANADIAN SYMPOSIUM ON HCV—FRIDAY, FEBRUARY 28, 2020
SESSION #1: BIOMEDICAL RESEARCH
Differential gene expression of circulating CD8 T cells of cirrhotic HCV-infected individuals identifies pathways associated with lasting dysfunction
The effect of apoptosis and inflammasome-mediated pyroptosis on HCV infection
Identifying the role played by the poly(rC)-binding protein 2 (PCBP2) in the HCV life cycle
Understanding NK and T cell dysfunction in chronic HCV patients with advanced liver fibrosis by immunoprofiling of inhibitory receptors
Precision cut liver slice (PCLS) culture: a model to examine HCV interactions with the liver microenvironment
Hepatitis C virus exploits cyclophilin A to evade PKR- and IRF1-dependent antiviral responses
Neutrophils are the major producers of the pro-fibrogenic cytokine IL-17A in non-alcoholic fatty liver disease (NAFLD)
Quantifying the relative contributions of the three roles of miR-122 in the HCV life cycle
Immune restoration of hepatitis C virus-specific T cells following direct acting antiviral therapy in acute hepatitis C virus–infected patients
The use of oncolytic measles-based vectors for targeted treatment of HCV-induced liver cancer
HCV 3’UTR and the host helicases DDX1 and DDX3X
The role of community-based specialized pharmacies in provincial hepatitis C elimination
SESSION #2: SOCIAL, CULTURAL, ENVIRONMENTAL, AND POPULATION HEALTH RESEARCH
Effect of sustained virologic response and opioid agonist therapy on mortality among people living with chronic hepatitis C
Anticipated timing of elimination of hepatitis C virus in Canada’s four most populous provinces
Community and corrections based point-of-care testing into a provincial hepatitis C elimination framework
Integrating community-based recruitment with data linkage to inform and enhance scale-up of HCV treatment among people who inject drugs in Canada: The VCCC study protocol
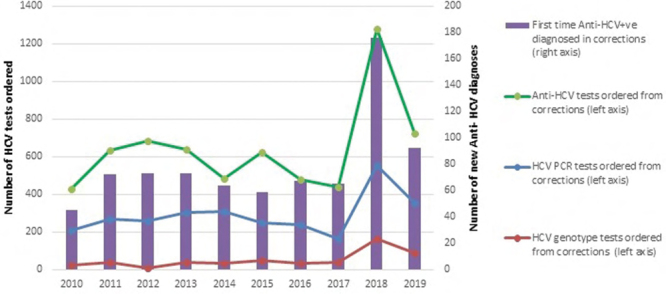
Increasing hepatitis C screening & new diagnoses in 10 British Columbia provincial correctional centres from 2010–2019
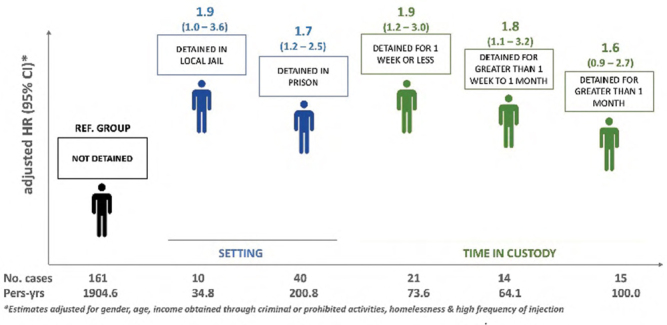
Diversity of detention patterns among people who inject drugs and the associated risk with incident hepatitis C virus (HCV) infection: Implications for hepatitis C prevention
Gender-specific associations between psychological distress and HCV risk behaviours among people who inject drugs in Montreal
Estimation of an individual-level deprivation index for HIV/HCV coinfected persons
Geographic distribution of people living with hepatitis C in British Columbia: An application of latent class analysis and disease mapping
Hepatitis C awareness, screening and linkage to care among the Pakistani community in Montreal: The Aagahi Project
Mapping the Immigrant population and cultural and community organizations to inform community outreach and HCV microelimination efforts in Montreal
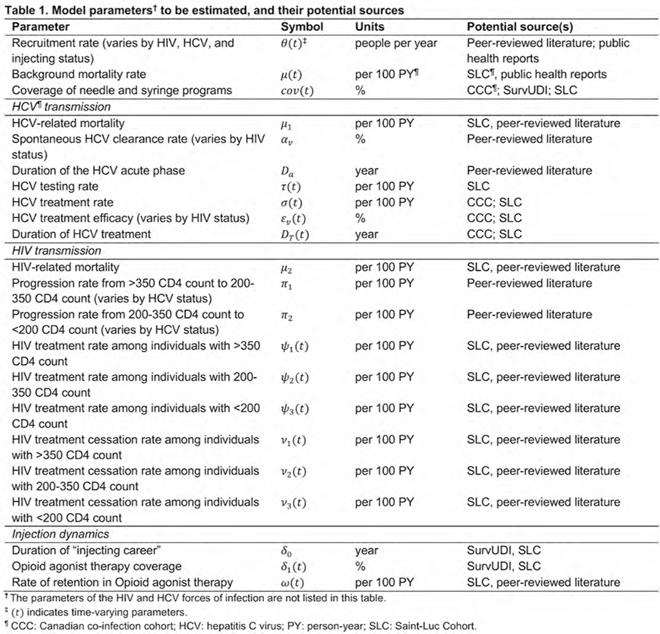
Reviewing, appraising, and synthesizing observational data to inform dynamic mathematical modeling of HCV and HIV transmission among people who inject drugs in Montréal, Canada
Potential impacts of closing low-threshold overdose prevention sites on HCV and HIV prevention efforts in Toronto, Canada
Perceptions of HCV treatment and reinfection risk among HIV-positive men who have sex with men in Sydney, Australia: A qualitative study
Toward the elimination of HCV vertical transmission: A targeted, patient-informed hepatitis C engagement program in persons who use drugs in their child bearing years in southern New Brunswick
Peer led point-of-care testing: The role of Atlantic Canada’s first overdose prevention site in hepatitis C elimination
Strengthening Canada’s hepatitis C response by producing culturally and linguistically relevant resources for Canadian immigrants and their service providers
Estimating direct-acting antiviral impact among key populations in Ontario: A research proposal
The synthesis and integration of hepatitis C clinical practice guidelines to facilitate low threshold access to evidence-based care in the outreach setting
HCV: The neurocognitive impact in the overdose epidemic
SESSION #3: CLINICAL RESEARCH
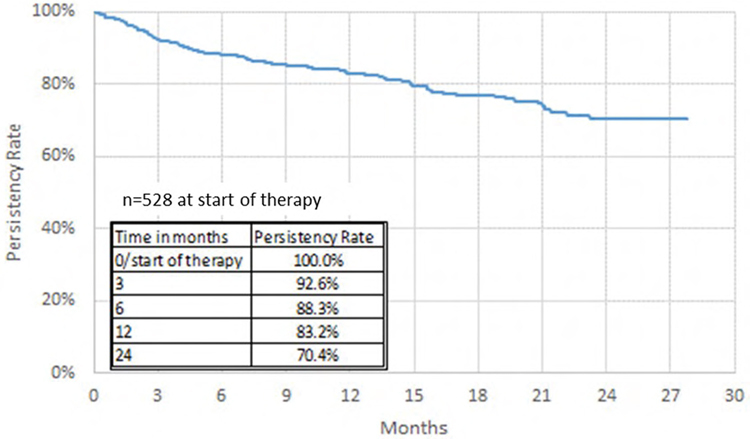
Efficacy of sofosbuvir/velpatasvir (S/V): impact of treatment adherence
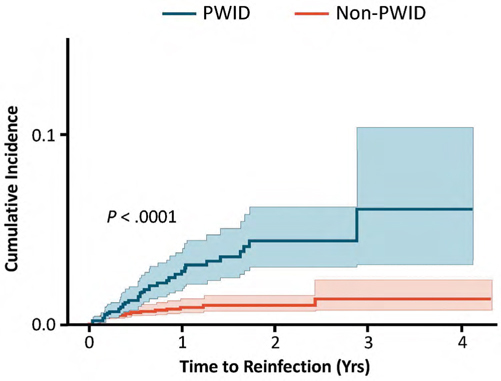
Reinfection following successful direct-acting antiviral therapy for hepatitis C infection among people who inject drugs
Non-invasive surrogates of portal hypertension predict decompensation in obese patients with compensated advanced chronic liver disease
Impact of treatment with tenofovir alafenamide (TAF) or tenofovir disoproxil fumarate (TDF) on hepatocellular carcinoma (HCC) incidence in patients with chronic hepatitis B (CHB)
Universal HCV and HIV screening in an emergency room – fewer new cases than expected
DAA treatment uptake or outcomes are not effected by alcohol use: A CANUHC analysis
SESSION #4: HEALTH SERVICES RESEARCH
Can we afford to screen and treat hepatitis C virus (HCV) infection in Canada? Latest insight from a Canadian policy model – A province-by-province analysis
HBV-HCV coinfection among immigrants in Ontario, Canada
A review of public reimbursement criteria for pan-genotypic HCV DAAs in Canada
Pilot peer-led hepatitis C screening leads to high testing uptake and rapid treatment initiation in a women’s residential recovery facility
Disparities in health utilities among hepatitis C patients receiving care in different settings
Health services impact analysis of simplifying HCV diagnosis and treatment decision in Canada
Hepatitis C (HCV) re-engagement strategy after loss to follow-up
Building capacity in hepatitis C management: progress and outcomes from the ECHO Ontario liver program
Examining patient complexity in ECHO: Results from a hepatitis C telementoring education program
The Canadian network on hepatitis C virtual cascade of care cohort (VCCC) feasibility study – Saskatchewan component
CANADIAN LIVER MEETING—SATURDAY, FEBRUARY 29–SUNDAY, MARCH 1, 2020
SESSION #1: VIRAL HEPATITIS
Achieving functional cure of HBV and HBV / HDV co-infection with REP 2139: Completed follow-up in the REP 401 and REP 301-LTF studies
Micro-elimination of hepatitis C in a population of opioid substitution clients – successful task-shifting of testing and treatment to a community-based nurse/pharmacist dyad
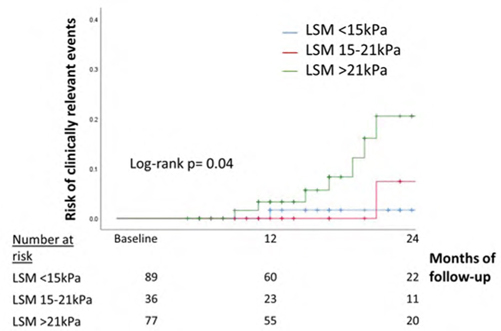
Role of pre- and post-treatment transient elastography measurements in predicting hepatocellular carcinoma (HCC) among hepatitis C patients treated with direct acting antivirals (DAA)
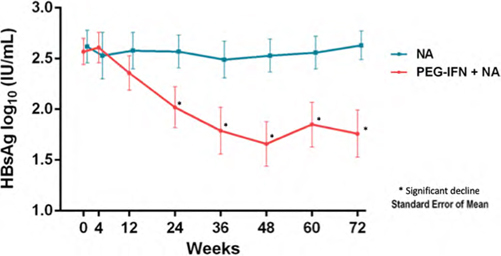
Addition of peginterferon alfa 2a increases HbsAg decline in HbeAg-negative chronic hepatitis B patients treated with long-term nucleos(t)ide analogue therapy: Results from a multicenter randomized controlled trial (PAS study)
Real-world drug resistance profile of hepatitis C patients who failed direct-acting antivirals – SHARED
Hepatitis C–positive organ transplantation to negative recipients at a multiorgan Canadian transplant centre: Ready for prime time
Reducing the burden of hepatitis C-related complications through genomics-guided treatment optimization
Outcomes of hepatitis C treatment are similar in Canadian Indigenous people compared to non-Indigenous patients
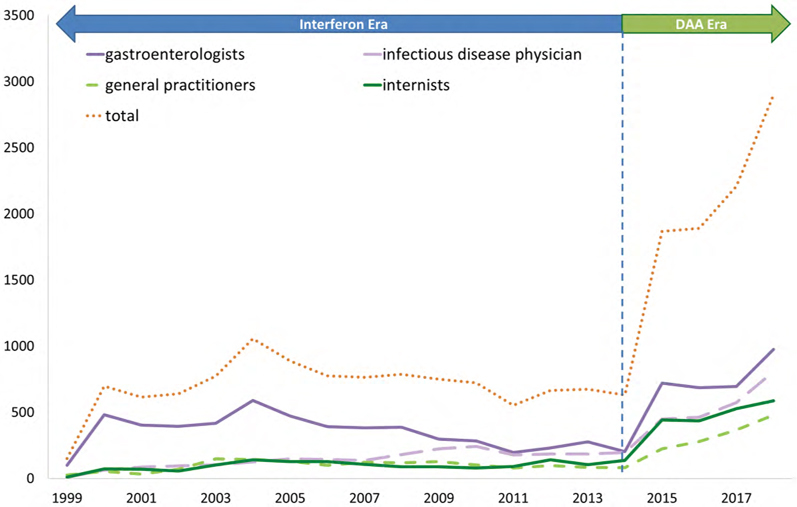
Treatment differential in HCV treatment prescribers in British Columbia over time
Characteristics of resistance-associated substitutions in “unusual” hepatitis C virus (HCV) subtypes
Comparison of hepatitis C prenatal screening approaches between provinces: Seroprevalence and infection rates in universal vs risk-based screening
Factors associated with on-demand HCV screening among Canadian provincial inmates
Real world single centre experience on the efficacy of stopping long term nucleos(t)ide analog therapy in patients with chronic hepatitis B (CHB)
Reticulon-3 modulates the loading of replication competent hepatitis C virus molecules for release inside infectious exosomes
Birth cohort screening for hepatitis C during routine outpatient endoscopy
Mechanistic analysis of miR-122 promotion of HCV replication
Identification of patients with compensated cirrhosis who can safely use protease inhibitor-based therapy for HCV infection
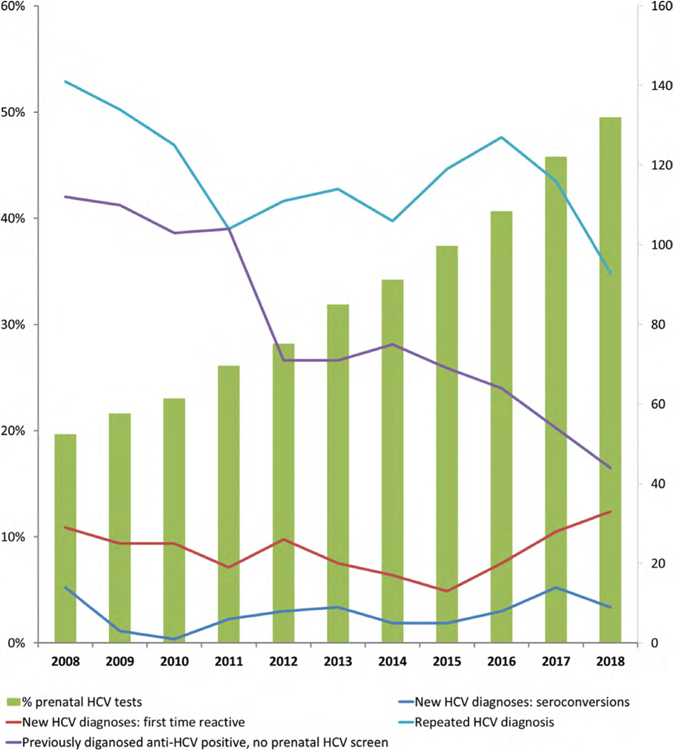
Prenatal hepatitis C screening and diagnoses in British Columbia, 2008–2018
Targeting Hepatitis B virus cccDNA in vitro
Exploiting cccDNA’s structural features to find new HBV targets
NAFLD and alcohol affect long-term liver fibrosis regression post-HCV eradication
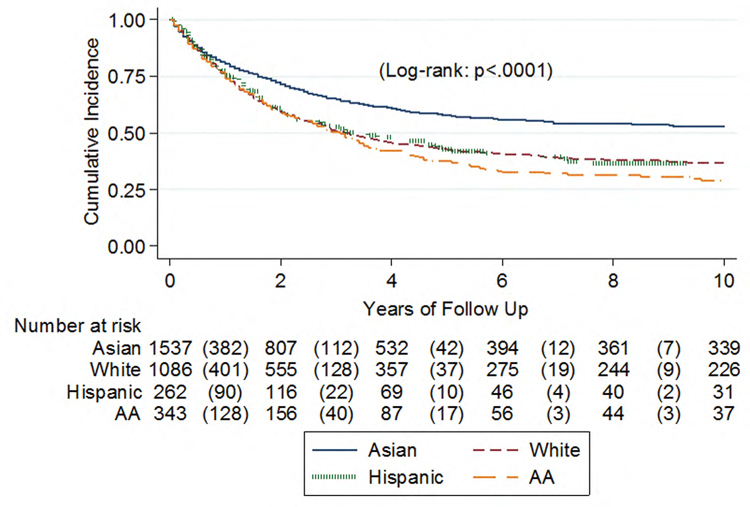
Ethnic differences in HCV-related HCC (HCV-HCC) outcomes: Report from the real-world evidence by the Asia Pacific Rim liver consortium for HCC (REAL-HCC)
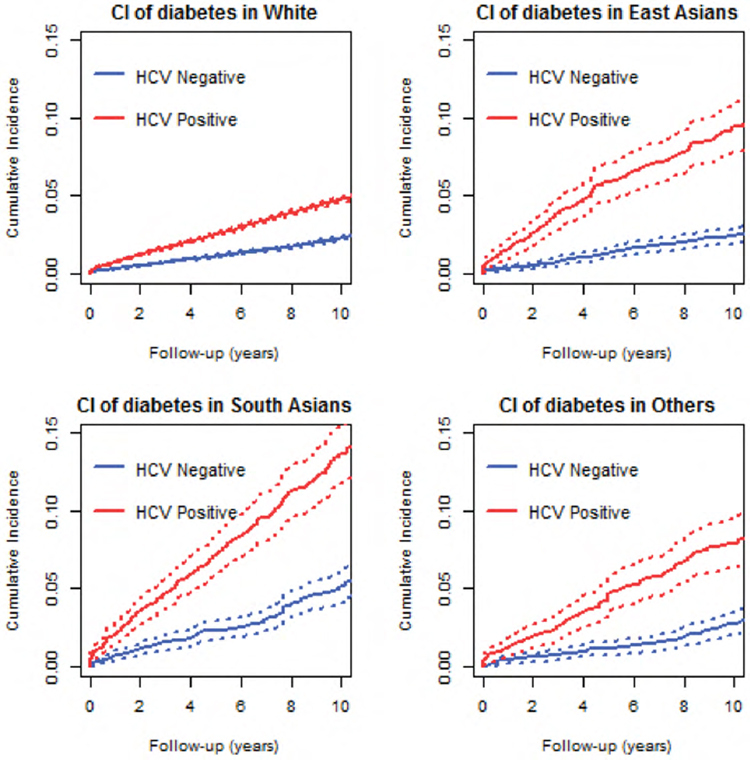
Ethnic disparities in the risk of hepatitis C virus-related diabetes in a large population-based cohort in Canada
Natural history of cirrhotic people who use drugs (PWUD) following successful HCV therapy in the direct-acting antiviral (DAA) era
Real-world effectiveness of sofosbuvir/velpatasvir/voxilaprevir as a hepatitis C virus infection salvage treatment
Current opioid agonist therapy is associated with hepatitis C virus treatment uptake among people who inject drugs in a population-based data linkage study
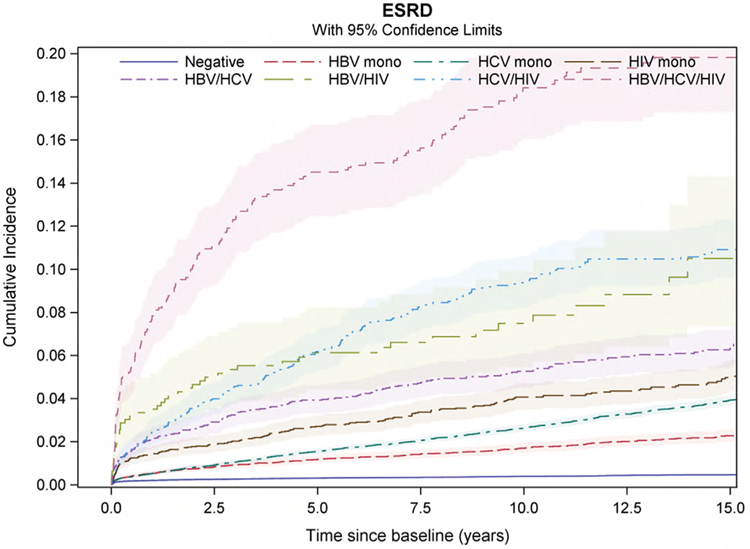
Syndemic of viral co-infections and incident end-stage renal disease
Genotype misclassification and its impact on treatment choices, outcomes and drug resistance
Association between prescription opioids and hepatitis C virus seroconversion among people who use injection drugs in British Columbia
Evidence for a striking failure in diagnosis and linkage to care for hepatitis C-infected patients in Alberta, Canada
Prescribing trends of direct acting antivirals (DAAs) for the treatment of hepatitis C in Ontario
CD8 T cell dysfunction and cancer development in a murine model of liver fibrosis
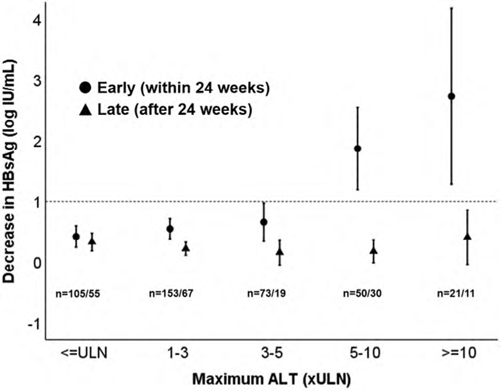
Early peg-interferon-related ALT flares of high magnitude lead to HbsAg decline and loss. A study of 639 chronic hepatitis B patients
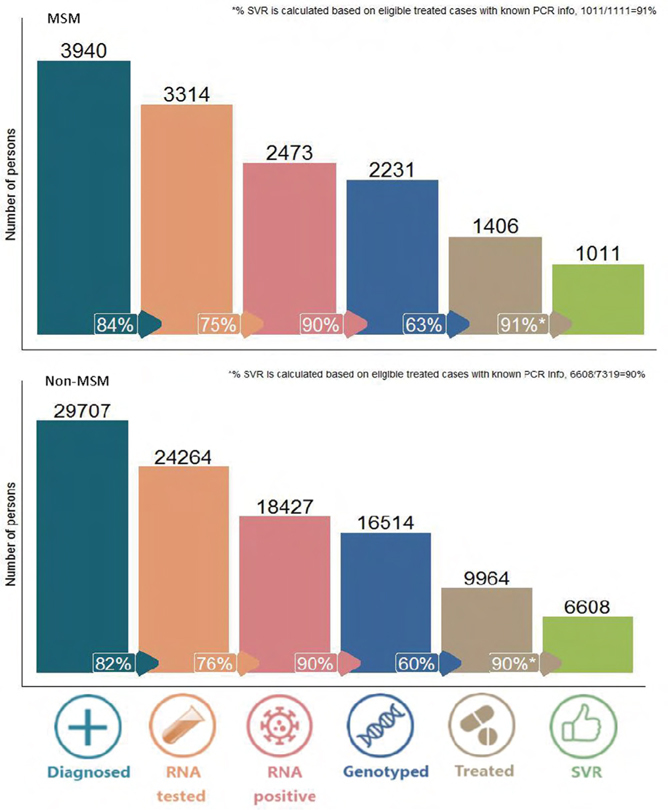
Population-level hepatitis C cascade of care among men who have sex with men in British Columbia, Canada
Live animal imaging of oncolytic virus infection in non-cancer cells; how this might affect therapeutic outcome
Differential hepatitis B virus (HBV) and hepatitis D virus (HDV) specific T cell response in HDV RNA positive and negative patients
Differences in surveillance for HCC in HIV infected patients with and without HCV/HBV coinfection: Insights from LIVEHIV cohort
Long-term follow-up of people who use drugs cured of hepatitis C infection: re-infection and re-treatment
Transaminase flares during HbsAg reduction to <1 IU/mL are correlated with the establishment of virologic control and functional cure of HBV following NAP-based combination therapy
Hepatitis C virus reinfection after successful treatment with direct acting antiviral therapy in British Columbia
Glecaprevir/pibrentasvir for the treatment of hepatitis C virus infection among active drug users: The GRAND PLAN study
Overdose events among active drug users successfully treated for HCV: The impact of homelessness
Prevalence and genotype of occult hepatitis B infection in a human immunodeficiency virus (HIV) positive patient cohort in Gondar, Ethiopia
Trends in hepatocellular carcinoma survival among individuals infected with HBV and/or HCV in British Columbia, Canada (2001–2016)
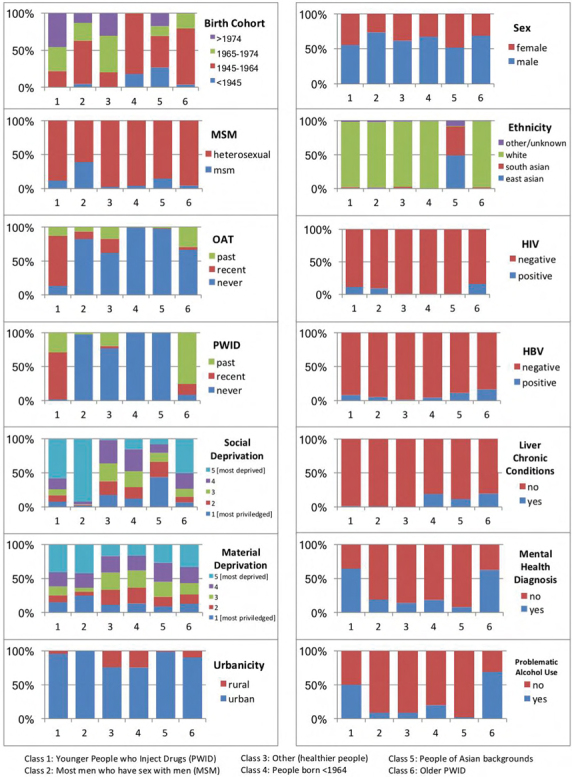
A population-level latent class analysis of people living with hepatitis C virus for effective program planning and health care resource distribution
Sofosbuvir/velpatasvir (S/V) for the treatment of chronic HCV in active drug users: The CHIME study
Clinical evaluation of cholesterolic metabolism on the background of biliary insufficiency in patients with chronic hepatitis C
Who are the real transformational leaders? From peer educators to peer navigators
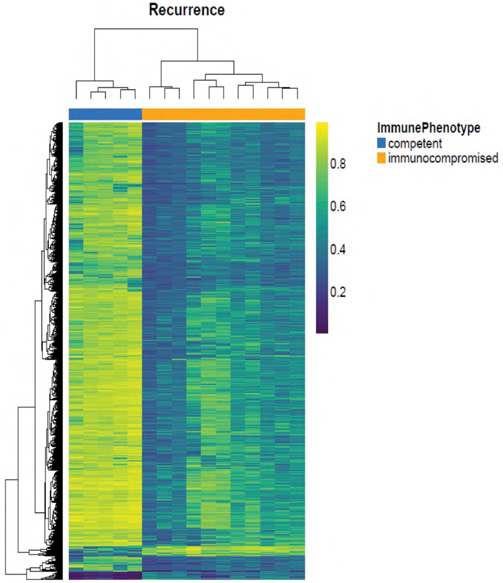
Transcriptomic analyses of the immune response during HCV re-infection
Association between hepatitis B virus infection and risk of non-alcoholic fatty liver disease: a meta-analytic synthesis of observational studies
MicroRNA-122 promotion of Hepatitis C Virus translation is important early in the HCV infection cycle to initiate an HCV infection
Improved linkage to care by targeting HCV RNA(+) persons in the BC-HCV network
The impact of small RNA binding on hepatitis C virus replication via structural changes within the 5’ untranslated region
Can neurological weakness be the first presentation of chronic hepatitis in Immunosuppressed population? Case report and literature review
SESSION #2: GENERAL HEPATOLOGY
Differences in clonal evolution of recurrent hepatocellular carcinoma depending on the immune environment
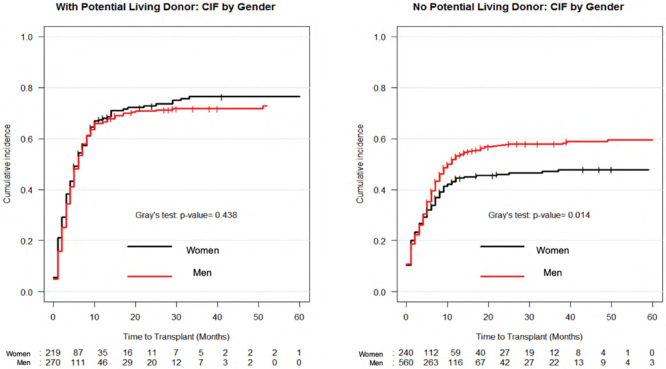
Women likely to benefit from having a potential living liver donor compared to men
Durable response in the markers of cholestasis through 5 years of open-label extension study of obeticholic acid in primary biliary cholangitis
Preventive effect of celecoxib in sorafenib-related hand-foot syndrome in hepatocellular carcinoma patients, a single-center, open-label, randomized, controlled clinical phase III trial
Noninvasive tests (NITs) may more accurately quantify fibrosis than liver histology in patients with advanced fibrosis due to NASH
Health-related quality of life —A rapid independent predictor of hospitalizations and mortality in cirrhosis
IL-16 as a new marker for the diagnosis of AIH/PBC overlap syndrome
Hypofibrinolysis as a contributing mechanism of cirrhotic portal vein thrombosis as evidenced by rotational thromboelastometry (ROTEM)
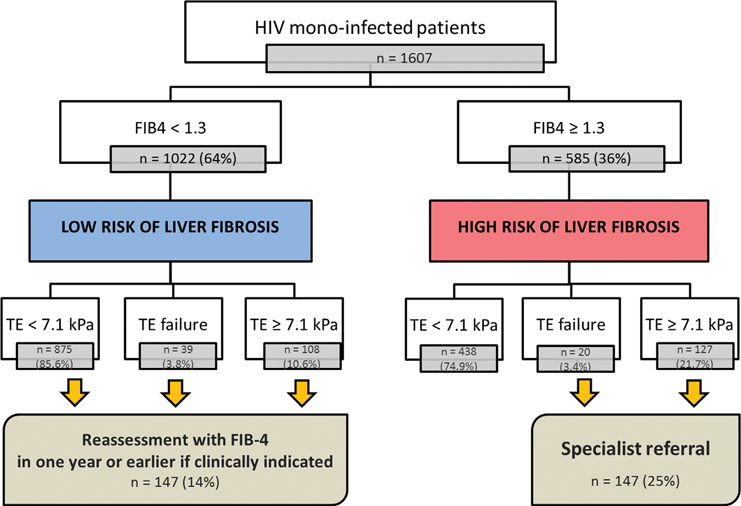
“FIB-4 First” strategy in a NAFLD assessment pathway for HIV mono-infected patients
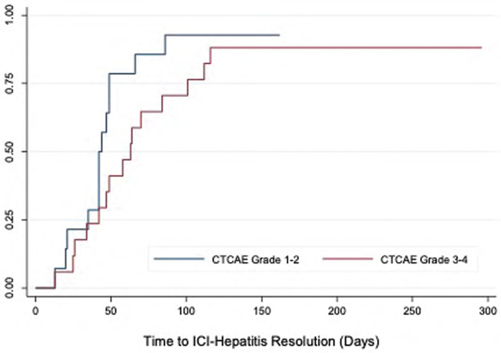
Combined regimen immune checkpoint inhibitor-associated hepatitis: Experience from a North American multicenter cohort
Hemojuvelin deficiency predisposes mice to hepatocellular carcinoma
Simple non-invasive prediction of advanced fibrosis in NAFLD—A stepwise approach and external validation study to reduce indeterminates and biopsy
Impaired hepatic leukocyte recruitment and increased thrombin generation during acute bacterial challenge in a mouse model of non-alcoholic fatty liver disease (NAFLD)
Modelling non-alcoholic fatty liver disease burden in Canada, 2019–2030
Lenvatinib for the first-line treatment of advanced or unresectable hepatocellular carcinoma: A cost-effectiveness analysis from a Canadian perspective
Reducing length of stay in patients following a liver transplant
The role of transferrin receptor 1 (Tfr1) in liver iron sensing and systemic iron homeostasis
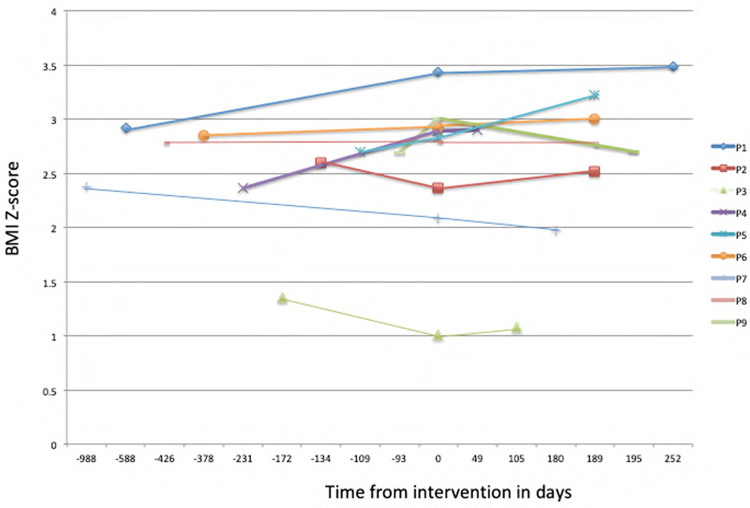
A new score including anthropometric measurement for weight Improved prediction of mortality of adolescents on the liver transplantation waiting list: US nationwide study
Performance of noninvasive fibrosis tests among NAFLD patients with normal ALT: Data from a large North American primary care NAFLD pathway
Impact of repeat transient elastography within 3 years on clinical management in chronic liver disease
Evolution of autoimmune cholangitis and primary sclerosing cholangitis in a pediatric cohort
Tolerogenic effect of pregnancy in autoimmune hepatitis
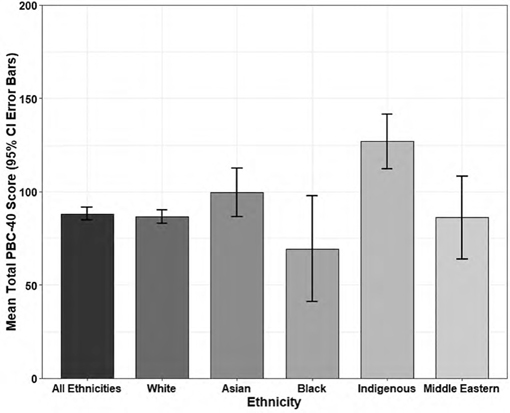
Symptom burden in patients living with primary biliary cholangitis: Indigenous Canadians report significantly higher PBC-40 quality of life scores
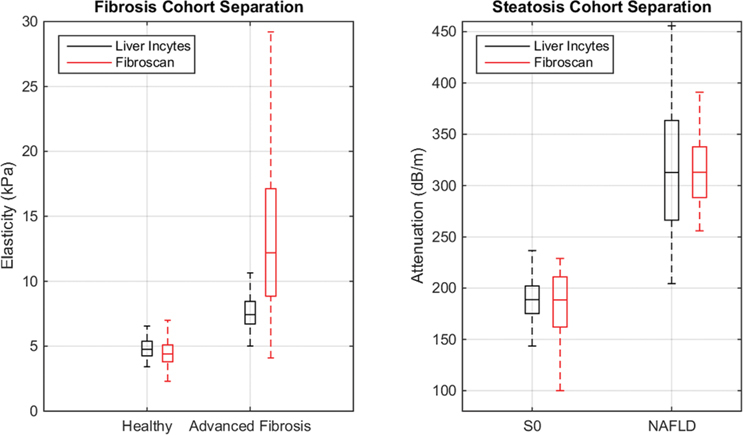
Assessment of fibrosis and steatosis in patients and healthy volunteers
The burden of cirrhosis on the Canadian health care system: A comparison between alcoholic and nonalcoholic cirrhosis patients
Impact of depression and antidepressant use on the development of chronic liver disease: a longitudinal UK population-based study
Baseline liver function and outcomes in the phase III REFLECT study in patients with unresectable hepatocellular carcinoma (uHCC) treated with lenvatinib (LEN) vs sorafenib (SOR)
Ultrasound surface and hepatic vein nodularity as predictors of histologic advanced fibrosis in chronic liver disease
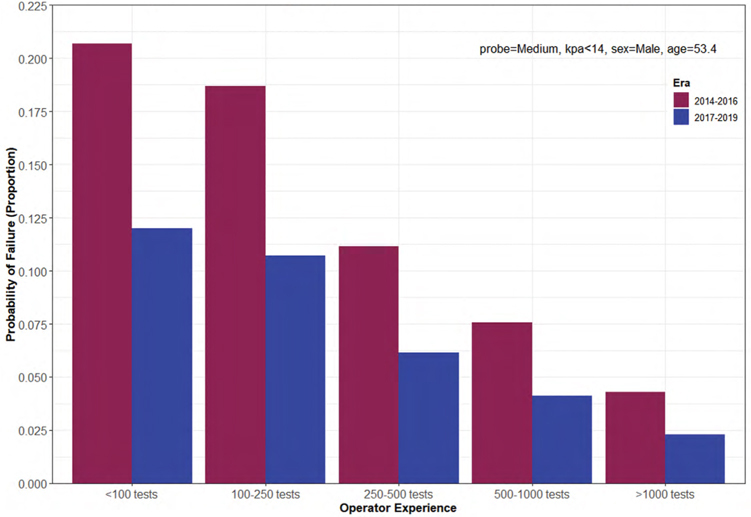
Factors associated with vibration controlled transient elastography failure in a high-volume North American liver clinic
Validation of a hierarchical algorithm to define chronic liver disease and cirrhosis etiology in administrative healthcare data
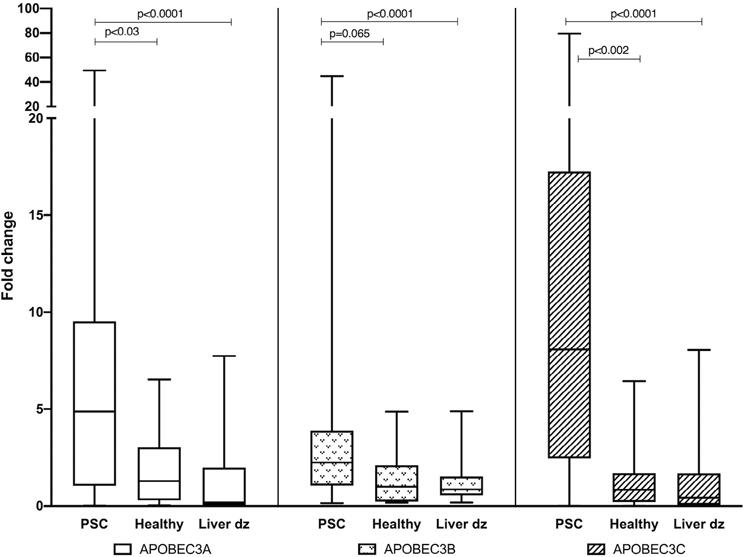
Retrovirus footprint in primary sclerosing cholangitis; APOBEC3 family expression
Optimising trial design in late-stage primary biliary cholangitis: evaluating options for composite clinical endpoint studies
Real-world experience with obeticholic acid (OCA) in Canada: A retrospective analysis of primary biliary cholangitis (PBC) patient characteristics and treatment patterns from the Canadian Patient Support Program
Metabolic alterations of human liver tissue occurring during biobanking procedures
Liver transplantation (LT) for hepatocellular carcinoma in Alberta: Patients assessed for LT in Calgary wait longer for listing and have increased mortality compared to those assessed in Edmonton
Serum ferritin is not associated with elevated elastography scores in non-alcoholic fatty liver disease
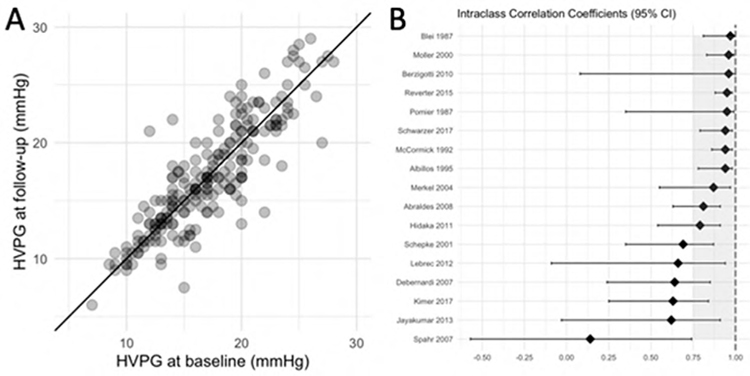
Test-retest reliability of hepatic venous pressure gradient: A study in 215 patients from the control arms of 17 randomized controlled trials
Preliminary results of the prevalence of fatty liver disease in the Greater Toronto population
FINCh: Fibroscan Impact on Non-alcoholic fatty liver disease in children—using randomized placebo-phase design trial
Outcomes of sarcopenic obesity and metabolic syndrome in liver transplant patients
Hepatic steatosis predicts fibrosis in long-term methotrexate use
Clinical evaluation of portal-hepatic blood flow at a low-invasive treatment of mechanical jaundice
Validation of FIB-4 scores and liver stiffness measurements (LSM) by VCTE as non-invasive modalities for detection of advanced fibrosis in NAFLD patients
Impact of depression and antidepressant usage on the clinical outcomes of chronic liver disease
Post-partum primary biliary cholangitis (PBC) after resolution of intrahepatic cholestasis of pregnancy (ICP) in First Nations patients of BC: A case series
NMR metabolic profiling can help discriminate between normal primary hepatocytes and diverging hepatic cancer cell lines
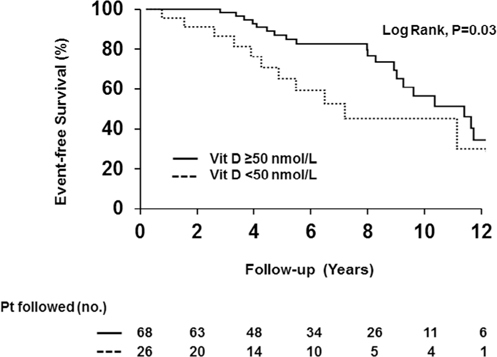
Vitamin D deficiency and its association with clinical outcomes in primary sclerosing cholangitis
Erythropoietic protoporphyria: An unusual presentation of advanced liver fibrosis during infancy
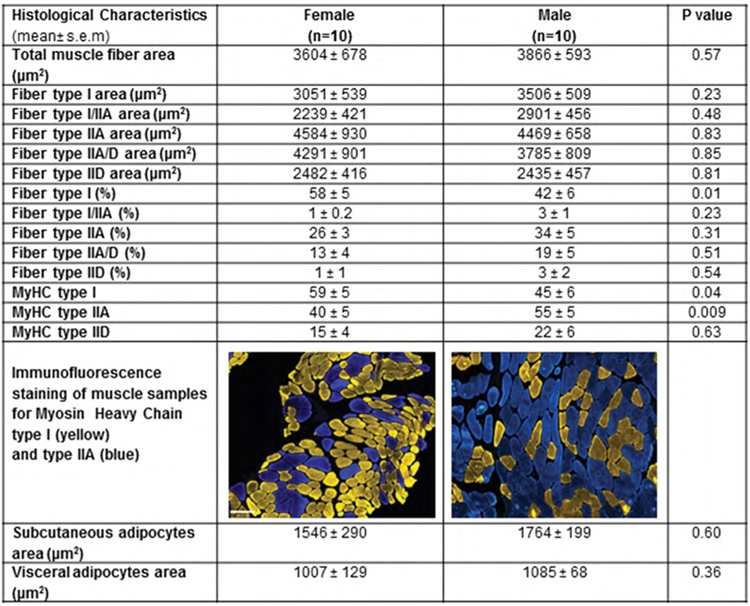
Histological characterization of muscle and adipose tissue in patients with cirrhosis receiving liver transplant
Role of glutamine in the tumorigenicity of the murine hepatocarcinoma cell line Dt81Hepa1-6
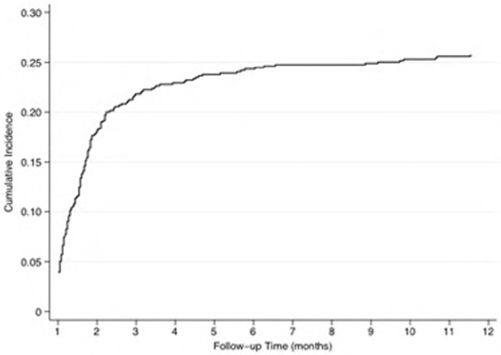
Factors associated with dialysis independence in patients with cirrhosis and acute kidney injury requiring dialysis: a population-based study
FIB4 or NFS can reliably predict fibrosis in sub-groups of NAFLD patients
Prevalence and associated factors of non-alcoholic fatty liver disease in South Asian women with polycystic ovary syndrome: A prospective study using transient elastography
Diabetes is associated with the development of hepatic encephalopathy in cirrhotic patients
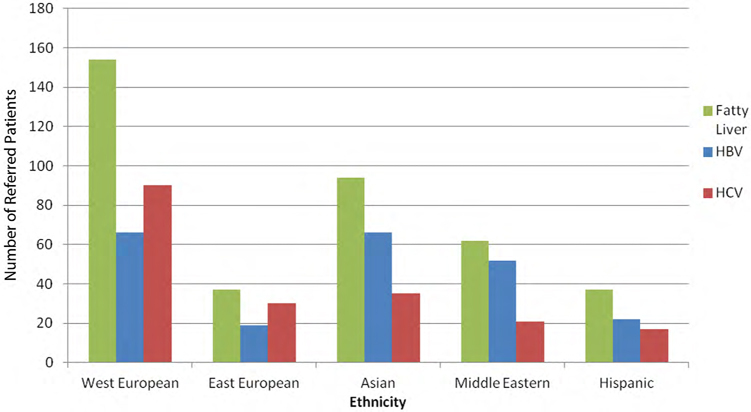
Prevalence of liver diseases in referred patients of varying ethnic backgrounds within the Toronto Liver Centre
Characteristics and outcomes of patients with primary sclerosing cholangitis at a Canadian tertiary care center
New insights on the impact of sex on chronic liver disease and hepatic encephalopathy