Abstract
BACKGROUND
We examined changes in hepatitis B virus (HBV) viral loads (VLs) in pregnancy, their association with hepatitis B e antigen (HBeAg), and the associated infant outcomes.
METHODS
We prospectively followed 132 mothers positive for hepatitis B surface antigen (HBsAg) and their 135 infants from 2011 to 2015 in Vancouver, British Columbia. Outcome measures included association between maternal HBeAg and high (>200,000 IU/mL) or low (≤200,000 IU/mL) HBV VL, changes in HBV VL through pregnancy, infant HBsAg status, and infant completion of the HBV vaccination series.
RESULTS
f the 91 participants with an available HBV VL, 13 (14.3%) had an HBV VL of more than 200,000 IU/mL. Of 59 participants with paired HBeAg and HBV VL in pregnancy, 6 had an HBV VL of more than 200,000 IU/mL; of interest, 2 of the 6 (33.3%) were HBeAg-negative. Thirty-eight participants had HBV VL results at both mid-trimester and delivery. For these 38 participants, Wilcoxon signed-ranks test for paired data found that an HBV VL remained stable (p = .58). We observed no perinatal transmissions. However, 20.7% of infants did not have a documented complete HBV vaccination series, 20.0% did not have post-vaccination HBsAg testing completed, and 18% did not have anti-HBs titres measured by age 12 months.
CONCLUSIONS
Our study demonstrates that HBeAg and HBV VL are not reliably predictive of each other. This supports the improved predictive value of VL measurement in pregnancy to risk stratify pregnant patients to offer antiviral treatment when indicated and further minimize the risk of perinatal transmission.
KeyWords: antivirals, hepatitis B virus, perinatal transmission, pregnancy, virus
Introduction
Worldwide, more than a quarter billion people are living with chronic hepatitis B virus (HBV) infection, putting them at a 20% lifetime risk of death from liver cirrhosis and hepatocellular carcinoma (1). In Canada, the prevalence of chronic HBV infection is less than 1%. However, the burden of disease falls disproportionately on immigrant populations, which have a seroprevalence of 5%–15% compared with 0.4% among the general population (2,3). British Columbia has the highest rate of chronic HBV infection in Canada (4) and a maternal HBV carrier rate of 1.3% (5).
Perinatally infected infants have a 90% risk of developing chronic HBV, compared with a 5% risk if infected as an adult (6). Post-exposure prophylaxis (PEP) for infants born to mothers with HBV prevents perinatal transmission in 85%–95% of cases (7). In British Columbia, PEP consists of hepatitis B immunoglobulin and the first dose of HB vaccine administered at birth, followed by doses at ages 2, 4, and 6 months (8).
PEP has been reported to fail in up to 15% of cases (5,9–11). Factors associated with PEP failure include positive hepatitis B envelope antigen (HBeAg) status and high maternal HBV viral load (VL), with HBV VL likely the strongest predictor of transmission (10,12). To minimize transmission risk, current recommendations are to consider treating pregnant women with an HBV VL of more than 200,000 IU/mL with antiviral therapy (13). Rates of PEP failure have been found to decrease by up to 70% with the use of antivirals (14). HBV VL screening with subsequent antiviral treatment when indicated, in addition to PEP, is more effective and cost saving than administration of PEP alone (15).
In the past, positive maternal HBeAg status was used as marker of high HBV VL and increased risk of perinatal transmission (16). However, high HBV VL can exist with negative HBeAg status. Because HBV VL assumes an important role in the management of prenatal HBV carriers, it becomes important to determine the stability of HBV VL in pregnancy. In this prospective cohort, we sought to characterize the association between HBeAg status and HBV VL, the natural history of HBV VL over the course of pregnancy, and the associated infant outcomes.
Methods
Study design
This prospective cohort study followed HBsAg-positive women and their infants in the greater Vancouver area from October 2011 through August 2015. This study was funded by a British Columbia Children’s Telethon Research Award and grants in kind from British Columbia Hepatitis and Gilead Sciences. All participants provided informed consent. Institutional approval for this project was obtained from the University of British Columbia clinical research ethics board (H11-00339).
Population recruitment
We recruited 132 women in the greater Vancouver area to participate in this study. Participants included in the study were HBsAg-positive pregnant women older than age 18 years who were residents of British Columbia. Excluded participants were those who planned to deliver outside of British Columbia or move out of province postpartum.
Pregnant women in British Columbia are screened for HBsAg early in pregnancy. All positive HBsAg test results are reported to public health, and a nurse contacts each HBsAg-positive pregnant woman by telephone to discuss HBV in pregnancy. Requests for permission to contact women regarding this study were made during this call. A study physician subsequently called and sought consent to participate. Participants were also recruited by research assistants from the Reproductive Infectious Disease Clinic and Labour and Delivery at British Columbia Women’s Hospital.
Participants were asked to provide two blood samples for HBV VL. One sample was obtained at 13–28 weeks gestational age (mid-trimester sample); the second was obtained within 1 month before or after delivery (delivery sample). Participants recruited in Labour and Delivery provided only the delivery sample. Participants were excluded from maternal analyses if they had no HBV VL results from the pregnancy or had results that fell outside of the gestational age inclusion range (Figure 1).
Figure 1:
Participant recruitment
HBsAg = hepatitis B surface antigen; HBV VL = hepatitis B virus viral load
Laboratory results
Participants’ HBsAg and HBeAg status was obtained from private laboratories and the British Columbia Centre for Disease Control Laboratory. Pregnancy and delivery blood samples were sent to the St. Paul’s Hospital Virology Laboratory for HBV VL testing. HBV DNA polymerase chain reaction testing was performed with the Abbott RealTime HBV Viral Load assay (Abbott Park, IL) until August 2013; it was then tested on the COBAS AmpliPrep/COBAS TaqMan HBV Test, Version 2.0 (Pleasanton, CA), for the remainder of the study.
Data collection
Participants enrolled in the study were contacted by phone 9–12 months postpartum by a public health nurse to collect information on (1) demographics, (2) HBV antiviral treatment, (3) birth parameters, (4) HBV PEP and vaccine administration to infants, and (5) infant HBV serology. Vaccine administration and serology results were verified via chart review by public health nurses.
Statistical analysis
Statistical analysis was conducted using Microsoft Excel 2016 (Microsoft Corp., Redmond, WA) and R (R Foundation for Statistical Computing, Vienna, Austria). Descriptive statistics were used to evaluate (1) demographics; (2) the proportion of HBeAg-positive and HBeAg-negative participants; (3) the proportion of participants with a high (>200,000 IU/mL) or low (≤200,000 IU/mL) HBV VL (because this is the currently recommended threshold for consideration of antiviral treatment in pregnancy); (4) correlation between HBeAg status and HBV VL; (5) changes in HBV VL from mid-trimester to delivery; and (6) characteristics of participants with high HBV VLs. Analytic statistics included a Cohen’s κ test for agreement to assess the relationship between HBeAg and HBV VL and a Wilcoxon signed-ranks test for paired data to assess the trend in changes in HBV VL through pregnancy.
The success of PEP in this population was evaluated by describing (1) the proportion of infants who received complete and timely PEP (hepatitis B immunoglobulin and the first dose of HB vaccine within 24 h of birth and a dose of HB vaccine at ages 2, 4, and 6 mo, ±1 mo), (2) the proportion of infants who received follow-up HBV serologic testing by age 9–12 months, and (3) the HBsAg status of all infants.
Results
Participant demographics
We followed 132 pregnancies and 135 infants (three twin pregnancies). Of all participants, 41 (31.1%) had no HBV VL results from mid-trimester or delivery or had HBV VL results that fell outside the gestational age inclusion range and were excluded from maternal analyses.
Of the 91 participants assessed, the median maternal age was 32 years, the median gestational age at delivery was 39 weeks, most (n = 56; 61.5%) delivered by spontaneous vaginal delivery, and most (n = 76; 83.5%) chose to breastfeed their infant. There were 38 (41.8%) participants with paired HBV VL results during mid-trimester and delivery, 28 (30.8%) participants with results during mid-trimester only, and 25 (27.4%) participants with results at delivery only. Of the 91 participants with at least one HBV VL result, 13 (14.3%) had one HBV VL of more than 200,000 IU/mL (Table 1).
Table 1:
Participant demographics for those with at least one HBV VL result (N = 91)
| Characteristic | n (%)* |
|---|---|
| Maternal age, y, median (IQR) [range] | 32 (30–36) [25–44] |
| Gestational age at delivery, wk, median (IQR) [range] | 39 (38–40) [29–44] |
| Mode of delivery | |
| Spontaneous vaginal delivery | 56 (61.5) |
| Cesarean section | 24 (26.4) |
| Assisted vaginal delivery (vacuum or forceps) | 11 (12.1) |
| Duration of ruptured membranes, h | |
| >18 | 7 (7.7) |
| ≤18 | 24 (26.4) |
| Unknown | 60 (65.9) |
| Breastfeeding | |
| Any breastfeeding | 76 (83.5) |
| No breastfeeding | 5 (5.5) |
| Unknown | 10 (11) |
| HBV VL samples available | |
| Delivery and mid-trimester sample | 38 (41.8) |
| Mid-trimester sample only | 28 (30.8) |
| Delivery sample only | 25 (27.4) |
| HBV VL results by level | |
| Any sample >200,000 IU/mL | 13 (14.3) |
| All samples ≤200,000 IU/mL | 78 (85.7) |
* Unless otherwise indicated
HBV VL = hepatitis B virus viral load; IQR = inter-quartile range
Eight participants had an HBV VL of more than 200,000 IU/mL at delivery (Table 4). Two of these participants had an HBV VL available in mid-trimester, one of more than 200,000 IU/mL and one 200,000 IU/mL or less. Of the eight participants, six had positive HBeAg status, one had negative HBeAg status, and one’s status was unknown. None of the participants with a delivery HBV VL of more than 200,000 IU/mL had been treated with antivirals.
Table 4:
Characteristics of participants with delivery HBV VL >200,000 IU/mL (n = 8)
| Participant | Year of delivery | HBeAg status | Mid-trimester gestational age (wk) | Mid-trimester HBV VL (IU/mL) | Delivery gestational age (wk) | Delivery HBV VL (IU/mL) | Antiviral treatment (yes or no) | Infant HBsAg status |
|---|---|---|---|---|---|---|---|---|
| 1 | 2011 | Negative | 22 | 1,570 | 38 | 1,232,032 | Unknown | Negative |
| 2 | 2012 | Positive (delivery sample) | N/A | N/A | 39 | 13,340,1162 | No | Negative |
| 3 | 2012 | Unknown | N/A | N/A | 37 | 20,745,970 | No | Negative |
| 4 | 2012 | Positive | 26 | 78,144 239 | 38 | 355,717,386 | Unknown | Unknown |
| 5 | 2012 | Positive | N/A | N/A | 40 | 290,815,045 | No | Negative |
| 6 | 2013 | Positive | N/A | N/A | 38 | 40,385,130 | Unknown | Unknown |
| 7 | 2013 | Positive | N/A | N/A | 38 | 8,159,631 | No | Negative |
| 8 | 2015 | Positive | N/A | N/A | 39 | 515,368 | No | Negative |
HBV = hepatitis B virus; VL = viral load; IU = international unit; HBeAg = hepatitis B envelop antigen; HBsAg = hepatitis B surface antigen; N/A = not available
Change in HBV viral load through pregnancy
Thirty-eight participants had an HBV VL available during mid-trimester and at delivery. The time elapsed between the two samples ranged from 9 to 27 weeks, with a median of 17 weeks (inter-quartile range 14–20 weeks).
A Wilcoxon signed-ranks test for paired data showed no significant directional change in HBV VL through pregnancy for these participants (p = .58). One participant demonstrated an increase in HBV VL from 1,570 IU/mL to 1,232 032 IU/mL, and one demonstrated a decrease in HBV VL from 3,689,997 IU/mL to 564 IU/mL, with documented antiviral treatment (Figure 2).
Figure 2:
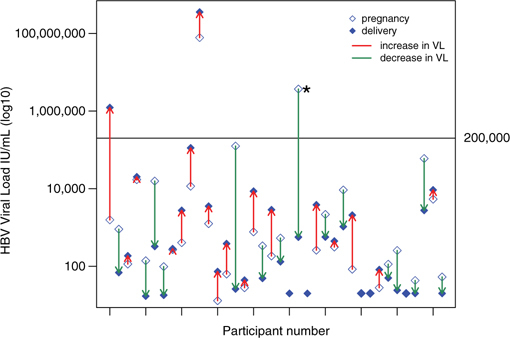
Individual changes in HBV VL from mid-trimester to delivery (n = 38)
* Participant who received antivirals in pregnancy. HBV = hepatitis B virus; VL = viral load
Infant vaccination and follow-up
Of the 135 infants, 107 (79.3%) received all four doses of HB vaccine, 6 (4.4%) did not receive a complete vaccine series, and 22 (16.3%) had incomplete or unavailable documentation of their HB vaccine series. Of all infants followed, 100 (74.1%) received all doses on time, 15 (11.1%) obtained the vaccine in a delayed fashion, and 20 (14.8%) had incomplete or unavailable documentation of the timing of their HBV vaccination. Post-vaccination serology was obtained for 113 (83.7%) infants but was not obtained for 15 (11.1%) infants. One hundred and ten (81.5%) infants had protective anti-HBs levels, although 24 (17.8%) did not have documented anti-HBs levels. All infants with a documented HBsAg result, tested HBsAg-negative, although 27 (20.0%) infants did not have documented HBsAg status, indicating that one-fifth of the newborns were not tested for perinatal infection by age 9–12 months (Table 5).
Table 5:
Infant outcomes, vaccination, and serological testing (N = 135)
| n (%) | |
|---|---|
| Delayed HBV vaccination series* | |
| No | 100 (74.1) |
| Yes | 15 (11.1) |
| Unknown | 20 (14.8) |
| All 4 doses of HBV vaccination series received | |
| No | 6 (4.4) |
| Yes | 107 (79.3) |
| Unknown | 22 (16.3) |
| Post-vaccination serology obtained | |
| No | 15 (11.1) |
| Yes | 113 (83.7) |
| Unknown | 7 (5.2) |
| Infant’s HBsAg status | |
| Negative | 108 (80.0) |
| Unknown | 27 (20.0) |
| Protective anti HBs (>10 mIU/mL) | |
| No | 1 (0.7) |
| Yes | 110 (81.5) |
| Unknown | 24 (17.8) |
Note: In British Columbia, the recommended vaccination series is one dose of HBV vaccine at birth and at ages 2, 4, and 6 months
* Delayed was defined as having received any dose at ≥1 month off schedule
HBV = hepatitis B virus; HBsAg = hepatitis B surface antigen; anti HBs = hepatitis B surface antibody
Interpretation
In this study, we observed a generally good correlation between HBeAg status and HBV VL but found that for some participants, HBeAg status and HBV VL were not predictive of one another. HBV VL among our participants remained relatively stable through pregnancy. We found that at mid-trimester, 14.0% of participants were candidates for antiviral therapy with an HBV VL of more than 200,000 IU/mL. Although we observed no transmissions, follow-up testing and documentation of complete vaccination was absent for as many as 20% of infants.
Although HBeAg is generally a reliable correlate of HBV VL, we observed instances in which this was not the case. Two of six (33.3%) of our participants with an HBV VL of more than 200,000 IU/mL at mid-trimester had negative HBeAg status. HBeAg status changes with the natural history of HBV infection and becomes negative in the immune-control phase of chronic HBV infection, which is usually accompanied by a decrease in HBV VL (16). However, 5%–15% of people with HBeAg-negative chronic HBV develop chronic active hepatitis, with associated high HBV VL, while HBeAg status remains negative because of pre-core (PC) or basal core promoter (BCP) mutations (16). One study assessing the correlation of HBeAg, HBsAg, and HBV VL in 149 patients with chronic HBV found that HBeAg titre correlated positively with HBV VL and that HBeAg titre decreased in participants with PC or BCP mutations after adjustment for HBV VL (17). Similarly, in a study of 943 pregnant Indonesian women, two-thirds of participants with elevated HBV VL and negative HBeAg status had either a PC or BCP mutation (18). The two participants in our study with negative HBeAg and high HBV VL likely fall into the HBeAg-negative, chronic active hepatitis category and may harbour PC or BCP mutations. This highlights the value of HBV VL versus HBeAg status as a means to properly identify those who should be considered for antiviral treatment in pregnancy.
An elevated HBV VL at delivery is a key risk factor for perinatal transmission (10,12). Few studies have assessed the natural history of HBV VL through pregnancy. However, we know that chronic hepatitis B infection can be affected by immune changes that occur with pregnancy (19). Plausibly, the immune suppression associated with pregnancy could result in increases in viral replication during pregnancy, and the immune reconstitution after delivery could potentiate hepatitis flares (20). Although we observed no significant directional pattern in change of HBV VL through the pregnancies of our participants, there were exceptions. Our findings are similar to a study of 29 pregnant patients with HBV in California, which also observed no significant pattern in the direction of change of HBV VL during pregnancy (21). A retrospective study on stored serum samples in Sweden observed a similar pattern of generally stable VL through pregnancy in its cohort (22). Our study, along with those described, suggests that for most women with chronic HBV, disease state and HBV VL remain relatively stable through pregnancy, although there can be outliers with potential important clinical implications for mother and infant.
In our cohort, 14.3% of participants had at least one HBV VL of more than 200,000 IU/mL, putting them above the currently recommended threshold for treatment with antivirals. Only two of these participants received treatment. This is likely because this study was conducted over a time period in which evidence for the use of antivirals for pregnant patients with HBV was still emerging. Extrapolating our findings to the British Columbia prenatal population, which includes approximately 350 HBsAg-positive pregnant patients each year (personal communication, British Columbia Centre for Disease Control, 2014), we estimate that 50 patients each year may benefit from antiviral treatment. For every 100 high-risk women receiving antiviral treatment, an additional 10 transmissions might be prevented beyond that achievable by PEP alone (15), suggesting that in our community we could prevent as many as five transmissions per year if we treat those at risk.
We observed no perinatal transmissions in our cohort. However, 21% of infants did not have a complete HB vaccine series documented. Although it is unclear whether absent documentation reflects failure to provide HBV PEP, this raises concern regarding the overall effectiveness of the current provincial HBV high-risk prenatal program in reaching all at risk. This proportion is similar to what was observed in a previous study conducted by this group in the same community (5). This observation may be due to loss to follow-up, lack of knowledge on the part of the care provider, or barriers in communication.
Strengths of our study include its prospective nature and our ability to obtain serial VL through a pregnancy. We were also able to follow a large proportion of the infants to 12 months postpartum. Limitations include the small sample size that likely reflects participants who are more clinically engaged. We also did not assess maternal HBV VL beyond delivery.
In summary, we observed that HBV VL remains stable through pregnancy, but the majority of women in our cohort who met indications for antiviral therapy did not receive it. Given that elevated HBV VL is highly predictive of transmission (10,12), we suggest that screening with HBV VL has more clinical utility than HBeAg status among women who are HBsAg-positive and should become the current standard of practice in pregnancy. This will allow clinicians to appropriately risk stratify pregnant patients into those who may benefit from antiviral treatment in pregnancy to further reduce the risk of perinatal transmission.
Acknowledgments:
The authors thank Dr Kathryn Dewar for research support and co-ordination conducted as an employee of the Women’s Health Research Institute.
Ethics Approval:
The study protocol was approved by an ethics committee and the ethics certificate information is available from the authors upon request.
Informed Consent:
Informed consent was obtained from the patient(s).
Registry and Registration No. of The Study/Trial:
N/A
Funding:
None to declare
Disclosures:
Dr Krajden reports grants from Roche, Hologic, and Siemens outside the submitted work; Dr Money reports grants from Merck, GSK, and Sanofi outside the submitted work.
Peer Review:
This article has been peer reviewed.
References
- World Health Organization (WHO). Hepatitis B fact sheet. Geneva: WHO; 2016. [Google Scholar]
- Rotermann M, Langlois K, Andonov A, Trubnikov M. Seroprevalence of hepatitis B and C virus infections: results from the 2007 to 2009 and 2009 to 2011 Canadian health measures survey. Health Rep. 2013;24(11):3. [PubMed] [Google Scholar]
- Wong W, Minuk G. A cross-sectional seroepidemiologic survey of chronic hepatitis B virus infections in southeast Asian immigrants residing in a Canadian urban centre. Clin Invest Med. 1994;17(5):443–7. [PubMed] [Google Scholar]
- Public Health Agency of Canada (PHAC). Report on Hepatitis B and C in Canada: 2013. Ottawa: PHAC; 2015 [Google Scholar]
- Van Schalkwyk J, Nourmoussavi M, Massey A, et al. Missed opportunities for prevention of perinatal transmission of hepatitis B: a retrospective cohort study. Can J Gastroenterol Hepatology. 2014;28(10):525–8. 10.1155/2014/549764. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmunds WJ, Medley GF, Nokes DJ, Hall AJ, Whittle HC. The influence of age on the development of the hepatitis B carrier state. Proc Biol Sci. 1993;253(1337):197–201. 10.1098/rspb.1993.0102. Medline: [DOI] [PubMed] [Google Scholar]
- McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology. 2009;49(Supplement 5), S45–55. 10.1002/hep.22898. Medline: [DOI] [PubMed] [Google Scholar]
- British Columbia Centre for Disease Control. BC routine immunization schedule: infant and child. Vancouver: British Columbia Centre for Disease Control; 2017 [Google Scholar]
- Wiseman E, Fraser MA, Holden S, Perinatal transmission of hepatitis B virus: an Australian experience. Med J Aust. 2009;190(9):489. 10.5694/j.1326-5377.2009.tb02828.x [DOI] [PubMed] [Google Scholar]
- Zou H, Chen Y, Duan Z, Zhang H, Pan C. Virologic factors associated with failure to passive–active immunoprophylaxis in infants born to HBsAg-positive mothers. J Viral Hepat. 2012;19(2):e18–25. 10.1111/j.1365-2893.2011.01492.x. Medline: [DOI] [PubMed] [Google Scholar]
- Kubo A, Shlager L, Marks AR, et al. Prevention of vertical transmission of hepatitis B: an observational study. Ann Intern Med. 2014; 160(12):828–35. 10.7326/M13-2529. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Sung J, Yang S, Choe YH, Chang YS, Park WS. Factors associated with immunoprophylaxis failure against vertical transmission of hepatitis B virus. Eur J Pediatr. 2007;166(8):813–8. 10.1007/s00431-006-0327-5. Medline: [DOI] [PubMed] [Google Scholar]
- Castillo E, Murphy K, van Schalkwyk J. No. 342-hepatitis B and pregnancy. J Obstet Gynaecol Can. 2017;39(3):181–90. 10.1016/j.jogc.2016.11.001. Medline: [DOI] [PubMed] [Google Scholar]
- Brown RS, McMahon BJ, Lok AS, et al. Antiviral therapy in chronic hepatitis B viral infection during pregnancy: a systematic review and meta-analysis. Hepatology. 2016;63(1):319–33. 10.1002/hep.28302. Medline: [DOI] [PubMed] [Google Scholar]
- Fan L, Owusu-Edusei K, Schillie SF, Murphy TV. Cost-effectiveness of active-passive prophylaxis and antiviral prophylaxis during pregnancy to prevent perinatal hepatitis B virus infection. Hepatology. 2016;63(5): 1471–80. 10.1002/hep.28310. Medline: [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO). Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. Geneva: WHO; 2015 [PubMed] [Google Scholar]
- Thompson AJ, Nguyen T, Iser D, et al. Serum hepatitis B surface antigen and hepatitis B e antigen titers: disease phase influences correlation with viral load and intrahepatic hepatitis B virus markers. Hepatology. 2010; 51(6):1933–44. 10.1002/hep.23571. Medline: [DOI] [PubMed] [Google Scholar]
- Fujiko M, Chalid MT, Ie SI, et al. Chronic hepatitis B in pregnant women: is hepatitis B surface antigen quantification useful for viral load prediction? Int J Infect Dis. 2015;41:83–9. 10.1016/j.ijid.2015.11.002. Medline: [DOI] [PubMed] [Google Scholar]
- Trowsdale J, Betz AG. Mother’s little helpers: mechanisms of maternal-fetal tolerance. Nat Immunol. 2006;7(3):241–6. 10.1038/ni1317. Medline: [DOI] [PubMed] [Google Scholar]
- Angel Garcia AL. Effect of pregnancy on pre-existing liver disease physiological changes during pregnancy. Ann Hepatol. 2006;5(3):184–6. 10.1016/S1665-2681(19)32007-1. [DOI] [PubMed] [Google Scholar]
- Nguyen G, Garcia R, Nguyen N, Trinh H, Keeffe E, Nguyen M. Clinical course of hepatitis B virus infection during pregnancy. Aliment Pharmacol Ther. 2009;29(7):755–64. 10.1111/j.1365-2036.2009.03932.x. Medline: [DOI] [PubMed] [Google Scholar]
- Söderström A, Norkrans G, Lindh M. Hepatitis B virus DNA during pregnancy and post partum: aspects on vertical transmission. Scand J Infect Dis. 2003;35(11-12):814–9. 10.1080/00365540310016547. Medline: [DOI] [PubMed] [Google Scholar]


