Abstract
Background
Textile industry has been widely implicated in environmental pollution. The health effects of residing near manufacturing industries are not well documented in India, especially in central India. Hence, a cross-sectional environmental monitoring and health assessment study was initiated as per directions of the local authorities.
Methods
Comprehensive exposure data about the concentrations of relevant pollutants in the ambient air and ground water samples in the study area will be collected over one year. Using stratified random sampling, 3003 apparently healthy adults will be selected from the study area. Sociodemographic and anthropometric information, relevant medical and family history, and investigations including spirometry, electrocardiogram, neurobehavioral tests, and laboratory investigations (complete blood count, lipid profile and random blood glucose) will be conducted. Finally Iodine azide test and heavy metal level detection in urine and blood samples respectively will be conducted in a subset of selected participants to assess individual pollution exposure. Ethics approval has been obtained from the Institutional Ethics Committee of the National Institute for Research in Environmental Health (No: NIREH/IEC-7-II/1027, dated 07/01/2021).
Discussion
This manuscript describes the protocol for a multi-disciplinary study that aims to conduct environmental monitoring and health assessment in residential areas near viscose rayon and associated chemical manufacturing industries. Although India is the second largest manufacturer of rayon, next only to China, and viscose rayon manufacturing has been documented to be a source of multiple toxic pollutants, there is a lack of comprehensive information about the health effects of residing near such manufacturing units in India. Therefore implementing this study protocol will aid in filling in this knowledge gap.
Introduction
Industrial development has contributed to economic growth but at the same time industrial activities constitute a major source of environmental pollution [1]. Adverse health effects posed by industrial pollution is well-established [1]. De Sario et al., 2018 [2] in their scoping review identified 762 epidemiological studies which conclude that living in industrial areas is associated with a wide range of diseases ranging from malignancies and respiratory diseases to adverse birth outcomes and even premature mortality. Of these studies, few were conducted in India that focussed on the public health and environmental effects of industrial emission. Chatham-Stephens et al., 2013 [3] using the Toxic Sites Identification Program developed by the Blacksmith Institute and the United Nations Industrial Development Organization identified 221 point-sources of pollution from industrial activities in India that pose risk to public health. The authors [3] calculated that exposure to pollutants generated in industrial regions in developing countries like India, Indonesia, and the Philippines pose equal or even greater risk to human health than widespread communicable diseases like malaria and account for almost eight million years of healthy life lost due to disease, disability or premature death (i.e. 828,722 Disability-adjusted Life Years) among the 8.6 million exposed population in the three countries. Therefore, research is needed to assess the environmental health effects of major industries in India.
India, being a rapidly developing economy, is home to multiple industries. Textile industry, plays a critical role in Indian economy as it is the second largest source of employment for a country which is counted amongst the top producers, exporters as well as consumers of textiles in the world [4]. However, the textile industry has been widely implicated in pollution of various environmental matrices like air, water and soil both in India [5, 6] and other countries [7, 8]. It accounts for almost twenty percent of global industrial activity induced water pollution [7] and is associated with emission of many gaseous pollutants into ambient air [9]. Multiple studies [10. 11] have documented the adverse health effects of exposure to the chemical pollutants that emanate from textile and associated chemical manufacturing. In India, research [12–14] on the health effects of such textile industry has mostly been focussed on individuals who are occupationally exposed to the chemical pollutants. The health effects of residing near manufacturing industries are not as well documented.
The industrial area of Birlagram Nagda in the central Indian state of Madhya Pradesh (MP), has been under scrutiny due to the pollution emanating from the multiple viscose rayon textile and associated chemical manufacturing industries operating in the area [15]. As per data compiled by the Central Pollution Control Board (CPCB) [16] in 2013, the apex government run agency monitoring environmental pollutant concentrations in the country, the area has a comprehensive environment pollution index score of 66.67, which indicates that it is among the “severely polluted” industrial clusters in India. Although recent statistics have shown improvement in the area’s pollution index, it is still listed in the top hundred polluted industrial clusters in India [17]. Major chemical pollution associated with viscose rayon textile manufacturing occur secondary to gaseous emission of carbon disulphide (CS2), Hydrogen Disulphide (H2S), Hydrogen Chloride (HCl), Chlorine (Cl2), Sulphur oxides (SO2 and SO3) and untreated discharge of viscose fibre wastewater effluent containing high concentration of heavy metals, and other toxic substances into natural water sources [9]. However, there is hardly any scientific evidence regarding the health effects of residing near the industries of Birlagram. Considering the growing health concerns of the local public, regulatory authorities in India directed the industries operational in the region to implement pollution control strategies as well as commissioned detailed environmental and health-oriented investigation of the issue [18]. The current study was initiated as per directions of the local authorities to evaluate the health effects of exposure to multiple pollutants emitted from the industrial activity in Birlagram. Specifically, the objectives of the current study include:
To measure the concentration of gaseous pollutants (i.e. CS2, H2S, HCl, Cl2, and SO2) in the ambient air in the study area.
To measure the concentrations of heavy metals and anions [i.e. lead (Pb), mercury(Hg), aluminium (Al), chloride (Cl-) and sulphate(SO42-)] in groundwater samples.
To determine the association between the concentration levels of pollutants in air and water samples with that in bio-samples (blood and urine).
To identify the association between exposure to pollutants and health effects in study participants.
Methods and analysis
Study design
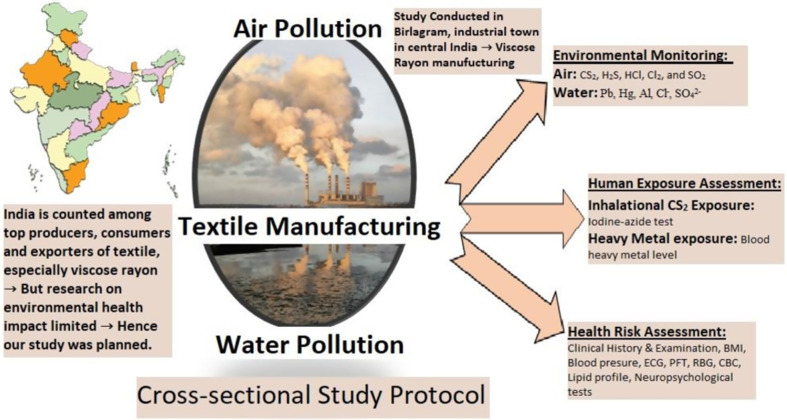
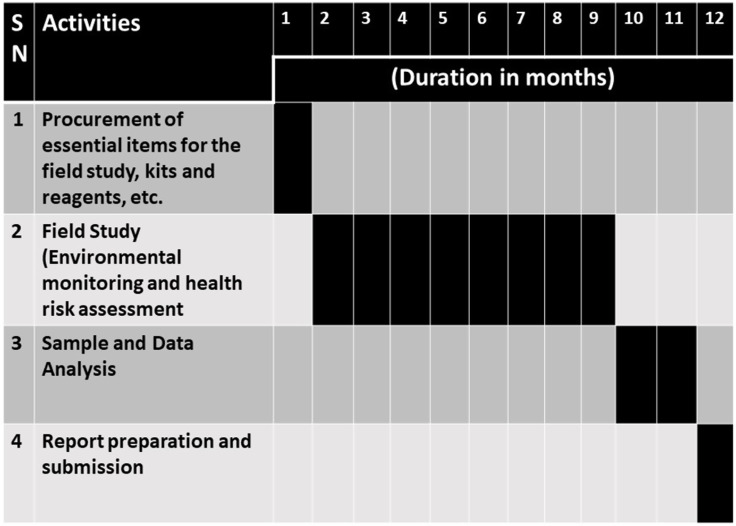
This is the protocol for a cross-sectional research study that aims to collect and correlate comprehensive exposure and health outcome information to ascertain objective achievement (see Fig 1). This study will be conducted during a duration of one year. The time schedule of various study methods of this protocol is described in Fig 2.
Fig 1. Graphical abstract: Overview of the study methodology.
[Note: carbon disulphide (CS2), Hydrogen sulphide (H2S), Hydrogen Chloride (HCl), Chlorine (Cl2), Sulphur oxides (SO2), lead (Pb), mercury(Hg), aluminium (Al), chloride (Cl-), sulphate (SO42-), body mass index (BMI), Electrocardiogram (ECG), Pulmonary function test (PFT), Random Blood glucose (RBG), Complete blood count (CBC)] (The graphical abstract image has been created by the research team using copyright-free images of Indian map and industry from https://pixabay.com).
Fig 2. Time schedule of activities.
[Note: Each numbered column represents one month of the project duration with the total duration of the project being 12 months].
Setting
This study will be conducted in and around the Birlagram industrial region located in Nagda city in the Ujjain district (administrative unit) in the central Indian state of MP. Almost 72 million individuals reside in MP, most (72%) of whom reside in rural areas and depend on agriculture for their livelihood [19]. However, industrial activity in MP is gradually increasing [20] and multiple large scale industries in the Ujjain district [21] constitute an emerging public health concern for the province that needs to be evaluated.
The Birlagram industrial area is situated within the borders of Nagda city which in turn is surrounded on all sides by villages [15]. The industries situated in Birlagram are directly or indirectly (through production of chemical raw materials) involved in synthetic textile (viscose rayon) manufacturing [22]. Including the population residing in Birlagram itself, Nagda city has a total population of more than 0.1 million. River Chambal is the main source of water for the region and previous research findings highlight the poor quality of its water [23]. Details on air quality of the area are lacking in published literature.
Participant selection and sampling strategy
Sample size calculation
As the health effects of residing near textile manufacturing industries in Birlagram, India are not previously documented, we calculated the sample size using the statistics reported by a previous study conducted in Taiwan where authors [24] found that 26.3% of the population exposed to emissions from a viscose rayon manufacturing industry had electrocardiogram (ECG) abnormalities. Since one of our outcomes is the identification of the proportion of exposed population in Birlagram, India with ECG abnormalities, we calculated sample size as follows:
Where, p = Expected population proportion = 0.26; q = 1-p = 0.74; z = z value for 5% level of significance is 1.96; R = Non-response rate (assumed to be 30% as pooled non-response rate reported by a systematic review of participation response in Indian industrial surveys [25]); Deff = Design effect(1.25 for systematic sampling); and d = Margin of error 2% (assumed to be 0.02). The calculated sample size was 3003.
Sampling strategy
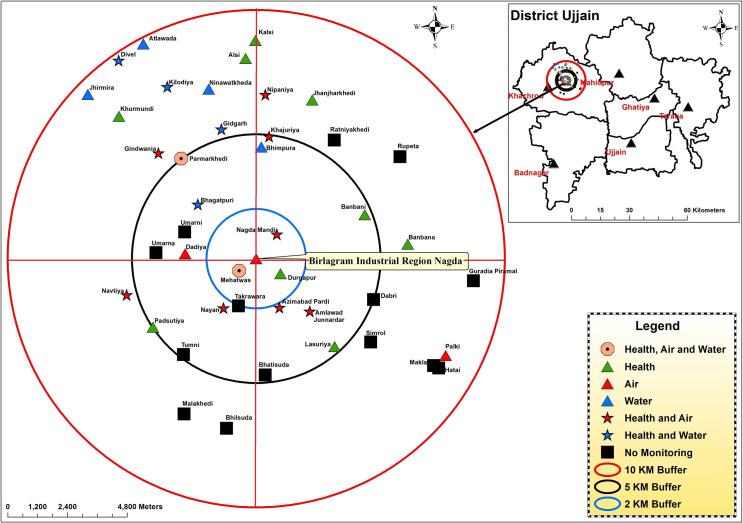
In this study stratified random sampling technique will be used for selection of participants where stratification will be done based on environmental factors that are likely to affect exposure. Since both distance from fixed contamination source as well as local environment features such as meteorology, topography, etc. are the major factors affecting the dispersion of both ambient air as well as water pollutants [26], a map of the study area was created to assist in selection of exposure monitoring locations and in participant sampling (see Fig 3). Briefly, taking Birlagram industrial region as the centre, villages located within consecutive circles of radius 2 kilometres (km), 5km and 10km were demarcated on the map using ArcInfo (version 10). This strategy was used as it is expected that gaseous pollutants’ dispersion occurs homogenously in all directions independent of anthropogenic roadways and that with increase in distance the pollutant concentration will become diluted enough to prevent adverse health effects [27]. Based on seasonal wind pattern data retrieved from local authorities, the north-eastern quadrant of the circular study area was seen to be the downwind area for most of the year. The downstream direction of River Chambal falls in the north-western quadrant of the circular study area. Therefore the majority of the pollutants are expected to disperse in the northern direction increasing the potential risk faced by the residents and so, we decided to over-represent the northern hemisphere.
Fig 3. Map of the study area created by research team to assist in selection of exposure monitoring locations and in participant sampling.
[Note: The inset in the map shows the Ujjain district of the central Indian province of MP. Within the Khachrod Tehsil of the Ujjain district, lies the study area of the current project. Considering the Birlagram industrial region as the centre, three concentric circular regions were demarcated based on distance (2, 5 and 10 km from the centre)].
Finally, 2000 participants will be selected from the villages in the northern hemisphere while 1003 will be taken from the villages in the southern hemisphere within the 10km radius comprising a total of 3003 participants. In each hemisphere, a fixed number of villages will be selected randomly from those situated in each of the 0-2km, 2-5km and 5-10km zones. Further in each selected village, using the list of all individuals residing in the villages that will be retrieved from the local authorities as the sampling frame, individuals meeting the eligibility criteria will be randomly invited for participation in the study. Total number of individuals sampled in each village will be proportionate to the total population of the selected village.
Participants will be selected for the study only if they fulfill all the following criteria:
Adults (≥18 years of age) of both genders (except pregnant women),
Should have been residing for at least one year in the selected village,
No history of neuropsychiatric conditions that might hinder completion of neurobehavioral tests,
No history of other conditions that are contraindications for performing Pulmonary function test (PFT) like presence of any active disease (e.g. pulmonary Tuberculosis, active haemoptysis, orofacial pain, acute respiratory infection including COVID-19 i.e. coronavirus disease caused by SARS-Cov-2 virus) or recent history (within previous 1 month) of myocardial infarction/aneurysm/surgery, etc.
Environmental monitoring of pollutants at study sites
Pollutants for assessment in both environmental and human matrices were selected based upon their relevance to viscose rayon and associated chemical manufacturing industry identified through review of previously published literature [9].
i) Monitoring of gaseous pollutants in ambient air. The ambient air monitoring will be carried out in the study area (see Fig 2) for identified gaseous pollutants viz., CS2, H2S, HCl, Cl2, and SO2 using a factory calibrated, electrochemical sensor based multi-gas monitor (Make: Swan® Environmental Pvt. Ltd. India, Model: GRI-IAT; Sensor make: Membrapor®, Switzerland). The monitoring will be carried out in post-monsoon season (October-January). The instrument has an inbuilt geographical positioning system to record the coordinates of sampling site. The ambient air monitoring will be carried out continuously for 24 hours in at identified sites. In case of cluster of sites, a representative site will be identified for gaseous monitoring. The monitor will be placed at more than 3 metres height from ground level at a place having uninterrupted power supply. The data loggers present in the instrument will store the data at set frequency and will be utilized for further statistical analysis. The sampling and monitoring of ambient air for said pollutants will be carried out following the existing guidelines of CPCB, Ministry of Environment, Forests and Climate Change, New Delhi, India [28].
ii) Monitoring of pollutants in groundwater. Levels of concerning heavy metals and ions, namely Al, Pb, Hg, Cl- and SO42- will be assessed in representative groundwater samples collected from sources including tube-wells, hand-pumps and open wells from the selected 10 downstream villages (Fig 2). 120 millilitre (mL) each of groundwater samples will be collected from all available and accessible sources in each village. Samples will be collected in high density polypropylene (HDPE) bottles which were pre-cleaned by soaking overnight in 2% nitric acid and washed with double distilled water. Water samples will be collected from handpumps, tube-wells, and open-wells after removing stagnant water using appropriate purging methods [29]. Also, a few water samples will be collected from the Chambal River at specified locations to assess metallic pollutants. Collected water samples will be labeled and transported to the laboratory at room temperature for initial measurement of physiological parameters including pH, total dissolved salts, conductivity, and salinity. Subsequently, the samples will be preserved with trace metal grade nitric acid (HNO3, pH <2) and stored at 4°C till analysis within a week.
Analysis of toxic metals, viz. Pb, Hg and Al in the collected groundwater samples will be carried out using inductively coupled plasma optical emission spectroscopy (iCAP® 7400 Duo ICP-OES, ThermoFisher Scientific® Pvt Ltd) using the EPA method 200.7 (revision 4.4) [30]. Briefly, 6-point calibration standards for each element (Al, Pb, and Hg) will be first prepared in the range of 1–200 parts per billion (ppb) by serially diluting the multi-element stock solution (ICP multi-element standard XIII, Merck, Germany) in 5% HNO3 in double distilled water. Specific run parameters and sample introduction system that will be used for analysis of these metals on ICP-OES instrument are summarized in Table 1. The concentration of individual metals in the unfiltered acid preserved groundwater samples (turbidity <1 Nephelometric Turbidity unit), will be determined after successful run of standards (e.g., linear graph with correlation coefficient > 0.999) in the axial view using standard sample introduction setup for Al and Pb, and hydride generation sample introduction system for Hg. The online hydride generation for Hg will be achieved with Enhanced Vapour System sample introduction kit using 0.5% mass by volume (m/v) sodium tetrahydroborate (NaBH4) stabilized in 0.5% m/v Sodium hydroxide (NaOH) and 50% volume/volume (v/v) HCl solution [31]. Emission acquisition will be done on the Qtegra® ISDS Software at interference free wavelengths of 220.353 nanometer (nm), 184.950 nm, and 396.152 nm for Pb, Hg and Al, respectively. The instrument performance will be checked in between the sample run using quality control samples containing metals of known concentration. Metal levels in each sample will be reported in ppb levels.
Table 1. The ICP OES instrument parameters that will be used during analysis of Pb, Al, and Hg in the groundwater and/or blood samples.
| Parameter | Aqueous sample introduction | EVS sample introduction |
|---|---|---|
| Pump Tubing | Sample = white/white | Sample = green/green |
| Drain = orange/blue | Drain = black/white | |
| Acid blank = orange/yellow | ||
| Reductant = black/black | ||
| Spray Chamber | Baffled cyclonic | Gas Liquid Separator |
| Nebulizer | V-groove | V-groove |
| Auxiliary Gas Flow | 0.5 Litre per minute | 0.5 Litre per minute |
| Coolant Gas Flow | 12 Litre per minute | 16 Litre per minute |
| Nebulizer Gas Flow | 0.5 Litre per minute | 0.3 Litre per minute |
| Pump Speed | 50 revolutions per minute | 30 revolutions per minute |
| Center Tube | 1.5 millimeter | 1.5 millimeter |
| RF Power | 1150 Watt | 1350 Watt |
| Exposure Time | Axial view: Ultraviolet light- 15 seconds, Visible light- 15 seconds | Axial view: Ultraviolet light- 15 seconds,Visible light- 15 seconds |
| Radial view: Ultraviolet light- 15 seconds, Visible light- 15 seconds | Radial View: Ultraviolet light- 15 seconds,Visible light- 15 seconds |
EVS: Enhanced Vapour System sample introduction, Acid blank: 50% v/v HCl; Reductant: 0.5% m/v NaBH4 in 0.5% m/v NaOH. Aqueous sample introduction kit was used for analysis of Al and Pb. Hydride generation kit (EVS) was used for analysis of Hg.
Analysis of chloride and sulphate ions will be performed through kit-based colorimetric (AQUACheck chloride testing kit, WT004A, Himedia Laboratories Pvt Ltd, India) and turbidimetric methods (AQUACheck sulphate testing kit, WT043, Himedia Laboratories Pvt Ltd, India), respectively [32, 33]. As per manufacturers’ instruction, detection of sulphate ions will be performed by taking 1 mL of water sample in a test jar to which 5 drops of supplied reagent 043A and one spoonful of reagent 043B will be added. After a gentle mixing, the solution will be incubated for 5–10 minutes at room temperature to allow the reaction to occur. Then, the solution will be diluted to 10 mL and the test jar will be placed on a circular control sulphate disk of colour chart to observe black disk from above and match with the standard sulphate disk to find out sulphate levels as milligram per litre or parts per million by turbidity comparison. Similarly, for detection of chloride, 2 mL of water sample will be taken to test jar and added with spoonful of reagent 04A-1 and two drops of reagent 04A-2. After gentle mixing, reagent 04A-3 will be added drop by drop to this solution until the colour of entire solution will changes to bluish violet. Chloride content of the water sample will then be calculated using the formula:
Human exposure assessment
Following biomonitoring will be done to assess exposure in the selected participants:
i) Inhalational CS2 exposure. Iodine-azide test will be carried out for estimation of CS2 exposure of the participants. The iodine-azide test is based on the fact that certain constituents in the urine of persons exposed to CS2 catalyze the reaction between iodine and sodium azide [34, 35]. Participants will be asked to provide approximately 50 mL of clean catch spot urine sample in a sterile container which will be stored at -20°C till analysis. Preparation of reagents and buffers as well as the test procedures will be carried out as previously described [34, 36]. The exposure coefficient will be calculated to estimate Carbon disulfide exposure.
ii) Metal (Pb, Hg and Al) exposure. Blood levels of Pb, Al, and Hg will be examined in a sub-group of the studied population (sample from every tenth participants). For this, 1 mL of whole blood from each participant will be mixed with 6 mL of freshly prepared mixture of concentrated trace metal grade HNO3 and hydrogen peroxide (H2O2) in a ratio 2:1 (v/v) in high-purity polytetrafluoroethylene (PTFE-TFM) vessels. After gentle mixing of reactants, the PTFE-TFM vessels will be arranged in the rotor (24HVT80, Anton PAAR) and digestion will be carried out in the Anton Paar, Multimicrowave PRO Reaction System at 200°C for 15 minutes [37]. After digestion, the solutions resulting from each digested samples will be cooled to 40°C and diluted to 30 mL with distilled water. Blank will also be prepared for each cycle of digestion using distilled water, nitric acid, and hydrogen peroxide mixture. Analysis of Pb, Al and Hg in the digested blood samples will be carried out on ICP-OES (iCAP® 7400 Duo ICP-OES, ThermoFisher Scientific® Pvt Ltd) system using same run parameters as used for groundwater analysis. Recorded metal levels in each sample will be tabulated in ppb levels.
Health assessment
Following health-related data will be collected from all selected participants:
i) Questionnaire-based collection of data on demography, exposure and lifestyle. A structured questionnaire which has been developed by the researchers will be pilot-tested and thereafter wards used for collection of socioeconomic and demographic details along with information regarding lifestyle factors, occupational and relevant exposure history. The questionnaire will be administered by trained investigators in the local language.
ii) Anthropometry and blood pressure monitoring. Height and weight will be measured using stadiometer and weighing scale, respectively and will be used to calculate body mass index (BMI). As per guidelines for Indian population [38], BMI in the range of 18.5–22.9 kilogram per square metre (kg/m2) will be considered as normal, 23–24.9 kg/m2 as overweight and more than or equal to 25 kg/m2 will be considered as obesity. Blood pressure will be measured as per the International Society of Hypertension’s 2020 Global Hypertension Practice Guidelines [39] in sitting position on the left arm using validated electronic blood pressure monitor (Omron®), after 5 minutes of rest. Systolic and diastolic blood pressure will be recorded as the mean of last two of three readings taken 1 minute apart. Hypertension will be defined as the study participants having raised systolic and/or diastolic blood pressure (more than or equal to 140 and/or 90 millimetres of mercury i.e. mm Hg, respectively) [38].
iii) Clinical history and examination. The information regarding current and previous cardiorespiratory and neurological symptoms will be taken by the physicians from the participants using a clinical proforma. The details regarding the onset, duration, severity of symptoms, diurnal variation, aggravating/relieving factors, and associated symptoms details will be noted. A general physical examination and detailed respiratory, cardiological and neurological examination of all participants will be conducted which will include higher mental function examination, speech assessment, cranial nerve examinations, examination of the motor system and muscle power, examination of the sensory system, reflexes and coordination, and gait assessment, etc. All abnormal findings will be recorded in a pre-designed the clinical proforma.
iv) Neurobehavioral tests. CS2, mainly used in the production of viscose rayon fibre, is one of the main air pollutant in Nagda Industrial town. Although overt CS2-induced neurotoxicity is no longer a serious problem nowadays, neurobehavioral problems due to chronic exposure to low levels of CS2 are not uncommon [40]. Previous studies, although mostly conducted in occupational set-up, have shown problems in cognition [41, 42], fine coordination [43], learning and memory [44], visual attention & concentration [45], sensory and motor functions [46], and other behavioral functions [47] in humans due to CS2 exposure. Hence a battery of neurobehavioral tests (see Table 2) will be employed in the present study to screen any known neuro-behavioral effects due to CS2 exposure.
Table 2. Details of neurobehavioral tests to be administered to study participants.
| Name of the test | Domain measured | Method/instructions | Interpretation |
|---|---|---|---|
| Hindi Mental State Examination (HMSE) | Cognition | This is a validated 23 items questionnaire and widely used for assessing the cognitive impairment in Indian population [48]. | Sum total of the correct responses. |
| Finger dexterity test | Fine coordination | We will adopt the method used by Tiwari R. R. et. al. (Tiwari RR, Raghvan S, Tripathi S. Cardiological and neurological health effects in viscose rayon workers exposed to carbon disulphide. 2012. [Unpublished]) to perform finger dexterity test. The subject is given the instructions to pick three pins at a time with his/her preferred hand and go on filling into the holes row wise as fast as he/she can do in 180 seconds. | The score is the number of holes filled in 180 seconds. The higher the score, the more is the efficiency in performance. |
| Tweezer dexterity test | Fine coordination using a tweezer | We will adopt the method used by Tiwari R. R. et. al. (Tiwari RR, Raghvan S, Tripathi S. Cardiological and neurological health effects in viscose rayon workers exposed to carbon disulphide. 2012. [Unpublished]) to perform tweezer dexterity test. The subject is given the instructions to pick up with the tweezer one pin at a time with his/her preferred hand and begin to fill into the holes row wise as fast as he/she can do in 180 seconds. | The score is the number of holes filled in 180 seconds. The higher the score, the more is the efficiency in performance. |
| Letter-digit substitution test (LDST) | Verbal learning | The LDST consists of a worksheet, which has 8 rows and 20 columns and randomly arranged letters in rows and columns. We will follow previously published method to perform LDST. The subject will be first given the instructions to substitute target letters with digits and practice on a separate sheet for 1–2 rows. Then he/she will be instructed to substitute as many letters with digits as possible in 180 seconds [49]. | The net score will be obtained by deducting wrong substitutions from the total substitutions attempted [50]. |
| Memory forward and backward test | Forward span: attention efficiency and capacity. Backward span: executive task particularly dependent on working memory. | We will follow previously published methodology [51] to perform this test. A list of random numbers is read aloud and the subject is asked to recall the numbers in the same order (forwards) and then in reverse order (backwards). | The score will be reported as the maximum number of digits correctly produced forwards (forward subscore) and backwards (backward subscore) by the subject. |
| Serial three subtraction test | Concentration and attention | We will follow previously published methodology [52] to perform this test. The subject will be asked to count the numbers by serial subtractions of three from 20 backwards. | The time to complete the task along with errors will be noted as score [53]. |
| Trail making test (TMT) | Trail making test A: visual attention and processing speed Trail making test B: executive abilities including set shifting and mental flexibility |
We will follow previously published method [54] to perform TMT. In Trail A, the subjects are asked to draw lines connecting circled numbers on a page in sequence as quickly as they can. In Trail B, one alternates between numbers and letters. | In each test, the primary score is the time to complete the task. |
| Finger tapping test (FTT) | Motor functioning, specifically, motor speed and lateralized coordination | We will use a shorter version of Finger tapping test [55]. In this test, the subject’s palm of dominant hand should be immobile and flat on the board, with fingers extended, and the index finder placed on the counting device. The subject taps his index finger on the lever as quickly as possible within a 10 seconds time interval, in order to increase the number on the counting device with each tap. Such five trials are performed with 10 seconds rest interval in-between each trial [55]. | The score is the mean score of trials 3–5. |
v) Clinical investigations (PFT and ECG). PFT will be conducted using portable spirometer (Cosmed® Pony FX) on comfortably seated participants by a trained technician, supervised by a trained physician, following national guidelines [56], to measure forced expiratory volume in first second (FEV1), forced vital capacity(FVC), and peak expiratory flow. Of three acceptable spirograms defined as the difference between the two largest FVC and the two largest FEV1 measurements are less than or equal to 0.150 litres, the best value for each parameter will be included for analysis. The predicted values for the FVC will be calculated for each subject using Kamat’s Regression equation [57]. For categorizing pulmonary function impairment, cut-off of 80% of predictive FVC and 70% of FEV1/FVC ratio will be taken [58].
A portable 12-lead Electrocardiogram device (BPL CardiArt® 9108D) will be used to measure electrical activity of the heart following American Heart Association guidelines [59]. Briefly, in a private screened area, ECG recording will be done by previously trained medical staff on the chest and limbs of supine and relaxed participants. Trained physicians will interpret the ECG recording as per standard guidelines [60].
vi) Laboratory investigations. A battery of clinical laboratory investigations comprising of Random Blood Glucose, Complete Blood Counts, and Lipid profile (Total cholesterol, High and low density lipoprotein cholesterol, and Triglyceride) will be carried out for all participants of the study. Blood sample collection will be carried out by trained technician according to standard operating procedure in compliance with WHO Best Practices [61].
To undertake the afore-mentioned three tests, briefly, a total of 5 mL sample of venous blood will be collected from the median cubital vein of the participants by trained technician maintaining sterile precautions. 2 mL blood will be collected in ethylene diamine tetra acetic acid vacutainer for complete blood count which will be processed within 4 hours using a fully automated hematology analyzer (Sysmex®). 3 mL blood sample will be collected in plain vacutainer from which serum will be separated and stored at -20°C till analysis. The serum will be used for estimation of lipid profile using semi-automated biochemistry analyzer (ERBA®). Random blood glucose will be estimated on the spot from a drop of blood at the time of sample collection, using a hand held glucometer (Accu-Chek®).
Quality control
Necessary measures as per the National guidelines for data quality in surveys [62] such as data collection by trained researchers, validation and pilot-testing of study instruments prior to use, meticulous checking and standardization of equipment, and reagents to be used, etc. will be taken to maintain data quality at the highest possible level.
Data management and analysis
All participants’ data will be coded by assigning unique identifiers. These codes will be used to identify and link various parameters of the individual participants. Data from the filled questionnaires/clinical proforma as well as the laboratory analysis values will be entered into the latest version of Microsoft Excel spreadsheets. Statistician of the team will supervise data entry and to ensure error-free data at least 10% of the entered data will be randomly verified.
Once data entry is complete, hard copies of questionnaire/proforma will be stored in a secure archive in the institute. Similarly, all entered data will be stored in password protected computer systems with access restricted to the research team alone. The data collected/generated in this study will be stored for a period of ten years. The primary investigator will be in-charge of data safety and back-up maintenance. Anonymized data set will be shared publicly during publication of study results.
All statistical analyses will be performed using IBM SPSS Statistics (version 25). For ordinal and continuous variables, the values will be given as descriptive statistics (like mean, median, standard deviation, 95% confidence interval, interquartile range); and categorical variables will be given as numbers (percentages) and proportions. Association for different health aspects with pollution level of various parameters of air/water will be made using Pearson’s chi-square test for independent categorical data. By considering the variability within and between groups; t-test and ANOVA will be used to analyze the mean differences between two and more groups respectively for various anthropological parameters, the concentration of air/water pollutants, etc. The Mann–Whitney U test and Wilcoxon signed ranks test will be used for independent and dependent for ordinal and continuous data with respect to different health aspects. Pearson and Spearman’s correlation coefficient will be used to examine the association between different variables. Multiple linear and logistic regression models will be used to estimate the relationship for different neurological assessments with a set of the independent variable. For all statistical tests, two-sided P-values <0.05 will be considered to be significant.
Ethics and dissemination
Ethics approval has been obtained from the Institutional (Human) Ethics Committee, National Institute for Research in Environmental Health (No: NIREH/IEC-7-II/1027, dated January 7 2021). Before the initiation of research activities, local authorities of the selected village will be approached for consent and aid in logistics. Prior to the start of data collection written informed consent will be taken from each participant. Dissemination of the study findings will be done through submission of study technical report to the funding agency, distribution of health reports to all the participants while maintaining their confidentiality and finally, through publications.
Discussion
This manuscript describes the protocol for a cross-sectional study being conducted to comprehensively assess the health effects of residing near viscose rayon and associated chemical manufacturing industries. Viscose rayon is one of the oldest man-made commercial fiber [63] that is still quite popular today with almost 4.5 million tons being manufactured annually worldwide [64]. It is manufactured by chemically dissolving natural cellulose, commonly in the form of wood pulp [63], using a NaOH / CS2 dissolution system [64]. However, during this process almost 25–30% of the CS2 used is not recovered and escapes as emission into the ambient air [64]. In their review, Jiang et al., 2020 [64] report that for every ton of viscose produced, almost 20kg of exhaust and 300–600 tons of waste water containing heavy metals and other toxic residues are generated. Our study will produce up-to-date evidence specific to the Indian context which is especially relevant considering the fact that India is the second largest manufacturer of rayon, next only to China [63].
Chronic low dose exposure to CS2, a major component of the viscose rayon industry exhaust, affects human physiology especially the cardiovascular and nervous system [10, 11. 65]. Studies have documented abnormalities in blood pressure [66], ECG findings [67], and lipid profile [68]. Similarly CS2 exposure has been linked to peripheral neuropathy [69], abnormalities in colour vision [70] and neurobehavioral pattern [71]. Though fewer studies have been done to assess the effects of CS2 on the respiratory system, Spyker et al., 1982 [72] showed alterations in pulmonary function. Effect on eyes, reproductive system and renal system have also been noted, although following prolonged heavy exposure [46]. Other gaseous pollutants emitted during viscose rayon and associated chemical manufacturing like H2S, HCl, Cl2, and SO2 are known to affect human health, particularly the cardiorespiratory and nervous system [73–75].
Similarly, water and soil contamination occurring due to effluents being discharged by viscose rayon manufacturing industries has been reported in literature [76]. Effluents of textile industries using high amounts of salts may promote groundwater salinization due to the high mobility of salts/ions in the soil, and thus deteriorate drinking and irrigation water quality that may impact human health and land fertility/ crop productivity [77, 78]. The viscose fibre wastewater has also been shown to have a wide variety of residues ranging from heavy metals to cellulose and lignin remnants, most of which resist biodegradation [9, 79]. Previously published studies [80] report exposure of workers in viscose rayon industries to heavy metals like mercury, lead, etc. Chronic heavy metal exposure is again known to cause multiple adverse cardiorespiratory and neurological effects [81].
Most of the evidence regarding health effects of exposure to pollution emanating from viscose rayon and associated chemical manufacturing originates from outside India with there being hardly any published epidemiological evidence focussing on either workers in Indian industries [82] or nearby residents. However, the difficulties perceived by the residents living in Nagda industrial region has been scientifically documented [15] as well as has been highlighted in multiple other communication media [83]. Previous reports by local regulatory authorities [18] showed substantial heavy metal contamination of groundwater in and around Nagda region. Furthermore, the chemical industries in Nagda region are engaged in production of various chlorinated and sulphur-containing product (e.g., poly-aluminium chloride, bleaching powder, calcium chloride, chloromethane, chlorosulphonic acid) [84] which can potentially lead to introduction of other heavy metals like Aluminium and ions like Cl- and SO4- ions into the surrounding groundwater. Therefore, this study is planned to collect pertinent and timely comprehensive information on the combined effect of the multiple toxic gases including H2S, Sulphur oxides, HCl, Cl2, etc. emanating as exhaust from the viscose rayon and associated chemical manufacturing industries as well as to assess the physiological parameters and heavy metal/ ion concentration in the groundwater in the study area.
Industrial pollution is a multidimensional issue since it occurs in multiple environmental matrices and in turn affects multiple organ systems in the human. Therefore, in our study we will focus on multiple objectives with an overarching goal to assess the potential health effects of exposure. A major strength of this study is therefore its multidisciplinary research team with expertise of investigators ranging from health sciences like epidemiology and neurology to environmental sciences and basic science. To strengthen the association between pollutant concentration in environmental matrices and health effects, human exposure assessment will be done in a subset of the population to ascertain dose-response relation. Therefore, exposure to CS2 will be confirmed using the urine azide test which has been reported to adequately reflect occupational CS2 inhalation by published literature [34–36]. Furthermore, blood levels of heavy metals will also be determined to estimate exposure of villagers to toxic metals, viz. Pb, Al and Hg from various environmental matrices, viz. air, water and food/soil. However, pertaining to resource limitation, biomonitoring will be conducted in a subset of the total study population.
The current study also faces certain limitations. Long term follow-up of the participants to establish a temporal relationship between exposure and outcome is not possible in our cross-sectional study. Considering the ongoing dispute between local residents, viscose rayon manufacturers and the local authorities, the current study prioritised timely delivery of baseline health effect information. Future research might therefore focus on longitudinal data collection on the environmental health effects of viscose rayon manufacturing. Furthermore, past exposure assessment will be done through questionnaire data and is therefore, prone to recall bias. Although data will be collected for multiple relevant confounders including other environmental/meteorological, socioeconomic and diet/lifestyle factors, the risk of residual confounding cannot be completely eliminated.
Acknowledgments
We would like to acknowledge the technical and field staff of ICMR-NIREH, participants as well as the local authorities and health workers working in the villages where we conducted our exposure assessment and health outcome assessment surveys. We would also like to thank Dr. Vivek Parashar, Research Officer, R.D. Gardi Medical College Ujjain, Madhya Pradesh, India, for his support in creating the study area maps.
Abbreviations
- Al
Aluminium
- ANOVA
Analysis Of Variance (Statistical Test)
- BMI
Body Mass Index
- Cl
Chloride Ion
- Cl2
Chlorine Gas
- CPCB
Central Pollution Control Board
- CS2
Carbon Disulphide
- ECG
Electrocardiogram
- FEV1
Forced Expiratory Volume In first Second
- FVC
Forced Vital Capacity
- H2S
Hydrogen Disulphide
- HCl
Hydrogen Chloride
- Hg
Mercury
- HNO3
nitric acid
- MP
Madhya Pradesh
- Pb
Lead
- PFT
Pulmonary Function Test
- pH
Potential Of Hydrogen (Measure Of Acidity/Basicity)
- ppb
parts per billion
- SO2
Sulphur Dioxide
- SO3
Sulphur Trioxide
- SO42
Sulphate Ion
- °C
Degrees Celsius
- km
Kilometre
- kg/m2
Kilogram Per Square Metre
- mL
Millilitre
- mgL-1
Milligram Per Litre
- mm Hg
Millimetre Of Mercury
Data Availability
No datasets were generated or analysed during the current study. All relevant data from this study will be made available upon study completion.
Funding Statement
The study on which this protocol is based was funded as a consultancy project by the Aditya Birla Grasim Industry Ltd (Service No. 4700221679/104; dated 10.11.2020) [Funding issued to Subroto Shambhu Nandi] which owns the major viscose rayon manufacturing unit in the study area (Birlagram Nagda) as per the directions of local pollution control authorities. The funders had and will not have a role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Rahman M. M., Alam K., and Velayutham E., “Is industrial pollution detrimental to public health? Evidence from the world’s most industrialised countries,” BMC Public Health, vol. 21, no. 1, p. 1175, Jun. 2021, doi: 10.1186/s12889-021-11217-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Sario M. et al., “A scoping review of the epidemiological methods used to investigate the health effects of industrially contaminated sites,” Epidemiol Prev, vol. 42, no. 5-6S1, pp. 59–68, Dec. 2018, doi: 10.19191/EP18.5-6.S1.P059.088 [DOI] [PubMed] [Google Scholar]
- 3.Chatham-Stephens K. et al., “Burden of disease from toxic waste sites in India, Indonesia, and the Philippines in 2010,” Environ Health Perspect, vol. 121, no. 7, pp. 791–796, Jul. 2013, doi: 10.1289/ehp.1206127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dixit P. and Lal R. C., “Inclusive Growth & Social Responsibility—A Critical Analysis of IndianTextile Industry,” MERC Global’s International Journal of Management, vol. 7, no. 2, pp. 202–210, 2019. [Google Scholar]
- 5.Bhatia D., Sharma N. R., Kanwar R., and Singh J., “Physicochemical assessment of industrial textile effluents of Punjab (India),” Applied Water Science, vol. 8, no. 3, p. 83, May 2018, doi: 10.1007/s13201-018-0728-4 [DOI] [Google Scholar]
- 6.Manikandan P., Palanisamy P. N., and Baskar R., “Physicochemical analayis of textile industrial effluents from Tirupur city, TN, India,” undefined, 2015, Accessed: Sep. 27, 2021. [Online]. Available: https://www.semanticscholar.org/paper/PHYSICO-CHEMICAL-ANALYSIS-OF-TEXTILE-INDUSTRIAL-TN%2C-Manikandan-Palanisamy/f7b02c20e197ce3bde89d48a197365d62ab869e7 [Google Scholar]
- 7.Aldalbahi A., El-Naggar M. E., El-Newehy M. H., Rahaman M., Hatshan M. R., and Khattab T. A., “Effects of Technical Textiles and Synthetic Nanofibers on Environmental Pollution,” Polymers, vol. 13, no. 1, 2021, doi: 10.3390/polym13010155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shirvanimoghaddam K., Motamed B., Ramakrishna S., and Naebe M., “Death by waste: Fashion and textile circular economy case,” Science of The Total Environment, vol. 718, p. 137317, May 2020, doi: 10.1016/j.scitotenv.2020.137317 [DOI] [PubMed] [Google Scholar]
- 9.Madhav S., Ahamad A., Singh P., and Mishra P. K., “A review of textile industry: Wet processing, environmental impacts, and effluent treatment methods,” Environmental Quality Management, vol. 27, no. 3, pp. 31–41, Mar. 2018, doi: 10.1002/tqem.21538 [DOI] [Google Scholar]
- 10.Pappuswamy M., Sundaram R., Kuppanna H. M., and Periyasamy T., “Carbon Disulfide (CS2) Induced Chromosomal Alterations and Apoptosis in Circulated Blood Lymphocytes of Personnel Working in Viscose Industry,” Asian Pacific Journal of Cancer Biology, vol. 3, no. 1, Art. no. 1, Feb. 2018, doi: 10.31557/apjcb.2018.3.1.17-24 [DOI] [Google Scholar]
- 11.Sieja K., von Mach-Szczypiński J., and von Mach-Szczypiński J., “Health effect of chronic exposure to carbon disulfide (CS 2) on women employed in viscose industry,” Med Pr, vol. 69, no. 3, pp. 329–335, May 2018, doi: 10.13075/mp.5893.00600 [DOI] [PubMed] [Google Scholar]
- 12.Bedi R., “Evaluation of occupational environment in two textile plants in Northern India with specific reference to noise,” Ind Health, vol. 44, no. 1, pp. 112–116, Jan. 2006, doi: 10.2486/indhealth.44.112 [DOI] [PubMed] [Google Scholar]
- 13.Sellappa S., Prathyumnan S., Joseph S., Keyan K. S., and Balachandar V., “Genotoxic effects of textile printing dye exposed workers in India detected by micronucleus assay,” Asian Pac J Cancer Prev, vol. 11, no. 4, pp. 919–922, 2010. [PubMed] [Google Scholar]
- 14.Singh M. B., Fotedar R., and Lakshminarayana J., “Occupational morbidities and their association with nutrition and environmental factors among textile workers of desert areas of Rajasthan, India,” J Occup Health, vol. 47, no. 5, pp. 371–377, Sep. 2005, doi: 10.1539/joh.47.371 [DOI] [PubMed] [Google Scholar]
- 15.Pinney C., “On living in the kal(i)yug: Notes from Nagda, Madhya Pradesh,” Contributions to Indian Sociology, vol. 33, no. 1–2, pp. 77–106, Feb. 1999, doi: 10.1177/006996679903300106 [DOI] [Google Scholar]
- 16.Central Pollution Control Board, “Comprehensive Environmental Assessment of Industrial Clusters,” Ministry of Environment and Forests, New Delhi, India, EIAS/5/2009–2010, Dec. 2009. Accessed: Aug. 09, 2021. [Online]. Available: https://cpcb.nic.in/displaypdf.php?id=Q1BBL05ld0l0ZW1fMTUyX0ZpbmFsLUJvb2tfMi5wZGY=
- 17.Central Pollution Control Board, “National Green Tribunal- CPCB to rank industrial Units on pollution level,” OA No 1038/2018, 2019. Accessed: Apr. 25, 2022. [Online]. Available: https://greentribunal.gov.in/sites/default/files/all_documents/Final%20Report%20in%20OA%20No1038%20of%202018%20The%20Asian%20Age.pdf
- 18.“Order of the National Green Tribunal regarding pollution by Grasim Chemical Division, Birlagram, Ujjain district, Madhya Pradesh.” India Environment Portal, Nov. 09, 2020. Accessed: Feb. 09, 2021. [Online]. Available: http://www.indiaenvironmentportal.org.in/files/file/Nagada-Grasim-industry-NGT-order-April7-2021.pdf
- 19.“Madhya Pradesh Population 2011–2020.” https://www.census2011.co.in/census/state/madhya+pradesh.html (accessed Aug. 03, 2020).
- 20.“Madhya Pradesh Presentation and Economy Growth Report | IBEF.” https://www.ibef.org/states/madhya-pradesh-presentation (accessed Aug. 13, 2021).
- 21.Br.MSME- Development Institute, “Brief Industrial Profile of Ujjain District Madhya Pradesh,” Ministry of MSME, Govt. of India, Rewa, Madhya Pradesh. Accessed: Aug. 13, 2021. [Online]. Available: http://dcmsme.gov.in/old/dips/Ujaain.pdf
- 22.“Report on Prevention & Control of pollution in River Chambal An Action Plan for Rejuvenation,” Regional Office M.P. Pollution control Board, Ujjain (M.P.), 2019. Accessed: Aug. 13, 2021. [Online]. Available: http://www.mppcb.nic.in/proc/NGT%20Chambal-River.pdf
- 23.Reddy P. B. and Baghel B. S., “Impact of Industrial Wastewaters on the Physicochemical Characteristics of Chambal River at Nagda, M. P., India,” Nature Environment and Pollution Technology, vol. 9, no. 3, p. 8, 2010. [Google Scholar]
- 24.Kuo H.-W., Lai J.-S., Lin M., and Su E.-S., “Effects of exposure to carbon disulfide (CS2) on electrocardiographic features of ischemic heart disease among viscose rayon factory workers,” International Archives of Occupational and Environmental Health, vol. 70, no. 1, pp. 61–66, Nov. 1997, doi: 10.1007/s004200050187 [DOI] [PubMed] [Google Scholar]
- 25.Krishnan T. N. and Poulose S., “Response rate in industrial surveys conducted in India: Trends and implications,” IIMB Management Review, vol. 28, no. 2, pp. 88–97, Jun. 2016, doi: 10.1016/j.iimb.2016.05.001 [DOI] [Google Scholar]
- 26.Martin-Olmedo P. et al., “Environmental and health data needed to develop national surveillance systems in industrially contaminated sites,” Epidemiol Prev, vol. 42, no. 5-6S1, pp. 11–20, Dec. 2018, doi: 10.19191/EP18.5-6.S1.P011.084 [DOI] [PubMed] [Google Scholar]
- 27.Sharan M., Yadav A. K., Singh M. P., Agarwal P., and Nigam S., “A mathematical model for the dispersion of air pollutants in low wind conditions,” Atmospheric Environment, vol. 30, no. 8, pp. 1209–1220, Apr. 1996, doi: 10.1016/1352-2310(95)00442-4 [DOI] [Google Scholar]
- 28.“National ambient air quality standards,” Central Pollution Control Board, New Delhi, 2009. Accessed: Sep. 30, 2021. [Online]. Available: https://cpcb.nic.in/openpdffile.php?id=UHVibGljYXRpb25GaWxlLzYzMF8xNDU3NTA2Mjk1X1B1YmxpY2F0aW9uXzUxNF9haXJxdWFsaXR5c3RhdHVzMjAwOS5wZGY=
- 29.“SESDPROC-301-R3, Groundwater Sampling procedure, U.S. EPA, 2013,” U.S. EPA, Georgia, 2013. Accessed: Sep. 24, 2021. [Online]. Available: https://www.epa.gov/sites/default/files/2015-06/documents/Groundwater-Sampling.pdf
- 30.T. D. Martin, C. A. Brockhoff, J. T. Creed, and EMMC Methods Work Group, “Method 200.7, Revision 4.4: Determination of Metals and Trace Elements in Water and Wastes by Inductively Coupled Plasma-Atomic Emission Spectrometry,” US Environmental Protection Agency, Cincinnati, 1994. Accessed: Sep. 30, 2021. [Online]. Available: https://www.epa.gov/sites/default/files/2015-08/documents/method_200-7_rev_4-4_1994.pdf
- 31.N. Bartsch, “Analysis of Hydride Forming Elements with the Thermo Scientific iCAP 7000 Plus Series ICP-OES and the Basic Hydride Generator Kit.” Thermo Fisher Scientific, 2016. Accessed: Mar. 29, 2022. [Online]. Available: https://assets.thermofisher.com/TFS-Assets/CMD/Application-Notes/AN-43374-ICP-OES-Hydride-Forming-Elements-AN43374-EN.pdf
- 32.IS 2035 (Part 24), “Method of Sampling and Test (Physical and Chemical) for Water and Wastewater, Part 24: Sulphates (First Revision). ICS 13.060.50.” Bureau of Indian Standards, Manak Bhavan, New Delhi, India, 2009. Accessed: Mar. 29, 2022. [Online]. Available: https://www.iitk.ac.in/ce/test/IS-codes/is.3025.24.1986.pdf
- 33.IS 2035 (Part 32), “Method of Sampling and Test (Physical and Chemical) for Water and Wastewater, Part 32: Chloride (First Revision). ICS 13.060.50.” Bureau of Indian Standards, Manak Bhavan, New Delhi, India, 2007. Accessed: Mar. 29, 2022. [Online]. Available: https://www.iitk.ac.in/ce/test/IS-codes/is.3025.32.1988.pdf
- 34.Djurić D., Surducki N., and Berkes I., “Iodine-azide test on urine of persons exposed to carbon disulphide,” Br J Ind Med, vol. 22, no. 4, pp. 321–323, Oct. 1965, doi: 10.1136/oem.22.4.321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vasak V., Vanecek M., and Kimmelova B., “[Assessment of the exposure of workers in areas contaminated by carbon disulfide vapors. ii. use of the iodine azide reaction for the detection and estimation of sulfide metabolites in the urine],” Prac Lek, vol. 15, pp. 145–149, May 1963. [PubMed] [Google Scholar]
- 36.Rosier J., Billemont G., Van Peteghem C., Vanhoorne M., Grosjean R., and Van de Walle A., “Relation between the iodine azide test and the TTCA test for exposure to carbon disulphide,” Br J Ind Med, vol. 41, no. 3, pp. 412–416, Aug. 1984, doi: 10.1136/oem.41.3.412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alrobaian M. and Arida H., “Assessment of Heavy and Toxic Metals in the Blood and Hair of Saudi Arabia Smokers Using Modern Analytical Techniques,” International Journal of Analytical Chemistry, vol. 2019, p. e7125210, Jun. 2019, doi: 10.1155/2019/7125210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Centre for Disease Control, “Module for Medical Officers- Population-based screening for Non-Communicable Diseases [National Programme for Prevention and Control of Diabetes, Cardiovascular Disease and Stroke],” Ministry of Health and Family Welfare, Government of India., New Delhi, 2017. Accessed: Apr. 25, 2022. [Online]. Available: https://extranet.who.int/ncdccs/Data/IND_D1_NCD%20Module%20For%20Medical%20Officers%20-%20Population%20based%20screening.pdf
- 39.Unger T. et al., “2020 International Society of Hypertension Global Hypertension Practice Guidelines,” Hypertension, vol. 75, no. 6, pp. 1334–1357, Jun. 2020, doi: 10.1161/HYPERTENSIONAHA.120.15026 [DOI] [PubMed] [Google Scholar]
- 40.Liang Y., Jing X., Shen G., Li Y., and Li R., “Health effects on low level CS2 exposure in viscose rayon workers—An epidemiological study on psychological effects,” Acta Academiae Medicinae Primae Shanghai, vol. 10, no. 1, pp. 15–20, 1983. [Google Scholar]
- 41.Kruse A., Borch-Johnsen K., and Pedersen L. M., “Cerebral Damage Following a Single High Exposure to Carbon Disulphide,” Occupational Medicine, vol. 32, no. 1, pp. 44–45, Jan. 1982, doi: 10.1093/occmed/32.1.44 [DOI] [PubMed] [Google Scholar]
- 42.Ku M.-C. et al., “Diffuse White Matter Lesions in Carbon Disulfide Intoxication: Microangiopathy or Demyelination,” European Neurology, vol. 50, no. 4, pp. 220–224, 2003, doi: 10.1159/000073863 [DOI] [PubMed] [Google Scholar]
- 43.De Fruyt F., Thiery E., De Bacquer D., and Vanhoorne M., “Neuropsychological Effects of Occupational Exposures to Carbon Disulfide and Hydrogen Sulfide,” null, vol. 4, no. 3, pp. 139–146, Jul. 1998, doi: 10.1179/oeh.1998.4.3.139 [DOI] [PubMed] [Google Scholar]
- 44.Krstev S., Peruničić B., Farkić B., and Banićević R., “Neuropsychiatric Effects in Workers with Occupational Exposure to Carbon Disulfide,” Journal of Occupational Health, vol. 45, no. 2, pp. 81–87, Mar. 2003, doi: 10.1539/joh.45.81 [DOI] [PubMed] [Google Scholar]
- 45.Cassitto M. G., Camerino D., Imbriani M., Contardi T., Masera L., and Gilioli R., “Carbon disulfide and the central nervous system: a 15-year neurobehavioral surveillance of an exposed population,” Environ Res, vol. 63, no. 2, pp. 252–263, Nov. 1993, doi: 10.1006/enrs.1993.1145 [DOI] [PubMed] [Google Scholar]
- 46.Newhook R., Meek M. E., Caldbrick D., World Health Organization, and International Programme on Chemical Safety, Carbon disulfide. Geneva: World Health Organization, 2002. Accessed: Apr. 25, 2022. [Online]. Available: https://apps.who.int/iris/handle/10665/42554 [Google Scholar]
- 47.Putz-Anderson V. et al., “A behavioral examination of workers exposed to carbon disulfide,” Neurotoxicology, vol. 4, no. 1, pp. 67–77, 1983. [PubMed] [Google Scholar]
- 48.Ganguli M. et al., “A hindi version of the MMSE: The development of a cognitive screening instrument for a largely illiterate rural elderly population in india,” International Journal of Geriatric Psychiatry, vol. 10, no. 5, pp. 367–377, May 1995, doi: 10.1002/gps.930100505 [DOI] [Google Scholar]
- 49.Pradhan B. and Nagendra H., “Normative data for the digit-letter substitution task in school children,” Int J Yoga, vol. 2, no. 2, pp. 69–72, Jul. 2009, doi: 10.4103/0973-6131.60047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Natu M. and Agarwal A., “Testing of stimulant effects of coffee on the psychomotor performance: an exercise in clinical pharmacology,” Indian Journal of Pharmacology, vol. 29, p. 11, 1997. [Google Scholar]
- 51.Fink H. A. et al., Cognitive Outcomes After Cardiovascular Procedures in Older Adults: A Systematic Review. Rockville (MD): Agency for Healthcare Research and Quality (US), 2014. Accessed: Nov. 24, 2021. [Online]. Available: http://www.ncbi.nlm.nih.gov/books/NBK285350/ [PubMed] [Google Scholar]
- 52.Kennedy D. O. and Scholey A. B., “Glucose administration, heart rate and cognitive performance: effects of increasing mental effort,” Psychopharmacology, vol. 149, no. 1, pp. 63–71, Mar. 2000, doi: 10.1007/s002139900335 [DOI] [PubMed] [Google Scholar]
- 53.Williams M. A., LaMarche J. A., Alexander R. W., Stanford L. D., Fielstein E. M., and Boll T. J., “Serial 7s and Alphabet Backwards as brief measures of information processing speed,” Archives of Clinical Neuropsychology, vol. 11, no. 8, pp. 651–659, Jan. 1996, doi: 10.1093/arclin/11.8.651 [DOI] [Google Scholar]
- 54.Corrigan J. D. and Hinkeldey N. S., “Relationships between Parts A and B of the Trail Making Test,” Journal of Clinical Psychology, vol. 43, no. 4, pp. 402–409, Jul. 1987, doi: [DOI] [PubMed] [Google Scholar]
- 55.Ashendorf L., Horwitz J. E., and Gavett B. E., “Abbreviating the Finger Tapping Test,” Archives of Clinical Neuropsychology, vol. 30, no. 2, pp. 99–104, Mar. 2015, doi: 10.1093/arclin/acu091 [DOI] [PubMed] [Google Scholar]
- 56.Aggarwal A. N. et al., “Joint Indian Chest Society-National College of Chest Physicians (India) guidelines for spirometry,” Lung India, vol. 36, no. Supplement, pp. S1–S35, Apr. 2019, doi: 10.4103/lungindia.lungindia_300_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kamat S. R. et al., “Indian norms for pulmonary function: observed values prediction equations and intercorrelations,” J Assoc Physicians India, vol. 25, no. 8, pp. 531–540, Aug. 1977. [PubMed] [Google Scholar]
- 58.Pellegrino R. et al., “Interpretative strategies for lung function tests,” Eur Respir J, vol. 26, no. 5, pp. 948–968, Nov. 2005, doi: 10.1183/09031936.05.00035205 [DOI] [PubMed] [Google Scholar]
- 59.Sandau K. E. et al., “Update to Practice Standards for Electrocardiographic Monitoring in Hospital Settings: A Scientific Statement From the American Heart Association,” Circulation, vol. 136, no. 19, pp. e273–e344, Nov. 2017, doi: 10.1161/CIR.0000000000000527 [DOI] [PubMed] [Google Scholar]
- 60.Hampton J. and Hampton J., The ECG Made Easy, 9th ed. Elsevier, 2019. [Google Scholar]
- 61.Best practice in phlebotomy and blood collection. Geneva: World Health Organization, 2010. Accessed: Sep. 24, 2021. [Online]. Available: https://www.ncbi.nlm.nih.gov/books/NBK138496/
- 62.ICMR-National Institute of Medical Statistics, National Guidelines for Data Quality in Surveys. New Delhi: ICMR-National Institute of Medical Statistics, 2021. Accessed: Sep. 27, 2021. [Online]. Available: https://ndqf.in/wp-content/uploads/2021/07/National-Guidelines-for-DATA-QUALITY-in-Surveys.pdf
- 63.Baker I., “Rayon,” in Fifty Materials That Make the World, Baker I., Ed. Cham: Springer International Publishing, 2018, pp. 195–197. doi: 10.1007/978-3-319-78766-4_37 [DOI] [Google Scholar]
- 64.Jiang X., Bai Y., Chen X., and Liu W., “A review on raw materials, commercial production and properties of lyocell fiber,” Journal of Bioresources and Bioproducts, vol. 5, no. 1, pp. 16–25, Feb. 2020, doi: 10.1016/j.jobab.2020.03.002 [DOI] [Google Scholar]
- 65.Gelbke H.-P., Göen T., Mäurer M., and Sulsky S. I., “A review of health effects of carbon disulfide in viscose industry and a proposal for an occupational exposure limit,” Crit Rev Toxicol, vol. 39 Suppl 2, pp. 1–126, Oct. 2009, doi: 10.1080/10408440902837967 [DOI] [PubMed] [Google Scholar]
- 66.Chang S.-J., Chen C.-J., Shih T.-S., Chou T.-C., and Sung F.-C., “Risk for hypertension in workers exposed to carbon disulfide in the viscose rayon industry,” Am J Ind Med, vol. 50, no. 1, pp. 22–27, Jan. 2007, doi: 10.1002/ajim.20409 [DOI] [PubMed] [Google Scholar]
- 67.Chang S.-J. et al., “Electrocardiographic abnormality for workers exposed to carbon disulfide at a viscose rayon plant,” J Occup Environ Med, vol. 48, no. 4, pp. 394–399, Apr. 2006, doi: 10.1097/01.jom.0000190302.35094.7f [DOI] [PubMed] [Google Scholar]
- 68.Takebayashi T. et al., “A Six Year Follow up Study of the Subclinical Effects of Carbon Disulphide Exposure on the Cardiovascular System,” Occupational and Environmental Medicine, vol. 61, no. 2, pp. 127–134, 2004, doi: 10.1136/oem.2002.006858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Corsi G., Maestrelli P., Picotti G., Manzoni S., and Negrin P., “Chronic peripheral neuropathy in workers with previous exposure to carbon disulphide,” Br J Ind Med, vol. 40, no. 2, pp. 209–211, May 1983, doi: 10.1136/oem.40.2.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ruijten M. W., Sallé H. J., Verberk M. M., and Muijser H., “Special nerve functions and colour discrimination in workers with long term low level exposure to carbon disulphide,” Br J Ind Med, vol. 47, no. 9, pp. 589–595, Sep. 1990, doi: 10.1136/oem.47.9.589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Godderis L., Braeckman L., Vanhoorne M., and Viaene M., “Neurobehavioral and clinical effects in workers exposed to CS (2),” International journal of hygiene and environmental health, vol. 209, pp. 139–50, Apr. 2006, doi: 10.1016/j.ijheh.2005.09.005 [DOI] [PubMed] [Google Scholar]
- 72.Spyker D. A., Gallanosa A. G., and Suratt P. M., “Health effects of acute carbon disulfide exposure,” J Toxicol Clin Toxicol, vol. 19, no. 1, pp. 87–93, Mar. 1982, doi: 10.3109/15563658208990369 [DOI] [PubMed] [Google Scholar]
- 73.White C. W. and Martin J. G., “Chlorine gas inhalation: human clinical evidence of toxicity and experience in animal models,” Proc Am Thorac Soc, vol. 7, no. 4, pp. 257–263, Jul. 2010, doi: 10.1513/pats.201001-008SM [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lewis R. J. and Copley G. B., “Chronic low-level hydrogen sulfide exposure and potential effects on human health: a review of the epidemiological evidence,” Crit Rev Toxicol, vol. 45, no. 2, pp. 93–123, Feb. 2015, doi: 10.3109/10408444.2014.971943 [DOI] [PubMed] [Google Scholar]
- 75.Wu Y., Li R., Cui L., Meng Y., Cheng H., and Fu H., “The high-resolution estimation of sulfur dioxide (SO2) concentration, health effect and monetary costs in Beijing,” Chemosphere, vol. 241, p. 125031, Feb. 2020, doi: 10.1016/j.chemosphere.2019.125031 [DOI] [PubMed] [Google Scholar]
- 76.Konstantinova E. et al., “Geochemical transformation of soil cover and vegetation in a drained floodplain lake affected by long-term discharge of effluents from rayon industry plants, lower Don River Basin, Southern Russia,” Environmental Geochemistry and Health, Aug. 2020, doi: 10.1007/s10653-020-00683-3 [DOI] [PubMed] [Google Scholar]
- 77.Kumar S. et al., “Assessment of heavy metal pollution in groundwater of an industrial area: a case study from Ramgarh, Jharkhand, India,” null, pp. 1–23, Oct. 2020, doi: 10.1080/03067319.2020.1828391 [DOI] [Google Scholar]
- 78.Shaji E., Gómez-Alday J. J., Hussein S., Deepu T. R., and Anilkumar Y., “Salinization and Deterioration of Groundwater Quality by Nitrate and Fluoride in the Chittur Block, Palakkad, Kerala,” Journal of the Geological Society of India, vol. 92, no. 3, pp. 337–345, Sep. 2018, doi: 10.1007/s12594-018-1017-4 [DOI] [Google Scholar]
- 79.Ding C.-Q. et al., “Study on community structure of microbial consortium for the degradation of viscose fiber wastewater,” Bioresources and Bioprocessing, vol. 4, no. 1, p. 31, Jul. 2017, doi: 10.1186/s40643-017-0159-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vanhoorne M., Berge L. V. D., Devreese A., Tijtgat E., Poucke L. V., and Peteghem C. V., “Survey of chemical exposures in a viscose rayon plant,” The Annals of Occupational Hygiene, vol. 35, no. 6, pp. 619–631, Dec. 1991, doi: 10.1093/annhyg/35.6.619 [DOI] [PubMed] [Google Scholar]
- 81.Briffa J., Sinagra E., and Blundell R., “Heavy metal pollution in the environment and their toxicological effects on humans,” Heliyon, vol. 6, no. 9, pp. e04691–e04691, Sep. 2020, doi: 10.1016/j.heliyon.2020.e04691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.“Employment Generated Through Textile Sector.” https://pib.gov.in/Pressreleaseshare.aspx?PRID=1575203 (accessed Apr. 25, 2022).
- 83.The Changing Markets Foundation, “Pollution and disease: report reveals disastrous impact of the viscose giant behind high street brands,” 2018. Accessed: Aug. 13, 2021. [Online]. Available: http://changingmarkets.org/wp-content/uploads/2018/02/Dirty-Fashion-2018-press-release-FINAL.pdf
- 84.Grasim Industries Limited, “Nagda Environment Clearance Compliance Report (Oct 20-Mar 21).” May 31, 2021. Accessed: Nov. 24, 2021. [Online]. Available: https://www.grasim.com/Upload/PDF/nagda-environment-clearance-compliance-report-oct-20-mar-21.pdf