Abstract
Cardiovascular disease is a leading cause of death among cancer survivors, second only to cancer recurrence or development of new tumors. Cardio-oncology has therefore emerged as a relatively new specialty focused on prevention and management of cardiovascular consequences of cancer therapies. Yet challenges remain regarding precision and accuracy with predicting individuals at highest risk for cardiotoxicity. Barriers such as access to care also limit screening and early diagnosis to improve prognosis. Thus, developing innovative approaches for prediction and early detection of cardiovascular illness in this population is critical. In this review, we provide an overview of the present state of machine learning applications in cardio-oncology. We begin by outlining some factors that should be considered while utilizing machine learning algorithms. We then examine research in which machine learning has been applied to improve prediction of cardiac dysfunction in cancer survivors. We also highlight the use of artificial intelligence (AI) in conjunction with electrocardiogram (ECG) to predict cardiac malfunction and also atrial fibrillation (AF), and we discuss the potential role of wearables. Additionally, the article summarizes future prospects and critical takeaways for the application of machine learning in cardio-oncology. This study is the first in a series on artificial intelligence in cardio-oncology, and complements our manuscript on echocardiography and other forms of imaging relevant to cancer survivors cared for in cardiology clinical practice.
Keywords: Precision, Cardio-oncology, Malignancy, Cancer, Cardiomyopathy, Prevention, Artificial intelligence, Electrocardiography
1. Introduction
Approximately 370,000 cancer survivors die from cardiovascular diseases each year [1]. Thus, cardio-oncology has been developed as a relatively new subspecialty in medicine that focuses on the prevention and management of adverse cardiovascular effects associated with cancer therapies [2], [3], [4]. A small subset of the field is devoted to the diagnosis and management of primary or secondary heart tumors [5]. The majority of the field is devoted to chemotherapy, radiotherapy, and immunotherapy-related cardiotoxicity. While anthracyclines are the most frequently investigated medications and are commonly associated with cardiomyopathy, new cardiotoxic pharmacologic agents are continuously being developed, associated with a variety of cardiovascular (CV) effects (Table 1, Table 2). Conventional chemotherapies, as well as endocrine therapies, and targeted or immunotherapies can all cause cardiovascular toxicities [6], [7], [8], [9], [10], [11], [12], [13], [14], as can radiation therapy [15], [16], [17].
Table 1.
Cardiovascular toxicities associated with traditional chemotherapies.
| Cancer type | Class | Chemotherapeutic agents | Cardiovascular effects |
|---|---|---|---|
| Breast cancer, sarcoma, leukemia, and lymphomas | Anthracyclines | Doxorubicin, daunorubicin, idarubicin, epirubicin, and mitoxantrone | Increase clinical cardiotoxicity [36]. |
| Breast, head and neck, and gastrointestinal cancers | Fluoropyrimidines/antimetabolites | 5-Fluorouracil (5-FU) and capecitabine | Angina, myocardial infarction, arrhythmias, and infrequently acute pulmonary edema [37]. |
| Breast, head and neck, and gastrointestinal cancers | Microtubule-targeting agents | Paclitaxel and docetaxel | Abnormal conduction (e.g., bradycardia and heart block) [38], [39]. |
| Breast cancers and lymphomas | Alkylating agents | Cyclophosphamide Cisplatin and busulfan |
Ventricular dysfunction and pericardial disease [40], [41]. Cisplatin and busulfan are associated with cardiovascular toxicity (conduction abnormalities and arteriovenous thromboembolism) [42], [43]. |
Table 2.
Cardiovascular toxicities associated with targeted, endocrine, immune, and cell therapies.
| Cancer type | Class | Chemotherapeutic agents | Cardiovascular effects |
|---|---|---|---|
| Human epidermal growth factor receptor 2 (HER2)-positive breast cancer | Monoclonal antibodies | Trastuzumab | Synergistic toxic effects on cardiomyocytes with concomitant use of HER2 monoclonal antibodies and anthracyclines [44]. |
| Chronic myelogenous leukemia and acute lymphocytic leukemia | Tyrosine kinase inhibitors (TKIs) | Bosutinib, dasatinib, ponatinib, and nilotinib | QT prolongation, heart failure, myocardial infarction, and potentially fatal thrombosis [45]. |
| B-cell malignancies (chronic lymphocytic lymphoma and indolent lymphomas) | Bruton tyrosine kinase inhibitors | Ibrutinib | Increase risk of atrial fibrillation or hypertension [45]. |
| Renal cell carcinoma | Angiogenesis inhibitors | Sunitinib, axitinib, sorafenib, and pazopanib | Hypertension, thrombosis, QT interval prolongation, or left ventricular dysfunction [46], [47], [48]. |
| Metastatic breast cancer | Cyclin-dependent kinase inhibitors | Palbociclib, ribociclib, abemaciclib | Ribociclib has been shown to prolong the QTc interval [50]. |
| Multiple myeloma | Proteasome inhibitors | Carfilzomib | Heart failure, systemic and pulmonary hypertension, arrhythmias, and acute coronary syndrome [51], [52]. |
| Hematological malignancies (lymphomas and multiple myeloma) | Histone deacetylase inhibitors | Vorinostat, romidepsin, and panobinostat | Cardiac ischemia, arrhythmias, and conduction abnormalities [53], [54], [55], [56]. |
| Breast cancers | Aromatase inhibitors | Anastrozole, letrozole, exemestane, and tamoxifen, an estrogen receptor selective modulator | May increase the risk of ischemic heart disease [57], [58], [59], [60]. |
| Breast cancer, head and neck, and lung cancers | Immune checkpoint inhibitors (monoclonal antibodies) | Anti–PD-1 and anti–PD-L1 | Myocarditis [61]. |
| Recalcitrant hematological cancers | Cellular therapy | Chimeric antigen receptor (CAR)-T cell therapy | Cardiovascular effects related to (CAR)-T cell therapy is primarily a result of cytokine release syndrome (CRS). Cardiovascular effects include sinus tachycardia, left ventricular systolic dysfunction, and hypotension [64]. |
| Multiple myeloma | Stem cell transplantation | – | Increased risk for cardiovascular toxicity including cardiomyopathy [69]. |
PD-1: Programmed death receptor-1; PD-L1: Programmed death receptor 1 ligand.
Cancer survivors are at increased risk for heart failure and cardiac mortality attributable to prior exposure to anthracycline chemotherapy and/or chest-directed radiation [18], [19], [20]. Early recognition of cardiomyopathy provides an opportunity for interventions that can potentially improve cardiac health and quality and length of survival [21], [22]. Consequently, clinical practice guidelines recommend echocardiogram screening and early detection of asymptomatic cardiomyopathy, with the goal of reducing progression to symptomatic or fatal heart failure.
Guidelines from the American Society of Clinical Oncology (ASCO) and National Comprehensive Cancer Network (NCCN) recommend post-anthracycline echocardiography for patients with elevated cardiovascular risk [23], [24], [25]. However, these guidelines are inconsistently followed, and predicting anthracycline cardiotoxicity continues to be relatively evasive. Furthermore, variable definitions have been used for cardiovascular toxicity over time and some have been rather differential such as a decline in left ventricular ejection fraction (LVEF) by more than 10% points if the LVEF remains ≥50%, or a LVEF decline by 5% points or more if the LVEF is <50%. In addition, pediatric cancer cohort studies have demonstrated disparities in access and barriers to adherence to follow-up care related to race/ethnicity, socioeconomic and insurance status, geographic location, behavioral factors, survivor knowledge and perceptions and provider knowledge [26], [27], [28], [29], [30]. These aspects make it inherently difficult to develop and validate prediction models and deep learning algorithms. Applying new methods of prediction and early recognition of cardiovascular diseases in this population is therefore important and can potentially inform antineoplastic regimen, cardioprotection, and surveillance decision-making. Nonetheless, prediction and early diagnosis remain a challenge.
Despite well-established clinical risk factors, predicting cardiotoxicity continues to be relatively evasive. Due to the large inter-individual variability and inadequacy of clinical predictors, our prediction of cardiovascular disease in cancer survivors is imprecise. Initiatives are needed to overcome these challenges and transform the cardiovascular care of cancer patients. There is great need to develop non-invasive, accessible, low-cost tools for early identification of survivors at risk for cardiovascular disease to enable optimal screening, early diagnosis, and timely interventions.
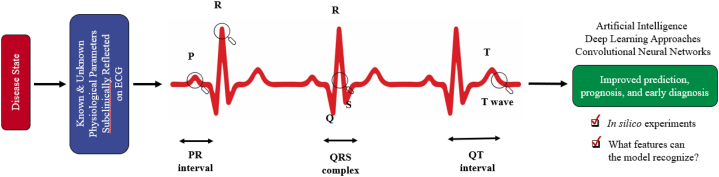
Although AI has its own challenges, machine learning (ML) and deep learning (DL) approaches applied to various features could be transformative for prediction and diagnosis in cardio-oncology. ML learns from cumulative data to generate predictive models and explore relationships among variables; DL utilizes deep or convoluted neural networks to recognize subtle patterns through the abstraction and synthesis of numerous layers of data for analysis and prediction [31]. AI, including ML and the subcategory DL, in early stages has been applied to various cardiovascular modalities [31], [32], [33], [34]. Notably, the ECG remains the most often used diagnostic tool for determining heart anatomy and electrical activity, and is a key prognostic and diagnostic tool in cardio-oncology. Parallel advancements in processing power, machine learning methods, and the availability of large-scale data, may significantly expand the clinical inferences obtained from the ECG while keeping interpretability for medical decision-making in cardio-oncology (Fig. 1).
Fig. 1.
The use of convolutional neural networks, a deep learning approach as a form of artificial intelligence, can be used to computationally predict cardiac dysfunction or atrial fibrillation in in silico experiments. Traditional multivariate discrimination approaches in statistics typically use known prespecified physiological parameters for analyses. In comparison, artificial intelligence methods can uncover and use subclinical unknown physiological parameters reflected in subtle changes on the ECG for improved prediction, early diagnosis, and prognosis of cardiac dysfunction and atrial fibrillation.
Here, we share our point of view with a brief survey of the current early state of machine learning applications in cardio-oncology. We begin by discussing some of the considerations for using machine learning algorithms. We then discuss studies in which machine learning was used to improve prediction of cardiac dysfunction in cancer survivors, and we explain that machine learning algorithms vary in sensitivity and specificity depending on the specific phenotype that they are used to identify (i.e., a rarer more severe cardiac issue vs. a more common less severe issue). We provide a spotlight on the use of AI with ECG for predicting cardiac dysfunction and also atrial fibrillation (Table 3, Table 4), and we mention a potential role for wearables. Additionally, future directions and key takeaways for the application of machine learning in cardio-oncology are summarized. This article is one of a series of publications on AI in cardio-oncology, complementing our manuscript on echocardiography and other forms of imaging salient to cancer survivors cared for in cardiology clinical practice. In our companion manuscript, titled “AI & Imaging in Cardio-Oncology,” we delve deeper into the application of AI to imaging modalities and their predictive power [35].
Table 3.
Prediction of cardiovascular diseases in AI-ECG studies.
| Number of patients | Predicted cardiovascular diseases | Predicted effect size | Cardiomyopathy study YES/NO | History of cancer YES/NO | Reference |
|---|---|---|---|---|---|
| 44,959 | Asymptomatic left ventricular dysfunction (ALVD), ejection fraction (EF) ≤35% | AUC of 0.93 Sensitivity: 86.3% Specificity: 85.7% Accuracy: 85.7% |
YES | NO | [100] |
| Subset of 16,056 | Left ventricular systolic dysfunction (LVSD), EF ≤35% | AUC of 0.918 (95% CI 0.902% - 0.934%) | YES | NO | [101] |
| 5680 | LVSD, EF ≤40% | AUC of 0.83 (95% CI 0.82–0.84) | YES | NO | [102] |
| 1606 | LVSD, EF ≤35% LVSD, EF <50% |
AUC of 0.89 (95% CI 0.86–0.91) AUC of 0.85 (95% CI 0.83–0.88) |
YES | NO | [103] |
| 14,613 | Heart failure (HF) | AUC of 0.76 (95% CI 0.72–0.80) | YES | YES | [104] |
| 22,641 adults (n = 11,573 intervention; n = 11,068 control) | Low EF ≤50% | Increased diagnosis of low EF in the intervention group than the control group (2.1% vs. 1.6%, OR 1.32, CI 1.01–1.61, p = 0.007). | YES | NO | [105] |
| Systematic review | Left ventricular (LV) systolic dysfunction (three reports) LV hypertrophy (one report) Ischemic heart disease (eight reports) |
AUC of 0.89–0.93 and accuracy: 98% AUC of 0.87 and accuracy: 87% AUC of 0.88–1.00 and accuracy: 83–99.9% |
NO | NO | [106] |
| Subset of 36,186 echocardiograms | Pulmonary arterial hypertension Hypertrophic cardiomyopathy Cardiac amyloid Mitral valve prolapse |
AUROC of 0.94 (95% CI 0.93–0.95) AUROC of 0.91 (95% CI 0.90–0.92) AUROC of 0.86 (95% CI 0.82–0.89) AUROC of 0.77 (95% CI, 0.76–0.78) |
YES | NO | [108] |
| MIT-BIH arrhythmia database | Premature ventricular contraction (PVC) | Accuracy: 96%–99% | NO | NO | [113] |
| MIT-BIH arrhythmia database | Heart rhythm | Accuracy: 99.26 Specificity: 99.14 |
NO | NO | [110] |
| MIT-BIH arrhythmia database | Atrial premature contraction (APC) Paced beat (PB) Premature ventricular contraction (PVC) Right bundle branch block (RBBB) Ventricular bigeminy (VB) Ventricular couplets (VCs) Ventricular tachycardia (VT) |
Support vector machine (SVM): F1 score of 0.8193a | NO | NO | [115] |
| 1217 | Cardiomyopathy | AUC of 0.87 (95% CI, 0.83 to 0.90) | YES | YES | [119] |
| 52,870 | Low EF ≤35% | Non-Hispanic white (n = 44,524, AUC 0.931) Asian (n = 557, AUC 0.961) Black/African American (n = 651, AUC 0.937) Hispanic/Latino (n = 331, AUC 0.937) American Indian/Native Alaskan (n = 223, AUC 0.938) |
YES | NO | [134] |
AI-ECG: artificial intelligence-enhanced electrocardiography; AUC: area under the curve; AUROC: area under the receiver operating characteristic curve; CI: confidence interval; EF: ejection fraction; F1: F-Score/F-Measure; LVSD: left ventricular systolic dysfunction; MIT-BIH: Massachusetts Institute of Technology-Beth Israel Hospital; OR: odds ratio.
A combined measure representing algorithm precision and recall based on ability to predict true positive.
Table 4.
Prediction of atrial fibrillation in AI-ECG studies.
| Number of patients | Predicted effect size for atrial fibrillation | History of cancer YES/NO | Reference |
|---|---|---|---|
| 180,922 | Main analysis: AUC of 0.87 (95% CI 0.86–0.88) Secondary analysis: AUC of 0.90 (95% CI 0.90–0.91) |
NO | [99] |
| 415,389 | AUROC of 0.909 (95% CI 0.903–0.914) | NO | [107] |
| 1936 | Concordance statistic (C statistic) of 0.72 (95% CI 0.69–0.75) for combined AI-ECG and clinical score | NO | [109] |
| Total: 12,186 ECG recordings Training dataset: 8528 ECG recordings Testing set: 3658 ECG recordings |
Overall F1 accuracy of 0.864 F1 accuracy 0.919 for normal rhythms F1 accuracy 0.858 for atrial fibrillation rhythms F1 accuracy 0.816 for other rhythms |
NO | [112] |
| 508 | Sensitivity of 93.7% (95% CI 89.8% - 96.4%) Specificity of 98.2% (95% CI 95.8% - 99.4%) Accuracy of 96.1% (95% CI 94.0% - 97.5%) |
NO | [126] |
AI-ECG: artificial intelligence-enhanced electrocardiography; AUC: area under the curve; AUROC: area under the receiver operating characteristic curve; CI: confidence interval; F1: F-Score/F-Measure.
2. Cardiotoxicities in cardio-oncology
2.1. Traditional chemotherapies
Anthracyclines are frequently used in the treatment of breast cancer, sarcoma, leukemia, and lymphomas. Anthracyclines are extremely effective against a variety of cancers but may cause cardiotoxicity [36] (Table 1). 5-fluorouracil (5-FU) and its metabolic precursor capecitabine are fluoropyrimidines or antimetabolites frequently used in multiple cancer treatments and typical associate with cardiotoxicity during the first cycle of chemotherapy [37]. Paclitaxel and docetaxel are microtubule-targeting agents are typically used to treat breast, head and neck, and gastrointestinal cancers, and have been shown to cause abnormal conduction [38], [39]. Cyclophosphamide is frequently used to treat breast cancers and lymphomas and also carries numerous cardiac adverse effects [40], [41]. Cisplatin and busulfan have been linked to additional cardiovascular toxicities [42], [43].
2.2. Targeted, endocrine, immune, and cell therapies
Trastuzumab is a monoclonal antibody to the human epidermal growth factor receptor 2 (HER2) receptors and is most frequently used to treat patients with HER2-positive breast cancer [44]. Concomitant use of anthracyclines and HER2 monoclonal antibodies is typically avoided, due to synergistic cardiotoxic effects (Table 2).
With excellent clinical outcomes, multitargeted TKIs such as bosutinib, dasatinib, ponatinib, and nilotinib have transformed the management of chronic myelogenous leukemia and acute lymphocytic leukemia, and these drugs are also linked to cardiotoxicity. Bruton tyrosine kinase inhibitors have been used to manage B-cell malignancies, and have been shown to increase cardiotoxicity [45] (Table 2). Angiogenesis inhibitors may cause adverse effects such as hypertension, thrombosis, Q-T interval prolongation, or left ventricular dysfunction [46], [47], [48].
Cyclin-dependent kinase inhibitors are used to treat metastatic breast cancer [49]. Ribociclib has been reported to prolong the QTC interval [50]. Proteasome inhibitors, such as carfilzomib, have resulted in unprecedented advances in the treatment of multiple myeloma [51], [52]. Carfilzomib has been associated with cardiovascular adverse events such as heart failure, systemic and pulmonary hypertension, arrhythmias, and acute coronary syndrome. Histone deacetylase inhibitors, such as vorinostat, romidepsin, and panobinostat are used to treat hematological malignancies, and have been linked to cardiac ischemia, arrhythmias, and conduction abnormalities [53], [54], [55], [56].
There is some evidence that estrogen deprivation caused by aromatase inhibitors (e.g., anastrozole, letrozole, exemestane or tamoxifen (an estrogen receptor selective modulator) used to treat early and advanced breast cancers may increase the risk of ischemic heart disease [57], [58], [59], [60].
Immune checkpoint inhibitors are monoclonal antibodies that activate T cells and initiate an adaptive immune response, enabling the immune system to launch an optimal immune response when exposed to cancerous cells, but this can associate with myocarditis [61].
Cellular therapy, more precisely chimeric antigen receptor (CAR)-T cell therapy, has demonstrated early success in patients with recalcitrant hematological cancers [62], [63]. Cardiovascular complications have been reported in this setting primarily as a result of cytokine release syndrome and complications [64]. The most common malignancy treated with autologous stem cell transplantation is multiple myeloma [65], which associates with cardiomyopathy and poor outcomes [55], [66], [67], [68], [69].
3. AI and cardio-oncology
Artificial intelligence (AI) advancements have gained traction in recent years, with the use of computer algorithms to simulate human intelligence [70], [71]. In common practice, AI is already being used to a limited extent in the form of computer-generated electrocardiograms (ECGs) [70]. Machine Learning (ML) in particular, which employs advanced computer algorithms to learn complex patterns [71], has the potential to improve cancer survivor prediction, early diagnosis, and ultimately prevention. Over the last decade, the idea of applying machine learning to cardiology has generated considerable interest, with over 3000 papers published on the subject in the last five years alone [70], [71].
Developing novel AI algorithms could provide an avenue for research to improve patient care for cancer patients and survivors at risk for cardiovascular disease related to cancer therapy. The term AI was first used in 1956 to refer to machines simulating human intelligence. AI comprises multiple techniques including Bayesian networks, ML, and hybrid intelligent systems that allow efficient data representations. AI processes and analyzes high-density data not otherwise feasible by traditional statistics. AI differentiates itself from traditional parametric statistical methods by capturing high dimensional and hierarchical relationships, which makes AI more applicable to real-world problems.
In cardio-oncology, the use of machine learning to predict cardiac dysfunction (cardiomyopathy, left ventricular systolic dysfunction) in cancer survivors is of particular interest. Cardiac dysfunction is one of the most frequently reported long-term adverse effects of cancer therapy. This is especially true for some patients receiving anthracyclines, one of the most frequently prescribed classes of cancer medications [13]. In these patients, every doubling of the time required to detect and treat cardiac dysfunction can result in a fourfold reduction in the likelihood of complete recovery of cardiac function [72].
4. AI opportunities for diagnosis, prognosis, and care delivery
The application of machine learning is contingent upon the ability of algorithms to process heterogeneous data within a learning dataset and to generate accurate and reliable predictions. After developing the algorithm, its performance must be validated against an alternate dataset to which the algorithm has never been exposed previously. The most frequently used validation parameters are those that assess the model's ability to 1) discriminate between different outcomes, which is commonly expressed as an area under the curve (AUC) or a c-statistic, and 2) calibrate the model-derived risk estimate to observed outcomes [73], [74]. Researchers evaluating machine learning algorithms work with a variety of datasets that are frequently consolidated retrospectively. The type of data collected varies between studies and may include baseline demographics, clinical characteristics, treatments, and outcome measures that may be adjudicated centrally. There is a dearth of data on the optimal type of machine learning algorithm to use or the parameters to set prior to initiating the learning process [75]. Nonetheless, some studies are demonstrating the utility of ML in cardio-oncology.
4.1. AI techniques used in cardiology, oncology, and cardio-oncology
Various AI techniques have been applied in cardiology [32], [33], [34], [71], [76], [77], [78], [79], [80], [81], [82], [83] and oncology [84], [85] and are now also being used in cardio-oncology (Table 5). The most frequently used techniques in ML and DL generally fall within the two primary categories of ‘supervised learning’ and ‘unsupervised learning’. A recent publication has delineated advantages, disadvantages, and example uses cases of common algorithm subclasses within supervised or unsupervised learning [32]. In supervised learning, both input and output data are provided to algorithms, which then determine complex mathematical relationships between the input data and the expected output data [31]. In unsupervised learning, on the other hand, input data (without expected output data) are provided to the algorithm, which extracts insights from the data based only on its internal structure and statistics [31].
Table 5.
Machine learning artificial intelligence techniques used in ECG studies.
| Cardiovascular pathology | Artificial intelligence techniques | Reference |
|---|---|---|
| N/A (Machine learning (ML)/Deep learning (DL) review) |
ML: decision tree, support vector machine (SVM), supervised ML, unsupervised ML, clustering, segmentation, reinforcement learning DL: neural network |
[70] |
| N/A (Artificial intelligence (AI) in cardiac imaging review) |
Supervised ML: regression analysis, SVM, random forest (RF), neural network, convoluted neural network (CNN), DL Unsupervised ML: principal component analysis, hierarchical clustering, partitioning algorithm, model-based clustering, grid-based algorithm, density-based spatial clustering of applications with noise |
[71] |
| N/A (Challenges of ML/DL models) |
ML/DL algorithms | [75] |
| N/A (ML review) |
ML algorithms | [98] |
| Coronary artery disease Atrial fibrillation (AF) Heart failure (HF) Stroke Myocardial infarction De novo cancer therapy–related cardiac dysfunction (CTRCD) |
ML: K-nearest neighbor (kNN), logistic regression (LR), SVM, RF, gradient tree boosting | [94] |
| CTRCD | ML, topology-based K-means clustering, hierarchical clustering | [93] |
| AF | AI-enabled electrocardiograph (ECG), CNN with Keras framework with a Tensorflow (Google; Mountain View, CA, USA) backend |
[99] |
| Asymptomatic left ventricular dysfunction (ALVD) | AI-enabled ECG, CNN with Keras framework with a Tensorflow (Google; Mountain View, CA, USA) backend | [100] |
| Left ventricular systolic dysfunction (LVSD), ejection fraction (EF) ≤35% | AI-augmented ECG, CNN with Keras framework with a Tensorflow (Google; Mountain View, CA, USA) backend | [101] |
| LVSD, EF ≤40% |
AI-augmented ECG | [102] |
| LVSD, EF ≤35% LVSD, EF <50% |
AI-enabled ECG, CNN with Keras framework with a Tensorflow (Google; Mountain View, CA, USA) backend | [103] |
| HF | ECG-AI model, CNN, Light Gradient Boosting (LGBoost) | [104] |
| Low EF ≤50% | AI-enabled ECG, neural network | [105] |
| LV systolic dysfunction LV hypertrophy Ischemic heart disease |
DL, CNN, deep neural network, RF, LR, SVM, classification and regression tree, multilayer perceptron (MLP), recurrent neural network (RNN), long-short term memory (LSTM), bilateral long-short term memory (BLSTM), multiple feature branch convolutional bidirectional recurrent (MFB-CBRNN), neural network, ensemble neural network | [106] |
| AF | Deep representation learning, RF classifier | [107] |
| Pulmonary arterial hypertension Hypertrophic cardiomyopathy Cardiac amyloid Mitral valve prolapse |
ML, combination of CNN and hidden Markov model | [108] |
| AF | AI-enabled ECG, CNN with Keras framework with a Tensorflow (Google; Mountain View, CA, USA) backend | [109] |
| Heart rhythm | LSTM recurrence network model with focal loss | [110] |
| N/A (ECG identification) |
Bidirectional (LSTM)-based deep RNN | [111] |
| AF | 21-layer 1D convolutional RNN (RhythmNet) | [112] |
| Premature ventricular contraction (PVC) | RNN with LSTM | [113] |
| N/A (Cardiac monitoring on wearable devices) |
Algorithm consisting of multiple LSTM recurrent neural networks and wavelet transform | [114] |
| Atrial premature contraction (APC) Paced beat (PB) Premature ventricular contraction (PVC) Right bundle branch block (RBBB) Ventricular bigeminy (VB) Ventricular couplets (VCs) Ventricular tachycardia (VT) |
LSTM with a second stage model including MLP, SVM and LR | [115] |
| Cardiomyopathy | DL, model consisting of a 12-layer 1D CNN and 2-layer dense neural network | [118] |
| Cardiomyopathy | DL, XGBoost, descriptive statistics, sample entropy, probabilistic symbolic pattern recognition, Fourier transformation, discrete wavelet transformation, continuous wavelet transformation, CNN | [119] |
| AF | Deep neural network | [126] |
| Cardiotoxicity | DL algorithms | [128] |
| Anthracycline cardiotoxicity | ML, RF classifier | [129] |
| Low EF ≤35% | CNN algorithms | [134] |
AF = atrial fibrillation; AI = artificial intelligence; CNN = convolutional neural network; CTRCD = cancer therapy–related cardiac dysfunction; DL = deep learning; ECG = electrocardiograph; EF = ejection fraction; HF = heart failure; kNN = k-nearest neighbor; LR = logistic regression; LSTM = long-short term memory; LV = left ventricular; LVSD = left ventricular systolic dysfunction; MACE = major adverse cardiac events; ML = machine learning; MLP = multilayer perceptron; RF = random forest; RNN = recurrent neural network; SVM = support vector machine; XGBoost = extreme gradient boosting.
In supervised learning, the objectives are outcome prediction, classification of observations, and parameter estimation [32]. Three common classes of algorithms in supervised learning are regularized regression, decision tree ensembles, and support vector machines [32]. Advantages of regularized regression include simple and automatic solutions to complex problems, and recognized interpretations of the relationship between variables and outcomes. Disadvantages include the use for groups of features that must correlate with one another, the arbitrary choosing of any single feature (least absolute shrinkage and selection operator). An example application has been the development of a model to predict acute myocardial infarction using proteomic and clinical data [86]. Advantages of decision tree ensembles include frequently being the best “off-the-shelf” algorithms for classification or prediction, and built-in methods for selecting features and determining the relative importance of variables. Disadvantages include being less useful for descriptive analysis than while being more useful for predictive analysis of datasets and variables, as well as a proclivity for overfitting data. An example application has been predicting the risk of cardiovascular events [87]. Advantages of support vector machines include utilizing the “kernel trick” to convert linear classifiers to nonlinear classifiers, and frequently making extremely accurate predictions. Disadvantages include by default using nonprobabilistic classification, and computations in high-dimensional spaces can be challenging. An example application has been using metabolites in the blood to predict in-stent restenosis [88]. There are other simple prototypes of machine learning algorithms that do not fall neatly into these categories. One of the most often used is the k-nearest neighbor approach, which sidesteps the typical model algorithm in favor of making predictions based on the outcomes of similar cases [89], [90]. For example, a k-nearest neighbor approach may be used to predict whether a patient may have a cardiovascular event, based on whether similar patients have had cardiovascular events. The distance between patients in multidimensional vector space is calculated, and those patients with the shortest distance between them are considered to be the nearest neighbors. This methodology is utilized in patient similarity algorithms, which can be used for this purpose in cardiology and oncology [91], [92], and in cardio-oncology [93], [94].
In unsupervised learning, the goals are discovery of hidden data structure, exploration of relationships between variables, and the tendency for features discovered by unsupervised learning often being available for incorporation into subsequent more explainable and interpretable supervised learning models [32]. Three common classes of algorithms in unsupervised learning are deep learning algorithms, tensor factorization, and topological data analysis [32]. Advantages of deep learning algorithms include the use of current state-of-the-art feature engineering techniques, and features are frequently then used as input to supervised learning models as described. Accordingly, the use of these algorithms is developing rapidly, with broad industry and academic interest. Disadvantages include computational cost to train the unsupervised algorithms, with large datasets needed to train the models, and interpretability of the model output can be challenging. An example application has been the unsupervised development of representative models predicting important patient characteristics using electronic health record data [95]. Advantages of tensor factorization include the ability to naturally incorporate both multidimensional and multimodal data. Disadvantages include small numbers of applications published in cardiovascular reports, and the factorization algorithm chosen is critical to the outcome. An example application has included evaluating subcategories of heart failure with preserved ejection fraction [96]. Advantages of topological data analysis include clustering and identification of variable relationships in an interpretable manner. Disadvantages include being less mature than other unsupervised learning methods, and the requirements for licensing agreements associated with frequently commercial algorithms. An example application has been determining subcategories of diabetes mellitus type 2 in data from electronic medical records [97].
4.2. Cardiac dysfunction in cancer survivors
ML has shown great promise in cardio-oncology research thus far. This is made possible in part by the availability of longitudinal clinical data, such as ECGs and cardiovascular imaging, which significantly improves performance when compared to static single timepoint data. Indeed, the robustness of the available data has an effect on the nature and quality of the algorithm that results [98]. In one such longitudinal study, researchers evaluated the use of machine learning algorithms to predict the risk of cardiac dysfunction (Table 3). In a longitudinal retrospective study of 4309 cancer patients, echocardiographic data were predictive of cardiac dysfunction, with laboratory data having limited additive value [94]. AUC of 0.85 was obtained from echocardiographic data alone, whereas AUC of 0.74 was obtained from laboratory data alone. Finally, the combined model incorporating both types of data demonstrated the best performance for diagnosing cardiac dysfunction (AUC 0.91). This analysis demonstrates the most robust cardio-oncology-specific risk estimates available in the published AI literature. After additional external validation, the algorithm will be made available in an online risk stratification tool.
In a subsequent study, this research group recently applied machine learning to large-scale institutional electronic medical records to predict which patients will have adverse cardiac outcomes [93]. They established a large longitudinal cardio-oncology cohort (with a maximum follow-up of >20 years, from March 1997 to January 2019) of >4600 cancer patients at Cleveland Clinic who had one of five cardiac diagnoses: atrial fibrillation, coronary artery disease, heart failure, myocardial infarction, or stroke. The population as a whole was composed of 84% white Americans and 11% black Americans, with a median age of ~65 years. They used a topology-based K-means clustering approach to conduct unbiased patient–patient network analyses using data from general demographics, echocardiography (over 25,000), laboratory testing, and clinical factors. Hazard ratio (HR) and Kaplan–Meier analyses were used to identify clinically actionable variables. Cox regression models were used to eliminate all confounding variables. Random-split and time-split training-tests were used to validate the model. They identified four clinically significant subgroups of cancer survivors with four levels of cardiac events incidence and mortality. They demonstrated in this study that machine learning algorithms focused on analyzing similarity among patients over several years may facilitate the identification of cancer survivors at increased risk of cardiac dysfunction.
5. ECG for prediction and prognostication in cardio-oncology
Recent findings associating AI findings in ECGs with severe cardiac conditions represent a giant step forward in cardiology [99]. More precisely, using 12-lead ECGs AI algorithms can predict reduced left ventricular ejection fraction (LVEF <40%) and a patient's predisposition to atrial fibrillation while in normal sinus rhythm (NSR) [99], [100]. Deviations from NSR on ECG are being identified via AI technologies to automate diagnoses. AI-based predictive tools utilizing low-cost, accessible, and potentially remote applicable ECG data may become useful for screening and diagnosis in cardio-oncology. In this section, we describe various studies assessing the potential utility of ECG to define or predict cardiac pathology in the general population. We then outline how AI-ECG can be applied in cardio-oncology, with a focus on cardiomyopathy and atrial fibrillation and looking toward the future.
5.1. Cardiac pathology on ECG
This statement is supported by recent studies utilizing deep learning on 12-Lead ECG data in accurately estimating echocardiogram parameters representing left ventricular systolic dysfunction [101], [102], [103]. Further, there is also literature suggesting that deep learning applied to ECG alone can identify patients with no heart failure yet in risk for developing in 10 years (AUC of 0.76) with a similar accuracy to Framingham Heart Study's risk calculator (AUC of 0.78) utilizing several clinical risk factors. Also, when AI-ECG results are combined with clinical risk factors, the accuracy was further increase (AUC of 0.83) confirming the added value of ECG in assessing cardiovascular risk [104]. Another study group determined whether AI-ECG could be used in a clinical decision support tool for early detection of cardiac dysfunction [105]. The study authors utilized a fast-paced low-cost pragmatic trial embedded in the electronic health record clinical workflow to generate real-world evidence in less than a two-year time period. They used existing and prospective real-time and longitudinal electronic health record data, incorporated with clinical decision support tools, throughout small and large hospital, academic, community, and rural practices. In the intervention arm, AI-ECG results were provided to 181 clinicians on 120 primary care teams from 45 clinics/hospitals; the control arm (standard care; 177 clinicians) did not receive the AI-ECG results. ECGs from >22,600 adults (n = 11,573 in the intervention group, n = 11,068 in the control group) without previously known left ventricular systolic dysfunction were obtained during routine visits. There was a greater number of subsequent early diagnoses of left ventricular systolic heart failure in the intervention group than the control group (2.1% vs. 1.6%, odds ratio (OR) 1.32, confidence interval (CI) 1.01–1.61, p = 0.007).
In another study, the authors evaluated the evidence for using DL to analyze resting electrocardiograms (ECG) in order to predict cardiac dysfunction, left ventricular hypertrophy, and coronary heart disease [106]. A systematic review was pursued, yielding 12 published reports on the application of DL algorithms to resting electrocardiogram signals. These algorithms were used for the detection of structural cardiac pathologies in the ambulatory setting, during stress testing, or from intracardiac/implantable devices; ECGs already clearly demonstrating arrhythmias were excluded. Three articles reported on the use of DL-ECG to detect cardiac systolic dysfunction, with an area under the curve (AUC) of 0.89–0.93 and a 98% accuracy. One study reported on the use of DL-ECG to detect cardiac hypertrophy (AUC 0.87, 87% accuracy). Six articles reported on the use of DL-ECG to detect ischemic heart disease and two articles reported on the use of DL-ECG to detect stable coronary heart disease (AUC 0.88–1.00, 83–99.9% accuracy). Algorithms for deep learning, particularly those based on convolutional neural networks, showed superior performance to rules-based and other ML algorithms. DL algorithms may provide promising methodologies for analyzing resting electrocardiogram signals to detect structural heart disease, including left ventricular dysfunction. This may have clinical utility for screening asymptomatic individuals and early diagnosis of symptomatic patients.
One group assessed >1 million raw 12-lead ECGs from >415,000 unique patients, paired with their clinical data, to predict the development of atrial fibrillation [107] (Table 4). Recordings were assigned to training, validation, and test sets, stratified by class, age, and gender. A random forest machine learning classifier was trained to estimate the risk of AF development within five years for a particular recording. Results indicated the highest accuracy of prediction with incorporation of heart rate variability, morphology of the ECG, and features derived from a variety of sources, including demographics, clinical data, designed features, and deep representation learning (AUR = 0.91). Another group applied a new combination of convolutional neural networks and hidden Markov models to >360 k 12-lead ECGs to diagnose pulmonary arterial hypertension, hypertrophic cardiomyopathy, cardiac amyloid, and mitral valve prolapse (AUC 0.94, 0.91, 0.86, and 0.77, respectively) [108]. In recent years, investigators have sought to characterize the utility of AI-ECG as a predictor of future developing atrial fibrillation in more than 1900 participants, compared to the Cohorts for Aging and Research in Genomic Epidemiology–AF (CHARGE-AF) score [109]. They found improved C statistics for AI-ECG model output (0.69, 95% confidence interval [CI] 0.66–0.72) and CHARGE-AF (0.69, 95% CI 0.66–0.71) when the two features were combined (0.72, 95% CI 0.69–0.75). Several other authors have reported on the use of AI-ECG to predict cardiac pathology (see review [78]).
Although the studies mentioned above were not specifically on patients with a history of cancer, they demonstrate promise for application in cancer survivors. One advantage of AI is specifically DL algorithm. In these algorithms, learned information and patterns in pre-existing AI models can be utilized via transfer learning. This can be helpful for customizing models for cancer survivors. These models can be used to detect and predict a variety of forms of cardiotoxicity. Further, these models mainly use convolutional neural network architecture, which is a specific type of deep learning; yet there are other types of deep learning such as recurrent neural networks and long-short term memory networks that can also be implemented in the analysis of ECGs [110], [111], [112], [113], [114], [115].
5.2. Cardiomyopathy
5.2.1. Children
In 2001, one of the first studies in this area evaluated ECG changes in children who developed a decline in cardiac function after receiving doses of various anthracyclines in the order of 198 to 737 mg/m2 cumulative doxorubicin equivalents (CDEs) for various hematologic cancers and solid tumors [116] (Table 6). The ECGs of 16 children with cardiomyopathy (fractional shortening < 28%) were compared to ECGs of 31 children without cardiomyopathy who also had received anthracycline therapy (CDE dose range 120–517 mg/m2). Age, body surface area, and time since anthracycline therapy were the same between the two groups. All ECGs were also compared to those of a second control group including 530 healthy children without cancer. A decrease in QRS duration was noted more frequently in children with cardiomyopathy compared to both control groups. In 2019, a study of 589 children treated with anthracyclines for various pediatric cancers demonstrated that a reduction of 0.6 mV in the sum of absolute QRS amplitude in the 6 limb leads (SQRS) and a 10 ms increase of QTc interval associated with a 17% (HR 1.174; p = 0.003) and 10% (HR 1.098, p < 0.006) increased risk of developing cardiomyopathy (defined as LVEF < 50%, fractional shortening < 26%, or left ventricular end-diastolic diameter z-score > 2.5), compared to those who did not develop cardiomyopathy (CDE median 236 mg/m2 and range 153–329 mg/m2 versus median 165 mg/m2 and range 92–232 mg/m2) [117]. ECG changes were more pronounced for those who received higher doses of anthracyclines [117]. Neither of these studies in children with cardiomyopathy had yet evaluated the use of AI-ECG to predict cardiomyopathy. However, a recent study indicated that artificial intelligence algorithms trained on the ECGs of 1217 childhood cancer survivors (65% with lymphoma/leukemia and 77% received anthracycline therapy with median dose 169 mg/m2 and range 35–734 mg/m2) can predict the development of late-onset cardiomyopathy (defined based on 2014 American Society for Echocardiography guidelines) compared to baseline in those at high risk among a test set of 244 individuals, with a sensitivity, specificity, and AUC of 76%, 79%, and 0.87 (95% CI 0.83–0.90), respectively [118], [119].
Table 6.
Potential utility of ECG parameters to predict cardiomyopathy in children and adults.
5.2.2. Adults
In 1977, a study assessed factors associated with anthracycline-induced cardiomyopathy (based on published clinically actionable thresholds) in 53 adult cancer patients treated with doxorubicin for a variety of cancers [120]. The investigators found that a 30% decrease in QRS amplitude in the limb leads was significantly more frequently noted on the ECGs of 17 individuals who developed cardiomyopathy compared to the ECGs of 36 individuals who did not develop cardiomyopathy despite receipt of similar doses of anthracycline (median 510 mg/m2, range 310–825 mg/m2 versus median 510 mg/m2, range 405–600 mg/m2). Subsequently, in 2009, a study revealed a 1 mV decrease in QRS amplitude and an approximately 15 ms increase in QTc as the two main ECG changes in 9 of 26 patients with leukemia who received anthracycline therapy (median 429 mg/m2, range 240–715 mg/m2), with a statistically significant correlation between these electrical abnormalities and left ventricular systolic dysfunction (LVEF ≤55%) compared to baseline [121]. While both of these studies identified associations between changes in left ventricular conduction and systolic function, neither study pursued a prospective analysis nor the use of AI-ECG for prediction.
A recent AI study demonstrated that the 12-lead ECG can be used to identify patients in the general population (without a cancer history) with reduced ejection fraction with quite favorable test performance characteristics [100]. Since the 12-lead ECG is low-cost, widely available, and minimally invasive, it may serve as an ideal method to screen patients who remain at risk of low ejection fraction after receiving cardiotoxic chemotherapeutics. While it is currently unknown whether AI-ECG can be used to identify adult cancer patients or survivors at risk for cancer therapy-induced cardiomyopathy, this approach is currently being tested in the ongoing TACTIC trial (ClinicalTrials.gov Identifier: NCT03879629). The main objectives of this trial are to define the need, timing, and duration of cardioprotection with the beta-blocker carvedilol in individuals with breast cancer who are commenced on human epidermal growth factor receptor 2 (HER2)-targeted therapy. One of the subaims of the study is to assess how an AI-ECG algorithm developed to detect an LVEF <35–40% in a community population performs in patients on HER2-directed therapies. In order to accomplish this aim, patients are asked to undergo serial ECGs in addition to troponins and echocardiography over a two-year period. Preliminary data are supportive of the concept, indicating a diagnostic performance of the AI-ECG algorithm for the detection of an LVEF <40% that is similar to the general population. The hope is that, if confirmed, AI-ECG could be used as a gatekeeper to the currently recommended more costly serial imaging-based cardiac function studies. This aspect of cost-effectiveness is very pertinent, especially in times of challenging health care delivery scenarios such as viral pandemics.
5.3. Atrial fibrillation
No studies have been reported on using AI-ECG to predict atrial fibrillation or other arrhythmias in cancer patients or survivors. Yet, atrial fibrillation has become a common cardiovascular toxicity of antineoplastic therapy, including during ibrutinib treatment for chronic lymphocytic leukemia (CLL), or after chest radiotherapy (which increases the risk of conduction abnormalities). Other contributors to atrial fibrillation in the cancer patients and survivors include underlying comorbidities, systemic inflammation or direct effect from the presence of the cancer itself, surgery, or pharmacologic cancer therapy [122]. The presence of AF in cancer patients and survivors confers an increased risk of CV complications, including a 3-fold risk of heart failure and a 5-fold risk of stroke, with prognostic implications [122]. AI-ECG could potentially identify cancer patients and survivors at high risk of atrial fibrillation and facilitate early individualized treatment choices or could inform shared decision-making.
5.4. Wearables and mobile devices
There has been an exponential increase in AI applications in medicine parallel to the technological advancements of the last decade. The integration of digitized medical data and electronic health records into daily living (e.g., wearables, smart phone apps) has produced massive data. These developments and their application hold promise for the transformation of medicine and are being increasingly available [123]. Recent technological advancements in biomedical engineering have enabled recording lead I of ECGs via smartwatches, conferring a tremendous opportunity for remote patient screening for detection and prediction of various cardiovascular risks facilitating earlier recognition and intervention. Moreover, recent literature shows that Lead I ECG collected via smartwatch is comparable to ECGs recorded via a Holter device or standard 12-Lead ECG in terms of known ECG characteristics including R peak location and P-wave amplitude [124], [125], [126].
Although current guidelines recommend serial echocardiography for patients who have received anthracyclines and other agents, clinical uptake of this practice is inconsistent, and many patients may not undergo surveillance due to the costs and cumbersome nature of screening. Therefore, the application of AI-ECG on convenient hand-held and mobile devices could improve screening rates without patients needing to present for in-person medical care. As part of the TACTIC study (Ongoing clinical trial, ClinicalTrials.gov Identifier: NCT03879629), ECGs from mobile devices (Kardia (AliveCor) and EKO device) are compared to in-clinic 12-lead ECGs and cardiac function parameters by echocardiography. Results will help us determine whether 12-lead ECGs, or even 1‑lead ECGs from mobile devices, can potentially serve as initial screening prior to echocardiography.
The idea of routine screening over time is interesting and particularly important in the setting of patients with current or prior treatment with cardiotoxic agents. AI-ECG has been shown to be effective in identifying patients at higher likelihood of low ejection fraction. This concept of using AI-ECG as a screening mechanism is being explicitly tested in the ongoing TACTIC trial (ClinicalTrials.gov Identifier: NCT03879629), which seeks to identify new systolic dysfunction among patients treated with chemotherapeutic agents for breast cancer. In this study, we are performing serial ECG and echocardiographic assessment of patients and we hope that some of these relationships will be clarified. Ongoing retrospective analyses of changes in model output over time are also ongoing and will help inform perspectives on the potential use of AI-ECG for screening in cancer patients and survivors. We will need to determine whether AI-ECG algorithms are useful for identifying changes in risk over time, rather than an isolated aggregate risk that remains relatively constant, while still important to identify.
In the general population, the detection of arrhythmias particularly atrial fibrillation can be feasible and effective. This has been demonstrated by studies in which ambulatory automated handheld ECG-based apps can accurately monitor heart rhythm with 71% sensitivity and 99% specificity [127]. Wearables ECG-monitoring devices compared to ECG-based apps have also improved detection of atrial fibrillation, facilitating optimal anticoagulation. Translating the work that has been done in the general population on the development and validation of ECG-monitoring wearables (which depend on AI) in addition to AI-ECG algorithms that predict AF risk [99], [127] to patients currently and previously treated for cancer is ongoing. Use of AI-ECG prior to treatment initiation to predict which of these patients will go on to develop an arrhythmia could facilitate targeted prevention and management strategies to improve outcomes.
6. AI in precision and translational cardio-oncology
6.1. Genomic and precision medicine using biologically relevant models
In a recent study, biologically relevant models capable of detecting drug-induced toxicity via phenotypic screening were developed [128]. Deep learning, high-content image analysis, and cardiomyocytes derived from induced pluripotent stem cells (iPSC-CMs) were used to rapidly screen for instigators of cardiotoxicity. Deep learning was used to determine a single-parameter score predicting cardiotoxicity induced by a library of ~1300 screened bioactive compounds. Compounds with potential cardiotoxic properties in iPSC-CMs were identified. DNA intercalators, epidermal growth factor receptor, cyclin-dependent kinase, and multi-kinase inhibitors were among the compounds that demonstrated cardiotoxicity in iPSC-CMs. Combining deep learning and iPSC technology in this way could be a powerful method for developing biologically relevant models for modeling cardiotoxicities from cancer therapies.
Another research group recently used machine learning algorithms to develop a clinical and genetic risk prediction model for anthracycline cardiotoxicity in survivors of childhood cancer [129]. The study authors sequenced the exomes of 289 childhood cancer survivors who had been exposed to anthracyclines for at least three years. 183 case patients with decreased left ventricular ejection fraction despite low-dose doxorubicin (≤250 mg/m2) and 106 control patients with preserved left ventricular ejection fraction despite doxorubicin >250 mg/m2 were chosen as extreme phenotypes in a nested case-control design. Rare/low-frequency variants were collapsed to identify genes that were differentially enriched for variants between case and control patients. The expression levels of five top-ranked genes were determined in cardiomyocytes derived from human induced pluripotent stem cells, and variant enrichment was confirmed in a replication cohort. A risk prediction model with genetic and clinical predictors was developed using random forest machine learning algorithms. Between case and control patients, 31 genes were significantly enriched for variants (p < 0.001). Only 42.6% of case patients and 89.6% of control patients had a variant in these genes (odds ratio: 0.09; 95% confidence interval: 0.04 to 0.17; p = 3.98 × 10−15). In comparison to a clinical-only model, a risk prediction model for cardiotoxicity that included clinical and genetic factors had a higher prediction accuracy and a lower misclassification rate. In vitro inhibition of gene-related pathways (PI3KR2, ZNF827) protected cardiomyocytes from cardiotoxicity. The study identified cardioprotective variants in cardiac injury pathway genes, aided in the development of a prediction model for delayed anthracycline cardiotoxicity, and identified novel targets in autophagy genes for the development of cardioprotective drugs.
Similar AI methods may potentially be applied to the study of electrophysiological parameters associated with adverse cardiovascular events from cancer therapy. The use of iPSC-CMs in cardio-oncology investigations is growing [130], [131], and these include electrophysiology studies in cardio-oncology [132], [133]. A translational study of acquired prolonged QT, or long QT syndrome (LQTS), and torsades de pointes (TdP) in iPSCs-CM and in more than 6 million male patients from the United Kingdom Vigibase database investigated clinical features and underlying mechanisms of LQTS associated with androgen deprivation therapy (ADT) used to treat prostate cancer [132]. Particularly, enzalutamide (an androgen receptor antagonist) was associated with a higher rate of death compared to other ADT drugs used to treat prostate cancer (17% versus 8.1%, p < 0.0001). In this same translational study, enzalutamide was shown to prolong action potential durations and induced afterdepolarizations in iPSCs, via inhibition of the delayed rectifier potassium current and activation of the late sodium current; administration of the androgen dihydrotestosterone counteracted the effects of enzalutamide. It would be interesting to evaluate and determine the utility of applying machine learning or subset deep learning AI algorithms to electrophysiological changes in iPSCs in response to cancer therapies to predict (and ultimately prevent) adverse events in cardio-oncology.
7. Challenges and limitations
Despite early proof-of-concept publications in cardio-oncology, there is an unmet need for additional investigation of machine learning-based approaches in dedicated cardio-oncology cohorts. Prospective studies examining the impact of this technology on patient outcomes may shed additional light on the utility of AI utility for cardio-oncology patients. The widespread use of machine learning approaches is constrained by their heterogeneity and logistical challenges associated with their seamless integration into contemporary clinical practice [9]. Additionally, the validity and efficacy of such methods have not been established in well-designed prospective studies [39]. Certain data challenges will need to be overcome through open access to algorithms and auto-population of research databases across multiple institutions to enable algorithms to be externally validated. Algorithms integrated as plugins into clinical systems may aid physicians by alerting them to subtle changes in their data interpretation that may require additional workup in high-risk patients.
We have discussed ECGs, echocardiograms, laboratory, and electronic health record data as input to algorithms. Additional publications on cardiac magnetic resonance, computed tomography, and nuclear imaging are also becoming available [6], [29], [40], [41], [42]. These cardio-oncology applications for patients at risk of coronary ischemia, myocarditis, hyperinflammatory response, and other conditions will almost certainly continue to benefit from ML research. Over the last decade, the use of AI applications in medicine has grown exponentially, particularly for remote monitoring and mobile and connected health, such as using smart devices (e.g. smartwatches) or smartphone apps to diagnose or manage various cardiac diseases [6], [7]. Along with cardiomyopathy, the use of smartwatches to predict other cardiovascular conditions such as atrial fibrillation is being studied and may become more prevalent in the future [6]. This has enormous potential for cardio-oncology patient monitoring.
Globally, significant advancements in cancer diagnosis and treatment have resulted in a continuous increase in the number of survivors. The application of machine learning to predict cardiac dysfunction (cardiomyopathy, left ventricular systolic dysfunction) in cancer survivors is of particular interest in cardio-oncology. Using machine learning to predict cardiac dysfunction in cancer survivors was more predictive than clinical laboratory data alone. However, combining both types of data resulted in the most precise prediction. In cardio-oncology, recent research indicates that machine learning can be used to analyze ECGs to detect subclinical cardiac dysfunction prior to major echocardiographic changes in patients receiving chemotherapy. Thus, machine learning algorithms have been used to detect subtle differences in cardiac mechanics in cancer survivors prior to the onset of overt cardiac dysfunction.
Additionally, efforts to apply AI in clinical practice must be directed toward overcoming rather than propagating health inequities and bias [43]. This is especially true in cardiology, oncology, and cardio-oncology, all of which are fields in which African Americans, for example, face a disproportionate risk of poor outcomes when compared to their Caucasian counterparts. For use in cardio-oncology, AI algorithms will need to be tested and validated in diverse populations, to ensure their appropriate and equitable use. Great care must be taken to ensure that AI models are generalizable to diverse populations, and that their implementation does not reflect or perpetuate healthcare disparities. The AI-ECG algorithms we have assessed in cardio-oncology so far show great promise for the detection of low ejection fraction across a range of ethnic and racial subgroups [134]. However, we must remain cognizant of the risk of AI models to reflect, perpetuate, and even promote bias in medicine.
Another challenge in developing and implementing AI-based clinical decision support tools on ECG is the infrastructural unpreparedness. The majority of AI-ECG models rely on using raw digital time-voltage ECG data. These raw data are typically not available through electronic health records and are typically underutilized/untouched in over 99% of institutions. Accessing these raw ECG data and integrating these data with clinical information requires technical knowledge and infrastructural investment. Further, in many cases, these raw ECG data are proprietary to vendors, making it more challenging to access ECG data for research purposes. These are not limitations specific to cancer survivors. However, studies involving cancer survivors often have small sample sizes. This compounds the limitations because large sample sizes are needed for training deep learning models.
We recognize that in general individual AI models can vary greatly depending on the architecture, data used to train, training methodology, and so on. Consequently, each model should be evaluated separately for generalizability and absence of propagation of bias. Additional challenges of AI-ECG include distinguishing signal data from noise (especially in wearables), recognizing and avoiding false positives or negatives, and optimizing the implementation of technologies to improve morbidity and mortality.
8. Conclusion
These studies add to the body of evidence demonstrating the utility of machine learning in predicting cardiac dysfunction in cancer survivors. As a result, AI has the potential to make a significant difference in predicting cardiac dysfunction in cancer survivors. In particular, over the course of half a century, ECG parameters have been evaluated and found to associate with (and perhaps predict) systolic dysfunction. With the dawn of the digital era, it will be prudent to advance our application of AI-ECG in cardio-oncology. Future studies should assess the ability of AI-ECG to predict the development of different cardiomyopathy phenotypes, AF, and other forms of cardiovascular toxicities in both children and adults, creating greater opportunity to pursue prophylactic and preventive cardioprotective measures, while facilitating cancer cure or palliation. One could imagine using AI-ECG to create an individualized profile of cardiotoxicity risk that could be used to tailor treatment choices and cardiovascular monitoring. Such applications are precocious at this point, but we anticipate that, with time, we will be able to leverage AI technologies to personalize care. This application of AI-ECG in cardio-oncology may be helpful to transform care in small and large hospital, academic, community, and rural practices, especially utilizing fast-paced low-cost pragmatic trial embedded in the electronic health records. Such incorporation of AI-ECG into cardio-oncology and other precision medicine programs may be beneficial to predict, preempt, and optimize cardiovascular health.
We did not obtain ethical/IRB approval for this literature review.
CRediT authorship contribution statement
Conception and design: SAB.
Drafting of the manuscript: SAB, DLM.
Interpretation of data: SAB, DLM, PN, OA, JH, KR, AH, RM, AS, RD, FG, JLJ.
Critical revision: SAB, DLM, PN, OA, JH, KR, AH, RM, AS, RD, FG, JLJ, SAB.
Final approval of manuscript: All authors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We are grateful to Dr. David Rayan from the Medical College of Wisconsin and Dr. Tarek Nafee from Boston University for their assistance with this manuscript.
Funding
This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Numbers UL1TR001436 and KL2TR001438. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- 1.Sturgeon K.M., Deng L., Bluethmann S.M., Zhou S., Trifiletti D.M., Jiang C., et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur. Heart J. 2019;40(48):3889–3897. doi: 10.1093/eurheartj/ehz766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chow E.J., Leger K.J., Bhatt N.S., Mulrooney D.A., Ross C.J., Aggarwal S., et al. Paediatric cardio-oncology: epidemiology, screening, prevention, and treatment. Cardiovasc. Res. 2019;115(5):922–934. doi: 10.1093/cvr/cvz031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hahn V.S., Lenihan D.J., Ky B. Cancer therapy-induced cardiotoxicity: basic mechanisms and potential cardioprotective therapies. J. Am. Heart Assoc. 2014;3(2) doi: 10.1161/JAHA.113.000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellinger A.M., Arteaga C.L., Force T., Humphreys B.D., Demetri G.D., Druker B.J., et al. Cardio-oncology: how new targeted cancer therapies and precision medicine can inform cardiovascular discovery. Circulation. 2015;132(23):2248–2258. doi: 10.1161/CIRCULATIONAHA.115.010484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maleszewski J.J., Bois M.C., Bois J.P., Young P.M., Stulak J.M., Klarich K.W. Neoplasia and the heart: pathological review of effects with clinical and radiological correlation. J. Am. Coll. Cardiol. 2018;72(2):202–227. doi: 10.1016/j.jacc.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 6.Herrmann J., Lerman A., Sandhu N.P., Villarraga H.R., Mulvagh S.L., Kohli M. Evaluation and management of patients with heart disease and cancer: cardio-oncology. Mayo Clin. Proc. 2014;89(9):1287–1306. doi: 10.1016/j.mayocp.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campia U., Moslehi J.J., Amiri-Kordestani L., Barac A., Beckman J.A., Chism D.D., et al. Cardio-oncology: vascular and metabolic perspectives: a scientific statement from the American Heart Association. Circulation. 2019;139(13):e579–e602. doi: 10.1161/CIR.0000000000000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu J.R., Florido R., Lipson E.J., Naidoo J., Ardehali R., Tocchetti C.G., et al. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc. Res. 2019;115(5):854–868. doi: 10.1093/cvr/cvz026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iliescu C.A., Grines C.L., Herrmann J., Yang E.H., Cilingiroglu M., Charitakis K., et al. SCAI expert consensus statement: evaluation, management, and special considerations of cardio-oncology patients in the cardiac catheterization laboratory (endorsed by the cardiological society of India, and sociedad latino Americana de Cardiologıa intervencionista) Catheter. Cardiovasc. Interv. 2016;87(5):E202–E223. doi: 10.1002/ccd.26379. [DOI] [PubMed] [Google Scholar]
- 10.Cameron A.C., Touyz R.M., Lang N.N. Vascular complications of cancer chemotherapy. Can J Cardiol. 2016;32(7):852–862. doi: 10.1016/j.cjca.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ederhy S., Cautela J., Ancedy Y., Escudier M., Thuny F., Cohen A. Takotsubo-like syndrome in cancer patients treated with immune checkpoint inhibitors. JACC Cardiovasc. Imaging. 2018;11(8):1187–1190. doi: 10.1016/j.jcmg.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 12.Blaes A.H., Thavendiranathan P., Moslehi J. Cardiac toxicities in the era of precision medicine: underlying risk factors, targeted therapies, and cardiac biomarkers. Am. Soc. Clin. Oncol. Educ. Book. 2018;38:764–774. doi: 10.1200/EDBK_208509. [DOI] [PubMed] [Google Scholar]
- 13.Chang H.M., Moudgil R., Scarabelli T., Okwuosa T.M., Yeh E.T.H. Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: part 1. J. Am. Coll. Cardiol. 2017;70(20):2536–2551. doi: 10.1016/j.jacc.2017.09.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang H.M., Okwuosa T.M., Scarabelli T., Moudgil R., Yeh E.T.H. Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: part 2. J. Am. Coll. Cardiol. 2017;70(20):2552–2565. doi: 10.1016/j.jacc.2017.09.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desai M.Y., Jellis C.L., Kotecha R., Johnston D.R., Griffin B.P. Radiation-associated cardiac disease: a practical approach to diagnosis and management. JACC Cardiovasc. Imaging. 2018;11(8):1132–1149. doi: 10.1016/j.jcmg.2018.04.028. [DOI] [PubMed] [Google Scholar]
- 16.Menezes K.M., Wang H., Hada M., Saganti P.B. Radiation matters of the heart: a mini review. Front Cardiovasc Med. 2018;5:83. doi: 10.3389/fcvm.2018.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sylvester C.B., Abe J.I., Patel Z.S., Grande-Allen K.J. Radiation-induced cardiovascular disease: mechanisms and importance of linear energy transfer. Front Cardiovasc Med. 2018;5:5. doi: 10.3389/fcvm.2018.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mulrooney D.A., Armstrong G.T., Huang S., Ness K.K., Ehrhardt M.J., Joshi V.M., et al. Cardiac outcomes in adult survivors of childhood cancer exposed to cardiotoxic therapy: a cross-sectional study. Ann. Intern. Med. 2016;164(2):93–101. doi: 10.7326/M15-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armstrong G.T., Chen Y., Yasui Y., Leisenring W., Gibson T.M., Mertens A.C., et al. Reduction in late mortality among 5-year survivors of childhood cancer. N. Engl. J. Med. 2016;374(9):833–842. doi: 10.1056/NEJMoa1510795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hawkins M., Brownsdon A., Reulen R. Falling risk of heart disease among survivors of childhood cancer. BMJ. 2020;368 doi: 10.1136/bmj.m58. [DOI] [PubMed] [Google Scholar]
- 21.Group CsO. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent and Young Adult Cancers, Version 5.0 Monrovia, CA: Children's Oncology Group; [Available from: www.survivorshipguidelines.org.
- 22.Armenian S.H., Hudson M.M., Mulder R.L., Chen M.H., Constine L.S., Dwyer M., et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the international late effects of childhood cancer guideline harmonization group. Lancet Oncol. 2015;16(3):e123–e136. doi: 10.1016/S1470-2045(14)70409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armenian S.H., Lacchetti C., Barac A., Carver J., Constine L.S., Denduluri N., et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 2017;35(8):893–911. doi: 10.1200/JCO.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- 24.Dent S.F., Kikuchi R., Kondapalli L., Ismail-Khan R., Brezden-Masley C., Barac A., et al. Optimizing cardiovascular health in patients with cancer: a practical review of risk assessment, monitoring, and prevention of cancer treatment-related cardiovascular toxicity. Am Soc Clin Oncol Educ Book. 2020;40:1–15. doi: 10.1200/EDBK_286019. [DOI] [PubMed] [Google Scholar]
- 25.National Comprehensive Cancer Network . 2014. NCCN Clinical Practice Guidelines in Oncology. Breast Cancer. Version 3.https://jnccn.org/abstract/journals/jnccn/12/4/article-p542.xml Available from: [Google Scholar]
- 26.Hudson M.M., Leisenring W., Stratton K.K., Tinner N., Steen B.D., Ogg S., et al. Increasing cardiomyopathy screening in at-risk adult survivors of pediatric malignancies: a randomized controlled trial. J. Clin. Oncol. 2014;32(35):3974–3981. doi: 10.1200/JCO.2014.57.3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casillas J., Oeffinger K.C., Hudson M.M., Greenberg M.L., Yeazel M.W., Ness K.K., et al. Identifying predictors of longitudinal decline in the level of medical care received by adult survivors of childhood cancer: a report from the childhood cancer survivor study. Health Serv. Res. 2015;50(4):1021–1042. doi: 10.1111/1475-6773.12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caplin D.A., Smith K.R., Ness K.K., Hanson H.A., Smith S.M., Nathan P.C., et al. Effect of population socioeconomic and health system factors on medical Care of Childhood Cancer Survivors: a report from the childhood cancer survivor study. J Adolesc Young Adult Oncol. 2017;6(1):74–82. doi: 10.1089/jayao.2016.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mueller E.L., Park E.R., Kirchhoff A.C., Kuhlthau K., Nathan P.C., Perez G.K., et al. Insurance, chronic health conditions, and utilization of primary and specialty outpatient services: a childhood cancer survivor study report. J. Cancer Surviv. 2018;12(5):639–646. doi: 10.1007/s11764-018-0700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan A.P., Chen Y., Henderson T.O., Oeffinger K.C., Hudson M.M., Gibson T.M., et al. Adherence to surveillance for second malignant neoplasms and cardiac dysfunction in childhood cancer survivors: a childhood cancer survivor study. J. Clin. Oncol. 2020;38(15):1711–1722. doi: 10.1200/JCO.19.01825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez-Jimenez F., Attia Z., Arruda-Olson A.M., Carter R., Chareonthaitawee P., Jouni H., et al. Artificial intelligence in cardiology: present and future. Mayo Clin. Proc. 2020;95(5):1015–1039. doi: 10.1016/j.mayocp.2020.01.038. [DOI] [PubMed] [Google Scholar]
- 32.Johnson K.W., Torres Soto J., Glicksberg B.S., Shameer K., Miotto R., Ali M., et al. Artificial intelligence in cardiology. J. Am. Coll. Cardiol. 2018;71(23):2668–2679. doi: 10.1016/j.jacc.2018.03.521. [DOI] [PubMed] [Google Scholar]
- 33.Souza Filho E.M., Fernandes F.A., Soares C.L.A., Seixas F.L., Santos A.A.S.M., Gismondi R.A., et al. Artificial intelligence in cardiology: concepts, tools and challenges - “The horse is the one who runs, you must be the Jockey”. Arq. Bras. Cardiol. 2019;114:718–725. doi: 10.36660/abc.20180431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Westcott R.J., Tcheng J.E. Artificial intelligence and machine learning in cardiology. JACC Cardiovasc Interv. 2019;12(14):1312–1314. doi: 10.1016/j.jcin.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 35.Madan N., Lucas J., Akhter N., Collier P., Cheng F., Guha A., et al. Artificial intelligence and imaging: opportunities in cardio-oncology. American Heart Journal Plus: Cardiology Research and Practice. 2022:100126. doi: 10.1016/j.ahjo.2022.100126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith L.A., Cornelius V.R., Plummer C.J., Levitt G., Verrill M., Canney P., et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer. 2010;10:337. doi: 10.1186/1471-2407-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saif M.W., Shah M.M., Shah A.R. Fluoropyrimidine-associated cardiotoxicity: revisited. Expert Opin. Drug Saf. 2009;8(2):191–202. doi: 10.1517/14740330902733961. [DOI] [PubMed] [Google Scholar]
- 38.Rowinsky E.K., McGuire W.P., Guarnieri T., Fisherman J.S., Christian M.C., Donehower R.C. Cardiac disturbances during the administration of taxol. J. Clin. Oncol. 1991;9(9):1704–1712. doi: 10.1200/JCO.1991.9.9.1704. [DOI] [PubMed] [Google Scholar]
- 39.Bissett D., Setanoians A., Cassidy J., Graham M.A., Chadwick G.A., Wilson P., et al. Phase I and pharmacokinetic study of taxotere (RP 56976) administered as a 24-hour infusion. Cancer Res. 1993;53(3):523–527. [PubMed] [Google Scholar]
- 40.Nieto Y., Cagnoni P.J., Bearman S.I., Shpall E.J., Matthes S., Jones R.B. Cardiac toxicity following high-dose cyclophosphamide, cisplatin, and BCNU (STAMP-I) for breast cancer. Biol. Blood Marrow Transplant. 2000;6(2A):198–203. doi: 10.1016/s1083-8791(00)70043-7. [DOI] [PubMed] [Google Scholar]
- 41.Brockstein B.E., Smiley C., Al-Sadir J., Williams S.F. Cardiac and pulmonary toxicity in patients undergoing high-dose chemotherapy for lymphoma and breast cancer: prognostic factors. Bone Marrow Transplant. 2000;25(8):885–894. doi: 10.1038/sj.bmt.1702234. [DOI] [PubMed] [Google Scholar]
- 42.Tomirotti M., Riundi R., Pulici S., Ungaro A., Pedretti D., Villa S., et al. Ischemic cardiopathy from cis-diamminedichloroplatinum (CDDP) Tumori. 1984;70(3):235–236. doi: 10.1177/030089168407000305. [DOI] [PubMed] [Google Scholar]
- 43.Perry M.C. Cancer and the Heart. Springer; 1986. Effects of chemotherapy on the heart; pp. 223–226. [Google Scholar]
- 44.Giordano S.H., Elias A.D., Gradishar W.J. NCCN guidelines updates: breast cancer. J. Natl. Compr. Cancer Netw. 2018;16(5S):605–610. doi: 10.6004/jnccn.2018.0043. [DOI] [PubMed] [Google Scholar]
- 45.Ganatra S., Sharma A., Shah S., Chaudhry G.M., Martin D.T., Neilan T.G., et al. Ibrutinib-associated atrial fibrillation. JACC Clin Electrophysiol. 2018;4(12):1491–1500. doi: 10.1016/j.jacep.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 46.Ranpura V., Pulipati B., Chu D., Zhu X., Wu S. Increased risk of high-grade hypertension with bevacizumab in cancer patients: a meta-analysis. Am. J. Hypertens. 2010;23(5):460–468. doi: 10.1038/ajh.2010.25. [DOI] [PubMed] [Google Scholar]
- 47.Hedhli N., Russell K.S. Cardiotoxicity of molecularly targeted agents. Curr. Cardiol. Rev. 2011;7(4):221–233. doi: 10.2174/157340311799960636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chu T.F., Rupnick M.A., Kerkela R., Dallabrida S.M., Zurakowski D., Nguyen L., et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370(9604):2011–2019. doi: 10.1016/S0140-6736(07)61865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gradishar W.J., Anderson B.O., Balassanian R., Blair S.L., Burstein H.J., Cyr A., et al. Breast cancer, version 4.2017, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2018;16(3):310–320. doi: 10.6004/jnccn.2018.0012. [DOI] [PubMed] [Google Scholar]
- 50.Muluneh B., Richardson D.R., Hicks C., Jensen B.C., Zeidner J.F. Trials and tribulations of corrected QT interval monitoring in oncology: rationale for a practice-changing standardized approach. J. Clin. Oncol. 2019;37(30):2719–2721. doi: 10.1200/JCO.19.00922. [DOI] [PubMed] [Google Scholar]
- 51.Cornell R.F., Ky B., Weiss B.M., Dahm C.N., Gupta D.K., Du L., et al. Prospective study of cardiac events during proteasome inhibitor therapy for relapsed multiple myeloma. J. Clin. Oncol. 2019;37(22):1946–1955. doi: 10.1200/JCO.19.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waxman A.J., Clasen S., Hwang W.T., Garfall A., Vogl D.T., Carver J., et al. Carfilzomib-associated cardiovascular adverse events: a systematic review and meta-analysis. JAMA Oncol. 2018;4(3) doi: 10.1001/jamaoncol.2017.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lane A.A., Chabner B.A. Histone deacetylase inhibitors in cancer therapy. J. Clin. Oncol. 2009;27(32):5459–5468. doi: 10.1200/JCO.2009.22.1291. [DOI] [PubMed] [Google Scholar]
- 54.Takizawa D., Kakizaki S., Horiguchi N., Tojima H., Yamazaki Y., Ichikawa T., et al. Histone deacetylase inhibitors induce cytochrome P450 2B by activating nuclear receptor constitutive androstane receptor. 2010;38(9):1493–1495. doi: 10.1124/dmd.110.032854. [DOI] [PubMed] [Google Scholar]
- 55.Chiengthong K., Lertjitbanjong P., Thongprayoon C., Bathini T., Sharma K., Prasitlumkum N., et al. Arrhythmias in hematopoietic stem cell transplantation: a systematic review and meta-analysis. Eur. J. Haematol. 2019;103(6):564–572. doi: 10.1111/ejh.13322. [DOI] [PubMed] [Google Scholar]
- 56.Schiattarella G.G., Sannino A., Toscano E., Cattaneo F., Trimarco B., Esposito G., et al. Cardiovascular effects of histone deacetylase inhibitors epigenetic therapies: systematic review of 62 studies and new hypotheses for future research. Int. J. Cardiol. 2016;219:396–403. doi: 10.1016/j.ijcard.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 57.EBCTCG Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386(10001):1341–1352. doi: 10.1016/S0140-6736(15)61074-1. [DOI] [PubMed] [Google Scholar]
- 58.Baum M., Buzdar A., Cuzick J., Forbes J., Houghton J., Howell A., et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer: results of the ATAC (Arimidex, tamoxifen alone or in Combination) trial efficacy and safety update analyses. Cancer. 2003;98(9):1802–1810. doi: 10.1002/cncr.11745. [DOI] [PubMed] [Google Scholar]
- 59.Coates A.S., Keshaviah A., Thürlimann B., Mouridsen H., Mauriac L., Forbes J.F., et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1–98. J. Clin. Oncol. 2007;25(5):486–492. doi: 10.1200/JCO.2006.08.8617. [DOI] [PubMed] [Google Scholar]
- 60.van de Velde C.J., Rea D., Seynaeve C., Putter H., Hasenburg A., Vannetzel J.M., et al. Adjuvant tamoxifen and exemestane in early breast cancer (TEAM): a randomised phase 3 trial. Lancet. 2011;377(9762):321–331. doi: 10.1016/S0140-6736(10)62312-4. [DOI] [PubMed] [Google Scholar]
- 61.Zhang L., Reynolds K., Lyon A., Palaskas N., Neilan T. The evolving immunotherapy landscape and the epidemiology, diagnosis, and Management of Cardiotoxicity. JACC CardioOncol. 2021;3(1):35–47. doi: 10.1016/j.jaccao.2020.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brown C.E., Mackall C.L. CAR T cell therapy: inroads to response and resistance. Nat Rev Immunol. 2019;19(2):73–74. doi: 10.1038/s41577-018-0119-y. [DOI] [PubMed] [Google Scholar]
- 63.Kochenderfer J.N. Genetic engineering of T cells in leukemia and lymphoma. Clin Adv Hematol Oncol. 2014;12(3):190–192. [PubMed] [Google Scholar]
- 64.Ghosh A.K., Chen D.H., Guha A., Mackenzie S., Walker J.M., Roddie C. CAR T cell therapy–related cardiovascular outcomes and management: systemic disease or direct cardiotoxicity? JACC CardioOncology. 2020;2(1):97–109. doi: 10.1016/j.jaccao.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mathur P., Thanendrarajan S., Paydak H., Vallurupalli S., Jambhekar K., Bhatti S., et al. Cardiovascular complications of multiple myeloma in the elderly. Expert. Rev. Cardiovasc. Ther. 2017;15(12):933–943. doi: 10.1080/14779072.2017.1409114. [DOI] [PubMed] [Google Scholar]
- 66.Mathur P., Paydak H., Thanendrarajan S., van Rhee F. Atrial fibrillation in hematologic malignancies, especially after autologous hematopoietic stem cell transplantation: review of risk factors, current management, and future directions. Clin Lymphoma Myeloma Leuk. 2016;16(2):70–75. doi: 10.1016/j.clml.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 67.Cardinale D., Colombo A., Sandri M.T., Lamantia G., Colombo N., Civelli M., et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006;114(23):2474–2481. doi: 10.1161/CIRCULATIONAHA.106.635144. [DOI] [PubMed] [Google Scholar]
- 68.Rotz S.J., Ryan T.D., Jodele S., Jefferies J.L., Lane A., Pate A., et al. The injured heart: early cardiac effects of hematopoietic stem cell transplantation in children and young adults. Bone Marrow Transplant. 2017;52(8):1171–1179. doi: 10.1038/bmt.2017.62. [DOI] [PubMed] [Google Scholar]
- 69.Armenian S.H., Sun C.L., Shannon T., Mills G., Francisco L., Venkataraman K., et al. Incidence and predictors of congestive heart failure after autologous hematopoietic cell transplantation. Blood. 2011;118(23):6023–6029. doi: 10.1182/blood-2011-06-358226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Quer G., Arnaout R., Henne M. Machine learning and the future of cardiovascular care: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2021;77(3):300–313. doi: 10.1016/j.jacc.2020.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dey D., Slomka P.J., Leeson P., Comaniciu D., Shrestha S., Sengupta P.P., et al. Artificial intelligence in cardiovascular imaging: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2019;73(11):1317–1335. doi: 10.1016/j.jacc.2018.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Awadalla M., Hassan M.Z.O., Alvi R.M., Neilan T.G. Advanced imaging modalities to detect cardiotoxicity. Curr. Probl. Cancer. 2018;42(4):386–396. doi: 10.1016/j.currproblcancer.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Steyerberg E.W., Vickers A.J., Cook N.R., Gerds T., Gonen M., Obuchowski N., et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21(1):128–138. doi: 10.1097/EDE.0b013e3181c30fb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alba A.C., Agoritsas T., Walsh M., Hanna S., Iorio A., Devereaux P.J., et al. Discrimination and calibration of clinical prediction models: Users' guides to the medical literature. JAMA. 2017;318(14):1377–1384. doi: 10.1001/jama.2017.12126. [DOI] [PubMed] [Google Scholar]
- 75.Beam A.L., Manrai A.K., Ghassemi M. Challenges to the reproducibility of machine learning models in health care. JAMA. 2020;323(4):305–306. doi: 10.1001/jama.2019.20866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dilsizian M.E., Siegel E.L. Machine meets biology: a primer on artificial intelligence in cardiology and cardiac imaging. Curr. Cardiol. Rep. 2018;20(12):139. doi: 10.1007/s11886-018-1074-8. [DOI] [PubMed] [Google Scholar]
- 77.Dorado-Díaz P.I., Sampedro-Gómez J., Vicente-Palacios V., Sánchez P.L. Applications of artificial intelligence in cardiology. The future is already here. Rev Esp Cardiol (Engl Ed) 2019;72(12):1065–1075. doi: 10.1016/j.rec.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 78.Feeny A.K., Chung M.K., Madabhushi A., Attia Z.I., Cikes M., Firouznia M., et al. Artificial intelligence and machine learning in arrhythmias and cardiac electrophysiology. Circ. Arrhythm. Electrophysiol. 2020;13(8) doi: 10.1161/CIRCEP.119.007952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gomez J., Doukky R. Artificial intelligence in nuclear cardiology. J. Nucl. Med. 2019;60(8):1042–1043. doi: 10.2967/jnumed.118.222356. [DOI] [PubMed] [Google Scholar]
- 80.Krittanawong C., Zhang H., Wang Z., Aydar M., Kitai T. Artificial intelligence in precision cardiovascular medicine. J. Am. Coll. Cardiol. 2017;69(21):2657–2664. doi: 10.1016/j.jacc.2017.03.571. [DOI] [PubMed] [Google Scholar]
- 81.Sandstedt M., Henriksson L., Janzon M., Nyberg G., Engvall J., De Geer J., et al. Evaluation of an AI-based, automatic coronary artery calcium scoring software. Eur. Radiol. 2020;30(3):1671–1678. doi: 10.1007/s00330-019-06489-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sardar P., Abbott J.D., Kundu A., Aronow H.D., Granada J.F., Giri J. Impact of artificial intelligence on interventional cardiology: from decision-making aid to advanced interventional procedure assistance. JACC Cardiovasc Interv. 2019;12(14):1293–1303. doi: 10.1016/j.jcin.2019.04.048. [DOI] [PubMed] [Google Scholar]
- 83.Xu B., Kocyigit D., Grimm R., Griffin B.P., Cheng F. Applications of artificial intelligence in multimodality cardiovascular imaging: a state-of-the-art review. Prog. Cardiovasc. Dis. 2020;63(3):367–376. doi: 10.1016/j.pcad.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 84.Luchini C., Pea A., Scarpa A. Artificial intelligence in oncology: current applications and future perspectives. Br. J. Cancer. 2022;126(1):4–9. doi: 10.1038/s41416-021-01633-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shimizu H., Nakayama K.I. Artificial intelligence in oncology. Cancer Sci. 2020;111(5):1452–1460. doi: 10.1111/cas.14377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kolek M.J., Graves A.J., Xu M., Bian A., Teixeira P.L., Shoemaker M.B., et al. Evaluation of a prediction model for the development of atrial fibrillation in a repository of electronic medical records. JAMA Cardiol. 2016;1(9):1007–1013. doi: 10.1001/jamacardio.2016.3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pavlou M., Ambler G., Seaman S.R., Guttmann O., Elliott P., King M., et al. How to develop a more accurate risk prediction model when there are few events. BMJ. 2015;351 doi: 10.1136/bmj.h3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Narula S., Shameer K., Salem Omar A.M., Dudley J.T., Sengupta P.P. Machine-learning algorithms to automate morphological and functional assessments in 2D echocardiography. J. Am. Coll. Cardiol. 2016;68(21):2287–2295. doi: 10.1016/j.jacc.2016.08.062. [DOI] [PubMed] [Google Scholar]
- 89.Deo R.C. Machine learning in medicine. Circulation. 2015;132(20):1920–1930. doi: 10.1161/CIRCULATIONAHA.115.001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cover T., Hart P. Nearest neighbor pattern classification. IEEE Trans. Inf. Theory. 1967;13(21–27) [Google Scholar]
- 91.Brown S.A. Patient similarity: emerging concepts in systems and precision medicine. Front. Physiol. 2016;7:561. doi: 10.3389/fphys.2016.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Parimbelli E., Marini S., Sacchi L., Bellazzi R. Patient similarity for precision medicine: a systematic review. J. Biomed. Inform. 2018;83:87–96. doi: 10.1016/j.jbi.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 93.Hou Y., Zhou Y., Hussain M., Budd G.T., Tang W.H.W., Abraham J., et al. Cardiac risk stratification in cancer patients: a longitudinal patient-patient network analysis. PLoS Med. 2021;18(8) doi: 10.1371/journal.pmed.1003736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou Y., Hou Y., Hussain M., Brown S.A., Budd T., Tang W.H.W., et al. Machine learning-based risk assessment for cancer therapy-related cardiac dysfunction in 4300 longitudinal oncology patients. J. Am. Heart Assoc. 2020;9(23) doi: 10.1161/JAHA.120.019628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Choi E., Schuetz A., Stewart W.F., Sun J. Using recurrent neural network models for early detection of heart failure onset. J. Am. Med. Inform. Assoc. 2017;24(2):361–370. doi: 10.1093/jamia/ocw112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kiranyaz S., Ince T., Gabbouj M. Real-time patient-specific ECG classification by 1-D convolutional neural networks. IEEE Trans. Biomed. Eng. 2016;63(3):664–675. doi: 10.1109/TBME.2015.2468589. [DOI] [PubMed] [Google Scholar]
- 97.Abdolmanafi A., Duong L., Dahdah N., Cheriet F. Deep feature learning for automatic tissue classification of coronary artery using optical coherence tomography. Biomed Opt Express. 2017;8(2):1203–1220. doi: 10.1364/BOE.8.001203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Obermeyer Z., Emanuel E.J. Predicting the future - big data, machine learning, and clinical medicine. N. Engl. J. Med. 2016;375(13):1216–1219. doi: 10.1056/NEJMp1606181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Attia Z.I., Noseworthy P.A., Lopez-Jimenez F., Asirvatham S.J., Deshmukh A.J., Gersh B.J., et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet (London, England). 2019;394(10201):861–867. doi: 10.1016/S0140-6736(19)31721-0. [DOI] [PubMed] [Google Scholar]
- 100.Attia Z.I., Kapa S., Lopez-Jimenez F., McKie P.M., Ladewig D.J., Satam G., et al. Screening for cardiac contractile dysfunction using an artificial intelligence-enabled electrocardiogram. Nat. Med. 2019;25(1):70–74. doi: 10.1038/s41591-018-0240-2. [DOI] [PubMed] [Google Scholar]
- 101.Attia Z.I., Kapa S., Yao X., Lopez-Jimenez F., Mohan T.L., Pellikka P.A., et al. Prospective validation of a deep learning electrocardiogram algorithm for the detection of left ventricular systolic dysfunction. J. Cardiovasc. Electrophysiol. 2019;30(5):668–674. doi: 10.1111/jce.13889. [DOI] [PubMed] [Google Scholar]
- 102.Jentzer J.C., Kashou A.H., Attia Z.I., Lopez-Jimenez F., Kapa S., Friedman P.A., et al. Left ventricular systolic dysfunction identification using artificial intelligence-augmented electrocardiogram in cardiac intensive care unit patients. Int. J. Cardiol. 2021;326:114–123. doi: 10.1016/j.ijcard.2020.10.074. [DOI] [PubMed] [Google Scholar]
- 103.Adedinsewo D., Carter R.E., Attia Z., Johnson P., Kashou A.H., Dugan J.L., et al. Artificial intelligence-enabled ECG algorithm to identify patients with left ventricular systolic dysfunction presenting to the emergency department with dyspnea. Circ. Arrhythm. Electrophysiol. 2020;13(8) doi: 10.1161/CIRCEP.120.008437. [DOI] [PubMed] [Google Scholar]
- 104.Akbilgic O., Butler L., Karabayir I., Chang P., Kritzman D., Alonso A., et al. Artificial intelligence applied to ECG improves heart failure prediction accuracy. J Am College Cardiol. 2021;77:3045. [Google Scholar]
- 105.Yao X., Rushlow D.R., Inselman J.W., McCoy R.G., Thacher T.D., Behnken E.M., et al. Artificial intelligence-enabled electrocardiograms for identification of patients with low ejection fraction: a pragmatic, randomized clinical trial. Nat. Med. 2021;27(5):815–819. doi: 10.1038/s41591-021-01335-4. [DOI] [PubMed] [Google Scholar]
- 106.Hinai G., Jammoul S., Vajihi Z., Afilalo J. Deep learning analysis of resting electrocardiograms for the detection of myocardial dysfunction, hypertrophy, and ischaemia: a systematic review. Eur Heart J Digit Health. 2021;2:416–423. doi: 10.1093/ehjdh/ztab048. ztab048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Biton S., Gendelman S., Ribeiro A., Miana G., Moreira C., Ribeiro A., et al. Atrial fibrillation risk prediction from the 12-lead ECG using digital biomarkers and deep representation learning. Eur Heart J Digit Health. 2021;2:576–585. doi: 10.1093/ehjdh/ztab071. ztab071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tison G.H., Zhang J., Delling F.N., Deo R.C. Automated and interpretable patient ECG profiles for disease detection, tracking, and discovery. Circ Cardiovasc Qual Outcomes. 2019;12(9) doi: 10.1161/CIRCOUTCOMES.118.005289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Christopoulos G., Graff-Radford J., Lopez C.L., Yao X., Attia Z.I., Rabinstein A.A., et al. Artificial intelligence-electrocardiography to predict incident atrial fibrillation: a population-based study. Circ. Arrhythm. Electrophysiol. 2020;13(12) doi: 10.1161/CIRCEP.120.009355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gao J., Zhang H., Lu P., Wang Z. An effective LSTM recurrent network to detect arrhythmia on imbalanced ECG dataset. J Healthc Eng. 2019;2019:6320651. doi: 10.1155/2019/6320651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim B.H., Pyun J.Y. ECG identification for personal authentication using LSTM-based deep recurrent neural networks. Sensors (Basel) 2020;20(11) doi: 10.3390/s20113069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xiong Z., Nash M.P., Cheng E., Fedorov V.V., Stiles M.K., Zhao J. ECG signal classification for the detection of cardiac arrhythmias using a convolutional recurrent neural network. Physiol. Meas. 2018;39(9) doi: 10.1088/1361-6579/aad9ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhou X., Zhu X., Nakamura K., Mahito N. Premature ventricular contraction detection from ambulatory ECG using recurrent neural networks. Annu Int Conf IEEE Eng Med Biol Soc. 2018;2018:2551–2554. doi: 10.1109/EMBC.2018.8512858. [DOI] [PubMed] [Google Scholar]
- 114.Saadatnejad S., Oveisi M., Hashemi M. LSTM-based ECG classification for continuous monitoring on personal wearable devices. IEEE J. Biomed. Health Inform. 2020;24(2):515–523. doi: 10.1109/JBHI.2019.2911367. [DOI] [PubMed] [Google Scholar]
- 115.Chauhan S., Vig L., Ahmad S. ECG anomaly class identification using LSTM and error profile modeling. Comput. Biol. Med. 2019;109:14–21. doi: 10.1016/j.compbiomed.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 116.Vaksmann G., Gutierrez R., Duhamel A., Nelken B., Francart C., Kouakam C., et al. Signal-averaged electrocardiography in children with anthracycline-induced cardiomyopathy. Pediatr. Cardiol. 2001;22(6):494–498. doi: 10.1007/s002460010282. [DOI] [PubMed] [Google Scholar]
- 117.Desai L., Balmert L., Reichek J., Hauck A., Gambetta K., Webster G. Electrocardiograms for cardiomyopathy risk stratification in children with anthracycline exposure. Cardio-Oncology. 2019;5:10. doi: 10.1186/s40959-019-0045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gunturkun F., Davis R.L., Armstrong G.T., Jefferies J.L., Ness K.K., Green D.M., et al. Deep learning for improved prediction of late-onset cardiomyopathy among childhood cancer survivors: a report from the st. Jude lifetime cohort (SJLIFE) J. Clin. Oncol. 2020;38(15):10545. [Google Scholar]
- 119.Güntürkün F., Akbilgic O., Davis R.L., Armstrong G.T., Howell R.M., Jefferies J.L., et al. Artificial intelligence-assisted prediction of late-onset cardiomyopathy among childhood cancer survivors. JCO Clin Cancer Inform. 2021;5:459–468. doi: 10.1200/CCI.20.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Minow R.A., Benjamin R.S., Lee E.T., Gottlieb J.A. Adriamycin cardiomyopathy–risk factors. Cancer. 1977;39(4):1397–1402. doi: 10.1002/1097-0142(197704)39:4<1397::aid-cncr2820390407>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 121.Horacek J.M., Jakl M., Horackova J., Pudil R., Jebavy L., Maly J. Assessment of anthracycline-induced cardiotoxicity with electrocardiography. Exp. Oncol. 2009;31(2):115–117. [PubMed] [Google Scholar]
- 122.Farmakis D., Parissis J., Filippatos G. Insights into onco-cardiology: atrial fibrillation in cancer. J. Am. Coll. Cardiol. 2014;63(10):945–953. doi: 10.1016/j.jacc.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 123.Han J.K. Smartphones, smartwatches, and CIED patient safety: so far, so good. JACC Clin Electrophysiol. 2020;6(9):1167–1170. doi: 10.1016/j.jacep.2020.05.016. [DOI] [PubMed] [Google Scholar]
- 124.Pierleoni P., Gambi E., Ricciuti M., Sbrollini A., Palma L., Belli A., et al. Simultaneously acquired data from contactless and wearable devices for direct and indirect heart-rate measurement. Data Brief. 2019;26 doi: 10.1016/j.dib.2019.104436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Isakadze N., Martin S.S. How useful is the smartwatch ECG? Trends Cardiovasc Med. 2020;30(7):442–448. doi: 10.1016/j.tcm.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 126.Dörr M., Nohturfft V., Brasier N., Bosshard E., Djurdjevic A., Gross S., et al. The WATCH AF trial: SmartWATCHes for detection of atrial fibrillation. JACC Clin Electrophysiol. 2019;5(2):199–208. doi: 10.1016/j.jacep.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 127.McConnell M.V., Turakhia M.P., Harrington R.A., King A.C., Ashley E.A. Mobile health advances in physical activity, fitness, and atrial fibrillation: moving hearts. J. Am. Coll. Cardiol. 2018;71(23):2691–2701. doi: 10.1016/j.jacc.2018.04.030. [DOI] [PubMed] [Google Scholar]
- 128.Grafton F., Ho J., Ranjbarvaziri S., Farshidfar F., Budan A., Steltzer S., et al. Deep learning detects cardiotoxicity in a high-content screen with induced pluripotent stem cell-derived cardiomyocytes. eLife. 2021;10 doi: 10.7554/eLife.68714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chaix M.-A., Parmar N., Kinnear C., Lafreniere-Roula M., Akinrinade O., Yao R., et al. Machine learning identifies clinical and genetic factors associated with anthracycline cardiotoxicity in pediatric cancer survivors. JACC CardioOncol. 2020;2(5):690–706. doi: 10.1016/j.jaccao.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Perry T.R., Roberts M.L., Sunkara B., Maddula R., McLeish T., Gomez J., et al. Modeling precision cardio-oncology: using human-induced pluripotent stem cells for risk stratification and prevention. Curr. Oncol. Rep. 2021;23(7):77. doi: 10.1007/s11912-021-01066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Asnani A., Moslehi J.J., Adhikari B.B., Baik A.H., Beyer A.M., de Boer R.A., et al. Preclinical models of cancer therapy-associated cardiovascular toxicity: a scientific statement from the American Heart Association. Circ. Res. 2021;129(1):e21–e34. doi: 10.1161/RES.0000000000000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Salem J.E., Yang T., Moslehi J.J., Waintraub X., Gandjbakhch E., Bachelot A., et al. Androgenic effects on ventricular repolarization: a translational study from the international pharmacovigilance database to iPSC-cardiomyocytes. Circulation. 2019;140(13):1070–1080. doi: 10.1161/CIRCULATIONAHA.119.040162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Millard D., Dang Q., Shi H., Zhang X., Strock C., Kraushaar U., et al. Cross-site reliability of human induced pluripotent stem cell-derived cardiomyocyte based safety assays using microelectrode arrays: results from a blinded CiPA pilot study. Toxicol. Sci. 2018;164(2):550–562. doi: 10.1093/toxsci/kfy110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Noseworthy P.A., Attia Z.I., Brewer L.C., Hayes S.N., Yao X., Kapa S., et al. Assessing and mitigating bias in medical artificial intelligence: the effects of race and ethnicity on a deep learning model for ECG analysis. Circ. Arrhythm. Electrophysiol. 2020;13(3) doi: 10.1161/CIRCEP.119.007988. [DOI] [PMC free article] [PubMed] [Google Scholar]