Abstract
BACKGROUND
Prostate-specific antigen screening has profoundly affected the epidemiology of prostate cancer in the United States. Persistent racial disparities in outcomes for Black men warrant re-examination of the harms of screening relative to its cancer-specific mortality benefits in this population.
METHODS
We estimated overdiagnoses and overtreatment of prostate cancer for men of all races and for Black men 50 to 84 years of age until 2016, the most recent year with treatment data available, using excess incidence relative to 1986 based on the Surveillance, Epidemiology, and End Results registry and U.S. Census data as well as an established microsimulation model of prostate cancer natural history. Combining estimates with plausible mortality benefit, we calculated numbers needed to diagnose (NND) and treat (NNT) to prevent one prostate cancer death.
RESULTS
For men of all races, we estimated 1.5 to 1.9 million (range between estimation approaches) overdiagnosed and 0.9 to 1.5 million overtreated prostate cancers by 2016. Assuming that half of the 270,000 prostate cancer deaths avoided by 2016 were attributable to screening, the NND and the NNT would be 11 to 14 and 7 to 11 for men of all races and 8 to 12 and 5 to 9 for Black men, respectively. Alternative estimates incorporating a lag between incidence and mortality resulted in a NND and a NNT for Black men that reached well into the low single digits.
CONCLUSIONS
Complementary approaches to quantifying overdiagnosis indicate a harm-benefit tradeoff of prostate-specific antigen screening that is more favorable for Black men than for men of all races considered together. Our findings highlight the need to account for the increased value of screening in Black men in clinical guidelines. (Funded by the Patient-Centered Outcomes Research Institute, the National Cancer Institute, the Bristol Myers Squibb Foundation, and the Damon Runyon Cancer Research Foundation.)
Introduction
The adoption of prostate-specific antigen (PSA) screening in the United States beginning around 1987 has profoundly changed the epidemiology of prostate cancer, with a rapid doubling of incidence and, by 2015, a 50% decrease in annual prostate cancer mortality.1 Randomized trial data support a significant mortality benefit to PSA screening.2,3 However, uncertainty remains regarding how much PSA screening (as opposed to advances in the therapeutic armamentarium) is responsible for declining mortality rates4 as well as how the benefits of screening measure up to the harms of finding and treating cancers that never would have caused morbidity or mortality (i.e., overdiagnosis and overtreatment). This uncertainty is even greater for Black men, who have historically been underrepresented in diagnostic and therapeutic clinical trials despite having nearly double the risk of prostate cancer death compared with the general population. This difference in mortality is one of the largest racial disparities in any cancer.5
In 2009, Welch and Albertsen,6 using data from the Surveillance, Epidemiology, and End Results (SEER) program and the U.S. Census, calculated that the number needed to diagnose (NND; defined as the number of men overdiagnosed with prostate cancer per prostate cancer death prevented) and the number needed to treat (NNT; defined as the number of patients with prostate cancer overtreated per prostate cancer death prevented) for men of all races were 23 and 18, respectively. Their calculations used the excess number of men diagnosed each year over the period from 1986 to 2005 relative to 1986 as proxies for the number overdiagnosed.6 For benefit, they assumed that all observed decreases in prostate cancer mortality were secondary to screening, which was an admittedly optimistic assumption.
With shorter follow-up, the excess incidence approximation can be problematic because, even if there is no overdiagnosis, incidence is expected to increase as a result of cases being diagnosed early, which is the goal of screening.7 In this study, we update the calculation by Welch and Albertsen6 with 11 more years of data. We also examine the sensitivity of the results to alternative estimates of overdiagnoses from a microsimulation model and assuming that only a fraction of prostate cancer deaths avoided were attributable to screening. Using an extended analysis for all races as a reference point, we examine the plausible harm-to-benefit tradeoffs of screening Black men, for whom race-specific guidelines are lacking5 but who disproportionately bear the burden of prostate cancer morbidity and mortality.
Methods
OVERDIAGNOSIS AND OVERTREATMENT
Overdiagnosis is not directly observable; therefore, indirect methods are needed to estimate its magnitude.8 We examined two widely used approaches that provide complementary assessments.9
The excess incidence in a screened group compared with an unscreened group is an intuitive approach for estimating overdiagnosis. The validity of this approach depends on the chosen metric (cumulative or annualized excess incidence) and the amount of follow-up under stable screening conditions.10 In our report, we first use data through 2016 to recapitulate the analysis by Welch and Albertsen.6 As in that study, we calculate cumulative excess incidence relative to 1986 using age- and year-specific data from the core nine catchment areas of the SEER database1 limited to men 50 to 84 years of age at diagnosis for consistency with the alternative estimation approach noted below.
An alternative approach to quantifying overdiagnosis is to model onset of subclinical disease and progression to clinical diagnosis in the absence of screening. By superimposing screening, the model is then used to predict the probability that screen-detected disease would not progress to clinical diagnosis before death from causes other than prostate cancer; this is known as “competing death.”7,11 The validity of this approach depends on the model’s assumptions about disease natural history and the quality of the incidence and screening data used to estimate the model. In this study, we use a model of prostate cancer natural history12 that was previously used to estimate overdiagnosis across a range of screening strategies13 and patient and tumor characteristics.14 The model simulates individual life histories and longitudinal PSA trajectories for men 50 to 84 years of age. Age- and grade-specific PSA patterns after subclinical onset are directly linked to the risk of diagnosis in the absence of screening (i.e., diagnosis following symptomatic presentation, rectal examination, or other pathways in the pre-PSA era). In the presence of a reconstruction of national PSA screening,15 the model approximately reproduces prostate cancer incidence in the SEER database by age, stage, and grade for all races (White, Black, other, and unknown race categories considered together) and for Black men.16,17 Race in the SEER database is determined by all resources in the participating facilities, including the medical record, face sheet, physician and nursing notes, photographs, and any other sources.18 For the report herein, the model was extended to approximate decreased incidence19 following the U.S. Preventive Services Task Force (USPSTF) recommendation against prostate cancer screening for men 75 years of age and older in 200820 and for all ages in 201221 (Supplementary Methods in the Supplementary Appendix).
To estimate the total numbers overdiagnosed by age, year, and race, we made the assumption that the approaches described earlier, which use data from the SEER population, could be applied to the entire U.S. population. To do so, we scaled up the cumulative incidence of overdiagnosis (using either estimation approach) to total population counts using demographic data reported by the U.S. Census.22 We calculated total numbers overtreated as the product of the number of men overdiagnosed and the corresponding age-, year-, and race-specific frequencies of definitive treatment (prostatectomy or radiation therapy) in the SEER database, assuming that these frequencies approximate those for the entire U.S. population.1
NUMBERS NEEDED TO DIAGNOSE AND TREAT
To relate total overdiagnoses and overtreatments to the potential benefit of screening, we first followed the approach used by Welch and Albertsen6 and assumed that all decreases in prostate cancer deaths since 1986 were attributable to screening. As in that study, we excluded years with an increased prostate cancer mortality rate (1987 to 1996) as an optimistic baseline assumption. We used year- and race-specific U.S. Vital Statistics data22 to calculate the total decrease in the number of prostate cancer deaths. We then calculated the NND and NNT as the ratio of overdiagnoses or overtreatments relative to decreases in prostate cancer deaths. Finally, we re-estimated NNDs and NNTs assuming that only half of these decreases were attributable to screening, a magnitude more consistent with estimates of 45% to 70% or 48% to 52% from two prior modeling studies.23,24
Furthermore, to explore the extent to which lower NNDs and NNTs are attributable to decreased prostate cancer incidence after the USPSTF recommendation against PSA screening in 2012,21 we recalculated results using the incidence and mortality rates from 2011 for each year in the period from 2012 to 2016.
Finally, we performed the same analysis accounting for the time it takes for the mortality benefit of early detection to fully manifest. For example, a prostate cancer detected early and cured by treatment in 2006 might have caused death 5 or 10 (or more) years later. In other words, it is misleading to use the same final year of follow-up for calculating harm and for calculating mortality benefit. To investigate this time dependence, we shortened the calendar period used to calculate overdiagnosis by 5 or 10 years.
Results
Figure 1 shows age-standardized prostate cancer incidence, considering the entire cohort 50 to 84 years of age, partitioned into overdiagnosed and nonoverdiagnosed components for each of the two estimation approaches we used (i.e., excess incidence or microsimulation modeling) for men of all races (Fig. 1A) and for Black men (Fig. 1B). The two estimation approaches produce similar age-standardized burdens of overdiagnosis over the period from 1986 to 2016. However, when the results are viewed stratified by age at diagnosis, differences appear. The excess incidence approach produces greater estimates of overdiagnoses at younger ages compared with older ages, whereas the microsimulation model produces greater estimates of overdiagnoses at older ages compared with younger ages (Fig. 2 and Table S1).
Figure 1.
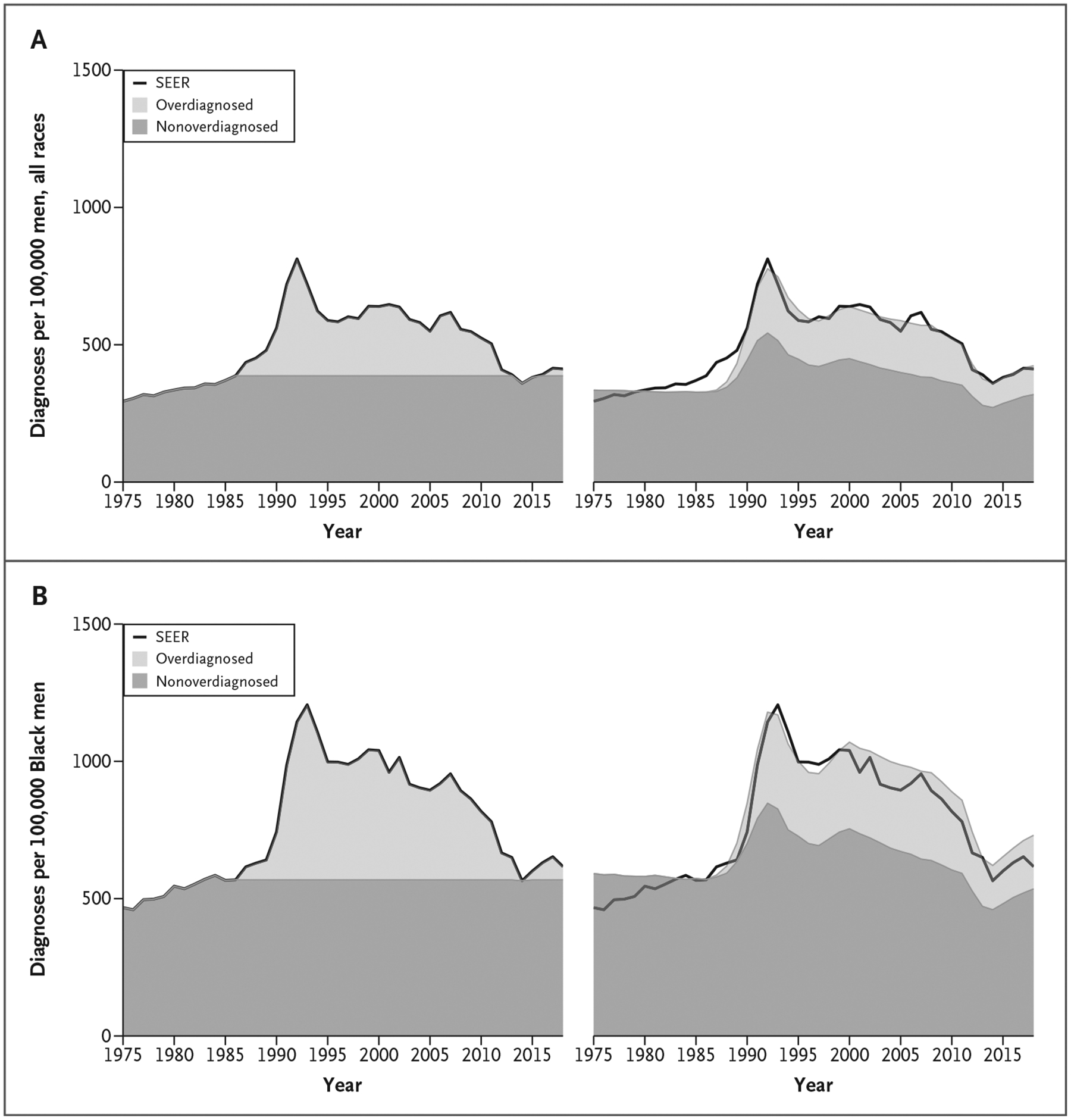
Age-Standardized Prostate Cancer Incidence Rates per 100,000 Men 50 to 84 Years of Age from SEER and Proportions Overdiagnosed and Nonoverdiagnosed Attributable to Prostate Cancer Screening by Estimation Approach and Race.
Excess incidence (left) and microsimulation model results (right) for men of all races (Panel A) and for Black men (Panel B). Age standardization used the U.S. standard million in 2000. SEER denotes Surveillance, Epidemiology, and End Results.
Figure 2.

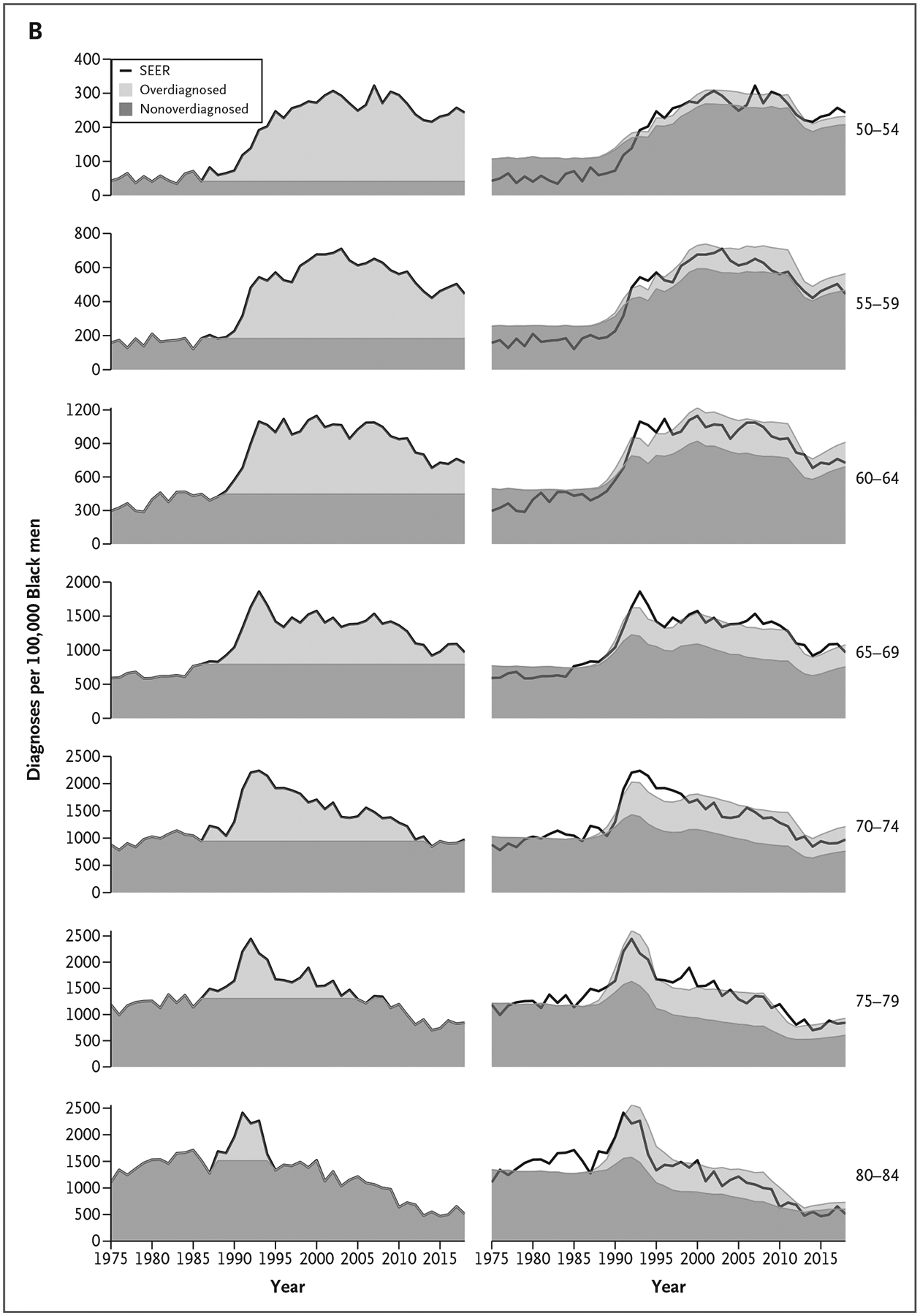
Age-Specific Prostate Cancer Incidence Rates per 100,000 Men 50 to 84 Years of Age from SEER and Proportions Overdiagnosed and Nonoverdiagnosed Attributable to Prostate Cancer Screening by Estimation Approach and Race.
Excess incidence (left) and microsimulation model results (right) for men of all races (Panel A) and for Black men (Panel B). The excess incidence method includes negative estimates of overdiagnosis when the incidence rate is below the rate in 1986 (not shown). SEER denotes Surveillance, Epidemiology, and End Results.
Age-specific frequencies of definitive treatment were similar for men of all races and for Black men over the period from 1986 to 2016, with markedly lower frequencies of treatment among men older than 70 years of age compared with men 50 to 69 years of age (Fig. S1). Over this period, prostate cancer mortality initially rose and then declined relative to 1986, with 270,000 fewer deaths among men of all races and 55,000 fewer deaths among Black men (Fig. 3 and Table 1).
Figure 3.

Age-Standardized Prostate Cancer Mortality Rates per 100,000 Men 50 to 84 Years of Age from SEER.
Age standardization used the U.S. standard million in the year 2000. SEER denotes Surveillance, Epidemiology, and End Results.
Table 1.
Estimated Numbers of Men 50 to 84 Years of Age Who Were Overdiagnosed and Overtreated, Assumed Numbers of Prostate Cancer Deaths Prevented by Screening, and Implied NND and NNT by Estimation Approach and Race.*
| Overdiagnosis Estimation Approach | Assumed Decrease in Prostate Cancer Deaths Attributable to PSA Screening, % | Follow-up for Overdiagnosis and Overtreatment | No. of Overdiagnosed Patients | No. of Overtreated Patients | No. of Deaths Prevented | NND | NNT |
|---|---|---|---|---|---|---|---|
| All races | |||||||
| Excess incidence | 100 | 1986–2016 | 1,921,112 | 1,499,487 | 271,033 | 7 | 6 |
| 100 | 1986–2011 | 1,829,008 | 1,400,750 | 271,033 | 7 | 5 | |
| 100 | 1986–2006 | 1,425,533 | 1,071,513 | 271,033 | 5 | 4 | |
| 50 | 1986–2016 | 1,921,112 | 1,499,487 | 135,517 | 14 | 11 | |
| 50 | 1986–2011 | 1,829,008 | 1,400,750 | 135,517 | 14 | 10 | |
| 50 | 1986–2006 | 1,425,533 | 1,071,513 | 135,517 | 11 | 8 | |
| Microsimulation model | 100 | 1986–2016 | 1,537,126 | 927,580 | 271,033 | 6 | 3 |
| 100 | 1986–2011 | 1,309,060 | 793,465 | 271,033 | 5 | 3 | |
| 100 | 1986–2006 | 979,105 | 586,062 | 271,033 | 3 | 2 | |
| 50 | 1986–2016 | 1,537,126 | 927,580 | 135,517 | 11 | 7 | |
| 50 | 1986–2011 | 1,309,060 | 793,465 | 135,517 | 10 | 6 | |
| 50 | 1986–2006 | 979,105 | 586,062 | 135,517 | 7 | 4 | |
| Black men | |||||||
| Excess incidence | 100 | 1986–2016 | 321,754 | 229,263 | 53,955 | 6 | 4 |
| 100 | 1986–2011 | 285,789 | 200,360 | 53,955 | 5 | 4 | |
| 100 | 1986–2006 | 212,407 | 145,526 | 53,955 | 4 | 3 | |
| 50 | 1986–2016 | 321,754 | 229,263 | 26,977 | 12 | 9 | |
| 50 | 1986–2011 | 285,789 | 200,360 | 26,977 | 11 | 7 | |
| 50 | 1986–2006 | 212,407 | 145,526 | 26,977 | 8 | 5 | |
| Microsimulation model | 100 | 1986–2016 | 220,484 | 131,558 | 53,955 | 4 | 2 |
| 100 | 1986–2011 | 179,511 | 107,012 | 53,955 | 3 | 2 | |
| 100 | 1986–2006 | 126,345 | 73,368 | 53,955 | 2 | 1 | |
| 50 | 1986–2016 | 220,484 | 131,558 | 26,977 | 8 | 5 | |
| 50 | 1986–2011 | 179,511 | 107,012 | 26,977 | 7 | 4 | |
| 50 | 1986–2006 | 126,345 | 73,368 | 26,977 | 5 | 3 |
The follow-up period for mortality benefit was 1986 to 2016 for all scenarios. NND denotes number needed to diagnose, NNT number needed to treat, and PSA prostate-specific antigen.
When we applied the estimates obtained using the excess incidence approach to the entire male U.S. population and summed over the period from 1986 to 2016, there were a total of 1.9 million overdiagnosed and 1.5 million overtreated men. Using the same assumptions as Welch and Albertsen6 (i.e., that all of the mortality benefit is due to screening), the NND was 7 and the NNT was 6 for men of all races (Table 1). Among Black men, corresponding estimates were 320,000 overdiagnosed and 230,000 overtreated, translating to a NND of 6 and a NNT of 4.
When we repeated these calculations using estimates from the microsimulation model for all races, the estimated number of overdiagnosed men was reduced by 400,000 to 1.5 million and the number overtreated by 600,000 to 900,000; this resulted in the calculated NND being reduced to 6 and the NNT being reduced to 3 (Table 1). Using the microsimulation model for Black men, the number overdiagnosed was 220,000, and the number overtreated was 130,000, translating to a NND of 4 and a NNT of 2.
If only half of the decrease in prostate cancer mortality were attributable to screening, the NND and NNT would be 14 and 11 (excess incidence) or 11 and 7 (model) for men of all races and would be 12 and 9 (excess incidence) or 8 and 5 (model) for Black men. The NND and NNT for all races are markedly lower than estimates in Welch and Albertsen6 through 2005, demonstrating that longer follow-up alone allows for the manifestation of relatively more benefits than harms, even when only half of the prostate cancer mortality decline is attributed to PSA screening. The reductions in the NND and NNT for the final years of follow-up ranging from 2005 to 2016 are shown in Figure S2.
To assess the plausible effect of the USPSTF 2012 guidelines, in which PSA screening was no longer recommended, on NND and NNT, we recalculated the results using the incidence and mortality rates observed in 2011 for each year in the period from 2012 to 2016. The estimated NND and NNT were similar to those obtained above for all races and Black men regardless of the estimation approach (Table S2). Thus, decreases in PSA screening and diagnoses after the revised USPSTF recommendations in 2012 do not explain the more favorable NND and NNT obtained under longer follow-up.
Finally, we calculated the NND and NNT assuming that only half of the mortality benefit is attributable to screening and accounting for a time lag between incidence and mortality benefit of 5 or 10 years (i.e., estimating overdiagnoses up to 2006 or 2011 while continuing to use mortality decreases up to 2016). For example, under the 10-year lag, the NND and NNT would be 11 and 8 (excess incidence) or 7 and 4 (model) for men of all races and 8 and 5 (excess incidence) or 5 and 3 (model) for Black men (Table 1). In short, the NND and NNT are sensitive to the specified time lag and are more favorable under longer time lags. In all scenarios, they are more favorable for Black men than for the general population.
Discussion
By using either an intuitive empirical approach or a formal statistical model to quantify overdiagnosis associated with PSA screening, we show that longer follow-up alone indicates a more favorable harm-benefit tradeoff than suggested previously. These tradeoffs are consistently more favorable for Black men across modeling assumptions and estimation methods. In all scenarios, the NND and NNT were estimated to be lower when the final year of follow-up was 2016 compared with 2005 or 2010. For example, using the excess incidence approach relative to 1986, simply accounting for another decade of follow-up lowered the NND from 20 to 6 and the NNT from 14 to 4 for Black men by 2016. If only half of the prostate cancer deaths avoided were attributable to screening, as previously suggested by modeling studies,23,24 the NND and NNT would be 12 and 9 for Black men by 2016. These reductions do not appear to be explained by the decreases in prostate cancer incidence after the USPSTF “D” recommendation in 2012. Rather, the apparent explanation for our findings is that mortality benefit accrues over many years and that the magnitude of benefit relative to harm was greater for Black men compared with the general population.
Black men have a higher incidence of and mortality from prostate cancer compared with men of other races.25 Although there is debate about the etiology of this disparity,26–28 the NND and NNT for screening in Black men are more favorable than those for the general population. Even under the least optimistic scenarios, the estimated NNTs for Black men were single-digit numbers. This finding is particularly important if we take into consideration that once a man is diagnosed with prostate cancer, Black race does not appear to be associated with inferior long-term outcomes as long as there is equal access to care and standardized treatment.27 Considering the poor representation of Black men in randomized PSA screening studies, our findings provide reason to rethink current guidelines on PSA screening in this population.
Our analysis does not take into consideration the potential benefit of increasingly common early detection strategies, such as prostate magnetic resonance imaging and other tests to triage men before prostate biopsy.29,30 The development of tools to improve risk stratification of patients for treatment, as well as the continued expansion of the use of active surveillance for low-risk disease, may also improve treatment decisions.31,32
Our work has several limitations. First, the excess incidence approach attributes all excess cases to PSA screening and does not distinguish increases attributable to overdiagnoses from increases resulting from early detections. Although intuitive, this approach implies lower overdiagnosis among older men compared with younger men and no (or negative) overdiagnosis in recent years for older men despite some continued screening. Interestingly, despite opposing age gradients, the two estimation approaches produce similar overall burdens of overdiagnosis and similar decreasing trends relative to accumulating mortality benefit over time. Second, the microsimulation model is based on data from multiple sources, relies on several assumptions, and does not perfectly replicate total incidence, particularly for Black men. Third, both approaches for estimating overdiagnosis assume that prostate cancer incidence would have remained constant at the 1986 level in the absence of screening. Fourth, receipt of definitive treatment was assumed to depend on patient race, age, and year of diagnosis but was independent of overdiagnosis status. This simplification followed previous analyses6,33; more granular analyses that account for tumor stage and grade require additional data and modeling assumptions.15,34 Finally, treatment frequencies in the SEER database were used in previous analyses,6,33 but these may not be representative of all subregions in the United States and do not include androgen deprivation monotherapy.
Conclusions
Given the long natural history of prostate cancer, we find substantially more favorable harm-benefit tradeoffs than what were implied a decade ago, which were largely revealed by the longer follow-up times. We also estimate that the net benefit of PSA screening is greater for Black men than the general population. The potential for overdiagnosis and overtreatment remains, although these harms may be mitigated by contemporary protocols for triaging men before biopsy and active surveillance for men with low-risk disease. These data should prompt policy makers to reconsider the utility of PSA-based prostate cancer screening, particularly for Black men.
Supplementary Material
Disclosures
Supported by the Patient-Centered Outcomes Research Institute, the National Cancer Institute, Bristol Myers Squibb Foundation, and the Damon Runyon Cancer Research Foundation.
We thank Dr. Ruth Etzioni and Ms. Xian Wu for their valuable input.
Footnotes
Author disclosures and other supplementary materials are available at evidence.nejm.org.
References
- 1.National Cancer Institute. Surveillance Epidemiology and End Results SEER data and software. 2020. (https://seer.cancer.gov/resources/).
- 2.Tsodikov A, Gulati R, Heijnsdijk EAM, et al. Reconciling the effects of screening on prostate cancer mortality in the ERSPC and PLCO trials. Ann Intern Med 2017;167:449–455. DOI: 10.7326/M16-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hugosson J, Roobol MJ, Månsson M, et al. A 16-yr follow-up of the European randomized study of screening for prostate cancer. Eur Urol 2019; 76:43–51. DOI: 10.1016/j.eururo.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Etzioni R, Gulati R, Tsodikov A, et al. The prostate cancer conundrum revisited: treatment changes and prostate cancer mortality declines. Cancer 2012;118:5955–5963. DOI: 10.1002/cncr.27594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Etzioni R, Nyame YA. Prostate cancer screening guidelines for Black men: spotlight on an empty stage. J Natl Cancer Inst 2021;13: 650–651. DOI: 10.1093/jnci/djaa172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welch HG, Albertsen PC. Prostate cancer diagnosis and treatment after the introduction of prostate-specific antigen screening: 1986–2005. J Natl Cancer Inst 2009;101:1325–1329. DOI: 10.1093/jnci/djp278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Etzioni R, Gulati R, Mallinger L, Mandelblatt J. Influence of study features and methods on overdiagnosis estimates in breast and prostate cancer screening. Ann Intern Med 2013;158:831–838. DOI: 10.7326/0003-4819-158-11-201306040-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brodersen J, Schwartz LM, Heneghan C, O’Sullivan JW, Aronson JK, Woloshin S. Overdiagnosis: what it is and what it isn’t. BMJ Evid Based Med 2018;23:1–3. DOI: 10.1136/ebmed-2017-110886. [DOI] [PubMed] [Google Scholar]
- 9.Etzioni R, Gulati R. Recognizing the limitations of cancer overdiagnosis studies: a first step towards overcoming them. J Natl Cancer Inst 2015;108:djv345. DOI: 10.1093/jnci/djv345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gulati R, Feuer EJ, Etzioni R. Conditions for valid empirical estimates of cancer overdiagnosis in randomized trials and population studies. Am J Epidemiol 2016;184:140–147. DOI: 10.1093/aje/kwv342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loeb S, Bjurlin MA, Nicholson J, et al. Overdiagnosis and overtreatment of prostate cancer. Eur Urol 2014;65:1046–1055. DOI: 10.1016/j.eururo.2013.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gulati R, Inoue L, Katcher J, Hazelton W, Etzioni R. Calibrating disease progression models using population data: a critical precursor to policy development in cancer control. Biostatistics 2010;11: 707–719. DOI: 10.1093/biostatistics/kxq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gulati R, Gore JL, Etzioni R. Comparative effectiveness of alternative prostate-specific antigen–based prostate cancer screening strategies: model estimates of potential benefits and harms. Ann Intern Med 2013;158:145–153. DOI: 10.7326/0003-4819-158-3-201302050-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gulati R, Inoue LY, Gore JL, Katcher J, Etzioni R. Individualized estimates of overdiagnosis in screen-detected prostate cancer. J Natl Cancer Inst 2014;106:djt367. DOI: 10.1093/jnci/djt367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mariotto AB, Etzioni R, Krapcho M, Feuer EJ. Reconstructing PSA testing patterns between black and white men in the US from Medicare claims and the National Health Interview Survey. Cancer 2007;109:1877–1886. [DOI] [PubMed] [Google Scholar]
- 16.Tsodikov A, Gulati R, de Carvalho TM, et al. Is prostate cancer different in Black men? Answers from 3 natural history models. Cancer 2017;123:2312–2319. DOI: 10.1002/cncr.30687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nyame YA, Gulati R, Tsodikov A, Gore JL, Etzioni R. Prostate-specific antigen screening and recent increases in advanced prostate cancer. JNCI Cancer Spectr 2021;5:pkaa098. DOI: 10.1093/jncics/pkaa098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Institutes of Health PHS, U.S. Department of Health and Human Services. SEER program coding and staging manual. 2021. (https://seer.cancer.gov/manuals/2021/SPCSM_2021_MainDoc.pdf).
- 19.Negoita S, Feuer EJ, Mariotto A, et al. Annual report to the nation on the status of cancer, part II: recent changes in prostate cancer trends and disease characteristics. Cancer 2018;124:2801–2814. DOI: 10.1002/cncr.31549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.US Preventive Services Task Force. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2008;149(3):185–191. DOI: 10.7326/0003-4819-149-3-200808050-00008. [DOI] [PubMed] [Google Scholar]
- 21.Moyer VA. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012;157: 120–134. DOI: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Census Estimates Request. CDC WONDER database (https://wonder.cdc.gov/bridged-race-v2006.html).
- 23.Gulati R, Tsodikov A, Etzioni R, et al. Expected population impacts of discontinued prostate-specific antigen screening. Cancer 2014; 120:3519–3526. DOI: 10.1002/cncr.28932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control 2008;19:175–181. DOI: 10.1007/s10552-007-9083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin 2019; 69:211–233. DOI: 10.3322/caac.21555. [DOI] [PubMed] [Google Scholar]
- 26.Riviere P, Luterstein E, Kumar A, et al. Survival of African American and non-Hispanic white men with prostate cancer in an equal-access health care system. Cancer 2020;126:1683–1690. DOI: 10.1002/cncr.32666. [DOI] [PubMed] [Google Scholar]
- 27.Dess RT, Hartman HE, Mahal BA, et al. Association of Black race with prostate cancer-specific and other-cause mortality. JAMA Oncol 2019;5:975–983. DOI: 10.1001/jamaoncol.2019.0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kensler KH, Pernar CH, Mahal BA, et al. Racial and ethnic variation in PSA testing and prostate cancer incidence following the 2012 USPSTF recommendation. J Natl Cancer Inst 2021;113:719–726. DOI: 10.1093/jnci/djaa171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eklund M, Jäderling F, Discacciati A, et al. MRI-targeted or standard biopsy in prostate cancer screening. N Engl J Med 2021;385: 908–920. DOI: 10.1056/NEJMoa2100852. [DOI] [PubMed] [Google Scholar]
- 30.Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 2018; 378:1767–1777. DOI: 10.1056/NEJMoa1801993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eggener SE, Rumble RB, Armstrong AJ, et al. Molecular biomarkers in localized prostate cancer: ASCO guideline. J Clin Oncol 2020;38: 1474–1494. DOI: 10.1200/JCO.19.02768. [DOI] [PubMed] [Google Scholar]
- 32.Mahal BA, Butler S, Franco I, et al. Use of Active Surveillance or Watchful Waiting for Low-Risk Prostate Cancer and Management Trends Across Risk Groups in the United States, 2010–2015. JAMA 2019;321:704–706. DOI: 10.1001/jama.2018.19941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Welch HG, Gorski DH, Albertsen PC. Trends in metastatic breast and prostate cancer—lessons in cancer dynamics. N Engl J Med 2015;373:1685–1687. DOI: 10.1056/NEJMp1510443. [DOI] [PubMed] [Google Scholar]
- 34.Heijnsdijk EA, der Kinderen A, Wever EM, Draisma G, Roobol MJ, de Koning HJ. Overdetection, overtreatment and costs in prostate-specific antigen screening for prostate cancer. Br J Cancer 2009; 101:1833–1838. DOI: 10.1038/sj.bjc.6605422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


