Abstract
Racial/ethnic disparities in childhood cancer survival persist despite advances in cancer biology and treatment. Survival rates are consistently lower among non-Hispanic Black and Hispanic children as compared with non-Hispanic White children across a range of hematologic cancers and solid tumors. We provide a framework for considering complex systems and social determinants of health in research examining the drivers of racial/ethnic disparities in childhood cancer survival, given that pediatric patients’ interactions with the healthcare system are filtered through their caregiver, family, and societal structure. Dismantling the multi-level (patient, family, healthcare system, and structural) barriers into modifiable drivers is critical to developing policies and interventions toward equitable health outcomes. This commentary highlights areas at the family, healthcare system, and society levels that merit closer examination and proposes actions and interventions to support improvements across these levels.
Hispanic and non-Hispanic Black (NHB) children have lower rates of survival compared with non-Hispanic White (NHW) children (1). In children with acute lymphoblastic leukemia (ALL), for example, the 5-year survival rates increased from 72.8% (1992–2000) to 82.1% (2001–2007) among NHB patients and from 85.9% to 89.0% among NHW patients; survival rates remain significantly lower among Black children than their White counterparts (P <0.01; ref. 2). Inequitable social conditions limit the reach and potency of medical advances (3), and thus, addressing drivers rooted in social structure is essential to close the racial/ethnic gap in childhood cancer survival.
Zhao and colleagues (4) quantified the role of health insurance coverage and social deprivation in mediating survival differences among children (<18 years) with newly diagnosed cancer. Their analysis of the National Cancer Database (NCDB) revealed reduced overall survival rates among NHB and Hispanic children compared with NHW children across a range of cancers (4). Although adjusting for differences in insurance coverage and zip-code level indicators of social deprivation attenuated disparities between groups, together these factors accounted for only 25.2% and 62.1% of the differences in survival rates between NHB and NHW children and between Hispanic and NHW children, respectively (4).
Zhao and colleagues’ research on socioeconomic drivers of health at the patient-level set the foundation for further research considering other modifiable, multi-level drivers of childhood cancer survival. We propose that for children with cancer, patient-level drivers are nested within family-level determinants, which in turn are nested within a healthcare system informed by discriminatory policies shaped by a history of racism and segregation (Fig. 1). Although there are numerous possible drivers at each level, we focus on key modifiable drivers in the areas of caregiver capacity and health literacy, delivery of quality care, structural racism, and public policy. A multi-level approach is needed to raise awareness and identify solutions targeting modifiable drivers that can reduce racial/ethnic differences in childhood cancer survival (Table 1).
Figure 1.
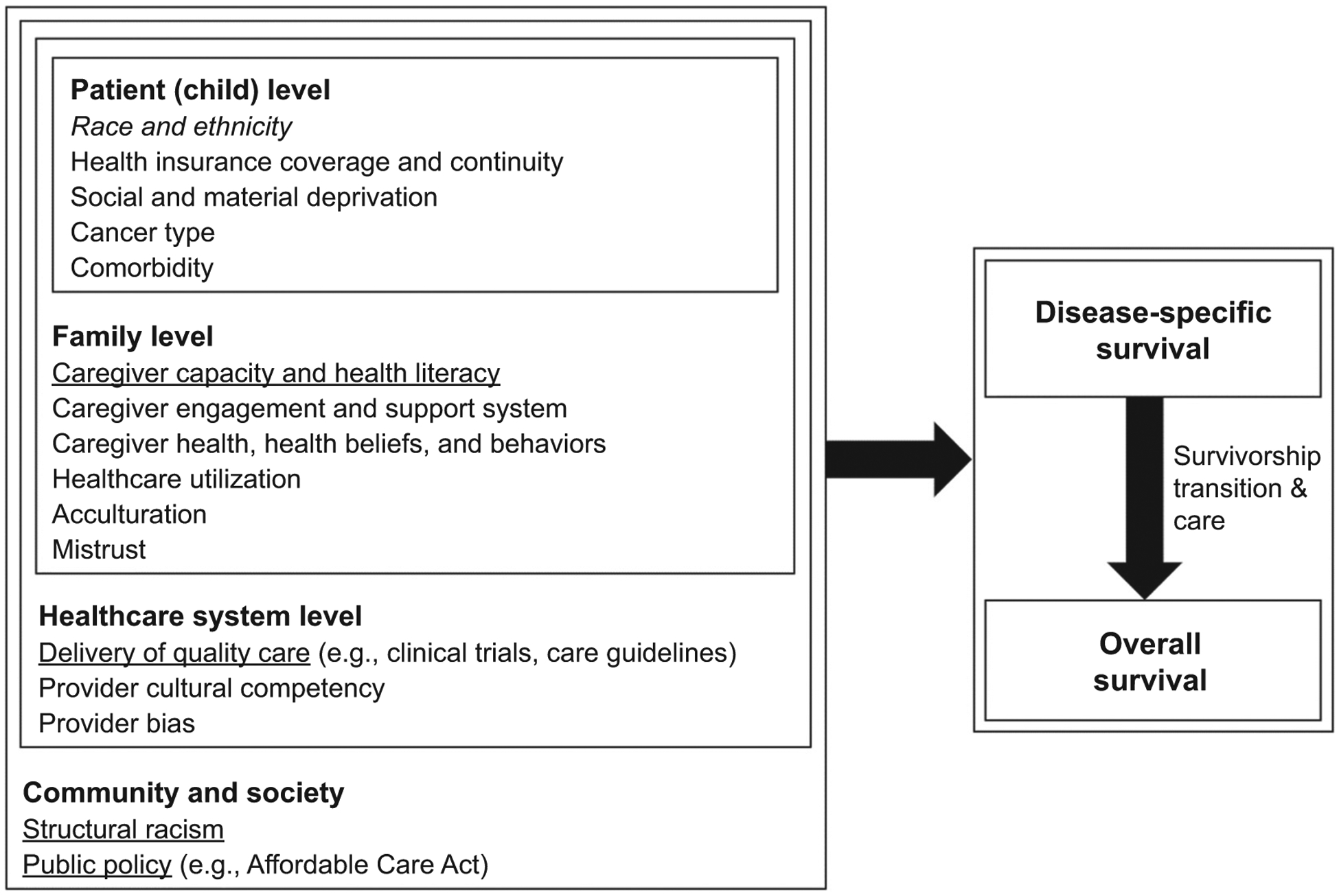
Multi-level drivers that affect the association between race/ethnicity and survival among children diagnosed with cancer. Notes: The multi-level drivers do not refer to a linear relationship, rather nesting relationships; patient-level drivers are nested within families, and patient-and family-level drivers are nested within the healthcare system, which are all nested within community and society.
Table 1.
Call for actions in key areas across multi-level drivers of disparate childhood cancer survival.
| Level | Proposed action or intervention |
|---|---|
| Family: | |
| Caregiver capacity Caregiver health literacy |
|
| Healthcare system: | |
| Delivery of quality care |
|
| Community and society: | |
| Structural racism |
|
| Public policy |
|
Abbreviations: DEI, Diversity, Equity, and Inclusion; RFA, Research Funding Announcement.
Evaluate at cancer diagnosis and through cancer survivorship.
Family Level: Caregiver Capacity and Health Literacy
Healthcare for children with cancer depends on their caregiver and family resources and functioning. Families endure demanding healthcare stressors spanning many years, with significant disruption in routines and in their social, occupational, and family roles (5). Care-givers are often required to manage complicated treatment regimens, including multiple medications with different dosing schedules, weekly or twice weekly outpatient appointments, acute care visits for treatment, off-treatment follow-up care, and specialty services and procedures (6). For example, patients with ALL have about 3 to 7 admissions with 24 to 55 hospital days in the first 6 months of therapy alone (7). Overall, 25% to 33% of children and their families acknowledge considerable challenges adapting to life with cancer (8). Complex, at-home, day-to-day disease management over 2 to 3 years further requires caregiver capacity and health literacy. The demands associated with having a chronically ill child and with navigating cancer care can have negative financial implications impacting work performance, absenteeism, and insurance access, particularly germane to families starting with limited resources. Research suggests that Hispanic ethnicity and living in a single mother household were associated with nonadherence to oral chemotherapy, with decreasing adherence correlated with a progressive increase in relapse (9). Higher caregiver burden is associated with lower survival for pediatric and adult patients (10). Thus, inequities in caregiver burden may be a key contributor to racial/ethnic disparities in childhood cancer survival. However, caregiver burden is difficult to measure and intervene upon, given wide variability across treatment centers and in the type and amount of psychosocial care offered to families. Furthermore, despite evidence suggesting lower health literacy among NHB and Hispanic parents compared with NHW parents (11) and lower parent literacy linked to inferior child health (12), the role of health literacy in racial/ethnic disparities in childhood cancer remains understudied. Health literacy is a potentially modifiable driver that may improve caregiver engagement, adaptation, and treatment adherence, an area that merits future exploration (12).
Recognizing that race and ethnicity are not monolithic, empowering caregivers, and developing sustainable infrastructure supporting healthcare delivery approaches may be effective in ensuring equitable access and closing gaps in childhood cancer survival (13). Actions may include: routine screening and standardized measurement of social determinants of health and household material hardship (e.g., food, energy, and housing insecurity; ref. 14–16); expansion of financial support and psychosocial resources for caregivers; and provision of educational and culturally tailored interventions that help identify burden early on, augment caregiver capacity, and address patients’/families’ unmet healthcare and social needs (see details in Table 1).
Healthcare System Level: Delivery of Quality Care
Translation of advances in diagnostics, biology, and therapy also relies on the delivery of quality care. Differential enrollment in clinical trials and in dissemination and implementation of intensive therapies for Hispanic and NHB children may explain survival differences. Non-White patients are significantly underrepresented in clinical trials testing novel agents and new regimens (17). Differential enrollment may be partially explained by a distrust of healthcare institutions, providers, and research among minoritized populations due to a history of unethical medical research and care (18). Proposed strategies to improve the diversity, equity, and inclusion of clinical trials range from aligning family trust with provider bias and cultural competence to addressing regulatory and language barriers that may prevail in low-resource settings; this requires institutional policies and investment with metrics that need continued evaluation and corrective actions (Table 1; ref. 19–21).
Non-White children are also less likely to receive life-saving postrelapse treatment, such as hematopoietic stem cell transplantation (HSCT), potentially leading to inferior overall survival compared with NHW children (22). HSCT requires parental work absence and family support for at least 6 months. Access to HSCT services can be further impaired by the limited number of centers specializing in such procedures, with resulting limitations of the geographic distance from a family’s home community. Strategies that incorporate families’ social circumstances require consideration by pediatric oncology practices when tailoring and delivering patient-centered care (Table 1). Notably, information on cancer relapse and cause of death are missing in the NCDB, limiting Zhao and colleagues’ evaluation of the overall survival rate. For pediatric lymphoma, overall survival was worse in non-White than NHW children, with lower postrelapse survival in non-White children driving the disparate overall survival (22). An in-depth understanding of the modifiable, multi-level drivers of racial/ethnic disparities in disease-specific survival, including postrelapse survival and noncancer mortality, will be an important future direction.
Community and Society Level: Structural Racism
Reducing childhood cancer disparities requires confronting the structural racism defined by historic economic, public policies, and institutional practices that perpetuate social inequities across minoritized race/ethnic groups (23). Racial discrimination in hiring and employment practices contributes to the underrepresentation of NHB in jobs that offer health benefits (24). Policies that restrict job mobility for undocumented or recent immigrants curtail access to employer-sponsored health coverage among caregivers of Hispanic children (25). Parents’ employment often dictates their child’s access to private insurance coverage. The public insurance options for children, such as Medicaid, have not fully closed the race/ethnic gap in coverage in part due to the exclusion of vulnerable immigrants (26). Furthermore, state Medicaid programs require patients to provide income and other documentation to prove their eligibility at initial application and at intermittent renewal time points. These requirements can impose high burden on patients/families, in terms of time and paperwork to obtain and maintain Medicaid coverage, particularly while families balance the time commitments of managing a child with cancer. Together, discriminatory employment practices and immigration policies contribute to inconsistent health insurance coverage for racial/ethnic minoritized children, leading to disparate care access and cancer survival (27).
Racial residential segregation is another product of structural racism applied through legislative and judicial systems (23). Racial residential segregation creates inequitable neighborhood conditions (28), which directly influences educational and employment opportunities, determines timely and geographic access to care, and forms material hardship and physical environments (29). NHB children bear the greatest share of the adverse impact of segregation policies whose enduring legacies continue to stymie generations’ achievements and outcomes in education, wealth, and health (23).
As implied in Zhao and colleagues’ research, the structural sources of racial/ethnic discrimination are particularly consequential for children with cancer, and investigations targeting the structural drivers of racial/ethnic inequities in cancer survival are warranted. Particularly, medical and health services research must engage community stake-holders and incorporate widely available geographic data on area-level deprivation that are orthogonal to patient/family characteristics. Area-level deprivation—including neighborhood access to employment, education, food/nutrition, healthcare, transportation, and environmental pollutants—can impact child health by itself, regardless of characteristics at the patient and family levels. Furthermore, directing research funding towards designing and implementing interventions that address the modifiable and unjust structural drivers of gaps in childhood cancer survival is essential.
Community and Society Level: Public Policy
Public policies to enhance insurance coverage and care access may attenuate racial/ethnic health disparities. The Affordable Care Act (ACA) contains multiple provisions designed to improve insurance coverage relevant for racial/ethnic minoritized children (30). Under the ACA, participating states expanded Medicaid eligibility to all parents with income ≤138% of the federal poverty level (30); these coverage gains can increase the proportion of Medicaid-insured children and decrease the uninsured rate among children (31). Other ACA provisions that benefit disadvantaged children include: enhanced funding for Children’s Health Insurance Program (CHIP); outreach to increase enrollment of eligible but previously uninsured children into Medicaid/CHIP; and subsidized private coverage options (30). We recently demonstrated that ACA insurance expansions were associated with a narrowing of racial/ethnic disparities in coverage and cancer stage at diagnosis in a young cohort (32, 33). Future research that demonstrates the impact of ACA provisions on disparities in childhood cancer survival is needed to inform policies designed to ensure universal and seamless healthcare coverage to improve access for children experiencing cancer survival disparities.
Conclusion
Identification of modifiable and unjust drivers of racial/ethnic disparities in childhood cancer is critically important to inform evidence-based policies and to develop interventions toward a sustained reduction in disparities. Zhao and colleagues’ research underscores health insurance and neighborhood social deprivation as two measurable and modifiable drivers. We highlight a combination of supports and interventions targeting modifiable drivers across multiple levels—including families, the healthcare system, and community and society—are imperative for future research and quality initiatives (Table 1), to facilitate meaningful changes towards decreasing structural racism, improving equitable care delivery and access, and reducing survival differences for children with cancer.
Acknowledgments
This work was supported in part by grants R03CA259665 (to X. Ji and S.M. Castellino) and R03CA267456 (to X. Ji and S.M. Castellino) from the National Cancer Institute of the NIH, grant R00HD096322 (to H. Sohn) from the National Institute of Child Health and Human Development of the NIH, and grant K23HL133457 (to S. Sil) from the National Heart, Lung, and Blood Institute of the NIH.
Footnotes
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Authors’ Disclosures
No disclosures were reported.
References
- 1.Bhatia S Disparities in cancer outcomes: lessons learned from children with cancer. Pediatr Blood Cancer 2011;56:994–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pui C-H, Pei D, Pappo AS, Howard SC, Cheng C, Sandlund JT, et al. Treatment outcomes in black and white children with cancer: results from the SEER database and St Jude Children’s Research Hospital, 1992 through 2007. J Clin Oncol 2012;30:2005–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang VW, Lauderdale DS. Fundamental cause theory, technological innovation, and health disparities: the case of cholesterol in the era of statins. J Health Soc Behav 2009;50:245–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao J, Han X, Zheng Z, Nogueira L, Lu AD, Nathan PC, et al. Racial/ethnic disparities in childhood cancer survival in the United States. Cancer Epidemiol Biomarkers Prev 2021;30:2010–7. [DOI] [PubMed] [Google Scholar]
- 5.Pai AL, Greenley RN, Lewandowski A, Drotar D, Youngstrom E, Peterson CC. A meta-analytic review of the influence of pediatric cancer on parent and family functioning. J Fam Psychol 2007;21:407–15. [DOI] [PubMed] [Google Scholar]
- 6.Pai AL, McGrady ME. Assessing medication adherence as a standard of care in pediatric oncology. Pediatr Blood Cancer 2015;62:S818–28. [DOI] [PubMed] [Google Scholar]
- 7.Leahy AB, Elgarten CW, Li Y, Huang YSV, Fisher BT, Delp D, et al. Evaluation of hospital admission patterns in children receiving treatment for acute lymphoblastic leukemia: What does a typical leukemia experience look like? Blood 2018;132:4763. [Google Scholar]
- 8.Kupst MJ, Bingen K. Stress and coping in the pediatric cancer experience. In: Brown RT, ed. Comprehensive handbook of childhood cancer and sickle cell disease. New York, NY: Oxford University Press. 2006:35–52. [Google Scholar]
- 9.Bhatia S, Landier W, Shangguan M, Hageman L, Schaible AN, Carter AR, et al. Nonadherence to oral mercaptopurine and risk of relapse in Hispanic and non-Hispanic white children with acute lymphoblastic leukemia: a report from the children’s oncology group. J Clin Oncol 2012;30:2094–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Junkins CC, Kent E, Litzelman K, Bevans M, Cannady RS, Rosenberg AR. Cancer across the ages: a narrative review of caregiver burden for patients of all ages. J Psychosoc Oncol 2020;38:782–98. [DOI] [PubMed] [Google Scholar]
- 11.Yin HS, Johnson M, Mendelsohn AL, Abrams MA, Sanders LM, Dreyer BP. The health literacy of parents in the United States: a nationally representative study. Pediatrics 2009;124:S289–98. [DOI] [PubMed] [Google Scholar]
- 12.DeWalt DA, Hink A. Health literacy and child health outcomes: a systematic review of the literature. Pediatrics 2009;124:S265–74. [DOI] [PubMed] [Google Scholar]
- 13.Minasian LM, Carpenter WR, Weiner BJ, Anderson DE, McCaskill-Stevens W, Nelson S, et al. Translating research into evidence-based practice: the National Cancer Institute Community Clinical Oncology Program. Cancer 2010;116:4440–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng DJ, Shyr D, Ma C, Muriel AC, Wolfe J, Bona K. Feasibility of systematic poverty screening in a pediatric oncology referral center. Pediatr Blood Cancer 2018;65:e27380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bilodeau M, Ma C, Al-Sayegh H, Wolfe J, Bona K. Household material hardship in families of children post-chemotherapy. Pediatr Blood Cancer 2018;65:e26743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koh HK, Piotrowski JJ, Kumanyika S, Fielding JE. Healthy people: a 2020 vision for the social determinants approach. Health Educ Behav 2011;38:551–7. [DOI] [PubMed] [Google Scholar]
- 17.Liu L, Krailo M, Reaman GH, Bernstein L. Childhood cancer patients’ access to cooperative group cancer programs: a population-based study. Cancer 2003;97:1339–45. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong K, Ravenell KL, McMurphy S, Putt M. Racial/ethnic differences in physician distrust in the United States. Am J Public Health 2007;97:1283–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graetz DE, Madni A, Gossett J, Kang G, Sabin JA, Santana VM, et al. Role of implicit bias in pediatric cancer clinical trials and enrollment recommendations among pediatric oncology providers. Cancer 2021;127:284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahn JM, Gray DM, Oliveri JM, Washington CM, DeGraffinreid CR, Paskett ED. Strategies to improve diversity, equity, and inclusion in clinical trials. Cancer 2022;128:216–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubin EH, Scroggins MJ, Goldberg KB, Beaver JA. Strategies to maximize patient participation in clinical trials. Am Soc Clin Oncol Educ Book 2017;37:216–21. [DOI] [PubMed] [Google Scholar]
- 22.Kahn JM, Kelly KM, Pei Q, Bush R, Friedman DL, Keller FG, et al. Survival by race and ethnicity in pediatric and adolescent patients with Hodgkin lymphoma: a Children’s Oncology Group Study. J Clin Oncol 2019;37:3009–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massey DS. American Apartheid: Segregation and the making of the underclass. Am J Sociol 1990;96:329–57. [Google Scholar]
- 24.Kalleberg AL, Reskin BF, Hudson K. Bad jobs in America: Standard and nonstandard employment relations and job quality in the United States. Am Sociol Rev 2000;65:256–78. [Google Scholar]
- 25.Asad AL, Clair M. Racialized legal status as a social determinant of health. Soc Sci Med 2018;199:19–28. [DOI] [PubMed] [Google Scholar]
- 26.Sommers BD. Stuck between health and immigration reform—care for undocumented immigrants. N Engl J Med 2013;369:593–5. [DOI] [PubMed] [Google Scholar]
- 27.Sohn H. Racial and ethnic disparities in health insurance coverage: dynamics of gaining and losing coverage over the life-course. Popul Res Policy Rev 2017;36:181–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams DR, Mohammed SA, Leavell J, Collins C. Race, socioeconomic status and health: Complexities, ongoing challenges and research opportunities. Ann NY Acad Sci 2010;1186:69–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Acevedo-Garcia D Residential segregation and the epidemiology of infectious diseases. Soc Sci Med 2000;51:1143–61. [DOI] [PubMed] [Google Scholar]
- 30.Rudowitz R, Artiga S, Arguello R. Children’s Health Coverage: Medicaid, CHIP and the ACA. Menlo Park, CA: The Henry J. Kaiser Family Foundation. March 2014. Available at: https://www.kff.org/wp-content/uploads/2014/03/8570-children_s-health-coverage-medicaid-chip-and-theaca1.pdf. Accessed August 10, 2020. [Google Scholar]
- 31.Dubay L, Kenney G. Expanding public health insurance to parents: effects on children’s coverage under medicaid. Health Serv Res 2003;38:1283–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ji X, Castellino SM, Mertens AC, Zhao J, Nogueira L, Jemal A, et al. Association of Medicaid expansion with cancer stage and disparities in newly diagnosed young adults. J Natl Cancer Inst 2021 May 21 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji X, Hu X, Castellino SM, Mertens AC, Yabroff KR, Han X. Narrowing insurance disparities among children and adolescents with cancer following the affordable care act. JNCI Cancer Spectrum 2022;6:pkac006.. [DOI] [PMC free article] [PubMed] [Google Scholar]


