Background:
The insulin-like growth factor 1 (IGF1) pathway is a key regulator of cellular metabolism and aging. Although its inhibition promotes longevity across species, the effect of attenuated IGF1 signaling on cardiac aging remains controversial.
Methods:
We performed a lifelong study to assess cardiac health and lifespan in 2 cardiomyocyte-specific transgenic mouse models with enhanced versus reduced IGF1 receptor (IGF1R) signaling. Male mice with human IGF1R overexpression or dominant negative phosphoinositide 3-kinase mutation were examined at different life stages by echocardiography, invasive hemodynamics, and treadmill coupled to indirect calorimetry. In vitro assays included cardiac histology, mitochondrial respiration, ATP synthesis, autophagic flux, and targeted metabolome profiling, and immunoblots of key IGF1R downstream targets in mouse and human explanted failing and nonfailing hearts, as well.
Results:
Young mice with increased IGF1R signaling exhibited superior cardiac function that progressively declined with aging in an accelerated fashion compared with wild-type animals, resulting in heart failure and a reduced lifespan. In contrast, mice with low cardiac IGF1R signaling exhibited inferior cardiac function early in life, but superior cardiac performance during aging, and increased maximum lifespan, as well. Mechanistically, the late-life detrimental effects of IGF1R activation correlated with suppressed autophagic flux and impaired oxidative phosphorylation in the heart. Low IGF1R activity consistently improved myocardial bioenergetics and function of the aging heart in an autophagy-dependent manner. In humans, failing hearts, but not those with compensated hypertrophy, displayed exaggerated IGF1R expression and signaling activity.
Conclusions:
Our findings indicate that the relationship between IGF1R signaling and cardiac health is not linear, but rather biphasic. Hence, pharmacological inhibitors of the IGF1 pathway, albeit unsuitable for young individuals, might be worth considering in older adults.
Keywords: aging, autophagy, cardiomyopathies, insulin-like growth factor 1, mitochondria, mouse, phosphatidylinositol 3-kinases
Clinical Perspective.
What Is New?
End-stage failing human hearts, but not those with compensated hypertrophy, display exaggerated insulin/insulin-like growth factor 1 receptor (IGF1R) expression and signaling activity.
Cardiomyocyte IGF1R overexpression in mice results in physiological hypertrophy and superior cardiac function in early life, but leads to accelerated cardiac aging, heart failure, and reduced lifespan in late life.
Increased cardiomyocyte IGF1R signaling accentuates cardiac dysfunction by reducing autophagy and mitochondrial oxidative capacity at old age.
What Are the Clinical Implications?
Pharmacological inhibition of cardiac IGF1R signaling in late life could suppress the age-related deterioration of cardiac performance and increase lifespan.
Age should be considered as a major outcome determinant in future clinical trials testing IGF1R/phosphoinositide 3-kinase inhibitors for cardiac benefits.
The insulin-like growth factor 1 (IGF1) pathway is a master regulator of cellular metabolism, growth, and aging.1 Previous genomic studies revealed that loss-of-function mutations in the IGF1 receptor (IGF1R) or its downstream effectors promote longevity in various model organisms.2 In mammals, however, the lifespan-extending effect appears limited to females,3,4 and its magnitude depends on the genetic background.5 Although these research efforts have provided important insights into the role of IGF1R signaling in the modulation of lifespan, the effect of diminished IGF1R signaling on healthspan (ie, the disease-free period of life), with the exception of cancer, remains largely controversial.6,7 Such uncoupling of lifespan and healthspan underscores the need to investigate whether IGF1R signaling regulates the pace of aging in a cell-autonomous manner. That said‚ only a few animal studies have examined the salutary effects of cell- or tissue-specific IGF1R signaling modulation throughout the course of life.8,9
In this context, IGF1R signaling is recognized to be most critical for cardiac homeostasis.2 As such, reduced cardiomyocyte IGF1R signaling has been shown to exert detrimental effects, whereas its activation is linked to enhanced cardiac contractility and physiological hypertrophy.10,11 However, recent studies reported that reduced IGF1R signaling attenuates adverse cardiac remodeling in genuinely aged mice (ie, >18 months old), whereas late-in-life treatment with IGF1R monoclonal antibodies improved cardiac function, at least in female mice.3,12 To reconcile these disparate observations, it has been postulated that the cardiac outcomes of IGF1R manipulation might be beneficial or detrimental depending on whether the heart is young or old, respectively.2 However, this assumption remains heretofore untested, and lifelong studies elucidating the underlying mechanisms of such biphasic and age-dependent cardiac effect of IGF1 are still lacking.
To address this knowledge gap, we performed a lifelong study, in which we comprehensively assessed cardiac health and lifespan of 2 transgenic mouse models with opposing effects on IGF1R signaling in cardiomyocytes. Here, we demonstrate that the cardiac advantages in young male mice with increased cardiomyocyte IGF1R signaling are lost and even inverted later in life, leading to accentuated age-related cardiac decline and heart failure. In contrast, low IGF1R signaling suppressed cardiac growth and function in young mice but preserved cardiac health in aging. Reduced cardiac autophagic flux and mitochondrial oxidative capacity mechanistically underlay the late-life detrimental effects of increased IGF1R signaling. Low IGF1R signaling consistently improved myocardial bioenergetics and protected the heart from age-related dysfunction in an autophagy-dependent manner. In humans, explanted failing hearts displayed increased IGF1R expression and pathway activity, supporting that these results may be clinically relevant.
Methods
The data supporting the results are available within the article and its Supplemental Material. On reasonable request, data, analytic methods, and study materials will be made available from the corresponding authors to other researchers for purposes of reproducing the results or replicating the procedure.
Mouse Models
Male mice overexpressing human IGF1R specifically in cardiomyocytes (IGF1Rtg mice)10 or male mice expressing a dominant negative phosphoinositide 3-kinase (PI3K) p110α mutant with impaired catalytic activity restricted to cardiomyocytes (dnPI3K mice)13 were used. In brief, a truncated p110 mutant with p85 binding domains, but lacking the kinase domain (p110Δkinase), was generated, and the p110Δkinase gene, together with FLAG epitope tag, was cloned into the αMyHC promoter construct to produce dnPI3K transgenic mice.13 Both transgenic mouse models and their wild-type (WT) littermates were generated on the FVB/N genetic background and used at 3, 6, 9, 12, or 20 months of age, and were euthanized for tissue analysis as indicated. In a separate set of studies, spermidine (No. S2626; Sigma-Aldrich) was administered orally (3 mmol/L in the drinking water) to 15-month-old IGF1Rtg mice for 5 months.14 A subgroup of dnPI3K and WT mice were assigned at the age of 19 months to receive daily intraperitoneal injections of hydroxychloroquine (60 mg/kg; Cayman Chemical) for 4 weeks.15 The experimenters were blinded to the genotype and treatment of mice.
Genotype was determined from ear biopsies by polymerase chain reaction–based analysis using the following primers: IGF1R or dnPI3K forward: αMHC-4, 5′GGC ACT TTA CAT GGA GTC CT3′ (Lot No.: 2355627, Microsynth); IGF1R reverse, 5′GAA CAG CAA GTA CTC GGT AAT3′ (Lot No.: 2355628); and p110-2R reverse, 5′TGGCCTCTCTGAACAGTTCAT3′ (Lot No.: 2355626). Mice were housed in a temperature- and humidity-controlled animal facility under specific pathogen-free or conventional conditions in 12-hour dark/light cycles with ad libitum access to water and food (standard chow, Ssniff V1534, ssniff-Spezialdiäten GmbH). Because of excessive fighting, we commonly housed 1 or 2 mice per cage that was enriched with autoclaved nest material and paper houses.
Details on mouse lifespan evaluation, echocardiography, hemodynamics, exercise tolerance testing and indirect calorimetry, mitochondrial respiration and ATP synthesis assays, autophagic flux assessment, immunoblotting and quantitative polymerase chain reaction analysis, metabolites profiling, and morphometric analysis of hypertrophy and fibrosis are in the Supplemental Material.
All animal experiments were performed according to the European ethical regulations (Directive 2010/63/EU) and were approved by the responsible national agencies (Bundesministerium für Wissenschaft, Forschung und Wirtschaft, BMWFW, Austria: BMWFW-66.010/0160-WF/V/3b/2014, BMWFW-66.010/0198-WF/V/3b/2017, and BMBWF-66.010/0042-V/3b/2018).
Human Samples
Myocardial samples were obtained from explanted donor hearts. On ice-cold cardioplegia, cardiac biopsies were harvested from the left ventricular free wall, quickly frozen in liquid nitrogen, and stored at –80 °C for future analysis. Donors of the failing hearts had severe contractile dysfunction and underwent cardiac transplantation surgery because of end-stage nonischemic dilated cardiomyopathy. Compensated hypertrophy samples were procured from deceased donors with echocardiographic evidence of moderate-to-severe hypertrophic remodeling, defined as interventricular septum thickness ≥13 mm, and preserved ejection fraction (EF) ≥50%.16 The nonfailing nonhypertrophied hearts were from donors with no clinical history of cardiac abnormalities, and echocardiography-derived interventricular septum ≤12 mm and EF ≥50%, as well. The human study protocol conformed to the Declaration of Helsinki. The requirement for informed consent was waived by the institutional ethics committee, which approved the collection and reporting of biological material and patients’ characteristics, respectively (Ethical votum No.: 24-224 ex 11/12 and 28-508 ex 15/16; Medical University of Graz).
Statistical Analysis
Data are presented as bar graphs with error bars showing mean and SEM, respectively, along with individual data points superimposed. Indicated sample sizes in figure legends refer to biological replicates (ie, individual hearts/animals). Comparisons between 2 groups were done by the Student t test, Welch t test, or Mann-Whitney test (continuous variables) and χ2 test (categorical variables), as appropriate. In case of multiple group comparisons, ANOVA followed by the Dunnett post hoc, Welch and Dunnett T3 post hoc or Kruskal-Wallis and Dunn post hoc tests were applied, as appropriate. For the statistical models‚ including multiple factors (eg, genotype, age, or treatment), 2-way ANOVA was used. In case of serial measurements, as in dnPI3K and WT mice treated with hydroxychloroquine (HCQ) or dobutamine, Greenhouse-Geisser–corrected 2-way repeated-measures ANOVA was applied. Whenever significant, the main effects of these factorial designs were reported on top of the respective panel. And, in general, this was followed by pairwise comparisons among different levels of a given factor. Data distribution was tested for normality using the Shapiro-Wilk test and depending on data distribution homogeneity of variances was verified using the Levene or Brown-Forsythe tests. Data violating these linear model assumptions were either transformed or the abovementioned nonparametric alternatives were used. Statistical analysis of survival data was detailed in the Supplemental Methods. A 2-sided P value of 0.05 was considered significant. GraphPad Prism 9 (GraphPad Software, LLC) or IBM SPSS statistics software (Version 25) were used to run the analysis.
Results
Cardiac Overexpression of IGF1 Receptor Promotes Cardiac Health in Early Adulthood, But Causes Heart Failure and Premature Mortality Late in Life
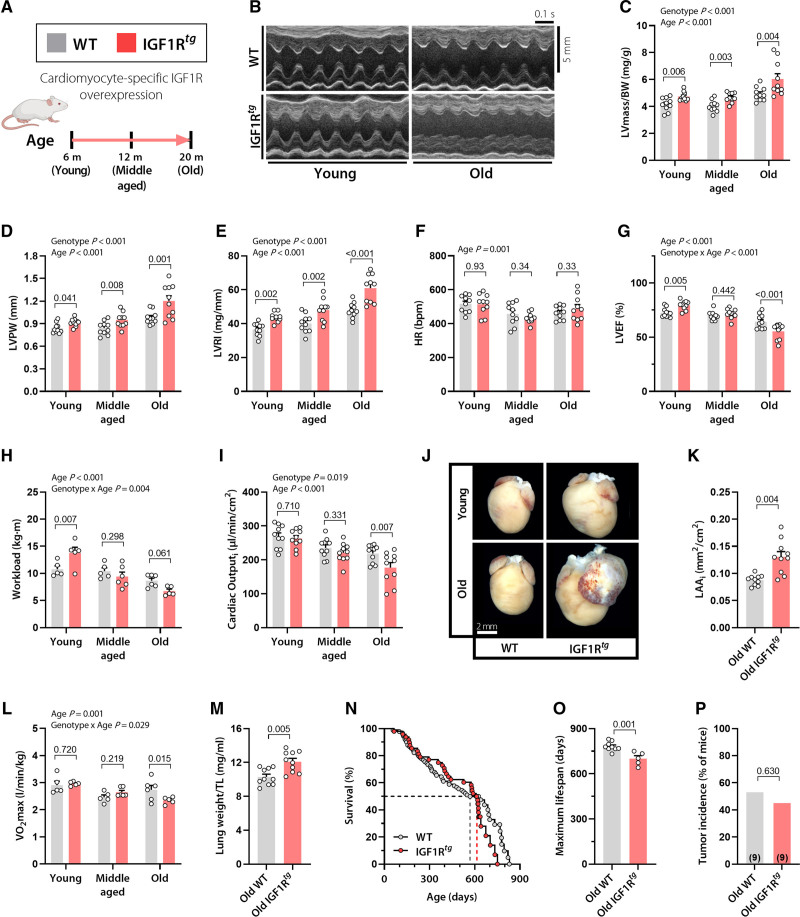
To determine the long-term effect of activated IGF1R signaling on the heart, we used male mice overexpressing the human IGF1R specifically in cardiac myocytes (IGF1Rtg).10 We focused on male mice because the controversial effect of IGF1R signaling mainly concerns males.3 Both IGF1Rtg mice and their WT littermates were subjected to a lifelong follow-up of survival, and cardiac structure and function, as well. To this end, a full cardiac workup composed of echocardiography, exercise tolerance testing, and cardiopulmonary capacity was conducted at 3 physiologically relevant life stages; early adulthood (3–6 months), midlife (12 months), and old age (20 months; Figure 1A). It is not surprising10,17 that IGF1Rtg mice exhibited pronounced cardiac hypertrophy across the 3 life stages, as denoted by higher left ventricular (LV) mass normalized to body weight, posterior wall thickness, and LV remodeling index (defined as the ratio between LV mass and internal diastolic diameter) than WT mice (Figure 1B–1E and Table S1). IGF1Rtg hearts exhibited signs of hypertrophy also at the cardiomyocyte level (Figure S1A). IGF1Rtg and WT mice had functionally similar heart rates, irrespective of age (Figure 1F). However, although IGF1Rtg mice showed superior cardiac contractility and better exercise capacity at young age (Figure 1G and 1H), the beneficial effects of IGF1R overexpression were lost by 12 months of age (Figure 1G and 1H). Strikingly‚ we found that 20-month-old IGF1Rtg mice developed clear signs of cardiomyopathy characterized by increased LV fibrosis (Figure S1B), reduced EF, lower cardiac output, and severe left atrial remodeling (Figure 1G and 1I–1K) in the absence of LV dilation (Table S1). These findings were further supported by invasive measures of cardiac function, including maximal LV pressure, the maximum and minimum rates of LV pressure change, and preload recruitable stroke work, which were all significantly reduced in aged IGF1Rtg mice (Table S2). Evident systolic and diastolic dysfunctions in aged IGF1Rtg mice indicated an age-related shift from physiological to pathological hypertrophy and resembled human nondilated cardiomyopathy that is commonly associated with hypertrophic remodeling.18 Consistent with this notion, exercise tolerance testing coupled to indirect calorimetry revealed compromised cardiopulmonary functional capacity in old IGF1Rtg mice (Figure 1L) accompanied by pulmonary congestion, as indicated by the increased tibia length–normalized lung weight (Figure 1M). Taken together, these findings indicate that, although cardiac IGF1R overexpression promotes cardiac health during early adulthood, it exacerbates age-related cardiac decline, leading to increased risk of heart failure with aging.
Figure 1.
Cardiac overexpression of IGF1R improves ejection fraction and effort tolerance in early adulthood, but causes heart failure and premature mortality late in life. A, Male mice with cardiomyocyte-specific overexpression of the human IGF1 receptor (IGF1Rtg) and their wild-type (WT) littermates (FVB/N background) were subjected to a comprehensive assessment of cardiac function and structure at the indicated age. B, Representative echocardiography-derived left ventricular (LV) M-mode tracings; C, LV mass normalized to body weight (BW); D, LV posterior wall thickness (LVPW); E, LV remodeling index (LVRI); F, heart rate (HR); and G, LV ejection fraction (LVEF) in 6-, 12-, and 20-month-old IGF1Rtg and WT mice (n=10 mice per group). H, Maximum workload during exercise tolerance testing in 3-, 12-, and 20-month-old IGF1Rtg and WT mice (n=5–6 mice per group). I, Body surface area–normalized cardiac output (cardiac index), derived by echocardiography, in IGF1Rtg and WT mice at the age of 6, 12, and 20 months (n=10 mice per group). J and K, Representative heart photomicrographs (J) and body surface area–normalized left atrial area (LAAi; K) in 20-month-old IGF1Rtg and WT mice (n=10 mice per group). L, Maximum oxygen consumption (Vo2max) during exercise tolerance testing in IGF1Rtg and WT mice at the age of 3, 12, and 20 months (n=5–6 mice per group). M, Lung weight normalized to tibia length (TL) in 20-month-old IGF1Rtg and WT mice (n=10 mice per group). N, Kaplan-Meier survival analysis of IGF1Rtg and WT mice (n=48/90 mice per group, respectively). Median survival is depicted by the dashed lines (please see also Table S3 for details). O, Maximum lifespan calculated as the average lifespan of the longest-lived decile in IGF1Rtg and WT mice (n=5/9 mice per group, respectively). P, Tumor incidence detected by gross necropsy in IGF1Rtg and WT mice (20/17 mice per group, respectively). Number in brackets indicates the number of mice that developed tumors. Indicated P values on top of panels (C–I and L) represent factor comparisons by 2-way ANOVA including genotype and age as fixed factors, followed by simple main effects analysis of pairwise comparisons between IGF1Rtg and their age-matched WT controls. Other P values were calculated by the Welch t test (K, M, and O) or χ2 test (P). Bars and error bars show means and SEM, respectively, with individual data points superimposed. IGF1 indicates insulin-like growth factor 1; IGF1R, IGF1 receptor; and IGF1Rtg mice, mice overexpressing human IGF1R specifically in cardiomyocytes.
To determine whether age-associated cardiac decline in IGF1Rtg mice reduces lifespan, we performed a longevity study which revealed that, despite exhibiting a median survival comparable to WT mice (Figure 1N and Table S3), IGF1Rtg mice had an obvious reduction in maximum lifespan (Figure 1O). We then evaluated various tissues by gross necropsy, which unveiled that shorter maximum survival in these mice cannot be attributed to increased tumorigenesis (Figure 1P). Although we acknowledge that determining the exact cause of death in mice is extremely challenging, this finding, along with the cardiac-specific nature of these mutants, might suggest that reduced longevity in IGF1Rtg mice is driven by cardiac insufficiency.
Aged IGF1R tg Mice Develop Heart Failure Attributable to Reduced Autophagic Activity and Mitochondrial Oxidative Capacity
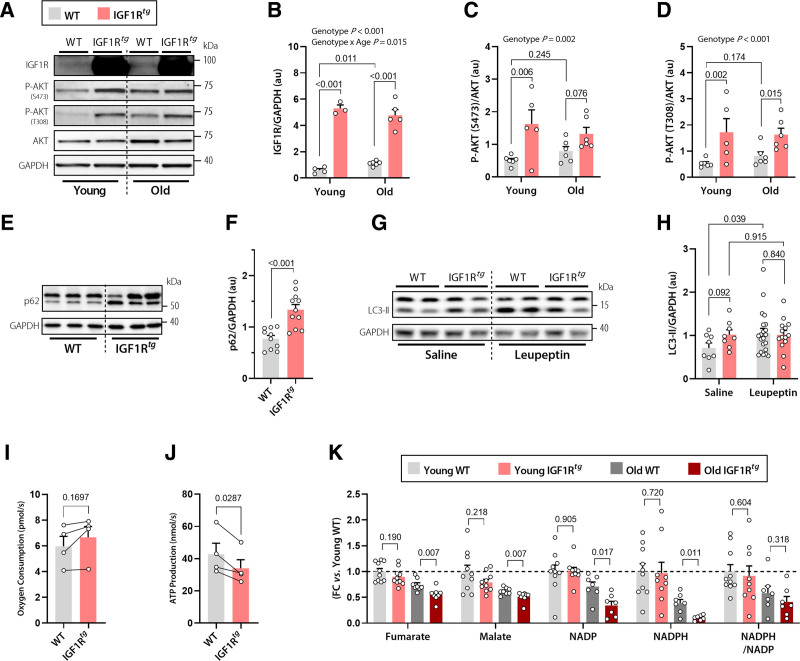
We next sought to evaluate age-dependent changes of IGF1R signaling by measuring circulating and cardiac concentrations of IGF1 and assessing downstream effectors of the IGF1R pathway. IGF1Rtg mice exhibited comparable plasma IGF1 concentrations, but significantly lower cardiac IGF1 levels than their WT littermates, perhaps due to receptor-mediated internalization (Figure S2). Regardless, higher cardiomyocyte IGF1R expression significantly increased the pathway signaling activity, as indicated by increased phosphorylation of the serine/threonine-protein kinase AKT at Ser473 and Thr308, both in young and old IGF1Rtg mice (Figure 2A–2D). Old WT mice also showed a significant, albeit slight, age-dependent increase in IGF1R expression, which did not appear to result in a significant activation of AKT, at least up to the age of 20 months (Figure 2A–2D). IGF1R stimulates protein synthesis and suppresses catabolic pathways, such as autophagy, through the mammalian target of rapamycin complex 1 (mTORC1).19 Therefore, we determined the extent of mTORC1 activation in IGF1Rtg mice by examining the phosphorylation of the mTORC1 subunit PRAS40 (proline-rich AKT1 substrate 1) and that of several mTORC1 substrates, including p70 S6K (p70 ribosomal S6 kinase 1), 4E-BP1 (eIF4E-binding protein 1), and ULK-1 (Unc-51-like kinase-1). IGF1Rtg mice exhibited increased phosphorylation of p70 S6K, 4E-BP1, and PRAS40 compared with nontransgenic controls, and this difference was particularly clear at old age (Figure S3). mTOR-dependent ULK-1 phosphorylation was significantly higher only in old IGF1Rtg mice (Figure S4). In the absence of differences in AMPK-dependent phosphorylation of ULK1 (Ser317; Figure S5), mTOR-dependent ULK1 (Ser757) phosphorylation might result in a more pronounced age-associated autophagy decline in IGF1Rtg mice.
Figure 2.
Impaired autophagy and mitochondrial oxidative capacity underlie the detrimental effect of cardiac IGF1R signaling in aged IGF1Rtg mice. A through D, Representative Western blots (A) and quantification (B) of cardiac IGF1 receptor (IGF1R) expression normalized to GAPDH, and AKT phosphorylation at Ser473 (C) and Thr308 normalized to total AKT expression (D) in 6-month-old (young) and 20-month-old (old) IGF1Rtg and WT mice (n=3–6 mice per group). E and F, Representative Western blots (E) and quantification of (F) the autophagy substrate p62 normalized to GAPDH in cardiac lysates of 12-month-old IGF1Rtg and WT mice (n=11/10 mice per group, respectively). G and H, Representative Western blots (G) and quantification of (H) the lipidated form of the autophagy marker LC3-II in the hearts of 12-month-old IGF1Rtg and WT mice that were treated with the protease inhibitor leupeptin (n=14/21 mice per group, respectively) or saline (n=8 mice per group). I and J, Paired assessment of oxygen consumption rate (I) and corresponding ATP production (J) in cardiac mitochondria isolated from 20-month-old IGF1Rtg and WT mice (n=4 mice per group). K, Relative difference in the tricarboxylic acid cycle intermediates, fumarate and malate, and the ratio between reduced and oxidized forms of the cofactor nicotinamide adenine dinucleotide phosphate (NADPH/NADP) in young (3 months old) and old (20 months old) IGF1Rtg and WT mice (n=7–10 mice per group). Indicated P values on top of panels (B–D and H) represent factor comparisons by 2-way ANOVA including genotype and age (B–D) or genotype and treatment (H) as fixed factors; the following simple main effects denote pairwise comparisons between IGF1Rtg and their respective WT controls within a specific age or treatment. Other P values were calculated by unpaired Welch t test or Mann-Whitney test (F and K) or paired Student t test (I and J). Bars and error bars show means and SEM, respectively, with individual data points superimposed. FC indicates fold change; IGF1, insulin-like growth factor 1; IGF1R, IGF1 receptor; IGF1Rtg mice, mice overexpressing human IGF1R specifically in cardiomyocytes; and WT, wild-type.
To test this hypothesis, we determined whether reduced autophagic activity underlies the detrimental cardiac phenotype of old IGF1Rtg mice, especially because autophagy is indispensable for cardiac homeostasis in aging.20,21 To this end, we evaluated autophagy markers in IGF1Rtg hearts that displayed a higher accumulation of the autophagic substrate p62 as early as at 12 months of age (Figure 2E and 2F). We also detected higher levels of the autophagy-related lipidated form of microtubule-associated protein 1A/1B-light chain 3B (LC3B-II) in IGF1Rtg hearts (Figure 2G and 2H) that could reflect either increased formation or reduced degradation of autophagosomes. To determine autophagic flux in vivo, we injected IGF1Rtg and WT mice with the protease inhibitor leupeptin (or saline) intraperitoneally, and measured LC3B-II.22 Unlike WT controls, 12-month-old IGF1Rtg mice failed to further increase LC3-II in response to leupeptin (Figure 2G and 2H), indicating that higher LC3-II levels at baseline result from blocked autophagic flux. These results collectively suggest that reduced cardiac autophagic flux precedes the development of an adverse cardiac phenotype in IGF1Rtg mice.
Because dysregulated autophagy is intimately linked to aberrant function of cellular organelles, including mitochondria,20,21 we speculated that aged IGF1Rtg hearts might be failing because of compromised mitochondrial function. To test this, we evaluated both respiratory function and ATP production capacity in mitochondria isolated from aged IGF1Rtg and WT hearts. Cardiac IGF1Rtg and WT mitochondria surprisingly had comparable respiratory capacity (Figure 2I). However, simultaneous measurement of ATP kinetics revealed uncoupled oxidative phosphorylation from ATP synthesis because IGF1Rtg mitochondria generated significantly lower amounts of ATP than WT mice (Figure 2J), implying impaired mitochondrial oxidative capacity and likely increased oxidative stress. In support of this notion, targeted metabolome analysis of aged IGF1Rtg hearts unveiled reduced levels of the tricarboxylic acid cycle intermediates, fumarate and malate, and reduced antioxidative potential, as measured by NADPH/NADP ratio (Figure 2K). Furthermore, we detected increased accumulation of the glycolytic intermediates, glucose 6-phosphate and glycerol 3-phosphate, that, in conjunction with lactate accumulation in IGF1Rtg myocardium (Figure S6), suggested increased anaerobic metabolism and glucose use for energy production. Hence, mitochondrial disturbance of IGF1Rtg hearts reduced oxidative phosphorylation and stress resistance, both of which might contribute to the pathogenesis of premature heart failure.
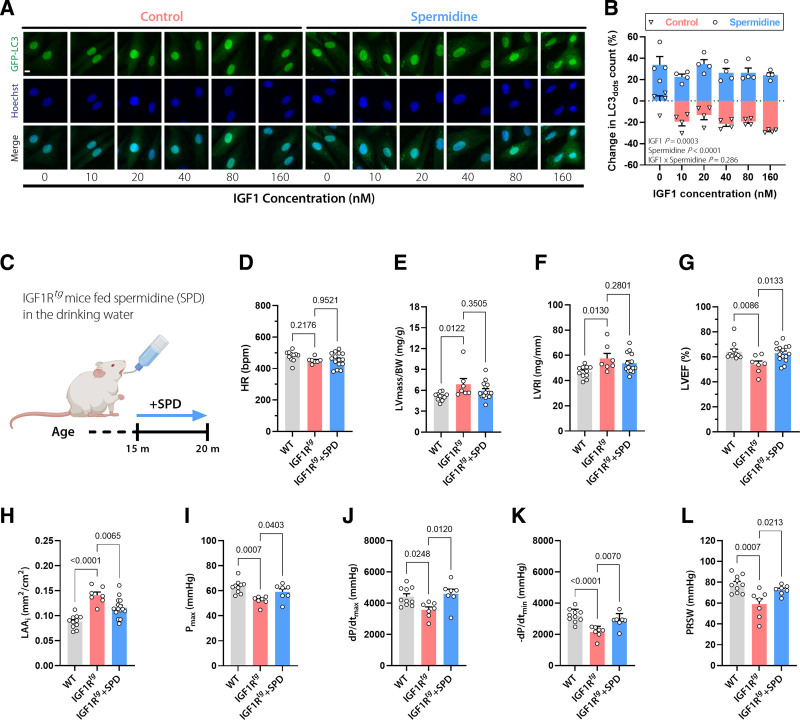
To establish a causal link between dysregulated autophagy and impaired cardiac function in old IGF1Rtgmice, we supplemented a subset of mice with spermidine that we have previously shown to enhance cardiac autophagy in aged mice.14 We confirmed that spermidine effectively prevents IGF1-induced suppression of autophagy in cardiac H9c2 cells (Figure 3A and 3B). After 5 months of spermidine feeding (3 mmol/L in the drinking water), IGF1Rtg mice were subjected to an extensive in vivo cardiac assessment (Figure 3C). Spermidine-fed IGF1Rtgmice exhibited heart rates, LV mass, and remodeling indices that were comparable to untreated IGF1Rtg mice (Figure 3D–3F). Although spermidine failed to reverse established cardiac hypertrophy, it ameliorated LV EF, left atrial remodeling, maximal LV pressure, and the maximum and minimum rates of LV pressure change, as well (Figure 3G–3K and Table S2). Preload recruitable stroke work, the gold standard measure of cardiac contractility, was also significantly higher in spermidine-treated than in nontreated IGF1Rtg mice (Figure 3L). Spermidine supplementation also improved, at least in part, the levels of tricarboxylic acid cycle and glycolytic intermediates in aged IGF1Rtg mice (Figure S7). In aggregate, these results suggest that reduced cardiac autophagy might be involved in accentuated cardiac dysfunction of aged IGF1Rtg mice.
Figure 3.
The autophagy inducer spermidine ameliorates the cardiac phenotype of aged IGF1Rtg mice. A and B, Representative confocal images (A) and quantification (B) of autophagic activity assessed by GFP-LC3 positive dots in GFP-LC3–expressing H9c2 cells treated with increasing concentrations of IGF1 in the presence or absence of spermidine (6.25 µmol/L) for 6 hours. Scale bar, 10 µm (n=4 biological replicates per condition). C, Schematic representation of spermidine (SPD) feeding protocol to male IGF1Rtg mice. SPD (3 mmol/L) was added to the drinking water starting at the age of 15 months, and after 5 months cardiac parameters were assessed. D through H, Echocardiography-derived (D) heart rate (HR), LV mass normalized to body weight (BW; E), LV remodeling index (LVRI; F), LV ejection fraction (LVEF; G), and body surface area–normalized left atrial area (LAAi; H) in 20-month-old spermidine-treated and control IGF1Rtg and WT mice (n=15/7/11 mice per group, respectively). I through L, Invasively measured left ventricular maximum pressure (Pmax; I), maximum rate of pressure rise (dP/dtmax; J), maximum rate of pressure decay (–dP/dtmin; K), and preload recruitable stroke work (PRSW; L) in 20-month-old spermidine-treated and control IGF1Rtg and WT mice (n=7/7/10 mice per group, respectively). Indicated P values on top of B represent factor comparisons by 2-way ANOVA including IGF1 concentration and spermidine treatment as fixed factors. Other P values were calculated by ANOVA with the Dunnett post hoc test. Bars and error bars show means and SEM, respectively, with individual data points superimposed. GFP-LC3 indicates Green Fluorescent Protein-Microtubule-associated Protein 1A/1B-Light Chain 3; IGF1, insulin-like growth factor 1; IGF1R, IGF1 receptor; IGF1Rtg mice, mice overexpressing human IGF1R specifically in cardiomyocytes; LV, left ventricular; and WT, wild-type.
Lower IGF1R Signaling Attributable to Reduced PI3K Activity Extends Cardiac Healthspan at the Expense of Cardiac Growth
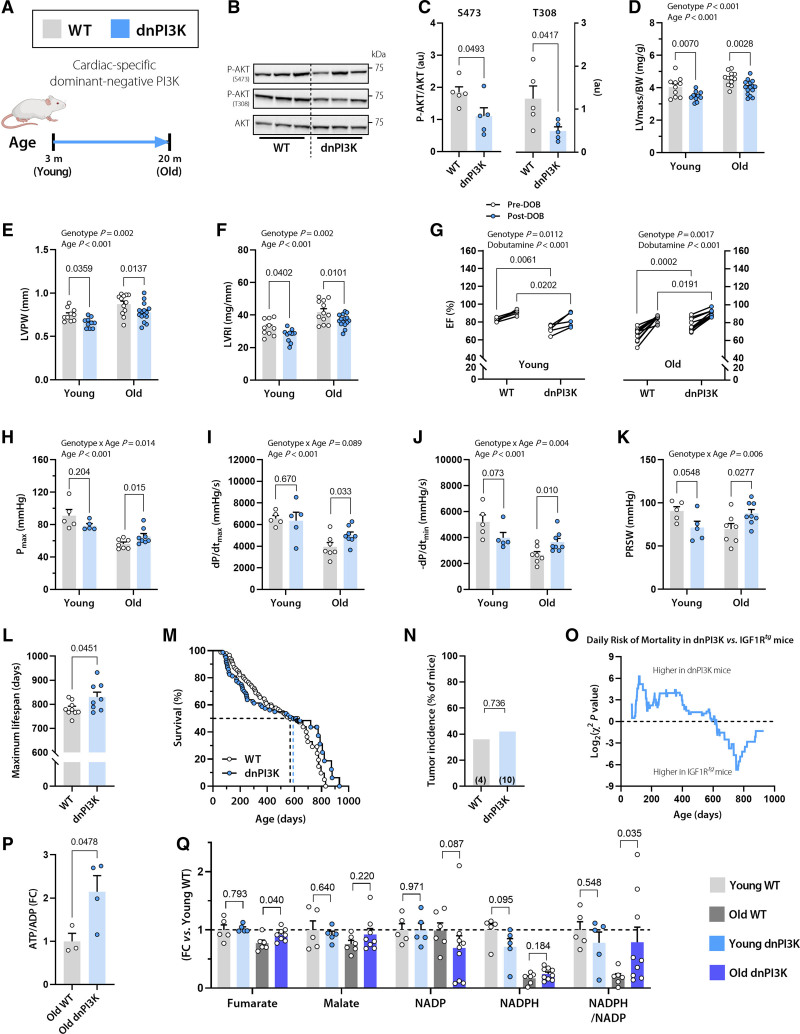
To gain further insight into the role of cardiac IGF1R signaling in early versus late life stages, we performed a second long-term study on mice with reduced IGF1R signaling. Because IGF1R deletion might cause embryonic defects and compromised perinatal survival,23,24 we instead used a mouse model harboring an inactivated (dominant negative, dn) p110α isoform of PI3K,13 which is a key IGF1R downstream effector (Figure 4A). Compared with WT controls, cardiomyocyte-specific dnPI3K mice had a lower cardiac IGF1 concentration (Figure S2) and clearly reduced IGF1R signaling, as evidenced by decreased phosphorylation of AKT at Ser473 and Thr308 (Figure 4B and 4C).
Figure 4.
Reduced cardiomyocyte IGF1R signaling in dnPI3K mice delays cardiac growth, but protects from age-related decline in cardiac function. A, Dominant negative PI3K (dnPI3K) male mice with reduced cardiomyocyte-specific IGF1R signaling and their WT littermates (FVB/N background) were subjected to a comprehensive assessment of cardiac function and structure at the age of 3 months (young) and 20 months (old). B and C, Representative Western blots (B) and quantification of (C) cardiac AKT phosphorylation at Ser473 and Thr308 normalized to total AKT expression in 10-month-old dnPI3K and WT mice (n=5 mice per group). D through F, Echocardiographic assessment of left ventricular (LV) mass normalized to body weight (BW; D), LV posterior wall thickness (LVPW; E), and LV remodeling index (LVRI; F) in young and old dnPI3K and WT mice (n=10 young and 12–15 old mice per genotype). G, LV ejection fraction (LVEF) before and after β-adrenergic stimulation by intraperitoneal injection of dobutamine (1.5 mg/kg IP) in young (Left) and old (Right) dnPI3K and WT mice (n=5 young and n=10 old mice per genotype). H through K, Invasive assessment of LV maximum pressure (Pmax; H), maximum rate of pressure rise (dP/dtmax; I), maximum rate of pressure decay (dP/dtmin; J), and preload recruitable stroke work (PRSW; K) in young and old dnPI3K and WT mice (n=5–8 mice per group). L, Maximum lifespan was calculated as the average lifespan of the longest-lived decile in dnPI3K and WT mice (n=8/9 mice per genotype, respectively). M, Kaplan-Meier survival analysis in dnPI3K and WT mice (n=80/90 mice per genotype, respectively). The same WT control group as in Figure 1N was used. Median survival is indicated by the corresponding dashed lines (also see Table S3 for further details). N, Tumor incidence detected by gross necropsy in dnPI3K and WT mice (26/11 mice per genotype, respectively). Number in brackets indicates the number of mice that developed tumors. O, Daily mortality risk in dnPI3K compared with IGF1Rtg mice (n=80/48 mice per genotype, respectively). P, Cardiac abundance of ATP/ADP ratio in 20-month-old dnPI3K and WT mice (n=4–5 mice per genotype). Q, Relative difference in the tricarboxylic acid cycle intermediates, fumarate and malate, and the ratio between reduced and oxidized forms of the cofactor nicotinamide adenine dinucleotide phosphate (NADPH/NADP) in young (3 months old) and old (20 months old) dnPI3K and WT mice (n=5–9 mice per group). Indicated P values on top of D through K represent factor comparisons by 2-way ANOVA including genotype and age as fixed factors (D–F, H–K) or genotype and dobutamine (G), followed by simple main effects analysis of pairwise comparisons between dnPI3K and their age- or treatment-matched WT controls. Other P values were calculated by the Welch t test or Mann-Whitney test, as appropriate (L, P through Q) or χ2 test (N). Bars and error bars show means and SEM, respectively, with individual data points superimposed. dnPI3K indicates mice harboring an inactivated (dominant negative, dn) p110α isoform of phosphoinositide 3-kinase; IGF1, insulin-like growth factor 1; IGF1R, IGF1 receptor; IGF1Rtg mice, mice overexpressing human IGF1R specifically in cardiomyocytes; and WT, wild-type.
Accordingly, dnPI3K mice exhibited lower body weight–normalized LV mass, posterior wall thickness, and remodeling index than WT mice at both 3 and 20 months of age (Figure 4D–4F). However, functional cardiac assessment revealed that young dnPI3K mice had slightly, but significantly lower EF than WT mice both at baseline and in response to β-adrenergic stimulation (Figure 4G), indicating that young dnPI3K mice might have suppressed cardiac growth, not attenuated remodeling. By contrast, aged dnPI3K mice had a significantly higher EF than WT mice, thereby exhibiting a conspicuously lower age-dependent decline in EF (2-way ANOVA interaction P=0.005; Table S4). Likewise, young, but not old, dnPI3K mice exhibited lower exercise capacity than WT controls (Figure S8). In fact, β-adrenergic stimulation revealed that aged dnPI3K mice display a significantly higher cardiac reserve capacity than aged WT mice (Figure 4G). These findings were further corroborated by invasive measurements of cardiac performance, including maximal LV pressure, the maximum and minimum rates of LV pressure change, and preload recruitable stroke work, which were all significantly ameliorated in aged dnPI3K mice (Figure 4H–4K), albeit without any change in left atrial area or lung weight (Figure S9). Thus, despite delayed cardiac growth early in life, partial inhibition of IGF1R signaling induced cardioprotective effects during aging. As a result, aged dnPI3K mice had a longer maximum lifespan than WT mice, with no apparent difference in tumor incidence (Figure 4L–4N). Although dnPI3K mice exhibited extended longevity compared with IGF1Rtg animals (18.5% longer maximum lifespan, P<0.001), they exhibited a higher risk of mortality at a young age (Figure 4O). These data collectively corroborate the essential role of cardiac IGF1R signaling in early life and its detrimental role in aging.
Considering that reduced autophagy contributed to exacerbated cardiac aging in IGF1Rtg mice, we sought to determine whether autophagy is also implicated in the extended cardiac healthspan of dnPI3K mice. We measured cardiac expression of the autophagy marker LC3-II in dnPI3K and WT mice that received an intraperitoneal injection of leupeptin or saline. Compared with saline administration, leupeptin induced significantly higher LC3-II accumulation in dnPI3K than WT hearts (Figure S10A and S10B), indicating higher autophagic activity. To determine whether increased autophagy inversely correlates with mTORC1 activity in dnPI3K mice, we analyzed several mTORC1 substrates. We found reduced phosphorylation of 4E-BP1 in dnPI3K mice (Figure S11). However, mTORC1 regulation appeared complex in dnPI3K mice as 4E-BP1 expression was increased, whereas both ULK1 (Ser757) phosphorylation and p70 S6K were not altered (Figure S11). Also, AMPK (AMP-activated protein kinase) expression and related ULK1 (Ser317) phosphorylation were not affected (Figure S12), suggesting that ULK1 phosphorylation is not involved in autophagy activation in dnPI3K mice. The TFEB (transcription factor EB) is another mTORC1 substrate that is intimately linked to lysosome biogenesis and autophagy,25 but has different phosphorylation kinetics from other mTORC1 substrates.26 TFEB phosphorylation by mTORC1 reduces the electrophoretic mobility of TFEB,27,28 resulting in an upward shift in the immunoblot signal (Figure S13A). dnPI3K mice exhibited a higher TFEB signal at the lower, unphosphorylated molecular weight (Figure S13A), suggesting reduced phosphorylation by mTORC1. Accordingly, in dnPI3K mice, TFEB target genes related to lysosome biogenesis were upregulated (Figure S13A),29 suggesting enhanced TFEB activity.
To examine whether a cause-effect relationship exists between increased autophagy and cardiac health in aged dnPI3K mice, 19-month-old transgenic mice and their WT littermates received daily intraperitoneal injections of HCQ (60 mg/kg) to chronically inhibit autophagy in vivo (Figure S10C).15 After 4 weeks of HCQ treatment, no significant alteration was detectable in the body weight–normalized LV mass of WT or dnPI3K mice (Figure S10D). However, the difference in cardiac function between WT and dnPI3K animals was entirely abolished after HCQ treatment. Before HCQ treatment, aged dnPI3K mice exhibited a significantly improved Doppler-derived myocardial performance index that is a global measure of LV function (best known as Tei index; Figure S10E). Aged dnPI3K animals also had a higher EF than WT mice, despite comparable heart rates (Figure S10F and S10G). However, after 4 weeks of HCQ administration, dnPI3K and WT mice had almost identical Tei indices and EF (Figure S10E and S10F), indicating that a functional autophagic flux is required for improved cardiac function in aged dnPI3K mice. Aged dnPI3K mice also exhibited a higher cardiac ATP/ADP ratio that was coupled to partial restoration of tricarboxylic acid cycle metabolites and NADPH/NADP ratio (Figure 4P and 4Q) without noticeable changes in glycolytic intermediates (Figure S14) compared with WT controls. Hence, these data suggest that aged dnPI3K hearts exhibit improved cardiac bioenergetics and mitochondrial metabolism in association with enhanced autophagy.
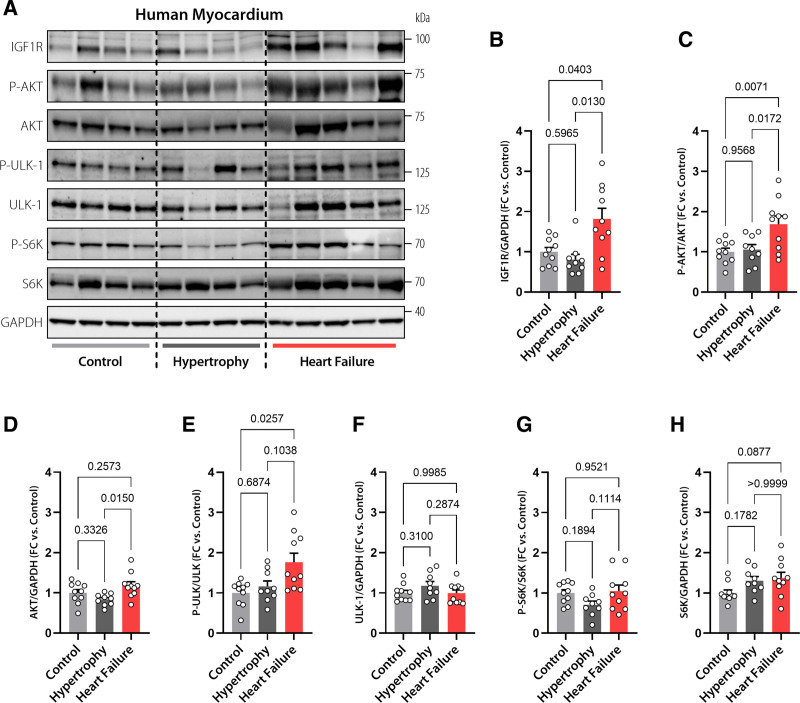
Human Failing Hearts Exhibit Exaggerated IGF1R Expression and Signaling Activity
Last, we examined the translational potential of our findings by evaluating the expression and signaling activity of IGF1R and its downstream effectors in aged human hearts with equivalent age to 20-month-old mice.30 We used LV biopsies obtained from explanted failing hearts and compared them with biopsies from control donors who had echocardiographic evidence of preserved cardiac function in the presence or absence of LV hypertrophy (Table S5). Of note, there was no increase in IGF1R expression in hypertrophic compared with control hearts (Figure 5A and 5B). Downstream to IGF1R, there were also no differences in the phosphorylation or expression levels of AKT, ULK-1, and S6K in hypertrophic compared with control hearts (Figure 5C–5H). In sharp contrast, IGF1R expression increased by almost 2-fold in failing compared with normal controls or nonfailing hypertrophic human myocardia (Figure 5A and 5B). Consistently, and in line with the results obtained from IGF1Rtg mice, failing hearts exhibited increased phosphorylation of AKT (Figure 5C and 5D). Compared with controls, failing hearts also exhibited elevated ULK-1 phosphorylation, perhaps reflecting increased mTORC1 activity (Figure 5E and 5F). However, the phosphorylation and expression of S6K were comparable among the groups (Figure 5G and 5H). In sum, these results indicate that heart failure is associated with exaggerated IGF1R signaling in humans.
Figure 5.
Increased IGF1R signaling in human failing hearts. Representative Western blots (A) and immunoblot analysis of cardiac IGF1 receptor (IGF1R) expression (B), AKT phosphorylation at Thr308 normalized to total AKT expression (C), AKT expression (D), ULK-1 phosphorylation normalized to total ULK-1 expression (E), ULK-1 expression (F), S6K phosphorylation normalized to total S6K expression (G), and S6K expression (H) in left ventricular samples obtained from failing and nonfailing human hearts with or without echocardiographic evidence of hypertrophy (n=9/10/10 hearts in Control, Hypertrophy, and Heart Failure, respectively). GAPDH was used as a loading control. Indicated P values were calculated by Welch test with Dunnett T3 post hoc (B and E), ANOVA with Tukey post hoc (C, D, F, G) or Kruskal-Wallis-test with Dunn post hoc (H). Bars and error bars show means and SEM, respectively, with individual data points superimposed. FC indicates fold change; IGF1, insulin-like growth factor 1; IGF1R, IGF1 receptor; and IGF1Rtg mice, mice overexpressing human IGF1R specifically in cardiomyocytes.
Discussion
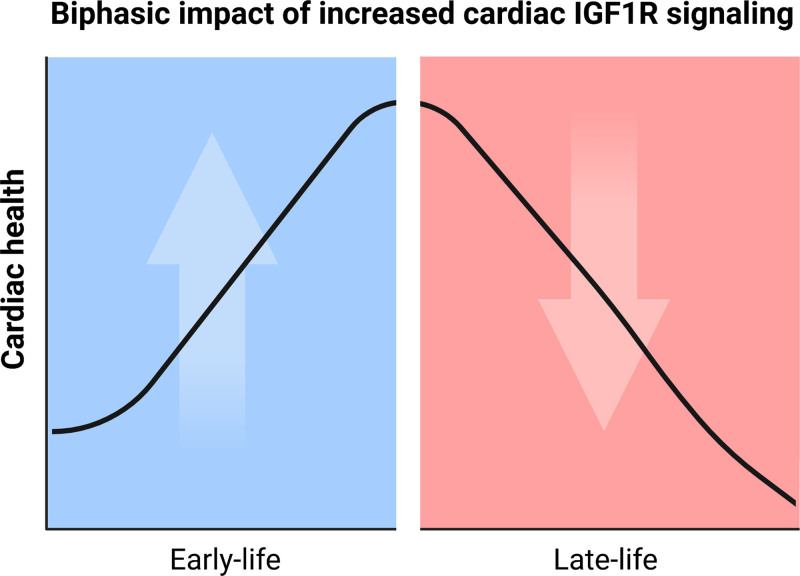
The combination of experimental and clinical evidence provided in this study consistently suggests that the relationship between IGF1R signaling and cardiac function is not linear, but rather biphasic and age-dependent (Figure 6), thereby bringing into question the current assertion that IGF1R-PI3K signaling is inherently cardioprotective. IGF1R signaling proved most crucial for cardiac structure and function during early life, because young mice exhibited cardiac benefits in association with increased IGF1R signaling. By contrast, attenuated cardiac growth and impaired heart function were evident in young dnPI3K mice with reduced IGF1R signaling. It is striking, however, that old dnPI3K mice showed a rejuvenated cardiac phenotype, whereas old IGF1Rtg mice manifested accelerated cardiac aging and signs of heart failure, suggesting that IGF1R-PI3K signaling is deleterious for the aging heart. Conversely, cardiac-specific inactivation of PI3K p110α was associated with an extension in maximum longevity, despite an increased risk of mortality in early life. These findings echo a similar survival advantage described for male mice with partial global deactivation of PI3K,31 yet clarify that heart-specific modulation of the IGF1R-PI3K pathway suffices to affect organismal lifespan.
Figure 6.
Graphical summary of the role of cardiomyocyte IGF1R signaling in regulating cardiac health during the course of life. IGF1R signaling proved crucial for cardiac homeostasis during early life as young IGF1Rtg mice exhibited cardiac benefits in association with increased IGF1R signaling, whereas IGF1R activity was deleterious for cardiac function in aged IGF1Rtg mice. Conversely, reduced cardiac IGF1R signaling in dnPI3K mice was associated with lower cardiac performance and increased risk of mortality in early life, but extended cardiac healthspan and maximum longevity later in life. dnPI3K indicates mice harboring an inactivated (dominant negative, dn) p110α isoform of phosphoinositide 3-kinase; IGF1, insulin-like growth factor 1; IGF1R, IGF1 receptor; and IGF1Rtg mice, mice overexpressing human IGF1R specifically in cardiomyocytes.
IGF1R signaling mechanistically controls cellular growth and metabolism through a wide network of downstream pathways.1 Among these, autophagy, a catabolic pathway essential for protein and organelle quality control, emerged as a major determinant of the cardiac response to IGF1R modulation. At young age, it appears that IGF1R signaling promotes growth by favoring anabolic over catabolic reactions, leading to improved cardiac performance. However, this comes at the expense of insufficient cardiomyocyte maintenance by autophagy, which progressively declines with aging.20 Because cardiomyocytes are terminally differentiated cells, they cannot dilute dysfunctional organelles through cell divisions, which means that they are particularly vulnerable to dysfunctional autophagy. Indeed, the autophagy-inducer spermidine rescued aged IGF1Rtg mice from heart failure. In contrast, reduced IGF1R signaling extended the cardiac healthspan of dnPI3K mice in an autophagy-dependent manner. In line with these observations, a growing body of evidence suggests that efficient autophagy promotes healthy cardiac aging with various autophagy-inducing interventions showing promising cardioprotective effects in aged mice.20,32 By contrast, dysfunctional autophagy in aged IGF1Rtg mice was associated with impaired mitochondrial function and oxidative metabolism, leading to inefficient cardiac bioenergetics and heart failure. Although a direct link between reduced mitochondrial oxidative metabolism and premature onset of heart failure was recently established in a number of studies using mice deficient in the mitochondrial pyruvate carrier,33,34 future studies will be required to determine whether specific targeting of mitochondrial metabolism can delay aging. At least in dnPI3K mice, reduced cardiac decline in aging was coupled to improved cardiac oxidative metabolism and energy reserves. Along similar lines, improved oxidative metabolism has been reported for adipose tissue–specific mutant mice with reduced insulin/IGF1 signaling.35
On the basis of our findings, we propose that age determines the cardiac effects of IGF1R signaling, which is essential for early-life cardiac growth but detrimental in late life. Thus, our study suggests that inhibition of cardiac IGF1R signaling in late life is likely to suppress the age-related deterioration of cardiac function and increase lifespan. In support of this idea, a recent study showed that late-life administration of IGF1R monoclonal antibody effectively promotes both cardiac health and lifespan, at least in female mice.3 Current evidence in humans also suggests a U-shape relationship between IGF1 levels and mortality.36 Although low serum IGF1 levels have been previously linked to a higher risk of heart failure,37 we found exaggerated IGF1R signaling activity in LV samples of patients with end-stage nonischemic heart failure. In line with our observation, a recent study reported IGF1R overexpression, at least at the transcriptional level, in 2 other forms of cardiomyopathy, ie, ischemic heart disease and heart failure with preserved EF.38 Because causality cannot be inferred from these observational studies, whether increased IGF1R signaling in the heart reflects maladaptive or compensatory mechanisms remains an open question.39 Hence, clinical evaluation of pharmacological IGF1R/PI3K inhibitors, which are already used against cancer,40 is required to determine their potential to improve the prognosis of elderly patients at risk of heart failure.
Limitations of the Study
Considering that the effect of IGF1R signaling is less controversial in female than in male mice,3 this study used only male mice as test subjects. However, future studies should clarify whether the dual role of IGF1R signaling is sex dependent. Because PI3K does not exclusively mediate IGF1R signaling, dnPI3K mice cannot be viewed as the exact opposite of IGF1Rtg mice. Thus, future long-term studies using a conditional IGF1R deletion model will be needed to corroborate the findings obtained in dnPI3K mice. Furthermore, we acknowledge that spermidine is a pleiotropic molecule that might affect cellular processes other than autophagy.41 Hence, future studies using more specific autophagy-inducing interventions (eg, overexpression of autophagy-related transgenes) are warranted to determine the exact contribution of autophagy to the avoidance of cardiac aging.
Article Information
Acknowledgments
The authors are grateful to F. Aprahamian, N. Nirmalathasan, and S. Schauer for their excellent technical assistance. We also acknowledge the support of the animal facility staff at the Institute of Biomedical Research (IBF), Medical University of Graz, for the well-being of our animals. Mouse clipart were created with Biorender.com. Drs Abdellatif and Sedej conceived and supervised the study. Drs Abdellatif, Trummer-Herbst, Heberle, Humnig, Pendl, Hofer, Voglhuber, Durand, Cerrato, Islam, Ramos Pittol, Schmidt, and Sedej performed experiments and analyzed the data. Drs von Lewinski, Rainer, and Scherr characterized patients and provided human cardiac tissue. Drs Kepp, Hoefler, Diwan, Bisping, McMullen, Eisenberg, Madeo, Thedieck, and Kroemer discussed data or gave conceptual advice. Drs Abdellatif and Sedej wrote the manuscript, including input from all coauthors.
Sources of Funding
This work was funded by the Austrian Science Fund (FWF) through grants P27637-B28 and I3301-MINOTAUR to Dr Sedej. Dr Abdellatif acknowledges support from the European Society of Cardiology (ESC basic research fellowship), Austrian Society of Cardiology (Präsidentenstipendium-ÖKG), Medical University of Graz (Start Fund), and the European Commission (H2020-MSCA-IF, Nr. 101025118). Dr Kroemer is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; AMMICa US23/CNRS UMS3655; Association pour la recherche sur le cancer (ARC); Association “Ruban Rose”; Cancéropôle Ile-de-France; Fondation pour la Recherche Médicale (FRM); a donation by Elior; Equipex Onco-Pheno-Screen; European Joint Programme on Rare Diseases (EJPRD); Gustave Roussy Odyssea, the European Union Horizon 2020 Projects Oncobiome and Crimson; Fondation Carrefour; High-end Foreign Expert Program in China (GDW20171100085), Institut National du Cancer (INCa); Inserm (HTE); Institut Universitaire de France; LabEx Immuno-Oncology (ANR-18-IDEX-0001); the Leducq Foundation; the RHU Torino Lumière; Seerave Foundation; SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); and SIRIC Cancer Research and Personalized Medicine (CARPEM). This study contributes to the IdEx Université de Paris ANR-18-IDEX-0001. Dr Thedieck is supported by the MESI-STRAT project (grant agreement 754688), the PoLiMeR Innovative Training Network (Marie Skłodowska-Curie grant agreement 812616), and the ARDRE COFUND Training Network (Marie Skłodowska-Curie grant agreement No. 847681) that all received funding from the European Union Horizon 2020 Research and Innovation Program; the German Tuberous Sclerosis Foundation and Stichting TSC Fonds. Drs Ramos Pittol and Heberle are supported by the Tyrolean Science Fund (TWF; grant agreements F.18896 and F.33468/7–2021).
Disclosures
Drs Madeo and Eisenberg have financial interests in TLL – The Longevity Labs GmbH. Dr Eisenberg conducts paid consultancies for TLL.
Supplemental Material
Supplemental Methods
Tables S1–S5
Figures S1–S14
Supplementary Material
Nonstandard Abbreviations and Acronyms
- 4E-BP1
- eIF4E-binding protein 1
- dnPI3K
- mice harboring an inactivated (dominant negative, dn) p110α isoform of PI3K
- EF
- ejection fraction
- HCQ
- hydroxychloroquine
- IGF1
- insulin-like growth factor 1
- IGF1Rtg
- mice overexpressing the human IGF1R specifically in cardiac myocytes
- LC3B-II
- autophagy-related lipidated form of microtubule-associated protein 1A/1B-light chain 3B
- LV
- left ventricle
- mTORC1
- mammalian target of rapamycin complex 1
- p70 S6K
- p70 ribosomal S6 kinase 1
- PI3K
- phosphoinositide 3-kinase
- PRAS40
- proline-rich AKT1 substrate 1
- ULK-1
- unc-51-like kinase-1
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.122.059863.
For Sources of Funding and Disclosures, see page 1865.
Circulation is available at www.ahajournals.org/journal/circ
Contributor Information
Viktoria Trummer-Herbst, Email: viktoria.herbst@medunigraz.at.
Alexander Martin Heberle, Email: Alexander.Heberle@uibk.ac.at.
Alina Humnig, Email: humnig.alina@gmx.net.
Tobias Pendl, Email: tobias.eisenberg@uni-graz.at.
Sylvère Durand, Email: sylvere.durand@gustaveroussy.fr.
Giulia Cerrato, Email: giulia.cerrato1@gmail.com.
Sebastian J. Hofer, Email: sebastian.hofer@uni-graz.at.
Moydul Islam, Email: mislam@wustl.edu.
Julia Voglhuber, Email: julia.voglhuber@medunigraz.at.
José Miguel Ramos Pittol, Email: Jose.Ramos-Pittol@uibk.ac.at.
Oliver Kepp, Email: captain.olsen@gmail.com.
Gerald Hoefler, Email: Gerald.Hoefler@uniklinikum.kages.at.
Albrecht Schmidt, Email: albrecht.schmidt@medunigraz.at.
Peter P. Rainer, Email: peter.rainer@medunigraz.at.
Daniel Scherr, Email: daniel.scherr@medunigraz.at.
Dirk von Lewinski, Email: dirk.von-lewinski@medunigraz.at.
Egbert Bisping, Email: egbert.bisping@medunigraz.at.
Julie R. McMullen, Email: julie.mcmullen@baker.edu.au.
Abhinav Diwan, Email: adiwan@wustl.edu.
Tobias Eisenberg, Email: tobias.eisenberg@uni-graz.at.
Frank Madeo, Email: frank.madeo@uni-graz.at.
Kathrin Thedieck, Email: Kathrin.Thedieck@uibk.ac.at.
Guido Kroemer, Email: kroemer@orange.fr.
References
- 1.Johnson SC. Nutrient sensing, signaling and ageing: the role of IGF-1 and mTOR in ageing and age-related disease. Subcell Biochem. 2018;90:49–97. doi: 10.1007/978-981-13-2835-0_3 [DOI] [PubMed] [Google Scholar]
- 2.Milman S, Huffman DM, Barzilai N. The somatotropic axis in human aging: framework for the current state of knowledge and future research. Cell Metab. 2016;23:980–989. doi: 10.1016/j.cmet.2016.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao K, Quipildor GF, Tabrizian T, Novaj A, Guan F, Walters RO, Delahaye F, Hubbard GB, Ikeno Y, Ejima K, et al. Late-life targeting of the IGF-1 receptor improves healthspan and lifespan in female mice. Nat Commun. 2018;9:2394. doi: 10.1038/s41467-018-04805-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bokov AF, Garg N, Ikeno Y, Thakur S, Musi N, DeFronzo RA, Zhang N, Erickson RC, Gelfond J, Hubbard GB, et al. Does reduced IGF-1R signaling in Igf1r+/– mice alter aging? PLoS One. 2011;6:e26891. doi: 10.1371/journal.pone.0026891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu J, Gontier G, Chaker Z, Lacube P, Dupont J, Holzenberger M. Longevity effect of IGF-1R+/– mutation depends on genetic background-specific receptor activation. Aging Cell. 2014;13:19–28. doi: 10.1111/acel.12145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toth P, Tarantini S, Ashpole NM, Tucsek Z, Milne GL, Valcarcel-Ares NM, Menyhart A, Farkas E, Sonntag WE, Csiszar A, et al. IGF-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell. 2015;14:1034–1044. doi: 10.1111/acel.12372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gong Z, Kennedy O, Sun H, Wu Y, Williams GA, Klein L, Cardoso L, Matheny RW, Jr, Hubbard GB, Ikeno Y, et al. Reductions in serum IGF-1 during aging impair health span. Aging Cell. 2014;13:408–418. doi: 10.1111/acel.12188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Araiz C, Yan A, Bettedi L, Samuelson I, Virtue S, McGavigan AK, Dani C, Vidal-Puig A, Foukas LC. Enhanced β-adrenergic signalling underlies an age-dependent beneficial metabolic effect of PI3K p110α inactivation in adipose tissue. Nat Commun. 2019;10:1546. doi: 10.1038/s41467-019-09514-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blüher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223 [DOI] [PubMed] [Google Scholar]
- 10.McMullen JR, Shioi T, Huang WY, Zhang L, Tarnavski O, Bisping E, Schinke M, Kong S, Sherwood MC, Brown J, et al. The insulin-like growth factor 1 receptor induces physiological heart growth via the phosphoinositide 3-kinase(p110alpha) pathway. J Biol Chem. 2004;279:4782–4793. doi: 10.1074/jbc.M310405200 [DOI] [PubMed] [Google Scholar]
- 11.Moellendorf S, Kessels C, Peiseler L, Raupach A, Jacoby C, Vogt N, Lindecke A, Koch L, Brüning J, Heger J, et al. IGF-IR signaling attenuates the age-related decline of diastolic cardiac function. Am J Physiol Endocrinol Metab. 2012;303:E213–E222. doi: 10.1152/ajpendo.00538.2011 [DOI] [PubMed] [Google Scholar]
- 12.Ock S, Lee WS, Ahn J, Kim HM, Kang H, Kim HS, Jo D, Abel ED, Lee TJ, Kim J. Deletion of IGF-1 receptors in cardiomyocytes attenuates cardiac aging in male mice. Endocrinology. 2016;157:336–345. doi: 10.1210/en.2015-1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shioi T, Kang PM, Douglas PS, Hampe J, Yballe CM, Lawitts J, Cantley LC, Izumo S. The conserved phosphoinositide 3-kinase pathway determines heart size in mice. EMBO J. 2000;19:2537–2548. doi: 10.1093/emboj/19.11.2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisenberg T, Abdellatif M, Schroeder S, Primessnig U, Stekovic S, Pendl T, Harger A, Schipke J, Zimmermann A, Schmidt A, et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med. 2016;22:1428–1438. doi: 10.1038/nm.4222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li M, Sala V, De Santis MC, Cimino J, Cappello P, Pianca N, Di Bona A, Margaria JP, Martini M, Lazzarini E, et al. Phosphoinositide 3-kinase gamma inhibition protects from anthracycline cardiotoxicity and reduces tumor growth. Circulation. 2018;138:696–711. doi: 10.1161/CIRCULATIONAHA.117.030352 [DOI] [PubMed] [Google Scholar]
- 16.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, et al. ; Chamber Quantification Writing Group; American Society of Echocardiography’s Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005 [DOI] [PubMed] [Google Scholar]
- 17.Santini MP, Tsao L, Monassier L, Theodoropoulos C, Carter J, Lara-Pezzi E, Slonimsky E, Salimova E, Delafontaine P, Song YH, et al. Enhancing repair of the mammalian heart. Circ Res. 2007;100:1732–1740. doi: 10.1161/CIRCRESAHA.107.148791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doumas A, Draper TS, Jr, Schick EC, Gaasch WH. Prevalence and clinical characteristics of nondilated cardiomyopathy and the effect of atrial fibrillation. Am J Cardiol. 2010;105:884–887. doi: 10.1016/j.amjcard.2009.10.068 [DOI] [PubMed] [Google Scholar]
- 19.Liu GY, Sabatini DM. mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol. 2020;21:183–203. doi: 10.1038/s41580-019-0199-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdellatif M, Sedej S, Carmona-Gutierrez D, Madeo F, Kroemer G. Autophagy in cardiovascular aging. Circ Res. 2018;123:803–824. doi: 10.1161/CIRCRESAHA.118.312208 [DOI] [PubMed] [Google Scholar]
- 21.Abdellatif M, Ljubojevic-Holzer S, Madeo F, Sedej S. Autophagy in cardiovascular health and disease. Prog Mol Biol Transl Sci. 2020;172:87–106. doi: 10.1016/bs.pmbts.2020.04.022 [DOI] [PubMed] [Google Scholar]
- 22.Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD, Adeli K, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy. 2016;12:1–222. doi: 10.1080/15548627.2015.1100356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 24.Epaud R, Aubey F, Xu J, Chaker Z, Clemessy M, Dautin A, Ahamed K, Bonora M, Hoyeau N, Fléjou JF, et al. Knockout of insulin-like growth factor-1 receptor impairs distal lung morphogenesis. PLoS One. 2012;7:e48071. doi: 10.1371/journal.pone.0048071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, et al. TFEB links autophagy to lysosomal biogenesis. Science. 2011;332:1429–1433. doi: 10.1126/science.1204592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Napolitano G, Di Malta C, Esposito A, de Araujo MEG, Pece S, Bertalot G, Matarese M, Benedetti V, Zampelli A, Stasyk T, et al. A substrate-specific mTORC1 pathway underlies Birt-Hogg-Dubé syndrome. Nature. 2020;585:597–602. doi: 10.1038/s41586-020-2444-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puertollano R, Ferguson SM, Brugarolas J, Ballabio A. The complex relationship between TFEB transcription factor phosphorylation and subcellular localization. EMBO J. 2018;37:e98804. doi: 10.15252/embj.201798804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cattelani C, Lesiak D, Liebscher G, Singer II, Stasyk T, Wallnöfer MH, Heberle AM, Corti C, Hess MW, Pfaller K, et al. The SZT2 interactome unravels new functions of the KICSTOR complex. Cells. 2021;10:2711. doi: 10.3390/cells10102711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alesi N, Akl EW, Khabibullin D, Liu HJ, Nidhiry AS, Garner ER, Filippakis H, Lam HC, Shi W, Viswanathan SR, et al. TSC2 regulates lysosome biogenesis via a non-canonical RAGC and TFEB-dependent mechanism. Nat Commun. 2021;12:4245. doi: 10.1038/s41467-021-24499-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flurkey K, Currer JM, Harrison DE, Fox JG. The mouse models in aging research. Fox JG, Quimby FW, Barthold SW, Newcomer CE, Smith AL, eds. In: The Mouse in Biomedical Research. 2nd ed. Elsevier; 2007:637–672. [Google Scholar]
- 31.Foukas LC, Bilanges B, Bettedi L, Pearce W, Ali K, Sancho S, Withers DJ, Vanhaesebroeck B. Long-term p110α PI3K inactivation exerts a beneficial effect on metabolism. EMBO Mol Med. 2013;5:563–571. doi: 10.1002/emmm.201201953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zimmermann A, Madreiter-Sokolowski C, Stryeck S, Abdellatif M. Targeting the mitochondria-proteostasis axis to delay aging. Front Cell Dev Biol. 2021;9:656201. doi: 10.3389/fcell.2021.656201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernandez-Caggiano M, Kamynina A, Francois AA, Prysyazhna O, Eykyn TR, Krasemann S, Crespo-Leiro MG, Vieites MG, Bianchi K, Morales V, et al. Mitochondrial pyruvate carrier abundance mediates pathological cardiac hypertrophy. Nat Metab. 2020;2:1223–1231. doi: 10.1038/s42255-020-00276-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Taufalele PV, Cochran JD, Robillard-Frayne I, Marx JM, Soto J, Rauckhorst AJ, Tayyari F, Pewa AD, Gray LR, et al. Mitochondrial pyruvate carriers are required for myocardial stress adaptation. Nat Metab. 2020;2:1248–1264. doi: 10.1038/s42255-020-00288-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katic M, Kennedy AR, Leykin I, Norris A, McGettrick A, Gesta S, Russell SJ, Bluher M, Maratos-Flier E, Kahn CR. Mitochondrial gene expression and increased oxidative metabolism: role in increased lifespan of fat-specific insulin receptor knock-out mice. Aging Cell. 2007;6:827–839. doi: 10.1111/j.1474-9726.2007.00346.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rahmani J, Montesanto A, Giovannucci E, Zand H, Barati M, Kopchick JJ, Mirisola MG, Lagani V, Bawadi H, Vardavas R, et al. Association between IGF-1 levels ranges and all-cause mortality: a meta-analysis. Aging Cell. 2022;21:e13540. doi: 10.1111/acel.13540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vasan RS, Sullivan LM, D’Agostino RB, Roubenoff R, Harris T, Sawyer DB, Levy D, Wilson PW. Serum insulin-like growth factor I and risk for heart failure in elderly individuals without a previous myocardial infarction: the Framingham Heart Study. Ann Intern Med. 2003;139:642–648. doi: 10.7326/0003-4819-139-8-200310210-00007 [DOI] [PubMed] [Google Scholar]
- 38.D’Assante R, Arcopinto M, Rengo G, Salzano A, Walser M, Gambino G, Monti MG, Bencivenga L, Marra AM, Åberg DN, et al. Myocardial expression of somatotropic axis, adrenergic signalling, and calcium handling genes in heart failure with preserved ejection fraction and heart failure with reduced ejection fraction. ESC Heart Fail. 2021;8:1681–1686. doi: 10.1002/ehf2.13067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jankowska EA, Biel B, Majda J, Szklarska A, Lopuszanska M, Medras M, Anker SD, Banasiak W, Poole-Wilson PA, Ponikowski P. Anabolic deficiency in men with chronic heart failure: prevalence and detrimental impact on survival. Circulation. 2006;114:1829–1837. doi: 10.1161/CIRCULATIONAHA.106.649426 [DOI] [PubMed] [Google Scholar]
- 40.Verret B, Cortes J, Bachelot T, Andre F, Arnedos M. Efficacy of PI3K inhibitors in advanced breast cancer. Ann Oncol. 2019;30(suppl 10):x12–x20. doi: 10.1093/annonc/mdz381 [DOI] [PubMed] [Google Scholar]
- 41.Madeo F, Eisenberg T, Pietrocola F, Kroemer G. Spermidine in health and disease. Science. 2018;359:eaan2788. doi: 10.1126/science.aan2788 [DOI] [PubMed] [Google Scholar]
- 42.Yuan R, Tsaih SW, Petkova SB, Marin de Evsikova C, Xing S, Marion MA, Bogue MA, Mills KD, Peters LL, Bult CJ, et al. Aging in inbred strains of mice: study design and interim report on median lifespans and circulating IGF1 levels. Aging Cell. 2009;8:277–287. doi: 10.1111/j.1474-9726.2009.00478.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abdellatif M, Trummer-Herbst V, Koser F, Durand S, Adão R, Vasques-Nóvoa F, Freundt JK, Voglhuber J, Pricolo MR, Kasa M, et al. Nicotinamide for the treatment of heart failure with preserved ejection fraction. Sci Transl Med. 2021;13:eabd7064. doi: 10.1126/scitranslmed.abd7064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdellatif M, Leite S, Alaa M, Oliveira-Pinto J, Tavares-Silva M, Fontoura D, Falcão-Pires I, Leite-Moreira AF, Lourenço AP. Spectral transfer function analysis of respiratory hemodynamic fluctuations predicts end-diastolic stiffness in preserved ejection fraction heart failure. Am J Physiol Heart Circ Physiol. 2016;310:H4–13. doi: 10.1152/ajpheart.00399.2015 [DOI] [PubMed] [Google Scholar]
- 45.Ayachi M, Niel R, Momken I, Billat VL, Mille-Hamard L. Validation of a ramp running protocol for determination of the true VO2max in mice. Front Physiol. 2016;7:372. doi: 10.3389/fphys.2016.00372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X, Spandidos A, Wang H, Seed B. PrimerBank: a PCR primer database for quantitative gene expression analysis, 2012 update. Nucleic Acids Res. 2012;40(Database issue):D1144–D1149. doi: 10.1093/nar/gkr1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Viltard M, Durand S, Pérez-Lanzón M, Aprahamian F, Lefevre D, Leroy C, Madeo F, Kroemer G, Friedlander G. The metabolomic signature of extreme longevity: naked mole rats versus mice. Aging (Albany NY). 2019;11:4783–4800. doi: 10.18632/aging.102116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sedej S, Schmidt A, Denegri M, Walther S, Matovina M, Arnstein G, Gutschi EM, Windhager I, Ljubojević S, Negri S, et al. Subclinical abnormalities in sarcoplasmic reticulum Ca2+ release promote eccentric myocardial remodeling and pump failure death in response to pressure overload. J Am Coll Cardiol. 2014;63:1569–1579. doi: 10.1016/j.jacc.2013.11.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.