Abstract
The localized application of the riboflavin/UV-A collagen cross-linking (UV-CXL) corneal treatment has been proposed to concentrate the stiffening process only in the compromised regions of the cornea by limiting the epithelium removal and irradiation area. However, current clinical screening devices dedicated to measuring corneal biomechanics cannot provide maps nor spatial-dependent changes of elasticity in corneas when treated locally with UV-CXL. In this study, we leverage our previously reported confocal air-coupled ultrasonic optical coherence elastography (ACUS-OCE) probe to study local changes of corneal elasticity in three cases: untreated, half-CXL-treated, and full-CXL-treated in vivo rabbit corneas (n = 8). We found a significant increase of the shear modulus in the half-treated (>450%) and full-treated (>650%) corneal regions when compared to the non-treated cases. Therefore, the ACUS-OCE technology possesses a great potential in detecting spatially-dependent mechanical properties of the cornea at multiple meridians and generating elastography maps that are clinically relevant for patient-specific treatment planning and monitoring of UV-CXL procedures.
1. Introduction
Quantifying corneal biomechanical properties is essential for understanding corneal disease onset and progression and treatment monitoring [1]. For instance, keratoconus is a degenerative disease that produces a local reduction of corneal stiffness leading to corneal deformation and loss of vision quality [2]. Keratoconus belongs to a group of disorders called corneal ectasia, the second leading cause of corneal transplants worldwide [3]. Riboflavin/UV-A collagen cross-linking (UV-CXL) was recently approved by the US Food and Drug Administration to treat keratoconus [4]. UV-CXL creates chemical bonds between collagen fibers in the corneal stroma to increase its overall strength [5]. Thus, UV-CXL stiffens the cornea and increases its resistance to further degeneration [4,5].
UV-CXL is generally applied globally in the whole extension of the cornea (i.e., covering an area of ∼9 mm diameter). Recently, the localized application of UV-CXL has been proposed in order to concentrate the stiffening process only in the compromised regions of the cornea by limiting the irradiation area and epithelium removal [6]. In this way, the postprocedural risk of complications, including potential damage of the stroma and infection, is reduced and outcomes are improved. However, current clinical screening devices dedicated to measuring corneal biomechanics (i.e., Corvis ST and the Oculus Response Analyzer) [7,8] cannot provide maps that could guide doctors during the assessment and monitoring of spatial-dependent changes of elasticity in corneas locally treated with UV-CXL.
Optical coherence elastography (OCE) [9] is the functional imaging modality based on optical coherence tomography (OCT) [10] dedicated to measuring the viscoelastic properties of tissues [11,12]. In particular, wave-based OCE leverages the relationship between tissue elasticity and the speed of mechanical waves propagating in such tissue [13]. Wave-based OCE has evolved into the study of the biomechanical properties of the ocular tunic (e.g., cornea, and sclera) and has extensively demonstrated the ability to distinguish untreated ex vivo corneas from UV-CXL treated corneas using different stimulation techniques: air-pulse [14], acoustic radiation force (ARF) [15], and air-coupled acoustic reflection-based radiation force [16]. Moreover, a previous in vivo study in normal and UV-CXL exposed corneas using air-pulse stimulation demonstrated a global elevation of Young’s modulus in the corneas after treatment [17]. However, the translation of these studies into the localized detection of untreated and UV-CXL treated regions in the same cornea in vivo using wave-based OCE has not yet been demonstrated.
In this study, we leverage our previously reported confocal air-coupled ultrasonic optical coherence elastography (ACUS-OCE) probe (Fig. 1(a)) [16] for the study of local changes of corneal elasticity in rabbits in vivo. A fully non-contact quasi-harmonic 2 kHz excitation is produced in untreated, half-CXL-treated, and full-CXL-treated in vivo corneas to generate wave speed maps. Statistical analysis of the measured average phase speed is conducted in each corneal region (superior, inferior, nasal, and temporal meridians) and for each UV-CXL treatment case (untreated, half-treated, and full-treated corneas). In addition, the modified Rayleigh-Lamb mechanical model is used to convert the Lamb wave speed into shear modulus considering the local thickness of each cornea. Finally, the average shear modulus in both regions of the half-CXL-treated corneas is compared against the typical cases: untreated and full-CXL-treated corneas.
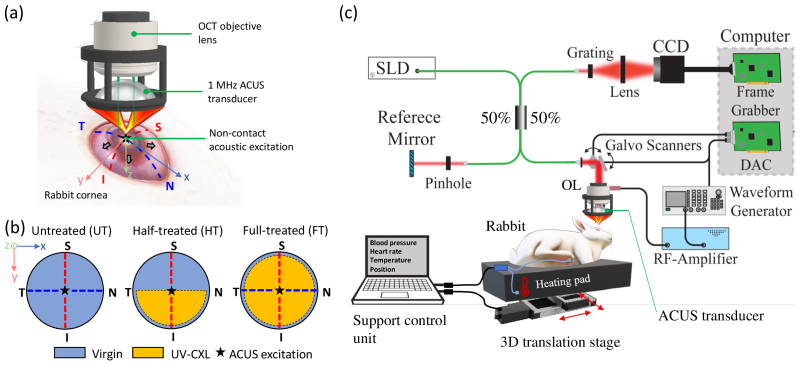
Fig. 1.
(a) ACUS-OCE probe producing acoustic excitation at the apex of the rabbit cornea. Outward-propagating Lamb waves are generated and analysed along four meridians: (N) nasal, (T) temporal, (S) superior, and (I) inferior. (b) Study cases of UV-CXL treatment in rabbit corneas: untreated, half-treated, and full-treated. Discontinuous lines represent measurement meridians. (c) Experimental setup including an SD-OCT system, the ACUS probe unit, and the support control system for the anesthetized rabbit. CCD: Charge-coupled device line camera, DAC: digital to analog converter, OL: objective lens, SLD: superluminescent diode.
2. Methods
2.1. Sample preparation and experimental design
ACUS-OCE measurements were made in four (n = 4) Dutch-Belted rabbits when the corneas were untreated (UT), half-treated (HT) and full-treated (FT) with UV-A/riboflavin cross-linking (UV-CXL) treatment (Fig. 1(b)) according to the standard “Dresden” clinical protocol [4]. According to this protocol, the epithelium was removed, and a solution of 0.1% riboflavin in 20% dextran was applied over a 30 min period at 5 minutes intervals on the surface of the stroma. Then, the solution was applied for another 30 min with the irradiation of UV light. The UV irradiation was spatially controlled with masks to constrain exposure to a full circle of 8 mm diameter (FT case) and a half-circle (HT case) in the cornea.
The first rabbit was measured with OCE in both untreated corneas, and the remaining three were measured under the following protocol: (1) the left cornea was measured with OCE before and after HT UV-CXL treatment, and (2) the right cornea was measured with OCE before and after FT UV-CXL.
In all cases and before any measurements or treatments, animals were anesthetized by a veterinarian with an intramuscular dose of 40 mg/kg ketamine and 5 mg/kg xylazine. After the rabbits had been anesthetized, they were placed in a lateral recumbent position in a custom-made rest covered with a heating pad to maintain a comfortable temperature, as shown in Fig. 1(c). Vital signals were monitored by the veterinarian and anesthesia maintenance doses of 10 mg/kg ketamine were administered as needed. Before conducting OCE measurements in the designated rabbit eye, the intraocular pressure (IOP) was measured using a handheld rebound tonometer (Tonovet, Icare Finland Oy, Vantaa, Finland). Finally, since the rabbits were anesthetized, they were unable to blink. Therefore, corneal hydration was ensured by applying 1x phosphate-buffered-saline (PBS) regularly. All the animal procedures were approved by the University of Houston Institutional Animal Care and Use Committee.
2.2. Experimental setup
The schematic of the OCE system and experimental setup is shown in Fig. 1(c). It includes a phase-sensitive spectral-domain OCT (SD-OCT) system with an excitation unit formed by a confocal air-coupled ultrasonic transducer (ACUS) demonstrated in our previous work [16]. The SD-OCT system used a superluminescent diode light source of ∼840 nm central wavelength and a ∼49 nm bandwidth. The axial and lateral resolutions of the OCT system was ∼9 µm and ∼8 µm, respectively, in air. The A-line acquisition speed was set to 50 kHz (temporal resolution µs). The optical phase stability was measured as ∼0.10 rad in a phantom, corresponding to a displacement sensitivity of ∼7 nm.
The ACUS transducer had a spherical shape with a focal distance of ∼ 20 mm, a central resonance frequency of ∼1 MHz, a diameter of ∼34 mm, and a central opening with a ∼10 mm diameter that allows for confocal and simultaneous OCT imaging and excitation. It produces a focused acoustic beam of ∼0.33 mm lateral spot size (measured as the full-width half-maximum of the pressure profile using a needle hydrophone [16]) with a depth-of-field of ∼0.3 mm at the focal position. The ACUS transducer was excited with five cycles of a 2 kHz square pulse (50% duty cycle) amplitude modulating a continuous 1 MHz signal. This modulated signal was then amplified using a radio-frequency amplifier (A150, Electronics & Innovation, Rochester, NY) before reaching the ACUS transducer.
2.3. OCE acquisition and processing
The M-B-mode protocol [13,18] was used for the acquisition of structural and motion 2D cross-sectional frames along two principal corneal directions: superior-inferior (y-axis), and nasal-temporal (x-axis), as shown in Fig. 1(a). The z-axis corresponds to the corneal depth direction. The OCT system captured M = 250 A-line repetitions (M-mode acquisition during 5 ms) after each mechanical excitation was produced in each measurement position. Each principal corneal direction consisted of 250 samples covering ∼7 mm ( ∼28 µm). The data were reorganized in 2D + time format resulting in an equivalent frame rate of 50 kHz. The total acquisition time was 2.5 s, including both principal directions.
In each study case of the cornea (before and after UV-CXL), a fast B-mode structural image (250 lateral samples, acquisition time: 5 ms) was acquired in each corneal meridian, followed by three repetitions of OCE measurements. Axial motion artifacts caused by the ocular saccades and respiration were suppressed at two levels: structural, and phase-sensitive data. First, in the structural B-mode images obtained with the OCE approach, speckle tracking of the corneal surface is applied to find the axial displacement shifts with respect to reference location of the corneal surface obtained from the fast B-mode image (hypothesized to be free of motion artifacts). These displacement shifts are obtained for every single lateral location along both meridional scans and used to locate and compensate the regions of higher distortion cause by the axial corneal movement. Secondly, phase-sensitive shifts are estimated in the positions associated to the distortion location (calculated in the previous step) before wave propagation is present (i.e., first 0.25 ms of the M-mode acquisition). Then, these phase shifts are subtracted from the whole M-mode acquisition at the selected locations, leaving only phase-sensitive information produced by wave propagation.
2.4. Speed maps and shear modulus
For every OCE acquisition, the ACUS transducer produced an acoustic reflection-based force at the corneal apex (within a region of ∼0.25 mm diameter) that generated a quasi-cylindrically propagating Lamb wave diverging towards the limbal region (x and y axes) and posterior side (z axis) of the cornea as seen in Fig. 1(a). The 2D phase-derivative method [13,19], with a kernel size of 0.2 × 0.2 mm2, was used over the particle velocity frames (obtained from phase-sensitive OCT A-lines) to calculate Lamb phase speed maps of the cornea (xz- and yz-planes) at 2 kHz excitation frequency. Average speed values along each corneal meridian (nasal, temporal, superior, and inferior) were calculated and compared in all cases of corneal UV-CXL treatment. Speed estimation within ∼0.5 mm of the excitation region within the apex (near field effect) was dismissed. Finally, because of thickness change before and after UV-CXL and the presence of aqueous humor in the posterior boundary (endothelium) of the cornea, the modified Rayleigh-Lamb frequency equation (mRLFE) wave model [19–21] was used to convert Lamb phase speed into shear elastic modulus for further comparison. Here, we assumed that corneal tissue is nearly incompressible and its Young’s modulus is three times its shear modulus.
3. Results
3.1. Axial motion compensation
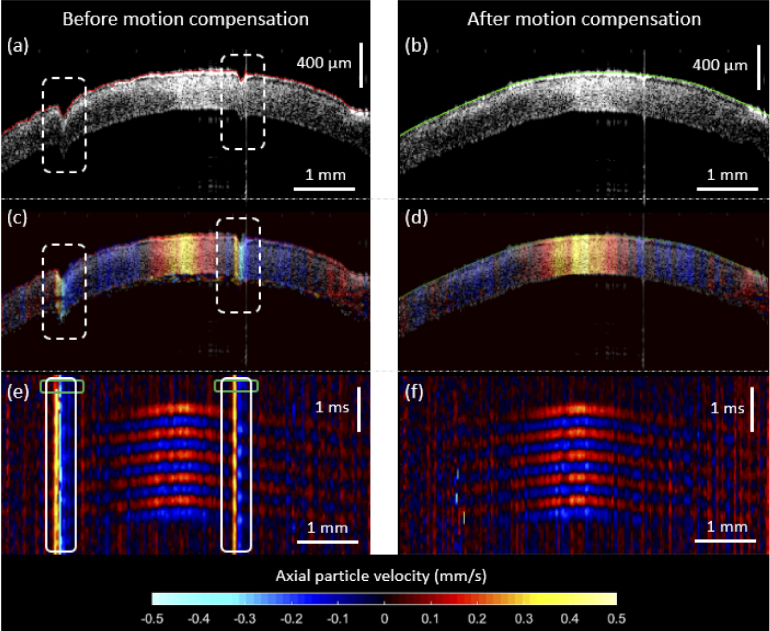
The results of our axial motion compensation approach are shown in Fig. 2 for a typical sample (left UT cornea from rabbit #1). In Fig. 2(a), two axial motion disruptions caused by the rabbit respiration are present in the B-mode structural image of the cornea. After the anterior surface of the cornea was segmented and subtracted from a reference surface obtained from a fast B-mode image (with no disruption), a lateral-dependent error vector was used to compensate for the jumps in the structural image, as shown in Fig. 2(b). Similarly, out-of-plane motion frames were also disrupted in the same locations of the axial motion artifacts as shown in Fig. 2(c), which diminishes the proper visualization of Lamb wave propagation and phase speed estimation. Therefore, the motion at those positions, at a time when the Lamb wave did not propagate yet (green rectangular regions in the space-time map of Fig. 2(e)), was measured and subtracted from the remaining time-dependent vector at the same locations (white rectangular regions in the space-time map of Fig. 2(e)). As a result, only the motion produced by the Lamb wave propagation remains in space and time, as shown in Fig. 2(d) and 2(f), respectively. This motion compensation mechanism is only valid for axial motion (it does not compensate for lateral movement) and assumes that the motion disruption is much slower than the excitation motion produced by the ACUS transducer.
Fig. 2.
Axial motion compensation results in an untreated in vivo rabbit cornea. Structural B-mode images with the anterior layer of the cornea segmented are shown before (a) and after (b) compensation. Jumps in (a) are due to respiration and saccades during OCE measurement. Axial particle velocity snapshots (at an instant . = 1.75 ms) of the same cornea depicting wave propagation are shown before (c) and after (d) compensation. Motion disruption is shown at the same locations of the jumps in (a). The color bar represents axial particle velocity (along the z-axis). Finally, space-time maps (lateral axis: the spatial propagating path along the corneal surface, axial axis: time) show outward Lamb wave propagation originate at the cornea apex before (e) and after (f) compensation. Green rectangular regions in (e) represent the locations in which the motion disruption was measured (before wave propagation has taken place) and then, later, subtracted to the time-dependent motion at the same locations (white rectangular regions), resulting in the (f) compensated version.
3.2. Lamb wave speed maps
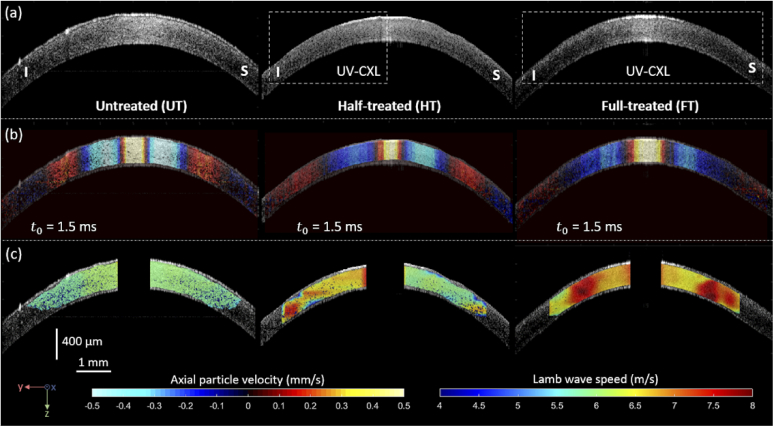
Structural B-mode images (Fig. 3(a)), motion snapshots depicting wave propagation (Fig. 3(b)), and Lamb wave phase speed maps at 2 kHz (Fig. 3(c)) along the inferior-superior direction are shown for the UT cornea (Fig. 3-left side), HT cornea (Fig. 3-center), and FT cornea (Fig. 3-right side) cases of in vivo UV-CXL treatments applied to rabbit #2. In Fig. 3(a), a decrement of central corneal thickness is observed from 400 µm (UT) to 310 µm (HT) and 350 µm (FT). Moreover, motion snapshots of wave propagation at an instant = 1.5 ms (Fig. 3(b)) show differentiated wavelength among cases: smaller and symmetric in the UT case, asymmetric in the HT case, and larger and symmetric in the FT case. As shown in Fig. 1(b) for the HT case along the inferior-superior direction, an asymmetry of wave propagation is expected due to the localization of the UV-CXL treatment along the inferior meridian. Finally, Lamb wave phase speed maps obtained at 2 kHz mechanical excitation are shown in Fig. 3(c) for all cases using the 2D phase-derivative method (color bar represents phase speed in m/s). As expected by the location of the UV-CXL treatment, the inferior meridian (left side) in the HT cornea case was stiffer (average speed ∼7.14 m/s) than its (right side) superior meridian (average speed ∼5.88 m/s). Moreover, speed maps for the UT and FT are symmetric and show a marked stiffness elevation from UT (average speed ∼5.31 m/s) to FT (average speed ∼7.66 m/s). These results demonstrate that the ACUS-OCE technique can map the localized UV-CXL treatment and associated stiffness change in rabbit in vivo corneas.
Fig. 3.
Structural B-mode images (a), motion snapshots depicting wave propagation (b), and Lamb wave phase speed maps (c) along the inferior-superior direction for the UT cornea (left side), HT cornea (center), and FT cornea (right side) cases of in vivo UV-CXL treatment applied to rabbit #2. Motion snapshots in (b) were taken at the instant = 1.5 ms and represent axial particle velocity in mm/s. Lamb wave phase speed maps (c) were taken at 2 kHz excitation frequency and are represented in m/s.
3.3. Statistical analysis
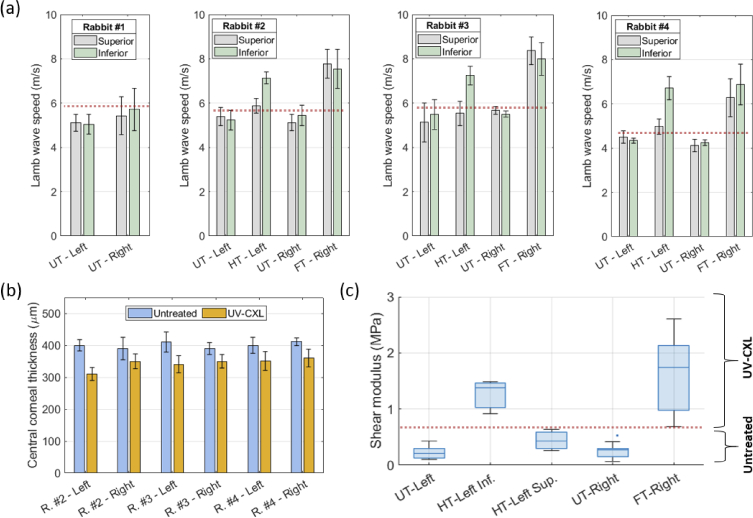
Lamb wave phase speed values for the superior and inferior meridians in all corneas are shown in Fig. 4(a). Each speed value and its error bar represent the average and standard deviation of three OCE measurement repetitions, respectively. For the HT corneas in rabbits #2, #3, and #4, it is evident a statistically significant increase in the wave speed in the inferior meridian (t-test, P = 0.0064) before (UT-left) and after (HT-left) UV-CXL compared to the superior meridian (P = 0.284) where no significant increase was found. Moreover, both superior and inferior meridians in the FT-right corneas show a statistically significant increase in wave speed (superior: P = 0.0312, inferior: P = 0.0116,) compared to the UT-right corneas for rabbits #2, #3, and #4. These results show that the in vivo UV-CXL treatment, when applied to a specific region, produces a very localized stiffening of the rabbit cornea that ACUS-OCE can detect. Then, our approach can discriminate corneal regions with UV-CXL from others where the treatment was not applied. The detailed report on the Lamb wave phase speed values measured in all meridians of all corneas, rabbits, and UV-CXL treatments cases is shown in Table 1.
Fig. 4.
Statistical analysis of Lamb wave phase speed, central corneal thickness, and shear modulus obtained with ACUS-OCE in the corneas of all rabbits. (a) Lamb wave speed measured in the superior and inferior meridians of the right and left corneas of all rabbits in all treatment cases. Error bars represent the standard deviation of n = 3 measurement repetitions. (b) Central corneal thickness measured from the B-mode structural images of all corneas with UV-CXL treatment cases in rabbits #2, #3, and #4. Error bars represent the standard deviation of n = 3 measurement repetitions. (c) Box plot of the estimated shear modulus (in MPa) for cases: UT-left (all rabbits and meridians, a total n = 16 meridians), HT-left Inf. (rabbits #2, #3, and #4, and only the inferior meridian, a total n = 3 meridians), HT-left Sup. (rabbits #2, #3, and #4, and only the superior meridian, a total n = 3 meridians), UT-right (all rabbits and meridians, a total n = 16 meridians), and FT-right (rabbits #2, #3, and #4, and all meridians, a total n = 12 meridians).
Table 1. Lamb wave speed values measured with ACUS-OCE in all corneas under different treatment conditions. Values represent mean ± standard deviation (n = 3) in m/s at 2 kHz excitation. Measurement meridians: S: superior, I: inferior, N: nasal, and T: temporal.
| Lamb wave phase speed (m/s) @ 2 kHz | |||||
|---|---|---|---|---|---|
| Left cornea | Right cornea | ||||
| Rabbit | Meridian | UT | HT | UT | FT |
| 1 | S | 5.12 ± 0.38 | 5.43 ± 0.85 | ||
| I | 5.05 ± 0.45 | 5.72 ± 0.95 | |||
| N | 5.47 ± 0.52 | 5.01 ± 0.59 | |||
| T | 5.45 ± 0.47 | 5.06 ± 0.41 | |||
|
| |||||
| 2 | S | 5.39 ± 0.41 | 5.88 ± 0.33 | 5.12 ± 0.37 | 7.78 ± 0.64 |
| I | 5.23 ± 0.45 | 7.14 ± 0.26 | 5.45 ± 0.46 | 7.54 ± 0.88 | |
| N | 4.37 ± 0.18 | 5.44 ± 0.22 | 7.73 ± 0.45 | ||
| T | 4.32 ± 0.28 | 5.39 ± 0.19 | 7.25 ± 0.11 | ||
|
| |||||
| 3 | S | 5.13 ± 0.87 | 5.54 ± 0.54 | 5.67 ± 0.18 | 8.37 ± 0.62 |
| I | 5.49 ± 0.68 | 7.25 ± 0.42 | 5.51 ± 0.15 | 8.01 ± 0.74 | |
| N | 4.24 ± 0.16 | 5.18 ± 0.13 | 7.93 ± 0.85 | ||
| T | 4.52 ± 0.31 | 5.12 ± 0.11 | 7.25 ± 0.47 | ||
|
| |||||
| 4 | S | 4.50 ± 0.28 | 4.98 ± 0.34 | 4.12 ± 0.27 | 6.28 ± 0.85 |
| I | 4.34 ± 0.12 | 6.71 ± 0.52 | 4.24 ± 0.13 | 6.88 ± 0.92 | |
| N | 4.92 ± 0.69 | 3.75 ± 0.15 | 6.71 ± 0.75 | ||
| T | 5.08 ± 0.27 | 3.80 ± 0.22 | 6.74 ± 0.84 | ||
Another evident effect of the UV-CXL treatment is the reduction of the central corneal thickness (CCT) due to the presence of 20% dextran in the solution applied to the cornea as demonstrated in other studies [22,23]. This effect takes place in the whole extent of the cornea where the solution was applied, and it was independent of the corneal location where the UV light was projected. Figure 4(b) shows a reduction of CCT in the range of 40 µm to 90 µm when the UV-CXL treatment is applied. Finally, the average shear modulus is shown in Fig. 4(c) for the cases: UT-left cornea (all rabbits and meridians, a total n = 16 meridians), HT-left inferior corneal meridian (rabbits #2, #3, and #4, and only the inferior meridian, a total n = 3 meridians), HT-left superior corneal meridian (rabbits #2, #3, and #4, and only the superior meridian, a total n = 3 meridians), UT-right cornea (all rabbits and meridians, a total n = 16 meridians), and FT-right cornea (rabbits #2, #3, and #4, and all meridians, a total n = 12 meridians). Finally, IOP values before and after UV-CXL are reported in Table 2. There was not a significant increase in IOP in corneas after HT (t-test, P = 0.101) and FT (P = 0.374) cases when measured within 30 minutes after the UV-CXL treatment application. The maximum IOP change detected from all corneal cases was found to be 2 mmHg.
Table 2. Intraocular pressure measurements of the cornea before and after the application of UV-CXL in the HT and FT corneal cases measured with a handheld rebound tonometer.
| Intraocular pressure (mmHg) | ||||
|---|---|---|---|---|
| Left cornea | Right cornea | |||
| Rabbit | UT | HT | UT | FT |
| 1 | 12 | 11 | ||
| 2 | 11 | 12 | 11 | 10 |
| 3 | 11 | 12 | 10 | 11 |
| 4 | 10 | 11 | 9 | 11 |
The shear modulus was calculated by incorporating the CCT and Lamb wave speed values in the mRLFE model [20,21] for each corneal case using the following parameters: excitation frequency 2 kHz, density of aqueous humor ∼1000 kg/m3, density of the cornea ∼1000 kg/m3, speed of sound in aqueous humor ∼1500 m/s, and speed of sound in the cornea ∼1540 m/s. There was a significant increase in the shear modulus in the inferior meridian of the left corneas (from 223.8 kPa to 1.259 MPa, P < 0.001, >450% increase) after half-treated UV-CXL was applied to this region, as shown in Fig. 4(c). Similarly, for the right corneas, there was a significant increase in shear modulus (from 246.4 kPa to 1.627 MPa, P < 0.001, >650% increase) in all meridians after full UV-CXL treatment is applied.
4. Discussion
In this work, we have demonstrated the capability of the confocal air-coupled ultrasonic optical coherence elastography (ACUS-OCE) technique for detection of stiffness changes in rabbit corneas in vivo before and after traditional UV-CXL and localized regions of stiffening from non-uniform UV-CXL. The importance of generating Lamb wave speed maps, as shown in Fig. 3, enables the possibility of doctors monitoring UV-CXL corneal treatments with various spatial distributions. Previous work, related to wave-based elastography of in vivo corneas [17,24] only reported average speed values of a single corneal region, preventing the characterization of non-uniform treatment cases. Moreover, existing medical equipment such as the ORA and Corvis ST only provide a single biomechanical representative value for the entire cornea. Therefore, ACUS-OCE possesses a great potential for detecting spatial-dependent mechanical properties of the cornea at multiple meridians, which has various clinically relevant applications including (1) the detection of softer corneal regions (i.e., cone location) of keratoconus including the development of biomarkers to detect pre-clinical stages of this disease; (2) the measurement of the spatio-temporal progression of other corneal diseases such as iatrogenic ectasia; (3) the possibility of monitoring the local and global biomechanical impact in the cornea after treatments such as refractive surgery [25], intra-stromal ring segments [26], and UV-CXL [6]; and (4) the development of patient-specific biomechanical models of the cornea for treatment planning [27].
In this experiment, the acoustic pressure amplitude at the transducer focus was measured to be ∼4 kPa (peak acoustic pressure), providing a spatial peak pulse average intensity, ISPPA, of ∼2 W/cm2 in air. Since the acoustic impedance mismatch between air and the cornea is high, almost all the acoustic intensity is reflected back (99.95%), leaving a ISPPA ∼1 × 10−3 W/cm2 in cornea (four orders or magnitude lower than the ophthalmic safety limit of 28 W/cm2 [28]). Moreover, given that the repeated excitation duty cycle is ∼25%, the spatial peak time average intensity, ISPTA, is ∼0.24 mW/cm2 which is much smaller than the ophthalmic safety limit of 17 mW/cm2 [28]. Therefore, the proposed ACUS excitation should not impose any safety concerns to ocular tissues.
In this experiment, wave propagation measured at four corneal meridians was obtained in 2.5 s. Even though this acquisition time is within the range of commercial ophthalmic devices and adequate for in vivo eye measurements, axial motion artifact produced by ocular movement and respiration can still be present. This problem can be tackled by the integration of the proposed ACUS-OCE setup with a fast pupil camera (to estimate the eye motion for further compensation), the projection of a fixation target to the patient (to reduce eye motion and improve eye alignment), and the use of signal post-processing approaches. The axial compensation approach presented here effectively eliminated most of these artifacts; however, it does not consider lateral and rotational eye motion. Finally, the presented ACUS-OCE technique has great potential for clinical translation since: (1) it does not produce any potential damage, discomfort, corneal dehydration, or pain, (2) it is fully non-contact (i.e. no need of coupling material between the eye and the system), and the excitation device is at least 15 mm away from the cornea, and, (3) it can measure spatial-dependent mechanical properties of the cornea in various meridians.
There are some limitations of this study. First, the ability to compensate the acquired Lamb wave speed results with IOP measurements is not a well-established procedure, and it was not applied in this study. Experimental ex vivo OCE results in porcine eyes demonstrated an increasing tendency of wave speed with the increase of IOP of at least ∼0.2 m/s/mmHg (IOP range of 10 - 20 mmHg, excitation frequency: 800 Hz) [19]. In this study, the maximum IOP change in all rabbit eyeballs before and after UV-CXL treatment was ∼2 mmHg as measured by a rebound tonometer, which may produce a small wave speed variation of ∼0.4 m/s that we consider as not significantly altering the main results of the statistical analysis. In addition, 3D acquisition in M-B-mode would take tens of seconds to minutes. Here, ultra-fast OCE methods could be applied to reduce imaging times [29–31]. Finally, the use of the mRLFE model for converting speed values into shear modulus assumes that the cornea is isotropic tissue. Nevertheless, recent studies demonstrated the anisotropy of the cornea [32] and its effects on Lamb wave propagation. Moreover, only a single frequency was utilized, and therefore, full assessment of corneal viscoelasticity was restricted. Future work may include more sophisticated wave propagation models that account for anisotropy and multi-frequency or impulse-based excitation for viscoelasticity quantification.
5. Conclusions
In this work, we have demonstrated the capability of wave-based OCE using a confocal air-coupled ultrasonic transducer for the localized detection of non-exposed and exposed regions to UV-CXL in the same cornea in vivo (significant increase of the shear modulus to >450%). Moreover, we compared these results with the typical full-treated UV-CXL cornea case observing an increase of >650% in shear modulus. This technology possesses great potential in detecting spatial-dependent mechanical properties of the cornea at multiple meridians and generating elastography maps that are clinically relevant for the treatment planning and monitoring of the UV-CXL procedures.
Acknowledgements
These studies were support by NIH grant R01EY022362 and P30EY007551.
Funding
National Institutes of Health10.13039/100000002 (P30EY07551, R01EY022362).
Disclosures
The authors declare no conflicts of interest.
Data availability
Data underlying the results presented in this paper are not publicly available at this time but may be obtained from the authors upon reasonable request.
References
- 1.Kirby M. A., Pelivanov I., Song S., Ambrozinski L., Yoon S. J., Gao L., Li D., Shen T. T., Wang R. K., O’Donnell M., “Optical coherence elastography in ophthalmology,” J. Biomed. Opt. 22(12), 1–28 (2017). 10.1117/1.JBO.22.12.121720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shajari M., Steinwender G., Herrmann K., Kubiak K. B., Pavlovic I., Plawetzki E., Schmack I., Kohnen T., “Evaluation of keratoconus progression,” Br. J. Ophthalmol. 103(4), 551–557 (2019). 10.1136/bjophthalmol-2017-311651 [DOI] [PubMed] [Google Scholar]
- 3.Gain P., Jullienne R., He Z., Aldossary M., Acquart S., Cognasse F., Thuret G., “Global survey of corneal transplantation and eye banking,” JAMA Ophthalmol 134(2), 167–173 (2016). 10.1001/jamaophthalmol.2015.4776 [DOI] [PubMed] [Google Scholar]
- 4.Wollensak G., Spoerl E., Seiler T., “Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus,” Am. J. Ophthalmol. 135(5), 620–627 (2003). 10.1016/S0002-9394(02)02220-1 [DOI] [PubMed] [Google Scholar]
- 5.Wollensak G., Spoerl E., Seiler T., “Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-a-induced cross-linking,” J. Cataract Refractive Surg. 29(9), 1780–1785 (2003). 10.1016/S0886-3350(03)00407-3 [DOI] [PubMed] [Google Scholar]
- 6.Seiler T. G., Fischinger I., Koller T., Zapp D., Frueh B. E., Seiler T., “Customized corneal cross-linking: one-year results,” Am. J. Ophthalmol. 166, 14–21 (2016). 10.1016/j.ajo.2016.02.029 [DOI] [PubMed] [Google Scholar]
- 7.Bak-Nielsen S., Pedersen I. B., Ivarsen A., Hjortdal J., “Dynamic scheimpflug-based assessment of keratoconus and the effects of corneal cross-linking,” J. Refract. Surg. 30(6), 408–414 (2014). 10.3928/1081597X-20140513-02 [DOI] [PubMed] [Google Scholar]
- 8.Luce D. A., “Determining in vivo biomechanical properties of the cornea with an ocular response analyzer,” J. Cataract. Refract. Surg. 31(1), 156–162 (2005). 10.1016/j.jcrs.2004.10.044 [DOI] [PubMed] [Google Scholar]
- 9.Schmitt J., “OCT elastography: Imaging microscopic deformation and strain of tissue,” Opt. Express 3(6), 199–211 (1998). 10.1364/OE.3.000199 [DOI] [PubMed] [Google Scholar]
- 10.Huang D., Swanson E. A., Lin C. P., Schuman J. S., Stinson W. G., Chang W., Hee M. R., Flotte T., Gregory K., Puliafito C. A., “Optical coherence tomography,” Science 254(5035), 1178–1181 (1991). 10.1126/science.1957169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kennedy B. F., Wijesinghe P., Sampson D. D., “The emergence of optical elastography in biomedicine,” Nat. Photonics 11(4), 215–221 (2017). 10.1038/nphoton.2017.6 [DOI] [Google Scholar]
- 12.Larin K. V., Sampson D. D., “Optical coherence elastography - OCT at work in tissue biomechanics [invited],” Biomed. Opt. Express 8(2), 1172–1202 (2017). 10.1364/BOE.8.001172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zvietcovich F., Larin K. V., “Wave-based optical coherence elastography: The 10-year perspective,” Prog Biomed Eng (2021). [DOI] [PMC free article] [PubMed]
- 14.Singh M., Li J., Han Z., Vantipalli S., Liu C. H., Wu C., Raghunathan R., Aglyamov S. R., Twa M. D., Larin K. V., “Evaluating the effects of riboflavin/uv-a and rose-bengal/green light cross-linking of the rabbit cornea by noncontact optical coherence elastography,” Invest. Ophthalmol. Visual Sci. 57(9), OCT112 (2016). 10.1167/iovs.15-18888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang H., Qian X., Chen R., Wodnicki R., Sun Y., Li R., Li Y., Shung K. K., Chen Z., Zhou Q., “2-d ultrasonic array-based optical coherence elastography,” IEEE Trans Ultrason Ferroelectr Freq Control 68(4), 1096–1104 (2021). 10.1109/TUFFC.2020.3033304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zvietcovich F., Nair A., Ambekar Y. S., Singh M., Aglyamov S. R., Twa M. D., Larin K. V., “Confocal air-coupled ultrasonic optical coherence elastography probe for quantitative biomechanics,” Opt. Lett. 45(23), 6567–6570 (2020). 10.1364/OL.410593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Y., Wang Y., Shen M., Jin Z., Chen Y., Zhou Y., Qu J., Zhu D., “In vivo evaluation of corneal biomechanical properties by optical coherence elastography at different cross-linking irradiances,” J. Biomed. Opt. 24(10), 1–7 (2019). 10.1117/1.JBO.24.10.105001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang S., Larin K. V., “Shear wave imaging optical coherence tomography (SWI-OCT) for ocular tissue biomechanics,” Opt. Lett. 39(1), 41–44 (2014). 10.1364/OL.39.000041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zvietcovich F., Nair A., Singh M., Aglyamov S. R., Twa M. D., Larin K. V., “Dynamic optical coherence elastography of the anterior eye: Understanding the biomechanics of the limbus,” Invest. Ophthalmol. Visual Sci. 61(13), 7 (2020). 10.1167/iovs.61.13.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han Z., Li J., Singh M., Wu C., Liu C. H., Raghunathan R., Aglyamov S. R., Vantipalli S., Twa M. D., Larin K. V., “Optical coherence elastography assessment of corneal viscoelasticity with a modified Rayleigh-Lamb wave model,” J Mech Behav Biomed Mater 66, 87–94 (2017). 10.1016/j.jmbbm.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han Z., Aglyamov S. R., Li J., Singh M., Wang S., Vantipalli S., Wu C., Liu C.-H., Twa M. D., Larin K. V., “Quantitative assessment of corneal viscoelasticity using optical coherence elastography and a modified Rayleigh-Lamb equation,” J. Biomed. Opt. 20(2), 020501 (2015). 10.1117/1.JBO.20.2.020501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaheer N., Khan W. A., Khan S., Khan M. A. M., “Comparison of changes in central corneal thickness during corneal collagen cross-linking, using isotonic riboflavin solutions with and without dextran, in the treatment of progressive keratoconus,” Cornea 37(3), 340–346 (2018). 10.1097/ICO.0000000000001496 [DOI] [PubMed] [Google Scholar]
- 23.Singh M., Han Z., Li J., Vantipalli S., Aglyamov S. R., Twa M. D., Larin K. V., “Quantifying the effects of hydration on corneal stiffness with noncontact optical coherence elastography,” J. Cataract Refractive Surg. 44(8), 1023–1031 (2018). 10.1016/j.jcrs.2018.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin Z., Khazaeinezhad R., Zhu J., Yu J., Qu Y., He Y., Li Y., Gomez Alvarez-Arenas T. E., Lu F., Chen Z., “In-vivo 3d corneal elasticity using air-coupled ultrasound optical coherence elastography,” Biomed. Opt. Express 10(12), 6272–6285 (2019). 10.1364/BOE.10.006272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shetty R., Francis M., Shroff R., Pahuja N., Khamar P., Girrish M., Nuijts R. M. M. A., Sinha Roy A., “Corneal biomechanical changes and tissue remodeling after smile and lasik,” Invest. Ophthalmol. Visual Sci. 58(13), 5703–5712 (2017). 10.1167/iovs.17-22864 [DOI] [PubMed] [Google Scholar]
- 26.Ariza-Gracia M. Á., Flecha-Lescún J., Büchler P., Calvo B., “Corneal biomechanics after intrastromal ring surgery: optomechanical in silico assessment,” Trans. Vis. Sci. Tech. 9(11), 26 (2020). 10.1167/tvst.9.11.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seven I., Vahdati A., De Stefano V. S., Krueger R. R., Dupps W. J., “Comparison of patient-specific computational modeling predictions and clinical outcomes of lasik for myopia,” Invest. Ophthalmol. Visual Sci. 57(14), 6287–6297 (2016). 10.1167/iovs.16-19948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.F. A. D. Administration , “Guidance for industry and food and drug administration staff,” in Marketing Clearance of Diagnostic Ultrasound Systems and Transducers , U. S. D. o. H. a. H. Services , ed. (Center for Devices and Radiological Health, 2019). [Google Scholar]
- 29.Singh M., Schill A. W., Nair A., Aglyamov S. R., Larina I. V., Larin K. V., “Ultra-fast dynamic line-field optical coherence elastography,” Opt. Lett. 46(19), 4742–4744 (2021). 10.1364/OL.435278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh M., Wu C., Liu C. H., Li J., Schill A., Nair A., Larin K. V., “Phase-sensitive optical coherence elastography at 1.5 million a-lines per second,” Opt. Lett. 40(11), 2588–2591 (2015). 10.1364/OL.40.002588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ambrozinski L., Song S., Yoon S. J., Pelivanov I., Li D., Gao L., Shen T. T., Wang R. K., O’Donnell M., “Acoustic micro-tapping for non-contact 4D imaging of tissue elasticity,” Sci. Rep. 6(1), 38967 (2016). 10.1038/srep38967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pitre J. J., Jr., Kirby M. A., Li D. S., Shen T. T., Wang R. K., O’Donnell M., Pelivanov I., “Nearly-incompressible transverse isotropy (NITI) of cornea elasticity: Model and experiments with acoustic micro-tapping OCE,” Sci. Rep. 10(1), 12983 (2020). 10.1038/s41598-020-69909-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data underlying the results presented in this paper are not publicly available at this time but may be obtained from the authors upon reasonable request.