Abstract
Objective
This study aimed to identify cases of coronavirus disease 2019 (COVID-19) vaccine–associated subacute thyroiditis (SAT) during the active vaccination period of the pandemic, analyze the characteristics of these cases, and compare them with cases of non-vaccine associated SAT diagnosed in the same period.
Methods
A total of 55 patients diagnosed with SAT in our outpatient clinic between February and October, 2021, were included in this retrospective single-center study.
Results
Of the study population, 16 (29.1%) were diagnosed with COVID-19 vaccine-associated SAT (10 with CoronaVac® and six with Pfizer-BioNTech® vaccine), with a median time to onset of symptoms after vaccination of 6.5 (range, 2–20) days. There was no statistically significant difference between the vaccine-associated (VA) and non-vaccine associated (NVA) groups in terms of age, gender, time to diagnosis, thyroid volumes, thyroid function tests, and acute phase reactants. Seven (43.8%) and 25 (64.1%) patients were treated with methylprednisolone in the VA group and NVA group, respectively (p = 0.16). Follow-up data of 45 patients (16/16 for VA and 29/39 for NVA) were available. The mean follow-up of these patients was 47.4 ± 19.4 days, and the follow-up periods of the VA group and NVA group were comparable (p = 0.24). There was no difference between the two groups in terms of the frequency of euthyroidism at the follow-up visit (12/16 vs.14/29, p = 0.08).
Conclusion
With the increase in COVID-19 vaccination rates during the current pandemic, VA SAT cases are seen more frequently. The present study demonstrated that these cases have similar diagnostic features and clinical course to that of classic forms of SAT. In addition, most patients with VA SAT had a mild clinical course that improved with non-steroidal anti-inflammatory drugs.
Keywords: COVID-19, Inactivated COVID-19 vaccine, mRNA vaccine, Thyroiditis, Thyrotoxicosis, Neck pain
Introduction
Subacute thyroiditis (SAT) is a self-limited, inflammatory disease of the thyroid gland that often affects middle-aged women [1, 2]. Symptoms such as fatigue, fever, palpitation, sweating, and weight loss may accompany the typical symptom of anterior neck pain that can radiate up to the jaw/ear [1, 3]. Although the etiology of SAT has not to date been clearly elucidated, viral pathogens are considered important causes, while SAT cases have also been reported after vaccinations against these viral pathogens (such as hepatitis B and influenza) [3–6]. Moreover, some studies suggest that certain types of human leukocyte antigens (HLAs) predispose to the development of SAT [7–9].
During the coronavirus disease 2019 (COVID-19) pandemic, which affected more than 250 million people over 2 years, some cases of SAT have been reported both associated with the disease itself and after vaccinations [10–38]. It is thought that COVID-19 vaccine-associated (VA) SAT occurs as a result of mechanisms that are not fully known (such as adjuvant effect and cross-reactivity resulting from molecular mimicry) in predisposed individuals [13, 14, 39].
The current study primarily aimed to identify cases of COVID-19 VA SAT during the active vaccination period of the pandemic. Secondly, it aimed to analyze the characteristics of these cases and compare them with non-vaccine associated (NVA) cases of SAT diagnosed during the same period.
Materials and methods
Patients and study design
This retrospective, single-center study was conducted in a university-based hospital, namely, University of Health Sciences, Diskapi Yildirim Beyazit Training and Research Hospital, Ankara, Turkey. During the 10 months from February 2021, when the COVID-19 vaccination program was initiated actively in Turkey, to November 2021, patients diagnosed with SAT in our outpatient clinic were screened and 58 patients were identified. Three of these patients were excluded from the study due to lack of COVID-19 vaccination data. Fifty-five patients who met the inclusion criteria were divided into two groups, namely, COVID-19 VA and NVA SAT (Fig. 1). Current and previous symptoms, vaccinations, COVID-19 RT-PCR (real-time reverse transcription-polymerase chain reaction) test history, laboratory tests, and thyroid ultrasonography (US) results of 55 patients were obtained from the hospital record system. A negative RT-PCR test for COVID-19 was required in order for patients to be included in the VA group. It was confirmed that COVID-19 RT-PCR tests performed in the 2 months before diagnosis of SAT were negative in all 16 patients in the VA group. Thirty-one of 39 patients included in the NVA group had COVID-19 RT-PCR data, and all of these patients also had negative tests.
Fig. 1.
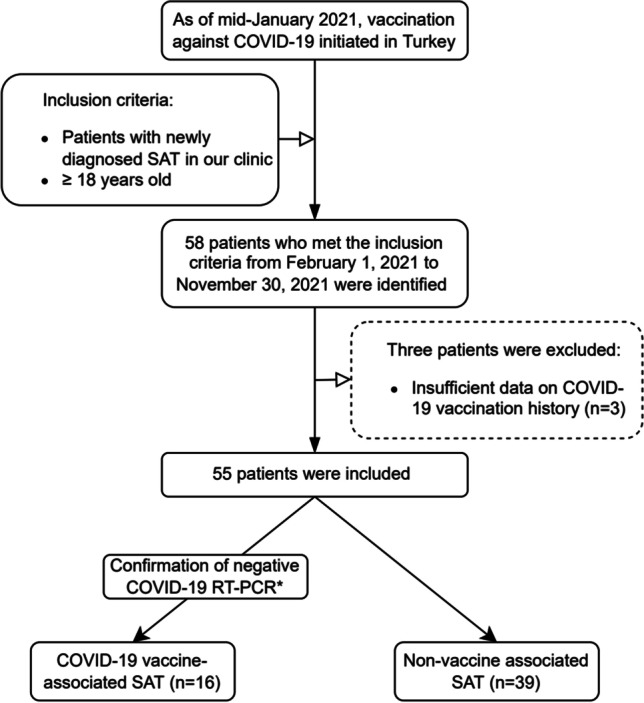
Flow diagram of study participants. COVID-19 coronavirus disease 2019, SAT subacute thyroiditis, RT-PCR real-time reverse transcription-polymerase chain reaction. *During the 2 months before diagnosis of SAT
Diagnosis of SAT and its relationship with vaccination
In the presence of typical clinical and physical examination findings, the diagnosis of SAT was made as recommended in the 2016 American Thyroid Association guidelines, with supportive laboratory and US findings [40]. The diagnosis of SAT was confirmed by the presence of a swollen, tender thyroid gland on physical examination, the detection of patchy hypoechoic areas with decreased vascularization in the painful areas on US examination, and the presence of supportive laboratory findings (elevated acute phase reactants and/or suppressed thyroid-stimulating hormone (TSH)/elevated free thyroxine (fT4) levels). In suspected cases, the diagnosis was confirmed by cytological verification. Pain in the anterior neck region radiating to the jaw/ear was considered the first symptom to determining the time from symptom to diagnosis and the duration of symptom onset after vaccination.
All patients diagnosed with SAT in our outpatient clinic were asked for data on their prior viral infections and COVID-19 vaccination history. The diagnosis of VA SAT was confirmed by the appearance of typical symptoms during the days following vaccination in patients with no history of COVID-19 or of any other viral infection and who did not have a positive RT-PCR test for COVID-19 in the 2 months before diagnosis. Patients who had not been vaccinated at the time of diagnosis or who had been vaccinated at least 2 months before were included in the NVA SAT group.
Biochemical and ultrasonographic parameters
All laboratory tests were performed in the Department of Biochemistry of our Hospital. TSH, fT4, hemogram, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) levels were examined both at the time of diagnosis and at the follow-up visit. Thyroid function was measured by direct chemiluminescence immunoassay. Normal reference ranges were defined as follows: TSH: 0.27–4.20 mIU/L, fT4: 11.97–21.88 pmol/L, ESR: 0–20 mL/h, CRP: 0–5 mg/L, and white blood cells (WBC): 3570–11,010 103/μL. An experienced endocrinologist in our clinic performed all ultrasonographic examinations of the patients at the time of diagnosis. US examinations were performed using a Hitachi® HI VISION Preirus unit (EUB 7000 HV; Hitachi, Tokyo, Japan) with a 13-MHz linear array transducer. The volume of the thyroid gland was calculated using the ellipsoid formula of (length (cm) × width (cm) × thickness (cm) × π/6).
Statistical analysis
Statistical analysis was performed using SPSS software (version 23.0, SPSS, IBM Corporation, NY, USA). Data distribution was assessed with the Shapiro–Wilk test. Data with normal distribution are reported using mean ± standard deviation, while non-normally distributed data are reported using median (min–max). Categorical variables were demonstrated with frequencies and percentages (%) and compared using the χ2 test. Continuous variables with normal distribution were compared using the independent samples t test, and those with non-normally distributed with the Mann–Whitney U test. The statistical significance level was set at p < 0.05.
Results
Characteristics of the study population
A total of 55 patients were included in the final analyses. The mean age of the study population was 46.4 ± 9.9 years. Most of the patients (67.3%) were women. At the time of diagnosis, 39 (70.9%) of the patients had overt hyperthyroidism. Bilateral involvement of the thyroid gland was present in 32 patients (58.2%), and the median thyroid volume was 19.25 (7.49–101.67) cm3. The median time to diagnosis was 29 (8–90) days. Thirty-two patients (58.2%) were treated with methylprednisolone and the rest with non-steroidal anti-inflammatory drugs (NSAIDs). In 16 (29.1%) of 55 patients, SAT diagnosis was found to be associated with COVID-19 vaccination. SAT developed in 10 patients after inactivated COVID-19 vaccine (CoronaVac®, Sinovac Life Sciences, Beijing, China) and in six patients after the COVID-19 messenger RNA (mRNA) (Pfizer-BioNTech®) vaccination. The median duration of symptom onset after vaccination was 6.5 (2–20) days. Baseline characteristics of the study population are depicted in Table 1.
Table 1.
Baseline characteristics of patients (n = 55)
| Variables* | Patients |
|---|---|
| N (%) | 55 (100) |
| Age, mean ± SD, years | 46.4 ± 9.9 |
| Female gender, n (%) | 37 (67.3) |
| Vaccination-associated cases, n (%) | 16 (29.1) |
| Inactivated COVID-19 vaccine-associated | 10 (62.5) |
| mRNA COVID-19 vaccine-associated | 6 (37.5) |
| Post-vaccination symptom onset time, median (range), days | 6.5 (2.0–20.0) |
| Time to diagnosis, median (range), days | 29 (8–90) |
| Thyroid hormone status at diagnosis, n (%) | |
| Hyperthyroid | 39 (70.9) |
| Subclinical hyperthyroid | 6 (10.9) |
| Euthyroid | 10 (18.2) |
| TSH, median (range), mIU/L | 0.02 (0.01–3.92) |
| fT4, median (range), pmol/L | 28.32 (12.10–88.04) |
| WBC, median (range), 103/mm3 | 9.30 (5.10–16.7) |
| ESR, mean ± SD, mm/h | 47.1 ± 18.8 |
| CRP, median (range), mg/L | 49.0 (5.7–224.0) |
| Disease involvement, n (%) | |
| Unilateral | 23 (41.8) |
| Bilateral | 32 (58.2) |
| Thyroid volume at diagnosis, median (range), cm3 | 19.25 (7.49–101.67) |
| Treatment modality, n (%) | |
| NSAIDs | 23 (41.8) |
| Methylprednisolon | 32 (58.2) |
| Follow-up time, mean ± SD, days (n = 45) | 47.4 ± 19.4 |
CRP C-reactive protein; ESR erythrocyte sedimentation rate; fT4 free, thyroxine; NSAIDs non-steroidal anti-inflammatory drugs; TSH thyroid-stimulating hormone; WBC white blood cells; SD standard deviation
*Normally distributed data are reported using mean ± standard deviation, while non-normally distributed data are reported using median (min–max)
Comparison of groups according to vaccine association
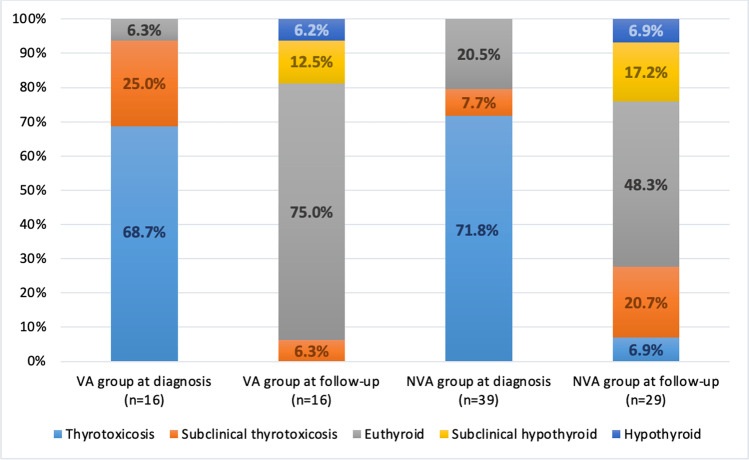
The mean age, gender frequency, and median time to diagnosis were comparable in both the VA group and NVA group (p > 0.05). There was no difference between the two groups in terms of the frequency of overt hyperthyroidism at the time of diagnosis, median TSH, fT4, CRP, and mean ESR levels (p > 0.05). Based on the ultrasonographic parameters at the time of diagnosis, both groups were comparable in terms of frequency of disease involvement and median thyroid volumes (Table 2). Approximately half (43.8%) of the patients in the VA group and 64.1% of the patients in the NVA group were treated with methylprednisolone (p = 0.16). Follow-up data were available for 45 patients (81.8%) (16/16 for VA, 29/39 for NVA). The mean follow-up time of these patients was 47.4 ± 19.4 days. During follow-up, 12 (75.0%) and 14 (48.3%) patients were euthyroid in the VA group and NVA group, respectively (Table 2). Thyroid hormone status at diagnosis and follow-up of these two groups are shown in Fig. 2.
Table 2.
Comparison of patient groups according to vaccine association
| Variables* | Vaccine-associated group (n = 16) | Non-vaccine associated group (n = 39) | p-value# |
|---|---|---|---|
| N (%) | 16 (29.1) | 39 (70.9) | - |
| Age, mean ± SD, years | 49.4 ± 12.0 | 45.2 ± 8.8 | 0.16 |
| Female gender, n (%) | 12 (75.0) | 25 (64.1) | 0.43 |
| Time to diagnosis, median (range), days | 30.5 (18.0–90.0) | 28.0 (8.0–60.0) | 0.51 |
| Overt hyperthyroidism at diagnosis, n (%) | 11 (68.7) | 28 (71.8) | 0.82 |
| The laboratory tests at diagnosis | |||
| TSH, median (range), mIU/L | 0.02 (0.01–1.50) | 0.02 (0.01–3.92) | 0.42 |
| fT4, median (range), pmol/L | 27.41 (12.10–88.04) | 29.86 (12.10–72.98) | 0.69 |
| WBC, median (range), 103/mm3 | 8.55 (5.27–16.70) | 9.65 (5.10–15.72) | 0.33 |
| ESR, mean ± SD, mm/h | 42.4 ± 18.7 | 49.0 ± 18.7 | 0.23 |
| CRP, median (range), mg/L | 25.5 (5.7–224.0) | 59.4 (7.3–190.5) | 0.15 |
| Disease involvement, n (%) | 0.43 | ||
| Unilateral | 8 (50.0) | 15 (38.5) | - |
| Bilateral | 8 (50.0) | 24 (61.5) | - |
| Thyroid volume at diagnosis, median (range), cm3 | 21.41 (7.49–38.05) | 19.08 (8.34–101.67) | 0.85 |
| Treatment modality, n (%)0.16 | |||
| NSAIDs | 9 (56.2) | 14 (35.9) | - |
| Methylprednisolon | 7 (43.8) | 25 (64.1) | - |
| Follow-up time, mean ± SD, days (n = 45) | 42.8 ± 17.0 | 49.9 ± 20.4 | 0.24 |
| Euthyroidism at follow-up, n (%) | 12/16 (75.0) | 14/29 (48.3) | 0.08 |
| The laboratory tests at follow-up | |||
| TSH, median (range), mIU/L | 2.94 (0.04–6.41) | 2.43 (0.01–11.21) | 0.38 |
| fT4, median (range), pmol/L | 12.48 (10.55–20.98) | 13.77 (10.81–29.35) | 0.19 |
CRP C-reactive protein, ESR erythrocyte sedimentation rate, fT4 free thyroxine, NSAIDs non-steroidal anti-inflammatory drugs, TSH thyroid-stimulating hormone, WBC white blood cells, SD standard deviation
*Normally distributed data are reported using mean ± standard deviation, while non-normally distributed data are reported using median (min–max)#In the comparison of continuous variables, p-values were calculated with the independent samples t-test (for normally distributed data) and the Mann–Whitney U test (for non-normally distributed data). In the comparison of categorical variables, p-values were calculated with the χ.2 test
Fig. 2.
Thyroid hormone status of the vaccine-associated (VA) and non-vaccine associated (NVA) subacute thyroiditis (SAT) patient groups at the time of diagnosis (n = 55) and at follow-up (n = 45) is shown in the graph. The frequency of euthyroidism during the follow-up of VA SAT cases appears similar to that of the NVA group. VA vaccine-associated group, NVA non-vaccine associated group
Comparison of groups according to vaccine type
When COVID-19 VA SAT cases were categorized as inactivated COVID-19 vaccine-associated (IVA) and mRNA COVID-19 vaccine-associated (mVA), the two groups were similar in terms of age, gender, time to diagnosis, laboratory and ultrasonographic parameters at the time of diagnosis, and treatments (p > 0.05) (Table 3). Median onset of symptoms after vaccination in the IVA group was 8 (4–20) days, while the median was 5 (2–15) days in the mVA group (p = 0.23). In 10 patients (five of the IVA group and five of the mVA group) (62.5%) with VA SAT, the disease developed after the first dose of vaccine. Three of these patients were vaccinated the second time (two with the inactivated and one with the mRNA COVID-19 vaccine). No clinical or laboratory findings suggesting recurrence were observed in these patients during the median follow-up period of 1 month after the revaccination. The frequency of euthyroidism was similar in the two groups at follow-up (p = 0.60) (Table 3). Detailed data of COVID-19 VA SAT cases are also presented in Table 4.
Table 3.
Comparison of vaccine-associated SAT cases by the vaccine type
| Variables* | Inactivated COVID-19 vaccine–associated group (n = 10) | mRNA COVID-19 vaccine–associated group (n = 6) | p-value# |
|---|---|---|---|
| N (%) | 10 (62.5) | 6 (37.5) | - |
| Age, median (range), years | 47.5 (32.0–71.0) | 44.0 (32.0–67.0) | 0.36 |
| Female gender, n (%) | 7 (70.0) | 5 (83.3) | 0.55 |
| Post-vaccination symptom onset time, median (range), days | 8.0 (4.0–20.0) | 5.0 (2.0–15.0) | 0.23 |
| Time to diagnosis, median (range), days | 32.5 (19.0–80.0) | 29.0 (18.0–90.0) | 0.79 |
| Overt hyperthyroidism at diagnosis, n (%) | 6 (60.0) | 5 (83.3) | 0.59 |
| The laboratory tests at diagnosis | |||
| TSH, median (range), mIU/L | 0.02 (0.01–1.50) | 0.01 (0.01–0.14) | 0.63 |
| fT4, median (range), pmol/L | 29.41 (12.10–88.04) | 26.73 (16.99–53.54) | 0.79 |
| WBC, median (range), 103/ mm3 | 8.55 (5.27–12.09) | 8.90 (5.88–16.70) | 0.87 |
| ESR, median (range), mm/h | 37.5 (19.0–66.0) | 43.5 (23.0–66.0) | 0.96 |
| CRP, median (range), mg/L | 38.5 (5.7–224.0) | 22.3 (13.0–136.0) | 0.91 |
| Disease involvement, n (%)1.00 | |||
| Unilateral | 5 (50.0) | 3 (50.0) | - |
| Bilateral | 5 (50.0) | 3 (50.0) | - |
| Thyroid volume at diagnosis, median (range), cm3 | 20.08 (7.49–33.81) | 27.18 (12.17–38.05) | 0.26 |
| Treatment modality, n (%) | 0.70 | ||
| Ibuprofen | 6 (60.0) | 3 (50.0) | - |
| Methylprednisolon | 4 (40.0) | 3 (50.0) | - |
| Follow-up time, median (range), days | 42.5 (31.0–59.0) | 34.0 (20.0–93.0) | 0.31 |
| Euthyroidism at follow-up, n (%) | 7 (70.0) | 4 (66.0) | 0.60 |
CRP C-reactive protein, ESR erythrocyte sedimentation rate, fT4 free thyroxine, NSAIDs non-steroidal anti-inflammatory drugs, TSH thyroid-stimulating hormone, WBC white blood cells*Non-normally distributed data are reported using median (min–max)#In the comparison of continuous variables, p-values were calculated with the Mann–Whitney U test. In the comparison of categorical variables, p-values were calculated with the χ.2 test
Table 4.
Characteristics of vaccine-associated subacute thyroiditis cases in the present study
| No | Age/Sex | Vaccine type/dose | Post-vaccination sypmtom onset (days) | COVID-19 history* | Time to diagnosis (days) | Thyroid hormone status at diagnosis | Follow-up time (days) | Thyroid hormone status at follow-up | Treatment |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 71/F | CoronaVac®/1st | 4 | No | 20 | O-Hyper | 31 | Euthyroid | MP |
| 2 | 68/M | CoronaVac®/2nd | 10 | No | 36 | O-Hyper | 57 | S-Hypo | Ibuprofen |
| 3 | 44/F | CoronaVac®/1st | 6 | No | 22 | O-Hyper | 42 | Euthyroid | MP |
| 4 | 62/M | CoronaVac®/1st | 6 | Yes | 19 | O-Hyper | 43 | S-Hypo | Ibuprofen |
| 5 | 32/F | Pfizer-BioNTech®/1st | 3 | No | 31 | O-Hyper | 93 | Euthyroid | MP |
| 6 | 39/F | CoronaVac®/2nd | 10 | No | 80 | S-Hyper | 59 | Euthyroid | Ibuprofen |
| 7 | 47/F | CoronaVac®/1st | 12 | No | 35 | O-Hyper | 48 | Euthyroid | MP |
| 8 | 45/F | Pfizer-BioNTech®/1st | 10 | No | 20 | O-Hyper | 49 | Euthyroid | MP |
| 9 | 43/F | Pfizer-BioNTech®/1st | 15 | Yes | 38 | O-Hyper | 35 | Euthyroid | Ibuprofen |
| 10 | 47/F | CoronaVac®/2nd | 20 | Yes | 60 | S-Hyper | 37 | Euthyroid | Ibuprofen |
| 11 | 48/M | CoronaVac®/1st | 6 | No | 30 | S-Hyper | 43 | Euthyroid | Ibuprofen |
| 12 | 67/M | Pfizer-BioNTech®/2nd | 2 | No | 18 | O-Hyper | 20 | S-Hyper | MP |
| 13 | 32/F | CoronaVac®/2nd | 6 | No | 25 | O-Hyper | 36 | Euthyroid | MP |
| 14 | 42/F | Pfizer-BioNTech®/1st | 2 | No | 27 | S-Hyper | 33 | O-Hypo | Ibuprofen |
| 15 | 53/F | CoronaVac®/2nd | 19 | No | 45 | Euthyroid | 35 | Euthyroid | Ibuprofen |
| 16 | 50/F | Pfizer-BioNTech®/1st | 7 | No | 90 | O-Hyper | 24 | Euthyroid | Ibuprofen |
MP methylprednisolone, S-Hyper subclinical hyperthyroidism, S-Hypo subclinical hypothyroidism, O-Hyper overt hyperthyroidism, O-Hypo overt hypothyroidism.*Diagnosis of COVID-19 2 or more months prior to the diagnosis of subacute thyroiditis
Literature review
SAT cases following COVID-19 vaccination published up to February 01, 2022, were reviewed. Fifty-four reported cases of COVID-19 VA SAT were identified [13–37]. More than half (57.4%) of these cases were associated with the COVID-19 mRNA vaccine, while 17 (31.5%) were associated with the inactivated and five (9.3%) with the adenovirus-vectored COVID-19 vaccine. The median age of these patients was 42 (26–82) years, and 41 were female (75.9%). The median age of the women was 41 (26–82) years and of the men 50 (32–75) years. The median symptom duration of these cases was 7 (1–21) days (6 days for inactivated, 7 days for mRNA, and 14 days for the adenovirus-vectored COVID-19 vaccine). Of the reported patients, 31 (57.4%) had been treated with NSAIDs (25 cases) or received no treatment (six cases), while 23 cases (42.6%) had received corticosteroid treatment.
Discussion
The aim of the current study was to investigate the effect of COVID-19 vaccines on the etiology of cases diagnosed with SAT during the active vaccination period in Turkey. Previously, VA SAT was described in case reports as occurring rarely after influenza and hepatitis B vaccines [5, 6]. During the last 2 years, several cases of COVID-19 VA SAT have been reported [13–37]. The first two VA SAT cases in our center were reported in 2021 [38]. The present retrospective study demonstrates that approximately three cases out of 10 among patients diagnosed with SAT in our outpatient clinic during the 10-month study period in 2021 were associated with COVID-19 vaccination. This increase in cases may be attributed to high vaccination rates and the attentional bias of clinicians and patients.
It was observed that there was no difference between the COVID-19 VA SAT group and the NVA SAT group in terms of demographic, laboratory, and ultrasonographic characteristics at the time of diagnosis. Although requirement for corticosteroids was less in VA SAT, there was no difference between the two groups in terms of treatment modality. In addition, although the frequency of euthyroidism tended to be higher in the VA SAT group during a mean follow-up period of 6 weeks, it was not statistically significant (Fig. 2). The present study demonstrated that COVID-19 VA SAT had diagnostic features similar to those of classic SAT and had a mild clinical course that mostly improved with NSAIDs. These results seem to be consistent with the results recorded in the literature. Recently, a study by Oğuz et al. reported that seven (46.7%) of 15 COVID-19 VA SAT cases were treated with corticosteroids, the authors stressing that a relatively lower percentage of these patients required treatment compared to that of patients with classic SAT [36]. In another similar study including six COVID-19 VA SAT cases published by Bahçecioğlu et al., it was stated that corticosteroids were required in a small portion of these cases and that their clinical presentations were milder [37]. Moreover, Ippolito et al. emphasized that SARS-CoV-2 VA SAT is usually of mild/moderate severity and easily treatable in most cases [41]. In our study, it was observed that patients with VA SAT were often symptomatic within the 7 days after vaccination and that the disease generally affected middle-aged women, as in classic SAT cases, these observations being confirmed by the literature review. Most importantly, as demonstrated in the current study, most cases of VA SAT presented mild to moderate symptoms and required relatively less corticosteroid treatment than that required in classic SAT.
The etiopathogenesis of VA SAT has not as yet been clearly elucidated. In the current study, there was no difference in terms of demographic, laboratory-ultrasonographic characteristics, and clinical course between the IVA group and mVA group (Table 3). However, the limited number of vaccine groups diminishes the reliability of these results. It has been reported that aluminum hydroxide, which is used as an adjuvant in the vaccine, might play a role in the development of SAT within the scope of ASIA (autoimmune/inflammatory syndrome induced by adjuvants) syndrome, especially in cases seen after the inactivated COVID-19 vaccine [13, 14]. In addition to the fact that adjuvants increase the immunogenicity of vaccines and enable the use of less viral protein, they can also cause adverse autoimmune/inflammatory syndromes [42, 43]. Recently, it has been shown that lipid nanoparticles (LNPs) in mRNA vaccines also act as adjuvants by causing immunostimulation [44]. Thus, ASIA syndrome may also develop with mRNA COVID-19 vaccines, even though they do not contain any known adjuvant. On the other hand, the behavior of the COVID-19 virus per se may play a role in the etiopathogenesis of VA SAT. Recent studies showed that the mRNA of angiotensin-converting enzyme 2 (ACE2) was expressed in thyroid follicular cells. Thus, it was hypothesized that direct entry of the pathogen into the cell may be effective in COVID-19-related SAT cases [12, 45, 46]. In addition, cross-reactivity, which occurs as a result of the similarity of virus spike proteins encoded in all COVID-19 vaccines, especially mRNA vaccines, with mammalian hexa and heptapeptides (e.g., TPO proteins), seems to be another effective mechanism [39, 47]. Besides, the fact that these mechanisms do not occur in every individual but only in some people indicates that genetic predisposition may also be important in the development of SAT [8]. The SAT cases associated with the COVID-19 vaccine reported in two sisters by Chatzi et al. further highlighted the importance of this relationship [16].
It has recently been reported that SAT is a disease that is often diagnosed with a delay due to lack of clinician awareness [48]. The current study likewise showed that patients whether with VA SAT or with NVA SAT were not diagnosed until 1 month had passed, this tardiness possibly seriously affecting the functional capacity and quality of life of patients. Clinicians thus need to increase their awareness of the need for prompt SAT diagnosis to avoid recurrent patient admissions and unnecessary antibiotic prescriptions. On the other hand, the reported cases of COVID-19 VA SAT should not discourage clinicians and patients from vaccinating against COVID-19. The current study shows that these cases are indistinguishable from classic SAT cases. Certainly, epidemiological studies with larger sample size and a better understanding of the effects of COVID-19 viral particles on thyroid cells are required to establish whether the COVID-19 vaccine increases SAT cases. Moreover, the second dose of vaccine was recommended for each patient who developed SAT after the first dose of vaccination, following the regression of signs and normalization of thyroid function tests. Recurrence of the disease was not experienced during the follow-up of the three patients who received the second dose of vaccine after the diagnosis of SAT. Likewise, Oğuz et al., having examined this issue with nine COVID-19 VA SAT patients, stated that revaccination appears to be safe in these patients [36]. The authors reported that symptoms that started after the first vaccination in two of the nine patients intensified after revaccination. It is noteworthy that while these two cases were revaccinated, one was still receiving NSAID and the other had neck discomfort [36]. Hence, based on our observation and the previous study, we believe that the second dose of the vaccine should be encouraged under appropriate conditions.
The most important limitations of the present study are its retrospective design and limited sample size. In addition, the lack of long-term follow-up of the patients is another limitation of this study.
Conclusion
With increasing rates of vaccination against COVID-19, VA SAT has become a more frequently encountered entity. Therefore, SAT should be considered in patients presenting with anterior neck pain following vaccination. The current study demonstrates that cases associated with the COVID-19 vaccine have similar diagnostic features and clinical course to those of classic SAT cases. However, in most cases of VA SAT, the relatively higher incidence of euthyroidism in the short-term treatment period and the less likely requirement of corticosteroids suggest that this novel entity could probably constitute the milder form of SAT. Nevertheless, further multicentric, prospective studies with large sample sizes are needed to better understand the dynamics of this entity.
Author contribution
H. B., S. K., and M. C. designed the article. H. B., S. K., M. C., S. H., and U. G. collected and interpreted the data. H. B. wrote the article. S. H., I. U. O., M. K., M. E. S., E. C., and B. U. reviewed the manuscript critically. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work.
Declarations
Ethics approval
The present study was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. Approval for the current study was granted by the Ethics Committee of the University of Health Sciences, Diskapi Yildirim Beyazit Training and Research Hospital, Ankara, Turkey (approval number: 123/07).
Informed consent
Written informed consent was obtained from the patients before the study.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Samuels MH. Subacute, silent, and postpartum thyroiditis. Med Clin North Am. 2012;96:223–233. doi: 10.1016/j.mcna.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Nishihara E, Ohye H, Amino N, Takata K, Arishima T, Kudo T, et al. Clinical characteristics of 852 patients with subacute thyroiditis before treatment. Intern Med. 2008;47:725–729. doi: 10.2169/internalmedicine.47.0740. [DOI] [PubMed] [Google Scholar]
- 3.Alfadda AA, Sallam RM, Elawad GE, Aldhukair H, Alyahya M. Subacute thyroiditis: clinical presentation and long term outcome. Int J Endocrinol. 2014;2014:794943. doi: 10.1155/2014/794943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desailloud R, Hober D. Viruses and thyroiditis: an update. Virol J. 2009;6:5. doi: 10.1186/1743-422X-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toft J, Larsen S, Toft H. Subacute thyroiditis after hepatitis B vaccination. Endocr J. 1998;45:135. [PubMed] [Google Scholar]
- 6.Altay FA, Güz G, Altay M. Subacute thyroiditis following seasonal influenza vaccination. Hum Vaccin Immunother. 2016;12:1033–1034. doi: 10.1080/21645515.2015.1117716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nyulassy S, Hnilica P, Buc M, Guman M, Hirschova V, Stefanovic J. Subacute (de Quervain’s) thyroiditis: association with HLA-Bw35 antigen and abnormalities of the complement system immunoglobulins and other serum proteins. J Clin Endocrinol Metab. 1977;45:270–274. doi: 10.1210/jcem-45-2-270. [DOI] [PubMed] [Google Scholar]
- 8.Stasiak M, Tymoniuk B, Michalak R, Stasiak B, Kowalski ML, Lewinski A. Subacute thyroiditis is associated with HLA-B* 18 01,-DRB1* 01 and-C* 04 01—the significance of the new molecular background. J Clin Med. 2020;9:534. doi: 10.3390/jcm9020534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohsako N, Tamai H, Sudo T, Mukuta T, Tanaka H, Kuma K, et al. Clinical characteristics of subacute thyroiditis classified according to human leukocyte antigen typing. J Clin Endocrinol Metab. 1995;80:3653–3656. doi: 10.1210/jcem.80.12.8530615. [DOI] [PubMed] [Google Scholar]
- 10.Brancatella A, Ricci D, Cappellani D, Viola N, Sgrò D, Santini F, et al. Is subacute thyroiditis an underestimated manifestation of SARS-CoV-2 infection? Insights from a case series. J Clin Endocrinol Metab. 2020;105:e3742–e3746. doi: 10.1210/clinem/dgaa537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christensen J, O’Callaghan K, Sinclair H, Hawke K, Love A, Hajkowicz K, et al. Risk factors, treatment and outcomes of subacute thyroiditis secondary to COVID-19: a systematic review. Intern Med J. 2021 doi: 10.1111/imj.15432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duntas LH, Jonklaas J. COVID-19 and thyroid diseases: a bidirectional impact. J Endocr Soc. 2021;5:bvab076. doi: 10.1210/jendso/bvab076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.İremli BG, Şendur SN, Ünlütürk U. Three cases of subacute thyroiditis following SARS-CoV-2 vaccine: post-vaccination ASIA syndrome. J Clin Endocrinol Metab. 2021;106:2600–2605. doi: 10.1210/clinem/dgab373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das L, Bhadada S, Sood A. Post-COVID-vaccine autoimmune/inflammatory syndrome in response to adjuvants (ASIA syndrome) manifesting as subacute thyroiditis. J Endocrinol Invest. 2022;45:465–467. doi: 10.1007/s40618-021-01681-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saygili ES, Karakilic E. Subacute thyroiditis after inactive SARS-CoV-2 vaccine. BMJ Case Rep. 2021;14:e244711. doi: 10.1136/bcr-2021-244711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chatzi S, Karampela A, Spiliopoulou C, Boutzios G. Subacute thyroiditis after SARS-CoV-2 vaccination a report of two sisters and summary of the literature. Hormones (Athens) 2022;21:177–179. doi: 10.1007/s42000-021-00332-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ŞahinTekin M, Şaylısoy S, Yorulmaz G. Subacute thyroiditis following COVID-19 vaccination in a 67-year-old male patient: a case report. Hum Vaccin Immunother. 2021;17:4090–4092. doi: 10.1080/21645515.2021.1947102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oyibo SO. Subacute thyroiditis after receiving the adenovirus-vectored vaccine for coronavirus disease (COVID-19) Cureus. 2021;13:e16045. doi: 10.7759/cureus.16045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bornemann C, Woyk K, Bouter C. Case report: two cases of subacute thyroiditis following SARS-CoV-2 vaccination. Front Med (Lausanne) 2021;8:737142. doi: 10.3389/fmed.2021.737142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel KR, Cunnane ME, Deschler DG. SARS-CoV-2 vaccine-induced subacute thyroiditis. Am J Otolaryngol. 2021;43:103211. doi: 10.1016/j.amjoto.2021.103211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soltanpoor P, Norouzi G. Subacute thyroiditis following COVID-19 vaccination. Clin Case Rep. 2021;9:e04812. doi: 10.1002/ccr3.4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sigstad E, Grøholt KK, Westerheim O. Subacute thyroiditis after vaccination against SARS-CoV-2. Tidsskr Nor Laegeforen. 2021;141:2021–14. doi: 10.4045/tidsskr.21.0554. [DOI] [PubMed] [Google Scholar]
- 23.Kyriacou A, Ioakim S, Syed AA. COVID-19 vaccination and a severe pain in the neck. Eur J Intern Med. 2021;94:95–96. doi: 10.1016/j.ejim.2021.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siolos A, Gartzonika K, Tigas S. Thyroiditis following vaccination against COVID-19 Report of two cases and review of the literature. Metabol Open. 2021;12:100136. doi: 10.1016/j.metop.2021.100136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ratnayake GM, Dworakowska D, Grossman AB. Can COVID-19 immunisation cause subacute thyroiditis? Clin Endocrinol (Oxf) 2021 doi: 10.1111/cen.14555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goindoo R, Vankayalapati P, Mohammadi A. COVID-19 AstraZeneca vaccination induced subacute thyroiditis. Endocr Abstr. 2021;77:CC7. doi: 10.1530/endoabs.77.CC7. [DOI] [Google Scholar]
- 27.Patel M, Shahid M, Khawaja A, Ejike C, Vemuri K (2021) Subacute thyroiditis secondary to Moderna COVID-19 vaccine: a case report of a rare manifestation. Adv Clin Med Res Healthc Deliv 1:9. 10.53785/2769-2779.1019
- 28.Jeeyavudeen MS, Patrick AW, Gibb FW, Dover AR. COVID-19 vaccine-associated subacute thyroiditis: an unusual suspect for de Quervain’s thyroiditis. BMJ Case Rep. 2021;14:e246425. doi: 10.1136/bcr-2021-246425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plaza-Enriquez L, Khatiwada P, Sanchez-Valenzuela M, Sikha A. A case report of subacute thyroiditis following mRNA COVID-19 vaccine. Case Rep Endocrinol. 2021;2021:8952048. doi: 10.1155/2021/8952048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pujol A, Gómez LA, Gallegos C, Nicolau J, Sanchís P, González-Freire M, et al. Thyroid as a target of adjuvant autoimmunity/inflammatory syndrome due to mRNA-based SARS-CoV2 vaccination: from Graves’ disease to silent thyroiditis. J Endocrinol Invest. 2022;45:875–882. doi: 10.1007/s40618-021-01707-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sözen M, Topaloğlu Ö, Çetinarslan B, Selek A, Cantürk Z, Gezer E, et al. COVID-19 mRNA vaccine may trigger subacute thyroiditis. Hum Vaccin Immunother. 2021;17:5120–5125. doi: 10.1080/21645515.2021.2013083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan F, Brassill MJ. Subacute thyroiditis post-Pfizer-BioNTech mRNA vaccination for COVID-19. Endocrinol Diabetes Metab Case Rep. 2021;2021:21–0142. doi: 10.1530/EDM-21-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pandya M, Thota G, Wang X, Luo H. Thyroiditis after coronavirus disease 2019 (COVID-19) mRNA vaccine a case series. AACE Clin Case Rep. 2021 doi: 10.1016/j.aace.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vasileiou V, Paschou SA, Tzamali X, Mitropoulou M, Kanouta F, Psaltopoulou T, et al. Recurring subacute thyroiditis after SARS-CoV-2 mRNA vaccine: a case report. Case Rep Womens Health. 2022;33:e00378. doi: 10.1016/j.crwh.2021.e00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.PlaPeris B, Merchante Alfaro AÁ, MaravallRoyo FJ, AbellánGaliana P, Pérez Naranjo S, González Boillos M. Thyrotoxicosis following SARS-COV-2 vaccination: a case series and discussion. J Endocrinol Invest. 2022 doi: 10.1007/s40618-022-01739-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oğuz SH, Şendur SN, İremli BG, Gürlek A, Erbas T, Ünlütürk U. SARS-CoV-2 vaccine-induced thyroiditis: safety of re-vaccinations and clinical follow-up. J Clin Endocrinol Metab dgac049. 2022 doi: 10.1210/clinem/dgac049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bahçecioğlu AB, Karahan ZC, Aydoğan BI, Kalkan IA, Azap A, Erdoğan MF. Subacute thyroiditis during the COVID-19 pandemic: a prospective study. J Endocrinol Invest. 2022;45:865–874. doi: 10.1007/s40618-021-01718-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bostan H, Unsal IO, Kizilgul M, Gul U, Sencar ME, Ucan B, et al. Two cases of subacute thyroiditis after different types of SARS-CoV-2 vaccination. Arch Endocrinol Metab. 2022;66:97–103. doi: 10.20945/2359-3997000000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanduc D, Shoenfeld Y. Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes: implications for the vaccine. Immunol Res. 2020;68:310–313. doi: 10.1007/s12026-020-09152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, et al. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26:1343–1421. doi: 10.1089/thy.2016.0229. [DOI] [PubMed] [Google Scholar]
- 41.Ippolito S, Gallo D, Rossini A, Patera B, Lanzo N, Fazzino GFM, et al. SARS-CoV-2 vaccine-associated subacute thyroiditis insights from a systematic review. J Endocrinol Invest. 2022 doi: 10.1007/s40618-022-01747-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shoenfeld Y, Agmon-Levin N. ‘ASIA’–autoimmune/inflammatory syndrome induced by adjuvants. J Autoimmun. 2011;36:4–8. doi: 10.1016/j.jaut.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Watad A, David P, Brown S, Shoenfeld Y. Autoimmune/inflammatory syndrome induced by adjuvants and thyroid autoimmunity. Front Endocrinol (Lausanne) 2017;7:150. doi: 10.3389/fendo.2016.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alameh MG, Tombácz I, Bettini E, Lederer K, Sittplangkoon C, Wilmore JR, et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity. 2021;54:2877–2892.e7. doi: 10.1016/j.immuni.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rotondi M, Coperchini F, Ricci G, Denegri M, Croce L, Ngnitejeu S, et al. Detection of SARS-COV-2 receptor ACE-2 mRNA in thyroid cells: a clue for COVID-19-related subacute thyroiditis. J Endocrinol Invest. 2021;44:1085–1090. doi: 10.1007/s40618-020-01436-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coperchini F, Ricci G, Croce L, Denegri M, Ruggiero R, Villani L, et al. Modulation of ACE-2 mRNA by inflammatory cytokines in human thyroid cells: a pilot study. Endocrine. 2021;74:638–645. doi: 10.1007/s12020-021-02807-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vojdani A, Vojdani E, Kharrazian D. Reaction of human monoclonal antibodies to SARS-CoV-2 proteins with tissue antigens: implications for autoimmune diseases. Front Immunol. 2021;11:617089. doi: 10.3389/fimmu.2020.617089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bostan H, Sencar ME, Calapkulu M, Hepsen S, Duger H, Unsal IO, et al. Two important issues in subacute thyroiditis management: delayed diagnosis and inappropriate use of antibiotics. Eur Thyroid J. 2021;10:323–329. doi: 10.1159/000513745. [DOI] [PMC free article] [PubMed] [Google Scholar]