Abstract
Background and objectives
Many mutations in variants for instance Delta and Alpha are associated with immune evasion and higher infectious potential. There are uncertainties regarding Omicron. In this regard, we aimed to compare the frequency of reinfection of SARS CoV-2 variants in our hospital between April 22, 2021 and January 26, 2022.
Method
The reinfection rates and demographic characteristics of a total of 27,487 COVID-19 patients infected with different SARS CoV-2 variants were examined.
Results
Reinfection was found in 26 (0.46%) of 5554 Alpha, 209 (1.16%) of 17,941 Delta, and 520 (13.0%) of 3992 Omicron variants. A statistically significant difference was observed between the reinfection rates of the variants (p = 0.000). The mean reinfection days were calculated as 204.4 ± 51.1 in the Alpha variant, 291.2 ± 58.2 in the Delta variant, and 361.2 ± 131.6 in the Omicron variant (p = 0.000). It was observed that 16.5% of reinfection cases caught COVID-19 for the second time 3–6 months after the first COVID-19 infection, 36.7% after 6–12 months, and 46.8% after more than 12 months. There was a significant difference between the times in reinfection cases. Most reinfections occurred more than 12 months apart. Among those with a reinfection time > 12 months, 0% had Alpha, 3.4% had Delta, and 96.6% had Omicron variants.
Conclusion
The highest reinfection rate was observed in the Omicron variant. Reinfection was approximately 30 times more frequent in the Omicron variant than in the Alpha variant and 10 times more frequent in the Delta variant.
Keywords: Alpha, Delta, Omicron, Reinfection, Variant
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been continuously mutating since the COVID-19 pandemic began in December 2019, with the emergence of many variants around the world [1]. The World Health Organization (WHO) has classified these variants into three categories to prioritize their monitoring and research: variants of concern (VOCs), variants of interest (VOIs), and variants under monitoring (VUMs) [2]. Five VOCs have been identified so far, namely Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2), and Omicron (B.1.1.529) variants. South Africa was the first to report the Omicron variant to the WHO on November 24, 2021. The day after the report was received, WHO classified it as VUM and named it the Omicron variant (B.1.1.529). Just 2 days later, WHO classified the Omicron variant as VOC faster than other variants. This situation has caused great concern in the public [2]. One of the first VOCs, Alpha (B.1.1.7), was first detected in the UK in November 2020. The Delta variant was first detected in the UK in March 2021 and designated VOC on May 6 2021 [3]. All of the variants have caused a new wave of pandemics and thousands of deaths in multiple countries and regions, and even around the world [4].
Many mutations in variants, for instance, Delta and Alpha are associated with immune evasion and higher infectious potential [5]. However, there are uncertainties about Omicron in this regard. The Omicron variant’s high number of mutations in the S protein may help the virus evade infection-blocking antibodies and other immune responses like the T-cell response [6]. Initial studies revealed that the variant has more than 30 mutations in the virus domain encoding the spike protein responsible for virus entry into human cells [7]. Compared to the mutations observed in the other four VOC variants, the number of spike mutations identified in Omicron is approximately 3–4 times higher [2]. The presence of the mutation group (H655Y, N679K, and P681H) increase the virus’s ability to enter the cell and the R203K and G204R mutations that increase the infection rates in the Omicron may cause a higher risk of transmission [7, 8]. Potentially, this new variant may be associated with high contagiousness, leading to increased reinfection rates [9]. The risk of reinfection with the Omicron variant appears to be higher than with other variants, according to preliminary evidence [5].
Today, an increase in reinfection cases is observed. Therefore, in this study, we aimed to compare the frequency of reinfection of Alpha, Delta, and Omicron variants detected in the microbiology laboratory of Siirt Training and Research Hospital between April 22, 2021, and January 26, 2022.
Material and methods
In our study, reinfection rates according to variant types of 27,487 COVID-19 patients infected with SARS CoV-2 Alpha, Delta, and Omicron variants between April 22, 2021, and January 26, 2022, and demographic characteristics of patients with reinfection were retrospectively examined. Patients who tested positive for COVID-19 twice, with an interval of more than ninety days, were considered for reinfection. Reinfections were grouped and compared as those that occurred 3–6 months, 6–12 months, and 12 months after the first infection.
Detection of SARS-CoV-2 in nasopharyngeal and oropharyngeal samples sent to Siirt Training and Research Hospital PCR laboratory for the diagnosis of COVID-19 was performed using the real-time reverse transcriptase-polymerase chain reaction (RT-PCR) method on the CFX 96 real-time PCR device (Biorad, USA). In this study, the Bio-Speedy SARS CoV-2 + VOC202012/01 RT-qPCR kit (Bioeksen, Istanbul, Turkey), was first obtained on April 22, 2021, and can detect the Alpha (B.1.1.7) variant, was used. As of this date, it was seen that the alpha variant was dominant. Later, with the spread of Beta (B.1.351), Gamma (P.1), and Delta (B.1.617.2) variants all over the world, as of July 1, 2021, the Bio-Speedy SARS CoV-2 Emerging Plus detection kit (Bioeksen, Istanbul, Turkey) was used to detect Alpha, Beta, Gamma, and Delta variants. As of this date, it was seen that the Delta variant was dominant in Siirt. Since January 1, 2022, only the Bio-Speedy SARS CoV-2 + Omicron RT-qPCR kit (Bioeksen, Istanbul, Turkey) has been used for variant analysis in our hospital, and the Omicron variant has become dominant in this period. Threshold cycle numbers (Cq) less than 33 in the FAM channel were considered positive. Detection of variants was made in accordance with the kit instructions.
In the study, data analysis was performed using the Statistical Package for the Social Sciences (SPSS) 26.0 statistical program. While evaluating the data, descriptive statistics were presented as the number of cases and percentages in categorical variables. In the analysis of continuous variables, normality analyzes were performed with the Shapiro–Wilk goodness-of-fit test. T-test and one-way analysis of variance in independent groups were used for the comparison of the normally distributed variables of the data, Mann–Whitney U test, and Kruskal–Wallis test was used for the comparison of the non-normally distributed variables, and the chi-square test was used for the comparison of the qualitative data. Means were presented with their standard deviations. Statistical significance was evaluated as p < 0.05.
Results
Of the 27,487 COVID-19 patients included in our study, 755 (2.7%) were defined as reinfection. Of the reinfected patients, 351 (46.5%) were male and 404 (53.5%) were female. The mean age of males was 38.9 ± 15.7, and the mean age of females was 36.9 ± 15.5. It was determined that 26 (3.4%) of 755 reinfected patients had the Alpha variant, 209 (27.7%) had the Delta variant, and 520 (68.9%) had the Omicron variant. The difference between the number of reinfected patients of the variants was found to be statistically significant (p = 0.000). The total number of people, mean age, and gender distribution of reinfections in each variant are shown in Table 1.
Table 1.
Number of people, mean age, and gender distribution of reinfections in each variant
| Alpha | Delta | Omicron | Test | P* | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||||
| Number of persons | 26 | (3.4) | 209 | (27.7) | 520 | (68.9) | Chi-square | 0.000 | |
| Mean of age** | 40.6 ± 14.8 | 35.5 | 35.1 ± 15.5 | 30.0 | 38.7 ± 15.6 | 35.0 | Kruskal–Wallis | 0.001 | |
| Gender | Male | 16 | (61.5) | 94 | (45.0) | 241 | (46.5) | Chi-square | 0.278 |
| Female | 10 | (38.5) | 115 | (55.0) | 279 | (53.5) | |||
| Mean of age | Male | 39.0 ± 14.4 | 32.5 | 35.5 ± 16.1 | 31.0 | 40.2 ± 15.5 | 37.0 | Kruskal–Wallis | 0.005 |
| Female | 43.2 ± 16.0 | 45.5 | 34.9 ± 15.1 | 30.0 | 37.5 ± 15.6 | 34.0 | Kruskal–Wallis | 0.067 | |
*p < 0.05 significant relationship and p > 0.05 no significant relationship; **In the measurement data, mean ± standard deviation was calculated instead of n, and median values were calculated instead of %
In the present study, Alpha variant was detected in 5554 (20.2%) of 27,487 COVID-19 patients, Delta variant in 17,941 (65.3%), and Omicron variant in 3992 (14.5%). Reinfection was detected in 26 (0.46%) of 5554 Alpha variants, 209 (1.16%) of 17,941 Delta variants, and 520 (13.0%) of 3992 Omicron variants. When the reinfection rates of the variants were compared, a statistically significant difference was found (p = 0.000) (Table 2).
Table 2.
Reinfection (Rİ) rates of variants
| Alpha | Delta | Omicron |
Test P |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rİ (n) | Total | % | Ri (n) | Total | % | Rİ (n) | Total | % | ||
| Reinfection rates | 26 | 5554 | (0.46) | 209 | 17,941 | (1.16) | 520 | 3992 | (13.0) | Chi-square 0.000 |
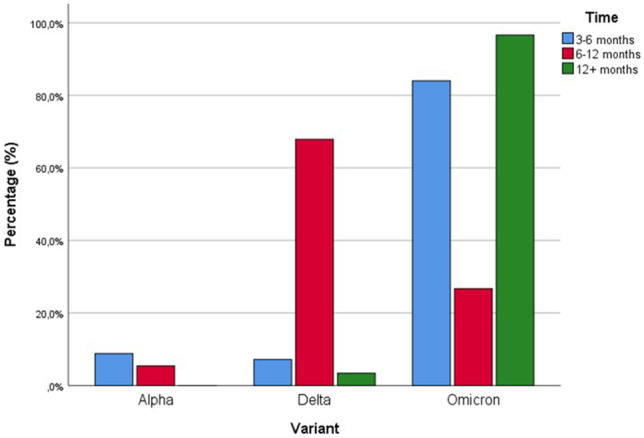
The mean and median reinfection days of 755 reinfection cases in this study were found to be 336.4 ± 120.5 days and 339.0, respectively. The mean reinfection days of the variants were calculated as 204.4 ± 51.1 in the Alpha variant, 291.2 ± 58.2 in the Delta variant, and 361.2 ± 131.6 in the Omicron variant (p = 0.000). The median reinfection days of the variants were found as 192 in the Alpha variant, 291 in the Delta variant, and 418 in the Omicron variant (Table 3). Of the reinfection cases in this study, 125 (16.5%) patients caught COVID-19 for the second time 3–6 months after the first COVID-19 infection, 277 (36.7%) after 6–12 months, and 353 (46.8%) after more than 12 months. A significant difference was found between these times in reinfection cases, and reinfections occurred at most with an interval of more than 12 months (p = 0.001). Among those with a reinfection period of 3–6 months, 11 (8.8%) Alpha variants, 9 (7.2%) Delta variants, and 105 (84.0%) Omicron variants were detected. Of those with a reinfection period of 6–12 months, 15 (5.4%) were Alpha variants, 188 (67.9%) were Delta variants, and 74 (26.7%) were Omicron variants. It was determined that 0 (0%) of those with reinfection duration > 12 months had the Alpha variant, 12 (3.4%) had the Delta variant, and 341 (96.6%) had the Omicron variant. When the reinfection times were compared between the variants, a statistically significant difference was found (p = 0.000) (Table 4, Fig. 1).
Table 3.
The mean and median days values of reinfection cases in the variants
| Genel | Alpha | Delta | Omicron | Test | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean + SD | Median | Mean + SD | Median | Mean + SD | Median | Mean + SD | Median | ||
|
Reenenf Gün Ort |
336.4 ± 120.5 | 339.0 | 204.4 ± 51.1 | 192.0 | 291.2 ± 58.2 | 291.0 | 361.2 ± 131.6 | 418.0 |
Kruskal–Wallis 0.000 |
Table 4.
Comparison of reinfection times between variants
| Variant | 3–6 months | 6–12 months | 12 + months | Test | P | |||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| Alpha | 11 | (8.8) | 15 | (5.4) | 0 | (0) | Chi-square | 0.000 |
| Delta | 9 | (7.2) | 188 | (67.9) | 12 | (3.4) | ||
| Omicron | 105 | (84.0) | 74 | (26.7) | 341 | (96.6) | ||
| Total | 125 | (16.5) | 277 | (36.7) | 353 | (46.8) | Chi-square | 0.001 |
Fig. 1.
Comparison of reinfection times between variants
Discussion
Since the beginning of the COVID-19 pandemic, the problem of potential reinfection has always existed. The number of reinfections globally has probably been greatly underestimated, in part because confirmation of reinfection necessitates viral genome sequencing to identify different SARS-CoV-2 strains in a primary and secondary infection [10]. While viral sequencing is the gold standard for minimizing false positives in identifying cases of reinfection, it is technically challenging and cannot identify cases of reinfection with the same viral strain [10]. The Centers for Disease Control and Prevention (CDC) recently released a guideline protocol for supporting public health laboratories to investigate suspected SARS-CoV-2 reinfections [11]. Specifically, the search criteria include a positive RT-PCR test at least 90 days after the initial test or a positive RT-PCR test at least 45 days after the initial test accompanied by the presence of symptoms and epidemiological exposure [11].
COVID-19 infection history may be as effective as SARS-CoV-2 vaccines in preventing reinfection [12, 13]. However, the duration and extent of SARS-CoV-2-induced immunity’s protective effect have not been adequately investigated. Immunity can last at least 5–6 months after infection, according to two studies conducted in the UK [14, 15]. Data from these studies showed that reinfection with SARS-CoV-2 is rare, occurring in less than 1% of people who had previously tested positive for SARS-CoV-2 [14, 15]. Protection against SARS-CoV-2 reinfection was observed for up to 10 months after initial infection in a systematic review [16]. A study that screened 43,000 people from Qatar with PCR suggested that 95% of individuals who caught COVID-19 had protection against recurrent infection lasting at least 7 months [17]. Another study of 12,541 healthcare workers in the UK reported 89% protection lasting at least 6 months [15]. In a study conducted in Italy, a reinfection rate of 0.31% was found in Lombardy, which was one of the most severely affected regions at the beginning of the pandemic, with an average follow-up interval of 230 days [18]. Perez et al. [19] reported that a total of 149,735 individuals had positive PCR tests between March 2020 and January 2021 in Israel. Of these, 154 people have reported two PCR tests as positive at least 100 days apart, and they included them in their study. They reported the reinfection rate as approximately 0.1%. In our study, the mean reinfection day of 755 reinfection cases was 336.4 ± 120.5. Of the reinfection cases in the current study, 125 (16.5%) were caught COVID-19 for the second time 3–6 months after the first COVID-19 infection, 277 (36.7%) after 6–12 months, and 353 (46.8%) after more than 12 months. A significant difference was found between these periods in reinfection cases. Most reinfections occurred more than 12 months apart (p = 0.001). Of the 27,487 COVID-19 patients included in our study, 755 (2.7%) were defined as reinfection. Since the patients included in our study consisted of only Alpha, Delta, and Omicron variants, the reinfection rate may be higher than in previous studies.
Available data from published reinfection cases, data from immune response studies after initial SARS-CoV-2 infection, and emerging new variants raise the possibility that reinfection may be widespread. In a UK study, of 36,509 people who had a positive PCR test before October 1, 2020, 249 people who reported two positive tests at least 90 days apart, and 7 symptom-free days between the two positive results were considered reinfections. The reinfection rate in the study was found to be 0.7%. In the study, this ratio was seen as a positive sign that vaccination against pre-existing variants or innate immunity may also be effective against the Alpha variant [20]. In a study that considered cases with laboratory-confirmed SARS-CoV-2 infection by positive nucleic acid amplification test (NAAT) and a positive NAAT result documented at least 90 days after the first positive test as reinfection, the median time to reinfection was found as 39.2 weeks. In the study, they reported that the percentage of SARS-CoV-2 reinfections among all NAAT-positive COVID-19 cases increased from 0.5% in the pre-Delta surge period to 1.8% during the fluctuation [21]. In another study that used genome sequencing to identify VOCs, 6 (1.2%) and 9 (1.8%) patients with Delta and Alpha variants were reported to have had previous natural exposure to SARS-CoV-2. In the study, the median (minimum–maximum) reinfection times were found to be 207 (115–529) days and 127 (9–453) days, respectively [22]. In our study, 5554 (20.2%) of 27,487 COVID-19 patients had Alpha variant, 17,941 (65.3%) Delta, and 3992 (14.5%) Omicron variant. We defined 26 (0.46%) of 5554 Alpha variants, 209 (1.16%) of 17,941 Delta variants, and 520 (13.0%) of 3992 Omicron variants as reinfections. The mean reinfection days were 204.4 ± 51.1 days in the Alpha variant, 291.2 ± 58.2 days in the Delta variant, and 361.2 ± 131.6 days in the Omicron variant. The median reinfection days of the variants were found as 192 in the Alpha variant, 291 in the Delta variant, and 418 in the Omicron variant. In a recent study conducted in Qatar by Altarawneh et al. [23], the median interval between previous infection and PCR testing among cases and controls were reported as 279, 254, and 314 days for analyzing the Alpha, Delta, and Omicron variants, respectively.
When the reinfection rates of the variants and the mean of reinfection days were compared, a statistically significant difference was found (p = 0.000). As can be seen, the variant with the highest reinfection rate in our study is the currently dominant Omicron variant (13.0%). Of those with a reinfection period of 3–6 months, 11 (8.8%) had Alpha variant, 9 (7.2%) Delta variant, and 105 (84.0%) Omicron variant. Of those with a reinfection period of 6–12 months, 15 (5.4%) had Alpha variant, 188 (67.9%) Delta variant, and 74 (26.7%) Omicron variant. Alpha variant was detected in 0 (0%) of those with reinfection duration > 12 months, Delta variant in 12 (3.4%), and Omicron variant in 341 (96.6%). When the reinfection times were compared between the variants, a statistically significant difference was found (p = 0.000). As can be seen, in our study, approximately half of the reinfection cases (46.8%) were reinfected with an interval of more than 12 months, and almost all (96.6%) of the reinfected patients in this period were infected with the Omicron variant. In addition, it is noteworthy that most of the patients (84.0%) with a reinfection period of 3–6 months were infected with the Omicron variant in our study. Available data indicate that the Omicron variant is highly transmissible and spreads several times faster than previous variants [5]. The Omicron variant may evade vaccines or innate immunity that occurred from previous infections and may blunt the potency of neutralizing antibodies, causing existing vaccines to be less effective against Omicron than other variants [24]. Recent research showed that the B.1.1.7 (Alpha), B.1.351 (Beta), and B.1.617.2 (Delta) variants of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) confer strong protection against reinfection [25–27]. Multiple mutations in the B.1.1.529 (Omicron) variant, on the other hand, can mediate immune evasion [23]. The reinfection risk profile of the Omicron variant is much higher than other variants [28]. The findings of our study also support these data.
Our study had some limitations. First, the analysis of variants in our study was done only by RT-PCR and was not confirmed by sequence analysis. Secondly, since our study was retrospective and we did not perform sequence analysis, we may have missed some reinfection cases since the valid time interval for the diagnosis of reinfection is considered to be at least 90 days. Third, there is a large number of patients with asymptomatic and undetected primary infection, which may have affected reinfection rates. Fourth, since the Omicron variant has been detectable in our laboratory for approximately 1 month, the follow-up period and the number of patients in terms of reinfection was relatively shorter compared to the Alpha and Delta variants.
Conclusion
In conclusion, the highest reinfection rate was found in the Omicron variant in our study. Reinfection was observed approximately 30 times more frequently in the Omicron variant than in the Alpha variant and 10 times more frequently than in the Delta variant. The rate of reinfection of the Omicron variant in the 3–6 months and > 12 months after the first infection was higher than the other variants. The observed increase in reinfections with the Omicron variant is likely due to reduced innate and vaccine immunity as well as being a highly contagious variant. These data may be useful in decisions regarding public health measures and vaccination strategies to struggle with the COVID-19 pandemic. Larger sample size studies are needed to fully understand the risk of reinfection of a new variant, the Omicron variant.
Author contribution
All authors contributed to the drafting of the manuscript.
Declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. The study was approved by the Siirt University Non-Interventional Clinical Research Ethics Committee (decision no: 2021/01.09) and the Republic of Turkey Ministry of Health as well.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Choi JY, Smith DM. SARS-CoV-2 variants of concern. Yonsei Med J. 2021;62(11):961. doi: 10.3349/ymj.2021.62.11.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He X, Hong W, Pan X, et al. SARS-CoV-2 Omicron variant: characteristics and prevention. MedComm. 2021;2(4):838. doi: 10.1002/mco2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Twohig KA, Nyberg T, Zaidi A, et al. Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B 1 617 2) compared with alpha (B 1 1 7) variants of concern a cohort study. Lancet Inf Dis. 2022;22(1):35–42. doi: 10.1016/S1473-3099(21)00475-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karim SSA, Karim QA. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. The Lancet. 2021;398(10317):2126–2128. doi: 10.1016/S0140-6736(21)02758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ren S-Y, Wang W-B, Gao R-D, et al. Omicron variant (B 1 1 529) of SARS-CoV-2 mutation infectivity transmission and vaccine resistance. World J Clin Cases. 2022;10:1–5. doi: 10.12998/wjcc.v10.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Shabasy RM, Nayel MA, Taher MM, et al. Three wave changes, new variant strains, and vaccination effect against COVID-19 pandemic. Int J Biol Macromol. 2022;204:161–168. doi: 10.1016/j.ijbiomac.2022.01.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Wang R, Gilby NB, et al. Omicron variant (B. 1.1. 529): infectivity, vaccine breakthrough, and antibody resistance. J Chem Inf Model. 2022;62:412–422. doi: 10.1021/acs.jcim.1c01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Explained: what we know so far about the Omicron variant of Covid-19 [Internet] (2021) [cited 2021 Dec 3]. Available from: https://www.indianexpresscom/article/explained/covid-variant-south-africa-explained-7642199/, (nd) 2021
- 9.Papanikolaou V, Chrysovergis A, Ragos V et al (2022) From Delta to Omicron: S1-RBD/S2 mutation/deletion equilibrium in SARS-CoV-2 defined variants. Gene 146134 [DOI] [PMC free article] [PubMed]
- 10.Siddiqui SM, Bowman KA, Zhu AL et al (2022) Serological markers of SARS-CoV-2 reinfection. mBio 13(1):e02141–02121 [DOI] [PMC free article] [PubMed]
- 11.Centers for Disease Control and Prevention (2020) Common investigation protocol for investigating suspected SARS-CoV-2 reinfection. https://www.cdc.gov/coronavirus/2019-ncov/php/reinfection.html. (accessed: 3 Nov 2020)
- 12.Kojima N, Roshani A, Brobeck M, Baca A, Klausner JD (2021) Incidence of severe acute respiratory syndrome coronavirus-2 infection among previously infected or vaccinated employees. medRxiv [DOI] [PMC free article] [PubMed]
- 13.Thompson MG, Burgess JL, Naleway AL, et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers—eight US locations, December 2020–March 2021. Morb Mortal Wkly Rep. 2021;70(13):495. doi: 10.15585/mmwr.mm7013e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poland GA, Ovsyannikova IG, Kennedy RB. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. The Lancet. 2020;396(10262):1595–1606. doi: 10.1016/S0140-6736(20)32137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall VJ, Foulkes S, Charlett A, et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN) The Lancet. 2021;397(10283):1459–1469. doi: 10.1016/S0140-6736(21)00675-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kojima N, Shrestha N, Klausner JD. A systematic review of the protective effect of prior SARS-CoV-2 infection on repeat infection. Eval Health Prof. 2021;44(4):327–332. doi: 10.1177/01632787211047932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lumley SF, O’Donnell D, Stoesser NE, Matthews PC, Howarth A, Hatch SB, Marsden BD, Cox S, James T, Warren F. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. 2021;384(6):533–540. doi: 10.1056/NEJMoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vitale J, Mumoli N, Clerici P, et al. Assessment of SARS-CoV-2 reinfection 1 year after primary infection in a population in Lombardy. Italy JAMA Intern Med. 2021;181(10):1407–1408. doi: 10.1001/jamainternmed.2021.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez G, Banon T, Gazit S et al (2021) A 1 to 1000 SARS-CoV-2 reinfection proportion in members of a large healthcare provider in Israel: a preliminary report. MedRxiv
- 20.Graham MS, Sudre CH, May A, et al. Changes in symptomatology, reinfection, and transmissibility associated with the SARS-CoV-2 variant B 1 1 7 an ecological study. The Lancet Public Health. 2021;6(5):e335–e345. doi: 10.1016/S2468-2667(21)00055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turabelidze G, Womack A, Mobley E, et al. Garikapaty V, Finley S: SARS-CoV-2 reinfections during the Delta variant surge-Missouri, June–October, 2021. Mo Med. 2021;118(6):539. [PMC free article] [PubMed] [Google Scholar]
- 22.Corrao G, Franchi M, Rea F, et al. Protective action of natural and induced immunization against the occurrence of delta or alpha variants of SARS-CoV-2 infection: a test-negative case-control study. BMC Med. 2022;20(1):1–10. doi: 10.1186/s12916-022-02262-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altarawneh HN, Chemaitelly H, Hasan MR et al (2022) Protection against the Omicron variant from previous SARS-CoV-2 infection. N Eng J Med [DOI] [PMC free article] [PubMed]
- 24.Callaway E. Omicron likely to weaken COVID vaccine protection. Nature. 2021;600(7889):367–368. doi: 10.1038/d41586-021-03672-3. [DOI] [PubMed] [Google Scholar]
- 25.Abu-Raddad LJ, Chemaitelly H, Ayoub HH et al (2021) Introduction and expansion of the SARS-CoV-2 B. 1.1. 7 variant and reinfections in Qatar: A nationally representative cohort study. PLoS Med 18(12) [DOI] [PMC free article] [PubMed]
- 26.Chemaitelly H, Bertollini R, Abu-Raddad LJ, et al. Efficacy of natural immunity against SARS-CoV-2 reinfection with the beta variant. N Eng J Med. 2021;385(27):2585–2586. doi: 10.1056/NEJMc2110300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim P, Gordon SM, Sheehan MM et al (2021) Duration of SARS-CoV-2 natural immunity and protection against the Delta variant: a retrospective cohort study. Clin Infect Dis XX(XX):1–6 [DOI] [PMC free article] [PubMed]
- 28.Brits E, Adepoju P. Omicron potential under close scrutiny. Nature. 2021;10:197–199. [Google Scholar]


