SUMMARY
An upsurge of fever cases of unknown origin, but resembling dengue and leptospirosis was reported in Havelock, Andaman & Nicobar Islands, an important tourism spot, during May 2014. Investigations were carried out to determine the aetiology, and to describe the epidemiology of the outbreak. The data on fever cases attending Primary Health Centre (PHC), Havelock showed that the average number of cases reporting per week over the last 2 years was 46·1 (95% confidence interval 19·4–72·9). A total of 27 (43·5%) patients out of the 62 suspected cases were diagnosed as having DENV infection based on a positive enzyme immunoassay or reverse transcriptase–polymerase chain reaction. The overall attack rate was 9·4 cases/1000 population and it ranged between 2·8 and 18·8/1000 in different villages. The nucleotide sequencing showed that the virus responsible was DENV-3. DENV-3 was first detected in the Andaman & Nicobar Islands in 2013 among wharf workers in Port Blair and within a year it has spread to Havelock Island which is separated from South Andaman by 36 nautical miles.
Key words: Dengue, DENV-3, Havelock Island, outbreak, South Andaman
INTRODUCTION
Dengue fever (DF) and dengue haemorrhagic fever (DHF) are mosquito-borne viral diseases of humans and are recognized as an important emerging public health problem throughout the world [1]. Epidemics of DF/DHF have occurred in tropical and sub-tropical countries, especially in the South East Asian region [2]. Dengue virus (DENV) is reported to be transmitted by Aedes mosquitoes, i.e. Ae. aegypti and Ae. albopictus. The infection is caused by any of the four dengue virus serotypes (DENV-1,-2,-3,-4), which belong to the genus Flavivirus. This virus causes a spectrum of manifestations, ranging from DF to DHF and dengue shock syndrome (DSS), which is fatal and are usually associated with secondary infections [3]. Due to environmental changes and rapid urbanization, a considerable increase in the spread of dengue cases has occurred in the past four decades, in several parts of India. The disease has been found to occur both in urban as well as in rural areas [4].
Andaman & Nicobar Islands is an archipelago of more than 500 islands and islets, stretching over 700 km from north to south, in the Bay of Bengal. Serologically confirmed DF was first detected in these islands during 2009, although dengue antibodies were reported earlier [5]. There is the possibility that dengue viral infection has been occurring silently in Andaman & Nicobar Islands during the past few years, and there have been reports from the South Pacific Islands [6]. Our previous report suggested that DF is emerging as an important public health problem in these islands, due to the widespread prevalence of Aedes vector mosquitoes [7, 8]. In addition, presence of multiple serotypes of the virus, i.e. DENV-1, -2 and -3 in the islands elevates the risk of DHF and DSS and therefore, future dengue outbreaks could lead to higher morbidity and mortality [9]. An upsurge of fever cases of unknown origin, but resembling dengue and leptospirosis was reported in Havelock, Andaman & Nicobar Islands (an important tourism spot), during May–June 2014. Investigations were carried out to determine the aetiology, and the role of DENV, if any, during the outbreak.
MATERIALS AND METHODS
An upsurge in the number cases of febrile illness with symptoms similar to DF was observed during May 2014 at the Primary Health Centre (PHC) of Havelock, Andaman & Nicobar. Blood samples were collected from suspected patients and transported to the Regional Medical Research Centre (RMRC), Port Blair, to investigate and identify the aetiological agent.
All the samples were tested for the presence of anti-DENV, anti-chikungunya virus (CHIKV) IgM antibodies by IgM capture ELISA kits developed by the National Institute of Virology, Pune [10]. The samples were also tested for the presence of anti-leptospiral antibodies by microscopic agglutination test (MAT) following standard procedures [11]. Reference strains belonging to 12 serogroups of Leptospira, common in the Andaman Islands and country, were included in the MAT panel as antigens. A subsample of the patients who reported to the hospital within 4 days of onset of symptoms and were negative for IgM anti-DENV antibodies was also tested for DENV RNA by reverse transcription–polymerase chain reaction (RT–PCR), followed by nested PCR, for detection of serotype. RNA was extracted from the serum samples using Qiagen Viral RNA extraction kit (Qiagen, USA) and RT–PCR was conducted following the standard protocol [12]. The first-round of PCR targeted a 511-bp fragment covering the capsid-protein C and pre-M regions of the viral genome, followed by nested multiplex PCR, to differentiate the serotypes of DENV, as the size of the amplified product was specific to each of the four serotypes. The PCR products were sequenced using the Big Dye Terminator Cycle Sequencing kit (Applied Biosystems, USA) according to the manufacturer's instructions. All DNA sequences were assembled using SeqMan II v. 5.03 (DNASTAR, USA). These sequences were then aligned with previously published sequences of DENV strains belonging to various combinations of serotypes and genotypes by the ClustalW multiple alignment and pair-wise alignment program of MEGA software v. 5 [13]. Genetic distances were estimated using Kimura's two-parameter algorithm and a phylogenetic tree was constructed by the neighbour-joining method [13]. The statistical significance of the relationships obtained was estimated by bootstrap resampling analysis (1000 repetitions). In addition, the aligned DNA sequences were BLAST-searched to assess their identity with previously characterized sequences of the DENV genome.
The epidemic curve was prepared using the data on number of cases reported. Number of fever cases reported to PHC during the week of reporting in the previous 2 years was plotted to assess any increase in fever cases beyond the expected occurrence. A spot map of cases was prepared using a copy of the map obtained from the Revenue Department. Attack rates in all the affected villages and for the whole island were estimated.
RESULTS AND DISCUSSION
Incidence of febrile illness in Havelock Island
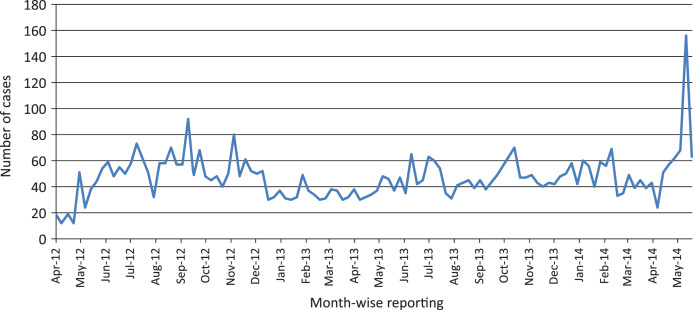
A sudden upsurge of fever cases was reported at PHC, Havelock in May 2014. During the week commencing 12 May 2014, a total of 156 fever cases attended the PHC with headache, rigors, and pain in the joints, back muscles and abdomen. This is an unusual scenario in the small island where, the average number of cases reported per week over the last 2 years was 46·1 (95% confidence interval 19·4–72·9), which was clearly in excess of the expected number of fever cases (Fig. 1). In order to confirm the existence of febrile illness in Havelock, the Public Health Department of Andaman & Nicobar Administration requested the RMRC, Port Blair to conduct an outbreak investigation in order to identify the infectious aetiology. A team comprising physician, laboratory technician and an entomologist visited the PHC and affected area between 15 May 2014 and 28 May 2014. Sixty-two suspected blood specimens were collected (on the basis of onset of symptoms, i.e. 1–5 days). Moreover, adult mosquito collection and Aedes larval infestation survey were conducted in the surrounding areas to confirm the transmission dynamics and the findings have been published recently [14].
Fig. 1.
Number of fever cases reported to Havelock Primary Health Centre during the week of reporting.
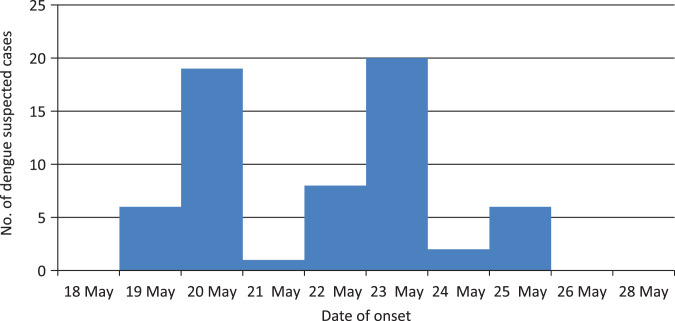
Dengue had not been reported in Havelock until May 2014, although there was an initial peak of febrile illness on 12 May followed by a lull during 15–18 May and again a peak in 19–20 May 2014 which initiated a local outbreak in the village leading to the initial peak. The epidemic curve constructed from the data on suspected cases of dengue attending the PHC is shown in Figure 2. Later, due to the abundance of vector mosquitoes [14] resulting in the dissemination of the virus to other villages, a second peak occurred during 22–23 May. Simultaneously mosquito control measures were initiated by healthcare workers during this period and these clearly showed the subsidence of febrile cases by the end of May 2014.
Fig. 2.
Epidemic curve of dengue outbreak in Havelock, May 2014.
Laboratory confirmation
Collected blood samples from the suspected patients attending the PHC and the household survey were screened for anti-DENV IgM antibodies by enzyme immunoassay (EIA) and two were found positive. All the samples were tested for DENV RNA, using RT–PCR and 25 were found positive. A total of 27 (43·5%) of 62 patients were diagnosed as having DENV infection, based on positive EIA or RT–PCR. There was no CHIKV IgM-positive and anti-leptospiral antibodies detected in the samples collected. The results confirmed the DENV aetiology for the febrile illness, which occurred as an outbreak in Havelock Island during May 2014.
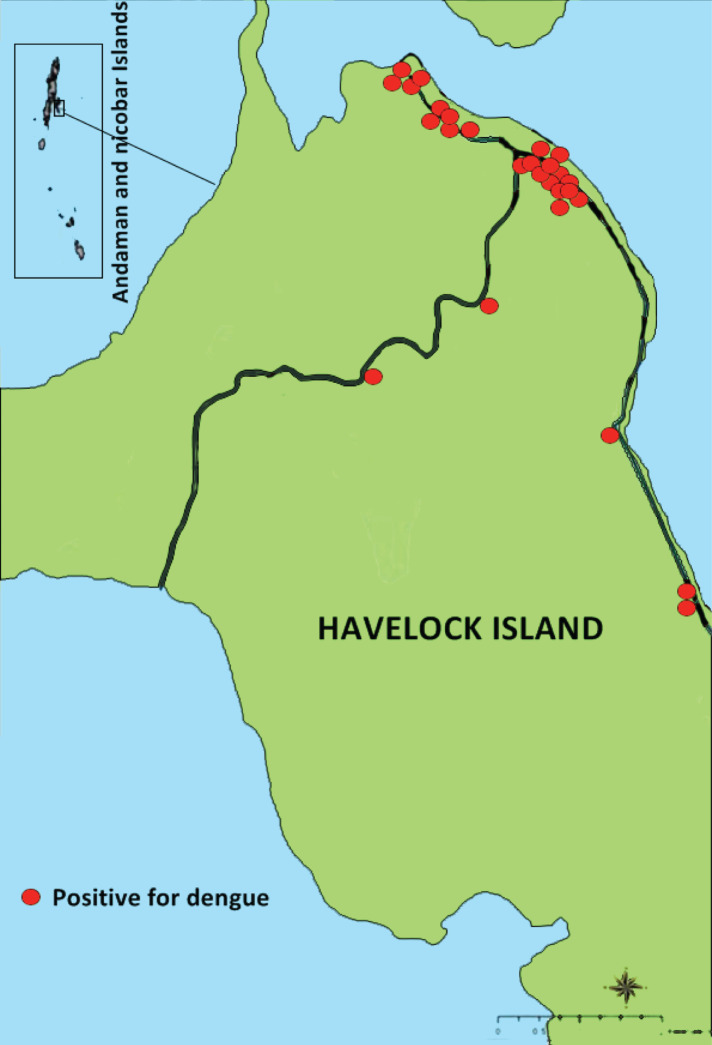
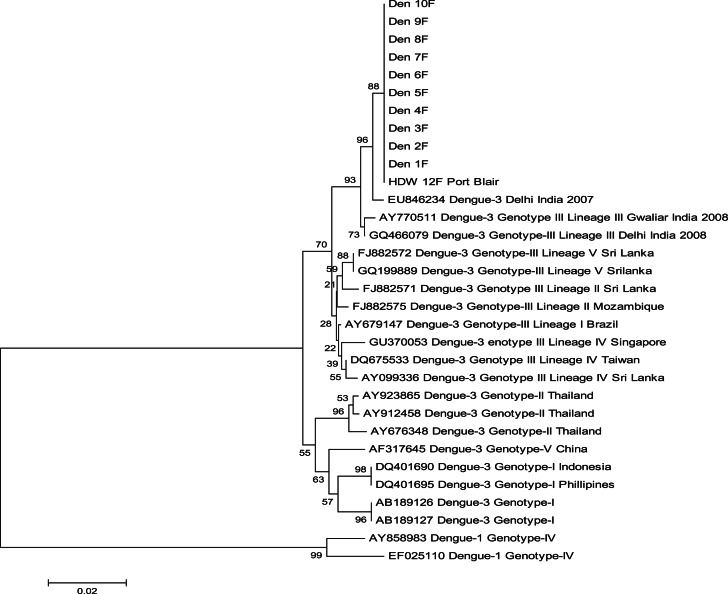
The total population of Havelock was 6613 and a total of 27 cases occurred during the outbreak (Fig. 3). Thus the attack rate was 9·4 cases/1000 population. The attack rates in villages are summarized in Table 1. The highest attack rate was observed in Havelock no. 3 village followed by Havelock no. 1. The nucleotide sequencing of the 511 bp amplicon confirmed that the virus sequence was homologous with DENV-3. Phylogenetic analysis of 10 strains from Havelock was compared with the sequences of representative strains of all serotypes of DENV. All the 10 capsid-protein C and pre-M region nucleotide sequences formed a single clade along with existing DENV serogroup 3 in Port Blair (Fig. 4). Dengue outbreaks with different serotypes have been reported in the mainland, India and also from Andaman & Nicobar Islands [9, 15]. Emergence of dengue serotype 3 in north and south India has been reported in the past [16, 17]. Earlier studies have reported the existence of two dengue serotypes, i.e. 1, 2 in these islands [9]. Recent investigations found the circulation of a third serotype of DENV in urban Port Blair during 2013 [8]. Interestingly, the recent entomological characteristic features indicate the existence of dengue serotype 3 during the outbreak in Havelock Island [14]. Therefore, the current findings envisaged that serotype 3 circulated in Havelock Island. According to antibody-dependent enhancement hypothesis, a secondary DENV infection could increase the risk factors for DHF/DSS [18]. Therefore, existence and circulation of multiple serotypes in these islands could precipitate the occurrence of DSS/DHF in the near future. Hence adequate precautionary measures need to be implemented both by the health authorities and the public to control the spread of dengue infection.
Fig. 3.
Map of Havelock Island depicting distribution of positive cases.
Table 1.
Attack rates of dengue in villages of Havelock
| Village | Population | Cases | Attack rate/1000 |
|---|---|---|---|
| Havelock no. 1 | 857 | 10 | 11·7 |
| Havelock no. 2 | 1257 | 9 | 7·2 |
| Havelock no. 3 | 1279 | 24 | 18·8 |
| Havelock no. 4 | 847 | 4 | 4·7 |
| Havelock no. 5 | 463 | 2 | 4·3 |
| Havelock no. 6 | 942 | 7 | 7·4 |
| Havelock no. 7 | 351 | 1 | 2·8 |
| Kalapathar | 617 | 5 | 8·1 |
| Total | 6613 | 62 | 9·4 |
Fig. 4.
Phylogenetic neighbour-joining tree showing 10 Havelock DENV sequences (Den 1F, Den 2F, Den 3F, Den 4F, Den 5F, Den 6F, Den 7F, Den 8F, Den 9F, Den 10F) that have been grouped with DENV serotype 3 and genotype III.
This investigation confirmed that the existence of an outbreak of febrile illness was due to DENV in Havelock during May 2014. The aetiological agent was identified as DENV serotype 3. This is the first outbreak of dengue reported from Havelock Island and the findings suggest that dengue, which appeared in Port Blair and adjoining areas a few years ago, is now spreading to other islands. DENV-3 infection was first identified in Andaman & Nicobar Islands in 2013 among wharf workers in Port Blair, South Andaman. Within a year, it has spread to Havelock Island, which is separated from South Andaman by about 36 nautical miles of open sea. As Havelock is an important tourist destination, frequent travellers between Port Blair and Havelock, particularly tourists, are likely to be infectious virus carriers to Havelock Island.
ACKNOWLEDGEMENTS
The current study formed a part of the activities under the Establishment of Grade I (Diagnostic) Virology at the Regional Medical Research Centre (ICMR), Port Blair which is supported by an extramural grant from the Indian Council of Medical Research (ref. letter no. 5/8/7/16/2010-ECD-I). The authors are grateful to the Directorate of Health Services, Andaman & Nicobar Administration for extending support during the conduct of study.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268816003423.
click here to view supplementary material
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Gubler DJ, Clark GG. Dengue/dengue hemorrhagic fever: the emergence of a global health problem. Emerging Infectious Diseases 1995; 1: 55–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pandey BD, et al. Dengue virus, Nepal. Emerging Infectious Diseases 2008; 14: 514–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guzman MG, Kouri G. Dengue diagnosis, advances and challenges. International Journal of Infectious Diseases 2004; 8: 69–80. [DOI] [PubMed] [Google Scholar]
- 4.Victor TJ. Detection of dengue viral infections in Aedes mosquitoes: an essential tool for epidemiological surveillance. Indian Journal of Medical Research 2009; 129: 634–636. [PubMed] [Google Scholar]
- 5.Padbidri VS, et al. A serological survey of arboviral diseases among the human population of the Andaman and Nicobar Islands, India. Southeast Asian Journal Tropical Medicine and Public Health 2002; 33: 794–800. [PubMed] [Google Scholar]
- 6.Steel A, Gubler DJ, Bennett SN. Natural attenuation of dengue virus type-2 after a series of island outbreaks: a retrospective phylogenetic study of events in the South Pacific three decades ago. Virology 2010; 405: 505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muruganandam N, et al. Dengue virus serotype-3 (subtype-III) in Port Blair, India. Journal of Vector Borne Diseases 2014; 51: 58–61. [PubMed] [Google Scholar]
- 8.Vijayachari P, et al. Emergence of dengue in Andaman & Nicobar archipelago: eco-epidemiological perspective. Indian Journal of Medical Research 2011; 134: 235–237. [PMC free article] [PubMed] [Google Scholar]
- 9.Chaaithanya IK, et al. Dengue: a newly emerging viral infection in Andaman and Nicobar Islands, India. Epidemiology and Infection 2012; 140: 1920–1924. [DOI] [PubMed] [Google Scholar]
- 10.Gadkari DA, Shaikh BH. IgM antibody capture ELISA in the diagnosis of Japanese encephalitis, West Nile and dengue virus infections. Indian Journal of Medical Research 1984; 80: 613–619. [PubMed] [Google Scholar]
- 11.Wolff JW. The laboratory diagnosis of leptospirosis. In: Dalldorf G, ed. American Lectures in Tests and Techniques. Springfield, IL: Charles C. Thomas, 1954. [Google Scholar]
- 12.Lanciotti RS, et al. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. Journal of Clinical Microbiology 1992; 30: 545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamura K, et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 2011; 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sivan A, et al. Natural transmission of dengue virus serotype 3 by Aedes albopictus (Skuse) during an outbreak in Havelock Island: entomological characteristics. Acta Tropica 2016; 156: 122–129. [DOI] [PubMed] [Google Scholar]
- 15.Kabilan L, et al. Dengue disease spectrum among infants in the 2001 dengue epidemic in Chennai, Tamil Nadu, India. Journal of Clinical Microbiology 2003; 41: 3919–3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dash PK, et al. Reemergence of dengue virus type-3 (subtype-III) in India: implications for increased incidence of DHF and DSS. Virology Journal 2006; 3: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paramasivan R, et al. Dengue fever caused by dengue virus serotype-3 (subtype-III) in a rural area of Madurai district, Tamil Nadu. Indian Journal of Medical Research 2010; 132: 339–342. [PubMed] [Google Scholar]
- 18.Halstead SB, O'Rourke EJ. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. Journal of Experimental Medicine 1977; 146: 201–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268816003423.
click here to view supplementary material