Abstract
Analysis of complex gene families in the lignin-degrading basidiomycete Phanerochaete chrysosporium has been hampered by the dikaryotic nuclear condition. To facilitate genetic investigations in P. chrysosporium strain BKM-F-1767, we isolated a homokaryon from regenerated protoplasts. The nuclear condition was established by PCR amplification of five unlinked genes followed by probing with allele-specific oligonucleotides. Under standard nitrogen-limited culture conditions, lignin peroxidase, manganese peroxidase, and glyoxal oxidase activities of the homokaryon were equivalent to those of the parental dikaryon. We used the homokaryon to determine the genomic organization and to assess transcriptional effects of a family of repetitive elements. Previous studies had identified an insertional mutation, Pce1, within lignin peroxidase allele lipI2. The element resembled nonautonomous class II transposons and was present in multiple copies in strain BKM-F-1767. In the present study, three additional copies of the Pce1-like element were cloned and sequenced. The distribution of elements was nonrandom; all localized to the same 3.7-Mb chromosome, as assessed by segregation analysis and Southern blot analysis of the homokaryon. Reverse transcription-PCR (RT-PCR) showed that Pce1 was not spliced from the lipI2 transcript in either the homokaryon or the parental dikaryon. However, both strains had equivalent lignin peroxidase activity, suggesting that some lip genes may be redundant.
The most efficient lignin-degrading microorganisms are the white-rot basidiomycetes, of which Phanerochaete chrysosporium strain BKM-F-1767 has been the most extensively characterized (18, 25). Under appropriate culture conditions, the fungus secretes multiple isozymes of lignin peroxidase (LiP) and manganese peroxidase (MnP) as well as of the cellulase cellobiohydrolase I. These isozymes are encoded by complex families of structurally related genes, although the precise number of genes and the relationships among the sequences have not been determined. Difficulties differentiating allelic variants from closely related genes and the lack of an accepted standardized nomenclature have complicated the issue (14).
Allelism could be experimentally resolved if cloning was routinely performed with cultures originating from single basidiospores, which are the homokaryotic products of meiosis. The haploid homokaryons possess a single nuclear type, in contrast to the two different nuclear types found in dikaryons. Analyses of single-basidiospore cultures have been used to differentiate alleles and to create genetic and physical maps of P. chrysosporium (10, 14, 16, 23, 27, 28, 31, 33, 36, 37). However, single-basidiospore strains typically exhibit reduced sporulation, growth rate, and enzyme yields relative to the parental strain (31, 43). Further, chromosome lengths and other aspects of genome organization are not maintained through meiotic recombination, limiting the experimental value of single-basidiospore strains (10, 13, 23, 37, 44). Homokaryons of nonmeiotic origin would greatly simplify analysis of complex gene families without the disadvantages incurred by recombination.
In addition to the intensively studied gene families, P. chrysosporium contains at least one family of repetitive elements. A 1,747-bp insertional element designated Pce1 was detected in LiP gene lipI2 (15). The element had features common to class II nonautonomous transposons (11, 20), such as inverted terminal repeats, and a putative target site duplication (TA), and it was present in multiple copies in BKM-F-1767 and in other strains of P. chrysosporium. However, Pce1 contains no extended open reading frames and shows no sequence similarity to known transposases. A single 3.7-Mb chromosome was observed on pulsed-field gel Southern blots probed with Pce1, suggesting a nonrandom distribution of Pce1-like sequences. The number, sequence, insertional context, and genomic organization of the additional Pce1-like sequences were not previously investigated.
Pce1 is inserted immediately adjacent to the fourth intron of lipI2, suggesting that it may be spliced from the mature transcript. Reverse transcription-PCR (RT-PCR) analysis of dikaryotic strain BKM-F-1767 identified a transcript from the wild-type allele, lipI1, but not from Pce1-disrupted lipI2 (15). Thus, in the heterozygous dikaryon, Pce1 transcriptionally inactivates lipI2 and is not spliced, as are certain transposons of higher plants (24). However, lipI2 processing in the absence of lipI1 (i.e., in a homokaryon containing only the lipI2 allele) was not previously assessed.
To simplify genetic analyses, we isolated and characterized a homokaryotic derivative of BKM-F-1767. The homokaryon was used to investigate the organization and transcriptional effects of Pce1-like elements. Four highly conserved elements were sequenced and mapped to a single chromosome. The homokaryon phenotype was similar to that of the parental dikaryon even though lipI was transcriptionally inactivated. The homokaryotic strain will simplify identification of new genes in libraries, enhance the resolution of pulsed-field gels, and aid investigations of chromosome-length polymorphisms.
MATERIALS AND METHODS
Fungal strains and isolation of homokaryons.
P. chrysosporium BKM-F-1767 was obtained from the Center for Forest Mycology Research, Forest Products Laboratory, Madison, Wis. Single-basidiospore progeny from BKM-F-1767 were described elsewhere (14).
Homokaryotic strains were isolated from regenerated protoplasts of the dikaryotic strain BKM-F-1767. Protoplasts were prepared essentially as described by Brody and Carbon (7), with minor modifications. A petri plate containing 25 ml of YEG medium (containing [per liter] 5 g of yeast extract and 20 g of glucose) or YMPG medium (containing [per liter] 2 g of yeast extract, 10 g of malt extract, 2 g of peptone, 10 g of glucose, 2 g of KH2PO4, 1 g of MgSO4 · 7H2O, and 1 mg of thiamine) was inoculated with 105 to 107 conidiospores and incubated at 37°C without shaking. After 22 h of incubation the medium was removed by aspiration and the mycelia were gently washed with 20 ml of MgOsm buffer (0.5 M MgSO4, 0.05 M maleic acid [pH 5.9]) (3). The mycelium was resuspended in approximately 12 ml of Novozyme 234 (10 mg of MgOsm buffer per ml; Novo Industries, Copenhagen, Denmark) and incubated at 37°C until protoplasts had developed (about 90 min). Protoplasts were filtered through two layers of Miracloth (Calbiochem, San Diego, Calif.), pelleted, and resuspended in approximately 10 ml of 1.2 M sorbitol–10 mM Tris-HCl, pH 7.4. Protoplasts were diluted to approximately 103 protoplasts ml−1 in MgOsm buffer, and 10- to 100-μl aliquots were plated on FMSS medium (17). Plates were incubated at 37°C until colonies were visible, and then the mycelium was transferred to YMPG plates, which were incubated at 37°C.
Dikaryotic and homokaryotic strains were differentiated by PCR amplification of specific genes. For the initial screening of 90 colonies, genomic DNA was amplified with lipI-specific primers flanking the Pce1 insertion site (15). The PCR products were size fractionated on agarose gels and stained with ethidium bromide. Dikaryons were recognized by bands at 2,236 bp and 479 bp, which correspond to lipI2 and lipI1, respectively. Putative homokaryons were further analyzed by PCR amplification of specific genes followed by Southern blot probing with allele-specific oligonucleotides (14). PCR primers and probes for differentiating alleles of lipE, lipJ, lipF, lipD, and glx were previously reported (14). The mnp2 alleles were amplified as described elsewhere (6) and differentiated with oligonucleotides 5′-GCGATCCTGTAATTGAA-3′ and 5′-GCGATGGTGCAACTGGA-3′. Two genes, lipE and lipJ, lie at opposite ends of a LiP gene cluster (36), whereas lipF, lipD, glx, and mnp2 are not linked to the cluster or to each other (14, 36).
Culture conditions and enzyme assays.
Nitrogen-limited cultures were grown statically at 39°C as described previously (8, 26) and harvested on day 5. LiP activities were assayed by veratryl alcohol oxidation (39). MnP activities were measured by monitoring the oxidation of 2,6-dimethoxyphenol (Aldrich, Milwaukee, Wis.) at 469 nm (41). Glyoxal oxidase activity was assayed as described elsewhere (21, 22) using methylglyoxal as the oxidase substrate and phenol red as the substrate for horseradish peroxidase in a coupled assay.
Isolation and mapping of Pce1-like sequences.
Pce1-like sequences were cloned from a pWE15-based cosmid library of P. chrysosporium (13) by probing with the full-length copy of Pce1. In some cases, the Universal Genome Walker kit (Clontech Laboratories, Palo Alto, Calif.) was used to clone adjacent regions (34).
Segregation analysis of the Pce1-like sequences was performed on 54 single-basidiospore isolates (14). The flanking regions of each element were unique, and element-specific PCR primer pairs were designed with one primer anchored in the element and the other in the flanking region. The sequences of these primers were as follows: Pce2 flanking primer, 5′-CGACAGGCTTGAACATGAC-3′; Pce3 flanking primer, 5′-GAGTGTTGACTGCAGCCTTGC-3′; Pce4 flanking primer, 5′-CTGCCAGGCAAGCTTCAGAGC-3′; Pce2 and Pce4 element-specific primer, 5′-TCAACCCAGGAGCATAG-3′; and Pce3 element-specific primer, 5′-AGCGCTGCAAGACGGTCTGGGATATTG-3′.
Southern blots were used to verify genetic segregation and to establish the nuclear origin of Pce1-like elements. Based on segregation analysis, the genomic DNA of single-basidiospore cultures harboring one, two, or four elements were digested with XhoI and probed with nick-translated Pce1 under conditions of high stringency (15). The number and identity of Pce1-like sequences in the homokaryon RP78 were established similarly. DNA of the parental dikaryon BKM-F-1767, which contains all the Pce1 copies, was included as a control.
Transcription of lipI2.
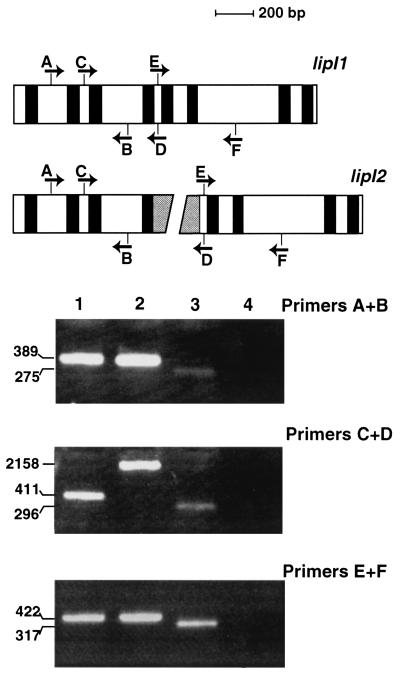
RT-PCR was used to identify lipI transcripts. Poly(A) RNA was derived from 2-week-old Aspen wood chip cultures, which favor lipI transcription (19). RT was primed with oligo(dT), and PCR conditions were as described previously (19, 40). Three sets of primers were designed to amplify different regions of lipI cDNA, including one set which flanked the Pce1 insert of lipI2 (Fig. 1). Primers were based on coding regions conserved in both alleles and were designated A through F, with sequences as follows: A, 5′-GCGCCTGGTTCGATGTTTTGGATG-3′; B, 5′-CGCCAGGGGTGACGTTGTGCTTCT-3′; C, 5′-TCTATCGCTATCTCTCCCGCT-3′; D, 5′-CGGTTCGGGAACAAGGCCATC-3′; E, 5′-CAGCTCCAGATGGCCTTGTTCC-3′; and F, 5′-CGCGGGCGATGGTGTGG-3′. Control PCR reactions contained cosmids carrying either lipI1 or lipI2. Reactions were run for 35 cycles, as previously reported (37), and the products were size fractionated in agarose gels. Similar studies were also performed using RNA from nitrogen-starved cultures, in which levels of lipI transcripts are relatively lower (37).
FIG. 1.
Analysis of lipI transcripts. At the top, a schematic representation of lipI alleles shows the locations of introns (black fill), Pce1 (gray fill), and the primers designated A through F (arrows indicate direction of transcription. Ethidium bromide-stained PCR products size fractionated on agarose gels are shown at the bottom. Lanes 1 and 2 are controls, showing amplification products from cosmid templates carrying lipI1 and lipI2, respectively. RT-PCR products from RNA derived from Aspen wood chip cultures inoculated with BKM-F-1767 and RP-78 are shown in lanes 3 and 4, respectively. No RT-PCR amplification product was obtained from the RP-78 RNA (lane 4). In contrast, BKM-F-1767 cultures contained the expected lipI transcript (lane 3). The sizes of the PCR products (in nucleotides) are shown on the left.
Sequence analysis.
Nucleotide sequences were determined with the ABI Prism Dye Terminator Cycle Sequencing kit (Perkin-Elmer Applied Biosystems, Foster City, Calif.) with an ABI373 DNA sequencer. Nucleotide and amino acid sequence similarity searches used the BLAST method (5) on the National Center for Biotechnology Information databases.
Nucleotide sequence accession numbers.
The elements designated Pce2, Pce3, and Pce4, together with a >900-bp flanking region for each, were assigned GenBank accession numbers AF134289, AF134290, and AF134291, respectively.
RESULTS
Isolation and characterization of homokaryon RP-78.
Homokaryotic derivatives of BKM-F-1767 were isolated from regenerated protoplasts, a strategy successfully used for other basidiomycetes (1, 12, 29, 35). In contrast to these previous studies, homokaryons and dikaryons cannot be simply differentiated because P. chrysosporium hyphae lack clamp connections (9) and the mating system is not well defined (4, 38).
We identified homokaryons through PCR amplification and allele-specific hybridization techniques. PCR amplification of the dimorphic lipI alleles (lipI2 is disrupted by Pce1 and is therefore 1,747 bp larger than lipI1) was used to distinguish between homokaryons and dikaryons. On this basis, approximately 50% of the 90 regenerated protoplasts were judged dikaryotic and discarded (data not shown). Allele-specific probing of five unlinked genes confirmed that the remaining isolates were all homokaryotic but of the same nuclear type. The existence of a deleterious or lethal allele(s) would most easily explain our inability to recover the second nuclear type.
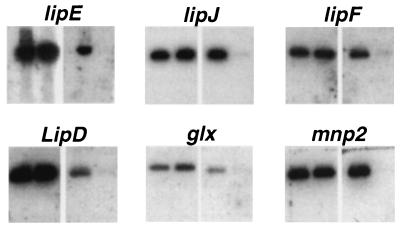
One homokaryotic isolate, RP-78, was characterized further. In addition to lipI, the precise alleles of five unlinked genes were determined using allele-specific oligonucleotide probes (Fig. 2). Under standard N-limited culture conditions, MnP, LiP, and glyoxal oxidase activities of RP-78 were similar to those of the parental strain, BKM-F-1767 (Table 1). On YMPG agar, RP-78 was slower growing but produced more asexual spores than the parent (Table 1).
FIG. 2.
Southern blot analysis of six PCR amplified genes, demonstrating the homokaryotic nature of RP-78. For each gene, two identical gels were run, with BKM-F-1767 PCR products in the left lanes and RP-78 products in the right. Each gel was then blotted to Nytran and probed with an allele-specific oligonucleotide. The blots show both alleles present in the dikaryotic strain BKM-F-1767 but only a single allele present in RP-78. PCR product sizes were as follows: lipE, 1,535 nucleotides (nt); lipJ, 675 nt; lipF, 1,480 nt; lipD, 440 nt; glx, 680 nt; and mnp2, 1,509 nt.
TABLE 1.
Enzyme activities and growth characteristics of dikaryotic (BKM-F-1767) and homokaryotic (RP-78) strains
| Enzyme activity or growth characteristic | Resultd for:
|
|
|---|---|---|
| BKM-F-1767 | RP-78 | |
| LiP activitya | 20 ± 9.4 | 37 ± 6.7 |
| MnP activitya | 220 ± 36 | 170 ± 29 |
| Glox activitya | 6.5 ± 1.6 | 7.6 ± 3.4 |
| Radial growth (mm)b | 12 ± 0.6 | 10 ± 0 |
| Spore productionc (spores/ml) | 3.5 × 107 ± 0.6 × 107 | 1.7 × 108 ± 0.6 × 108 |
Enzyme activities are given in nanomoles per minute per milliliter. Glox, glyoxal oxidase.
Radial growth was measured on centrally inoculated YMPG plates after 24 h at 37°C.
Harvested from YMPG plates after 2 weeks at 37°C.
Values are means ± standard deviations.
Genomic organization of the Pce1 family.
We cloned three additional Pce1-like sequences designated Pce2, Pce3, and Pce4. Analysis of flanking regions revealed no extended open reading frames and no significant similarity to database sequences. Surprisingly, the three new elements had at least 98% nucleotide identity with Pce1. The inverted terminal repeats were identical among the four Pce1 sequences, but they were imperfect, with 25 of 32 bases conserved in Pce2, Pce3, and Pce4. In short, all four sequences appeared to be the same nonautonomous class II element.
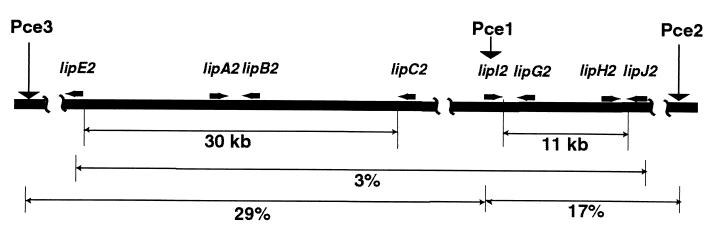
Pce1, Pce2, and Pce3 were linked (Fig. 3), but except for Pce1, none were closely linked to genes involved in lignocellulose degradation. Pce4 was not linked to the other three elements. Because the nucleotide sequences are highly conserved, all would be expected to strongly cross-hybridize under moderate stringency. Thus, the pattern of a previously published pulsed-field gel blot showing hybridization solely to a 3.7-Mb band (15) can be attributed to localization of all Pce1-like sequences to a single sister chromosome. Further confirming these results, Southern blots of XhoI-digested genomic DNA from RP-78 showed the presence of all four Pce1-like elements (data not shown).
FIG. 3.
Genetic linkage of Pce1, Pce2, and Pce3 and their relationship to the LiP gene cluster. Linkage was based on analyses of 54 single-basidiospore cultures. Vertical arrows indicate positions of elements. Thickened horizontal arrows show transcriptional orientation of LiP genes (36). Pce4 segregated independently.
Transcription of lipI2.
Under conditions favoring lipI expression (19), RT-PCR showed the presence of lipI transcripts in the dikaryotic parent BKM-F-1767 but not in RP-78 (Fig. 1). Conceivably, a Pce1-containing transcript might not be efficiently reverse transcribed and PCR amplified. To minimize this possibility, three sets of primers were used, including two which did not flank the insertion (results for primers A plus B and E plus F are shown in Fig. 1). The identity of the lipI cDNAs (Fig. 1, lane 3) was confirmed by direct sequencing, and identical results were obtained when RP-78 was grown in nitrogen-starved defined medium (data not shown).
DISCUSSION
Protoplast-derived homokaryons, termed “protoclones” by Sonnenberg et al. (35), have been identified by other means from other basidiomycetes (1, 12, 29, 35). The nucleus of P. chrysosporium protoclone RP-78 is genetically identical to a single nucleus of the dikaryotic strain BKM-F-1767, from which it was derived. RP-78 is genetically distinct from single-basidiospore cultures, which are also homokaryotic but have undergone meiotic recombination. The use of RP-78 should facilitate gene cloning, mutagenesis studies, and additional studies on the genetic mechanisms controlling variation in ligninolytic activity. For example, identifying new members of gene families, either from conventional libraries or by PCR amplification, will be greatly simplified because questions of allelism can be excluded. Pulsed-field gel analysis using RP-78 could avoid dimorphic sister chromosomes (10, 13, 23, 37) and thereby result in improved band resolution.
RP-78 is similar to BKM-F-1767 in its ligninolytic enzyme activity (Table 1) and biomechanical pulping performance (2; D. Dietrich, J. Gaskell, M. Akhtar, R. Blanchette, and D. Cullen, unpublished data). Interestingly, RP-78 LiP activity is approximately the same as that of BKM-F-1767 although it lacks a transcriptionally active lipI allele. This observation suggests that LiP activity is influenced by multiple factors and supports the hypothesis that LiP genes are redundant.
Previous Southern blot analysis of BKM-F-1767 suggested multiple Pce1-like sequences, and a strong signal was assigned to a single chromosome band of 3.7 Mb (15). Eight lip genes also reside on this chromosome and its dimorphic homologue of 3.5 Mb (36). Because of the Pce family's unusual distribution, the prospect of isolating an active transposon, and the possibility that other functional genes might be disrupted, three additional Pce1-like elements were cloned, sequenced, and mapped.
All four members of the Pce family were over 98% identical to each other, an unexpected result because none have extended open reading frames (the largest being 155 amino acids). The observed sequence conservation suggests these elements have arisen relatively recently. The Pce family may represent the inactive remnants of a transposable element. Approximately 15 copies of the 437-bp element, vader, are present in the Aspergillus niger genome, while their progenitor, Tan1, a 2.3-kb active transposon, is present as a single copy (30). Although no active progenitor of Pce was detected in P. chrysosporium, the possibility of some biological activity for the Pce sequences cannot be excluded.
Segregation analysis showed that Pce1, Pce2, and Pce3 were linked. While Pce4 was localized to the same chromosome, the distance was such that it appeared unlinked. Formally, the possibility of comigrating 3.7-Mb chromosomes (one with Pce4 and the other with Pce1, Pce2, and Pce3) cannot be dismissed. However, this seems unlikely because none of the more than 25 genes that have been genetically and physically mapped to date (14; S. Alsop, B. Janse, J. Gaskell, A. Vanden Wymelenberg, M. Vallim, and D. Cullen, unpublished data) reside on nonhomologous 3.7-Mb chromosomes. Moreover, Pce4 clearly cosegregates in the homokaryon RP-78.
The nonrandom distribution of eukaryotic repetitive sequences has been explained by a variety of mechanisms, including site-specific insertions and unequal crossover events between repetitive elements (32, 42). Site-specific insertion, either by specific sequences or by recognition of particular physical characteristics of certain regions of the genome, is not compatible with the available data for the Pce family. Over 900 bp flanking each Pce insertion site were sequenced, and no obvious similarity was detected.
Unequal crossover between repetitive sequences may be the simplest explanation for the unusual organization of the Pce family. Repetitive sequences (such as transposable elements or highly similar gene families) may function as small regions of homology for recombination to occur (44). Unequal crossover between sister chromosomes would generate a duplication in one and a deletion in the other. Thus, mechanisms that may enrich the Pce family on a single sister chromosome may also contribute to the observed chromosome length dimorphism. Arguing against this hypothesis, however, the regions surrounding insertion sites for all members of the Pce family appear to be conserved on both homologues (data not shown). No duplications or deletions were detected on either homologue. Nevertheless, the Pce1 family directly contributes to the observed length dimorphism, albeit a small proportion (∼8 of ∼200 kb). Conceivably, other repetitive sequences may be distributed on the 3.7-Mb homologue.
In conclusion, homokaryon RP-78 was isolated from regenerated protoplasts and shown to be phenotypically similar to its dikaryotic parent, BKM-F-1767. Both RP-78 and BKM-F-1767 contain four copies of the nonautonomous class II transposon Pce, all of which mapped to a 3.7-Mb chromosome. The lipI2 gene of RP-78 has been transcriptionally inactivated by Pce1 insertion but retains LiP activity equivalent to that of the dikaryon, suggesting that some lip genes are redundant. We anticipate that RP-78 will be useful for a variety of genetic analyses, especially differentiating new members of complex gene families.
ACKNOWLEDGMENT
This work was supported by Department of Energy grant DE-FG02-87ER13712.
REFERENCES
- 1.Addleman K, Archibald F. Kraft pulp bleaching and delignification by dikaryons and monokaryons of Trametes versicolor. Appl Environ Microbiol. 1993;59:266–273. doi: 10.1128/aem.59.1.266-273.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akhtar M, Blanchette R, Myers G, Kirk T K. An overview of biomechanical pulping research. In: Young R, Akhtar M, editors. Environmentally friendly technologies for the pulp and paper industry. New York, N.Y: John Wiley and Sons; 1998. pp. 309–340. [Google Scholar]
- 3.Alic M, Kornegay J R, Pribnow D, Gold M H. Transformation by complementation of an adenine auxotroph of the lignin-degrading basidiomycete Phanerochaete chrysosporium. Appl Environ Microbiol. 1989;55:406–411. doi: 10.1128/aem.55.2.406-411.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alic M, Letzring C, Gold M H. Mating system and basidiospore formation in the lignin-degrading basidiomycete Phanerochaete chrysosporium. Appl Environ Microbiol. 1987;53:1464–1469. doi: 10.1128/aem.53.7.1464-1469.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 6.Bogan B, Schoenike B, Lamar R, Cullen D. Manganese peroxidase mRNA and enzyme activity levels during bioremediation of polycyclic aromatic hydrocarbon-contaminated soil with Phanerochaete chrysosporium. Appl Environ Microbiol. 1996;62:2381–2386. doi: 10.1128/aem.62.7.2381-2386.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brody H, Carbon J. Electrophoretic karyotype of Aspergillus nidulans. Proc Natl Acad Sci USA. 1989;86:6260–6263. doi: 10.1073/pnas.86.16.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown J A, Glenn J K, Gold M H. Manganese regulates expression of manganese peroxidase by Phanerochaete chrysosporium. J Bacteriol. 1990;172:3125–3130. doi: 10.1128/jb.172.6.3125-3130.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burdsall H H, Eslyn W E. A new Phanerochaete with a chrysosporium imperfect state. Mycotaxon. 1974;1:123–133. [Google Scholar]
- 10.Covert S, Bolduc J, Cullen D. Genomic organization of a cellulase gene family in Phanerochaete chrysosporium. Curr Genet. 1992;22:407–413. doi: 10.1007/BF00352442. [DOI] [PubMed] [Google Scholar]
- 11.Daboussi M J. Fungal transposable elements and genome evolution. Genetica. 1997;100:253–260. [PubMed] [Google Scholar]
- 12.Eichlerova-Volakova I, Homolka L. Variability of ligninolytic enzyme activities in basidiospore isolates of the fungus Pleurotus ostreatus in comparison with that of protoplast-derived isolates. Folia Microbiol. 1997;42:583–588. [Google Scholar]
- 13.Gaskell J, Dieperink E, Cullen D. Genomic organization of lignin peroxidase genes of Phanerochaete chrysosporium. Nucleic Acids Res. 1991;19:599–603. doi: 10.1093/nar/19.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaskell J, Stewart P, Kersten P, Covert S, Reiser J, Cullen D. Establishment of genetic linkage by allele-specific polymerase chain reaction: application to the lignin peroxidase gene family of Phanerochaete chrysosporium. Bio/Technology. 1994;12:1372–1375. doi: 10.1038/nbt1294-1372. [DOI] [PubMed] [Google Scholar]
- 15.Gaskell J, Vanden Wymelenberg A, Cullen D. Structure, inheritance, and transcriptional effects of Pce1, an insertional element within Phanerochaete chrysosporium lignin peroxidase gene lipI. Proc Natl Acad Sci USA. 1995;92:7465–7469. doi: 10.1073/pnas.92.16.7465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaskell J, Vanden Wymelenberg A, Stewart P, Cullen D. Method for identifying specific alleles of a Phanerochaete chrysosporium encoding a lignin peroxidase. Appl Environ Microbiol. 1992;58:1379–1381. doi: 10.1128/aem.58.4.1379-1381.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gessner M, Raeder U. A histone promoter for expression of a phleomycin-resistance gene in Phanerochaete chrysosporium. Gene. 1994;142:237–241. doi: 10.1016/0378-1119(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 18.Gold M, Alic M. Molecular biology of the lignin-degrading basidiomycete Phanerochaete chrysosporium. Microbiol Rev. 1993;57:605–622. doi: 10.1128/mr.57.3.605-622.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janse B, Gaskell J, Ahktar M, Cullen D. Phanerochaete chrysosporium genes encoding lignin peroxidases, manganese peroxidases, and glyoxal oxidase in wood. Appl Environ Microbiol. 1998;64:3536–3538. doi: 10.1128/aem.64.9.3536-3538.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kempken F, Kuck U. Transposons in filamentous fungi—facts and perspectives. Bioessays. 1998;20:652–659. doi: 10.1002/(SICI)1521-1878(199808)20:8<652::AID-BIES8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 21.Kersten P J. Glyoxal oxidase of Phanerochaete chrysosporium; its characterization and activation by lignin peroxidase. Proc Natl Acad Sci USA. 1990;87:2936–2940. doi: 10.1073/pnas.87.8.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kersten P J, Kirk T K. Involvement of a new enzyme, glyoxal oxidase, in extracellular H2O2 production by Phanerochaete chrysosporium. J Bacteriol. 1987;169:2195–2201. doi: 10.1128/jb.169.5.2195-2201.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kersten P J, Witek C, Vanden Wymelenberg A, Cullen D. Phanerochaete chrysosporium glyoxal oxidase is encoded by two allelic variants: structure, genomic organization, and heterologous expression of glx1 and glx2. J Bacteriol. 1995;177:6106–6110. doi: 10.1128/jb.177.21.6106-6110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kidwell M G, Lisch D. Transposable elements as sources of variation in animals and plants. Proc Natl Acad Sci USA. 1997;94:7704–7711. doi: 10.1073/pnas.94.15.7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirk T K, Cullen D. Enzymology and molecular genetics of wood degradation by white-rot fungi. In: Young R A, Akhtar M, editors. Environmentally friendly technologies for the pulp and paper industry. New York, N.Y: John Wiley and Sons; 1998. pp. 273–308. [Google Scholar]
- 26.Kirk T K, Schultz E, Conners W J, Lorentz L F, Zeikus J G. Influence of culture parameters on lignin metabolism by Phanerochaete chrysosporium. Arch Microbiol. 1978;117:277–285. [Google Scholar]
- 27.Li B, Nagalla S, Renganathan V. Cellobiose dehydrogenase from Phanerochaete chrysosporium is encoded by two allelic variants. Appl Environ Microbiol. 1997;63:796–799. doi: 10.1128/aem.63.2.796-799.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li B, Renganathan V. Gene cloning and characterization of a novel cellulose-binding β-glucosidase from Phanerochaete chrysosporium. Appl Environ Microbiol. 1998;64:2748–2754. doi: 10.1128/aem.64.7.2748-2754.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsumoto T, Fukumasa-Nakai Y, Komatsu M. Efficient dedikaryotization of higher basidiomycetes by the protoplast regeneration method. Rep Tottori Mycol Inst. 1995;33:29–33. [Google Scholar]
- 30.Nyyssonen E, Amutan M, Enfield L, Stubbs J, Dunn-Coleman N. The transposable element Tan1 of Aspergillus niger var. awamori, a new member of the Fot1 family. Mol Gen Genet. 1996;253:50–56. doi: 10.1007/s004380050295. [DOI] [PubMed] [Google Scholar]
- 31.Raeder U, Thompson W, Broda P. Genetic factors influencing lignin peroxidase activity in Phanerochaete chrysosporium ME446. Mol Microbiol. 1989;3:919–924. doi: 10.1111/j.1365-2958.1989.tb00241.x. [DOI] [PubMed] [Google Scholar]
- 32.SanMiguel P, Tikhonov A, Jin Y-K, Motchoulskaia N, Zakharov D, Melake-Berhan A, Springer P S, Edwards K J, Lee M, Avramova Z, Bennetzen J L. Nested retrotransposons in the intergenic regions of the maize genome. Science. 1996;274:765–768. doi: 10.1126/science.274.5288.765. [DOI] [PubMed] [Google Scholar]
- 33.Schalch H, Gaskell J, Smith T L, Cullen D. Molecular cloning and sequences of lignin peroxidase genes of Phanerochaete chrysosporium. Mol Cell Biol. 1989;9:2743–2747. doi: 10.1128/mcb.9.6.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siebert P, Chenchik D, Kellogg D, Lukyanov K, Lukyanov S. An improved PCR method for walking in uncloned genomic DNA. Nucleic Acids Res. 1995;23:1087–1088. doi: 10.1093/nar/23.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sonnenberg A, Wessels J G, van Griensven L J. An efficient protoplasting/regeneration system for Agaricus bisporus and Agaricus bitorquis. Curr Microbiol. 1988;17:285–291. [Google Scholar]
- 36.Stewart P, Cullen D. Organization and differential regulation of a cluster of lignin peroxidase genes of Phanerochaete chrysosporium. J Bacteriol. 1999;181:3427–3432. doi: 10.1128/jb.181.11.3427-3432.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stewart P, Kersten P, Vanden Wymelenberg A, Gaskell J, Cullen D. The lignin peroxidase gene family of Phanerochaete chrysosporium: complex regulation by carbon and nitrogen limitation, and the identification of a second dimorphic chromosome. J Bacteriol. 1992;174:5036–5042. doi: 10.1128/jb.174.15.5036-5042.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson W, Broda P. Mating behavior in an isolate of Phanerochaete chrysosporium. Trans Br Mycol Soc. 1987;89:285–294. [Google Scholar]
- 39.Tien M, Kirk T K. Lignin-degrading enzyme from Phanerochaete chrysosporium: purification, characterization, and catalytic properties of a unique H2O2-requiring oxygenase. Proc Natl Acad Sci USA. 1984;81:2280–2284. doi: 10.1073/pnas.81.8.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vallim M, Janse B, Gaskell J, Pizzirani-Kleiner A, Cullen D. Phanerochaete chrysosporium cellobiohydrolase and cellobiose dehydrogenase transcripts in wood. Appl Environ Microbiol. 1998;64:1924–1928. doi: 10.1128/aem.64.5.1924-1928.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wariishi H, Valli K, Gold M H. Mn oxidation by manganese peroxidase from Phanerochaete chrysosporium: kinetic mechanisms and role of chelators. J Biol Chem. 1992;267:23688–23695. [PubMed] [Google Scholar]
- 42.Wichman H A, Van Den Bussche R A, Hamilton M J, Baker R J. Transposable elements and the evolution of genome organization in mammals. Genetica. 1992;86:287–293. doi: 10.1007/BF00133727. [DOI] [PubMed] [Google Scholar]
- 43.Wyatt A M, Broda P. Informed strain improvement for lignin degradation by Phanerochaete chrysosporium. Microbiology. 1995;141:2811–2822. doi: 10.1099/13500872-141-11-2811. [DOI] [PubMed] [Google Scholar]
- 44.Zolan M. Chromosome-length polymorphisms in fungi. Microbiol Rev. 1995;59:686–698. doi: 10.1128/mr.59.4.686-698.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]