SUMMARY
A nationwide study of Shiga toxin-producing Escherichia coli (STEC) was performed to determine the prevalence, characteristics and risk factors for fecal shedding of STEC among cattle in Japan. Information on rearing practices was also collected to identify risk factors for fecal shedding of STEC. STEC was isolated from 24·1% of samples (133/551) collected from 59·1% of farms (65/110). Bayesian clustering using the virulence marker profiles of the isolates subdivided the isolates into four genetically distinct groups, two of which corresponded to eae- or saa-positive STEC, which can cause severe disease in human. Both STEC groups exhibited characteristic phylogeny and virulence marker profiles. It is noteworthy that the tellurite resistance gene was not detected in all saa-positive STEC isolates, suggesting that the standard isolation method using tellurite might lead to an underestimation of the prevalence of saa-positive STEC. A multivariate logistic regression model using epidemiological information revealed a significantly (P < 0·01) high odds ratio on STEC fecal shedding in tie-stall housing and a low odds ratio in flat feed box and mechanical ventilation. Information on isolate characteristics of the two major pathotypes and risk factors in rearing practices will facilitate the development of preventative measures for STEC fecal shedding from cattle.
Key words: Cattle, Japan, prevalence, Shiga toxin-producing E. coli, virulence factor
Introduction
Shiga toxin-producing Escherichia coli (STEC) is an important cause of foodborne disease and causes diarrhea, hemorrhagic colitis and hemolytic uremic syndrome (HUS) in humans [1]. Large outbreaks of STEC are often linked to limited O serogroups, such as O157 and O26, and are caused by the consumption of contaminated food or water. By contrast, direct contact with cattle is important as a cause of sporadic cases [2].
Cattle are the principal reservoir of STEC. STEC prevalence in cattle ranges from 0·4 to 74·0% [3, 4]. This variation would be attributable to differences in farm environments and isolation methods. Temporal factors also contribute to fecal shedding of the bacterium [5]. Identifying risk factors that affect the prevalence of STEC can lead to developing intervention strategies for decreasing the fecal shedding. In other countries, various risk factors have been identified, including age of cattle, drinking water and feed ingredients [6–8]. However, in Japan, nationwide studies of the prevalence of STEC and associated rearing practices to identify risk factors for fecal shedding are lacking with only surveillance reports on the prevalence of a limited number of serogroups and regions being available [9, 10]. Therefore, the current study attempted to identify the risk factors for developing the intervention strategies.
As well as the prevalence, the strain characteristics are important. The principal virulence factor of STEC is Shiga toxin (Stx), which plays an important role in developing bloody diarrhea and HUS [11]. STEC secretes Stx1, Stx2 or both, and there are multiple Stx subtypes [11, 12]. Stx1a, Stx2a, Stx2c, and Stx2d are frequently detected from the human clinical isolates. However, the role in pathogenicity remains unclear. In addition to Stx, certain characteristics of STEC isolates are important for the development of vaccines, as well as elucidating its pathogenicity. Effector proteins secreted by a type III secretion system in STEC are essential for attachment to the intestinal epithelium in the initial stage of infection and thus are used in the vaccine against STEC O157 [13]. The type III secretion system is encoded by a genomic island known as the locus of enterocyte and effacement (LEE) region. This secretion system is responsible for the development of the characteristic attaching/effacing (AE) lesion, and STEC harboring this lesion is called AE-STEC [14]. In addition to AE-STEC, STEC can be divided into several other pathotypes according to their adhesins: Agg-STEC (which produces aggregative adherence fimbria, i.e., AAF/Hda adhesins) and Saa-STEC (which produces STEC auto-agglutinating adhesion, i.e., Saa) [14]. Most of severe cases in humans are attributable to these pathotypes. In addition, E. coli immunoglobulin-binding (Eib) protein was identified as an adhesin in LEE-negative STEC isolated from HUS patients [15]. Because STEC carrying more than two of these adhesins has not been observed, these pathotypes would be phylogenetically distinct. The potential relationships between phylogeny and various virulence factors are of great interest because adhesins themselves and other related markers can be potential targets for vaccine development. However, the relationships among the pathotypes, phylogeny and various virulence markers of STEC [16–18] in cattle isolates have not been fully elucidated.
In this study, we performed a nationwide investigation of the prevalence of STEC and rearing practices in cattle farms in Japan for the following purposes: (i) to characterize virulence factors and phylogenies of STEC isolates among cattle in Japan, and (ii) to identify the risk factors for fecal shedding based on the rearing practices of farms. This information will facilitate the development of techniques to reduce fecal shedding of important STEC isolates from cattle.
Methods
Sample collection
Japan consists of 47 prefectures located in eight districts. Cattle feces were collected from 110 farms in 15 prefectures, which were selected randomly, located in seven districts in Japan from May 2013 to February 2014 by veterinarians at local livestock hygiene service centers. One-third of domestic cattle population in Japan are kept in these 15 prefectures. Two to 15 farms from each prefecture were selected by convenience sampling and each farm was selected not to be close to other sampling farms. Because the reported STEC prevalence in cattle ranges from 0·4 to 74·0% [3, 4], we assumed that the prevalence of STEC in each farm was 50% in this study. Under this assumption, the sample size required to detect at least one case of STEC in each farm with a probability of 95% was four or five [19]. Therefore, five or six fecal samples were collected from each farm. In total, 551 fecal samples were collected. During sampling, a questionnaire (Table S1) was used to collect information about the farms and cattle for epidemiological analyses as described below.
STEC isolation
Because a previous study suggested that vancomycin and cefsulodin do not suppress the growth of STEC belonging to various serogroups [20], these antibiotics were added to the enrichment medium. Nine milliliters of mEC broth (Becton, Dickinson and Company, Franklin Lakes, New Jersey, USA) containing 10 mg/L vancomycin (Wako Pure Chemical Industries, Osaka, Japan) and 3 mg/L cefsulodin (Sigma Aldrich, St. Louis, Missouri, USA) was added to one gram of a fecal sample and incubated for 20 h at 42 °C. One milliliter of the culture was subjected to DNA extraction by an alkaline boiling method as described by Beige et al. [21]. Using this template DNA, stx1, stx2, an O157-specific gene (rfbEO157) and an O26-specific gene (wzxO26) were amplified by PCR [12, 22].
In stx-positive samples, STEC isolation was attempted by the colony hybridization method. Briefly, the culture medium was spread onto a MacConkey agar plate (Becton, Dickinson and Company) and incubated at 37 °C overnight. The plate was then precooled to 4 °C, and a positively charged nylon membrane (Hybond N+, GE health care, Little Chalfont, UK) was placed on the plate. The membrane was then transferred to a fresh LB agar plate (Becton, Dickinson and Company) and incubated at 37 °C for an hour. The cultured membrane was placed on a filter paper soaked with lysis solution (0·5 M NaOH, 1·5 M NaCl), followed by soaking with neutralization solution (3 M NaCl, 0·5 M Tris-HCl). The membrane was then gently shaken in 3 × SSC solution with 0·1% SDS at 68 °C for an hour. The membrane was then baked at 80 °C for 2 h. The membrane was hybridized in PerfectHyb hybridization solution (Toyobo, Osaka, Japan) with PCR-generated digoxigenin (DIG)-labeled stx probes, stx1, stx2, and stx2f [12] according to the manufacturer's instructions (PCR DIG Labeling Mix, Roche, Basel, Switzerland). The DIG signal was detected by a DIG Nucleic Acid Detection Kit (Roche), and the positive colonies were picked to obtain pure culture.
In rfbEO157- or wzxO26-positive samples, isolation of STEC O157 and O26 was attempted using Dynabeads anti-E. coli O157 and Dynabeads EPEC/VTEC O26 (Life Technologies, Carlsbad, CA, USA), respectively according to the manufacturer's instructions. Isolates were identified as E. coli by production of the β-galactosidase, β-glucuronidase and indole. Isolates that exhibited atypical characteristics were identified as E. coli by further biochemical tests using API ID 32E (bioMérieux, Marcy l'Etoile, France).
Serotyping, determination of the phylogenetic lineage and virulence marker profiling
Serogroup identification was performed by O-genotyping PCR [23] followed by slide agglutination using corresponding antisera obtained from the Statens Serum Institute (Copenhagen, Denmark) or from Denka Seiken Co., Ltd. (Tokyo, Japan). Major phylogenetic groups (A, B1, B2 and D) were determined by PCR as described by Doumith et al. [24]. Subtypes of stx were determined by the method of Scheutz et al. [12]. PCR was employed for the detection of 21 virulence markers (Table S2), including adhesins and its regulator (eae, saa, bfpA, aggR, lpfAO113, lpfAO157/OI-141, f5, f17, f41, fedA, clpG), enterohemolysin (ehxA), plasmid-borne virulence markers (stcE and katP), and markers of pathogenicity islands (OI-43/48, OI-50, OI-57, OI-122, locus of proteolysis activity (LPA), pathogenicity island of CL3 (PAI ICL3), HPI (high-pathogenicity island)). Eib was detected by Western blotting as described previously [15]. Subtypes of eae were determined by sequencing [25].
Antimicrobial susceptibility test
The Kirby-Bauer disc diffusion test was performed using Mueller-Hinton agar plates (Becton, Dickinson and Company) according to the manufacturer's instructions using the following antimicrobials: ampicillin (10 µg), cefoxitin (30 µg), cefotaxime (30 µg), kanamycin (30 µg), streptomycin (10 µg), tetracycline (30 µg), chloramphenicol (30 µg), fosfomycin (50 µg), trimethoprim–sulfamethoxazole (1·25/23·75 µg), nalidixic acid (30 µg), gentamicin (10 µg), and ciprofloxacin (5 µg) (Becton, Dickinson and Company).
Bayesian clustering
To group isolates according to their virulence marker profiles, a Bayesian clustering method implemented in the STRUCTURE program [26] was used. Markov Chain Monte Carlo searches consisting of 100 000 ‘burn-in’ steps followed by 1 000 000 iterations were employed. The number of clusters (K) was evaluated from 1 to 20, with 20 replicate runs each under the admixture model with correlated allele frequencies. Inference for the best K was obtained by the Δ K method [27].
Epidemiological analysis
For epidemiological analyses, a questionnaire (Table S1) was designed to identify risk factors on farm management described in previous studies, including herd size [28], farm type [6], housing type [28, 29], ventilation [30], and other animals in the same farm [9, 30]. We assumed that inappropriate management of feces and feed could lead to continuous infection and transmission of STEC to other animals. Therefore, type of bedding, feedbox, and watering were also asked. As potential control measures, use of probiotics and several biosecurity measures [9] were investigated. In addition, because individual factors can influence the STEC prevalence [31], information on sex, age, and breed of individual cattle was collected. The collected information was subjected to logistic regression models. First, univariate logistic regression analysis was performed using the glm function of R version 3·1·2 [32]. The presence of STEC in the farm or cattle was considered a binary outcome variable. Data on potential risk factors collected through the questionnaire were used as explanatory variables in the models. Continuous data were transformed to categorical data (i.e., farm size was categorized into <50, <100, <300, ⩾300 head per farm; cattle <8 months old was regarded as juvenile and the other was regarded as adult.). Second, all the categorical data were transformed to dichotomous data and subjected to logistic regression analyses using glm function of R and determination coefficient between all variables was calculated by R. Third, explanatory variables that generated P values of <0·2 in univariate logistic regression analyses were subjected to multivariate logistic regression with a generalized linear mixed model using the glmmML function of R. Explanatory variables were selected for the final model to generate minimum variance by analysis of variance. Absence of collinearity among variables to be included in the multivariable models was investigated and if two variables were collinear only the variable with the lowest P value in the univariable' model was included in the analysis.
Results
Prevalence of STEC
STEC was isolated from 133 samples (24·1%) collected from 65 farms (59·1%). In STEC-positive farms, the mean prevalence was 40·9%. At this prevalence, the calculated probability to detect at least one STEC in five samples was 98·9%, which indicated that the sample size for each farm was valid. Up to three and four isolates were detected in one sample and one farm, respectively. More than two isolates were isolated from 31 farms (27·9%). A total of 112 STEC isolates were used for further analyses, as isolates with the same O serogroup and stx subtype originating from the same farm were regarded as the same clone.
Serogroups and phylogenetic groups of STEC isolates
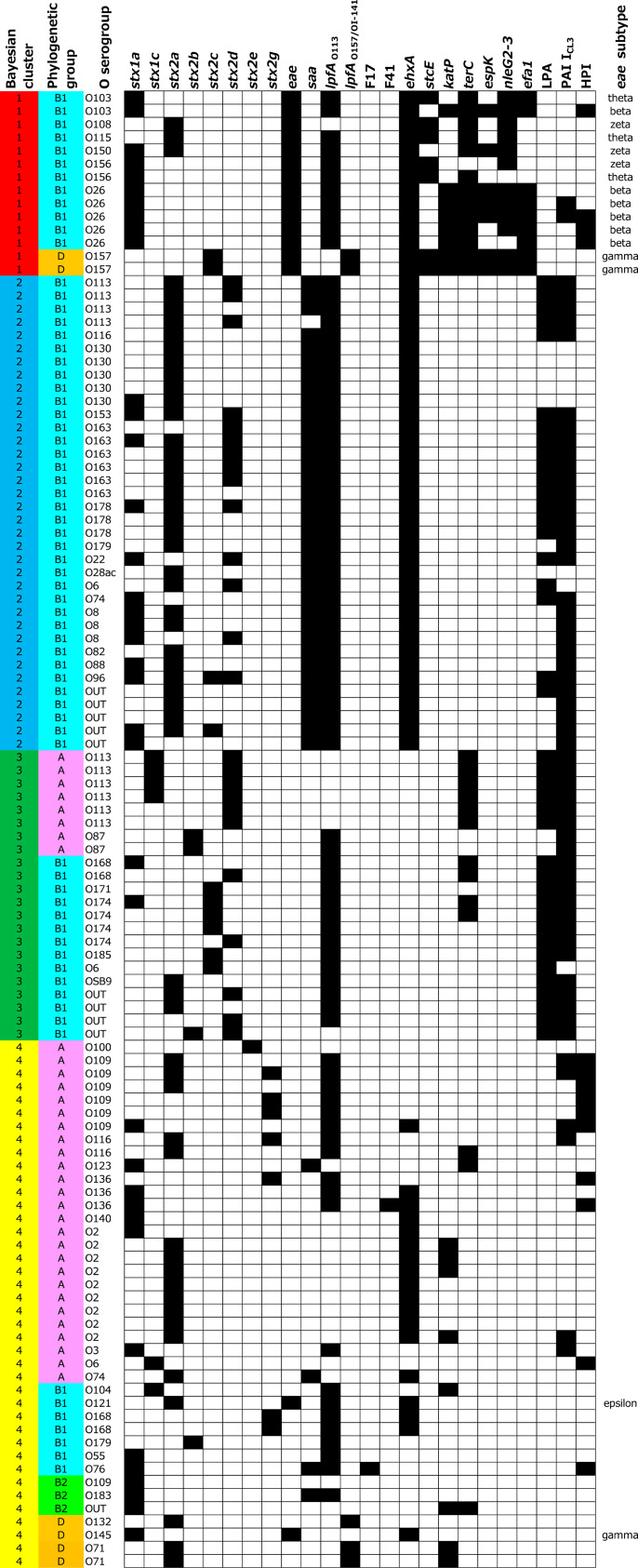
Among the 112 STEC isolates, 51 O serogroups were identified (Table 1). Among them, the common O serogroups observed in human infections were O157, O26, O103, O121, and O145. STEC O157 was isolated from four samples (0·7%) collected from two farms (1·8%). STEC O26 was isolated from nine samples (1·6%) collected from five farms (4·5%). The most prevalent phylogenetic group was B1 (69 isolates, 61·6%), followed by A (34 isolates, 30·4%) (Fig. 1). The serogroup and phylogenetic group mostly correlated. However, in some serogroups, including O6, O74, O109, O113, and O116, the isolates belonged to two phylogenetic groups (Fig. 1). One and two E. coli isolates of O157 and O26, respectively, without stx were isolated by immunomagnetic separation. Among them, one eae-positive O157 and O26 isolate each belonged to phylogenetic groups D and B1, respectively, whereas the eae-negative O26 isolate belonged to phylogenetic group B2. These isolates were not studied further.
Table 1.
Distribution of O serogroups in STEC isolates
| O serogroup | No. |
|---|---|
| O113 | 10 |
| O2 | 9 |
| O109 | 7 |
| O163 | 6 |
| O26, O130 | 5 |
| O168, O174 | 4 |
| O6, O8, O116, O136, O178 | 3 |
| O71, O74, O87, O103, O156, O157, O179 | 2 |
| O3, O22, O28ac, O55, O76, O82, O88, O96, O100, O104, O108, O115, O121, O123, O132, O140, O145, O150, O153, O171, O185, O183, OSB9* | 1 |
| OUT | 10 |
OSB9: Shigella boydii O serogroup 9.
Fig. 1.
Bayesian clustering results of STEC isolated from cattle in Japan. Each cluster or phylogenetic group is highlighted by a different color in the first and second columns. The distribution of virulence markers is shown in columns 4–27; black = presence; white = absence. The results for stx1d, stx2f, bfpA, aggR, f5, fedA, clpG, and Eib are omitted because no isolate was positive for these markers.
Prevalence and subtypes of stx and adhesins
Various stx subtypes, including stx1a, stx1c, stx2a, stx2b, stx2c, stx2d, stx2e, and stx2g, were detected among the isolates (Fig. 1). The most prevalent subtypes in stx1 and stx2 were stx1a (86·4%) and stx2a (64·7%), respectively. Several types of adhesins were detected in the isolates. Sixteen isolates (14·3%) were positive for eae, and 39 isolates (34·8%) were positive for saa. Isolates with both eae and saa positive were not found. Most of the eae-positive isolates belonged to serogroups commonly involved in human infections, including O157, O26, O103, O121, and O145. The subtypes of eae in these isolates were beta, epsilon, or gamma (Fig. 1), as reported in human isolates [33, 34], while isolates in uncommon serogroups, O108, O115, O150, and O156, possessed theta and zeta. Most the isolates (73·2%) possessed lpfAO113, whereas five isolates that belonged to phylogenetic group D were positive for lpfAO157/OI-141. Only one isolate each was positive for f17 or f41. Other adhesins investigated in this study, including bfpA, aggR, f5, fedA, clpG, and Eib, were not detected in any isolates (Fig. 1).
Virulence marker profiles and Bayesian clustering
To determine significant clusters based on the distribution of virulence markers, the virulence marker profiles were subjected to a Bayesian clustering approach. The Δ K method revealed that the most appropriate number of clusters was four (Fig. S1).
The results of clustering and the distribution patterns of the virulence markers are shown in Fig. 1. Bayesian cluster 1 consisted of eae-positive STECs, and their phylogenetic group was either B1 or D. In this group, lpfAO113, ehxA, terC, nleG2-3, and efa1 were highly prevalent. The virulence marker profiles of isolates belonging to the O121 and O145 serotypes, which were also eae positive, differed from those in cluster 1, and thus these isolates fell into cluster 4. Bayesian cluster 2 consisted of phylogenetic group B1 and exhibited highly homogeneous virulence marker profiles. All but one isolate possessed saa. Most of the isolates possessed stx2a, lpfAO113, and ehxA and two pathogenicity islands, LPA and PAI ICL3. Bayesian cluster 3 was similar to cluster 2 in that LPA and PAI ICL3 were prevalent. This cluster could be subdivided into two groups. One was a group belonging to phylogenetic group B1. This group exhibited a virulence marker profile similar to that of cluster 2 except for the absence of saa and ehxA. The other group consisted of mainly O113 isolates belonging to phylogenetic group A. This group exhibited a high prevalence of stx2d and terC. Bayesian cluster 4 was the most heterogeneous group. This cluster consisted of the isolates with various stx subtypes and were from all four phylogenetic groups. A few isolates carried eae or saa but had different virulence marker profiles than clusters 1 or 2, respectively.
Antimicrobial resistance
Antimicrobial resistance was observed for 7 of 12 antimicrobials used in this study. The resistance rate in the STEC isolates was highest against tetracycline (31·3%), followed by streptomycin (24·1%) (Fig. S2). When the results were stratified by the Bayesian clusters, differences were apparent. No antimicrobial-resistant isolates were present in cluster 2, whereas a relatively high rate of resistance was observed in cluster 3.
Risk factors associated with fecal shedding of STEC
Several risk factors for fecal STEC shedding were identified (Table 2). In the final model, high STEC prevalence was associated with tie-stall housing, and low prevalence was associated with flat feed boxes and mechanical ventilation (P < 0·01). Weak associations (0·01 ⩽ P < 0·05) were observed between high prevalence and free barn housing or female cattle and between low prevalence and manure bedding or adult cattle. Meanwhile, associations between STEC shedding and other known risk factors, including season, breed and water equipment, was not significant.
Table 2.
Risk factors for STEC fecal shedding in multivariable logistic regression analyses with mixed models
| Variable | No. of positive farm/sample | Total | Prevalence | Odds ratio (95% confidence interval) | P value |
|---|---|---|---|---|---|
| Farm level | |||||
| Housing: tie stall | 26 | 36 | 0·72 | 3·5 (1·7–7·1) | 0·001 |
| Feed box: flat | 17 | 35 | 0·49 | 0·3 (0·1–0·6) | 0·001 |
| Ventilation: mechanical | 39 | 72 | 0·54 | 0·4 (0·2–0·8) | 0·005 |
| Housing: free barn | 23 | 35 | 0·66 | 2·1 (1·1–4·1) | 0·032 |
| Bedding: manure | 2 | 7 | 0·29 | 0·2 (0·0–1·0) | 0·045 |
| Presence of pigs on the same farm | 2 | 3 | 0·67 | 4·5 (0·8–25·6) | 0·093 |
| Sample level | |||||
| Female | 117 | 415 | 0·28 | 2·5 (1·1–5·5) | 0·021 |
| Adult | 119 | 501 | 0·24 | 0·3 (0·1–0·9) | 0·034 |
Discussion
Our nationwide investigation revealed that more than half of the farms were contaminated by STEC in Japan. Kobayashi et al. [10] isolated STEC from 19% to 31% of samples collected from 69% of sampling farms in a limited area of Japan, consistent with our results. In another nationwide study, the prevalence of O157 and O26 was much higher than that in the present study [9]. This discrepancy could be due to differences in isolation methods. The cited study used novobiocin, cefixime, and potassium tellurite in the enrichment broth and agar plates, whereas we used mEC broth supplemented with cefsulodin and vancomycin and MacConkey agar plates without antimicrobials. Although our method might under-represent the prevalence of O157, it should have contributed to the isolation of a wide variety of STEC. In fact, 76·8% of the isolates did not possess terC, which is responsible for tellurite resistance. If potassium tellurite had been used in this study, many of these ter-negative isolates would not have been isolated.
Virulence marker profiling and Bayesian clustering revealed that STEC in the cattle were genetically heterogeneous, although AE- and Saa-STEC isolates showed similar virulence marker profiles. This result is important because the majority of severe infection in humans is responsible for these pathotypes. In AE-STEC, most of the isolates belonged to the common O serogroups isolated in human infection, including O157. The other O serogroups, O108, O115, O150, and O156, are not commonly isolated in human infection. The eae subtypes of these isolates, eae-theta and eae-zeta, are often detected in animal isolates [35]. Most of the Saa-STEC isolates fell into cluster 2 via Bayesian clustering in this study (Fig. 1). This cluster has a characteristic high prevalence of stx2a, lpfAO113, ehxA, LPA, and PAI ICL3 and a low prevalence of other virulence markers. LPA and PAI ICL3 encode homologs of adherence-mediating Iha [36] and Yersinia pestis-like adhesion [37], respectively. Because these pathogenicity islands are more likely to be detected in animal isolates, genes in the islands may have a role in the colonization of animal guts. No saa-positive isolates were resistant to any of the antimicrobials used in this study (Fig. S2). These results suggest that the Saa-STEC observed in this study might consist of phylogenetically related isolates. In Saa-STEC, O113:H21, O48:H21, and O91:H21 have been implicated as causative agents of HUS [38]. Some of our Saa-STEC isolates displayed virulence marker profiles similar to those of the above-mentioned serotypes, such as the presence of ehxA. These Saa-STEC isolates might have the virulence potential to cause severe disease in humans. These findings could be useful to select candidate markers for vaccine development against AE- and Saa-STEC. Vaccines against type III secreted proteins [13] and siderophore receptor/porin proteins [39] have been reported for STEC O157, but no successful vaccine against Saa-STEC is available. Thus, immunological responses against Saa or other virulence markers should be further studied to improve vaccine development. Surprisingly, all Saa-STEC isolates but one did not possess terC. This result implies that a standard isolation method for STEC using cefixime and potassium tellurite would fail to isolate the Saa-STEC isolates as described above.
The virulence potential of the eae- and saa-negative STEC isolates in this study remains unclear. Isolates in cluster 3 were genetically homogeneous, with a high prevalence of LPA and PAI ICL3 (Fig. 1). Cluster 3 differed from cluster 2 by the absence of saa and ehxA, and thus acquisition of plasmids carrying those genes may lead to a gain in virulence. In contrast to the other clusters, the virulence marker profiles of the isolates from cluster 4 were highly heterogeneous. In this cluster, non-pathogenic E. coli might acquire stx occasionally and play a role as a reservoir of stx.
The prevalence of antimicrobial-resistant isolates was comparable to that observed in previous investigations of commensal E. coli and STEC (Fig. S2) [40, 41]. The relatively higher prevalence of isolates resistant against tetracycline and streptomycin can be attributed to frequent usage of those agents in cattle [42]. However, risk against public health of antimicrobial resistance STEC would be limited so far because no isolates showed resistance against fosfomycin, which is commonly used as first-line treatment for human STEC infection. Interestingly, the Bayesian clustering also elucidate biased distribution of antimicrobial resistance. In cluster 2, no isolates exhibited resistance, whereas isolates in cluster 3 exhibited a higher prevalence of resistance to tetracycline and streptomycin, regardless of the phylogenetic group. The isolates in these clusters may have different mechanisms in acquisition of resistance genes. These results support the hypothesis that the Bayesian clusters reflect not only the phylogeny of STEC but also their distinct phenotypes.
In epidemiological analyses, several risk factors associated with the fecal shedding were identified. The results were in accordance with previous studies indicating that the incidence rates of diseases are lower in free stall than in tie-stall [28, 29] housing and that the prevalence of STEC O157 is lower in farms with mechanical ventilation compared with natural ventilation (Table 2) [30]. Flat feed boxes also contributed to a lower prevalence of STEC, possibly due to the ease of cleaning compared with other feeding mechanisms, including indent and box types. We expected the bedding substance to be important for the persistence of STEC in the environment, but only manure appeared to have a weak (P = 0·045), negative association with STEC shedding. Because only seven farms used manure as bedding, this association requires further investigation. Interestingly, female cattle displayed a higher odds ratio for STEC shedding in this study. Female breeding cattle were previously identified as a risk factor for fecal shedding in Scottish farms [31]. Calves <8 months old had a weak association with fecal shedding. Because some serogroups can cause diarrhea in calves [43, 44], calves may be more likely to shed STEC. In contrast, statistical significances were not detected from some known risk factors, including the presence of other animals and farm type (cow or beef) [9]. The aim of our study was to investigate risk factors on different types of cattle farm. However, management practices would differ widely in farm type and region. To confirm risk factors revealed in our results, intervention studies are essential. Studies on intervention strategies such as a management of fecal wastes may be helpful to find out effective control measures, because feces and hides or environment contaminated by feces can promote continuous infection and transmission of STEC to other animals in cattle farms [45].
In conclusion, unbiased sampling and isolation method contributed to the effective isolation of a wide variety of STEC from more than half of sampled farms in Japan. Clustering analysis of the isolates revealed that AE- and Saa-STEC are widely distributed among cattle in Japan. These adhesins might play an important role in the colonization of cattle as well as severe disease in humans. The virulence characteristics of the AE- and Saa-STEC isolates presented may provide insight into controlling STEC on farms and for developing detection methods and vaccines.
ACKNOWLEDGEMENTS
This study was supported by grants-in-aid from the Japan Society for the Promotion of Science fellows (25–5234), and the Japan Agency for Medical Research and Development, AMED, a Grant-in-Aid for the Research Program on Emerging and Re-emerging Infectious Diseases (15fk0108021h0002). The authors are grateful to the veterinarians at the local livestock hygiene service centers for collecting fecal samples and questionnaires. They are also grateful to Atsushi Iguchi, University of Miyazaki, for O-genotyping.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268817000474.
click here to view supplementary material
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Gyles CL. Shiga toxin-producing Escherichia coli: an overview. Journal of Animal Science 2007; 85: E45–E62. [DOI] [PubMed] [Google Scholar]
- 2.Strachan NJC, et al. Escherichia coli O157: burger bug or environmental pathogen? International Journal of Food Microbiology 2006; 112: 129–137. [DOI] [PubMed] [Google Scholar]
- 3.Hussein HS, Bollinger LM. Prevalence of Shiga toxin-producing Escherichia coli in beef cattle. Journal of Food Protection 2005; 68: 2224–2241. [DOI] [PubMed] [Google Scholar]
- 4.Hussein HS, Sakuma T. Prevalence of Shiga toxin-producing Escherichia coli in dairy cattle and their products. Journal of Dairy Science 2005; 88: 450–465. [DOI] [PubMed] [Google Scholar]
- 5.Lammers GA, et al. Synchronization of E. coli O157 shedding in a grass-fed beef herd: a longitudinal study. Epidemiology and Infection 2015; 143: 3244–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Venegas-Vargas C, et al. Factors associated with Shiga toxin-producing Escherichia coli shedding by dairy and beef cattle. Applied and Environmental Microbiology 2016; 82: 5049–5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hussein HS, Lake SL, Ringkob TP. Review: cattle as a reservoir of Shiga-like toxin-producing Escherichia coli including O157:H7 – pre- and post-harvest control measures to assure beef safety. Professional Animal Scientist 2001; 17: 1–16. [Google Scholar]
- 8.Kuhnert P, et al. Prevalence and risk-factor analysis of Shiga toxigenic Escherichia coli in faecal samples of organically and conventionally farmed dairy cattle. Veterinary Microbiology 2005; 109: 37–45. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki Y, et al. Prevalence and characterization of Shiga toxin-producing Escherichia coli O157 and O26 in beef farms. Veterinary Microbiology 2011; 150: 140–145. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi H, et al. Prevalence and characteristics of Shiga toxin-producing Escherichia coli from healthy cattle in Japan. Applied and Environmental Microbiology 2001; 67: 484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayer CL, et al. Shiga toxins and the pathophysiology of hemolytic uremic syndrome in humans and animals. Toxins (Basel) 2012; 4: 1261–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheutz F, et al. Multicenter evaluation of a sequence-based protocol for subtyping Shiga toxins and standardizing Stx nomenclature. Journal of Clinical Microbiology 2012; 50: 2951–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith DR, et al. A two-dose regimen of a vaccine against type III secreted proteins reduced Escherichia coli O157:H7 colonization of the terminal rectum in beef cattle in commercial feedlots. Foodborne Pathogens and Disease 2009; 6: 155–161. [DOI] [PubMed] [Google Scholar]
- 14.Pierard D, et al. O157:H7 and O104:H4 Vero/Shiga toxin-producing Escherichia coli outbreaks: respective role of cattle and humans. Veterinary Research 2012; 43: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu Y, et al. A new immunoglobulin-binding protein, EibG, is responsible for the chain-like adhesion phenotype of locus of enterocyte effacement-negative, Shiga toxin-producing Escherichia coli. Infection and Immunity 2006; 74: 5747–5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Renter DG, et al. Prevalence, risk factors, O serogroups, and virulence profiles of Shiga toxin-producing bacteria from cattle production environments. Journal of Food Protection 2005; 68: 1556–1565. [DOI] [PubMed] [Google Scholar]
- 17.Cho S, et al. Prevalence of Shiga toxin-encoding bacteria and Shiga toxin-producing Escherichia coli isolates from dairy farms and county fairs. Veterinary Microbiology 2006; 118: 289–298. [DOI] [PubMed] [Google Scholar]
- 18.Lee K, et al. Multivariate analyses revealed distinctive features differentiating human and cattle isolates of Shiga toxin-producing Escherichia coli O157 in Japan. Journal of Clinical Microbiology 2011; 49: 1495–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thrusfield M. Survey. In: Veterinary Epidemiology, 3rd edn. Oxford, UK: Blackwell Science Ltd., 2009, pp. 228–246. [Google Scholar]
- 20.Gill A, et al. Development of a method for the detection of verotoxin-producing Escherichia coli in food. Journal of Food Protection 2012; 75: 827–837. [DOI] [PubMed] [Google Scholar]
- 21.Beige J, et al. Clinical evaluation of a Mycobacterium tuberculosis PCR assay. Journal of Clinical Microbiology 1995; 33: 90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paddock Z, et al. Applicability of a multiplex PCR to detect O26, O45, O103, O111, O121, O145, and O157 serogroups of Escherichia coli in cattle feces. Veterinary Microbiology 2012; 156: 381–388. [DOI] [PubMed] [Google Scholar]
- 23.Iguchi A, et al. Escherichia coli O-Genotyping PCR: a comprehensive and practical platform for molecular O serogrouping. Journal of Clinical Microbiology 2015; 53: 2427–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doumith M, et al. Improved multiplex PCR strategy for rapid assignment of the four major Escherichia coli phylogenetic groups. Journal of Clinical Microbiology 2012; 50: 3108–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramachandran V, et al. Distribution of intimin subtypes among Escherichia coli isolates from ruminant and human sources. Journal of Clinical Microbiology 2003; 41: 5022–5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics 2000; 155: 945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Molecular Ecology 2005; 14: 2611–2620. [DOI] [PubMed] [Google Scholar]
- 28.Valde JP, et al. Comparison of ketosis, clinical mastitis, somatic cell count, and reproductive performance between free stall and tie stall barns in Norwegian dairy herds with automatic feeding. Acta Veterinaria Scandinavica 1997; 38: 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simensen E, et al. Housing system and herd size interactions in Norwegian dairy herds; associations with performance and disease incidence. Acta Veterinaria Scandinavica 2010; 52: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berends I, et al. Prevalence of VTEC O157 in dairy and veal herds and risk factors for veal herds. Preventive Veterinary Medicine 2008; 87: 301–310. [DOI] [PubMed] [Google Scholar]
- 31.Chase-Topping ME, et al. Risk factors for the presence of high-level shedders of Escherichia coli O157 on Scottish farms. Journal of Clinical Microbiology 2007; 45: 1594–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.R Core Team. R: a Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2014. [Google Scholar]
- 33.Blanco JE, et al. Serotypes, virulence genes, and intimin types of Shiga toxin (verotoxin)-producing Escherichia coli isolates from human patients: prevalence in Lugo, Spain, from 1992 through 1999. Journal of Clinical Microbiology 2004; 42: 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mora A, et al. Seropathotypes, phylogroups, Stx subtypes, and intimin types of wildlife-carried, Shiga toxin-producing Escherichia coli strains with the same characteristics as human-pathogenic isolates. Applied and Environmental Microbiology 2012; 78: 2578–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cookson AL, et al. Intimin subtyping of Escherichia coli: concomitant carriage of multiple intimin subtypes from forage-fed cattle and sheep. FEMS Microbiology Letters 2007; 272: 163–171. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt H, et al. Identification and characterization of a novel genomic island integrated at selC in locus of enterocyte effacement-negative, Shiga toxin-producing Escherichia coli. Infection and Immunity 2001; 69: 6863–6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen S, et al. Evidence for a hybrid genomic island in verocytotoxin-producing Escherichia coli CL3 (serotype O113:H21) containing segments of EDL933 (serotype O157:H7) O islands 122 and 48. Infection and Immunity 2004; 72: 1496–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paton AW, et al. Characterization of Saa, a novel autoagglutinating adhesin produced by locus of enterocyte effacement-negative Shiga-toxigenic Escherichia coli strains that are virulent for humans. Infection and Immunity 2001; 69: 6999–7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fox JT, et al. Efficacy of Escherichia coli O157:H7 siderophore receptor/porin proteins-based vaccine in feedlot cattle naturally shedding E. coli O157. Foodborne Pathogens and Disease 2009; 6: 893–899. [DOI] [PubMed] [Google Scholar]
- 40.Sasaki Y, et al. Antimicrobial resistance in Shiga toxin-producing Escherichia coli O157 and O26 isolates from beef cattle. Japanese Journal of Infectious Diseases 2012; 65: 117–121. [PubMed] [Google Scholar]
- 41.Kijima-Tanaka M, et al. A national surveillance of antimicrobial resistance in Escherichia coli isolated from food-producing animals in Japan. Journal of Antimicrobial Chemotherapy 2003; 51: 447–451. [DOI] [PubMed] [Google Scholar]
- 42.Hosoi Y, et al. Sales of veterinary antimicrobial agents for therapeutic use in food-producing animal species in Japan between 2005 and 2010. Scientific and Technical Review 2014; 33: 1007–1015. [DOI] [PubMed] [Google Scholar]
- 43.Sandhu KS, Gyles CL. Pathogenic Shiga toxin-producing Escherichia coli in the intestine of calves. Canadian Journal of Veterinary Research 2002; 66: 65–72. [PMC free article] [PubMed] [Google Scholar]
- 44.Dorn CR, et al. Characteristics of Vero cytotoxin producing Escherichia coli associated with intestinal colonization and diarrhea in calves. Veterinary Microbiology 1993; 36: 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams KJ, et al. Risk factors for Escherichia coli O157 shedding and super-shedding by dairy heifers at pasture. Epidemiology and Infection 2015; 143: 1004–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268817000474.
click here to view supplementary material