SUMMARY
Changes in respiratory pathogen testing can affect disease burden estimates. Using linked data, we describe changes in respiratory virus testing among children born in Western Australia in 1996–2012. We extracted data on respiratory specimens from these children from birth to age 9 years. We estimated testing rates by age, year, Aboriginal status and geographical location. Predictors of testing among children hospitalised at least once before their 10th birthday were identified using logistic regression. We compared detection methods for respiratory viruses from nasal/nasopharyngeal (NP) specimens by age and year. Of 83 199 virology testing records in 2000–2012, 80% were nasal/NP specimens. Infants aged <1 month had the highest testing rates. Testing rates in all children increased over the study period with considerable yearly fluctuations. Among hospitalised children, premature children <32 weeks gestation had over three times the odds of being tested (95% CI 3·47–4·13) than those born at term. Testing using molecular methods increased from 5% to 87% over the study period. Proportion of positive samples increased from 36·3% to 44·4% (P < 0·01); this change was greatest in children aged 2–9 years. These findings will assist in interpreting results from future epidemiology studies assessing the pathogen-specific burden of disease.
Key words: Diagnostic testing, record linkage, respiratory infections, virology (human) and epidemiology
INTRODUCTION
Globally, acute lower respiratory infections (ALRI) cause significant childhood morbidity [1]. ALRI are associated with numerous bacterial and viral pathogens including Streptococcus pneumoniae, influenza and respiratory syncytial virus (RSV). A shift towards testing using molecular methods including polymerase chain reaction (PCR) to detect respiratory viruses has occurred due to its improved sensitivity and specificity over traditional methods [2, 3]. This shift may have resulted in artificial increases in estimates of disease incidence and prevalence over time [4]. A detailed understanding of changes in testing for respiratory pathogens is needed when interpreting longitudinal data on the burden of ALRI, particularly in children severe enough to warrant admission to hospital.
Western Australia (WA) is in a unique position to describe changes in pathogen testing using linked data via a state-wide record linkage system [5]. Record linkage is a method of connecting data from multiple sources relating to the same person, time or place [5]. Datasets available for linkage in WA include administrative registries managed by the state, geocoding information and selected datasets managed by other organisations such as Silver Chain Nursing and St John Ambulance [6]. We have previously demonstrated the feasibility and validity of using linked data obtained via this system to combine and analyse laboratory data alongside clinical data on respiratory infections [7, 8].
Within Australia's publicly funded healthcare system, PathWest Laboratory Medicine WA (PathWest) provides reference pathology services to the single tertiary paediatric hospital and all but three of the other hospitals in WA that admit children [9]. In addition, most virology testing is referred from private laboratories to PathWest. Therefore, testing changes observed from PathWest virology data are indicative of overall changes in hospital-based testing practices throughout the state.
As a precursor to future work investigating the pathogen-specific burden of ALRI, we sought to describe the changes over time in the frequency of and detection methods for (primarily hospital-based) respiratory virus testing in a cohort of WA-born children aged less than 10 years at the time of testing. We also aimed to identify demographic factors present at birth that are associated with increased likelihood of testing among a subset of these children who were hospitalised.
METHODS
WA covers a geographical area of two and a half million square kilometres with a population of over two and a half million people, of whom an estimated 4% identify as Aboriginal or Torres Strait Islander (hereafter referred to as Aboriginal) [10]. Data used for this study were part of a record linkage study established to investigate the pathogen-specific burden of ALRI in children born in WA between 1996 and 2012. The birth cohort was identified using data extracted from the Midwives Notifications System, Birth Registrations and Death Registrations provided via the WA Data Linkage Branch.
As Australia does not have a unique identifier for each person, probabilistic matching of identifiers from each dataset (e.g. name, address and date of birth) was used by the WA Data Linkage Branch to identify the same person across multiple datasets. These links are manually reviewed if the identifiers differ between datasets, with an estimated false negative rate of 2–3% for most routine linkages [11].
Data pertaining to in-patient hospitalisations from children in the cohort were derived from the Hospital Morbidity Data Collection. Only hospital stays that included both an admission and separation during the study period (1996–2012) were included in these analyses and are henceforth referred to as hospital admissions. Records from PathWest concerning routine respiratory specimens collected from children in the birth cohort were then extracted and analysed. Approximately 95% of PathWest records extracted for the study were successfully linked to children in the birth cohort.
Demographic data
Aboriginal children were identified using a derived flag for Aboriginal status provided by the WA Data Linkage Branch [12]. Non-Aboriginal children were further classified as Caucasian or other, based on the ethnicity of their mother. Residential postcode of the mother at the time of her child's birth was used to allocate children to metropolitan, rural or remote regions. Children born between December and February were classified as born in summer, between March and May as autumn, between June and August as winter and between September and November as spring.
Gestational age was calculated using Midwives Notifications System data based on a combination of variables [13]. These were grouped into very preterm (less than 32 weeks), preterm (32–36 weeks) and term (more than 36 weeks). Data on maternal smoking during pregnancy, mode of delivery and special care at birth were sourced from the Midwives Notifications System. Mode of delivery was coded as per a prior study [14]. A child was considered to have required special care if they spent at least one day in a level 2 or 3 neonatal nursery.
We used the Socio-Economic Indexes for Areas (SEIFA) [15] scores to measure socioeconomic status. SEIFA scores were calculated at the collection district level based on the boundaries used on the child's year of birth. Each collection district comprises of approximately 250 households in an urban area and is the smallest area from which SEIFA scores may be calculated [15]. Of the four slightly differing SEIFA scores available, we chose to use the Index of Relative Socio-Economic Advantage and Disadvantage, which is derived from education, occupation, income, housing, disability and family measures collected from the Census of Population and Housing [15]. Age of the child was recorded at the time of specimen collection and specimens collected at 10 years old or older were ignored as there were so few.
Laboratory data
As there were no PathWest data available pre-2000 and no coded mortality data available post-2012, all outcome datasets used for this study were restricted to the period between January 2000 and December 2012. All records of testing, regardless of whether a pathogen was detected or if the child was ever hospitalised, were included. Records were combined if they related to the same specimen collected from the same child on the same date.
Respiratory specimens were defined as nasal/nasopharyngeal (NP), throat, tracheal, bronchial, sputum, lung, pleural and serum. Nasal/NP specimens included combined nose and throat, NP, post-nasal swabs and per-nasal aspirates. Specimens collected on or after the date of death were considered to be post-mortem specimens and excluded from subsequent analyses.
Viruses were tested using one or more methods including culture, antigen detection, PCR or serology. Test protocols have changed over time within PathWest Laboratories. The most frequently utilised methods have included viral culture (Madin-Darby canine kidney cells and diploid human lung fibroblasts with confirmation using immunofluorescent (IF) antibodies (Oxoid Microbiology; ThermoFisher, Waltham, MA)), antigen detection using direct IF antibodies (Chemicon; Millipore Corporation, Billerica, MA) and multiplex tandem PCR. Serology for respiratory viruses is most frequently undertaken using complement fixation tests. A specimen was considered to have tested positive if a known viral respiratory pathogen was detected by any method. These include detections of human adenovirus, coronavirus, influenza virus (any subtype), human metapneumovirus, parainfluenza virus (any subtype), rhinovirus (or unspecified picornavirus from the respiratory tract) or RSV. Multiplex PCR and antigen detection assays were used to test for adenovirus, influenza virus, parainfluenza virus and RSV simultaneously. Human metapneumovirus was added to the multiplex PCR assay in 2003 and to the antigen detection assay utilised from 2008.
Statistical analysis and ethical approvals
Using person-time-at-risk as the denominator (derived by dates of birth, death and the end of the study period), we estimated the testing rate per 1000 child-years and incidence rate ratios (IRR) in the PathWest dataset among Aboriginal and non-Aboriginal children by age, geographical region of birth and year of testing. Testing rates were presented separately for Aboriginal children and non-Aboriginal children due to differences in risk and incidence of respiratory infections [16, 17].
Given the similarities in testing trends over time for some age groups, we chose to divide children into age groups of less than a month; 1–5 months; 6–23 months; and 2–9 years. The two youngest age groups were combined for some analyses for the same reason. Segregating by year, we calculated the proportions of nasal/NP specimens that underwent testing using culture, PCR and direct IF antibody detection, as well as the proportion with at least one respiratory virus detected. These analyses were restricted to nasal/NP specimens only as they represented the bulk of respiratory specimens collected among this cohort.
To identify demographic predictors of testing, we returned to the birth cohort and identified children admitted as in-patients to hospital between 2000 and 2012. We then merged this with the PathWest dataset to determine the number of children admitted as in-patients who were tested at least once for respiratory viruses before they turned 10 years old. Logistic regression with demographic variables at birth was used to obtain univariable and multivariable models of odds of being tested for respiratory viruses. Individuals with missing data on any of the variables included in the multivariable model were dropped. Demographic variables were selected based on known risk factors for hospital admission and respiratory infections. These included region at birth, season and year of birth, gender, ethnicity, gestational age, maternal smoking during pregnancy, mode of delivery, multiple births, SEIFA scores and special care requirements at birth. All variables were included as categorical variables except for year of birth.
Data cleaning and descriptive analyses were performed in IBM SPSS Statistics (version 23). IRR with exact 95% confidence intervals (CI) were calculated using EpiBasic [18]. Changes in proportions over time were compared using trend tests in EpiBasic [18]. Logistic regression analyses were performed in Stata (version 14·1). Ethical approvals were obtained from the WA Department of Health Human Research Ethics Committee, the WA Aboriginal Health Ethics Committee and the University of Western Australia Human Research Ethics Committee.
RESULTS
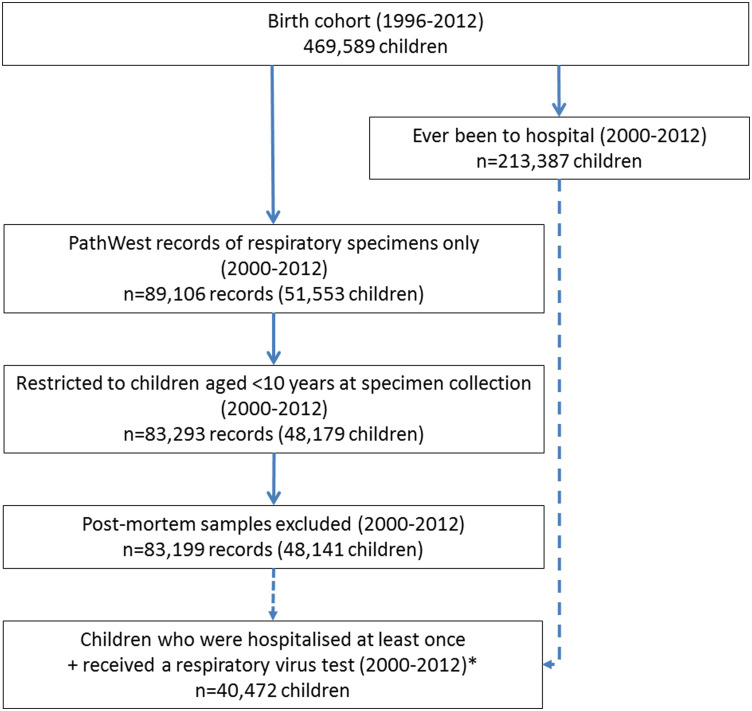
The birth cohort comprised 469 589 children born in WA from 1996 to 2012, of whom approximately half (51·2%, n = 240 237) were male and 6·7% (n = 31 348) were Aboriginal. Of these, 48 179 children were less than 10 years old when they underwent testing for respiratory viruses (Fig. 1). These children contributed 83 293 test records, of which 0·1% (n = 94) related to specimens collected on or after the date of death. These were mainly lung and/or tracheal specimens (n = 33).
Fig. 1.
Flow chart of data cleaning and cohort. Legend: solid arrow denotes that the latter dataset is a subgroup of the prior dataset. Dashed arrows denotes that the prior dataset feeds into the latter dataset. *Only data from this subgroup of children were used to generate results for Table 2. PathWest, PathWest Laboratory Medicine WA Database.
Excluding records relating to post-mortem specimens, there were 83 199 PathWest records remaining from 48 141 children (Fig. 1). Males accounted for 55·7% (n = 26 825) of this cohort, 11·0% (n = 5277) were Aboriginal and 76·8% (n = 36 958) were born in metropolitan Perth. Of all the specimens collected from these children, 80·1% (n = 66 606) were nasal/NP specimens, while the next most common specimen, throat samples, accounted for 8·2% (n = 6836).
Changes in frequency of testing
The overall rate of respiratory virus testing in children aged less than 10 years was 28·4 per 1000 child-years, although there were significant differences in the rate of testing by age, region at birth, Aboriginality and year of specimen collection. Despite the small number of infants aged less than a month, this group had the highest rates of testing (Table 1). Among non-Aboriginal children, infants aged less than a month received virology tests two times more than children aged 1–5 months (95% CI 2·05–2·17), five times more than children aged 6–23 months (95% CI 4·89–5·16) and 22 times more than children aged 2–9 years (Table 1). A similar pattern was observed among Aboriginal children and across different locations.
Table 1.
Overall frequency and rate per 1000 child-years of respiratory viral testing, 2000–2012
| <1 month | 1–5 months | 6–23 months | 2–9 years | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Description | n | rate | IRR | (95% CI) | n | rate | IRR | (95% CI) | n | rate | IRR | (95% CI) | n | rate | IRR |
| Aboriginal status | |||||||||||||||
| Non-Aboriginal | 7109 | 250·8 | 22·2 | (21·6–22·8) | 16 861 | 119·0 | 10·5 | (10·3–10·7) | 24 928 | 49·9 | 4·4 | (4·3–4·5) | 23 349 | 11·3 | Ref. |
| Aboriginal | 944 | 465·7 | 28·5 | (26·4–30·7) | 3385 | 334·0 | 20·4 | (19·4–21·5) | 4153 | 115·3 | 7·1 | (6·7–7·4) | 2470 | 16·4 | Ref. |
| Region at birth | |||||||||||||||
| Metropolitan | 6246 | 273·4 | 22·8 | (22·2–23·5) | 15 862 | 138·9 | 11·6 | (11·4–11·8) | 22 542 | 56·4 | 4·7 | (4·6–4·8) | 19 441 | 12·0 | Ref. |
| Rural | 1066 | 216·2 | 21·2 | (19·8–22·7) | 2267 | 92·0 | 9·0 | (8·6–9·5) | 3468 | 39·2 | 3·8 | (3·7–4·0) | 3931 | 10·2 | Ref. |
| Remote | 712 | 144·4 | 23·0 | (21·2–25·1) | 2083 | 84·5 | 13·5 | (12·7–14·3) | 3047 | 34·4 | 5·5 | (5·2–5·8) | 2415 | 6·3 | Ref. |
Note: IRR with children aged 2–9 years as the reference group. CI, confidence intervals; IRR, incidence rate ratio; Ref, reference.
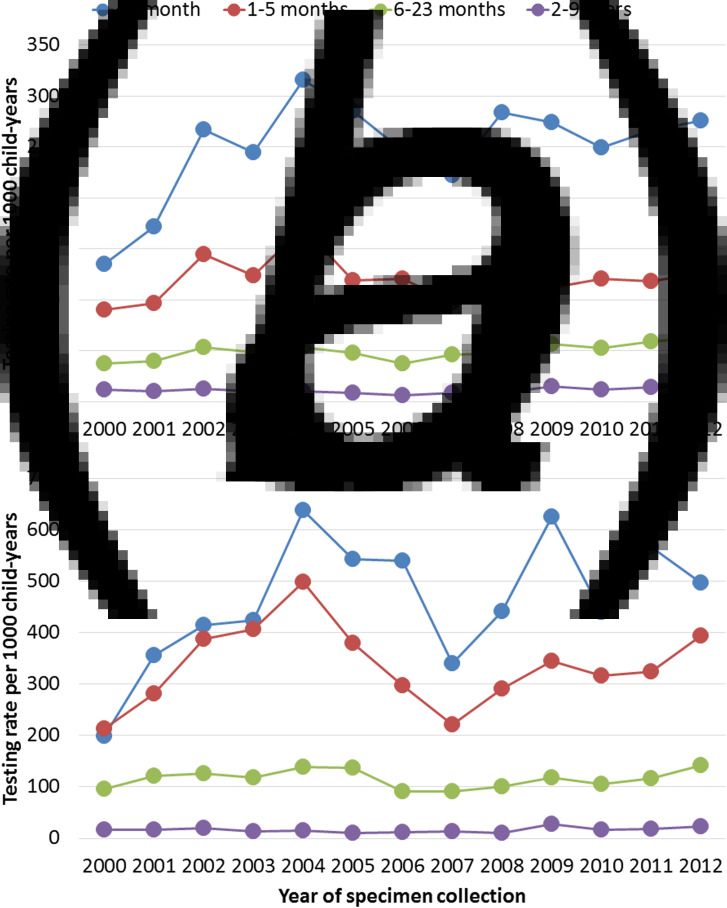
Testing rates in non-Aboriginal children aged <6 months peaked in 2004 before declining until 2007 and then gradually rose again from 2008 onwards, with a similar pattern observed among their Aboriginal peers (Fig. 2, Supplementary Tables S1–S2). Compared with these children, lower testing rates were observed in both Aboriginal and non-Aboriginal children aged 6–23 months, with testing increasing slightly over the study period (Fig. 2, Supplementary Tables S1–S2). All children aged 2–9 years showed a sudden increase in the testing rates in 2009 compared with 2008; from 8·4 to 14·9 per 1000 child-years in non-Aboriginal children and from 10·4 to 27·5 per 1000 child-years in Aboriginal children.
Fig. 2.
Yearly changes in testing rates per 1000 child-years by age groups in (a) non-Aboriginal and (b) Aboriginal children. Note the doubling in scale between Figures 2a and b.
Changes in testing methods and frequency of detection
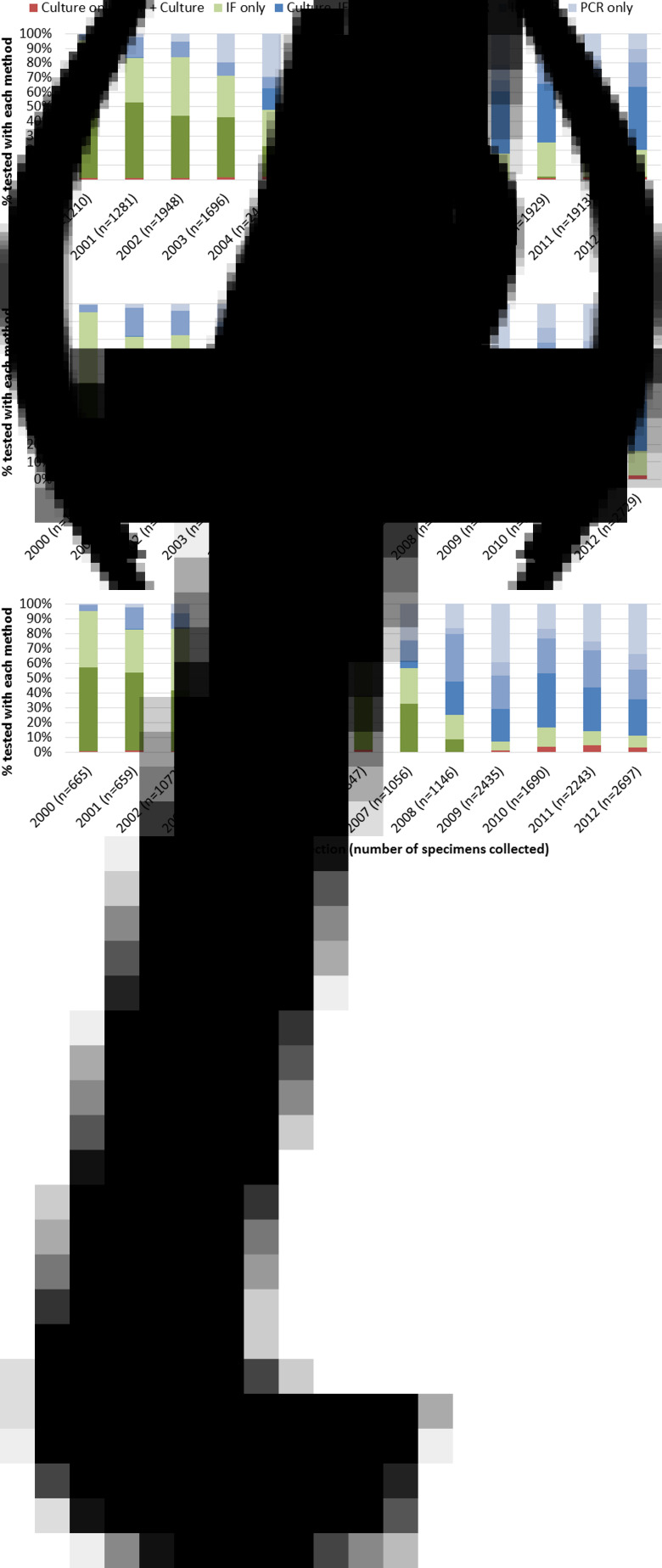
Over the study period, 23 414 nasal/NP specimens were collected in children aged <6 months, 25 281 from those aged 6–23 months and 17 911 from those aged 2–9 years. Overall, the proportion of these specimens undergoing testing using two or more of culture, PCR and IF ranged between 46·6% and 66·4%. Culture together with direct IF was the most common combination between 2000 and 2004, after which testing using all three methods was most common.
Although PCR was in use throughout the study period, less than a quarter of all nasal/NP specimens from children aged 6 months or more were tested using PCR by 2003 (Figs 3b and c). From 2004 onwards, PCR testing in all age groups increased and was used for over 80% of all nasal/NP specimen testing by 2012 (Fig. 3). In particular, 39·5% of all nasal/NP specimens collected from children aged 2–9 years in 2009 were tested using PCR only.
Fig. 3.
Proportion of nasal/NP specimens tested using culture, IF and PCR among children aged (a) <6 months, (b) 6–23 months and (c) 2–9 years at time of specimen collection. NP, nasopharyngeal; IF, immunofluorescence; PCR, polymerase chain reaction.
Overall, the proportion of nasal/NP specimens with at least one virus detected rose from 36·3% (n = 1218) in 2000 to 44·4% in 2012 (n = 3391; P for trend <0·01). This increase in the frequency of pathogen detection was greatest among children 2–9 years, increasing from 34·3% in 2000 to 49·9% in 2012 (P for trend <0·01; Fig. 4, Supplementary Table S3). Children aged 6–23 months had a similar, albeit less pronounced, increase over the same period (P for trend <0·01; Fig. 4, Supplementary Table S3). By contrast, the proportion of samples positive in children under 6 months of age showed limited change in the frequency of pathogen detection in 2000 compared with 2012 (P = 0·57; Fig. 4, Supplementary Table S3).
Fig. 4.
Yearly changes in frequency of virus detection from nasal/NP specimens by age groups. NP, nasopharyngeal.
Predictors of testing among children admitted to hospital
Approximately 45·4% of the birth cohort (n = 213 387) were hospitalised as in-patients at least once between 2000 and 2012 for any reason. Of these, 19·0% (n = 40 472) were tested for respiratory viruses at least once before their 10th birthday (Fig. 1).
Univariable analyses suggested that region at birth, ethnicity, gestational age, special care requirements at birth and multiple births were the strongest predictors of being tested for respiratory viruses (Table 2). With the exception of multiple births, these factors remained significant after adjusting for all other covariates. In particular, children who were born at less than 32 weeks gestation had more than three times higher odds of being tested for respiratory viruses compared with children born at term (more than 36 weeks; Table 2). Children born in the most disadvantaged areas, those born by elective caesarean, or whose mothers smoked during pregnancy also had higher odds of receiving a virology test (Table 2).
Table 2.
Logistic regression models of demographic factors at birth predicting likelihood of testing among those who were admitted as in-patients at least once (2000–2012)
| Hospitalised children (N = 213 387) | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| Factor | n | OR | (95% CI) | aOR | (95% CI) |
| Region at birth | |||||
| Metropolitan | 154 019 | Ref. | |||
| Rural | 38 250 | 0·64 | (0·62–0·66) | 0·59 | (0·55–0·63) |
| Remote | 20 782 | 0·96 | (0·93–1·00) | 0·76 | (0·69–0·85) |
| Season of birth | |||||
| Summer | 51 413 | Ref. | |||
| Spring | 52 427 | 1·00 | (0·97–1·03) | 1·04 | (0·98–1·10) |
| Autumn | 56 203 | 1·11 | (1·08–1·14) | 1·17 | (1·10–1·23) |
| Winter | 53 344 | 1·08 | (1·05–1·11) | 1·15 | (1·09–1·22) |
| Year of birth (per year) | 1·09 | (1·09–1·09) | 1·06 | (1·05–1·06) | |
| Male | 120 383 | 1·04 | (1·02–1·06) | 1·03 | (0·99–1·07) |
| SEIFA at birth | |||||
| 0–10% (most disadvantaged) | 21 460 | 1·13 | (1·08–1·19) | 1·28 | (1·15–1·42) |
| 11–25% | 33 285 | 1·08 | (1·03–1·13) | 1·25 | (1·14–1·38) |
| 26–75% | 97 484 | 1·02 | (0·98–1·07) | 1·16 | (1·07–1·27) |
| 76–90% | 28 364 | 0·98 | (0·93–1·03) | 1·05 | (0·95–1·16) |
| 91–100% (most advantaged) | 13 523 | Ref. | |||
| Ethnicity | |||||
| Caucasian | 173 669 | Ref. | |||
| Aboriginal | 19 832 | 1·52 | (1·48–1·58) | 1·51 | (1·42–1·61) |
| Other | 19 755 | 1·23 | (1·19–1·28) | 1·00 | (0·95–1·05) |
| Gestational age | |||||
| <32 weeks | 4036 | 4·89 | (4·60–5·19) | 3·79 | (3·47–4·13) |
| 32–36 weeks | 20 234 | 1·55 | (1·50–1·60) | 1·21 | (1·14–1·28) |
| >36 weeks | 188 938 | Ref. | |||
| Special care at birth | |||||
| No | 58 484 | Ref. | |||
| Yes | 24 161 | 2·04 | (1·98–2·11) | 1·22 | (1·16–1·28) |
| Mother smoked during pregnancy | |||||
| No | 149 727 | Ref. | |||
| Yes | 38 111 | 1·17 | (1·14–1·20) | 1·22 | (1·16–1·28) |
| Mode of delivery | |||||
| Emergency caesarean | 31 045 | 0·89 | (0·86–0·93) | 0·99 | (0·94–1·05) |
| Elective caesarean | 35 253 | 1·17 | (1·14–1·21) | 1·14 | (1·07–1·20) |
| Instrumental (vacuum or forceps) | 20 536 | 1·40 | (1·36–1·44) | 0·85 | (0·79–0·91) |
| Spontaneous vaginal | 126 374 | Ref. | |||
| Multiple birth | |||||
| No | 205 851 | Ref. | |||
| Yes | 7536 | 1·52 | (1·44–1·60) | 0·92 | (0·85–0·99) |
Note: Sum of number of children for each variable may not equal 213 387 due to missing data for some variables. Multivariable model adjusted for all other variables listed in the table. Year of birth was included as a continuous variable in both models. (a)OR, (adjusted) odds ratio; CI, confidence intervals; Ref, reference.
DISCUSSION
WA is one of the few jurisdictions globally that has access to respiratory pathogen testing data linked to perinatal and hospital data. Using these data, we described the testing patterns for respiratory pathogens in a total population cohort of children, 45% of whom were hospitalised before their 10th birthday. Approximately 10% of the birth cohort received a virology test before their 10th birthday, with the highest rate of testing among infants aged less than 1 month old. Testing rates in all children rose from 2008 but with considerable year-to-year variability. On average, over half of nasal/NP specimens were tested using multiple methods of detection. The proportions undergoing multiple testing that included PCR increased over time. While the proportions of nasal/NP specimens yielding a positive result increased over time, this was largely driven by children aged 2–9 years. Among hospitalised children, those born at less than 32 weeks gestation had over three times the odds of being tested than those born at term.
We are fortunate that PathWest is the sole public pathology provider for WA and, unlike notification data, records both positive and negative test results. These characteristics allowed us to investigate patterns of respiratory virus testing that are representative of the total hospitalised paediatric population in the state. We are unaware of other studies retrospectively investigating testing patterns for respiratory viruses in hospitalised children and so are unable to compare the frequency of testing observed here to other paediatric populations. We found considerable year-to-year variability in rates of testing across age groups within Aboriginal and non-Aboriginal children, some of which may be partly explained by external changes.
Potential explanations for the variability in pathogen testing over time include significant changes to the PathWest database structure in 2006–2007. We postulate that consultation and piloting of the new database structure may have affected data retrieval from the database and, in turn, partially contributed to sudden drops in testing between 2004 and 2007. At a local level, yearly variations in the severity of influenza and RSV seasons as well as changes to healthcare providers’ perceptions of testing may have further influenced the number of tests performed. At a global level, the emergence of pandemic influenza A/(H1N1) in 2009 [19] was likely to have driven the rise in virology tests, particularly among the older age groups. We are unaware of any further changes in education or practice that may have influenced the results observed. Documenting overall changes in testing frequency are important in contextualising changes in disease burden (especially during epidemics of certain pathogens) and assessing effectiveness of preventative programs such as immunisation.
The proportion of PCR-tested nasal/NP specimens increased across all age groups since 2004, particularly in combination with other methods. This mirrors broader trends in respiratory virus testing around Australia [20] and globally. Concurrent to this, the frequency of positive detection increased, particularly among children aged 2 years or older. This is likely due to the improved sensitivity of PCR compared with antigen detection methods, the latter of which has been shown to have lower sensitivity, particularly in older children and adults [21].
Over half of all nasal/NP specimens were tested using multiple methods during the study period. Despite other laboratories moving towards exclusively using molecular methods, PathWest has continued to use both antigen detection and viral culture. Continued use of antigen detection is largely influenced locally by faster turnaround times, which are critical for local hospital infection control activities (e.g. patient isolation and de-isolation), while culture is maintained to ensure its availability for viral surveillance and novel viral discovery. As new commercial platforms capable of detecting multiple pathogens become available, the additional cost of multiple testing methods, particularly with only marginal improvement in pathogen detection, needs to be reassessed in WA as well as in other regions with similar practices. Furthermore, with the limited clinical impact of multiple viral detection in low risk, immunocompetent children [22, 23], the breadth of routinely undertaken viral detection should also be reassessed.
We have identified a number of important predictors of testing that are known at birth including ethnicity, gestational age, maternal smoking during pregnancy and special care requirements at birth. These are in addition to factors at the time of admission, including hospital location, age at admission [8], season of admission, length of stay and diagnosis (data not shown). These results highlight the many complex factors that are influencing the likelihood of a child receiving a microbiology test. Combining findings from this and other studies will help to inform models assessing the viral burden of ALRI when using routinely collected data, which will ultimately provide more accurate estimates. We acknowledge that there are additional factors, such as physician preference, likely to influence testing that we are unable to explore here.
We provide, for the first time in WA, a comprehensive population-based view of how testing for respiratory pathogens has changed in the state. Future studies into the burden of respiratory infections will need to be interpreted with consideration for the increased sensitivity and specificity of the detection methods used. Findings from this study will be used to account for temporal changes in testing patterns and test sensitivities in planned analyses investigating the temporal changes in pathogen-specific admission rates of ALRI in children.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Peter Jacoby for his assistance in the person-time-at-risk calculations and Parveen Fathima for her assistance in cleaning the data from the Hospital Morbidity Data Collection. They thank the Linkage and Client Services Teams at the WA Data Linkage Branch, in particular Alexandra Merchant, Diana Rosman and Mikhalina Dombrovskaya, as well as custodians of all datasets used. They also thank Charmaine Tonkin and Brett Cawley from PathWest Laboratory Medicine for their support for this study as well as members of the Triple I Scientific Steering Committee for their input into this study. This study was funded through a National Health and Medical Research Council (NHMRC) Project Grant (APP1045668) and formed part of F.J.L.’s studies towards a Doctor of Philosophy, funded by a University of Western Australia Postgraduate Award. C.C.B. is supported by a NHMRC Career Development Fellowship (APP1111596). H.C.M. is supported by NHMRC Early Career Fellowship (APP1034254). The funding bodies had no part in the design, conduct, analysis or interpretation of this study.
AUTHOR CONTRIBUTIONS
H.C.M., C.C.B. and F.J.L. conceptualised the study. C.C.B. provided clinical expertise, C.C.B. and A.D.K. provided background information on PathWest and virology, while H.C.M. and N.d.K. provided statistical and record linkage expertise. F.J.L. wrote the first draft of this manuscript, F.J.L. performed the analyses for this study under supervision of H.C.M., C.C.B. and N.d.K. All authors have critically reviewed the manuscript and approve of the final version as submitted.
ETHICAL STANDARDS
Ethical approvals were obtained from the WA Department of Health Human Research Ethics Committee, the WA Aboriginal Health Ethics Committee and the University of Western Australia Human Research Ethics Committee.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268817000413.
click here to view supplementary material
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Nair H, et al. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet 2013; 381: 1380–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rabon-Stith KM, et al. Laboratory testing trends for respiratory syncytial virus, 2007–2011. Journal of Clinical Virology 2013; 58: 575–8. [DOI] [PubMed] [Google Scholar]
- 3.Stroparo E, et al. Adenovirus respiratory infection: significant increase in diagnosis using PCR comparing with antigen detection and culture methods. Revista do Instituto de Medicina Tropical de Sao Paulo 2010; 52: 317–21. [DOI] [PubMed] [Google Scholar]
- 4.Drews SJ. The role of clinical virology laboratory and the clinical virology laboratorian in ensuring effective surveillance for influenza and other respiratory viruses: points to consider and pitfalls to avoid. Current Treatment Options in Infectious Diseases 2016; 8: 165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holman CDJ, et al. Population-based linkage of health records in Western Australia: development of a health services research linked database. Australian and New Zealand Journal of Public Health 1999; 23: 453–9. [DOI] [PubMed] [Google Scholar]
- 6.Data Linkage WA. Core Data Collections [Internet]. 2016 [cited 2016 Jan 11] http://www.datalinkage-wa.org/data-linkage/data-collections.
- 7.Moore HC, et al. Use of data linkage to investigate the aetiology of acute lower respiratory infection hospitalisations in children. Journal of Paediatrics and Child Health 2012; 48: 520–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim FJ, et al. Optimization is required when using linked hospital and laboratory data to investigate respiratory infections. Journal of Clinical Epidemiology 2016; 69: 23–31. [DOI] [PubMed] [Google Scholar]
- 9.PathWest Laboratory Medicine WA. PathWest Laboratory Medicine – About us [Internet]. [Cited 2015 Feb 18] http://www.pathwest.com.au/about_what.asp.
- 10.Codde J. Rates Calculator. 9·5·5. Perth, Western Australia: Health Information Centre, Department of Health; 2013. [Google Scholar]
- 11.Data Linkage WA. Linkage Quality [Internet]. [Cited 2016 Dec 15] http://www.datalinkage-wa.org.au/about-us/linkage-quality.
- 12.Christensen D, et al. Evidence for the use of an algorithm in resolving inconsistent and missing Indigenous status in administrative data collections. Australian Journal of Social Issues 2014; 49: 423–43. [Google Scholar]
- 13.Blair EM, Liu Y, Cosgrove P. Choosing the best estimate of gestational age from routinely collected population-based perinatal data. Paediatric and Perinatal Epidemiology 2004; 18: 270–6. [DOI] [PubMed] [Google Scholar]
- 14.Moore HC, et al. Hospitalisation for bronchiolitis in infants is more common after elective caesarean delivery. Archives of Disease in Childhood 2012; 97: 410–4. [DOI] [PubMed] [Google Scholar]
- 15.Australian Bureau of Statistics. Socio-Economic Indexes for Areas (SEIFA) [Internet]. 2013. [Cited 2016 May 25] http://www.abs.gov.au/websitedbs/censushome.nsf/home/seifa?opendocument&navpos=260.
- 16.Moore HC, et al. Diverging trends for lower respiratory infections in non-Aboriginal and Aboriginal children. Journal of Paediatrics and Child Health Blackwell Publishing Asia; 2007; 43: 451–7. [DOI] [PubMed] [Google Scholar]
- 17.Bailey EJ, et al. Risks of severity and readmission of Indigenous and non-Indigenous children hospitalised for bronchiolitis. Journal of Paediatrics and Child Health Blackwell Publishing Asia; 2009; 45: 593–7. [DOI] [PubMed] [Google Scholar]
- 18.Juul S, Frydenberg M. EpiBasic. Aarhus University, Denmark; 2011. [Google Scholar]
- 19.World Health Organization. Evolution of a Pandemic – A(H1N1) 2009. Geneva, Switzerland; 2013. [Google Scholar]
- 20.Kaczmarek MC, Ware RS, Lambert SB. The contribution of PCR testing to influenza and pertussis notifications in Australia. Epidemiology and Infection 2016; 144: 306–14. [DOI] [PubMed] [Google Scholar]
- 21.Chan MCW, et al. Clinical and virologic factors associated with reduced sensitivity of rapid influenza diagnostic tests in hospitalized elderly patients and young children. Journal of Clinical Microbiology 2014; 52: 497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asner SA, et al. Clinical disease severity of respiratory viral co-infection versus single viral infection: a systematic review and meta-analysis. PLoS ONE 2014; 9: e99392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim FJ, et al. Systematic review and meta-analysis of respiratory viral coinfections in children. Respirology 2016; 21: 648–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268817000413.
click here to view supplementary material