Abstract
This study aimed at exploring the association between detectable cardiac and pulmonary involvement in long-term juvenile dermatomyositis (JDM) and to assess if patients with cardiac and pulmonary involvement differ with regard to clinical characteristics. 57 JDM patients were examined mean 17.3 (10.5) years after disease onset; this included clinical examination, myositis specific/associated autoantibodies (immunoblot), echocardiography, pulmonary function tests and high-resolution computed tomography. Cardiac involvement was defined as diastolic and/or systolic left ventricular dysfunction and pulmonary involvement as low diffusing capacity for carbon monoxide, low total lung capacity and/or high-resolution computed tomography abnormalities. Patients were stratified into the following four groups: (i) no organ involvement, (ii) pulmonary only, (iii) cardiac only, and (iv) co-existing pulmonary and cardiac involvement. Mean age was 25.7 (12.4) years and 37% were males. One patient had coronary artery disease, seven had a history of pericarditis, seven had hypertension and three had known interstitial lung disease prior to follow-up. There was no association between cardiac (10/57;18%) and pulmonary (41/57;72%) involvement (p = 0.83). After stratifying by organ involvement, 21% of patients had no organ involvement; 61% had pulmonary involvement only; 7% had cardiac involvement only and 11% had co-existing pulmonary or cardiac involvement. Patients with co-existing pulmonary or cardiac involvement had higher disease burden than the remaining patients. Patients with either cardiac or pulmonary involvement only, differed in clinical and autoantibody characteristics. We found no increased risk of developing concomitant cardiac/pulmonary involvement in JDM. Our results shed light upon possible different underlying mechanisms behind pulmonary and cardiac involvement in JDM.
Keywords: Juvenile dermatomyositis/polymyositis, Cardiovascular disease, Lung disease, Echocardiography, Pulmonary fibrosis
Introduction
Juvenile dermatomyositis (JDM) is a rheumatic disease of childhood that mainly targets skin and muscles, but may also affect internal organs, such as the heart and lungs [1]. Clinical manifest lung disease, including interstitial lung disease (ILD), is rare in JDM, but associates with high morbidity and mortality [2]. Our group previously reported a high frequency of mostly subclinical pulmonary involvement (PI) in JDM patients comprehensively examined after median 16.8 years disease duration [3]. It is not known if these patients later will develop manifest lung disease. Although very rare, serious cardiac involvement (CI) has been reported in JDM [4]. However, in our cohort, subclinical CI is relatively frequent in patients with medium- to long-term JDM [5, 6], also found in patients after median 4.6y disease duration [7]. It is not known if patients with JDM later will develop manifest cardiac disease.
The coexistence of CI and PI has become more acknowledged during recent years; in the general population, subclinical impairment of lung function is related to mild cardiac dysfunction [8]. This relationship is also found in adults with connective tissue diseases, where ILD is associated with cardiovascular complications [9]. However, it is unknown whether JDM patients with PI are at higher risk of developing cardiac dysfunction and vice versa. Thus, the aim of our study was to explore the association between CI and PI in JDM patients; also, to assess if patients with CI and/or PI differ with regard to clinical characteristics.
Methods
Patients
Inclusion criteria were a probable/definitive diagnosis of DM [10], disease onset before 18y, minimum 24 m disease duration and age ≥ 6y. There were no exclusion criteria. A retrospective inception cohort of Norwegian JDM patients (diagnosed between 1970 and 2006, n = 59) was identified [11]. Patients with complete cardiac and pulmonary data (for details see below) were included in the present study. Informed consents were obtained from patients (if > 16 years) or their parents (if ≤ 16 years) at the time of follow-up examination. The study was approved by the regional committee of health and medical research ethics in South-East Norway (S-05144) (Aug 2005—June 2023).
Data collection
Clinical examination was performed at a comprehensive follow-up visit of the identified patients at Oslo University Hospital from September 2005 to May 2009. In addition to lung and cardiac measures (see below), the examination included muscle strength/endurance measured by manual muscle test (MMT-8)/child myositis assessment scale (CMAS) and health-related quality of life (HRQOL) by the Short Form-36 physical and mental component summary scores (SF-36 PCS and MCS) in patients > 13y [12]. Cumulative prednisolone dose was calculated [11]. Inactive disease was defined according to the revised PRINTO criteria [13]. Nailfold capillary density was assessed as previously described [14]. Disease activity and cumulative organ damage were assessed at the follow-up visit and also scored retrospectively (by chart review) at 1 year post diagnosis using the Disease Activity Score for JDM (DAS) and the Myositis Damage Index (MDI), respectively, as previously described [11, 15].
Cardiac measures
Two-dimensional, M-mode, and Doppler echocardiography was performed at the follow-up visit as previously described [5, 6]. Low early diastolic tissue velocity (e’) reflects left ventricular (LV) diastolic dysfunction [16] and low long axis strain (LAS) reflects LV systolic dysfunction [6]. Cardiac involvement was defined as LV diastolic or systolic dysfunction; both defined as mean of control subjects – 2SD from one of our previous studies [6]. Heart rate variability (expressed as cSDNN) was measured by Holter ECG [17]. Total cholesterol (TC) and high-density lipoprotein (HDL) were analyzed according to hospital routine; TC:HDL ratio was calculated (superior to other lipid parameters in predicting ischemic heart disease [18].
Pulmonary measures
Pulmonary function tests (PFTs) were performed at the follow-up visit as previously described in detail [3]. For the present study, diffusing capacity for carbon monoxide (DLCO) (adjusted for Hgb) and total lung capacity (TLC) (both expressed as percentage of predicted) were included. Low TLC and DLCO were defined as less than the fifth percentile of the predicted values [19]. High-resolution computer tomography (HRCT) was carried out in all patients; HRCT-detected abnormalities includes ILD (reticular pattern, ground glass opacity) and airways disease (bronchiectasis, air trapping, micronodules) [3]. Pulmonary involvement was defined as low TLC, low DLCO, or HRCT- detected abnormalities.
Stratification of patients
We stratified our patients into four groups based on the presence of detectable CI and PI at follow-up as follows: group 1: neither PI nor CI; group 2: PI only; group 3: CI only; and, group 4: co-existing PI and CI.
Immunological analyses
Sera were thawed at the follow-up visit and later (2014) subjected to the following tests: (a) ANA screening by indirect immunofluorescence (IIF) on HEp-2 cells at serum dilution 1:160. Only nuclear fluorescence patterns were considered positive. Detection of myositis specific autoantibodies (MSA) and myositis associated autoantibodies (MAA) [20] using (b) the myositis line immunoassay A1 which included: MDA5, TIF1-γ, NXP-2, SAE1, SAE2, HMGCR-S (Sigma), HMGCR-E (EUROIMMUN AG), Mup44 and (c) the myositis line immunoassay A2 (Myositis Profile 3 Euroline) which included: Jo1, PL-7, PL-12, EJ, OJ, SRP, Mi-2, PM-Scl75, PM-Scl-100 Ku and Ro52; both from Euroimmun AG Lübeck, Germany. The immunoblot strips were scanned and evaluated digitally, using the Euroline scan. Signal intensities below 11 were regarded as negative.
Statistical analysis
Data were expressed as mean (SD), median (IQR) or numbers (%) as appropriate. Differences between patient groups were tested by one-way ANOVA with Tukey post-hoc test, Kruskal–Wallis test with Dunn post-hoc test or Chi-square (no post-hoc test applied due to small numbers). Due to the hypothesis generating nature of our study, we did not correct for multiple comparisons. Correlations were determined by the Spearman correlation coefficient (rsp). Chi-square goodness of fit test was used to explore whether the number of patients in the clinical groups were as predicted based on the fraction with CI and PI in our cohort. Due to small numbers, no statistics was performed for MSA/MAA between groups. IBM SPSS Statistics v. 25.0 (IBM, Armonk, NY, USA) was used for statistical analyses.
Results
Clinical characteristics
The JDM cohort included 57 patients, representing 97% of the eligible patients. One patient had coronary artery disease, seven had pericarditis during disease course, seven had hypertension and three had known ILD prior to follow-up. There were no cases of diabetes, heart failure or cardiac arrhythmia. In total, 10/57 (18%) of patients had CI and 41/57 (72%) had PI; the organ involvement was mostly subclinical.
Associations between CI and PI
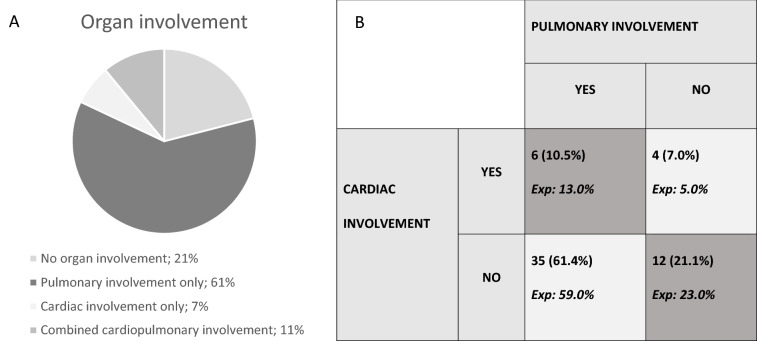
After stratification into groups, group 1 (neither PI nor CI) consisted of 12(21%) patients; group 2 (PI only) 35(61%); group 3: (CI only) 4(7%) and, group 4: co-existing PI and CI 6(11%) (Fig. 1A) (Table 1). No associations between CI and PI were found when comparing with the expected distribution (Fig. 1B, p = 0.83). We then correlated the variables defining PI (TLC, DLCO as continuous variables and HRCT abnormalities) and variables defining CI (e´ and LAS as continuous variables) with each other; no significant correlations were found (data not shown).
Fig. 1.
A Distribution of patients in the four clinical groups; B Distribution of patients in the four clinical groups compared to expected frequencies based the fraction with lung and cardiac involvement in our cohort; Exp expected distribution; p = 0.83 (Chi-square goodness of fit test)
Table 1.
Patient and disease characteristics and clinical variables stratified across the four groups
| Patients total | Group 1 No organ involvement | Group 2 Pulmonary only | Group 3 cardiac only | Group 4 coexisting pulmonary and cardiac | Significant p-values (between groups) | |
|---|---|---|---|---|---|---|
| Number of pts, n (%) | 57 (100) | 12 (21) | 35 (61) | 4 (7) | 6 (11) | NA |
| Age at follow-up, y | 25.7 (12.4) | 26.2 (11.9) | 22.5 (11.3) | 35.2 (7.6) | 37.7 (13.5) | 2 vs 4: p = 0.021 |
| Disease duration, y | 17.3 (10.5) | 16.9 (11.5) | 14.7 (9.1) | 28.5 (5.2) | 25.5 (11.7) | 2 vs 3: p = 0.047 |
| Male sex, n (%) | 21 (37) | 2(17) | 11 (31) | 3 (75) | 5 (83) | P = 0.014b |
| Smokers daily disease course; n (%)a | 14 (30) | 2 (18) | 7 (27) | 1 (25) | 4 (67) | NS |
| DAS 1 y | 5.9 (3.9) | 5.9 (4.2) | 4.8 (3.5) | 10.1 (4.1) | 9.0 (3.0) | 2 vs 3: p = 0.039 |
| MDI 1 y | 1.0 (0.0–2.0) | 1.0 (0.0–2.5) | 0.0 (0.0–2.0) | 2.5 (1.5–3.5) | 2.0 (1.0–5.0) | NS |
| Inactive Disease, n (%) | 28 (49) | 6 (50) | 18 (51) | 3 (75) | 1 (17) | NS |
| Calcinosis, n (%) | 21 (37) | 5 (42) | 10 (29) | 2 (50) | 4 (67) | NS |
| Lipodystrophy, n (%) | 10 (18) | 1 (8) | 2 (6) | 2 (50) | 5 (83) | P < 0.001 b |
| Hypertension, disease course, n (%) | 7 (12) | 1(8) | 2 (6) | 2 (50) | 2 (33) | P = 0.025 b |
| TC:HDL ratio N = 50 | 3.9 (2.0) | 3.1 (0.4) | 3.5 (1.3) | 7.4 (3.2) | 5.5 (2.8) |
1 vs 3: p < 0.001 1 vs 4: p = 0.044 2 vs 3: p < 0.001 2 vs 4: p = 0.049 |
| HRV, cSDNN, N = 55 | 39.7 (16.7) | 50.5 (21.8) | 36.8 (13.6) | 42.7 (2.3) | 29.5 (17.6) | 1 vs 4: p = 0.050 |
| NCD, cap/mm | 6.4 (2.1) | 7.1 (1.8) | 6.1 (2.3) | 7.5 (0.6) | 6.4 (2.1) | NS |
| Pred/DMARD, n (%) | 17 (30) | 4 (33) | 10 (29) | 1 (25) | 2 (33) | NS |
| Cum Prednisolone during disease course, g | 7.9 (3.6–12.6) | 8.9 (7.6–11.3) | 4.8 (2.5–10.6) | 17.9 (12.6–26.6) | 14.4 (7.9–27.3) |
2 vs 3; p = 0.008 2 vs 4; p = 0.005 |
| DAS | 4.7 (3.0) | 4.0 (2.4) | 4.5 (2.8) | 3.4 (2.5) | 8.5 (2.8) |
1 vs 4: p = 0.008 2 vs 4: p = 0.008 3 vs 4: p = 0.025 |
| MDI | 4.3 (3.1) | 4.3 (2.2) | 3.5 (2.8) | 5.2 (3.3) | 8.2 (3.8) |
1 vs 4: p = 0.036 2 vs 4: p = 0.003 |
| MMT-8 | 76.5 (4.7) | 77.2 (3.1) | 76.7 (4.1) | 80.0 (0.0) | 71.3 (8.5) |
1 vs 4: p = 0.048 2 vs 4: p = 0.040 3 vs 4: p = 0.019 |
| CMAS | 48.3 (5.4) | 48.8 (3.2) | 48.8 (4.7) | 51.0 (1.4) | 42.8 (10.5) | NS |
| SF-36, PCS a | 50.8 (9.0) | 51.0 (9.7) | 52.3 (7.3) | 56.0 (3.4) | 40.2 (10.9) |
2 vs 4: p = 0.011 3 vs 4: p = 0.023 |
| SF-36, MCSa | 53.4 (7.7) | 51.8 (6.4) | 53.6 (8.1) | 52.7 (5.7) | 55.4 (10.1) | NS |
Variables are assessed at follow-up if not otherwise stated; values are mean (SD) or median (25th—75th percentile) if not otherwise stated; NA not assessed, NS non-significant, DAS Disease activity score, MDI myositis damage index, FU follow-up, TC Total cholesterol, HDL high-density lipoprotein, NCD nailfold capillary density, HRV heart rate variability, NCD Nail fold capillary density, DMARD disease modifying antirheumatic drugs, CMAS child myositis assessment scale, SF-36 Short Form-36, PCS physical assessment scale, MCS mental component scale, Post-hoc tests not run for categorical variables due to low n in several groups. aassessed in patients ≥ 14y at follow-up, n = 47; bpost hoc tests not assessed
Variables defining CI and PI across the four clinical groups
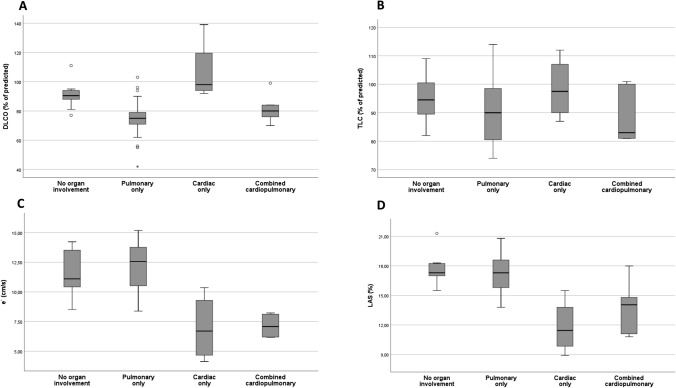
Naturally, DLCO was lower in group 2 and 4 (which include patients with PI) compared with the other two groups (p’s < 0.007) except for group 1 vs 4, NS) (Fig. 2A). For TLC, there were similar, albeit non-significant trends (Fig. 2B). Naturally, e´ and LAS were lower in group 3 and 4 (which include patients with CI) compared with the other two groups (p’s < 0.002) (Fig. 2C and D).
Fig. 2.
Variables defining cardiac and pulmonary involvement across the four clinical groups; p-values based on one-way ANOVA with Tukey post-hoc tests; A DLCO: Diffusing capacity for carbonmonoxide, % of predicted; B TLC: Total lung capacity; % of predicted; C e´: early diastolic tissue velocity, cm/s; D LAS: long-axis strain, %
Clinical variables across the four clinical groups
Group 4 (co-existing CI and PI) differed the most from the other groups; they were older at follow-up (only statistically significant compared to group 2), had numerically more males (5/6, NS), and 4/6 were daily smokers (Table 1). Also, group 4 had higher DAS at follow-up compared with the remaining sub-groups, as well as higher MDI than group 1 and 2. Group 4 had numerically more calcinosis, lipodystrophy as well as higher TC:HDL ratio compared with groups 1 and 2. They also had lower heart rate variability (cSDNN) than group 1. Group 4 also had more impaired SF-36 PCS than both groups 2 and 3. Group 3 (CI only) had higher early DAS, longer disease duration and higher cumulative prednisolone dose compared with group 2; also, they had higher TC:HDL ratio compared to both group 1 and 2. On the other hand, group 3 had less impaired SF-36 PCS than group 2 (Table 1).
MSA and MAA are shown in Table 2 as background data. 31(54%) had no detectable ANA. 19(33%) of patients had at least one detectable MSA/MAA; of those 13(68%) had one autoantibody and 6(32%) had > 1 autoantibody (Table 2). MSA known to be associated with lung involvement (PL-7, Jo-1 and MDA5) were only found in group 2 (PI only).
Table 2.
Myositis specific- and myositis associated autoantibodies stratified across the four JDM groups
| Patients total | Group 1 no organ involvement | Group 2 pulmonary only | Group 3 cardiac only | Group 4 coexisiting pulmonary and cardiac | |
|---|---|---|---|---|---|
| n | 57 | 12 | 35 | 4 | 6 |
| ANA IIF | 26 (46) | 6 (50) | 16 (46) | 1 (25) | 3 (50) |
| No MSA/ MAA | 38 (67) | 7 (58) | 23 (66) | 3 (75) | 5 (83) |
| Myositis specific autoantibodies | |||||
| Jo-1 | 1 (2) | 0 | 1 (3) | 0 | 0 |
| PL-7 | 1 (2) | 0 | 1 (3) 4 | 0 | 0 |
| SRP | 1 (2) | 1 (8) | 0 | 0 | 0 |
| Mi-2 | 3 (5) | 1 (8)1 | 2 (6) | 0 | 0 |
| NXP-2 | 5 (9) | 1 (8)3 | 3 (9)4 | 0 | 1 (25) |
| TIF1- γ | 1 (2) | 0 | 0 | 1 (25) | 0 |
| MDA5 | 2 (4) | 0 | 2 (6)5 | 0 | 0 |
| SAE-1 | 1 (2) | 1 (8)2 | 0 | 0 | 0 |
| HMGCR | 1 (2) | 1 (8)1 | 0 | 0 | 0 |
| Myositis associated autoantibodies | |||||
| PMScl75 | 3 (5) | 0 | 3 (9)6 | 0 | 0 |
| PMScl100 | 2 (4) | 0 | 2 (6)5 | 0 | 0 |
| Ku | 2 (4) | 1 (8) | 1 (3) | 0 | 0 |
| Ro52 | 3 (5) | 2 (17) 2,3 | 1 (3) 6 | 0 | 0 |
MSA Myositis specific autoantibodies, MAA Myositis associated autoantibodies, Numbers are n (%); No patients had detectable EJ, PL-12, OJ, SAE-2 or Mup44; 1,2,3,4,and 5denotes that more than one MSA and/or MAA are present in the same patient. IIF indirect immunofluorescence
Discussion
Based on previous studies in the general population [8] and connective tissue disease [9], we hypothesized that there was an increased risk of concomitant detectable cardiac and pulmonary involvement in JDM. No evidence for a higher risk of CI given PI and vice versa was detected in our study. However, due to small sample size our results should be interpreted with caution.
Special attention should be drawn to group 4 (combined CI and PI). Despite being small (n = 6/11% of the total cohort), several trends and statistically significant differences were found when compared with the other groups. This group displayed a higher disease burden (including higher disease activity and damage and also unfavorable lipid profile and heart rate variability) and experienced a reduced quality of life (SF-36 PCS) compared with the other groups. They were characterized by older age, more likely to be males and daily smokers. Importantly, the high MDI observed in this group is not explained by subclinical PI and CI.
Second, group 3 (CI only) had higher disease activity at 1 year post-diagnosis, unfavorable lipid profile, and higher cumulative prednisolone dose than group 2 (PI only). The higher age found in patients with cardiac involvement might reflect that they were diagnosed in a time with less aggressive treatment; also, it is known that cardiac involvement increases with age.
Interestingly, the patients with CI, either isolated (group 3) or combined (group 4) bear little resemblance with the patients with PI. Possibly, abnormalities in the heart and lung may represent two different mechanisms/phenotypes. This may explain the lack of correlation between subclinical heart and lung involvement in JDM patients. Although no statistical difference in NCD across clinical groups was found in the present study, we have previously reported an association between microvascular findings and PI, but no association between microvascular findings and CI [14].
Data on MSA and MAA was used as background information. 67% has no detectable MSA or MAA which is lower than reported in the literature [20]. This might be explained by methodological differences between assay methods [21]. Interestingly, MSA known to be associated with pulmonary involvement in JDM (MDA5, PL-7 and Jo-1)[20], were only found in patients with PI.
Strengths of our study include that our patients come from a restrospective inception cohort and they were comprehensively examined after medium- to long term follow-up. Limitations include that we did not calculate sample size (due to the hypothesis-generating nature of our study) and the cross-sectional design.
To conclude, no increased risk for concomitant heart and lung involvement was found in JDM. Our results may suggest different mechanisms underlying pulmonary and cardiac involvement in JDM; this requires further study. Based on our results, clinicians should be especially aware of the risk detectable organ involvement in patients with high disease activity and damage after medium- to long-term disease. Follow-studies are needed to see if patients with subclinical PI and CI later develop clinical manifest lung and/or cardiac disease.
Statement on open data sharing
The data will not be deposited, due to guidelines from the regional committee of health and medical research ethics in South-East Norway.
Acknowledgements
We thank professor Øyvind Molberg, MD, PhD at department of Rheumatology, Oslo University hospital, for critical review of the manuscript and Randi Karlsen, Senior Biomedical Laboratory Scientist at the Department of Immunology, Oslo University Hospital, Ullevål for help with the immunological analyses.
Author contributions
BNW, IS and HS: Conception of the study, data collection, analysis and interpretation, drafting of manuscript. TS: Data collection and interpretation, critical review of the manuscript. ZB: Data analysis and interpretation, critical review of the manuscript. MBL, TMA, ET and BF: Data interpretation, critical review of the manuscript. All co-authors have read and approved the final manuscript and take full responsibility for the correctness and integrity of the work.
Funding
Open access funding provided by University of Oslo (incl Oslo University Hospital). We thank the following foundations for financial support: Anders Jahre’s Foundation for the promotion of science, Simon Fougner Hartmann’s Family Found, Rakel and Otto Bruun’s Foundation.
Declarations
Conflict of interest
None of the authors have any conflicts of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Birgit Nomeland Witczak, Email: birgit.witczak@gmail.com.
Thomas Schwartz, Email: thomasschwartz1410@gmail.com.
Zoltan Barth, Email: zoltan.barth@gmail.com.
Eli Taraldsrud, Email: etaralds@ous-hf.no.
May Brit Lund, Email: m.b.samersaw-lund@medisin.uio.no.
Trond Mogens Aaløkken, Email: trond.mogens.aalokken@ous-hf.no.
Berit Flatø, Email: berit.flato@medisin.uio.no.
Ivar Sjaastad, Email: ivar.sjaastad@medisin.uio.no.
Helga Sanner, Email: helga.sanner@medisin.uio.no.
References
- 1.Papadopoulou C, McCann LJ. The Vasculopathy of Juvenile Dermatomyositis. Front Pediatr. 2018;6:284. doi: 10.3389/fped.2018.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathiesen PR, Buchvald F, Nielsen KG, Herlin T, Friis T, Nielsen S. Pulmonary function and autoantibodies in a long-term follow-up of juvenile dermatomyositis patient. Rheumatology (Oxford) 2014;53:644–649. doi: 10.1093/rheumatology/ket380. [DOI] [PubMed] [Google Scholar]
- 3.Sanner H, Aalokken TM, Gran JT, Sjaastad I, Johansen B, Flato B. Pulmonary outcome in juvenile dermatomyositis: a case-control study. Ann Rheum Dis. 2011;70:86–91. doi: 10.1136/ard.2010.131433. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz T, Diederichsen LP, Lundberg IE, Sjaastad I, Sanner H. Cardiac involvement in adult and juvenile idiopathic inflammatory myopathies. RMD Open. 2016;2:e000291. doi: 10.1136/rmdopen-2016-000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz T, Sanner H, Husebye T, Flato B, Sjaastad I. Cardiac dysfunction in juvenile dermatomyositis: a case-control study. Ann Rheum Dis. 2011;70:766–771. doi: 10.1136/ard.2010.137968. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz T, Sanner H, Gjesdal O, Flato B, Sjaastad I. In juvenile dermatomyositis, cardiac systolic dysfunction is present after long-term follow-up and is predicted by sustained early skin activity. Ann Rheum Dis. 2014;73:1805–1810. doi: 10.1136/annrheumdis-2013-203279. [DOI] [PubMed] [Google Scholar]
- 7.Diniz MFR, Kozu KT, Elias AM, Lianza AC, Sawamura KSS, Menezes CRB, Ferreira J, Silva CA, Leal GN. Echocardiographic study of juvenile dermatomyositis patients: new insights from speckle-tracking-derived strain. Clin Rheumatol. 2021;40:1497–1505. doi: 10.1007/s10067-020-05418-4. [DOI] [PubMed] [Google Scholar]
- 8.Baum C, Ojeda FM, Wild PS, Rzayeva N, Zeller T, Sinning CR, Pfeiffer N, Beutel M, Blettner M, Lackner KJ, Blankenberg S, Munzel T, Rabe KF, Schnabel RB, Gutenberg Health Study Subclinical impairment of lung function is related to mild cardiac dysfunction and manifest heart failure in the general population. Int J Cardiol. 2016;218:298–304. doi: 10.1016/j.ijcard.2016.05.034. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Lou M, Li Y, Ye W, Zhang Z, Jia X, Shi H, Zhu X, Wang L. Cardiovascular involvement in connective tissue disease: the role of interstitial lung disease. PLoS ONE. 2015;10:e0121976. doi: 10.1371/journal.pone.0121976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975;292:344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 11.Sanner H, Gran JT, Sjaastad I, Flato B. Cumulative organ damage and prognostic factors in juvenile dermatomyositis: a cross-sectional study median 16.8 years after symptom onset. Rheumatology (Oxford) 2009;48:1541–1547. doi: 10.1093/rheumatology/kep302. [DOI] [PubMed] [Google Scholar]
- 12.Rider LG, Werth VP, Huber AM, Alexanderson H, Rao AP, Ruperto N, Herbelin L, Barohn R, Isenberg D, Miller FW. Measures of adult and juvenile dermatomyositis, polymyositis, and inclusion body myositis: Physician and Patient/Parent Global Activity, Manual Muscle Testing (MMT), Health Assessment Questionnaire (HAQ)/Childhood Health Assessment Questionnaire (C-HAQ), Childhood Myositis Assessment Scale (CMAS), Myositis Disease Activity Assessment Tool (MDAAT), Disease Activity Score (DAS), Short Form 36 (SF-36), Child Health Questionnaire (CHQ), physician global damage, Myositis Damage Index (MDI), Quantitative Muscle Testing (QMT), Myositis Functional Index-2 (FI-2), Myositis Activities Profile (MAP), Inclusion Body Myositis Functional Rating Scale (IBMFRS), Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI), Cutaneous Assessment Tool (CAT), Dermatomyositis Skin Severity Index (DSSI), Skindex, and Dermatology Life Quality Index (DLQI) Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S118–157. doi: 10.1002/acr.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Almeida B, Campanilho-Marques R, Arnold K, Pilkington CA, Wedderburn LR, Nistala K, Juvenile Dermatomyositis Research Analysis of Published Criteria for Clinically Inactive Disease in a Large Juvenile Dermatomyositis Cohort Shows That Skin Disease Is Underestimated. Arthritis Rheumatol. 2015;67:2495–2502. doi: 10.1002/art.39200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barth Z, Schwartz T, Flato B, Aalokken TM, Koller A, Lund MB, Sjaastad I, Sanner H. Association between nailfold capillary density and pulmonary and cardiac involvement in medium to longstanding juvenile dermatomyositis. Arthritis Care Res (Hoboken) 2019;71:492–497. doi: 10.1002/acr.23687. [DOI] [PubMed] [Google Scholar]
- 15.Sanner H, Sjaastad I, Flato B. Disease activity and prognostic factors in juvenile dermatomyositis: a long-term follow-up study applying the Paediatric Rheumatology International Trials Organization criteria for inactive disease and the myositis disease activity assessment tool. Rheumatology (Oxford) 2014;53:1578–1585. doi: 10.1093/rheumatology/keu146. [DOI] [PubMed] [Google Scholar]
- 16.Okada K, Mikami T, Kaga S, Onozuka H, Inoue M, Yokoyama S, Nishino H, Nishida M, Matsuno K, Iwano H, Yamada S, Tsutsui H. Early diastolic mitral annular velocity at the interventricular septal annulus correctly reflects left ventricular longitudinal myocardial relaxation. Eur J Echocardiogr. 2011;12:917–923. doi: 10.1093/ejechocard/jer154. [DOI] [PubMed] [Google Scholar]
- 17.Barth Z, Nomeland Witczak B, Schwartz T, Gjesdal K, Flato B, Koller A, Sanner H, Sjaastad I. In juvenile dermatomyositis, heart rate variability is reduced, and associated with both cardiac dysfunction and markers of inflammation: a cross-sectional study median 13.5 years after symptom onset. Rheumatology (Oxford) 2016;55:535–543. doi: 10.1093/rheumatology/kev376. [DOI] [PubMed] [Google Scholar]
- 18.Prospective Studies C, Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, Qizilbash N, Peto R, Collins R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370:1829–1839. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 19.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 20.Tansley SL, McHugh NJ. Serological subsets of juvenile idiopathic inflammatory myopathies–an update. Expert Rev Clin Immunol. 2016;12:427–437. doi: 10.1586/1744666X.2016.1131126. [DOI] [PubMed] [Google Scholar]
- 21.Tansley SL, Li D, Betteridge ZE, McHugh NJ. The reliability of immunoassays to detect autoantibodies in patients with myositis is dependent on autoantibody specificity. Rheumatology (Oxford) 2020;59:2109–2114. doi: 10.1093/rheumatology/keaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]