Abstract
Objective
This study aimed to compute the pooled prevalence of diabetes mellitus and other underlying conditions in patients with coronavirus disease 2019 associated rhino-orbito-cerebral mucormycosis.
Method
A systematic literature review was performed in PubMed, Scopus, Web of Science, Embase and Google Scholar. The cross-sectional studies that reported the frequency of diabetes mellitus in patients with coronavirus disease 2019 associated rhino-orbito-cerebral mucormycosis were included.
Results
Eighteen eligible studies with a total number of 3718 patients were included in the current study. The pooled prevalence of diabetes in patients with coronavirus disease 2019 associated rhino-orbito-cerebral mucormycosis was 89 per cent and with new-onset diabetes was 32 per cent. The pooled prevalence of steroid use was high (79 per cent) too. The all-cause mortality rate was 24 per cent.
Conclusion
Diabetes mellitus was the most frequent underlying condition in patients with coronavirus disease 2019 associated rhino-orbito-cerebral mucormycosis. The second most frequent underlying condition was steroid use during coronavirus disease 2019 infection. The appropriate control of hyperglycaemia and rational prescription of steroids during the treatment of coronavirus disease 2019 associated rhino-orbito-cerebral mucormycosis is recommended.
Key words: COVID-19, Diabetes Mellitus, Prevalence, Mucormycosis
Introduction
Mucormycosis is an opportunistic life-threatening infection, caused by fungi class zygomycetes. Based on the anatomical site of involvement and clinical presentation, mucormycosis is divided into rhino-orbito-cerebral, pulmonary, cutaneous, gastrointestinal, renal and disseminated forms.1 The most common form of mucormycosis is rhino-orbito-cerebral.
The prevalence of mucormycosis significantly increased in the coronavirus disease 2019 (Covid-19) era; the highest prevalence of mucormycosis before the Covid-19 pandemic outbreak was reported from India, with 0.14 cases per 1000 diabetic patients.2 Recently, a study performed by Hussain et al. reported that the prevalence of Covid-19-associated mucormycosis was 7 cases per 1000 patients, which is 50 times higher than the highest reported prevalence before the Covid-19 pandemic outbreak.3
Multiple theories were supposed to explain the pathogenesis of mucormycosis in the Covid-19 era; severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection induces lymphocytopenia, decreased CD4+ and CD8+ cells and cytokine storm.4 Endothelial damage and endothelialitis happen in severe Covid-19. Glucose-regulated protein (GRP 78) and the mucorales adhesin spore coat protein homologs (CotH) are endothelial receptors that facilitate the endothelial adhesion of fungus to the endothelial cells. Acidosis and hyperglycaemia induce the expression of these two receptors.5 On the other hand, prolonged hospitalisation, use of broad-spectrum antibiotics, oxygen therapy and mechanical ventilation during the course of Covid-19 predisposes patients to fungal infections like mucormycosis.6
The presence of diabetes mellitus makes patients susceptible to mucormycosis; the innate immune system is impaired in hyperglycaemic states, resulting in decreased migration, chemotaxis and phagocytosis of neutrophils, which are the primary responses in fungus inoculation.7 Besides, the hyperglycaemic state leads to overexpression of glucose-regulated protein 78 and CotH3 receptors and impairment of oxidative and non-oxidative pathways.7 Diabetic ketoacidosis can exacerbate the condition by inhibition of iron sequestration proteins and increasing the free iron that promotes fungal growth.8 However, Covid-19 infection can induce hyperglycaemia by direct impairment of the insulin-secreting cells in the pancreas.9 Corticosteroid use for the treatment of Covid-19 can induce hyperglycaemia too.10
The reported prevalence of diabetes mellitus in the patients with rhino-orbito-cerebral mucormycosis was varied in the literature, with numbers between 51 to 88 per cent,11,12 and to the best of our knowledge, no study reported the prevalence of diabetes mellitus and other underlying conditions in patients with Covid-19-associated rhino-orbito-cerebral mucormycosis. Therefore, this study aimed to evaluate the pooled prevalence of diabetes mellitus in patients with Covid-19-associated rhino-orbito-cerebral mucormycosis. The secondary goal of the current study was to evaluate the prevalence of the other underlying conditions such as hypertension, cardiovascular disorders, chronic renal disease and corticosteroid use in patients with Covid-19-associated rhino-orbito-cerebral mucormycosis. Awareness of the prevalence of the risk factors and underlying conditions can improve the clinical suspicion and lead to early diagnosis and prompt treatment of Covid-19-associated rhino-orbito-cerebral mucormycosis, improving outcomes for this life-threatening infection.
Materials and methods
Inclusion and exclusion criteria
The studies included in this systematic review were peer-reviewed cross-sectional studies that reported rhino-orbito-cerebral mucormycosis that developed in Covid-19 positive patients of any age. Only patients with a proven diagnosis of Covid-19 before or at the time of development of mucormycosis were added. There was no language restriction. The exclusion criteria were: case series, case reports, reviews, non-peer reviewed and pre-print articles. In the studies that reported the patients with aspergillosis or candidiasis besides the mucormycosis, only the data of cases with mucormycosis were extracted if it was possible. Otherwise, the studies that reported the cumulative data were excluded. The studies that discussed mucormycosis of other sites of the body (other than the rhino-orbito-cerebral cases) were excluded when the extraction of the data of the rhino-orbito-cerebral patients was not possible. The primary objective of the current study was to estimate the pooled prevalence of diabetes mellitus; therefore studies that did not report the number of patients with Covid-19-associated rhino-orbito-cerebral mucormycosis who were suffering from diabetes mellitus were excluded.
Search strategy and data source
The literature search was conducted in several databases including PubMed (Medline), Scopus, Web of Science, Embase and Google Scholar from 1 December 2019 to 31 August 2021. A hand search of the references of the included studies was additionally performed. The search strategy was implemented using the following medical subject heading terms and free-text terms: ‘mucormycosis’ OR ‘Mucorales’ OR ‘zygomycosis’ OR ‘Rhizopus’ OR ‘phycomycosis’ OR ‘black fungus’ AND ‘Covid-19’ OR ‘SARS-CoV-2’ OR ‘coronavirus’ OR ‘2019-nCoV’.
Data collection and extraction
Two authors (MMS and HZ) reviewed the titles and abstracts of the obtained articles independently. The discrepancies were resolved by the final decision of the third author (MM). The same two authors evaluated the full texts of the retrieved articles by eligibility criteria. The eligible articles’ data were extracted independently by two researchers (MMS and HZ). The following data were extracted: first author, year of publication, country, number of participants, mean age, sex, orbital or cerebral involvement, number of patients with each co-morbidity, history of steroid use, and mortality.
Risk of bias assessment
The quality of the included studies was evaluated by OM using the Newcastle–Ottawa Scale adapted for cross-sectional studies.13 The Newcastle–Ottawa Scale was used to evaluate the observational studies in the three fields of selection, comparability and outcome assessment. It includes 7 questions with a maximum score of 10. Higher scores indicate a higher quality of study.
Statistical analysis
The number of patients with orbital or cerebral involvement, co-morbidities, steroid use and all-cause mortality was used for meta-analysis. All statistical analysis was performed by Stata statistical analysis software (version 14.0; Stata Corp LP, College Station, USA). The effect size was estimated using pooled prevalence with its 95 per cent confidence interval (CI). The heterogeneity was calculated using the inconsistency (I2) test and categorised based on a study performed by Higgins et al. to low, moderate and high correspondence to I2 values of 25 per cent, 50 per cent and 75 per cent, respectively.14 The significance level was set as a p-value less than 0.05. The random-effect model was used for effect size calculation.
Results
Literature search and baseline characteristics
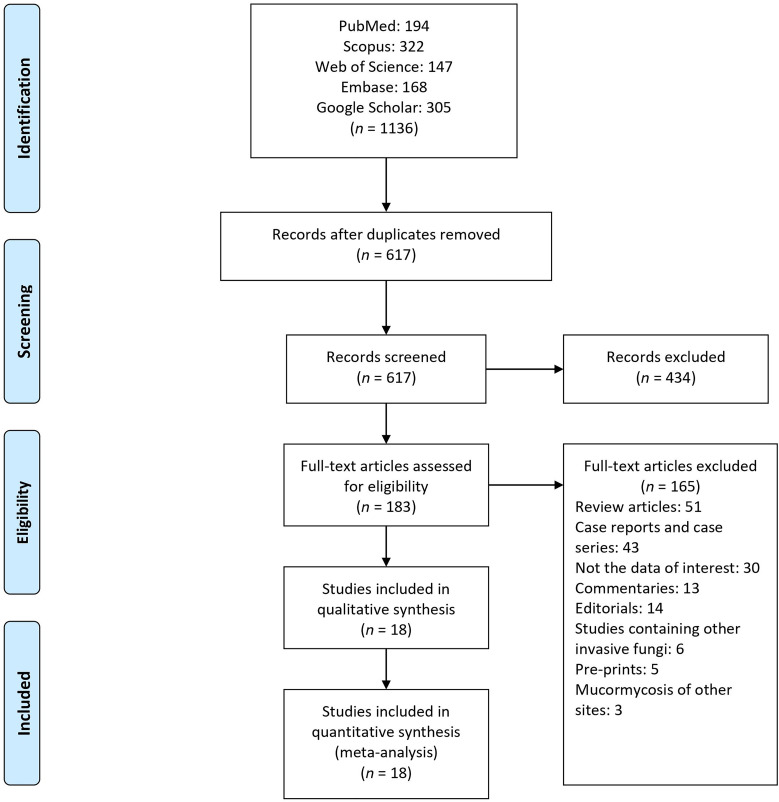
At the initial stage, we found 1136 articles; among them, 519 articles were duplicated and removed. The titles and abstracts of the 617 remaining articles were evaluated for eligibility criteria, and among them, 183 articles were selected for full-text evaluation. Finally, 18 articles were included in the study (Figure 1 and Table 1).
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow-chart of evaluated studies.
Table 1.
Characteristics of included studies
| First author | Patients (n) | Male/female (n) | Diabetes mellitus (n) | HTN | CVD (n) | CRD (n) | Other co-morbidities (n) |
Steroid use (n) | Mortality (n) | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|
| Arora et al.15 | 60 | 45/15 | 59 | 14 | 6 | 2 | Hypothyroidism: 4; malignancy: 1; organ transplant: 1 |
38 | N/A | 7 |
| Bhanuprasad et al.16 | 132 | 101/31 | 129 | N/A | 5 | 7 | Cerebrovascular disease: 2; HIV: 2 | 73 | 13 | 8 |
| Dave et al.17 | 58 | 55/11 | 56 | N/A | N/A | N/A | Malignancy: 1 | 48 | 20 | 7 |
| Desai et al.18 | 100 | 64/36 | 80 | 33 | 9 | 4 | Hypothyroidism: 2 | N/A | 20 | 6 |
| Desai et al.19 | 50 | 29/21 | 41 | 17 | 7 | 6 | Malignancy: 1 | 42 | 15 | 4 |
| Dubey et al.20 | 55 | 35/20 | 55 | N/A | N/A | N/A | N/A | 33 | N/A | 4 |
| Gupta et al.21 | 70 | 47/23 | 70 | N/A | N/A | N/A | Malignancy: 2; organ transplant: 5; immunosuppressive drug: 7 | N/A | 4 | 5 |
| Joshi et al.22 | 25 | 16/9 | 22 | N/A | N/A | N/A | HIV: 2 | 25 | 14 | 4 |
| Kumari et al.23 | 20 | 11/9 | 16 | N/A | N/A | 1 | Chronic liver disease: 1 | 16 | 6 | 4 |
| Mangal et al.24 | 67 | 44/23 | 67 | N/A | N/A | N/A | N/A | 56 | N/A | 5 |
| Mishra et al.25 | 32 | 17/15 | 28 | 16 | 2 | N/A | N/A | 30 | 4 | 6 |
| Pakdel et al.26 | 15 | 10/5 | 13 | 7 | 2 | N/A | Hypothyroidism: 1; malignancy: 2; cirrhosis: 1 | 7 | 7 | 6 |
| Paras et al.27 | 35 | 21/14 | 28 | N/A | N/A | N/A | N/A | 21 | N/A | 4 |
| Ramaswami et al.28 | 70 | 42/28 | 49 | 17 | 4 | 6 | Organ transplant: 2; immunosuppressive drugs: 4 | 49 | N/A | 7 |
| Selarka et al.29 | 47 | 35/12 | 36 | 27 | 6 | N/A | Hypothyroidism: 6; rheumatoid arthritis: 1 | 45 | 11 | 5 |
| Sen et al.30 | 2826 | 1993/833 | 2194 | 690 | 16 | 88 | Hypothyroidism: 4; malignancy: 2; organ transplant: 4; immunosuppressive drugs: 4; cerebrovascular disease: 8; liver disease: 2 | 2073 | 305 | 9 |
| Sharma et al.31 | 23 | 15/8 | 21 | 14 | N/A | 1 | N/A | 23 | N/A | 4 |
| Thayyil et al.32 | 33 | 23/10 | 28 | N/A | N/A | N/A | N/A | 26 | N/A | 6 |
HTN = hypertension; CVD = cardiovascular disease; CRD = chronic renal disease NOS = Newcastle–Ottawa Scale; HIV = human immunodeficiency virus; N/A = not available
A total of 3718 patients were included in this study. Among these 3718 patients, 2592 patients were male, and 1126 patients were female. The mean age ranged from 44.5 to 58.3 years. Among 18 studies, 17 studies with 3703 patients were reported from India, and 1 study with 15 patients was from Iran.
Risk of bias assessment
The risk of bias assessment was performed for all 18 studies, and the results are reported in Table 1 and supplementary Table 1 (see Table 1 in the supplementary material, available on The Journal of Laryngology & Otology website). The median quality score was 5.5 (range: 4 to 9).
Pooled prevalence of orbital and cerebral involvement
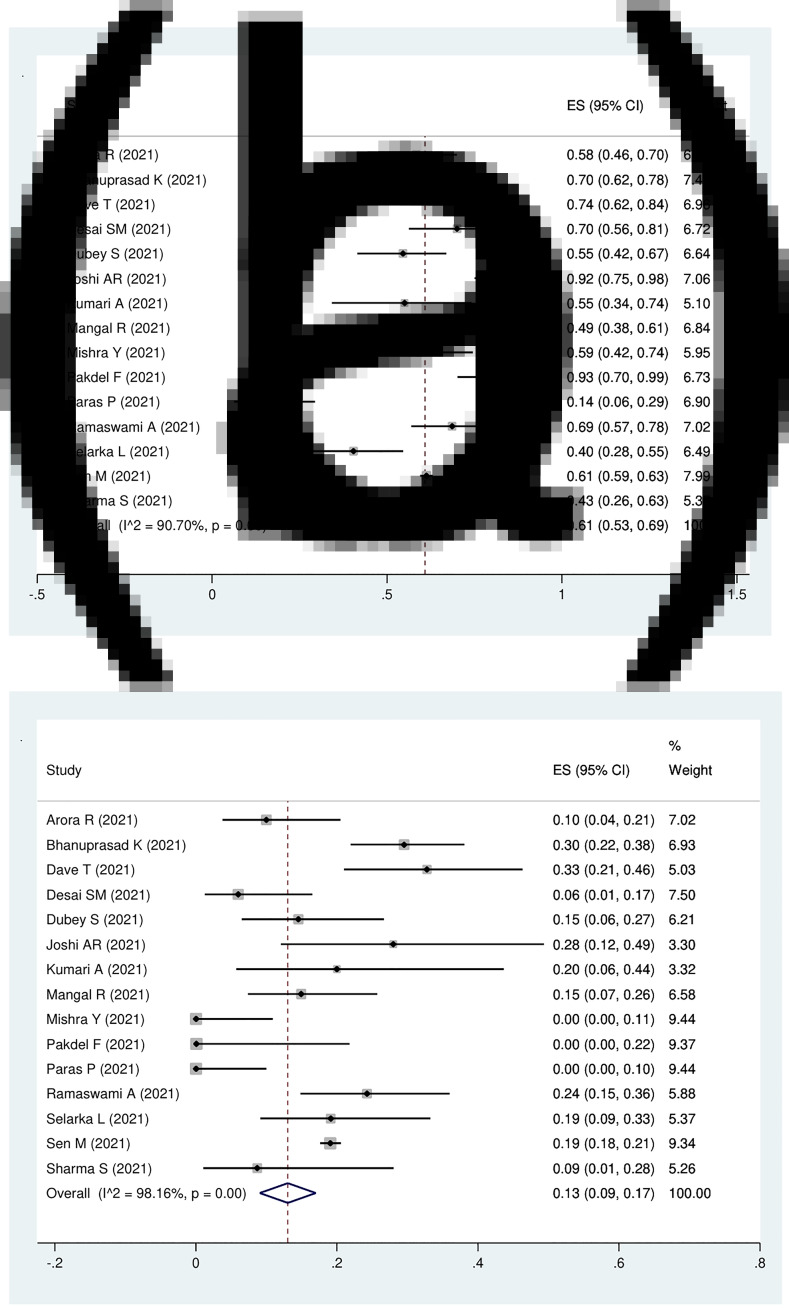
Fifteen studies were eligible for the analysis of the pooled prevalence of orbital involvement among Covid-19-associated rhino-orbito-cerebral mucormycosis patients. The pooled prevalence of orbital involvement was 61 per cent (95 per cent CI, 53 to 69 per cent). The model demonstrated high heterogeneity (I2 = 90.7 per cent, p < 0.01; Figure 2a).
Fig. 2.
Forest plot of pooled prevalence of (a) orbital involvement and (b) cerebral involvement patients with Covid-19-associated rhino-orbito-cerebral mucormycosis. ES = effect size; CI = confidence interval
Cerebral involvement was reported in 15 studies. The pooled prevalence of cerebral involvement in Covid-19-associated rhino-orbito-cerebral mucormycosis patients was 13 per cent (95 per cent CI, 9 to 17 per cent). The model demonstrated high heterogeneity (I2 = 98.2 per cent, p < 0.01; Figure 2b).
Pooled prevalence of diabetes mellitus
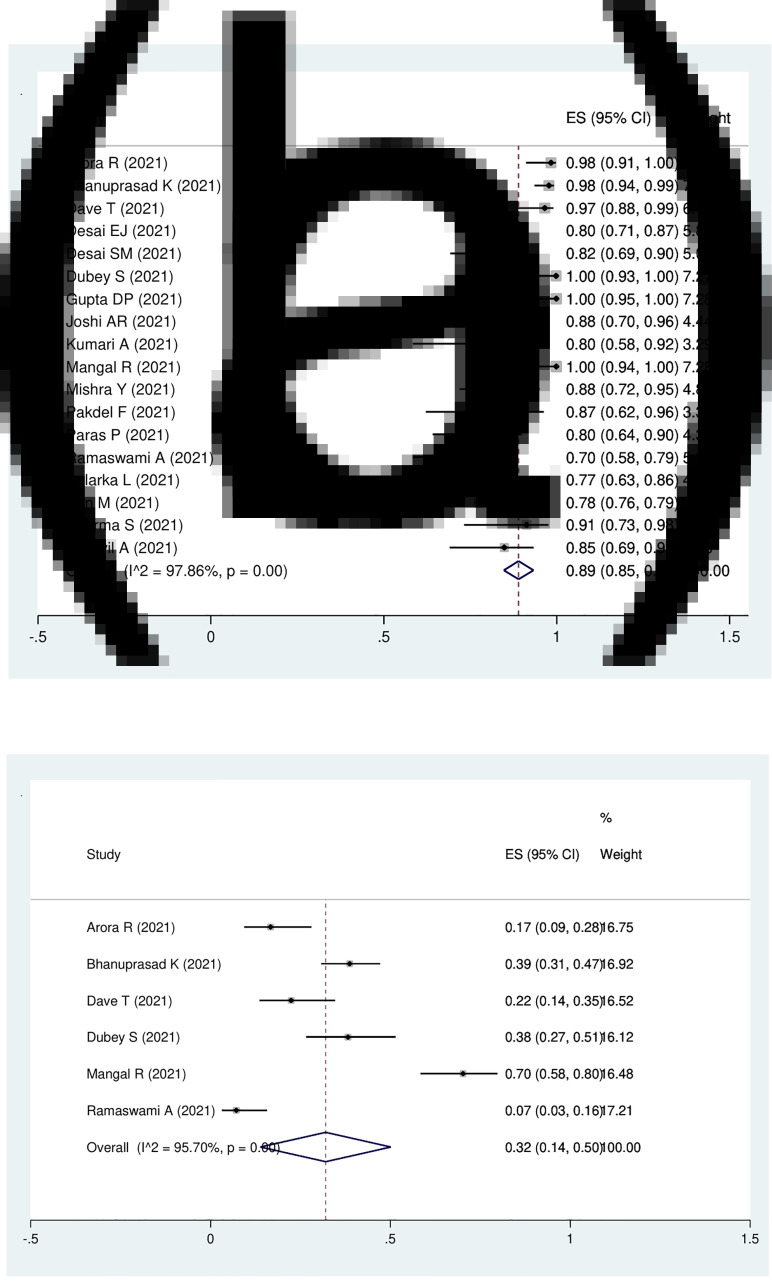
Eighteen studies with 3718 patients were eligible for the analysis of the prevalence of diabetes mellitus in the patients with Covid-19-associated rhino-orbito-cerebral mucormycosis, which was 89 per cent (95 per cent CI, 85 to 93 per cent). The model demonstrated high heterogeneity (I2 = 97.9 per cent, p < 0.01; Figure 3a).
Fig. 3.
Forest plot of pooled prevalence of (a) diabetes mellitus and (b) new-onset diabetes mellitus in the patients with Covid-19-associated rhino-orbito-cerebral mucormycosis. ES = effect size; CI = confidence interval
New-onset diabetes mellitus was reported in 6 studies composed of 442 patients. The pooled prevalence of new-onset diabetes mellitus in patients with Covid-19-associated rhino-orbito-cerebral mucormycosis was 32 per cent (95 per cent CI, 14 to 50 per cent; I2 = 97.5 per cent; p < 0.01; Figure 3b).
Pooled prevalence of other underlying conditions
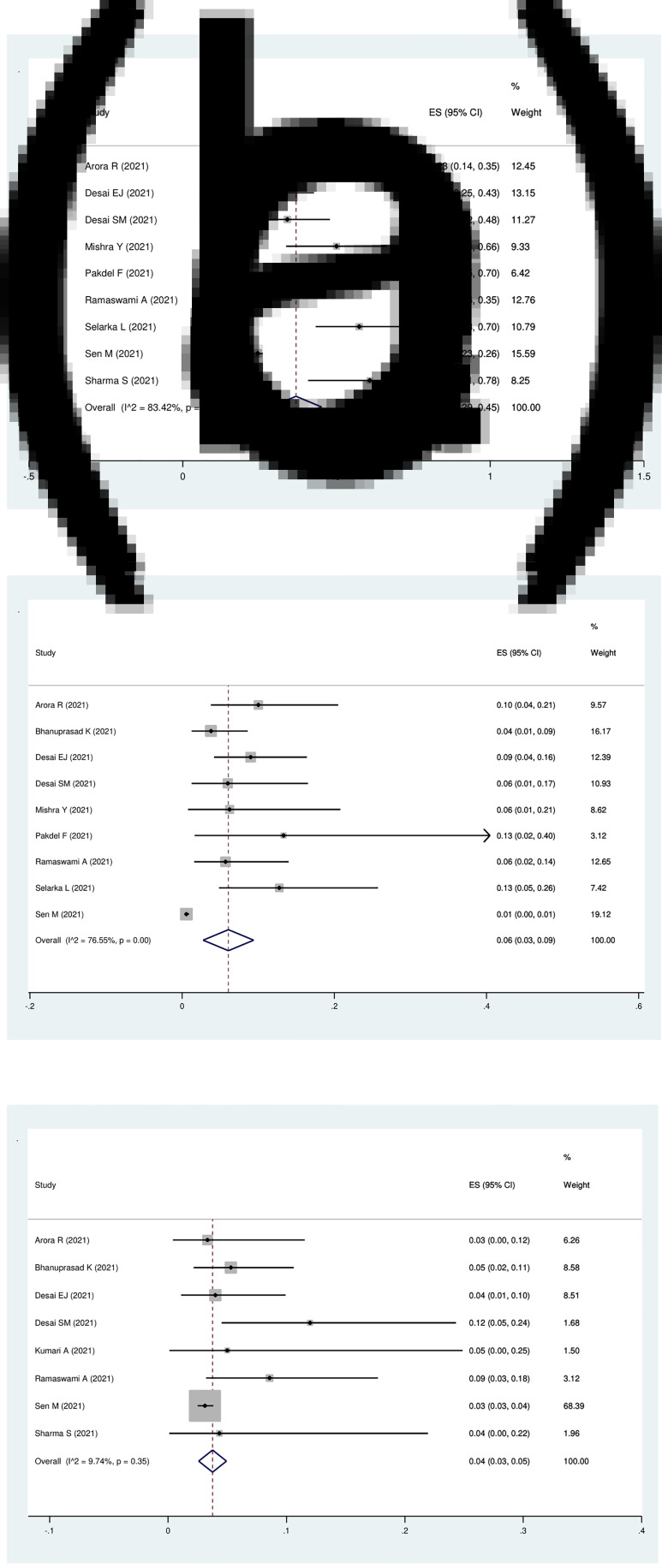
Nine studies were eligible for the analysis of the pooled prevalence of hypertension in patients with Covid-19-associated rhino-orbito-cerebral mucormycosis, which was 37 per cent (95 per cent CI, 29 to 45 per cent) with high heterogeneity (I2 = 83.4 per cent; p < 0.01; Figure 4a).
Fig. 4.
Forest plot of pooled prevalence of (a) hypertension, (b) cardiovascular disorders and (c) renal disease in patients with Covid-19-associated rhino-orbito-cerebral mucormycosis. ES = effect size; CI = confidence interval
Nine studies with 3332 patients were eligible for evaluation of the pooled prevalence of cardiovascular disorder in Covid-19-associated rhino-orbito-cerebral mucormycosis patients, which was 6 per cent (95 per cent CI, 3 to 9 per cent) with high heterogeneity (I2 = 76.6 per cent; p < 0.01; Figure 4b).
Eight studies with 3281 patients were eligible for the analysis of the pooled prevalence of chronic renal disease in patients with Covid-19-associated rhino-orbito-cerebral mucormycosis, which was 4 per cent (95 per cent CI, 3 to 5 per cent) with low heterogeneity (I2 = 9.7 per cent; p = 0.35; Figure 4c).
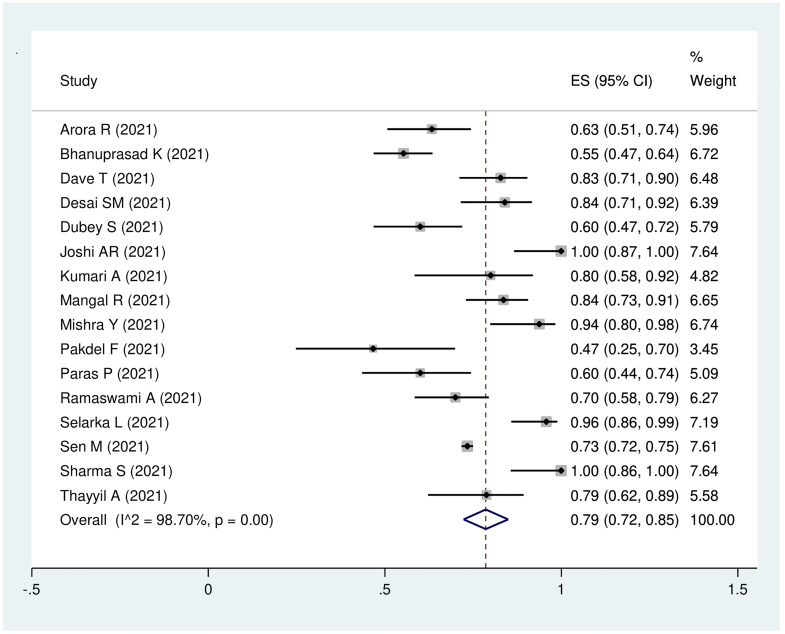
Sixteen studies with 3548 patients were eligible for analysis of the pooled prevalence of steroid use during Covid-19, which was 79 per cent (95 per cent CI, 72 to 85 per cent). The model demonstrated high heterogeneity (I2 = 98.7 per cent; p < 0.01; Figure 5).
Fig. 5.
Forest plot of pooled prevalence of steroid use in the patients with Covid-19-associated rhino-orbito-cerebral mucormycosis. ES = effect size; CI = confidence interval
Pooled prevalence of all-cause mortality
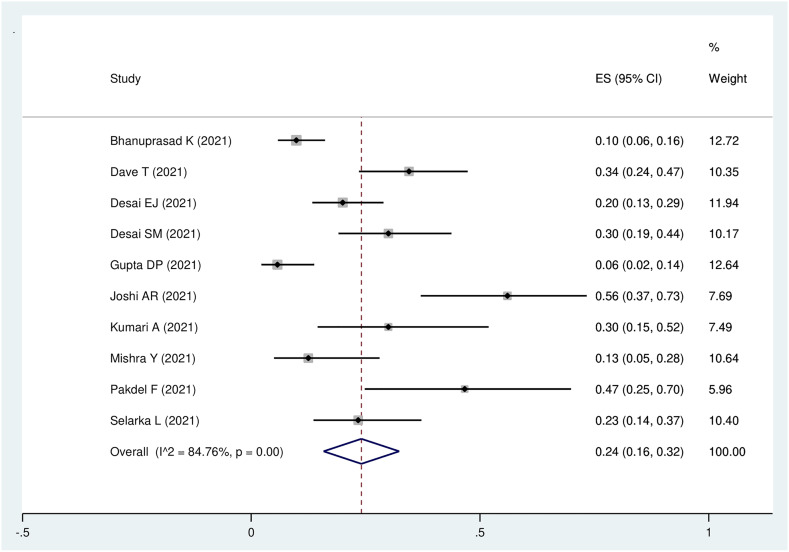
All-cause mortality was reported in 10 studies with 549 participants. The pooled prevalence of mortality was 24 per cent (95 per cent CI, 16 to 32 per cent; I2 = 84.8 per cent; p < 0.01; Figure 6).
Fig. 6.
Forest plot of pooled prevalence of all-cause mortality in the patients with Covid-19-associated rhino-orbito-cerebral mucormycosis. ES = effect size; CI = confidence interval
Discussion
This study was a comprehensive systematic review, composed of cross-sectional studies with a large number of participants which explored the pooled prevalence of underlying disorders in patients with Covid-19-associated rhino-orbito-cerebral mucormycosis. In the current study, the focus is on rhino-orbito-cerebral Covid-19-associated mucormycosis. The incidence of orbital involvement in cases with rhino-orbito-cerebral mucormycosis was reported to be 76 to 80 per cent in previous studies.33 In the current study, the prevalence of orbital involvement was 61 per cent. The frequency of cerebral involvement in Covid-19-associated rhino-orbito-cerebral mucormycosis was reported to be 22 per cent.34,35 The pooled prevalence of intracranial involvement was 13 per cent in the current study.
Mucormycosis is an opportunistic fungal infection with a prevalence of 0.14 per 1000 in diabetic patients, as reported from studies in India, which is 80 times more than the prevalence of mucormycosis in developed countries.2 This can explain why the majority of patients (3703 out of 3718 cases) were reported from India in the current study. The pooled prevalence of Covid-19-associated mucormycosis is 2.7 to 7 per 1000 cases, which is higher than the highest prevalence of Covid-19-associated mucormycosis reported before.3,36 Covid-19-associated mucormycosis is predominantly seen in males (56.3 to 80 per cent), which is compatible with the frequency of males in Covid-19-associated rhino-orbito-cerebral mucormycosis patients in the current study (2592 patients of 3718 cases).37–39
Uncontrolled diabetes mellitus with or without diabetic ketoacidosis is a predisposing condition for mucormycosis.7 Innate immunity is altered in diabetes mellitus because of dysfunction of the polymorphonuclear cells.40 Adaptive immunity and synthesis of some kinds of cytokines are compromised in diabetes mellitus too.41 Decreased innate and adaptive immunity responses play a key role in mucormycosis involvement.42 Additionally, during diabetic ketoacidosis, free unbounded serum iron increases. Iron overload is another predisposing factor for mucormycosis infection.43
The prevalence of diabetes mellitus in the patients with rhino-orbito-cerebral mucormycosis without Covid-19 varies in different studies: in a study performed in India by Nithyanandam et al., uncontrolled diabetes mellitus was present in 30 of 34 patients with rhino-orbito-cerebral mucormycosis (88 per cent).12 Another study performed in the USA reported that 34 of 41 patients with rhino-orbito-cerebral mucormycosis (83 per cent) were diabetic.44 Jeong et al. performed a systematic review and meta-analysis on the epidemiology of mucormycosis and reported that rhino-orbito-cerebral mucormycosis is more commonly observed in patients with diabetes mellitus (173 cases out of 340 patients; 51 per cent).11 In a meta-analysis performed by Vaughan et al., 112 out of 175 patients with rhino-orbito-cerebral mucormycosis were diabetic (64 per cent).45 Neither of these two meta-analyses reported the pooled prevalence of diabetes mellitus in patients with rhino-orbito-cerebral mucormycosis. Within the Covid-19 era, a systematic review and meta-analysis was performed by Hussain et al. on Covid-19-associated mucormycosis of all anatomical sites of the body; it reported a pooled prevalence of diabetes mellitus of 74.5 per cent.3 The pooled prevalence of diabetes mellitus in Covid-19-associated rhino-orbito-cerebral mucormycosis has not been reported before, to the best of our knowledge. In the current study, the pooled prevalence of diabetes mellitus in patients with Covid-19-associated rhino-orbito-cerebral mucormycosis was 89 per cent. Rhino-orbito-cerebral mucormycosis is more prevalent in patients with diabetes mellitus compared with the other forms of mucormycosis.1 Corzo-León et al. reported the prevalence of diabetes mellitus in different forms of mucormycosis; they reported diabetes mellitus in 72 per cent of the patients with mucormycosis and 88 per cent of the cases with rhino-orbito-cerebral mucormycosis.46 This can explain the higher prevalence of diabetes mellitus in the current study, compared with the study performed by Hussain et al.3
SARS-CoV-2 infection can increase the risk of new-onset diabetes mellitus. The proposed mechanism is the binding of the virus to the angiotensin-converting enzyme-2 receptors in the pancreas, which causes overexpression of angiotensin II, which can impede insulin secretion. On the other hand, SARS-CoV-2 can be inserted into beta-cells of the pancreas and causes impairment of cells that secrete insulin.9,47 The administration of corticosteroids for the treatment of Covid-19 can cause drug-induced hyperglycaemia. The pooled prevalence of new-onset diabetes mellitus was 32 per cent of the patients with Covid-19-associated rhino-orbito-cerebral mucormycosis in the current study. In a study performed in India, new-onset diabetes mellitus was reported in 110 out of 300 patients with Covid-19-associated rhino-orbito-cerebral mucormycosis (37 per cent),48 which is compatible with the results of our study.
The pooled prevalence of hypertension in patients with Covid-19-associated rhino-orbito-cerebral mucormycosis was 37 per cent in the current study. Hypertension was reported in 23 to 75 per cent of patients with Covid-19-associated mucormycosis in the literature.39,49–54 Cardiovascular disease was present in 6 of 72 patients with Covid-19-associated mucormycosis (8 per cent) in a study performed by Pal et al.53 The pooled prevalence of cardiovascular disorder in the current study was 6 per cent. Recently, chronic renal disease was considered a predisposing factor for rhino-orbito-cerebral mucormycosis.55 The frequency of chronic renal disease in Covid-19-associated mucormycosis patients was reported to be 1 to 11 per cent in the literature.39,51,53,54 The pooled prevalence of chronic renal disease in Covid-19-associated rhino-orbito-cerebral mucormycosis patients was 4 per cent in the current study. Although the presence of underlying conditions like hypertension, cardiovascular disorders and chronic renal failure is accompanied by a higher risk of mortality in patients with Covid-19,56,57 a study performed by Riad et al. could not find a significant risk of increased mortality in patients with Covid-19-associated mucormycosis.50
Based on a trial performed by the Recovery Collaborative Group, corticosteroids are recommended in patients with Covid-19 who require mechanical ventilation or supplemental oxygen in order to decrease the mortality rate.58 Although steroids have no benefit in patients without respiratory support, it is widely used in these patients. Long-term, high-dose steroid therapy is a risk factor for rhino-orbito-cerebral mucormycosis.55 Corticosteroids can impair the function of polymorphonuclear cells and macrophages, can exacerbate hyperglycaemia in patients with diabetes mellitus and induce hyperglycaemia in predisposed patients.59 However, the role of short-course corticosteroid therapy in the pathogenesis of rhino-orbito-cerebral mucormycosis is controversial. The history of corticosteroid use was reported in 84 to 90.5 per cent of the patients with Covid-19-associated mucormycosis.37,51,60 Hussain et al., in a systematic review and meta-analysis, reported that the pooled prevalence of steroid use in patients with Covid-19-associated mucormycosis was 94 per cent.3 The pooled prevalence of corticosteroid use in patients with Covid-19-associated rhino-orbito-cerebral mucormycosis was 79 per cent in the current study.
The all-cause mortality rate was between 30 to 38 per cent in patients with Covid-19-associated mucormycosis in previous systematic reviews.3,39,53 The mortality rate is dependent on the anatomical location of the involvement. In rhino-orbito-cerebral mucormycosis patients, the mortality rate is dependent on the extension of infection: it is higher in cerebral involvement (56 per cent), 20 per cent in rhino-orbital forms and nearly zero in rhinosinusal infection.61 In the current study, the pooled prevalence of all-cause mortality in patients with Covid-19-associated rhino-orbito-cerebral mucormycosis was 24 per cent.
The current study is a comprehensive systematic review with a large number of participants, which included cross-sectional studies with a special focus on Covid-19-associated rhino-orbito-cerebral mucormycosis. However, this study has some limitations: 17 out of 18 included studies were from India, which decreases the generalisability of the results, and interpretation of the results should be performed with caution. On the other hand, the data of co-morbidities was not reported in all studies, especially for malignancies and organ transplantation which are well-known risk factors for mucormycosis.62
Conclusion
In conclusion, the results of the current meta-analysis reported diabetes mellitus as the most frequent underlying disorder in patients with Covid-19-associated rhino-orbito-cerebral mucormycosis with a prevalence of 89 per cent. The second most prevalent underlying condition was steroid use. The prevalence of other co-morbidities such as hypertension, cardiovascular disorders and chronic renal disease was reported, which can increase the awareness and clinical suspicion in the presence of underlying conditions to accelerate the diagnosis of Covid-19-associated rhino-orbito-cerebral mucormycosis. The appropriate control of hyperglycaemia and judicious use of steroids in the treatment of Covid-19 is recommended.
Competing interests
None declared
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0022215122001074.
click here to view supplementary material
References
- 1.Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 2005;41:634–53 [DOI] [PubMed] [Google Scholar]
- 2.Chander J, Kaur M, Singla N, Punia RPS, Singhal SK, Attri AK et al. Mucormycosis: battle with the deadly enemy over a five-year period in India. J Fungi 2018;4:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hussain S, Riad A, Singh A, Klugarová J, Antony B, Banna H et al. Global prevalence of Covid-19-associated mucormycosis (CAM): living systematic review and meta-analysis. J Fungi 2021;7:985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu J, Li S, Liu J, Liang B, Wang X, Wang H et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 2020;55:102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 2020;383:120–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rawson TM, Moore LSP, Zhu N, Ranganathan N, Skolimowska K, Gilchrist M et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support Covid-19 antimicrobial prescribing. Clin Infect Dis 2020;71:2459–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis 2012;54:23–34 [DOI] [PubMed] [Google Scholar]
- 8.Gebremariam T, Lin L, Liu M, Kontoyiannis DP, French S, Edwards JE et al. Bicarbonate correction of ketoacidosis alters host-pathogen interactions and alleviates mucormycosis. J Clin Invest 2016;126:2280–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu C-T, Lidsky PV, Xiao Y, Lee IT, Cheng R, Nakayama T et al. SARS-CoV-2 infects human pancreatic β cells and elicits β cell impairment. Cell Metab 2021;33:1565–76.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moorthy A, Gaikwad R, Krishna S, Hegde R, Tripathi KK, Kale PG et al. SARS-CoV-2, uncontrolled diabetes and corticosteroids-an unholy trinity in invasive fungal infections of the maxillofacial region? A retrospective, multi-centric analysis. J Maxillofac Oral Surg 2021;20:418–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeong W, Keighley C, Wolfe R, Lee WL, Slavin MA, Kong DCM et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect 2019;25:26–34 [DOI] [PubMed] [Google Scholar]
- 12.Nithyanandam S, Jacob MS, Battu RR, Thomas RK, Correa MA, D'Souza O. Rhino-orbito-cerebral mucormycosis. A retrospective analysis of clinical features and treatment outcomes. Indian J Ophthalmol 2003;51:231–6 [PubMed] [Google Scholar]
- 13.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5 [DOI] [PubMed] [Google Scholar]
- 14.Higgins JPT. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arora R, Goel R, Khanam S, Kumar S, Shah S, Singh S et al. Rhino-orbito-cerebral-mucormycosis during the Covid-19 second wave in 2021 - a preliminary report from a single hospital. Clin Ophthalmol 2021;15:3505–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhanuprasad K, Manesh A, Devasagayam E, Varghese L, Cherian LM, Kurien R et al. Risk factors associated with the mucormycosis epidemic during the Covid-19 pandemic. Int J Infect Dis 2021;111:267–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dave TV, Gopinathan Nair A, Hegde R, Vithalani N, Desai S, Adulkar N et al. Clinical presentations, management and outcomes of rhino-orbital-cerebral mucormycosis (rocm) following Covid-19: a multi-centric study. Ophthalmic Plast Reconstr Surg 2021;37:488–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desai EJ, Pandya A, Upadhya I, Patel T, Banerjee S, Jain V. Epidemiology, clinical features and management of rhino orbital mucormycosis in post COVID 19 patients. Indian J Otolaryngol Head Neck Surg 2021;74:103–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desai SM, Gujarathi-Saraf A, Agarwal EA. Imaging findings using a combined MRI/CT protocol to identify the “entire iceberg” in post-Covid-19 mucormycosis presenting clinically as only “the tip.” Clin Radiol 2021;76:784.e27–784.e33 [DOI] [PubMed] [Google Scholar]
- 20.Dubey S, Mukherjee D, Sarkar P, Mukhopadhyay P, Barman D, Bandopadhyay M et al. Covid-19 associated rhino-orbital-cerebral mucormycosis: an observational study from Eastern India, with special emphasis on neurological spectrum. Diabetes Metab Syndr Clin Res Rev 2021;15:102267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta DP, Gupta S, Shah CK, Sreevidya SR. Clinical study of surge of mucormycosis in Covid-19 pandemic: a tertiary care center study. Indian J Otolaryngol Head Neck Surg. 10.1007/s12070-021-02784-6. Epub 2021 Aug 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joshi AR, Muthe MM, Patankar SH, Athawale A, Achhapalia Y. CT and MRI findings of invasive mucormycosis in the setting of Covid-19: experience from a single center in India. AJR Am J Roentgenol 2021;217:1431–2 [DOI] [PubMed] [Google Scholar]
- 23.Kumari A, Rao NP, Patnaik U, Malik V, Tevatia MS, Thakur S et al. Management outcomes of mucormycosis in Covid-19 patients: a preliminary report from a tertiary care hospital. Med J Armed Forces India 2021;77:S289–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mangal R, Gehlot PS, Bang A, Kaushal A, Kolare R. Covid-19 associated rhino-orbito-cerebral mucormycosis: clinical profile and imaging spectrum. J Clin Diagnostic Res 2021;15:TC01–6 [Google Scholar]
- 25.Mishra Y, Prashar M, Sharma D, Akash Kumar VP, Tilak TVSVGK. Diabetes, Covid 19 and mucormycosis: Clinical spectrum and outcome in a tertiary care medical center in Western India. Diabetes Metab Syndr Clin Res Rev 2021;15:102196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pakdel F, Ahmadikia K, Salehi M, Tabari A, Jafari R, Mehrparvar G et al. Mucormycosis in patients with Covid-19: a cross-sectional descriptive multicentre study from Iran. Mycoses 2021;64:1238–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paras P, Purvi P, Brijeshkumar S, Arunima B, Drasti P, Maitri B. Expanding the spectrum of fatal necrotizing fungal infections presented as sinonasal and rhino orbital mucormycosis and aspergillosis in post coronavirus disease. Eur J Mol Clin Med 2021;8:101–10 [Google Scholar]
- 28.Ramaswami A, Sahu AK, Kumar A, Suresh S, Nair A, Gupta D et al. Covid-19-associated mucormycosis presenting to the emergency department—an observational study of 70 patients. QJM An Int J Med 2021;114:464–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selarka L, Sharma S, Saini D, Sharma S, Batra A, Waghmare VT et al. Mucormycosis and Covid-19: an epidemic within a pandemic in India. Mycoses 2021;64:1253–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sen M, Honavar SG, Bansal R, Sengupta S, Rao R, Kim U et al. Epidemiology, clinical profile, management, and outcome of Covid-19-associated rhino-orbital-cerebral mucormycosis in 2826 patients in India - Collaborative OPAI-IJO Study on Mucormycosis in Covid-19 (COSMIC), Report 1. Indian J Ophthalmol 2021;69:1670–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma S, Grover M, Bhargava S, Samdani S, Kataria T. Post-coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J Laryngol 2021;135:442–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thayyil J, Divakaran A, Anikkady N. Covid-19 associated mucormycosis during the second wave of pandemic in South India. Int J Res Med Sci 2021;9:1 [Google Scholar]
- 33.Pai V, Sansi R, Kharche R, Bandili SC, Pai B. Rhino-orbito-cerebral mucormycosis: pictorial review. Insights Imaging 2021;12:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chouhan M, Solanki B, Shakrawal N. Rhino-orbital-cerebral mucormycosis: fungal epidemic in a viral pandemic. J Laryngol Otol 2021;135:981–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pradhan P, Shaikh Z, Mishra A, Preetam C, Parida PK, Sarkar S et al. Predisposing factors of rhino-orbital-cerebral mucormycosis in patients with Covid 19 infection. Indian J Otolaryngol Head Neck Surg 2021; 10.1007/s12070-021-02875-4. Epub 2021 Sep 25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel A, Agarwal R, Rudramurthy SM, Shevkani M, Xess I, Sharma R et al. Multicenter epidemiologic study of coronavirus disease – associated methods. Emerg Infect Dis 2021;27: 2349–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh AK, Singh R, Joshi SR, Misra A. Mucormycosis in Covid-19: a systematic review of cases reported worldwide and in India. Diabetes Metab Syndr Clin Res Rev 2021;15:102146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muthu V, Rudramurthy SM, Chakrabarti A, Agarwal R. Epidemiology and pathophysiology of Covid-19-associated mucormycosis: India versus the rest of the world. Mycopathologia 2021;186:739–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hussain S, Baxi H, Riad A, Klugarová J, Pokorná A, Slezáková S et al. Covid-19-associated mucormycosis (CAM): an updated evidence mapping. Int J Environ Res Public Health 2021;18:10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alba-Loureiro TC, Munhoz CD, Martins JO, Cerchiaro GA, Scavone C, Curi R et al. Neutrophil function and metabolism in individuals with diabetes mellitus. Brazilian J Med Biol Res 2007;40:1037–44 [DOI] [PubMed] [Google Scholar]
- 41.Morales-Franco B, Nava-Villalba M, Medina-Guerrero EO, Sánchez-Nuño YA, Davila-Villa P, Anaya-Ambriz EJ et al. Host-pathogen molecular factors contribute to the pathogenesis of rhizopus spp. in diabetes mellitus. Curr Trop Med Reports. 2021;8:6–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghuman H, Voelz K. Innate and adaptive immunity to mucorales. J Fungi 2017;3:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Narayanan S, Chua J V, Baddley JW. Coronavirus disease 2019–associated mucormycosis: risk factors and mechanisms of disease. Clin Infect Dis 2021;74:1279–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reed C, Bryant R, Ibrahim AS, Edwards JJ, Filler SG, Goldberg R et al. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis 2008;47:364–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaughan C, Bartolo A, Vallabh N, Leong SC. A meta-analysis of survival factors in rhino-orbital-cerebral mucormycosis—has anything changed in the past 20 years? Clin Otolaryngol 2018;43:1454–64 [DOI] [PubMed] [Google Scholar]
- 46.Corzo-León DE, Chora-Hernández LD, Rodríguez-Zulueta AP, Walsh TJ. Diabetes mellitus as the major risk factor for mucormycosis in Mexico: epidemiology, diagnosis, and outcomes of reported cases. Med Mycol 2018;56:29–43 [DOI] [PubMed] [Google Scholar]
- 47.Shrestha DB, Budhathoki P, Raut S, Adhikari S, Ghimire P, Thapaliya S et al. New-onset diabetes in Covid-19 and clinical outcomes: a systematic review and meta-analysis. World J Virol 2021;10:275–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goddanti N, Reddy YM, Kumar MK, Rajesh M, Reddy LS. Role of Covid 19 inflammatory markers in rhino-orbito-cerebral mucormycosis: a case study in predisposed patients at a designated nodal centre. Indian J Otolaryngol Head Neck Surg 2021; 10.1007/s12070-021-02970-6. Epub 2021 Nov 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gupta S, Ahuja P. Risk factors for procurence of mucormycosis and its manifestations post Covid-19: a single arm retrospective unicentric clinical study. Indian J Otolaryngol Head Neck Surg 2021; 34567997. Epub 2021 Sep 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riad A, Shabaan AA, Issa J, Ibrahim S, Amer H, Mansy Y et al. Covid-19-associated mucormycosis (CAM): case-series and global analysis of mortality risk factors. J Fungi 2021;7:837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dilek A, Ozaras R, Ozkaya S, Sunbul M, Sen EI, Leblebicioglu H. Covid-19-associated mucormycosis: case report and systematic review. Travel Med Infect Dis 2021;44:102148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zirpe K, Pote P, Deshmukh A, Gurav SK, Tiwari AM, Suryawanshi P. A retrospective analysis of risk factors of Covid-19 associated mucormycosis and mortality predictors: a single-center study. Cureus 2021;13:e18718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pal R, Singh B, Bhadada SK, Banerjee M, Bhogal RS, Hage N et al. Covid-19-associated mucormycosis: an updated systematic review of literature. Mycoses 2021;64:1452–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pippal SK, Kumar D, Ukawat L. Management challenge of rhino-orbito-cerebral mucormycosis in Covid 19 era: a prospective observational study. Indian J Otolaryngol Head Neck Surg 2021;34722223. Epub 2021 Oct 23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prakash H, Ghosh AK, Rudramurthy SM, Singh P, Xess I, Savio J et al. A prospective multicenter study on mucormycosis in India: epidemiology, diagnosis, and treatment. Med Mycol 2019;57:395–402 [DOI] [PubMed] [Google Scholar]
- 56.Tian W, Jiang W, Yao J, Nicholson CJ, Li RH, Sigurslid HH et al. Predictors of mortality in hospitalized Covid-19 patients: a systematic review and meta-analysis. J Med Virol 2020;92:1875–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ssentongo P, Ssentongo AE, Heilbrunn ES, Ba DM, Chinchilli VM. Association of cardiovascular disease and 10 other pre-existing comorbidities with Covid-19 mortality: a systematic review and meta-analysis. PLoS One 2020;15:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Recovery collaborative group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021;384:693–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lionakis MS, Kontoyiannis DP. Glucocorticoids and invasive fungal infections. Lancet 2003;362:1828–38 [DOI] [PubMed] [Google Scholar]
- 60.Vare A, Yellambkar S, Farheen A, Nandedkar V, Bhombe S, Shah R. Incidence, cumulative mortality and factors affecting the outcome of Covid-19-associated mucormycosis from Western India. Indian J Ophthalmol 2021;69:3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lanternier F, Dannaoui E, Morizot G, Elie C, Garcia-Hermoso D, Huerre M et al. A global analysis of mucormycosis in France: the retrozygo study (2005–2007). Clin Infect Dis 2012;54:35–43 [DOI] [PubMed] [Google Scholar]
- 62.Skiada A, Pavleas I, Drogari-Apiranthitou M. Epidemiology and diagnosis of mucormycosis: an update. J Fungi 2020;6:1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0022215122001074.
click here to view supplementary material