SUMMARY
Antimicrobial drugs are used to treat pathogenic bacterial infections in animals and humans. The by-stander enteric bacteria of the treated host's intestine can become exposed to the drug or its metabolites reaching the intestine in antimicrobially active form. We consider which processes and variables need to be accounted for to project the antimicrobial concentrations in the host's intestine. Those include: the drug's fraction (inclusive of any active metabolites) excreted in bile; the drug's fractions and intestinal segments of excretion via other mechanisms; the rates and intestinal segments of the drug's absorption and re-absorption; the rates and intestinal segments of the drug's abiotic and biotic degradation in the intestine; the digesta passage time through the intestinal segments; the rates, mechanisms, and reversibility of the drug's sorption to the digesta and enteric microbiome; and the volume of luminal contents in the intestinal segments. For certain antimicrobials, the antimicrobial activity can further depend on the aeration and chemical conditions in the intestine. Model forms that incorporate the inter-individual variation in those relevant variables can support projections of the intestinal antimicrobial concentrations in populations of treated host, such as food animals. To illustrate the proposed modeling framework, we develop two examples of treatments of bovine respiratory disease in beef steers by oral chlortetracycline and injectable third-generation cephalosporin ceftiofur. The host's diet influences the digesta passage time, volume, and digesta and microbiome composition, and may influence the antimicrobial loss due to degradation and sorption in the intestine. We consider two diet compositions in the illustrative simulations. The examples highlight the extent of current ignorance and need for empirical data on the variables influencing the selective pressures imposed by antimicrobial treatments on the host's intestinal bacteria.
Key words: Antimicrobial concentration in intestine, antimicrobial pharmacokinetics, antimicrobial resistance, cattle, drug degradation, population pharmacokinetics
INTRODUCTION
Antimicrobial therapies in animals and humans impose antimicrobial pressures on the host's enteric bacteria, promoting antimicrobial resistance (AMR) in these by-standers [1, 2]. The antimicrobials and resistant bacteria excreted in feces of the treated hosts contribute to AMR in the environment [3–5]. Little research has focused on the intestinal concentrations of antimicrobials [6, 7]. In human pharmacokinetic (PK) modeling, the drug intestinal transit time to the small intestine has been considered in view of adsorption to the central circulation of orally administered drugs [8–10]. Veterinary pharmacology has largely focused on the PK modeling related to the antimicrobial therapeutic effects, and on preventing drug residues in edible tissues from treated food animals [11, 12]. A recent review draws attention to the drug properties influencing its intestinal antimicrobial impacts [13]. However, applied modeling studies of antimicrobial treatments’ impacts on intestinal bacteria of food animals thus far lack to model explicitly the intestinal drug concentrations [14, 15].
Antimicrobial drugs or their active metabolites can reach the host intestine following administration via either oral or parenteral routes (as is detailed below and in [13, 16, 17]). We propose a modeling framework for projecting the antimicrobial concentrations in the host's intestine. The framework outlined in Figure 1a encompasses the processes influencing active concentrations of the antimicrobial drug (inclusive of any active metabolites) in the host's intestine. The processes that have been included in the earlier PK models for the drug concentrations pertinent to the antimicrobial therapeutic effects or residues in tissues are listed in Figure 1a in simple font. These include the drug's: absorption, distribution, metabolism, tissue deposition, elimination from the central circulation, and organ-specific barriers. The drug's intestinal transit time to the small intestine has been considered in some of those models in view of the absorption [8, 9, 18]. These processes determine the drug entering intestine [1] in bile (depending on the drug fraction eliminated from the central circulation via bile vs. urine) or [2] in secretion via the intestinal wall; and [3] drug absorption or [4] re-absorption (via enterohepatic circulation) from the intestine to the central circulation [19]. Further relevant processes (not included in the PK models for the therapeutic effects or residues in tissues) are listed in Figure 1a in Italics. These include the drug's: [5] transit time throughout the intestinal segments; [6] abiotic and [7] biotic degradation during the intestinal transit; sorption to the [8] digesta and [9] microbiome; [10] defecation (the rate and pattern of the fecal masses leaving the intestine); and [11] volume of the luminal contents in intestinal segments (the denominator for the antimicrobial concentrations present).
Fig. 1.
(a) Generalized schematic of modeling the intestinal concentrations of antimicrobials in the host's intestine. In simple font are the processes that have been characterized in PK models related to therapeutic effects of antimicrobial drugs. In Italics and underlined are further processes that need to be characterized. (b) Adaptation of the framework for oral CTC treatment in cattle. (c) Adaptation of the framework for parenteral treatment by cephalosporin ceftiofur in cattle.
To illustrate the importance of the variables [5–11] above, least understood processes influencing the antimicrobial fate in the intestine (i.e. the selective pressures on intestinal bacteria), we provide two illustrative examples of an oral and parenteral antimicrobial treatments in a major food animal species, cattle. The illustrative models are formulated for an individual and incorporating inter-individual variation. The model form incorporating inter-individual variation is simulated with random sampling the values of the variables relevant to the antimicrobial's intestinal fate from their assigned distributions expected among the hosts (i.e. each simulation represents one of the hosts, and outputs from multiple simulations are summarized). Hence, this model form can support projections of the intestinal antimicrobial concentrations in the treated host populations.
ILLUSTRATIVE EXAMPLES
Host and treatment model
We used a 12-month 300-kg beef steer as a treated host model. BRD (bovine respiratory disease) in feedlot cattle may be treated by either an oral or injectable antimicrobial; the choices in the U.S. include an oral chlortetracycline (CTC) and injectable third-generation cephalosporin ceftiofur [20]. The treatment protocols are: CTC fed in dosage 22 mg per kg of body weight (BW) per day for 5 days; and ceftiofur injected once in a sustained-release formulation in dosage 6·6 mg per kg BW.
Example 1: CTC per os
Model structure
From the framework outlined in Figure 1a, we chose the applicable processes and variables for modeling intestinal concentrations of CTC after oral administration (Figure 1b). We used a corresponding earlier deterministic model for CTC intestinal concentrations in cattle as the start [6]. Following that model, CTC was administered in the cattle's daily feed ration, and ingested by the animal in equal portions hourly during 12-h day-time. The drug underwent abiotic degradation to antimicrobially inactive compounds at the same rate through all segments of the gastrointestinal (GI) tract and other body compartments. The degradation dynamics was exponential decay. A fraction of the drug was absorbed into the central circulation from the upper 1/3 small intestine, distributed to and from tissues, and eliminated via bile to the upper 1/3 small intestine. Thus, the sources of CTC in the small and large intestines were the downward movements of the unabsorbed and biliary excreted drug portions. From the large intestine, a fraction of CTC was continuously excreted with feces. The hourly defecation volume was modeled based on the BW. We extended the model [6] to incorporate that a fraction of CTC may be adsorbed to the digesta or microbiome (reversibly or irreversibly) and be antimicrobially inactive. The model equations are included in the Supplementary Materials. The deterministic model's projections agreed well with the CTC measurements in feces and manure from treated cattle [21–23], as was detailed in [6].
Distributions of variables related to CTC intestinal fate
Our intention was to obtain the distribution of each relevant variable (Fig. 1b) by assembling a set of its empirical estimates, and finding the best-fit distribution model. However, the empirical data appeared scarce. A variable for which only two published estimates were available was assigned a Uniform distribution with the minimum and maximum being the estimates. If only a single estimate was available, the variable was assigned a Uniform on an interval ±0·25 of the estimate (as in [24, 25]).
Distributions assigned to the variables related to CTC intestinal concentrations are summarized in Table 1. The fractions of CTC excreted in bile vs. urine have been mostly studied in laboratory but not food animals [27]. Based on a 0·50 estimate available for cattle [26], the CTC fraction excreted in bile was assigned a Uniform (0·39, 0·64). For the rate of CTC abiotic degradation, which is pH and temperature dependent [6], several studies conducted at the pH range of animal feces and near-physiological temperatures in cattle manure, manure-contaminated soil, or dog urine were located [5, 23, 26, 28, 29]. An approach used in meta-analyses to parameterize population PK models for antimicrobial therapeutic effects [30] was adopted: the mean degradation rate estimates in the experiments were extracted into a dataset (n = 8) (one outlier of 0·2 h was removed); no weighting was applied. The best-fit model was chosen by fitting appropriate models to the dataset using PROC CAPABILITY, SAS® 9·2 software for Windows (SAS Institute Inc., Cary, NC, USA). The best fit was a Beta distribution (0·54, 37·4) (best visual fit; P-value for Anderson–Darling test >0·250 and for χ2 test 0·052, indicating a statistically acceptable but relatively poor fit). Notably, the Beta distribution is used in environmental sciences to model the biodegradation and other kinetic rates bound on the interval [0; 1] [31, 32].
Table 1.
Parameters proposed to be related to the drug intestinal concentrations, and the parameter distributions used to model variability in possible concentrations of CTC in the small and large intestines of beef cattle during the 5-day per os treatment
| Parameter | Definition | Distribution | Data used to derive distribution |
|---|---|---|---|
 |
Rate of CTC abiotic degradation in GI tract and other body parts per hour | Beta (0·54, 37·4) | [5, 23, 26, 28–29] |
 |
Fraction of CTC excreted in bile | Uniform (0·39, 0·64) | ±25% estimate [26] |
 |
Fraction of CTC adsorbed to the digesta in the lower 2/3 of small intestine | Uniform (0·69, 0·89) | [33–34] |
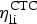 |
Fraction of CTC adsorbed to the digesta in large intestine | Uniform (0·69, 0·89) | [33–34] |
 |
Fraction of CTC adsorbed by the microbiome in the lower 2/3 of small intestinea | – | Not separately included in model simulations |
 |
Fraction of CTC adsorbed by the microbiome in large intestinea | – | Not separately included in model simulations |
 |
Fractional digesta flowb through stomachs to small intestine per hour | Ac: 0·0715 B: 0·0588 |
[35–36] |
 |
Fractional digesta flow through the upper 1/3 of small intestine per hour | A: 0·3333 B: 0·3077 |
[35, 37] |
 |
Fractional digesta flow through the lower 2/3 of small intestine per hour | A: 0·1330 B: 0·1330 |
[35, 37] |
 |
Fractional digesta flow through large intestine per hour (to defecation) | A: 0·1330 B: 0·2222 |
[35] |
| Vrest_si | Volume of digesta in the lower 2/3 of small intestine, litre | Uniform (4, 23) | [38] |
| Vli | Volume of digesta in large intestine, litre | Uniform (6, 22) | [38] |
The animals ingested CTC in equal portions during each 12 h of day time per day of the 5-day therapy. The animals consumed feed and water at similar intervals to the drug.
The values of the parameters relevant for the drug concentrations in the central circulation were kept constant in all simulations, and were as in (Cazer et al. [6]).
A single variable – fraction of CTC adsorbed to the digesta or microbiome – was included in the simulations.
Intestinal transit time of CTC was set as the average of the liquid and solid digesta phases.
Forage scenarios were diets: A – grain based, and B – long-form hay based.
For the fraction of CTC adsorbed to the digesta, we located a study indicating 89% of CTC in spiked sterile rat feces is not bioavailable; this appeared independent of the drug concentration [33]. The digesta is composed of fiber and protein contents; CTC extensively binds to proteins in serum, e.g., 69% on average in dogs [34]. In the absence of other data, the fraction of CTC adsorbed to the digesta and the enteric microbiome in cattle was assigned a Uniform (0·69, 0·89). Since CTC may degrade throughout the GI tract, the net degradation depends on the degradation rate and the transit time. As in the earlier model [6], CTC transit rate (reciprocal of transit time) was set as the average between that of the solid and liquid digesta phases. Because the digesta transit rates are diet-dependent, two comparative diet scenarios were included in the model simulations: based on grain and based on long-form hay. The corresponding rates of digesta intestinal transit adopted from literature [35–37] are summarized in Table 1. The transit time dependency on feed intake was not explicitly included in the simulations.
The small intestine content weight ranges 3–8 kg and the large intestine content weight ranges 3–5 kg in steers (n = 12) with final empty BW 246–302 kg [38]. We estimated the volume to weight ratio of 1·3–2·8 l/kg for fresh fecal pads (n = 32) (the pads were collected at the Kansas State University's beef cattle research facilities). Given the water absorption rates throughout the large intestine [39], the average digesta water content was assumed to be 1·5 times greater than feces water content. This provided the estimated average of 17 l and a Uniform (4, 23) variation of the volume of digesta contents in the lower 2/3 of small intestine below biliary in-flow (approximated as 2/3 of the estimated contents in small intestine). This also provided the estimated average of 11 l, and a uniform (6, 22) variation of the volume of large intestine contents. In the model simulations, CTC consumption and parameter values related to the drug concentration in the central circulation [6] were kept constant. We focused on simulating the influence of variation in the variables related to the CTC intestinal fate (above and Fig. 1b) on the outcome – the concentrations of antimicrobially active CTC, undegraded and unsorbed, in the small and large intestines during the treatment and the intestinal elimination period.
Example 2: cephalosporin ceftiofur by injection
Model structure
From the framework outlined in Figure 1a, we chose the applicable processes and variables for modeling intestinal concentrations of ceftiofur metabolites (Figure 1c). Ceftiofur was taken to be administered parenterally in a sustained-release formulation. It is released from the injection site over 10 days [40] and is rapidly metabolized. Activity of main metabolites is close to the parent drug activity; the total of drug and metabolites is termed ceftiofur equivalents (CE) [41]. We adopted the CE concentration dynamics in the central circulation estimated by the manufacturer [40], and kept those the same in all model simulations. We used as the start an earlier deterministic model for the CE intestinal concentrations in cattle [7]. The source of CE in the cattle intestine was the downward passage after biliary excretion into the upper 1/3 small intestine. The drug is not thought to undergo enterohepatic circulation [42]. The model was extended to incorporate that the metabolites undergo a biotic degradation to inactive compounds by enzymes of enteric bacteria in both the small and large intestines; the biodegradation dynamics was exponential decay [7, 43–45]. We considered but declined inclusion of the CE abiotic degradation: ceftiofur undergoes abiotic degradation in aqueous matters to desfuroylceftiofur, but this has a similar antimicrobial activity to the parent drug [43, 46, 47]. Cephalosporins adsorbed to human feces in vitro are antimicrobially inactive [48, 49]. We considered but declined inclusion of the CE binding to digesta or microbiome in cattle, because this led to a lower agreement between the model's projections and experimental data (see below). Further in the model, a fraction of the CE was continuously excreted with feces. The model equations are included in the Supplementary Materials. Based on the relatively short time post-treatment of the occurrence and the amounts of CE as a fraction of the injected ceftiofur in cattle feces [41], we assumed that CE transited with the liquid digesta phase. For this reason, the diet was not varied for the simulations.
The average projections by the ceftiofur model corresponded well to the CE measurements in recent experiments that implanted fluid chambers in the ileum and at the entrance to the large intestine in calves. The comparisons are approximate because the experimental calves were injected once by a non-sustained-release ceftiofur formulation at dosage 2·2 mg/kg BW [17]; the scenario modeled was of an injection by a sustained-release ceftiofur formulation at dosage 6·6 mg/kg BW. Drug release from the injection site would be more gradual with the sustained-release formulation. Also, the measurements were taken in the beginning of each intestinal segment, than the model projected the average CE concentration throughout the segment. The peak CE concentrations measured in the ileum were approximately 6 µg/ml [17], and the median projected peak CE concentrations in the lower 2/3 of small intestine (after the partial biodegradation) were 4–4·5 µg/ml (Fig. 3a) (also, 6 µg/ml was within the interquartile range of the projections). The peak CE concentrations measured at the entrance to the large intestine were 2–3 µg/ml [17], and the median projected CE concentrations throughout the large intestine (after a further biodegradation) were 1–2 µg/ml (Fig. 3b). The correspondence between the predictions and data has improved with this ceftiofur model formulation (incorporating the metabolite biotic degradation in small intestine and updated estimates of the digesta contents volumes); the earlier deterministic model formulation predicted lower CE concentrations in large intestine [7].
Fig. 3.
Simulated distributions of possible concentrations of antimicrobially active ceftiofur metabolites in (a) small and (b) large intestines of beef steers during a treatment by injection of a sustained-release ceftiofur formulation and the intestinal elimination period, allowing variation in the variables related to the metabolite intestinal fate (1000 model simulations). In the current model the metabolites transit with the liquid digesta phase (no dependency on the host's diet is included).
Distributions of variables related to intestinal fate of ceftiofur metabolites
Distributions assigned to the variables related to the CE intestinal concentrations are summarized in Table 2. Variation in the CE fraction excreted in bile in cattle was parameterized from experimental data [41], a Uniform (0·24, 0·45). Studies of ceftiofur inactivation by cattle fecal bacteria and of ceftriaxone (a structurally close cephalosporin) inactivation in human feces [50, 51] suggest anaerobes Bacteroides and Bifidobacteria, and in cattle also Bacilli, may be prominent contributors of the degradation enzymes. Since hosts vary in the composition of enteric bacteria [52–54], the CE biodegradation rate may have inter-individual variation. Indeed, the ceftriaxone fraction degraded by enzymatic preparations from human feces varies by donor, and for a donor by day [55]. For the biodegradation in cattle, we located three studies. The first plotted the time dynamics of ceftiofur loss in spiked cattle feces following initial sorption [44]. The second estimated ceftiofur inactivation in cultures of 21 strains of four bacterial genera [56], and the third of 71 strains of 17 genera [51]. The inactivation of ceftiofur varied significantly by bacterial strain. We assembled a dataset of the biodegradation rates by bacterial genera and strains, and the one for total in feces (n = 93). We considered the unweighted data and also weighting the estimates by reported relative abundance of the bacterial phyla in cattle feces [53]. For either of the datasets, we were unable to identify an acceptably fit distribution model (e.g., P-value for Anderson–Darling test >0·05, using PROC CAPABILITY in SAS® 9·2 software). Therefore, based on a 0·20 estimate of the total ceftiofur degradation rate in spiked cattle feces [7, 44, 45], and considering that all cattle would have some enteric bacteria producing the degradation enzymes, the CE hourly biodegradation rate in the intestine was assigned a Uniform (0·15, 0·25).
Table 2.
Parameters proposed to be related to the metabolite intestinal concentrations, and the parameter distributions used to model variability in possible concentrations of antimicrobially active ceftiofur metabolites in the small and large intestines of cattle treated with ceftiofur parenterally
| Parameter | Definition | Distribution | Data used to derive distribution |
|---|---|---|---|
 |
Rate of ceftiofur metabolites biotic degradation in small and large intestines per hour (possible dependency of β-lactamase production by enteric bacteria on the CE concentration was not included) | Uniform (0·15, 0·25) | [44–45] |
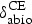 |
Rate of metabolites abiotic degradation in GI tract through hydrolysis | – | Antimicrobial activity of main product is close to parent drug |
 |
Fraction of metabolites excreted in bile | Uniform (0·24, 0·45) | [1] |
 |
Fraction of metabolites adsorbed to the digesta in the lower 2/3 of small intestine | – | Not included in model simulations |
 |
Fraction of metabolites adsorbed to the digesta in large intestine | – | Not included in model simulations |
 |
Fractional digesta flow through the upper 1/3 of small intestine per hour | 0·3237 | [35] |
 |
Fractional digesta flow through the lower 2/3 of small intestine per hour | 0·1619 | [35] |
 |
Fractional digesta flow through large intestine per hour (to defecation) | 0·3030 | [35] |
 |
Volume of digesta in the lower 2/3 of small intestine, litre | Uniform (4, 23) | [38] |
| Vli | Volume of digesta in large intestine, litre | Uniform (6, 22) | [38] |
Deterministic estimates of the dynamics of ceftiofur metabolites in the central circulation published by the drug manufacturer were used in all simulations. Intestinal transit time of ceftiofur metabolites was as for the liquid digesta phase.
We could not locate an estimate of CE sorption to cattle digesta or feces. Two reports of sorption of five cephalosporins to human feces (48, 49) experimented with higher drug concentrations (62–1000 µg/ml) than those in cattle intestine (<10 µg/ml) [17]. Because of non-linear relationships between the concentration and sorption in the reports [48, 49], an extrapolation to the lower range of concentrations was not attempted. Further in the model, the CE excreted in bile were taken to transit with the liquid digesta phase at the rates summarized in Table 1. Volumes of the small and large intestine contents were modeled as above for CTC. We simulated the influence of variation in the variables related to the CE intestinal fate (above and Fig. 1c) on the outcome – the CE concentrations, undegraded (and assumed to remain unsorbed) antimicrobially active ceftiofur metabolites, in the small and large intestines during the treatment and the intestinal elimination period.
Simulation and analysis of the models
Each model was simulated 1000 times in Vensim® Professional software (Ventana Systems, Inc; Harvard, MA, USA), with Latin Hypercube Sampling [57] of the value of each variable related to the drug/metabolite intestinal fate from the assumed distribution, except for the digesta transit rates that were explicitly defined for the two diet scenarios for the CTC model. Statistical analysis of the simulated outputs was done using PROC REG in SAS® 9·2 software. The strength of a variable's influence upon the active drug/metabolite concentration in the small or large intestine was inferred based on (i) significance of the correlation between the variable value and the maximum concentration during treatment (Spearman correlation coefficient's P-value ⩽0·01), after accounting for variability in that concentration due to influence of the other relevant variables; and (ii) fractional contribution of the variable to the maximum concentration's variance (adjusted R2 statistics from a linear regression of the maximum drug/metabolite concentration on the variables). The figures were made in SigmaPlot™ (Systat Software, San Jose, CA, USA) and Microsoft Office Power Point® 2013 (Microsoft, Redmond, WA, USA) software.
RESULTS AND DISCUSSION
The list of variables relevant for the active intestinal concentrations differed between the oral and parenteral antimicrobials considered (Fig. 1b vs. c). The limited current knowledge of the variables’ values led to relatively wide projections of the possible selective pressures on enteric bacteria of treated animals (Figs 2 and 3), highlighting the importance of those variables. Several variables were relevant for both the antimicrobials and associated administration routes; however, the possible strength of influence of individual variables upon the active drug/metabolite concentration in the large intestine varied (Tables 3–6).
Fig. 2.
Simulated distributions of possible antimicrobially active CTC concentrations in the intestines of beef steers during a 5-day oral CTC treatment and the intestinal elimination period, allowing variation in the variables related to the drug intestinal fate (1000 model simulations). Concentrations in (a) small and (b) large intestines with a grain-based diet. Concentrations in (c) small and (d) large intestines with a hay-based diet.
Table 3.
Parameters associated with the maximum concentrations of CTC in the lower 2/3 of small intestines of beef cattle during the 5-day per os treatment
| Parameter | Definition | Distribution | Fractional contribution to the variance of maximum CTC concentrations | ρ between the variable value and maximum CTC concentration, given variability due to all other variables related to CTC intestinal fate |
|---|---|---|---|---|
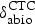 |
Rate of CTC abiotic degradation in GI tract and other body parts per hour | Beta (0·54, 37·4) | A: 7% B: 9% |
A: −0·30, P-value ⩽ 0·01 B: −0·34, P-value ⩽ 0·01 |
 |
Fraction of CTC excreted in bile | Uniform (0·39, 0·64) | A: 0·08% B: 0·38% |
A: 0·02, P-value = 0·566 B: 0·04, P-value = 0·159 |
 |
Fraction of CTC adsorbed to the digesta or microbiome in the lower 2/3 of small intestine | Uniform (0·69, 0·89) | A: 21% B: 14% |
A: −0·54, P-value ⩽ 0·01 B: −0·43, P-value ⩽ 0·01 |
| Vrest_si | Volume of digesta in the lower 2/3 of small intestine, litre | Uniform (4, 23) | A: 54% B: 50% |
A: −0·78, P-value ⩽ 0·01 B: −0·77, P-value ⩽ 0·01 |
The model was simulated 1000 times, assuming the animals consumed: A – grain-based diet, or B – long-form hay-based diet. The model outputs were subjected to the statistical analyses. The maximum CTC concentration was projected at hour 115 since the start of the 5-day treatment. ρ, Spearman correlation coefficient. Because of the scarcity of data for model parameterization, these results should be interpreted as a hypothesis.
Table 4.
Parameters associated with the maximum concentrations of CTC in the large intestines of beef cattle during the 5-day per os treatment
| Parameter | Definition | Distribution | Fractional contribution to the variance of maximum CTC concentrations | ρ between the variable value and maximum CTC concentration, given variability due to all other variables related to CTC intestinal fate |
|---|---|---|---|---|
 |
Rate of CTC abiotic degradation in GI tract and other body parts per hour | Beta (0·54, 37·4) | A: 15% B: 18% |
A: −0·42, P-value ⩽ 0·01 B: −0·47, P-value ⩽ 0·01 |
 |
Fraction of CTC excreted in bile | Uniform (0·39, 0·64) | A: 0% B: 0% |
A: 0, P-value = 0·972 B: 0, P-value = 0·576 |
 |
Fraction of CTC adsorbed to the digesta or microbiome in large intestine | Uniform (0·69, 0·89) | A: 25% B: 23% |
A: −0·54, P-value ⩽ 0·01 B: −0·53, P-value ⩽ 0·01 |
| Vli | Volume of digesta in large intestine, litre | Uniform (6, 22) | A: 44% B: 43% |
A: −0·67, P-value ⩽ 0·01 B: −0·65, P-value ⩽ 0·01 |
The model was simulated 1000 times, assuming the animals consumed: A – grain-based diet, or B – long-form hay-based diet. The model outputs were subjected to the statistical analyses. The maximum CTC concentration was projected at hour 119 since the start of the 5-day treatment. ρ, Spearman correlation coefficient. Because of the scarcity of data for model parameterization, these results should be interpreted as a hypothesis.
Table 5.
Parameters associated with the maximum concentrations of antimicrobially active ceftiofur metabolites in the lower 2/3 of small intestines of beef cattle administered ceftiofur parenterally
| Parameter | Definition | Distribution | Fractional contribution to the variance of maximum CE concentrations | ρ between the variable value and maximum CE concentration, given variability due to all other variables related to CE intestinal fate |
|---|---|---|---|---|
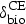 |
Rate of ceftiofur metabolites biotic degradation in small and large intestines per hour | Uniform (0·15, 0·25) | 7% | −0·31, P-value ⩽ 0·01 |
 |
Fraction of metabolites excreted in bile | Uniform (0·24, 0·45) | 8% | 0·32, P-value ⩽ 0·01 |
 |
Volume of digesta in the lower 2/3 of small intestine, litre | Uniform (4, 23) | 62% | −0·85, P-value ⩽ 0·01 |
The model was simulated 1000 times, assuming the metabolites passed through intestine with liquid digesta phase. The model outputs were subjected to the statistical analyses. CE – total of ceftiofur and its antimicrobial active metabolites. The maximum CE concentration was projected at hour 14 post-injection. ρ, Spearman correlation coefficient. Because of the scarcity of data for model parameterization, these results should be interpreted as a hypothesis.
Table 6.
Parameters associated with the maximum concentrations of antimicrobially-active ceftiofur metabolites in the large intestines of beef cattle administered ceftiofur parenterally
| Parameter | Definition | Distribution | Fractional contribution to the variance of maximum CE concentrations | ρ between the variable value and maximum CE concentration, given variability due to all other variables related to CE intestinal fate |
|---|---|---|---|---|
 |
Rate of ceftiofur metabolites biotic degradation in small and large intestines per hour | Uniform (0·15, 0·25) | 23% | −0·50, P-value ⩽ 0·01 |
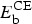 |
Fraction of metabolites excreted in bile | Uniform (0·24, 0·45) | 14% | 0·41, P-value ⩽ 0·01 |
| Vli | Volume of digesta in large intestine, litre | Uniform (6, 22) | 53% | −0·76, P-value ⩽ 0·01 |
The model was simulated 1000 times, assuming the metabolites passed through intestine with liquid digesta phase. The model outputs were subjected to the statistical analyses. CE – total of ceftiofur and its antimicrobial active metabolites. The maximum CE concentration was projected at hour 17 post-injection. ρ, Spearman correlation coefficient. Because of the scarcity of data for model parameterization, these results should be interpreted as a hypothesis.
The maximum projected CTC concentration in the small intestine was at hour 115 (Fig. 2a, c) and in the large intestine at hour 119 (Fig. 2b, d) of the peroral 5-day treatment irrespectively of the diet. The statistical analyses were performed for the CTC concentrations assuming a grain-based diet or a long-form hay-based diet. With the grain-based diet, variation in the CTC fraction sorbed to the digesta or microbiome, the CTC abiotic degradation, and the luminal contents volume contributed to the variance of the CTC concentration in the small intestine at its maximum hour during treatment (Table 3). The contribution of variation in the CTC fraction excreted in bile was negligible. The magnitude of CTC concentration at its maximum hour in the small intestine was negatively correlated (beyond variability due to influence of the other relevant variables) with the CTC abiotic degradation rate, the CTC fraction bound to digesta or microbiome, and the luminal contents volume (Table 3). The results were similar for the CTC concentration at its maximum in the large intestine, except for the CTC abiotic degradation rate in the animal body (Table 4). The degradation rate stronger influenced the CTC concentration reaching the large intestine (a 15% contribution to the variance in the maximum concentration in the large compared with a 7% in the small intestines). The relative influence of the variables on the antimicrobially active CTC concentrations in the small and large intestines were similar in the model simulations assuming a hay-based diet (Tables 3–4). The simulated distributions showed that a longer digesta transit via the upper GI with a hay-based diet may result in a greater degradation, and hence lower CTC concentrations in the small (Fig. 2a vs. c) and especially in the large (Fig. 2b vs. d) intestines, compared with those with a grain-based diet.
The maximum projected CE concentration in the small intestine was at hour 14 and in the large intestine at hour 17 post-injection of the sustained-release ceftiofur formulation (Fig. 3a, b). Variation in the CE fraction excreted in bile, the rate of CE biotic (enzymatic) degradation in intestines, and the luminal contents volume significantly contributed to the variance of the maximum CE concentration in the small intestine (Table 5). The magnitude of CE concentration at its maximum hour in the small intestine was positively correlated (beyond variability due to influence of the other relevant variables) with the drug fraction excreted in bile, and negatively correlated with the CE biotic degradation rate and the luminal contents volume (Table 5). The results were overall similar for the CE concentration at its maximum in the large intestine (Table 6). However, first, the rate of CE biotic degradation throughout the intestines stronger influenced the CE concentration reaching this lower intestinal segment (a 23% contribution to the variance in the maximum CE concentration in the large compared with a 7% in the small intestines). Also, the dilution effect of the digesta contents volume was comparatively stronger on the maximum CE concentration in the small than in the large intestine (a 62% contribution to the variance in the maximum concentration in the small compared with a 53% in the large intestines).
Here we discuss the relevance and implications of some of the key assumptions made in the illustrative models. First, the models explicitly incorporated the digesta transit time. The time is specific to the host species and size. For a given animal species and size (age), and a given drug formulation and its administration route, the antimicrobial intestinal transit time will vary with the water consumption, feed provided (source, processing, roughage), and feed intake. The relative timing of the feed and oral drug consumption or parenteral administration will further affect the drug transit time. The daily defecation pattern (drug excreted out of intestine) was also incorporated in the models (assumed continuous for cattle). The pattern is also specific to the animal species, age, and in some cases diet. In addition to the transit time, digesta composition can affect the drug's sorption to the digesta, abiotic degradation rate (due to its dependency on the chemical conditions such as pH), and potentially its biodegradation rate and sorption to the microbiome (these two may depend on the microbiome structure, which is influenced by the diet [53, 58]). These latter diet-specific dependencies were not included.
Second, in our illustrative simulations, we assumed that the drug/metabolite sorbed to the digesta or microbiome was antimicrobially inactive. The activity loss, complete or partial, may vary between drugs depending on the sorption mechanism, and in some cases the sorbent's structure. Activity of tetracycline against susceptible Gram-negative bacilli depends on the type of soil to which the drug is or was bound if re-suspended [59, 60]. Partial activity of soil-bound tetracycline is higher when combined with agitated, dynamic bacterial culture [60]. Similarly, digesta motion in the intestine could create opportunities for exposure of the luminal bacteria to antimicrobials sorbed onto the digesta or facilitate desorption. Third, a feedback may exist between the CE concentration and their biodegradation due to an upregulation of bacterial genes encoding the enzyme production [61]; this was not incorporated in the ceftiofur model due to scarcity of related data.
In the illustrative examples, we included inter-individual variation in the variables’ values related to the intestinal antimicrobial concentrations. Including this variation could enable the host population-level projections of the concentrations. The distributions of inter-individual variation in relevant PK variables could be estimated using in vivo experimental studies and the methods of population PK [30, 62, 63]. The other relevant variables need to be evaluated via alternative approaches. For example, variability in a drug's abiotic degradation in animal digesta could be evaluated via in vitro experiments, or a meta-analysis of the experimental data (e.g., as above for derivation of the distribution of CTC abiotic degradation rate). The proposed models can be used to adjust the estimates of animal- or herd-level antimicrobial drug use to project the active antimicrobial concentrations to which enteric bacteria in the treated animal populations are exposed (Y. T. Gröhn, C. Carson, C. Lanzas, L. Pullum, M. J. Stanhope, V. Volkova. Animal Health Research Reviews. 2017 Accepted). This could support evaluations of how antimicrobial drug use practices affect resistance in foodborne bacteria.
In this manuscript, we drafted the lists of processes and variables related to the active concentrations in the host's intestine of antimicrobial drug (inclusive of its active metabolites) administered orally or parenterally. In short, these include the drug's fractions excreted in bile or via intestinal wall and re-absorbed, the drug's abiotic and biotic degradation in the intestine, the rates and fractions of the drug's sorption to the digesta and enteric microbiome, as well as the digesta contents volume and passage time. As we illustrate here with peroral CTC and injected cephalosporin ceftiofur in cattle, the most influential variables will differ among antimicrobial drug classes and routes of administration. For certain drugs, there will also be dependency of the drug's activity on the chemical and aeration conditions in the intestine. This exercise highlighted the significance of current ignorance about the processes and variables related to the intestinal fate and concentrations of antimicrobials.
ACKNOWLEDGEMENTS
V.V.V. is supported via the Kansas Bioscience Authority funding for the Institute of Computational Comparative Medicine, Kansas State University. The authors thank Dr James Drouillard, Dr Charley Cull, Andrea Stallbaumer, and Christian Muller for help with the samples for estimating the weight to volume ratio of fresh beef cattle feces. C.L.C. was supported by Pfizer Inc. and Albert C. Bostwick Foundation through the Leadership Program for Veterinary Scholars, Cornell University College of Veterinary Medicine. Y.T.G. was supported by the USDA NIFA, under award number 2010-51110-21083.
DECLARATION OF INTEREST
None to declare.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S095026881700084X.
click here to view supplementary material
REFERENCES
- 1.Salyers AA, Gupta A, Wang Y. Human intestinal bacteria as reservoirs for antibiotic resistance genes. Trends in Microbiology 2004; 12(9): 412–416. [DOI] [PubMed] [Google Scholar]
- 2.Boerlin P, Reid-Smith RJ. Antimicrobial resistance: its emergence and transmission. Animal Health Research Reviews 2008; 9 (Special Issue 02): 115–126. [DOI] [PubMed] [Google Scholar]
- 3.Call DR, et al. Do antibiotic residues in soils play a role in amplification and transmission of antibiotic resistant bacteria in cattle populations? Frontiers in Antimicrobials, Resistance and Chemotherapy 2013; 4: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aust MO, et al. Distribution of sulfamethazine, chlortetracycline and tylosin in manure and soil of Canadian feedlots after subtherapeutic use in cattle. Environmental Pollution 2008; 156(3): 1243–1251. [DOI] [PubMed] [Google Scholar]
- 5.Carlson JC, Mabury SA. Dissipation kinetics and mobility of chlortetracycline, tylosin, and monensin in an agricultural soil in Northumberland County, Ontario, Canada. Environmental Toxicology and Chemistry 2006; 25(1): 1–10. [DOI] [PubMed] [Google Scholar]
- 6.Cazer CL, Volkova VV, Grohn YT. Use of pharmacokinetic modeling to assess antimicrobial pressure on enteric bacteria of beef cattle fed chlortetracycline for growth promotion, disease control, or treatment. Foodborne Pathogens and Disease 2014; 11(5): 403–411. [DOI] [PubMed] [Google Scholar]
- 7.Volkova VV, et al. Mathematical model of plasmid-mediated resistance to ceftiofur in commensal enteric Escherichia coli of cattle. PLoS ONE 2012; 7(5): e36738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimura T, Higaki K. Gastrointestinal transit and drug absorption. Biological and Pharmaceutical Bulletin 2002; 25(2): 149–164. [DOI] [PubMed] [Google Scholar]
- 9.Yu LX, Amidon GL. A compartmental absorption and transit model for estimating oral drug absorption. International Journal of Pharmaceutics 1999; 186(2): 119–125. [DOI] [PubMed] [Google Scholar]
- 10.Yu LX, Crison JR, Amidon GL. Compartmental transit and dispersion model analysis of small intestinal transit flow in humans. International Journal of Pharmaceutics 1996; 140(1): 111–118. [Google Scholar]
- 11.Giguere S, Prescott JF, Dowling PM, eds. Antimicrobial Therapy in Veterinary Medicine, 5th edn. Ames, IA: John Wiley & Sons Inc., 2013. [Google Scholar]
- 12.Riviere JE. Comparative Pharmacokinetics: Principles, Techniques, and Applications, 2nd edn. Ames, IA: Iowa State University Press: Wiley-Blackwell, a John Wiley and Sons, Inc., 2011. [Google Scholar]
- 13.Toutain PL, et al. Veterinary medicine needs new green antimicrobial drugs. Frontiers in Microbiology 2016; 7: 1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmad A, et al. Pharmacokinetic-pharmacodynamic model to evaluate intramuscular tetracycline treatment protocols to prevent antimicrobial resistance in pigs. Antimicrobial Agents and Chemotherapy 2015; 59(3): 1634–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmad A, et al. Modeling the growth dynamics of multiple Escherichia coli strains in the pig intestine following intramuscular ampicillin treatment. BMC Microbiology 2016; 16(1): 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanwar N, et al. Impact of treatment strategies on cephalosporin and tetracycline resistance gene quantities in the bovine fecal metagenome. Scientific Reports 2014; 4: 5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster DM, et al. Pharmacokinetics of enrofloxacin and ceftiofur in plasma, interstitial fluid, and gastrointestinal tract of calves after subcutaneous injection, and bactericidal impacts on representative enteric bacteria. Journal of Veterinary Pharmacology and Therapeutics 2016; 39(1): 62–71. [DOI] [PubMed] [Google Scholar]
- 18.Haruta S, et al. Prediction of plasma concentration-time curve of orally administered theophylline based on a scintigraphic monitoring of gastrointestinal transit in human volunteers. International Journal of Pharmaceutics 2002; 233(1–2): 179–190. [DOI] [PubMed] [Google Scholar]
- 19.Volkova VV, KuKanich B, Riviere JE. Exploring post-treatment reversion of antimicrobial resistance in enteric bacteria of food animals as a resistance mitigation strategy. Foodborne Pathogens and Disease 2016; 13(11): 610–617. [DOI] [PubMed] [Google Scholar]
- 20.USDA APHIS NAHMS. Beef Feedlot 2011: Part IV: Health and Health Management on U.S. Feedlots with a Capacity of 1000 or More Head Report. (http://www.Aphis.Usda.Gov/Animal_Health/Nahms/Feedlot/Downloads/Feedlot2011/Feed11_Dr_Partiv.Pdf). Accessed 1 June 2016.
- 21.Zhang M, Zhang H. Thermal degradation of chlortetracycline in animal manure and soil. In: Xu J-M, Huang PM, eds. Molecular Environmental Soil Science at the Interfaces in the Earth's Critical Zone. Berlin, Hangzhou, China: Germany jointly with Zhejiang University Press, Springer-Verlag, 2010. [Google Scholar]
- 22.Rumsey TS, Miller RW, Dinius DA. Residue content of beef feedlot manure after feeding diethylstilbestrol, chlortetracycline and ronnel and use of stirofos to reduce population of fly larvae in feedlot manure. Archives of Environmental Contamination and Toxicology 1977; 6(2–3): 203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arikan OA. Degradation and metabolization of chlortetracycline during the anaerobic digestion of manure from medicated calves. Journal of Hazardous Materials 2008; 158(18353545): 485–490. [DOI] [PubMed] [Google Scholar]
- 24.Volkova VV, et al. Evaluating targets for control of plasmid-mediated antimicrobial resistance in enteric commensals of beef cattle: a modelling approach. Epidemiology and Infection 2013; 141(11): 2294–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanzas C, et al. The effect of heterogeneous infectious period and contagiousness on the dynamics of salmonella transmission in dairy cattle. Epidemiology and Infection 2008; 136(11): 1496–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eisner HJ, Wulf RJ. The metabolic fate of chlortetracycline and some comparisons with other tetracyclines. Journal of Pharmacology and Experimental Therapeutics 1963; 142(14076514): 122–131. [PubMed] [Google Scholar]
- 27.Wells RJ. Analytical Laboratories Australian Government. Chlortetracycline. First Draft of Fao Food and Nutrition Paper 41/8. Rome, 1996. (Ftp://Ftp.Fao.Org/Ag/Agn/Jecfa/Vetdrug/41-8-Chlortetracycline.Pdf). Accessed 29 August 2013. [Google Scholar]
- 28.Arikan OA, Mulbry W, Rice C. Management of antibiotic residues from agricultural sources: use of composting to reduce chlortetracycline residues in beef manure from treated animals. Journal of Hazardous Materials 2009; 164(18829164): 483–489. [DOI] [PubMed] [Google Scholar]
- 29.Dolliver H, Gupta S, Noll S. Antibiotic degradation during manure composting. Journal of Environmental Quality 2008; 37(18453444): 1245–1253. [DOI] [PubMed] [Google Scholar]
- 30.Craigmill AL, et al. Meta-analysis of pharmacokinetic data of veterinary drugs using the food animal residue avoidance databank: oxytetracycline and procaine penicillin G. Journal of Veterinary Pharmacology and Therapeutics 2004; 27(5): 343–353. [DOI] [PubMed] [Google Scholar]
- 31.Vahatalo AV, Aarnos H, Mantyniemi S. Biodegradability continuum and biodegradation kinetics of natural organic matter described by the beta distribution. Biogeochemistry 2010; 100(1–3): 227–240. [Google Scholar]
- 32.Raschke M. Empirical behaviour of tests for the beta distribution and their application in environmental research. Stochastic Environmental Research and Risk Assessment 2011; 25(1): 79–89. [Google Scholar]
- 33.Bahl MI, et al. In vivo detection and quantification of tetracycline by use of a whole-cell biosensor in the rat intestine. Antimicrobial Agents and Chemotherapy 2004; 48(4): 1112–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pindell MH, et al. Absorption and excretions studies on tetracycline. Journal of Pharmacology and Experimental Therapeutics 1959; 125(4): 287–294. [PubMed] [Google Scholar]
- 35.Shaver RD, et al. Influence of amount of feed intake and forage physical form on digestion and passage of prebloom alfalfa hay in dairy cows. Journal of Dairy Science 1986; 69(6): 1545–1559. [Google Scholar]
- 36.Zebeli Q, et al. Effects of varying dietary forage particle size in two concentrate levels on chewing activity, ruminal mat characteristics, and passage in dairy cows. Journal of Dairy Science 2007; 90(17369233): 1929–1942. [DOI] [PubMed] [Google Scholar]
- 37.Martin C, Philippeau C, Michalet-Doreau B. Effect of wheat and corn variety on fiber digestion in beef steers fed high-grain diets. Journal of Animal Science 1999; 77(8): 2269–2278. [DOI] [PubMed] [Google Scholar]
- 38.Murray DM, Tulloh NM, Winter WH. Effect of three different growth-rates on some offal components of cattle. Journal of Agricultural Science 1977; 89(1): 119–128. [Google Scholar]
- 39.Hecker JF, Grovum WL. Rates of passage of digesta and water absorption along large intestines of sheep, cows and pigs. Australian Journal of Biological Sciences 1975; 28(2): 161–167. [DOI] [PubMed] [Google Scholar]
- 40.Pfizer Animal Health. Graph of plasma concentrations of ceftiofur equivalents following a single injection of a sustained-release ceftiofur formulation in beef (http://www.Excede.Com/Excede.Aspx?Country=Us&Drug=Xt&Species=Bf&Sec=300). Accessed 1 October 2016.
- 41.Beconi-Barker MG, et al. Ceftiofur sodium: absorption, distribution, metabolism, and excretion in target animals and its determination by high-performance liquid chromatography. Veterinary Drug Residues 1996; 636: 70–84. [Google Scholar]
- 42.Bakken JS, Cavalieri SJ, Gangeness D. Influence of plasma exchange pheresis on plasma elimination of ceftriaxone. Antimicrobial Agents and Chemotherapy 1990; 34(6): 1276–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li XL, et al. Degradation kinetics and mechanism of antibiotic ceftiofur in recycled water derived from a beef farm. Journal of Agricultural and Food Chemistry 2011; 59(18): 10176–10181. [DOI] [PubMed] [Google Scholar]
- 44.Gilbertson TJ, et al. Environmental fate of ceftiofur sodium, a cephalosporin antibiotic – role of animal excreta in its decomposition. Journal of Agricultural and Food Chemistry 1990; 38(3): 890–894. [Google Scholar]
- 45.Hornish RE, Kotarski SF. Cephalosporins in veterinary medicine – ceftiofur use in food animals. Current Topics in Medicinal Chemistry 2002; 2(7): 717–731. [DOI] [PubMed] [Google Scholar]
- 46.Sunkara G, Navarre CB, Kompella UB. Influence of pH and temperature on kinetics of ceftiofur degradation in aqueous solutions. Journal of Pharmacy and Pharmacology 1999; 51(3): 249–255. [DOI] [PubMed] [Google Scholar]
- 47.Salmon SA, Watts JL, Yancey RJ. In vitro activity of ceftiofur and its primary metabolite, desfuroylceftiofur, against organisms of veterinary importance. Journal of Veterinary Diagnostic Investigation 1996; 8(3): 332–336. [DOI] [PubMed] [Google Scholar]
- 48.Jansen G, et al. The non-enzymatic inactivation of thirteen beta-lactam antibiotics in human faeces. Infection 1992; 20(6): 355–359. [DOI] [PubMed] [Google Scholar]
- 49.de Vries-Hospers H, et al. The in vitro inactivation of thirteen beta-lactam antibiotics by other mechanisms than adsorption to faecal substance. Infection 1993; 21(2): 127–130. [DOI] [PubMed] [Google Scholar]
- 50.Welling GW, et al. The effect of ceftriaxone on the anaerobic bacterial flora and the bacterial enzymatic activity in the intestinal tract. Infection 1991; 19(5): 313–316. [DOI] [PubMed] [Google Scholar]
- 51.Wagner RD, et al. Bovine intestinal bacteria inactivate and degrade ceftiofur and ceftriaxone with multiple beta-lactamases. Antimicrobial Agents and Chemotherapy 2011; 55(11): 4990–4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berg G, Krause R, Mendes R. Cross-kingdom similarities in microbiome ecology and biocontrol of pathogens. Frontiers in Microbiology 2015; 6: 1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shanks OC, et al. Community structures of fecal bacteria in cattle from different animal feeding operations. Applied and Environmental Microbiology 2011; 77(9): 2992–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yatsunenko T, et al. Human gut microbiome viewed across age and geography. Nature 2012; 486(7402): 222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Welling GW, et al. Inactivation of ceftriaxone by faecal enzyme preparations during ceftriaxone treatment. Journal of Antimicrobial Chemotherapy 1992; 30(2): 234–236. [DOI] [PubMed] [Google Scholar]
- 56.Rafii F, et al. Isolation of bacterial strains from bovine fecal microflora capable of degradation of ceftiofur. Veterinary Microbiology 2009; 139(1–2): 89–96. [DOI] [PubMed] [Google Scholar]
- 57.Mckay MD, Beckman RJ, Conover WJ. A comparison of three methods for selecting values of input variables in the analysis of output from a computer code. Technometrics 1979; 21(2): 239–245. [Google Scholar]
- 58.Frese SA, et al. Diet shapes the gut microbiome of pigs during nursing and weaning. Microbiome 2015; 3: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Subbiah M, et al. Beta-lactams and florfenicol antibiotics remain bioactive in soils while ciprofloxacin, neomycin, and tetracycline are neutralized. Applied and Environmental Microbiology 2011; 77(20): 7255–7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chander Y, et al. Antibacterial activity of soil-bound antibiotics. Journal of Environmental Quality 2005; 34(6): 1952–1957. [DOI] [PubMed] [Google Scholar]
- 61.Livermore DM. Beta-lactamases in laboratory and clinical resistance. Clinical Microbiology Reviews 1995; 8(4): 557–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martin-Jimenez T, Riviere JE. Population pharmacokinetics in veterinary medicine: potential use for therapeutic drug monitoring and prediction of tissue residues. Journal of Veterinary Pharmacology and Therapeutics 1998; 21(3): 167–189. [DOI] [PubMed] [Google Scholar]
- 63.Wu H, et al. Use of population pharmacokinetic modeling and Monte Carlo simulation to capture individual animal variability in the prediction of flunixin withdrawal times in cattle. Journal of Veterinary Pharmacology and Therapeutics 2013; 36(3): 248–257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S095026881700084X.
click here to view supplementary material





